Antioxidant Systematic Alteration Was Responsible for Injuries Inflicted on the Marine Blue Mussel Mytilus edulis Following Strontium Exposure
Abstract
1. Introduction
2. Materials and Methods
2.1. Cultivation of Mussels
2.2. Experimental Setup
2.2.1. Simulation of Sr Exposure
2.2.2. Bioaccumulation of Sr in M. edulis
2.2.3. Effects of Sr on Hemolymph Composition in M. edulis
2.2.4. Effects of Sr on Hemocyte Structure and Function in M. edulis
2.2.5. Effects of Sr on Oxidation–Reduction Homeostasis and Antioxidant System Activity in M. edulis
2.3. Statistical Analysis
3. Results
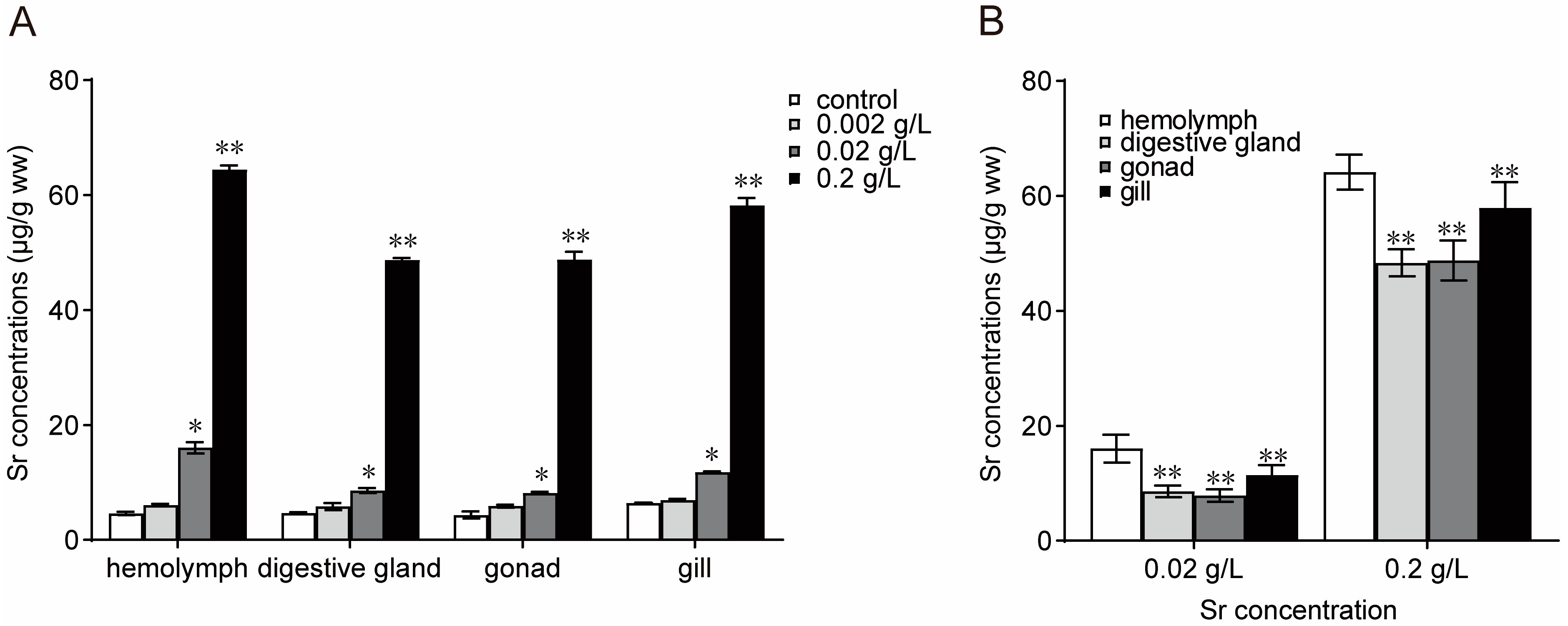
3.1. Bioaccumulation of Sr in M. edulis
3.2. Effects of Sr on Hemolymph Composition in M. edulis
3.3. Effects of Sr on Hemocyte Structure and Function in M. edulis
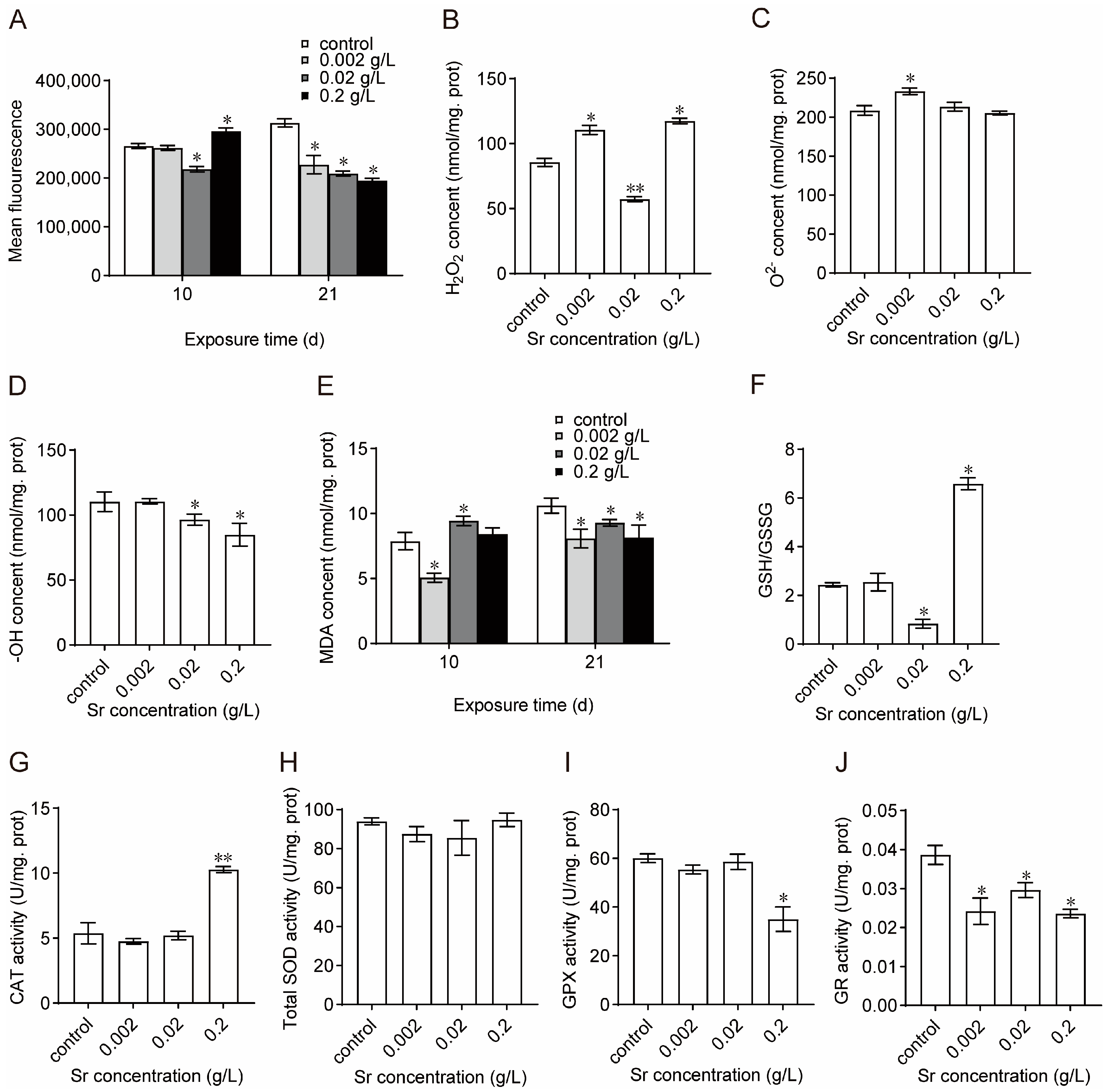
3.4. Effects of Sr on Oxidation–Reduction Homeostasis and Antioxidant System Activity in M. edulis
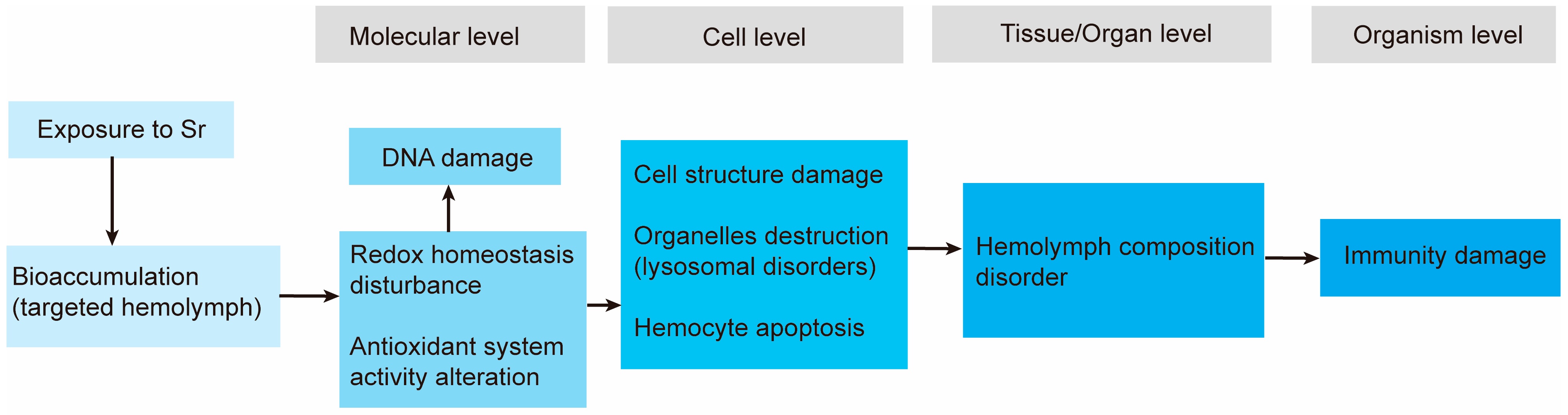
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Behrens, E.; Schwarzkopf, F.U.; Lübbecke, J.F.; Böning, C.W. Model simulations on the long-term dispersal of 137Cs released into the Pacific Ocean off Fukushima. Environ. Res. Lett. 2012, 7, 34004–34013. [Google Scholar] [CrossRef]
- Povinec, P.P.; Hirose, K.; Aoyama, M. Radiostrontium in the Western North Pacific: Characteristics, behavior, and the Fukushima impact. Environ. Sci. Technol. 2012, 46, 10356–10363. [Google Scholar] [CrossRef] [PubMed]
- Hiyama, A.; Nohara, C.; Kinjo, S.; Taira, W.; Gima, S.; Tanahara, A.; Otaki, J.M. The biological impacts of the Fukushima nuclear accident on the pale grass blue butterfly. Sci. Rep. 2012, 2, 570. [Google Scholar] [CrossRef] [PubMed]
- Luckey, T.D. Radiation Hormesis: The Good, the Bad, and the Ugly. Dose-Response 2006, 4, 169–190. [Google Scholar] [CrossRef] [PubMed]
- Bebianno, M.J.; Barreira, L.A. Polycyclic aromatic hydrocarbons concentrations and biomarker responses in the clam Ruditapes decussatus transplanted in the Ria Formosa lagoon. Ecotoxicol. Environ. Saf. 2009, 72, 1849–1860. [Google Scholar] [CrossRef]
