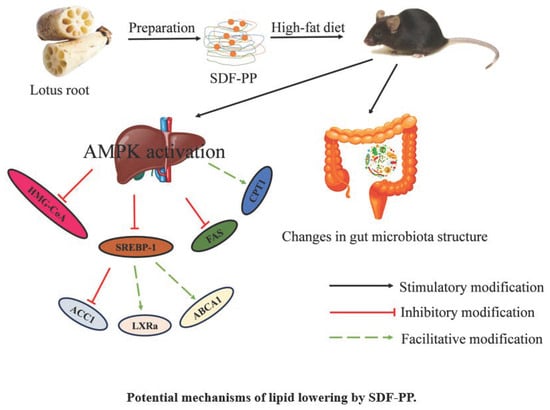
Complexes of Soluble Dietary Fiber and Polyphenols from Lotus Root Regulate High-Fat Diet-Induced Hyperlipidemia in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Animal Experiment Design
2.3. Hematological Analysis
2.4. Determination of Enzyme Activity and Pro-Inflammatory Cytokines in Liver
2.5. Histopathology Analysis
2.6. AMPK Pathway-Related Protein and Gene Expression Assays
2.6.1. Western Blot Analysis
2.6.2. Real-Time Quantitative Polymerase Chain Reaction Analysis
2.7. Gut Microbiota Analysis
2.8. Statistical Analysis
3. Results
3.1. Effects of SDF-PPs Complexes on the Weight Gain, Food Intake, and Organ Indices of HFD Mice
3.2. Effect of SDF-PPs Complexes on the Blood Lipids of HFD Mice
3.3. Oxidative Stress Indicators and Inflammatory Factors of HFD Mouse Liver
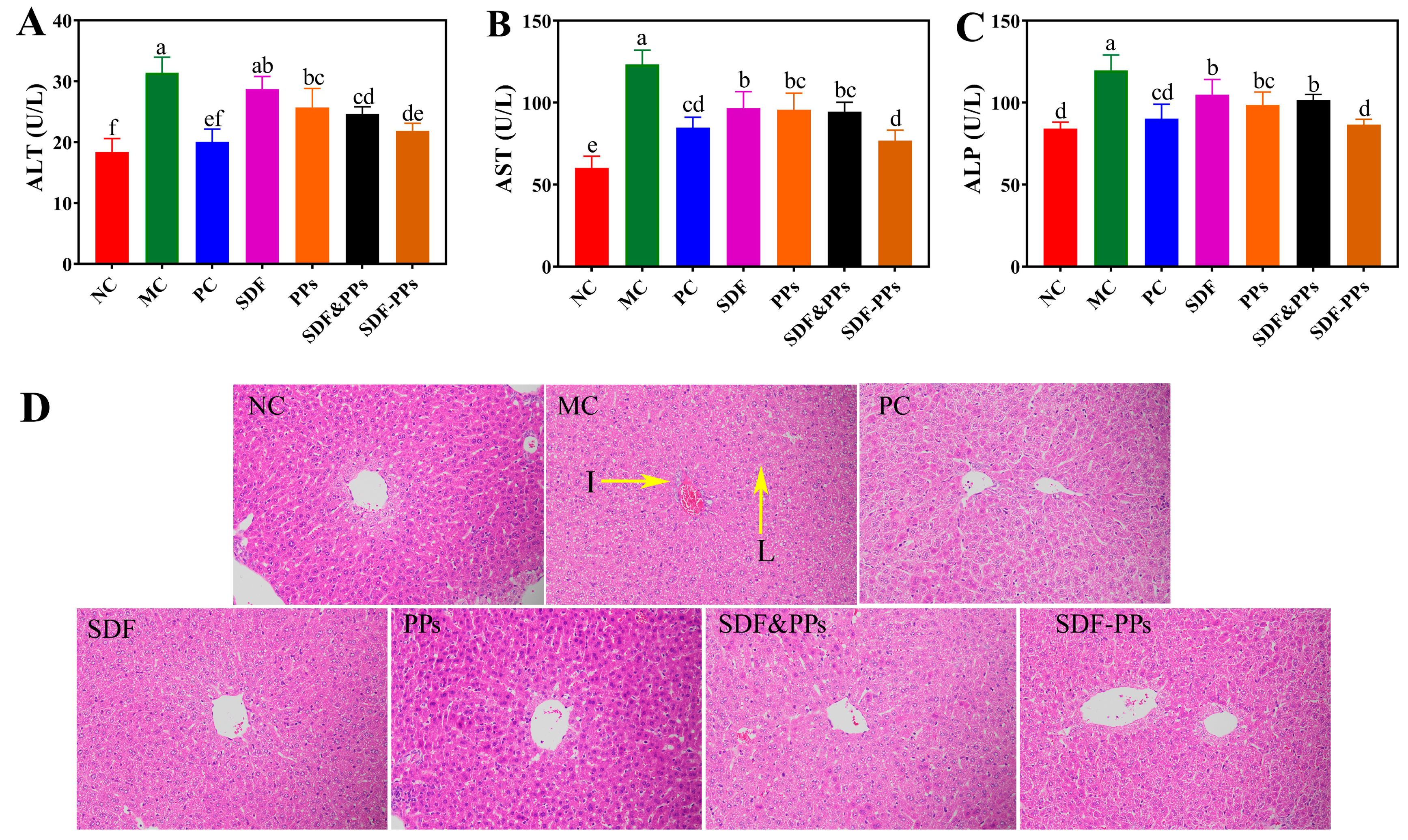
3.4. Enzyme Activities and Histopathologic Analysis of the HFD Mouse Livers
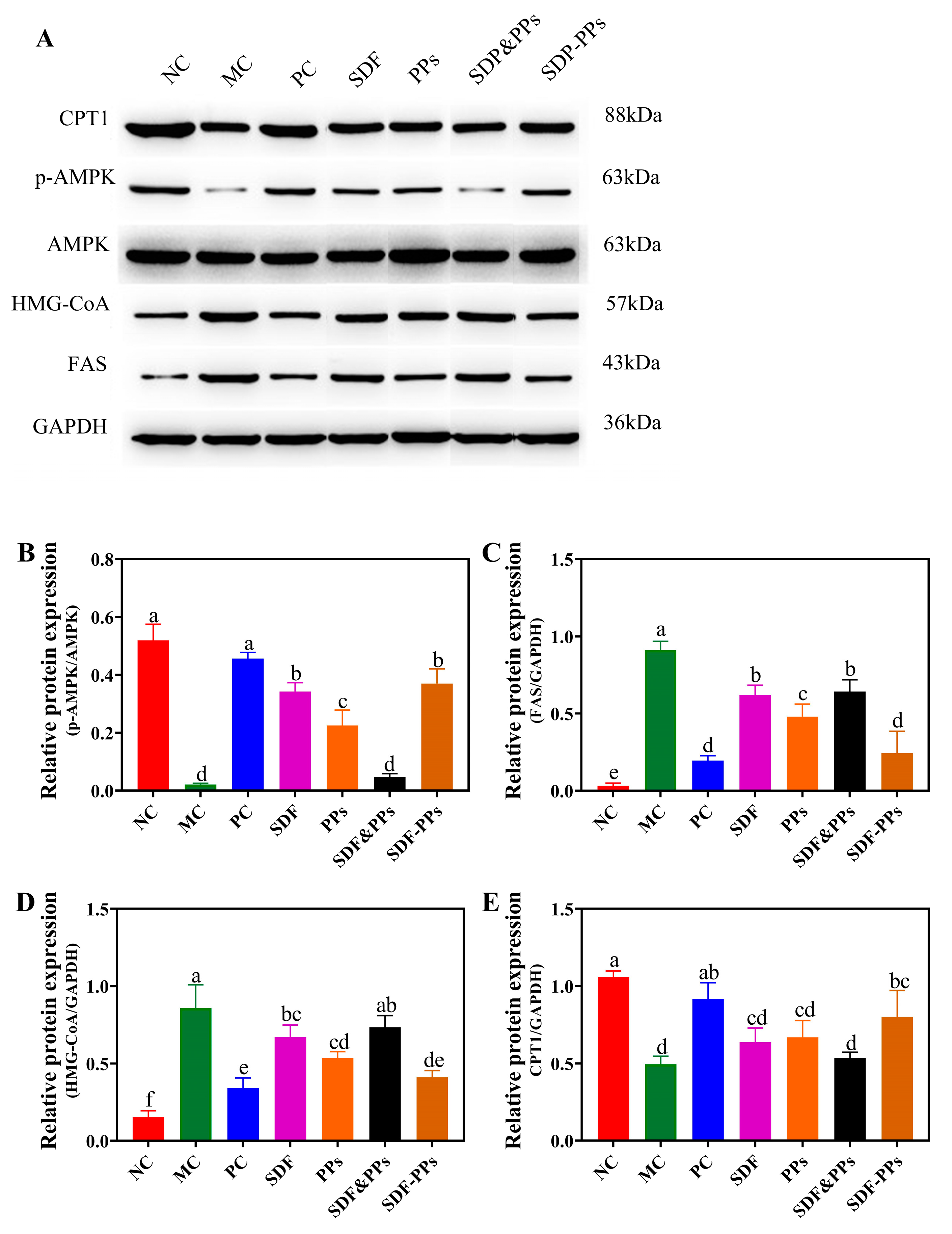
3.5. Effects of SDF-PPs Complexes on AMPK Phosphorylation and Lipid Metabolism in HFD Mice
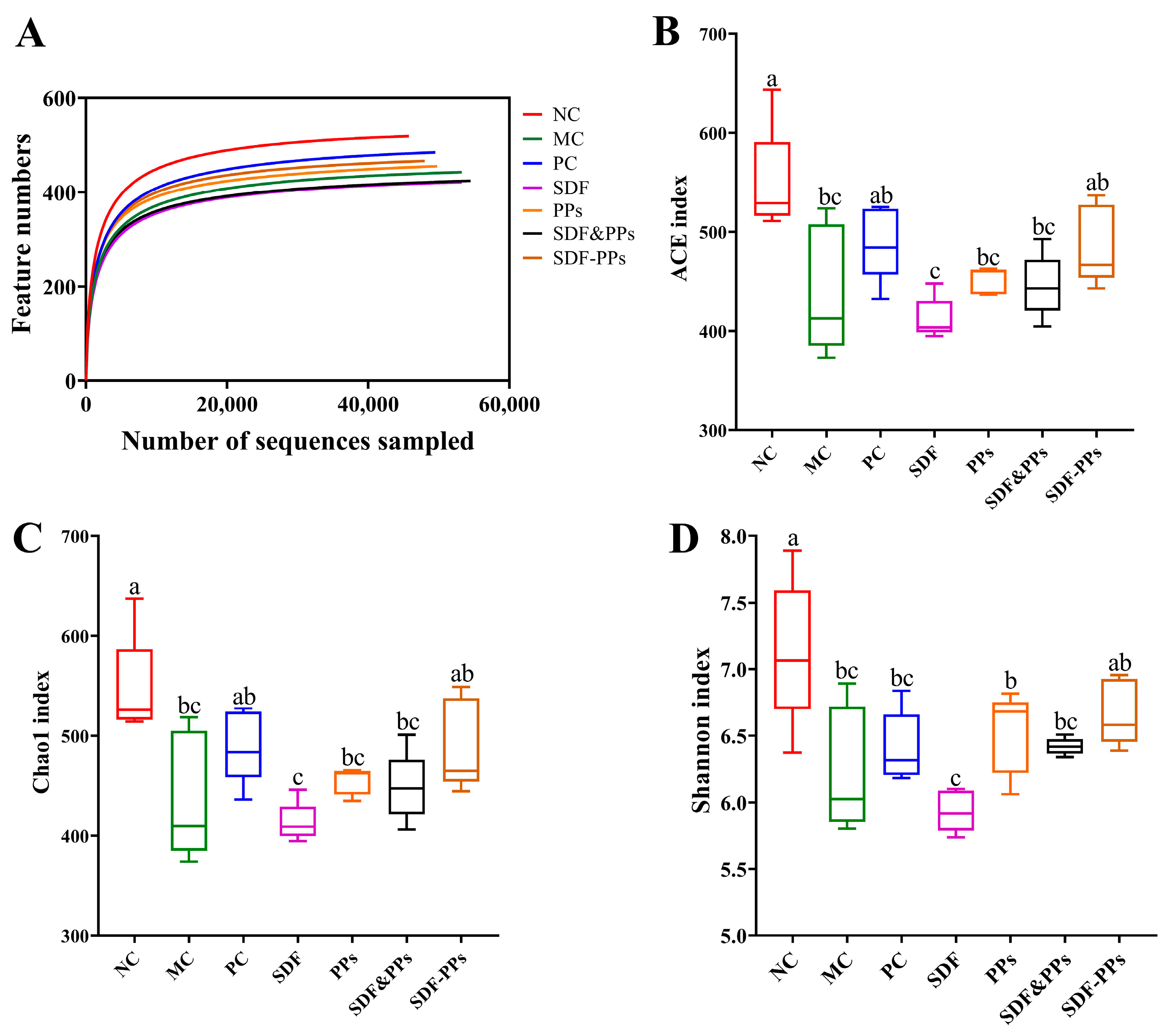
3.6. Gut Microbiology Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, W.; She, L.; Yu, K.; Yan, S.; Zhang, X.; Tian, X.; Ma, S.; Zhang, X. Jatrorrhizine hydrochloride attenuates hyperlipidemia in a high-fat diet-induced obesity mouse model. Mol. Med. Rep. 2016, 14, 3277–3284. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-J.; Lin, C.-S.; Lu, C.-C.; Martel, J.; Ko, Y.-F.; Ojcius, D.M.; Tseng, S.-F.; Wu, T.-R.; Chen, Y.-Y.M.; Young, J.D.; et al. Ganoderma lucidum reduces obesity in mice by modulating the composition of the gut microbiota. Nat. Commun. 2015, 6, 7489. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.Y.; Hong, M.H.; Kim, K.W.; Yoon, J.J.; Lee, J.E.; Kang, D.G.; Lee, H.S. Improvement of Hypertriglyceridemia by Roasted Nelumbinis folium in High Fat/High Cholesterol Diet Rat Model. Nutrients 2020, 12, 3859. