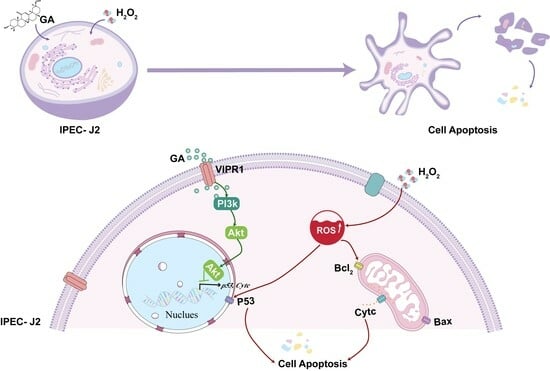
18Beta-Glycyrrhetinic Acid Attenuates H2O2-Induced Oxidative Damage and Apoptosis in Intestinal Epithelial Cells via Activating the PI3K/Akt Signaling Pathway
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Detection of IPEC-J2 Cell Viability
2.3. Assessment of Cellular Barrier
2.4. Apoptosis Analysis
2.5. Intracellular ROS Levels
2.6. Redox Status Analysis
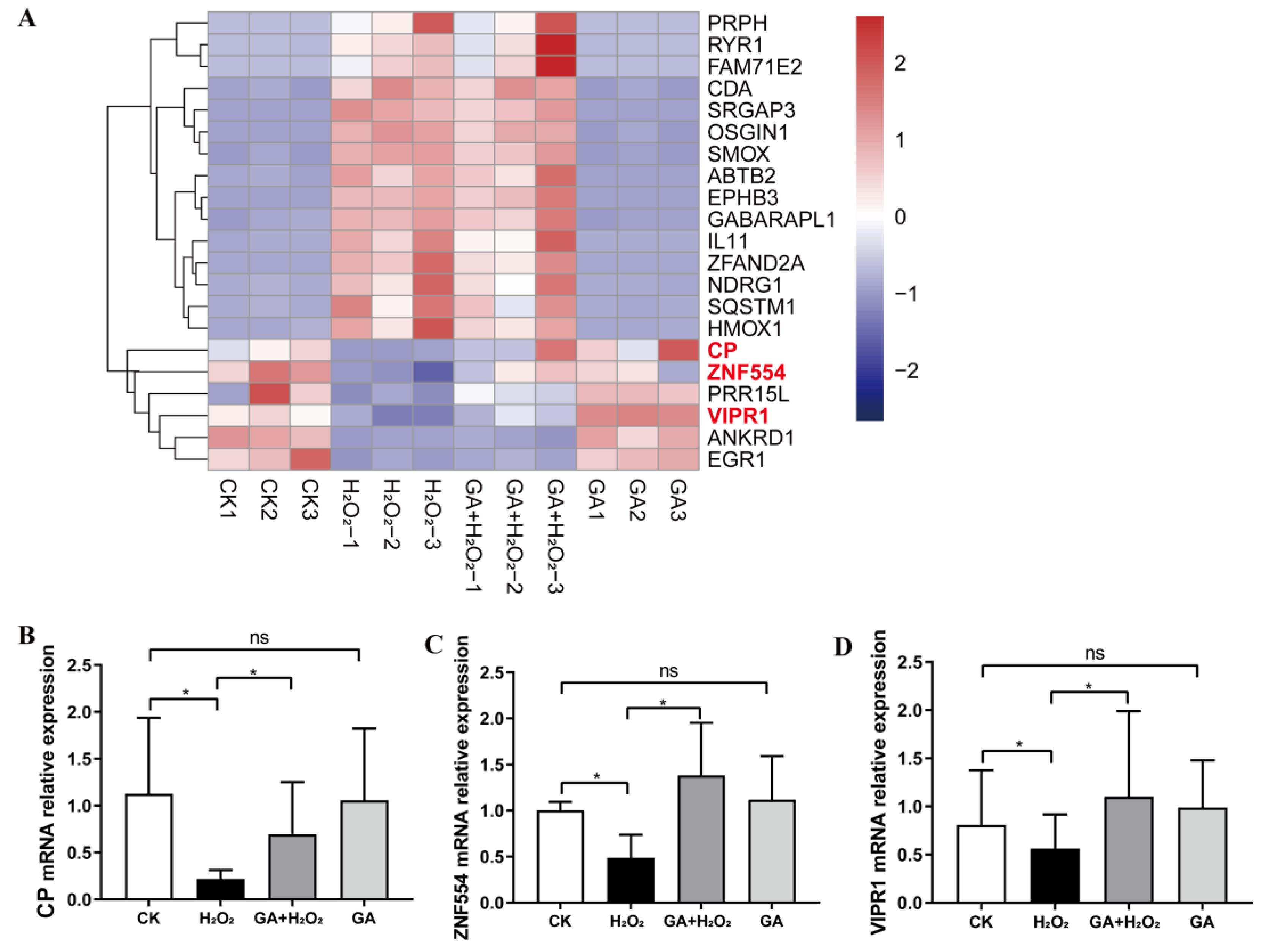
2.7. RNA-Seq Library Construction and Analysis
2.8. Quantitative Real-Time PCR Assay
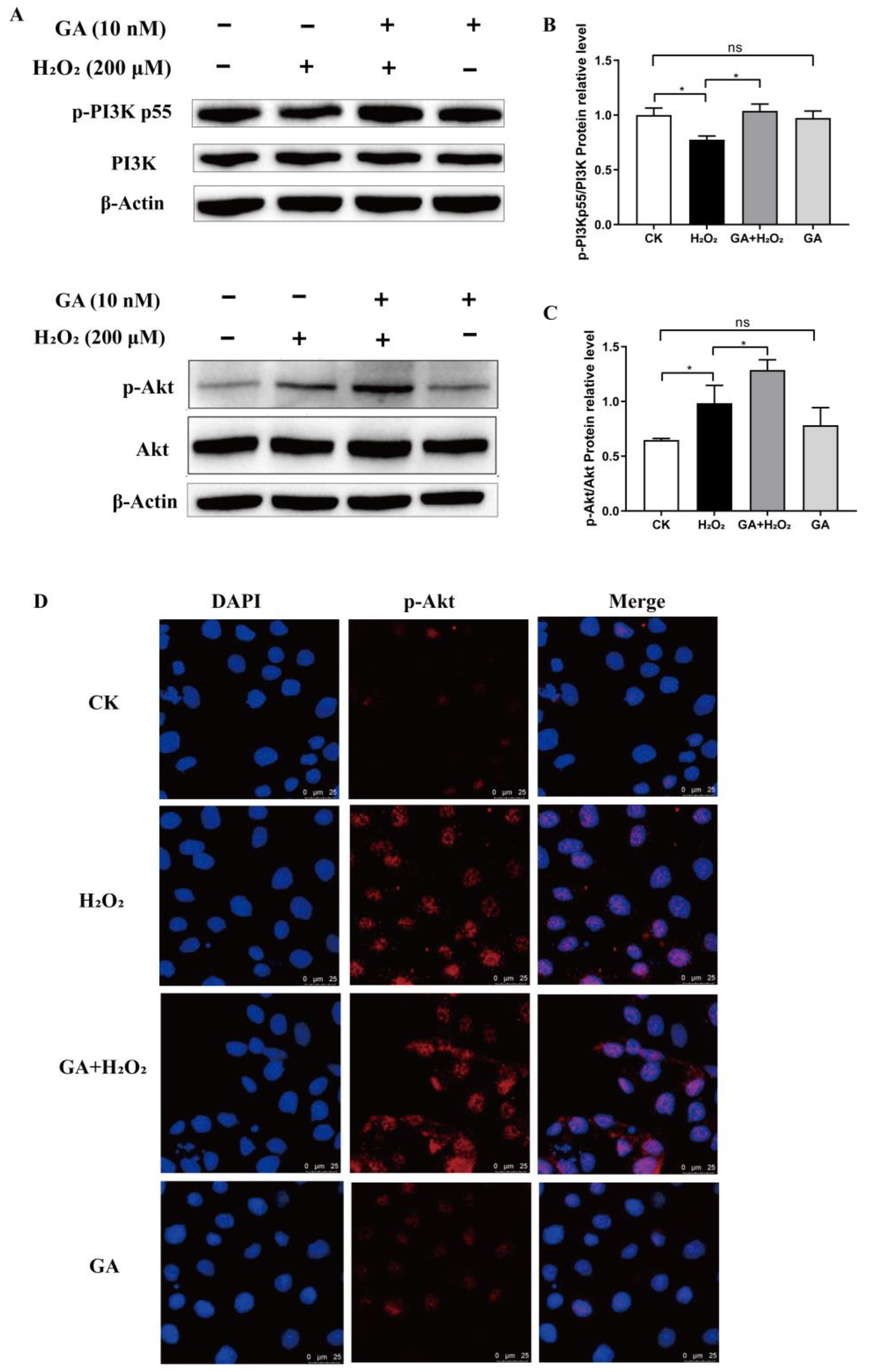
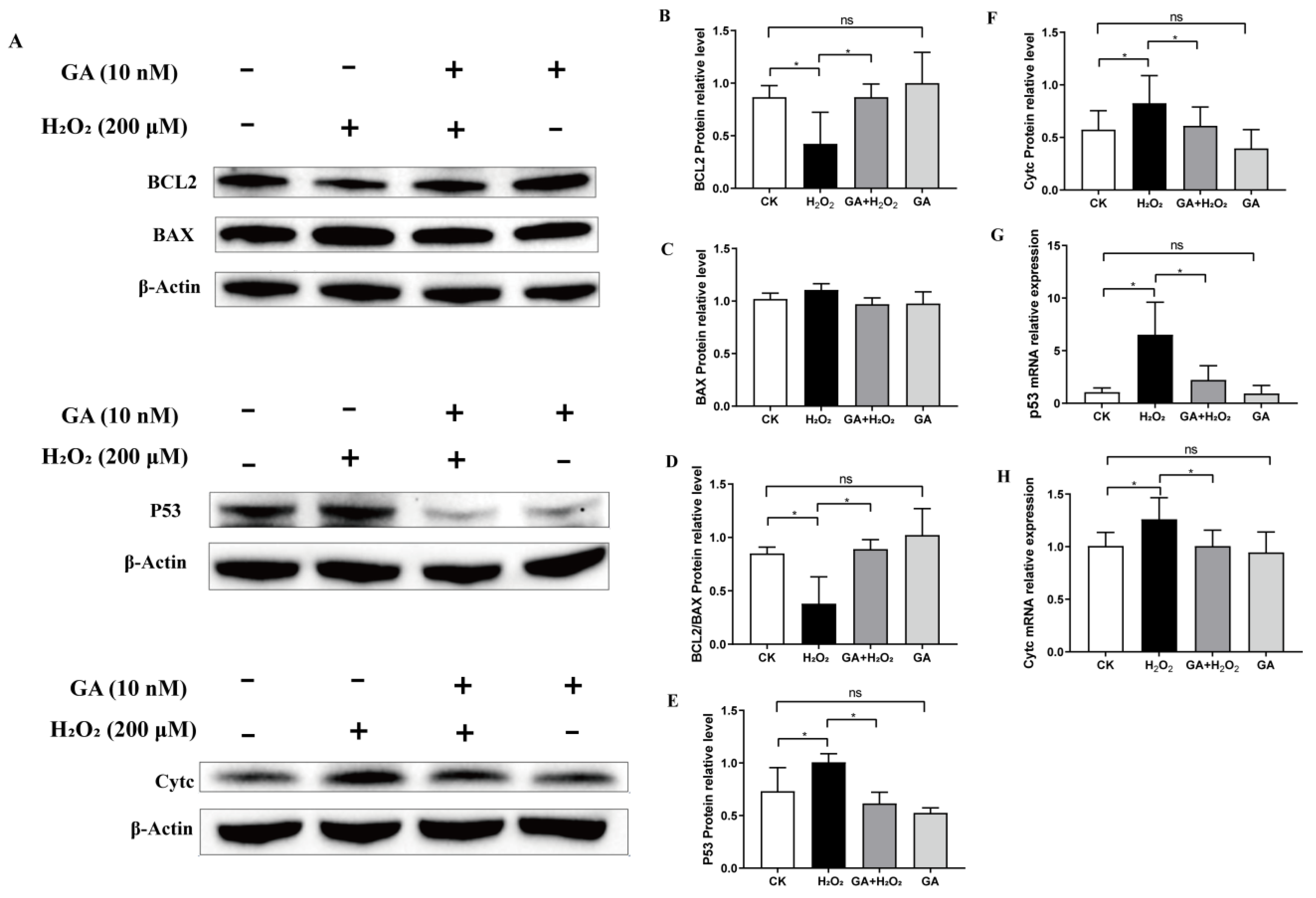
2.9. Western Blot Analysis
2.10. Immunofluorescence and Confocal Microscopy
2.11. Molecular Docking
2.12. Statistical Analysis
3. Results
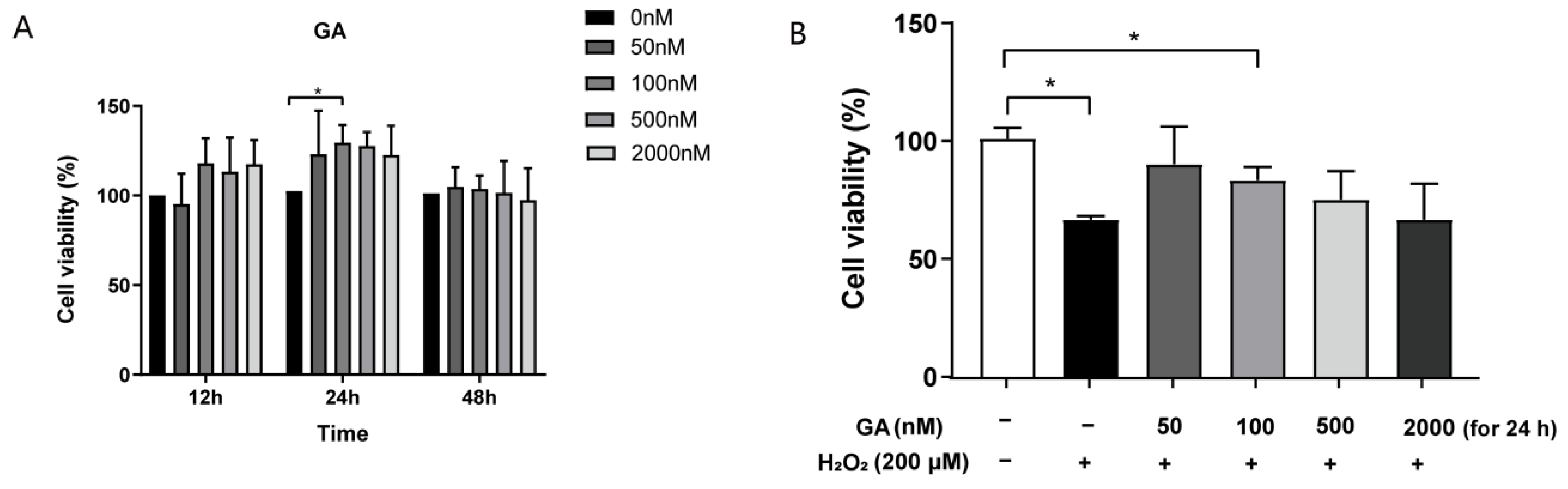
3.1. GA Restores H2O2-Induced Cell Viability in IPEC-J2 Cells
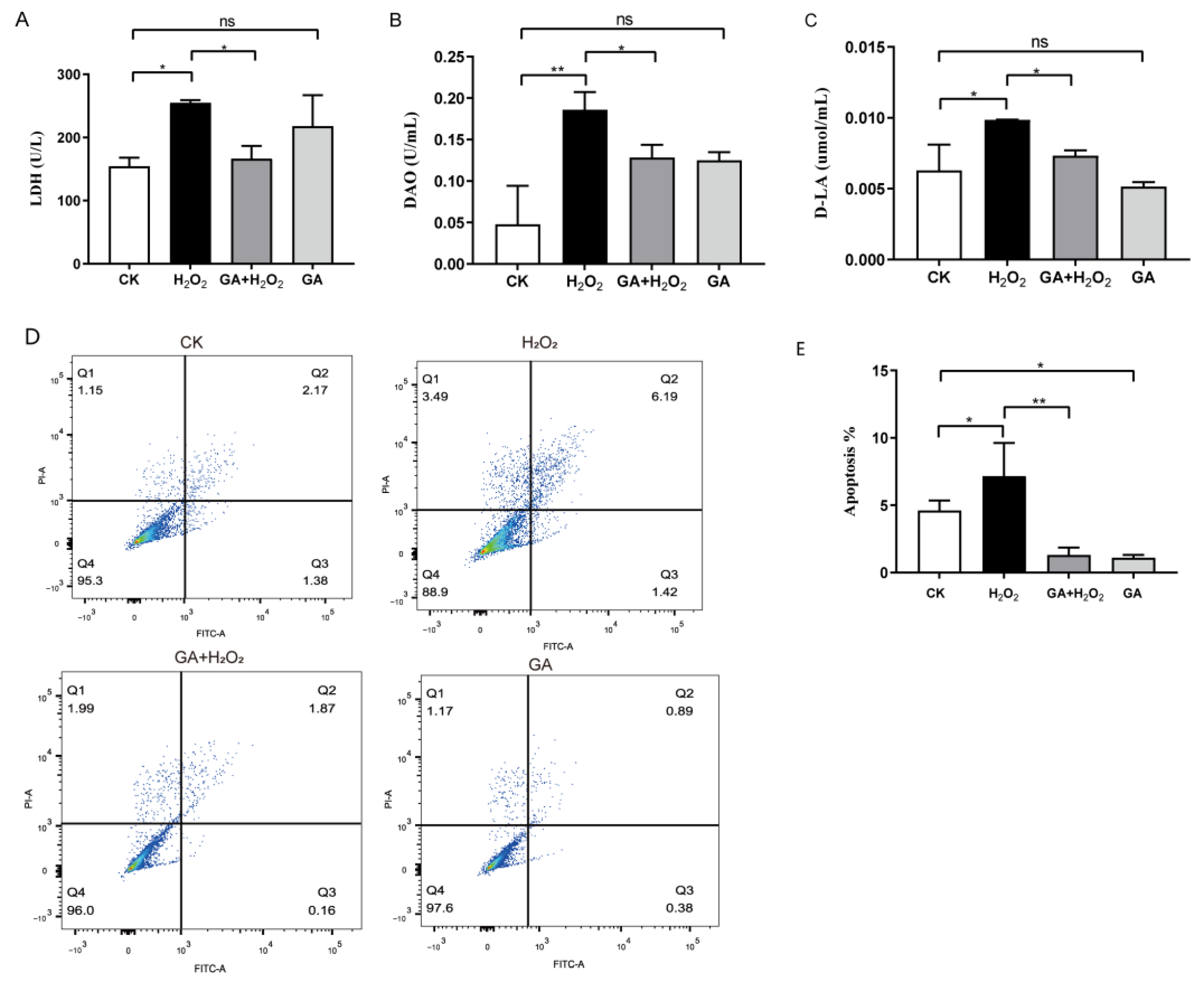
3.2. GA Protects IPEC-J2 Cells from Cell Injury and Apoptosis under Oxidative Stress
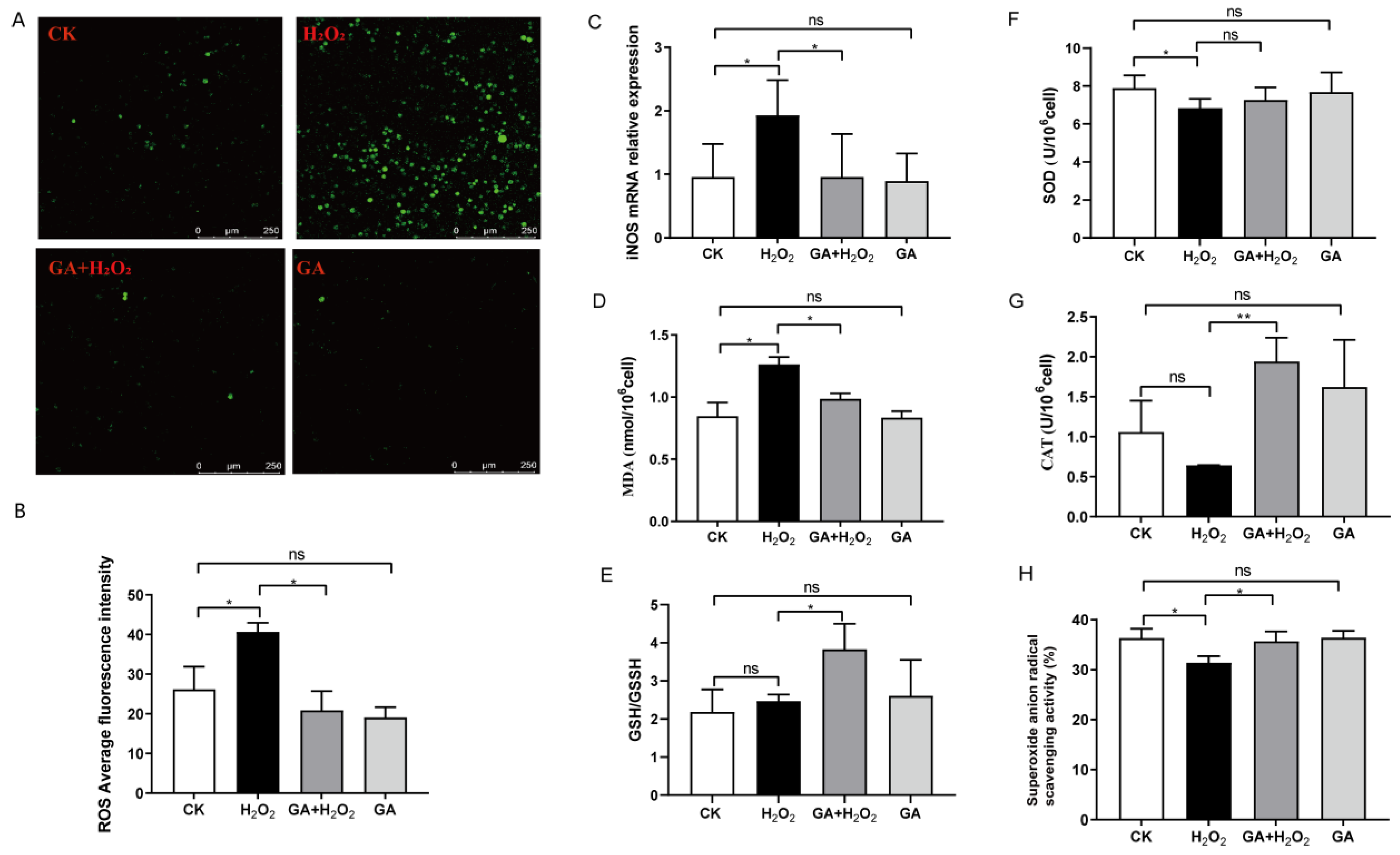
3.3. GA Reduces Cellular ROS Production and Increases Antioxidant Enzyme Secretion in IPEC-J2 Cells
3.4. GA Alleviates H2O2-Induced Oxidative Stress by Activating the PI3K/Akt Pathway
3.5. Molecular Binding between GA and Potential Target Proteins
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Camilleri, M.; Madsen, K.; Spiller, R.; Greenwood-Van Meerveld, B.; Verne, G.N. Intestinal barrier function in health and gastrointestinal disease. Neurogastroenterol. Motil. 2012, 24, 503–512. [Google Scholar] [CrossRef]
- Xu, S.; Li, X.; Zhang, S.; Qi, C.; Zhang, Z.; Ma, R.; Xiang, L.; Chen, L.; Zhu, Y.; Tang, C.; et al. Oxidative stress gene expression, DNA methylation, and gut microbiota interaction trigger Crohn’s disease: A multi-omics Mendelian randomization study. BMC Med. 2023, 21, 179. [Google Scholar] [CrossRef]
- Li, D.K.; Chaudhari, S.N.; Lee, Y.; Sojoodi, M.; Adhikari, A.A.; Zukerberg, L.; Shroff, S.; Barrett, S.C.; Tanabe, K.; Chung, R.T.; et al. Inhibition of microbial deconjugation of micellar bile acids protects against intestinal permeability and liver injury. Sci. Adv. 2022, 8, eabo2794. [Google Scholar] [CrossRef] [PubMed]
- Birben, E.; Sahiner, U.M.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative stress and antioxidant defense. World Allergy Organ J. 2012, 5, 9–19. [Google Scholar] [CrossRef]
