S-Allylmercapto-N-Acetylcysteine (ASSNAC) Attenuates Osteoporosis in Ovariectomized (OVX) Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Animals
2.3. OVX Surgery
2.4. Treatment Regimen
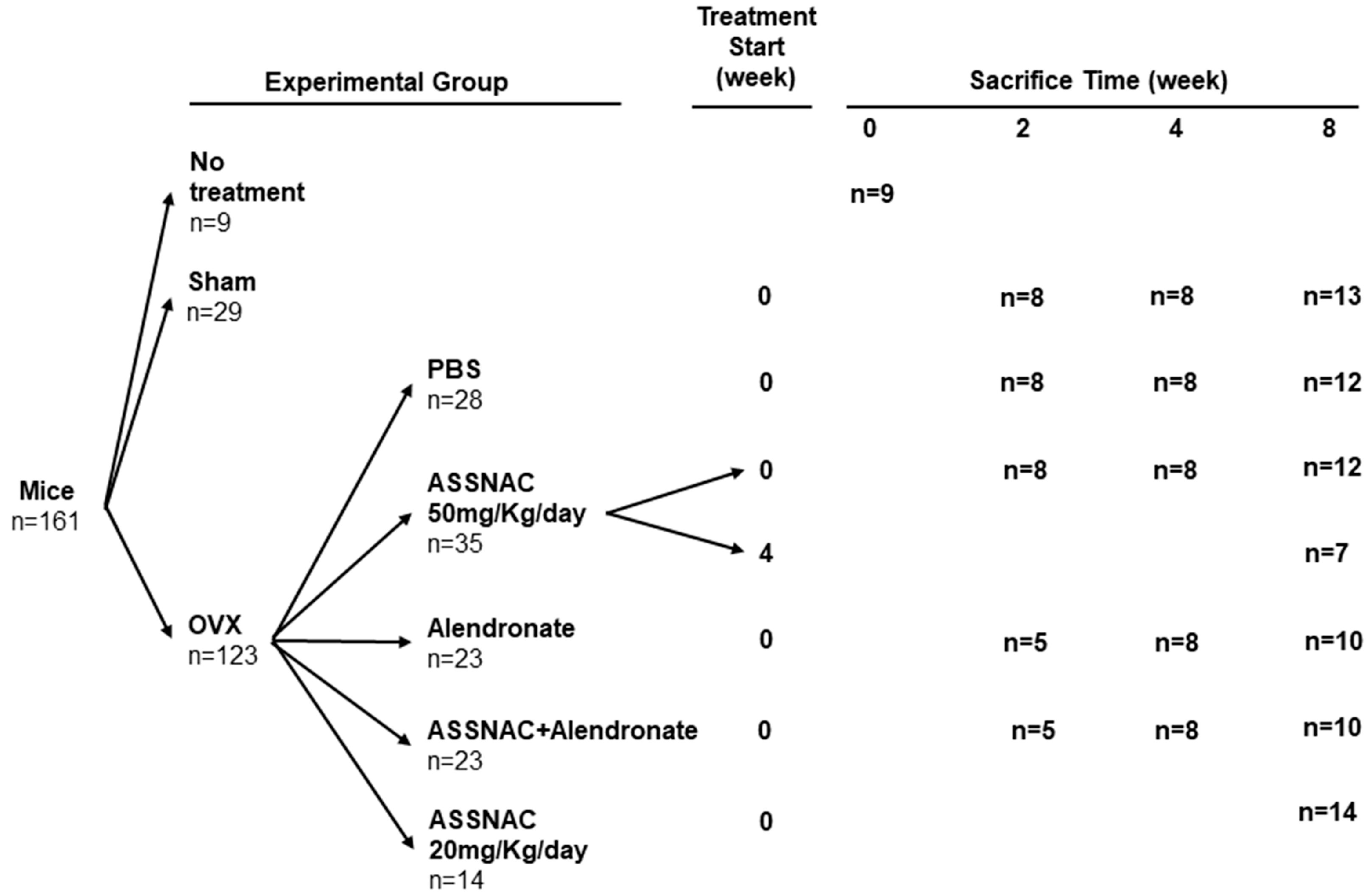
2.5. Experimental Groups
2.6. Femur and Vertebra Collection
2.7. Femur and Vertebra µCT Analysis
2.8. Determination of Procollagen I N-Terminal Propeptide (P1NP) and C-terminal Cross-Linked Telopeptide of Type I Collagen (CTX)
2.8.1. Blood Sample Collection
2.8.2. CTX and P1NP Assays