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Yu, S.; Liu, J.; Zhou, Y.; Cao, R.; Zhou, Y.; Shi, L.; Du, J. Effects of Lactobacillus on hyperlipidemia in high-fat diet-induced mouse model. Arch. Med. Sci. 2020, 16, 792–799. [Google Scholar] [CrossRef] [PubMed]
- Pan, S.; Gao, R.; Wu, S. Preparation, characterization and hypolipidaemic activity of Astragalus membranaceus polysaccharide. J. Funct. Foods 2017, 39, 264–267. [Google Scholar] [CrossRef]
- Yi, Y.; Sun, J.; Xie, J.; Min, T.; Wang, L.-M.; Wang, H.-X. Phenolic Profiles and Antioxidant Activity of Lotus Root Varieties. Molecules 2016, 21, 863. [Google Scholar] [CrossRef]
- Gu, Y.; Niu, L.; Song, J.; Liu, C.; Zhang, Z.; Liu, C.; Li, D.; Xiao, L. Effect of Pretreatment and High Hydrostatic Pressure on Soluble Dietary Fiber in Lotus Root Residues. J. Food Qual. 2022, 2022, 5565538. [Google Scholar] [CrossRef]
- Jakobek, L.; Matić, P. Non-covalent dietary fiber—Polyphenol interactions and their influence on polyphenol bioaccessibility. Trends Food Sci. Technol. 2019, 83, 235–247. [Google Scholar] [CrossRef]
- Fernandes, A.; Mateus, N.; de Freitas, V. Polyphenol-Dietary Fiber Conjugates from Fruits and Vegetables: Nature and Biological Fate in a Food and Nutrition Perspective. Foods 2023, 12, 1052. [Google Scholar] [CrossRef] [PubMed]
- Herzig, S.; Shaw, R.J. AMPK: Guardian of metabolism and mitochondrial homeostasis. Nat. Rev. Mol. Cell Biol. 2018, 19, 121–135. [Google Scholar] [CrossRef]
- Han, H.-J.; Song, X.; Yadav, D.; Hwang, M.S.; Lee, J.H.; Lee, C.H.; Kim, T.H.; Lee, J.J.; Kwon, J. Ulmus macrocarpa Hance modulates lipid metabolism in hyperlipidemia via activation of AMPK pathway. PLoS ONE 2019, 14, e0217112. [Google Scholar] [CrossRef] [PubMed]