- Cho, Y.E.; Yu, L.R.; Abdelmegeed, M.A.; Yoo, S.H.; Song, B.J. Apoptosis of enterocytes and nitration of junctional complex proteins promote alcohol-induced gut leakiness and liver injury. J. Hepatol. 2018, 69, 142–153. [Google Scholar] [CrossRef]
- Mo, F.; Zhou, X.; Yang, M.; Chen, L.; Tang, Z.; Wang, C.; Cui, Y. Trehalose Attenuates Oxidative Stress and Endoplasmic Reticulum Stress-Mediated Apoptosis in IPEC-J2 Cells Subjected to Heat Stress. Animals 2022, 12, 2093. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.J.; Wang, X.Q. Deoxynivalenol induces intestinal injury: Insights from oxidative stress and intestinal stem cells. Environ. Sci. Pollut. Res. Int. 2023, 30, 48676–48685. [Google Scholar] [CrossRef]
- Cao, S.; Wu, H.; Wang, C.; Zhang, Q.; Jiao, L.; Lin, F.; Hu, C.H. Diquat-induced oxidative stress increases intestinal permeability, impairs mitochondrial function, and triggers mitophagy in piglets. J. Anim. Sci. 2018, 96, 1795–1805. [Google Scholar] [CrossRef]
- Pastorino, G.; Cornara, L.; Soares, S.; Rodrigues, F.; Oliveira, M.B.P.P. Liquorice (Glycyrrhiza glabra): A phytochemical and pharmacological review. Phytother. Res. PTR 2018, 32, 2323–2339. [Google Scholar] [CrossRef]
- Abd El-Twab, S.M.; Hozayen, W.G.; Hussein, O.E.; Mahmoud, A.M. 18β-Glycyrrhetinic acid protects against methotrexate-induced kidney injury by up-regulating the Nrf2/ARE/HO-1 pathway and endogenous antioxidants. Ren. Fail. 2016, 38, 1516–1527. [Google Scholar] [CrossRef]
- Huo, H.Z.; Wang, B.; Liang, Y.K.; Bao, Y.Y.; Gu, Y. Hepatoprotective and antioxidant effects of licorice extract against CCl₄-induced oxidative damage in rats. Int. J. Mol. Sci. 2011, 12, 6529–6543. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, A.M.; Hussein, O.E.; Hozayen, W.G.; Abd El-Twab, S.M. Methotrexate hepatotoxicity is associated with oxidative stress, and down-regulation of PPARγ and Nrf2: Protective effect of 18β-Glycyrrhetinic acid. Chem. Biol. Interact. 2017, 270, 59–72. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Bei, B.; Feng, Y.; Li, X.; Jiang, Z.; Si, J.Y.; Qing, D.G.; Zhang, J.; Li, N. Glycyrrhetinic Acid Maintains Intestinal Homeostasis via HuR. Front. Pharmacol. 2019, 10, 535. [Google Scholar] [CrossRef] [PubMed]