2.9. Glutathione and MDA Determination
2.9.1. BM Cell Collection
2.9.2. Glutathione Assay
2.9.3. MDA Assay
2.10. Statistical Analysis
3. Results
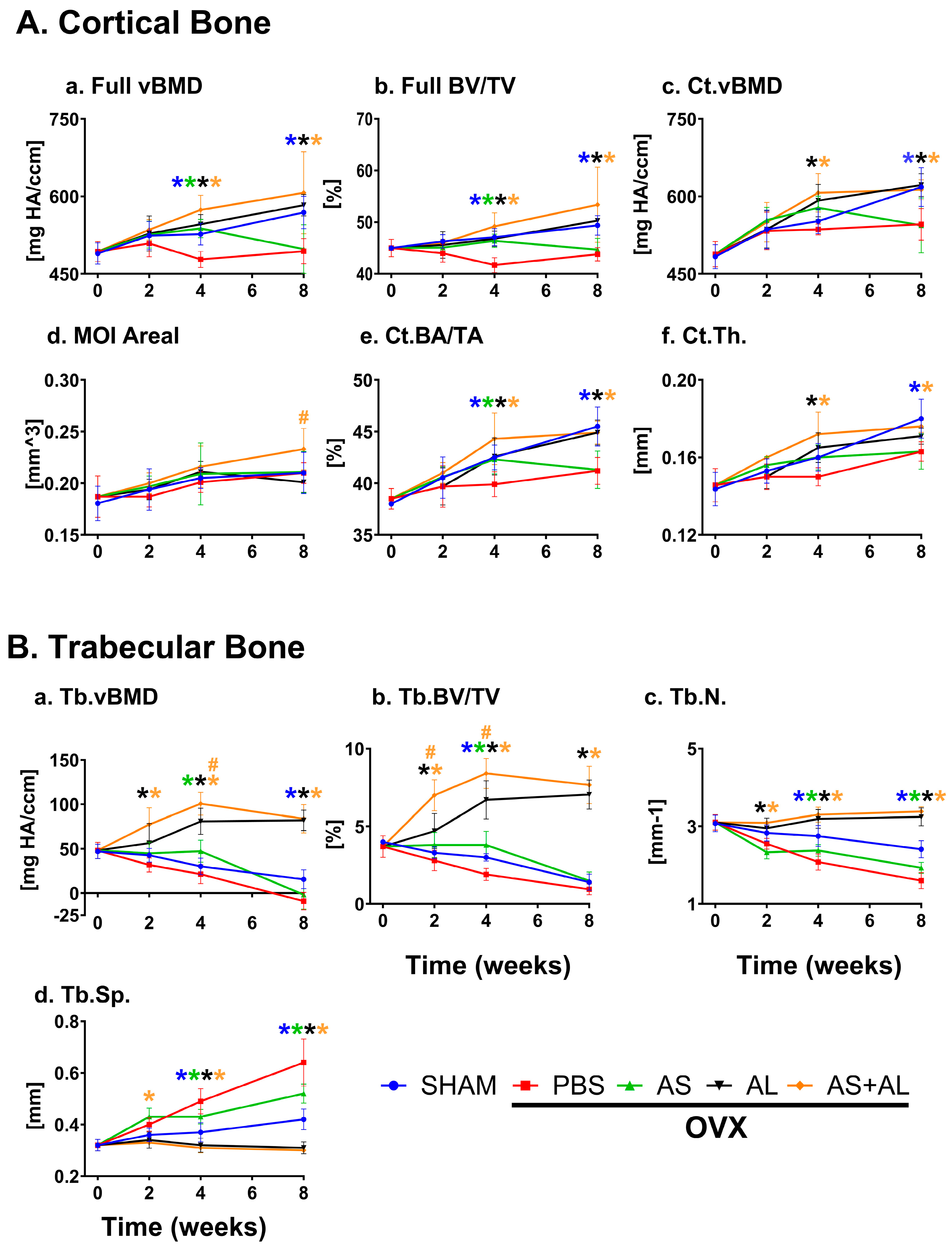
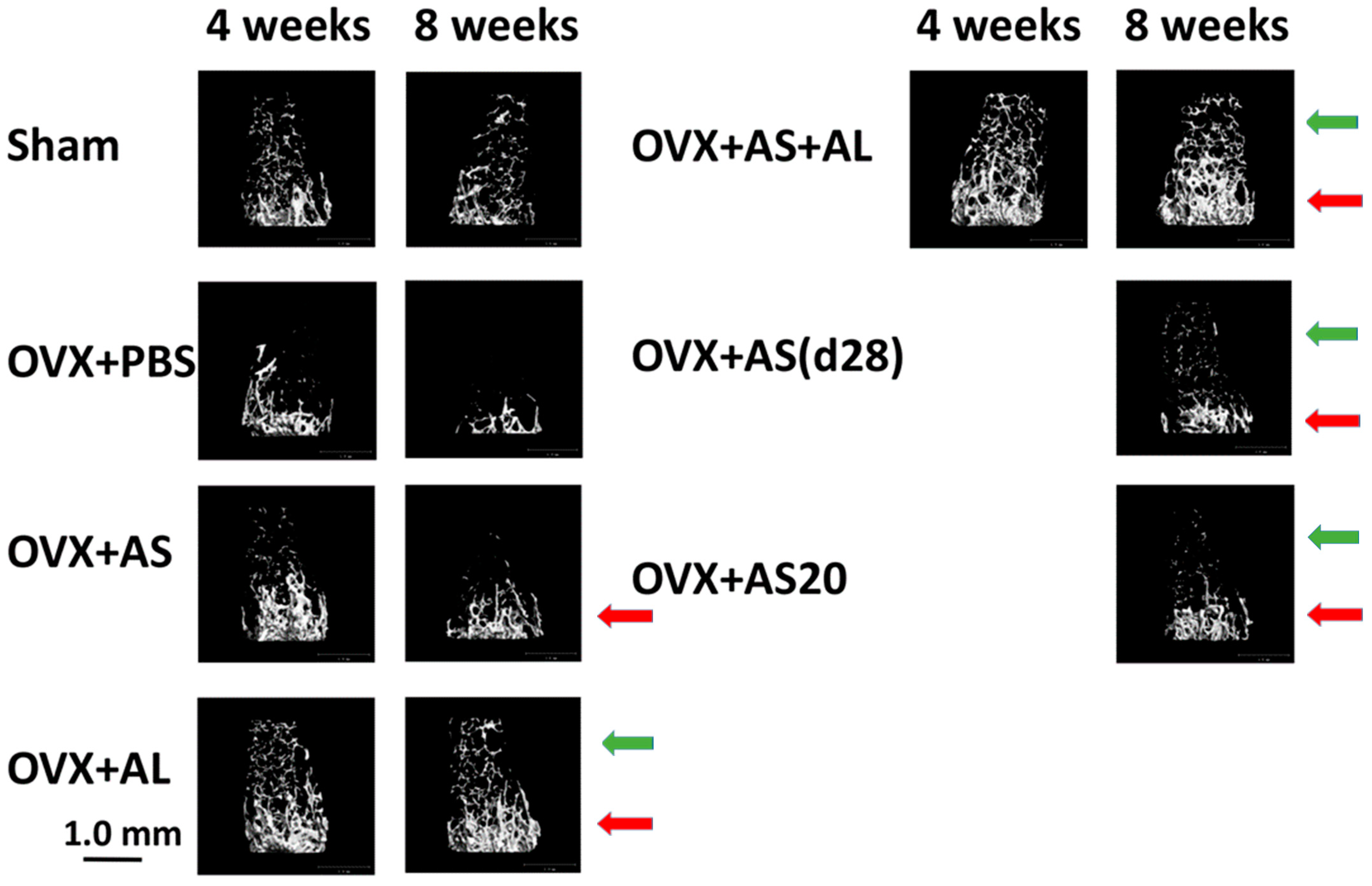
3.1. Effect of ASSNAC and Alendronate on Femur Microarchitecture in OVX Mice
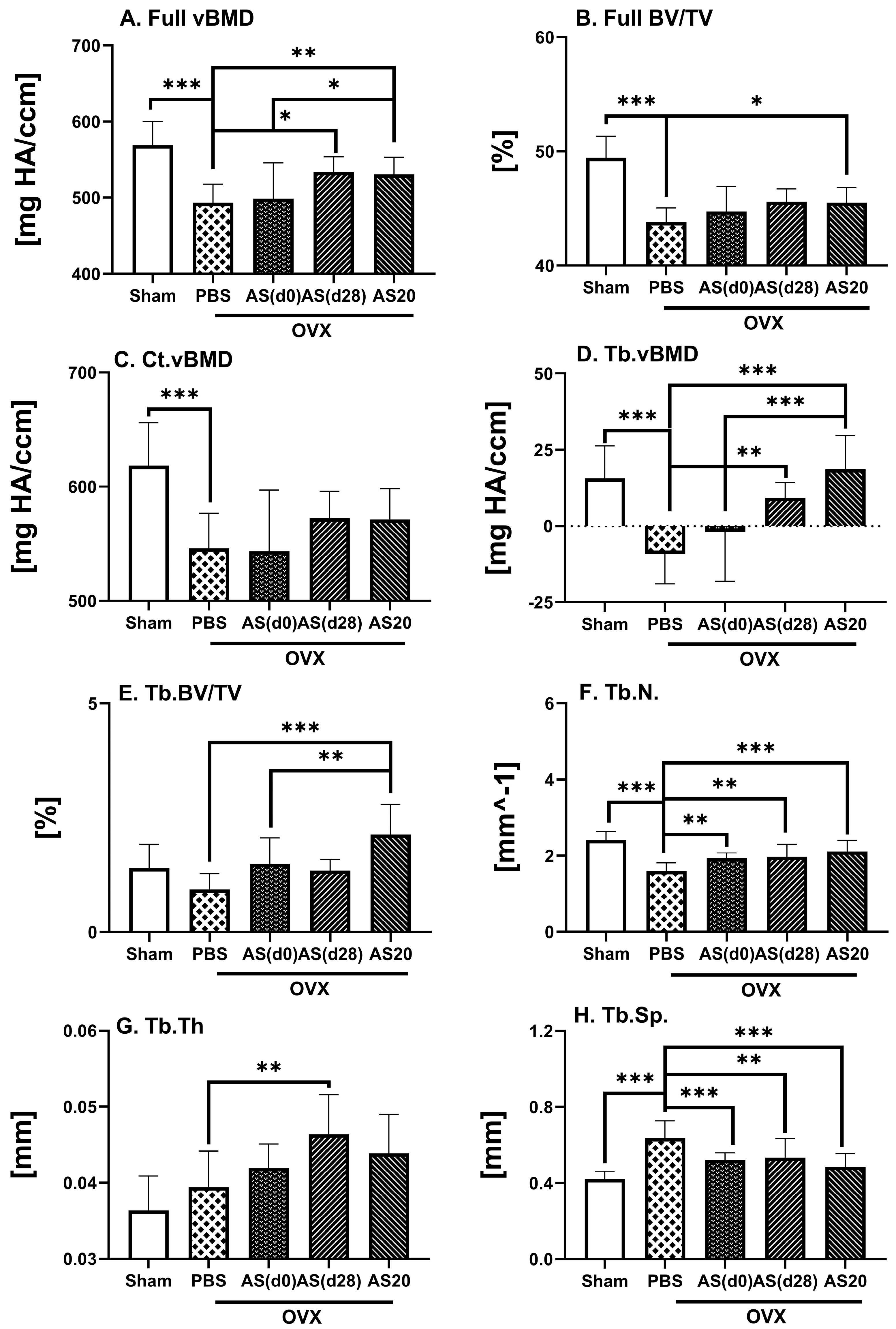
3.2. Effect of ASSNAC and Alendronate on Vertebra Microarchitecture in OVX Mice
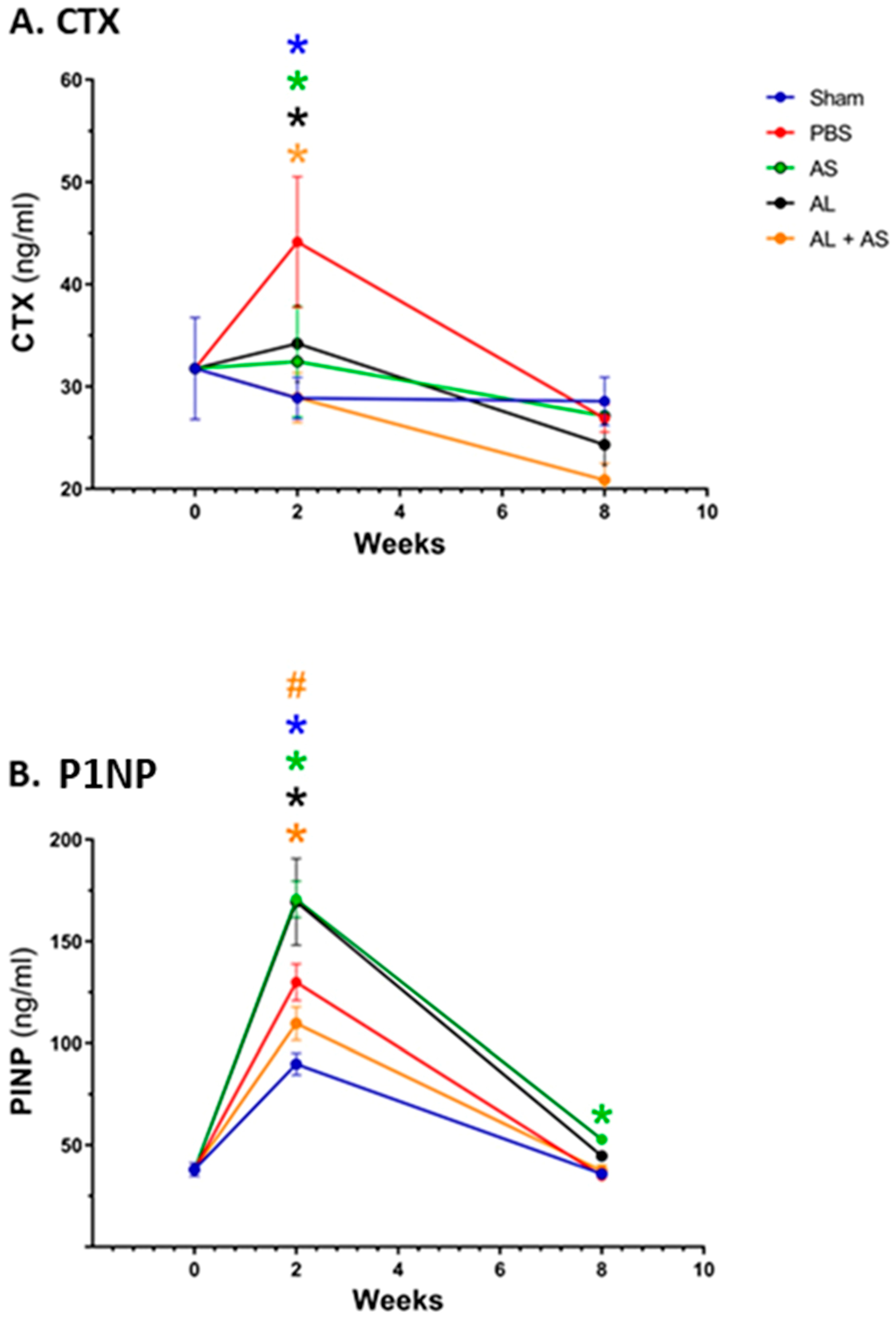
3.3. Effect of ASSNAC and Alendronate on Collagen Metabolism in OVX Mice
