Inflammatory Cytokines and Chemokines Are Synergistically Induced in a ROS-Dependent Manner by a Co-Culture of Corneal Epithelial Cells and Neutrophil-like Cells in the Presence of Particulate Matter
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Cells
2.2. Cell Cultures, Cells’ Differentiation into Neutrophils, and Flow Cytometry
2.3. Holotomography
2.4. Enzyme-Linked Immunosorbent Assay (ELISA)
2.5. Co-Culture HCE-T with HL-60 Using a Transwell
2.6. Measurement of ROS Production
2.7. Western Blotting
2.8. Reporter Assay
2.9. Ultraviolet (UV) Exposure
2.10. Statistics
3. Results
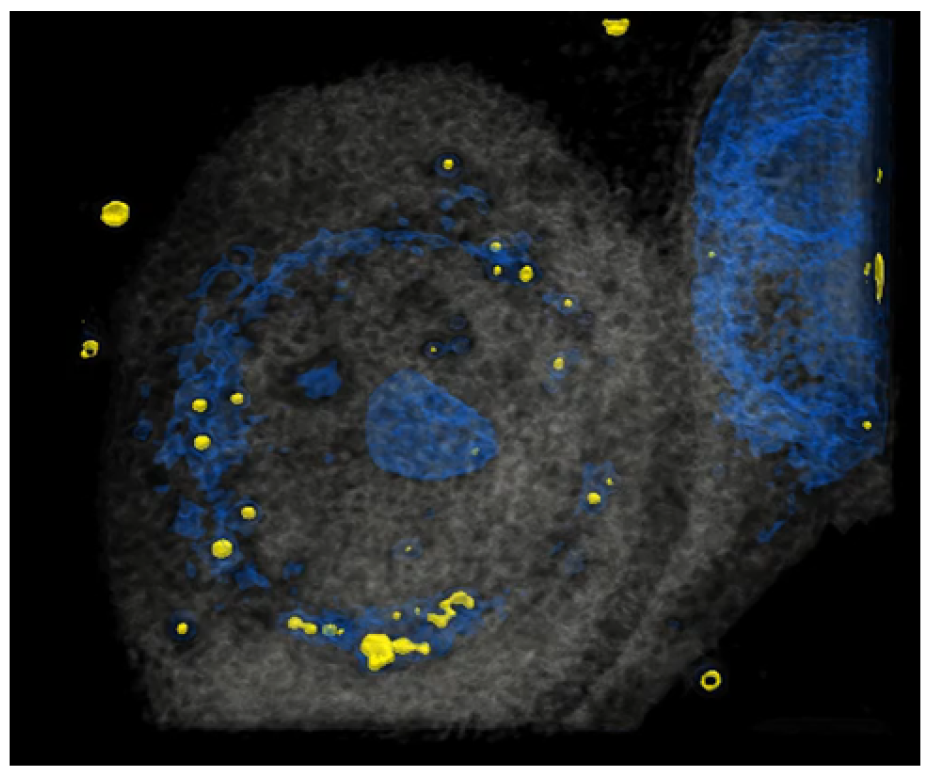
3.1. HCE-T’s Ability to Take Up PM Is Analyzed Using Holotomography Technology
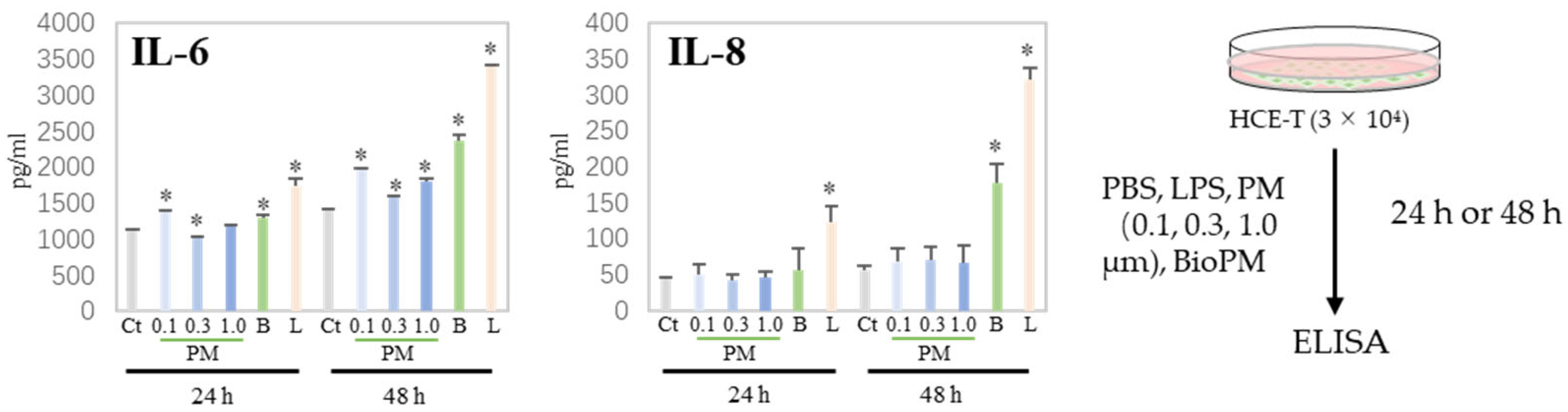
3.2. HCE-T Produces IL-6 and IL-8 Due to PM Treatment
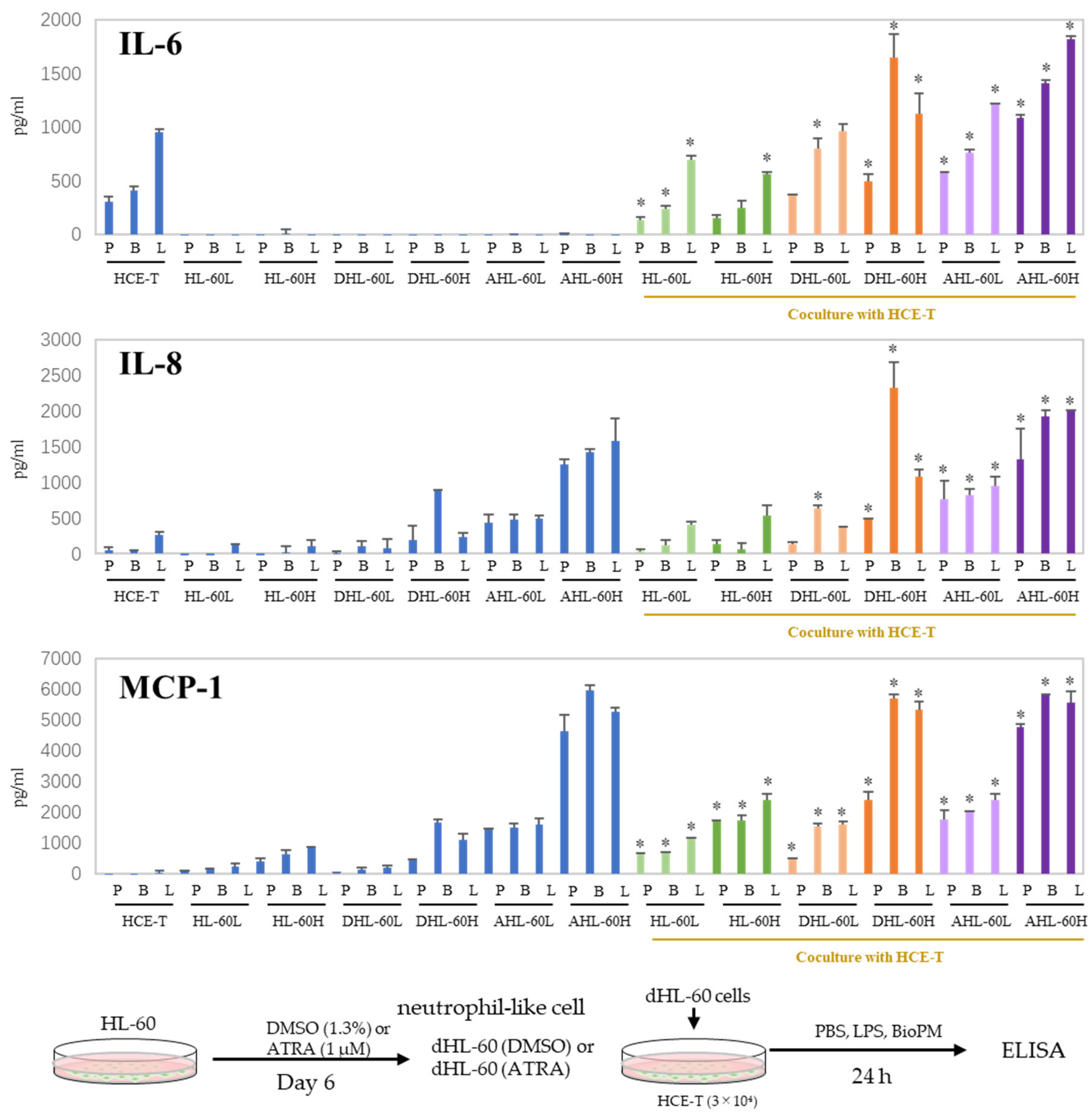
3.3. Co-Culture with Differentiated HL-60 Cells Enhances the IL-6, IL-8, and MCP-1 Production in HCE-T
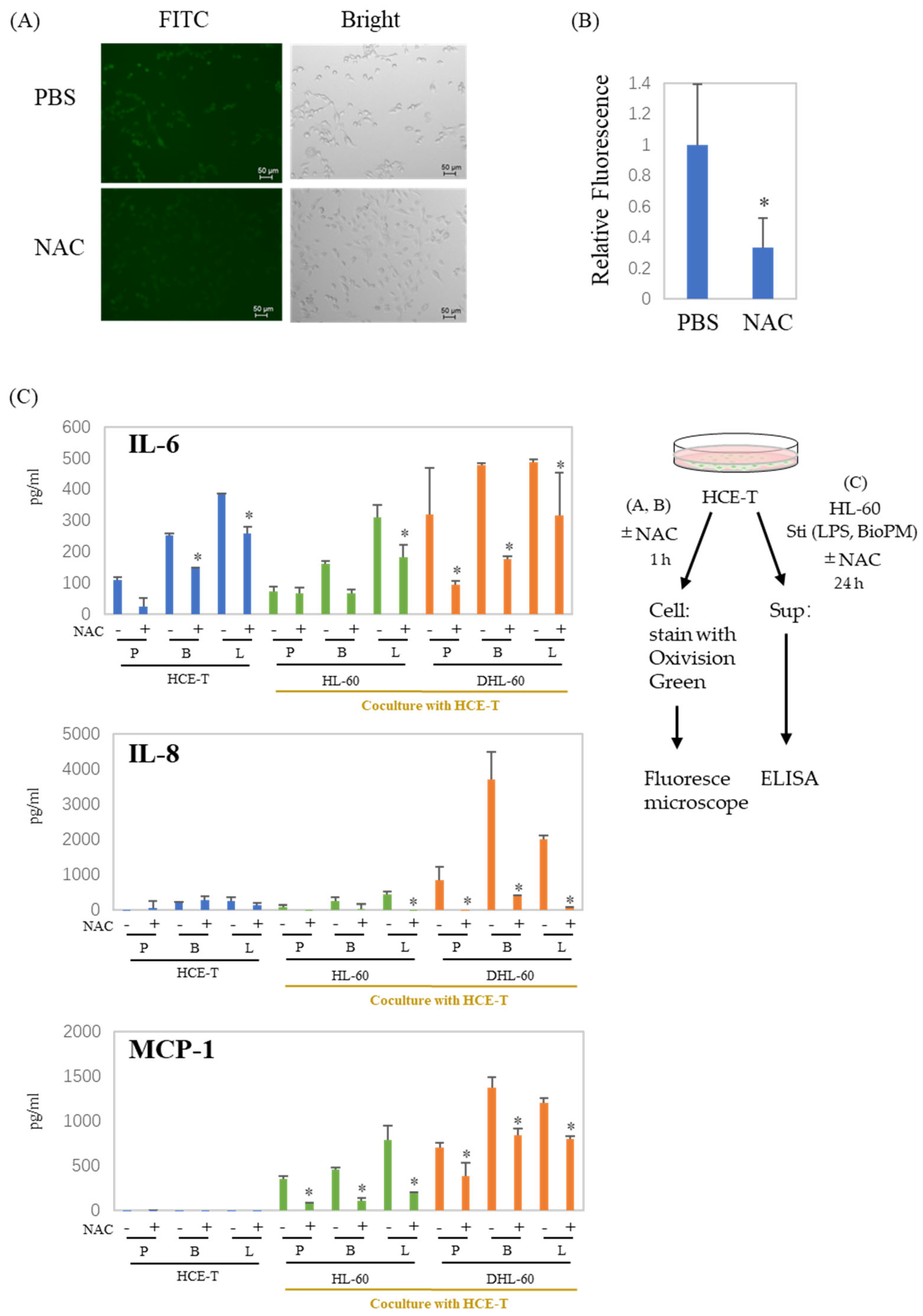
3.4. ROS Are Involved in the Production of Cytokines and Chemokines in Co-Culture Systems
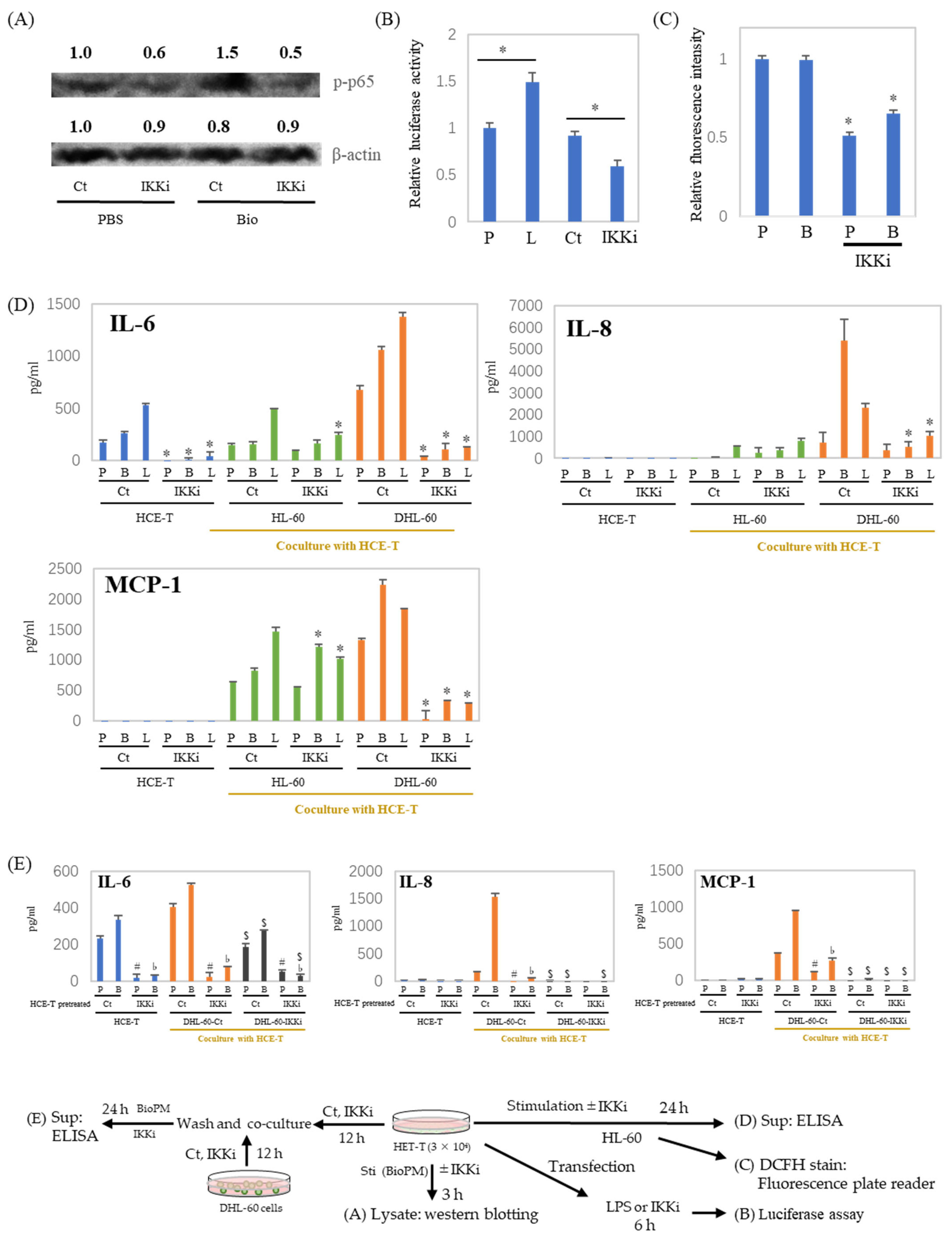
3.5. IL-6, IL-8, and MCP-1 Production Are Inhibited by an IKK Inhibitor after HCE-T Are Co-Cultured with Neutrophil-like Cells
3.6. Cell–Cell Interactions Affect Cytokines and Chemokines’ Production
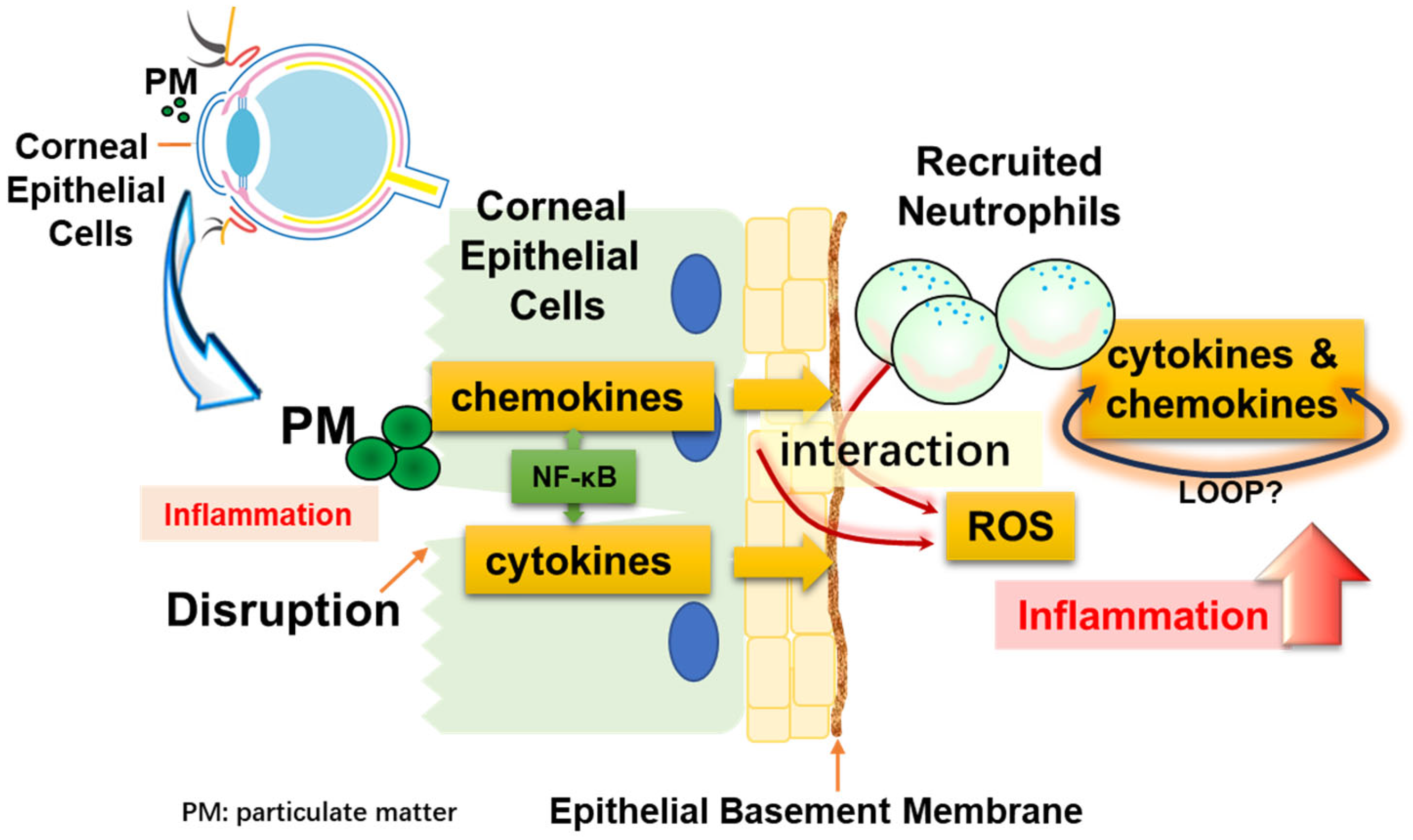
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rajaiya, J.; Zhou, X.; Barequet, I.; Gilmore, M.S.; Chodosh, J. Novel model of innate immunity in corneal infection. In Vitro Cell. Dev. Biol. Anim. 2015, 51, 827–834. [Google Scholar] [CrossRef]