- Malekinejad, M.; Pashaee, M.R.; Malekinejad, H. 18β-Glycyrrhetinic acid altered the intestinal permeability in the human Caco-2 monolayer cell model. Eur. J. Nutr. 2022, 61, 3437–3447. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.Y.; Wang, W.J.; Dou, J.H.; Gong, L.K. Research progress on the protective effects of licorice-derived 18β-glycyrrhetinic acid against liver injury. Acta Pharmacol. Sin. 2021, 42, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Xue, X. Detection of Total Reactive Oxygen Species in Adherent Cells by 2′,7′-Dichlorodihydrofluorescein Diacetate Staining. J. Vis. Exp. JoVE 2020, 160, e60682. [Google Scholar] [CrossRef]
- Xiao, K.; Liu, C.; Tu, Z.; Xu, Q.; Chen, S.; Zhang, Y.; Wang, X.; Zhang, J.; Hu, C.A.; Liu, Y. Activation of the NF-κB and MAPK Signaling Pathways Contributes to the Inflammatory Responses, but Not Cell Injury, in IPEC-1 Cells Challenged with Hydrogen Peroxide. Oxidative Med. Cell. Longev. 2020, 2020, 5803639. [Google Scholar] [CrossRef] [PubMed]
- Pfaffl, M.W.; Horgan, G.W.; Dempfle, L. Relative expression software tool (REST) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic. Acids Res. 2002, 30, e36. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Ming, D.; Wang, W.; Wang, Z.; Hu, Y.; Ma, X.; Wang, F. Pyrroloquinoline Quinone Alleviates Jejunal Mucosal Barrier Function Damage and Regulates Colonic Microbiota in Piglets Challenged with Enterotoxigenic Escherichia coli. Front. Microbiol. 2020, 11, 1754. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Liu, F.; Yao, Y.; Zhang, Q.; Lu, Z.; Zhang, R.; Liu, C.; Lin, C.; Zhu, C. Network pharmacology-based mechanism prediction and pharmacological validation of Xiaoyan Lidan formula on attenuating alpha-naphthylisothiocyanate induced cholestatic hepatic injury in rats. J. Ethnopharmacol. 2021, 270, 113816. [Google Scholar] [CrossRef]
- Seeliger, D.; de Groot, B.L. Ligand docking and binding site analysis with PyMOL and Autodock/Vina. J. Comput.-Aided Mol. Des. 2010, 24, 417–422. [Google Scholar] [CrossRef]
- Chen, Z.; Yuan, Q.; Xu, G.; Chen, H.; Lei, H.; Su, J. Effects of Quercetin on Proliferation and H₂O₂-Induced Apoptosis of Intestinal Porcine Enterocyte Cells. Molecules 2018, 23, 2012. [Google Scholar] [CrossRef]
- Wu, Z.; Wang, H.; Fang, S.; Xu, C. Roles of endoplasmic reticulum stress and autophagy on H2O2-induced oxidative stress injury in HepG2 cells. Mol. Med. Rep. 2018, 18, 4163–4174. [Google Scholar] [CrossRef] [PubMed]