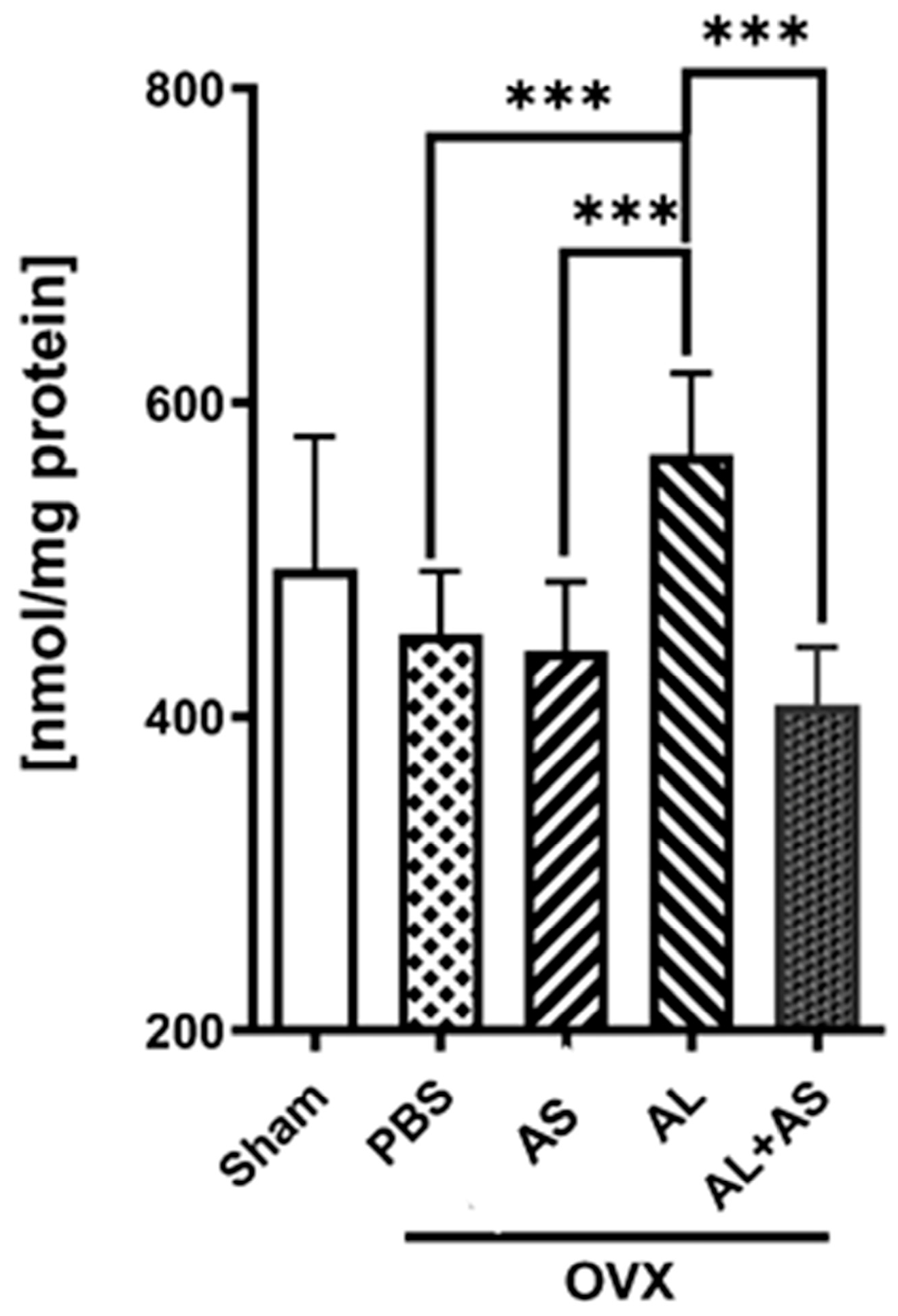
3.4. Effect of ASSNAC and Alendronate on Glutathione and MDA Levels in Femur-Derived BM in OVX Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Raisz, L.G. Pathogenesis of Osteoporosis: Concepts, Conflicts, and Prospects. J. Clin. Investig. 2005, 115, 3318–3325. [Google Scholar] [CrossRef]
- Kenkre, J.S.; Bassett, J.H.D. The Bone Remodelling Cycle. Ann. Clin. Biochem. 2018, 55, 308–327. [Google Scholar] [CrossRef] [PubMed]