- Daheshia, M.; Kanangat, S.; Rouse, B.T. Production of key molecules by ocular neutrophils early after herpetic infection of the cornea. Exp. Eye Res. 1998, 67, 619–624. [Google Scholar] [CrossRef] [PubMed]
- Hazlett, L.D. Corneal response to Pseudomonas aeruginosa infection. Prog. Retin. Eye Res. 2004, 23, 1–30. [Google Scholar] [CrossRef]
- Chintakuntlawar, A.V.; Astley, R.; Chodosh, J. Adenovirus type 37 keratitis in the C57BL/6J mouse. Investig. Ophthalmol. Vis. Sci. 2007, 48, 781–788. [Google Scholar] [CrossRef] [PubMed]
- Miyake, T.; Wang, D.; Matsuoka, H.; Morita, K.; Yasuda, H.; Yatera, K.; Kanazawa, T.; Yoshida, Y. Endocytosis of particulate matter induces cytokine production by neutrophil via Toll-like receptor 4. Int. Immunopharmacol. 2018, 57, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Pelletier, M.; Savoie, A.; Girard, D. Activation of human neutrophils by the air pollutant sodium sulfite (Na(2)SO(3)): Comparison with immature promyelocytic HL-60 and DMSO-differentiated HL-60 cells reveals that Na(2)SO(3) is a neutrophil but not a HL-60 cell agonist. Clin. Immunol. 2000, 96, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Verdon, R.; Gillies, S.L.; Brown, D.M.; Henry, T.; Tran, L.; Tyler, C.R.; Rossi, A.G.; Stone, V.; Johnston, H.J. Neutrophil activation by nanomaterials in vitro: Comparing strengths and limitations of primary human cells with those of an immortalized (HL-60) cell line. Nanotoxicology 2021, 15, 1–20. [Google Scholar] [CrossRef]