- Magenta, A.; Cencioni, C.; Fasanaro, P.; Zaccagnini, G.; Greco, S.; Sarra-Ferraris, G.; Antonini, A.; Martelli, F.; Capogrossi, M.C. miR-200c is upregulated by oxidative stress and induces endothelial cell apoptosis and senescence via ZEB1 inhibition. Cell Death Differ. 2011, 18, 1628–1639. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Tang, G.; Jiao, Y.; Yuan, Y.; Zheng, Y.; Chen, Y.; Xiao, J.; Li, C.; Chen, Z.; Cao, P. Propionibacterium acnes Induces Intervertebral Disc Degeneration by Promoting iNOS/NO and COX-2/PGE(2) Activation via the ROS-Dependent NF-κB Pathway. Oxidative Med. Cell. Longev. 2018, 2018, 3692752. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Li, C.; Chen, S.; Li, Z.; Jia, X.; Wang, K.; Bao, J.; Liang, Y.; Wang, X.; Chen, M.; et al. Berberine protects against 6-OHDA-induced neurotoxicity in PC12 cells and zebrafish through hormetic mechanisms involving PI3K/AKT/Bcl-2 and Nrf2/HO-1 pathways. Redox Biol. 2017, 11, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Than, N.G.; Romero, R.; Tarca, A.L.; Kekesi, K.A.; Xu, Y.; Xu, Z.; Juhasz, K.; Bhatti, G.; Leavitt, R.J.; Gelencser, Z.; et al. Integrated Systems Biology Approach Identifies Novel Maternal and Placental Pathways of Preeclampsia. Front. Immunol. 2018, 9, 1661. [Google Scholar] [CrossRef]
- Gates, K.C.; Goetzmann, L.N.; Cantlon, J.D.; Jeckel, K.M.; Anthony, R.V. Effect of proline rich 15-deficiency on trophoblast viability and survival. PLoS ONE 2017, 12, e0174976. [Google Scholar] [CrossRef]
- Yu, R.; Zhang, H.; Huang, L.; Liu, X.; Chen, J. Anti-hyperglycemic, antioxidant and anti-inflammatory effects of VIP and a VPAC1 agonist on streptozotocin-induced diabetic mice. Peptides 2011, 32, 216–222. [Google Scholar] [CrossRef]
- Zibara, K.; Zeidan, A.; Mallah, K.; Kassem, N.; Awad, A.; Mazurier, F.; Badran, B.; El-Zein, N. Signaling pathways activated by PACAP in MCF-7 breast cancer cells. Cell. Signal. 2018, 50, 37–47. [Google Scholar] [CrossRef]
- Asano, S.; Yamasaka, M.; Ozasa, K.; Sakamoto, K.; Hayata-Takano, A.; Nakazawa, T.; Hashimoto, H.; Waschek, J.A.; Ago, Y. Vasoactive intestinal peptide-VIPR2 signaling regulates tumor cell migration. Front. Oncol. 2022, 12, 852358. [Google Scholar] [CrossRef] [PubMed]