- Han, H.-S.; Lee, H.-H.; Gil, H.-S.; Chung, K.-S.; Kim, J.-K.; Kim, D.-H.; Yoon, J.; Chung, E.K.; Kil Lee, J.; Yang, W.M.; et al. Standardized hot water extract from the leaves of Hydrangea serrata (Thunb.) Ser. alleviates obesity via the AMPK pathway and modulation of the gut microbiota composition in high fat diet-induced obese mice. Food Funct. 2021, 12, 2672–2685. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Huang, H.; Zhang, Y.; Chen, H.; Zhou, X.; He, Y.; Meng, X.; Zhao, X. Effect of Lactobacillus fermentum HFY06 Combined with Arabinoxylan on Reducing Lipid Accumulation in Mice Fed with High-Fat Diet. Oxidative Med. Cell. Longev. 2022, 2022, 1068845. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Zhou, Y.; Qi, G.; Liu, D.; Cao, X.; Yu, J.; Zhang, R.; Lin, W.; Guo, P. Asperlin Stimulates Energy Expenditure and Modulates Gut Microbiota in HFD-Fed Mice. Mar. Drugs 2019, 17, 38. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Zhao, W.; Xie, B.; Liu, H. Effects of Auricularia auricula and its polysaccharide on diet-induced hyperlipidemia rats by modulating gut microbiota. J. Funct. Foods 2020, 72, 104038. [Google Scholar] [CrossRef]
- Anhê, F.F.; Roy, D.; Pilon, G.; Dudonné, S.; Matamoros, S.; Varin, T.V.; Garofalo, C.; Moine, Q.; Desjardins, Y.; Levy, E.; et al. A polyphenol-rich cranberry extract protects from diet-induced obesity, insulin resistance and intestinal inflammation in association with increased Akkermansia spp. population in the gut microbiota of mice. Gut 2015, 64, 872–883. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Zhao, J.; Xu, Z.; Chen, Y.; Shao, T.; Long, X.; Liu, Z.; Gao, X.; Rengel, Z.; Shi, J.; et al. Inulin from Jerusalem artichoke tubers alleviates hyperlipidemia and increases abundance of bifidobacteria in the intestines of hyperlipidemic mice. J. Funct. Foods 2018, 40, 187–196. [Google Scholar] [CrossRef]