- Brugge, D.; Buchner, V. Health effects of uranium: New research findings. Rev. Environ. Health 2011, 26, 231–249. [Google Scholar] [CrossRef] [PubMed]
- Cantillo, A.Y. Comparison of results of Mussel Watch Programs of the United States and France with Worldwide Mussel Watch Studies. Mar. Pollut. Bull. 1998, 36, 712–717. [Google Scholar] [CrossRef]
- Ibarguren, I.; Diaz-Enrich, M.J.; Cao, J.; Fernandez, M.; Barcia, R.; Villamarin, J.A.; Ramos-Martinez, J.I. Regulation of the futile cycle of fructose phosphate in sea mussel. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2000, 126, 495–501. [Google Scholar] [CrossRef]
- Cheng, T.C.; Sullivan, J.T. Effects of heavy metals on phagocytosis by Molluscan hemocytes. Mar. Environ. Res. 1984, 14, 305–315. [Google Scholar] [CrossRef]
- Donaghy, L.; Hong, H.K.; Lambert, C.; Park, H.S.; Shim, W.J.; Choi, K.S. First characterisation of the populations and immune-related activities of hemocytes from two edible gastropod species, the disk abalone, Haliotis discus discus and the spiny top shell, Turbo cornutus. Fish Shellfish Immunol. 2010, 28, 87–97. [Google Scholar] [CrossRef]
- Kimber, I.; Dearman, R.J.; Basketter, D.A.; Boverhof, D.R. Chemical respiratory allergy: Reverse engineering an adverse outcome pathway. Toxicology 2014, 318, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Ankley, G.T.; Bennett, R.S.; Erickson, R.J.; Hoff, D.J.; Hornung, M.W.; Johnson, R.D.; Mount, D.R.; Nichols, J.W.; Russom, C.L.; Schmieder, P.K.; et al. Adverse outcome pathways: A conceptual framework to support ecotoxicology research and risk assessment. Environ. Toxicol. Chem. 2010, 29, 730–741. [Google Scholar] [CrossRef] [PubMed]
- Vinken, M. The adverse outcome pathway concept: A pragmatic tool in toxicology. Toxicology 2013, 312, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Ding, K.; Liu, S.; He, Y.; Yan, D.; Zhang, F.; Wang, S.; Guo, J.; Zhang, W.; Wang, X.; Jiang, X. Simulating the Transfer of Strontium-90 from Soil to Leafy Vegetables by Using Strontium-88. Water Air Soil Pollut. 2016, 227, 414. [Google Scholar] [CrossRef]