- Shou, X.; Wang, Y.; Zhang, X.; Zhang, Y.; Yang, Y.; Duan, C.; Yang, Y.; Jia, Q.; Yuan, G.; Shi, J.; et al. Network Pharmacology and Molecular Docking Analysis on Molecular Mechanism of Qingzi Zhitong Decoction in the Treatment of Ulcerative Colitis. Front. Pharmacol. 2022, 13, 727608. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.J.; Li, H.D.; Yan, M.; Li, Z.H.; Gong, H.; Jiang, P.; Deng, Y.; Fang, P.F.; Zhang, B.K. The Protective Effects of Isoliquiritigenin and Glycyrrhetinic Acid against Triptolide-Induced Oxidative Stress in HepG2 Cells Involve Nrf2 Activation. Evid. Based Complement. Altern. Med. Ecam 2016, 2016, 8912184. [Google Scholar] [CrossRef]
- Zhang, D.; Sun, J.; Chang, S.; Li, X.; Shi, H.; Jing, B.; Zheng, Y.; Lin, Y.; Qian, G.; Pan, Y.; et al. Protective effect of 18β-glycyrrhetinic acid against H2O2-induced injury in Schwann cells based on network pharmacology and experimental validation. Exp. Ther. Med. 2021, 22, 1241. [Google Scholar] [CrossRef] [PubMed]
- Sheng, Y.; Liu, G.; Wang, M.; Lv, Z.; Du, P. A selenium polysaccharide from Platycodon grandiflorum rescues PC12 cell death caused by H2O2 via inhibiting oxidative stress. Int. J. Biol. Macromol. 2017, 104, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Deng, F.; Zhao, B.C.; Yang, X.; Lin, Z.B.; Sun, Q.S.; Wang, Y.F.; Yan, Z.Z.; Liu, W.F.; Li, C.; Hu, J.J.; et al. The gut microbiota metabolite capsiate promotes Gpx4 expression by activating TRPV1 to inhibit intestinal ischemia reperfusion-induced ferroptosis. Gut Microbes 2021, 13, 1902719. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Ye, H.; Du, M.; Yu, Q.; Chen, Y.; Shen, M. Mung Bean Protein Hydrolysates Protect Mouse Liver Cell Line Nctc-1469 Cell from Hydrogen Peroxide-Induced Cell Injury. Foods 2019, 9, 14. [Google Scholar] [CrossRef] [PubMed]
- Del Rio, D.; Stewart, A.J.; Pellegrini, N. A review of recent studies on malondialdehyde as toxic molecule and biological marker of oxidative stress. Nutr. Metab. Cardiovasc. Dis. NMCD 2005, 15, 316–328. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.W.; Yin, J.; Ge, Q.; Jiang, Z.L.; Gong, J.Y. In vitro antioxidant activities of polysaccharides extracted from Moso Bamboo-Leaf. Int. J. Biol. Macromol. 2013, 55, 1–5. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, W.; Wang, S.; Wang, Y.; Chu, X.; Ji, H. Lactobacillus plantarum Exhibits Antioxidant and Cytoprotective Activities in Porcine Intestinal Epithelial Cells Exposed to Hydrogen Peroxide. Oxidative Med. Cell. Longev. 2021, 2021, 8936907. [Google Scholar] [CrossRef]