- Wang, D.; Sennari, Y.; Shen, M.; Morita, K.; Kanazawa, T.; Yoshida, Y. ERK is involved in the differentiation and function of dimethyl sulfoxide-induced HL-60 neutrophil-like cells, which mimic inflammatory neutrophils. Int. Immunopharmacol. 2020, 84, 106510. [Google Scholar] [CrossRef]
- Wang, D.; Zeng, Z.; Shen, M.; Okazaki, R.; Miyata, H.; Yonezawa, T.; Yoshida, Y. ATP Consumption Is Coupled with Endocytosis in Exudated Neutrophils. Int. J. Mol. Sci. 2023, 24, 9039. [Google Scholar] [CrossRef]
- Fu, Q.; Lyu, D.; Zhang, L.; Qin, Z.; Tang, Q.; Yin, H.; Lou, X.; Chen, Z.; Yao, K. Airborne particulate matter (PM2.5) triggers autophagy in human corneal epithelial cell line. Environ. Pollut. 2017, 227, 314–322. [Google Scholar] [CrossRef]
- Lee, M.; Lee, S.Y.; Bae, Y.S. Emerging roles of neutrophils in immune homeostasis. BMB Rep. 2022, 55, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Niu, L.; Li, L.; Xing, C.; Luo, B.; Hu, C.; Song, M.; Niu, J.; Ruan, Y.; Sun, X.; Lei, Y. Airborne particulate matter (PM(2.5)) triggers cornea inflammation and pyroptosis via NLRP3 activation. Ecotoxicol. Environ. Saf. 2021, 207, 111306. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.X.; Song, X.L.; Li, S.S.; Lai, X.R.; Yang, Y.L.; Yang, G.; Li, Z.J.; Cui, Y.H.; Pan, H.W. Assessment of DNA Damage and Cell Senescence in Corneal Epithelial Cells Exposed to Airborne Particulate Matter (PM2.5) Collected in Guangzhou, China. Investig. Ophthalmol. Vis. Sci. 2016, 57, 3093–3102. [Google Scholar] [CrossRef] [PubMed]