- Tsuruta, Y.; Nagao, K.; Kai, S.; Tsuge, K.; Yoshimura, T.; Koganemaru, K.; Yanagita, T. Polyphenolic extract of lotus root (edible rhizome of Nelumbo nucifera) alleviates hepatic steatosis in obese diabetic db/db mice. Lipids Health Dis. 2011, 10, 202. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Li, J.; Zhu, Z.; Cheng, S.; He, J.; Lamikanra, O. Soluble dietary fiber and polyphenol complex in lotus root: Preparation, interaction and identification. Food Chem. 2020, 314, 126219. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Wang, J.; Li, J.; Xiong, L.; Chen, H.; Liu, X.; Wang, N.; Ouyang, K.; Wang, W. Antihyperlipidemic and hepatoprotective activities of polysaccharide fraction from Cyclocarya paliurus in high-fat emulsion-induced hyperlipidaemic mice. Carbohydr. Polym. 2018, 183, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Shen, Y.; Tang, N.; Zhu, J.; Xiao, J.; Cong, R.; Zhang, H.; Wu, G.; Qi, X. Ameliorative Role of Cabernet sauvignon Seed Oil on Hyperlipidemia, Inflammation, and Oxidative Stress in Mice. Eur. J. Lipid Sci. Technol. 2019, 121, 1800454. [Google Scholar] [CrossRef]
- Ta, N.; Erdunduleng, E.; Qi, R.; Mu, X.; Feng, L.; Ba, G.; Li, Y.; Zhang, J.; Bai, L.; Fu, M. Metabolomics analysis reveals amelioration effects of yellowhorn tea extract on hyperlipidemia, inflammation, and oxidative stress in high-fat diet-fed mice. Front. Nutr. 2023, 10, 1087256. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zheng, F.; Li, Z.; Uribarri, J.; Ren, B.; Hutter, R.; Tunstead, J.R.; Badimon, J.; Striker, G.E.; Vlassara, H. Reduced acute vascular injury and atherosclerosis in hyperlipidemic mice transgenic for lysozyme. Am. J. Pathol. 2006, 169, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Kong, Y.; Liang, Y.; Niu, M.; Feng, N.; Zhang, C.; Qi, Y.; Guo, Z.; Xiao, J.; Zhou, M.; et al. Protective mechanism of fruit vinegar polyphenols against AGEs-induced Caco-2 cell damage. Food Chem. X 2023, 19, 100736. [Google Scholar] [CrossRef] [PubMed]