- Lai, J.-L.; Luo, X.-G. High-efficiency antioxidant system, chelating system and stress-responsive genes enhance tolerance to cesium ionotoxicity in Indian mustard (Brassica juncea L.). Ecotoxicol. Environ. Saf. 2019, 181, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Tang, X.; Zhou, B.; Wang, Y. Comparative studies on the effects of seawater acidification caused by CO2 and HCl enrichment on physiological changes in Mytilus edulis. Chemosphere 2016, 144, 2368–2376. [Google Scholar] [CrossRef]
- Konovalenko, L.; Bradshaw, C.; Andersson, E.; Lindqvist, D.; Kautsky, U. Evaluation of factors influencing accumulation of stable Sr and Cs in lake and coastal fish. J. Environ. Radioact. 2016, 160, 64–79. [Google Scholar] [CrossRef] [PubMed]
- Goedken, M.; De Guise, S. Flow cytometry as a tool to quantify oyster defence mechanisms. Fish Shellfish Immunol. 2004, 16, 539–552. [Google Scholar] [CrossRef]
- Jiang, Y.; Tang, X.; Sun, T.; Wang, Y. BDE-47 exposure changed the immune function of haemocytes in Mytilus edulis: An explanation based on ROS-mediated pathway. Aquat. Toxicol. 2017, 182, 58–66. [Google Scholar] [CrossRef]
- Hégaret, H.; Wikfors, G.H.; Soudant, P. Flow-cytometric analysis of haemocytes from eastern oysters, Crassostrea virginica, subjected to a sudden temperature elevation: I. Haemocyte types and morphology. J. Exp. Mar. Biol. Ecol. 2003, 293, 237–248. [Google Scholar] [CrossRef]
- Gagnaire, B.; Thomas-Guyon, H.; Burgeot, T.; Renault, T. Pollutant effects on Pacific oyster, Crassostrea gigas (Thunberg), hemocytes: Screening of 23 molecules using flow cytometry. Cell Biol. Toxicol. 2006, 22, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Regoli, F.; Frenzilli, G.; Bocchetti, R.; Annarumma, F.; Scarcelli, V.; Fattorini, D.; Nigro, M. Time-course variations of oxyradical metabolism, DNA integrity and lysosomal stability in mussels, Mytilus galloprovincialis, during a field translocation experiment. Aquat. Toxicol. 2004, 68, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Hervio, D.; Chagot, D.; Godin, P.; Grizel, H.; Mialhe, E. Localisation and characterization of acid phosphate activity in Bonamia ostreae (Ascetospora), an intrahemocytic protozoan parasite of the flat oyster Ostrea edulis (Bivalvia). Dis. Aquat. Org. 1992, 12, 67–70. [Google Scholar] [CrossRef]