- Guha, M.; Mackman, N. LPS induction of gene expression in human monocytes. Cell Signal. 2001, 13, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, Y.; Kumar, A.; Koyama, Y.; Peng, H.; Arman, A.; Boch, J.A.; Auron, P.E. Interleukin 1 activates STAT3/nuclear factor-kappaB cross-talk via a unique TRAF6- and p65-dependent mechanism. J. Biol. Chem. 2004, 279, 1768–1776. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Verma, I.M. NF-kappaB regulation in the immune system. Nat. Rev. Immunol. 2002, 2, 725–734. [Google Scholar] [CrossRef]
- Bao, Y.; Cao, X. The immune potential and immunopathology of cytokine-producing B cell subsets: A comprehensive review. J. Autoimmun. 2014, 55, 10–23. [Google Scholar] [CrossRef] [PubMed]
- Bert, B.; Dorendahl, A.; Leich, N.; Vietze, J.; Steinfath, M.; Chmielewska, J.; Hensel, A.; Grune, B.; Schonfelder, G. Rethinking 3R strategies: Digging deeper into AnimalTestInfo promotes transparency in in vivo biomedical research. PLoS Biol. 2017, 15, e2003217. [Google Scholar] [CrossRef]
- Schechtman, L.M. Implementation of the 3Rs (refinement, reduction, and replacement): Validation and regulatory acceptance considerations for alternative toxicological test methods. ILAR J. 2002, 43 (Suppl. S1), S85–S94. [Google Scholar] [CrossRef]
- Abdalkader, R.K.; Fujita, T. Corneal epithelium models for safety assessment in drug development: Present and future directions. Exp. Eye Res. 2023, 237, 109697. [Google Scholar] [CrossRef]
- Song, Y.; Okazaki, R.; Yoshida, Y. Senescence-associated secretory phenotype and activation of NF-kappaB in splenocytes of old mice exposed to irradiation at a young age. Dev. Comp. Immunol. 2021, 122, 104124. [Google Scholar] [CrossRef]