- Zhan, T.; Han, Y.; Tang, C.; Zhao, Q.; Sun, D.; Li, Y.; Jia, X.; Zhou, L.; Zhang, J. Metabolism and biological activity of α-tocopherol derived from vitamin E-enriched transgenic maize in broilers. J. Sci. Food Agric. 2020, 100, 4319–4328. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Liu, L.; Wang, Z.; Jiang, C.P.; Zhu, Z.; Li, H.; Zeng, Q.; Xue, Y.; Wu, Y.; Wang, Y.; et al. Network pharmacology, molecular docking and in vivo and in vitro experiments to explore the molecular mechanism of licorice green tea beverage to scavenge oxygen free radicals. J. Food Biochem. 2022, 46, e14315. [Google Scholar] [CrossRef] [PubMed]
- Röhrdanz, E.; Kahl, R. Alterations of antioxidant enzyme expression in response to hydrogen peroxide. Free. Radic. Biol. Med. 1998, 24, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.L.; Wang, Y.Z.; Guo, J.; Liu, J.X.; Feng, J. Comparison of age-related differences in expression of antioxidant enzyme mRNA and activity in various tissues of pigs. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2007, 147, 445–451. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, B.A.; Gwon, Y.; Mishra, A.; Peng, J.; Nakamura, H.; Zhang, K.; Kim, H.J.; Taylor, J.P. Ubiquitination is essential for recovery of cellular activities after heat shock. Science 2021, 372, eabc3593. [Google Scholar] [CrossRef] [PubMed]
- Zhong, W.; Qian, K.; Xiong, J.; Ma, K.; Wang, A.; Zou, Y. Curcumin alleviates lipopolysaccharide induced sepsis and liver failure by suppression of oxidative stress-related inflammation via PI3K/AKT and NF-κB related signaling. Biomed. Pharmacother. Biomed. Pharmacother. 2016, 83, 302–313. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Han, X.; Han, X. The cardioprotection of the insulin-mediated PI3K/Akt/mTOR signaling pathway. Am. J. Cardiovasc. Drugs Drugs Devices Other Interv. 2014, 14, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Harris, M.H.; Thompson, C.B. The role of the Bcl-2 family in the regulation of outer mitochondrial membrane permeability. Cell Death Differ. 2000, 7, 1182–1191. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Jiang, H.; Shen, Z.; Wang, X. Activation of mitochondrial protease OMA1 by Bax and Bak promotes cytochrome c release during apoptosis. Proc. Natl. Acad. Sci. USA 2014, 111, 14782–14787. [Google Scholar] [CrossRef]