- Venier, P.; Maron, S.; Canova, S. Detection of micronuclei in gill cells and haemocytes of mussels exposed to benzo(a)pyrene. Mutat. Res./Genet. Toxicol. Environ. Mutagen. 1997, 390, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Lambert, C.; Soudant, P.; Choquet, G.; Paillard, C. Measurement of Crassostrea gigas hemocyte oxidative metabolism by flow cytometry and the inhibiting capacity of pathogenic vibrios. Fish Shellfish. Immunol. 2003, 15, 225–240. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Zhang, Y.; Cao, S.; Li, Y.; Wang, J.; Dong, H.; Wang, Y. A simulated toxic assessment of cesium on the blue mussel Mytilus edulis provides evidence for the potential impacts of nuclear wastewater discharge on marine ecosystems. Environ. Pollut. 2023, 316 Pt 1, 120458. [Google Scholar] [CrossRef] [PubMed]
- Bervoets, L.; Voets, J.; Covaci, A.; Chu, S.; Qadah, D.; Smolders, R.; Schepens, P.; Blust, R. Use of transplanted Zebra mussels (Dreissena polymorpha) to assess the bioavailability of microcontaminants in Flemish surface waters. Environ. Sci. Technol. 2005, 39, 1492–1505. [Google Scholar] [CrossRef] [PubMed]
- Rainbow, P.S. Trace metal concentrations in aquatic invertebrates: Why and so what? Environ. Pollut. 2002, 120, 497–507. [Google Scholar] [CrossRef]
- Wadige, C.P.M.; Maher, W.A.; Taylor, A.M.; Krikowa, F. Exposure-dose-response relationships of the freshwater bivalve Hyridella australis to cadmium spiked sediments. Aquat. Toxicol. 2014, 152, 361–371. [Google Scholar] [CrossRef]
- Li, J.R.; Li, X.P.; Wang, L.; Duan, Q. Advances in Uptake, Transportation and Bioaccumulation of Heavy Metal Ions in Bivalves. Fish. Sci. 2007, 26, 51–55. [Google Scholar]
- Wang, W.X.; Fisher, N.S. Modeling the influence of body size on trace element accumulation in the mussel Mytilus edulis. Mar. Ecol. Prog. Ser. 1997, 161, 103–115. [Google Scholar] [CrossRef]
- Zhang, B.; Shi, Z.-R.; Wang, X.-L.; Deng, S.-G.; Lin, H.-M. Depuration of cadmium from blue mussel (Mytilus edulis) by hydrolysis peptides and chelating metal elements. Food Res. Int. 2015, 73, 162–168. [Google Scholar] [CrossRef]