- Yoshida, Y.; Liu, J.; Sugiura, T.; Ishidao, T.; Ueno, S.; Yanagita, H.; Fueta, Y.; Kunugita, N.; Hori, H.; Yamashita, U. The indoor air pollutant 2-ethyl-hexanol activates CD4 cells. Chem. Biol. Interact. 2009, 177, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Tan, G.; Li, J.; Yang, Q.; Wu, A.; Qu, D.Y.; Wang, Y.; Ye, L.; Bao, J.; Shao, Y. Air pollutant particulate matter 2.5 induces dry eye syndrome in mice. Sci. Rep. 2018, 8, 17828. [Google Scholar] [CrossRef] [PubMed]
- Fang, D.; Wang, Q.; Li, H.; Yu, Y.; Lu, Y.; Qian, X. Mortality effects assessment of ambient PM2.5 pollution in the 74 leading cities of China. Sci. Total Environ. 2016, 569, 1545–1552. [Google Scholar] [CrossRef]
- Karottki, D.G.; Spilak, M.; Frederiksen, M.; Gunnarsen, L.; Brauner, E.V.; Kolarik, B.; Andersen, Z.J.; Sigsgaard, T.; Barregard, L.; Strandberg, B.; et al. An indoor air filtration study in homes of elderly: Cardiovascular and respiratory effects of exposure to particulate matter. Environ. Health 2013, 12, 116. [Google Scholar] [CrossRef] [PubMed]
- Mimura, T.; Ichinose, T.; Inoue, K.; Yoshida, Y.; Fujishima, H. Airborne Suspended Particulate Matter and the Prevalence of Allergic Conjunctivitis in Japan. Cureus 2024, 16, e53292. [Google Scholar] [CrossRef] [PubMed]
- Stepp, M.A.; Menko, A.S. Immune responses to injury and their links to eye disease. Transl. Res. 2021, 236, 52–71. [Google Scholar] [CrossRef]