- Roche, H.M. Dietary modulation of energy homoeostasis and metabolic-inflammation. Proc. Nutr. Soc. 2019, 78, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Zhang, J.; Su, Y.; Zhang, X.; Li, J.; Tu, L.; Yu, J.; Zheng, Y.; Wang, M. Monascus vinegar-mediated alternation of gut microbiota and its correlation with lipid metabolism and inflammation in hyperlipidemic rats. J. Funct. Foods 2020, 74, 104152. [Google Scholar] [CrossRef]
- Kim, B.; Woo, M.; Park, C.; Lee, S.; Kim, J.; Kim, B.; An, S.; Kim, S. Hovenia Dulcis Extract Reduces Lipid Accumulation in Oleic Acid-Induced Steatosis of Hep G2 Cells via Activation of AMPK and PPARα/CPT-1 Pathway and in Acute Hyperlipidemia Mouse Model. Phytotherapy Res. 2017, 31, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Lew, L.-C.; Hor, Y.-Y.; Jaafar, M.-H.; Lau, A.-S.; Lee, B.-K.; Chuah, L.-O.; Yap, K.-P.; Azlan, A.; Azzam, G.; Choi, S.-B.; et al. Lactobacillus Strains Alleviated Hyperlipidemia and Liver Steatosis in Aging Rats via Activation of AMPK. Int. J. Mol. Sci. 2020, 21, 5872. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Hu, G.; Li, D.; Sun, M.; Mou, D. Anti-hyperlipidemia effect of sea buckthorn fruit oil extract through the AMPK and Akt signaling pathway in hamsters. J. Funct. Foods 2020, 66, 103837. [Google Scholar] [CrossRef]
- Liu, B.; Yang, T.; Luo, Y.; Zeng, L.; Shi, L.; Wei, C.; Nie, Y.; Cheng, Y.; Lin, Q.; Luo, F. Oat β-glucan inhibits adipogenesis and hepatic steatosis in high fat diet-induced hyperlipidemic mice via AMPK signaling. J. Funct. Foods 2018, 41, 72–82. [Google Scholar] [CrossRef]
- Yang, H.; Xie, J.; Wang, N.; Zhou, Q.; Lu, Y.; Qu, Z.; Wang, H. Effects of Miao sour soup on hyperlipidemia in high-fat diet-induced obese rats via the AMPK signaling pathway. Food Sci. Nutr. 2021, 9, 4266–4277. [Google Scholar] [CrossRef] [PubMed]
- Ren, C.-Y.; Zhang, S.; Hong, B.; Guan, L.-J.; Huang, W.-G.; Feng, J.-R.; Sha, D.-X.; Yuan, D.; Li, B.; Ji, N.-N.; et al. Germinated brown rice relieves hyperlipidemia by alleviating gut microbiota dysbiosis. J. Integr. Agric. 2023, 22, 945–957. [Google Scholar] [CrossRef]