- Ghatei, N.; Nabavi, A.S.; Toosi, M.H.B.; Azimian, H.; Homayoun, M.; Targhi, R.G.; Haghir, H. Evaluation of bax, bcl-2, p21 and p53 genes expression variations on cerebellum of BALB/c mice before and after birth under mobile phone radiation exposure. Iran J. Basic Med. Sci. 2017, 20, 1037–1043. [Google Scholar] [CrossRef]
- Su, Z.; Burchfield, J.G.; Yang, P.; Humphrey, S.J.; Yang, G.; Francis, D.; Yasmin, S.; Shin, S.Y.; Norris, D.M.; Kearney, A.L.; et al. Global redox proteome and phosphoproteome analysis reveals redox switch in Akt. Nat. Commun. 2019, 10, 5486. [Google Scholar] [CrossRef] [PubMed]
- Suresh, P.S.; Kumar, A.; Kumar, R.; Singh, V.P. An in silico [correction of insilico] approach to bioremediation: Laccase as a case study. J. Mol. Graph. Model. 2008, 26, 845–849. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Huang, B.X.; Guo, M. Current advances in screening for bioactive components from medicinal plants by affinity ultrafiltration mass spectrometry. Phytochem. Anal. PCA 2018, 29, 375–386. [Google Scholar] [CrossRef] [PubMed]
- Couvineau, A.; Laburthe, M. VPAC receptors: Structure, molecular pharmacology and interaction with accessory proteins. Br. J. Pharmacol. 2012, 166, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Delgado, M.; Munoz-Elias, E.J.; Gomariz, R.P.; Ganea, D. Vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide prevent inducible nitric oxide synthase transcription in macrophages by inhibiting NF-kappa B and IFN regulatory factor 1 activation. J. Immunol. 1999, 162, 4685–4696. [Google Scholar] [CrossRef] [PubMed]
- Kojima, M.; Ito, T.; Oono, T.; Hisano, T.; Igarashi, H.; Arita, Y.; Kawabe, K.; Coy, D.H.; Jensen, R.T.; Nawata, H. VIP attenuation of the severity of experimental pancreatitis is due to VPAC1 receptor-mediated inhibition of cytokine production. Pancreas 2005, 30, 62–70. [Google Scholar] [PubMed]
- Steingart, R.A.; Solomon, B.; Brenneman, D.E.; Fridkin, M.; Gozes, I. VIP and peptides related to activity-dependent neurotrophic factor protect PC12 cells against oxidative stress. J. Mol. Neurosci. MN 2000, 15, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, N. Vascular endothelial growth factor receptors: Molecular mechanisms of activation and therapeutic potentials. Exp. Eye Res. 2006, 83, 1005–1016. [Google Scholar] [CrossRef] [PubMed]