- He, C.; Song, Y.; Ichinose, T.; He, M.; Morita, K.; Wang, D.; Kanazawa, T.; Yoshida, Y. Lipopolysaccharide levels adherent to PM2.5 play an important role in particulate matter induced-immunosuppressive effects in mouse splenocytes. J. Appl. Toxicol. 2018, 38, 471–479. [Google Scholar] [CrossRef]
- Nagai, N.; Ogata, F.; Otake, H.; Nakazawa, Y.; Kawasaki, N. Energy-dependent endocytosis is responsible for drug transcorneal penetration following the instillation of ophthalmic formulations containing indomethacin nanoparticles. Int. J. Nanomed. 2019, 14, 1213–1227. [Google Scholar] [CrossRef]
- Sung, M.; Kim, J.H.; Min, H.S.; Jang, S.; Hong, J.; Choi, B.K.; Shin, J.; Chung, K.S.; Park, Y.R. Three-dimensional label-free morphology of CD8 + T cells as a sepsis biomarker. Light Sci. Appl. 2023, 12, 265. [Google Scholar] [CrossRef]
- Yu, D.; Cai, W.; Shen, T.; Wu, Y.; Ren, C.; Li, T.; Hu, C.; Zhu, M.; Yu, J. PM(2.5) exposure increases dry eye disease risks through corneal epithelial inflammation and mitochondrial dysfunctions. Cell Biol. Toxicol. 2023, 39, 2615–2630. [Google Scholar] [CrossRef]
- Otsu, W.; Yako, T.; Sugisawa, E.; Nakamura, S.; Tsusaki, H.; Umigai, N.; Shimazawa, M.; Hara, H. Crocetin protects against mitochondrial damage induced by UV-A irradiation in corneal epithelial cell line HCE-T cells. J. Pharmacol. Sci. 2022, 150, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Ishida, K.; Yako, T.; Tanaka, M.; Otsu, W.; Nakamura, S.; Shimazawa, M.; Tsusaki, H.; Hara, H. Free-Radical Scavenger NSP-116 Protects the Corneal Epithelium against UV-A and Blue LED Light Exposure. Biol. Pharm. Bull. 2021, 44, 937–946. [Google Scholar] [CrossRef]