- Wu, C.; Tian, Y.; Yu, J.; Zhang, R.; Zhang, X.; Guo, P. The pandanus tectorius fruit extract (PTF) modulates the gut microbiota and exerts anti-hyperlipidaemic effects. Phytomedicine 2019, 58, 152863. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, X.; Yu, J.; Tan, Y.; Guo, P.; Wu, C. The gut microbiota confers the lipid-lowering effect of bitter melon (Momordica charantia L.) In high-fat diet (HFD)-Induced hyperlipidemic mice. Biomed. Pharmacother. 2020, 131, 110667. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Xu, C.; Liu, H.; Liu, M.; Wang, M.; Jiang, J.; Zhang, G.; Yang, C.; Huang, J.; Lou, Z. Linderae Radix Ethanol Extract Alleviates Diet-Induced Hyperlipidemia by Regulating Bile Acid Metabolism Through gut Microbiota. Front. Pharmacol. 2021, 12, 627920. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, J.; Zhang, Y.; Shi, Y.; Feng, D.; Zuo, Y.; Hu, P. Effect and Correlation of Rosa roxburghii Tratt Fruit Vinegar on Obesity, Dyslipidemia and Intestinal Microbiota Disorder in High-Fat Diet Mice. Foods 2022, 11, 4108. [Google Scholar] [CrossRef] [PubMed]
- Shen, F.; Zhuang, J.; Wang, Q.; Zhang, J.; Huang, Y.; Mo, Q.; Zhao, M.; Wang, J.; Zhong, H.; Feng, F. Enhancement in the metabolic profile of sea buckthorn juice via fermentation for its better efficacy on attenuating diet-induced metabolic syndrome by targeting gut microbiota. Food Res. Int. 2022, 162, 111948. [Google Scholar] [CrossRef] [PubMed]
- Larsen, J.M. The immune response to Prevotella bacteria in chronic inflammatory disease. Immunology 2017, 151, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Deng, Q.; Wang, W.; Zhang, L.; Chen, L.; Zhang, Q.; Zhang, Y.; He, S.; Li, J. Gougunao tea polysaccharides ameliorate high-fat diet-induced hyperlipidemia and modulate gut microbiota. Food Funct. 2023, 14, 703–719. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.-R.; Chou, T.-S.; Huang, C.-Y.; Hsiao, J.-K. A potential probiotic-Lachnospiraceae NK4A136 group: Evidence from the restoration of the dietary pattern from a high-fat diet. Res. Sq. 2020. [Google Scholar] [CrossRef]