- Cantley, L.C. The phosphoinositide 3-kinase pathway. Science 2002, 296, 1655–1657. [Google Scholar] [CrossRef]
- Feng, Z.; Sun, R.; Cong, Y.; Liu, Z. Critical roles of G protein-coupled receptors in regulating intestinal homeostasis and inflammatory bowel disease. Mucosal. Immunol. 2022, 15, 819–828. [Google Scholar] [CrossRef]
- Wang, Z.; Ma, J.; He, Y.; Miu, K.K.; Yao, S.; Tang, C.; Ye, Y.; Lin, G. Nrf2-mediated liver protection by 18β-glycyrrhetinic acid against pyrrolizidine alkaloid-induced toxicity through PI3K/Akt/GSK3β pathway. Phytomed. Int. J. Phytother. Phytopharm. 2022, 102, 154162. [Google Scholar] [CrossRef] [PubMed]

| Protein | Residue | AA | C | Distance H-A | Binding Energy/(kcal mol−1) |
|---|---|---|---|---|---|
| VIPR1 | 189A | ARG | C3 | 1.9 | −6.58 |
| 196A | LYS | C30 | 1.7 | −6.35 | |
| PI3K | 179A | ARG | C3 | 1.9 | −5.39 |
| 531A | LYS | C30 | 1.8 | −5.39 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, C.; Wang, F.; Zhu, J.; Wang, S.; Liu, Y.; Xu, J.; Zhao, Q.; Qin, Y.; Si, W.; Zhang, J. 18Beta-Glycyrrhetinic Acid Attenuates H2O2-Induced Oxidative Damage and Apoptosis in Intestinal Epithelial Cells via Activating the PI3K/Akt Signaling Pathway. Antioxidants 2024, 13, 468. https://doi.org/10.3390/antiox13040468
Ma C, Wang F, Zhu J, Wang S, Liu Y, Xu J, Zhao Q, Qin Y, Si W, Zhang J. 18Beta-Glycyrrhetinic Acid Attenuates H2O2-Induced Oxidative Damage and Apoptosis in Intestinal Epithelial Cells via Activating the PI3K/Akt Signaling Pathway. Antioxidants. 2024; 13(4):468. https://doi.org/10.3390/antiox13040468
Chicago/Turabian StyleMa, Cui, Fuxi Wang, Jiawei Zhu, Shiyi Wang, Yaqing Liu, Jianfang Xu, Qingyu Zhao, Yuchang Qin, Wei Si, and Junmin Zhang. 2024. "18Beta-Glycyrrhetinic Acid Attenuates H2O2-Induced Oxidative Damage and Apoptosis in Intestinal Epithelial Cells via Activating the PI3K/Akt Signaling Pathway" Antioxidants 13, no. 4: 468. https://doi.org/10.3390/antiox13040468
APA StyleMa, C., Wang, F., Zhu, J., Wang, S., Liu, Y., Xu, J., Zhao, Q., Qin, Y., Si, W., & Zhang, J. (2024). 18Beta-Glycyrrhetinic Acid Attenuates H2O2-Induced Oxidative Damage and Apoptosis in Intestinal Epithelial Cells via Activating the PI3K/Akt Signaling Pathway. Antioxidants, 13(4), 468. https://doi.org/10.3390/antiox13040468