- Benko, S.; Tozser, J.; Miklossy, G.; Varga, A.; Kadas, J.; Csutak, A.; Berta, A.; Rajnavolgyi, E. Constitutive and UV-B modulated transcription of Nod-like receptors and their functional partners in human corneal epithelial cells. Mol. Vis. 2008, 14, 1575–1583. [Google Scholar] [PubMed]
- Cloutier, A.; Guindi, C.; Larivee, P.; Dubois, C.M.; Amrani, A.; McDonald, P.P. Inflammatory cytokine production by human neutrophils involves C/EBP transcription factors. J. Immunol. 2009, 182, 563–571. [Google Scholar] [CrossRef]
- An, Z.; Li, J.; Yu, J.; Wang, X.; Gao, H.; Zhang, W.; Wei, Z.; Zhang, J.; Zhang, Y.; Zhao, J.; et al. Neutrophil extracellular traps induced by IL-8 aggravate atherosclerosis via activation NF-kappaB signaling in macrophages. Cell Cycle 2019, 18, 2928–2938. [Google Scholar] [CrossRef]
- Klein, M.B.; Hu, S.; Chao, C.C.; Goodman, J.L. The agent of human granulocytic ehrlichiosis induces the production of myelosuppressing chemokines without induction of proinflammatory cytokines. J. Infect. Dis. 2000, 182, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Baz, A.A.; Hao, H.; Lan, S.; Li, Z.; Liu, S.; Chen, S.; Chu, Y. Neutrophil extracellular traps in bacterial infections and evasion strategies. Front. Immunol. 2024, 15, 1357967. [Google Scholar] [CrossRef] [PubMed]
- Papayannopoulos, V. Neutrophil extracellular traps in immunity and disease. Nat. Rev. Immunol. 2018, 18, 134–147. [Google Scholar] [CrossRef]