- Amachree, D.; Moody, A.J.; Handy, R.D. Comparison of intermittent and continuous exposures to cadmium in the blue mussel, Mytilus edulis: Accumulation and sub-lethal physiological effects. Ecotoxicol. Environ. Saf. 2013, 95, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Wootton, E.C.; Dyrynda, E.A.; Ratcliffe, N.A. Bivalve immunity: Comparisons between the marine mussel (Mytilus edulis), the edible cockle (Cerastoderma edule) and the razor-shell (Ensis siliqua). Fish Shellfish Immunol. 2003, 15, 195–210. [Google Scholar] [CrossRef] [PubMed]
- Estrada, N.; Velázquez, E.; Rodríguez-Jaramillo, C.; Ascencio, F. Morphofunctional study of hemocytes from lions-paw scallop Nodipecten subnodosus. Immunobiology 2013, 218, 1093–1103. [Google Scholar] [CrossRef]
- Le Foll, F.; Rioult, D.; Boussa, S.; Pasquier, J.; Dagher, Z.; Leboulenger, F. Characterisation of Mytilus edulis hemocyte subpopulations by single cell time-lapse motility imaging. Fish Shellfish Immunol. 2010, 28, 372–386. [Google Scholar] [CrossRef]
- Cajaraville, M.P.; Abascal, I.; Etxeberria, M.; Marigómez, I. Lysosomes as cellular markers of environmental pollution: Time- and dose-dependent responses of the digestive lysosomal system of mussels after petroleum hydrocarbon exposure. Environ. Toxicol. Water Qual. 1995, 10, 1–8. [Google Scholar] [CrossRef]
- Moore, C.A.; Beckmann, N.; Morse, M.P. Cytoskeletal structure of diseased and normal hemocytes of Mya arenaria. J. Invertebr. Pathol. 1992, 60, 141–147. [Google Scholar] [CrossRef]
- Dixon, D.R. Marine invertebrate eco-genotoxicology: A methodological overview. Mutagenesis 2002, 17, 495–507. [Google Scholar] [CrossRef]
- A Steinert, S.; Streib-Montee, R.; Leather, J.M.; Chadwick, D.B. DNA damage in mussels at sites in San Diego Bay. Mutat. Res./Fundam. Mol. Mech. Mutagen. 1998, 399, 65–85. [Google Scholar] [CrossRef]
- Wrisberg, M.N.; Rhemrev, R. Detection of Genotoxins in the Aquatic Environment with the Mussel Mytilus edulis. Water Sci. Technol. 1992, 25, 317–324. [Google Scholar] [CrossRef]