| NC | MC | PC | SDF | PPs | SDF&PPs | SDF-PPs | |
|---|---|---|---|---|---|---|---|
| Starting weight (g) | 17.02 ± 1.47 a | 17.18 ± 0.56 a | 17.21 ± 0.78 a | 17.11 ± 1.45 a | 17.16 ± 0.89 a | 17.10 ± 1.21 a | 17.28 ± 0.57 a |
| Final weight (g) | 20.02 ± 0.99 c | 22.11 ± 0.28 a | 20.48 ± 1.00 bc | 21.26 ± 0.95 bc | 21.15 ± 1.17 ab | 20.94 ± 0.63 bc | 20.77 ± 0.99 ab |
| Weight gain (g) | 3.00 ±0.83 c | 4.93 ± 0.71 a | 3.26 ± 0.86 bc | 4.14 ± 0.85 bc | 3.99 ± 0.99 ab | 3.84 ± 0.83 bc | 3.49 ± 0.67 ab |
| Food intake (g/d) | 15.31 ± 1.32 a | 13.76 ± 0.90 b | 13.93 ± 0.73 b | 13.61 ± 1.49 b | 13.10 ± 2.22 b | 13.63 ± 1.31 b | 13.96 ± 1.62 b |
| Energy intake (Kcal/d/g) | 46.59 ± 4.02 a | 54.23 ± 3.56 b | 54.90 ± 2.88 b | 53.70 ± 5.17 b | 53.65 ± 5.85 b | 55.02 ± 6.39 b | 51.61 ± 8.76 b |
| Liver index (%) | 3.30 ± 0.19 c | 4.09 ± 0.19 a | 3.97 ± 0.25 ab | 3.83 ± 0.18 b | 3.79 ± 0.27 ab | 3.71 ± 0.26 c | 3.61 ± 0.29 b |
| NC | MC | PC | SDF | PPs | SDF&PPs | SDF-PPs | |
|---|---|---|---|---|---|---|---|
| Enzyme Activity | |||||||
| TAOC (mM) | 0.46 ± 0.03 a | 0.26 ± 0.05 d | 0.38 ± 0.04 b | 0.29 ± 0.03 cd | 0.36 ± 0.04 b | 0.34 ± 0.03 bc | 0.44 ± 0.04 a |
| GSH-Px (U/mgprot) | 49.52 ± 4.95 a | 34.38 ± 1.72 c | 43.10 ± 2.90 b | 34.55 ± 1.26 c | 37.68 ± 2.74 c | 37.56 ± 2.30 c | 43.51 ± 2.80 b |
| MDA (nmol/mgprot) | 2.96 ± 0.51 d | 7.26 ± 0.33 a | 4.65 ± 0.48 bc | 4.12 ± 0.23 c | 4.14 ± 0.56 c | 5.05 ± 0.44 b | 3.24 ± 0.67 d |
| SOD (U/mgprot) | 9.52 ± 0.54 a | 7.03 ± 0.34 c | 8.52 ± 0.28 b | 6.59 ± 0.53 c | 7.15 ± 0.22 c | 6.70 ± 0.23 c | 8.04 ± 0.37 b |
| AGEs (pg/g) | 2052.70 ± 72.32 e | 4125.66 ± 89.95 a | 2319.23 ± 139.53 d | 3634.31 ± 148.72 b | 3577.50 ± 158.84 b | 3469.02 ± 219.11 b | 3211.87 ± 83.15 c |