- Vaseruk, A.; Bila, G.; Bilyy, R. Nanoparticles for stimulation of neutrophil extracellular trap-mediated immunity. Eur. J. Immunol. 2024, 54, e2350582. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, Z.; Yoshida, Y.; Wang, D.; Fujii, Y.; Shen, M.; Mimura, T.; Tanaka, Y. Inflammatory Cytokines and Chemokines Are Synergistically Induced in a ROS-Dependent Manner by a Co-Culture of Corneal Epithelial Cells and Neutrophil-like Cells in the Presence of Particulate Matter. Antioxidants 2024, 13, 467. https://doi.org/10.3390/antiox13040467
Zeng Z, Yoshida Y, Wang D, Fujii Y, Shen M, Mimura T, Tanaka Y. Inflammatory Cytokines and Chemokines Are Synergistically Induced in a ROS-Dependent Manner by a Co-Culture of Corneal Epithelial Cells and Neutrophil-like Cells in the Presence of Particulate Matter. Antioxidants. 2024; 13(4):467. https://doi.org/10.3390/antiox13040467
Chicago/Turabian StyleZeng, Zirui, Yasuhiro Yoshida, Duo Wang, Yuri Fujii, Mengyue Shen, Tatsuya Mimura, and Yoshiya Tanaka. 2024. "Inflammatory Cytokines and Chemokines Are Synergistically Induced in a ROS-Dependent Manner by a Co-Culture of Corneal Epithelial Cells and Neutrophil-like Cells in the Presence of Particulate Matter" Antioxidants 13, no. 4: 467. https://doi.org/10.3390/antiox13040467
APA StyleZeng, Z., Yoshida, Y., Wang, D., Fujii, Y., Shen, M., Mimura, T., & Tanaka, Y. (2024). Inflammatory Cytokines and Chemokines Are Synergistically Induced in a ROS-Dependent Manner by a Co-Culture of Corneal Epithelial Cells and Neutrophil-like Cells in the Presence of Particulate Matter. Antioxidants, 13(4), 467. https://doi.org/10.3390/antiox13040467