- Demirci-Çekiç, S.; Özkan, G.; Avan, A.N.; Uzunboy, S.; Çapanoğlu, E.; Apak, R. Biomarkers of Oxidative Stress and Antioxidant Defense. J. Pharm. Biomed. Anal. 2022, 209, 114477. [Google Scholar] [CrossRef] [PubMed]
- Pires, A.; Almeida, Â.; Calisto, V.; Schneider, R.J.; Esteves, V.I.; Wrona, F.J.; Soares, A.M.; Figueira, E.; Freitas, R. Hediste diversicolor as bioindicator of pharmaceutical pollution: Results from single and combined exposure to carbamazepine and caffeine. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2016, 188, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Sifuentes-Franco, S.; Padilla-Tejeda, D.E.; Carrillo-Ibarra, S.; Miranda-Díaz, A.G. Oxidative Stress, Apoptosis, and Mitochondrial Function in Diabetic Nephropathy. Int. J. Endocrinol. 2018, 2018, 1875870. [Google Scholar] [CrossRef]
- Mauchline, J.; Templeton, W.L. Strontium, Calcium and Barium in Marine Organisms from the Irish Sea. ICES J. Mar. Sci. 1966, 30, 161–170. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, Z.; Xu, M.; Cao, Q.; Chi, W.; Cao, S.; Zhou, Z.; Wang, Y. Antioxidant Systematic Alteration Was Responsible for Injuries Inflicted on the Marine Blue Mussel Mytilus edulis Following Strontium Exposure. Antioxidants 2024, 13, 464. https://doi.org/10.3390/antiox13040464
Cheng Z, Xu M, Cao Q, Chi W, Cao S, Zhou Z, Wang Y. Antioxidant Systematic Alteration Was Responsible for Injuries Inflicted on the Marine Blue Mussel Mytilus edulis Following Strontium Exposure. Antioxidants. 2024; 13(4):464. https://doi.org/10.3390/antiox13040464
Chicago/Turabian StyleCheng, Zihua, Mengxue Xu, Qiyue Cao, Wendan Chi, Sai Cao, Zhongyuan Zhou, and You Wang. 2024. "Antioxidant Systematic Alteration Was Responsible for Injuries Inflicted on the Marine Blue Mussel Mytilus edulis Following Strontium Exposure" Antioxidants 13, no. 4: 464. https://doi.org/10.3390/antiox13040464
APA StyleCheng, Z., Xu, M., Cao, Q., Chi, W., Cao, S., Zhou, Z., & Wang, Y. (2024). Antioxidant Systematic Alteration Was Responsible for Injuries Inflicted on the Marine Blue Mussel Mytilus edulis Following Strontium Exposure. Antioxidants, 13(4), 464. https://doi.org/10.3390/antiox13040464