| Inflammation Indicators | |||||||
| IL-6 (pg/mg) | 426.06 ± 20.93 d | 1184.50 ± 68.42 a | 471.68 ± 34.60 d | 808.31 ± 72.31 b | 682.77 ± 85.36 c | 652.48 ± 40.11 c | 587.58 ± 74.46 c |
| IL-1β (pg/mg) | 390.03 ± 15.04 e | 1018.36 ± 73.31 a | 417.75 ± 42.04 e | 727.49 ± 30.29 b | 529.49 ± 92.99 cd | 544.45 ± 28.64 c | 455.84 ± 20.80 de |
| TNF-α (pg/mg) | 2610.47 ± 123.48 c | 6626.71 ± 230.65 a | 2828.00 ± 281.35 c | 3805.20 ± 277.90 b | 3877.26 ± 580.97 b | 3812.87 ± 182.58 b | 3387.50 ± 366.31 b |
| TGF-β (pg/mg) | 6610.59 ± 251.67 a | 2559.06 ± 201.59 f | 5327.29 ± 103.40 b | 3875.05 ± 186.15 c | 4511.18 ± 149.53 d | 4556.29 ± 252.70 cd | 4827.86 ± 155.42 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, Z.; Gao, W.; Zhu, Z.; Li, S.; Chen, X.; Cravotto, G.; Sui, Y.; Zhou, L. Complexes of Soluble Dietary Fiber and Polyphenols from Lotus Root Regulate High-Fat Diet-Induced Hyperlipidemia in Mice. Antioxidants 2024, 13, 466. https://doi.org/10.3390/antiox13040466
Zheng Z, Gao W, Zhu Z, Li S, Chen X, Cravotto G, Sui Y, Zhou L. Complexes of Soluble Dietary Fiber and Polyphenols from Lotus Root Regulate High-Fat Diet-Induced Hyperlipidemia in Mice. Antioxidants. 2024; 13(4):466. https://doi.org/10.3390/antiox13040466
Chicago/Turabian StyleZheng, Zhan, Weilan Gao, Zhenzhou Zhu, Shuyi Li, Xueling Chen, Giancarlo Cravotto, Yong Sui, and Lei Zhou. 2024. "Complexes of Soluble Dietary Fiber and Polyphenols from Lotus Root Regulate High-Fat Diet-Induced Hyperlipidemia in Mice" Antioxidants 13, no. 4: 466. https://doi.org/10.3390/antiox13040466
APA StyleZheng, Z., Gao, W., Zhu, Z., Li, S., Chen, X., Cravotto, G., Sui, Y., & Zhou, L. (2024). Complexes of Soluble Dietary Fiber and Polyphenols from Lotus Root Regulate High-Fat Diet-Induced Hyperlipidemia in Mice. Antioxidants, 13(4), 466. https://doi.org/10.3390/antiox13040466