- Väänänen, H.K.; Zhao, H.; Mulari, M.; Halleen, J.M. The Cell Biology of Osteoclast Function. J. Cell Sci. 2000, 113 Pt 3, 377–381. [Google Scholar] [CrossRef]
- Khosla, S.; Oursler, M.J.; Monroe, D.G. Estrogen and the Skeleton. Trends Endocrinol. Metab. 2012, 23, 576–581. [Google Scholar] [CrossRef]
- Frost, H.M.; Jee, W.S.S. On the Rat Model of Human Osteopenias and Osteoporosis. Bone Miner. 1992, 18, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Dempster, D.W.; Birchman, R.; Xu, R.; Lindsay, R.; Shen, V. Temporal Changes in Cancellous Bone-Structure of Rats Immediately After Ovariectomy. Bone 1995, 16, 157–161. [Google Scholar] [CrossRef]
- Kalu, D.N. The Ovariectomized Rat Model of Postmenopausal Bone Loss. Bone Miner. 1991, 15, 175–191. [Google Scholar] [CrossRef]
- Komori, T. Animal Models for Osteoporosis. Eur. J. Pharmacol. 2015, 759, 287–294. [Google Scholar] [CrossRef]
- Abuohashish, H.M.; Ahmed, M.M.; Al-Rejaie, S.S.; Eh Eltahir, K. Antidepressant Bupropion Exerts Alleviating Properties in an Ovariectomized Osteoporotic Rat Model. Acta Pharmacol. Sin. 2015, 36, 209–220. [Google Scholar] [CrossRef]
- Thompson, D.D.; Simmons, H.A.; Pirie, C.M.; Ke, H.Z. FDA guidelines and animal-models for osteoporosis. Bone 1995, 17, S125–S133. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.-A.; Kwak, M.-K. The Nrf2 System as a Potential Target for the Development of Indirect Antioxidants. Molecules 2010, 15, 7266–7291. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Heredia, A.; Kensicki, E.; Mohney, R.P.; Rull, A.; Triguero, I.; Marsillach, J.; Tormos, C.; Mackness, B.; Mackness, M.; Shih, D.M.; et al. Paraoxonase-1 Deficiency Is Associated with Severe Liver Steatosis in Mice Fed a High-Fat High-Cholesterol Diet: A Metabolomic Approach. J. Proteome Res. 2013, 12, 1946–1955. [Google Scholar] [CrossRef] [PubMed]
- Vaananen, H.K.; Laitala-Leinonen, T. Osteoclast Lineage and Function. Arch. Biochem. Biophys. 2008, 473, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Zhu, L.; Zhang, D.; Li, N.; Li, Q.; Dai, P.; Mao, Y.; Li, X.; Ma, J.; Huang, S. Oxidative Stress-Related Biomarkers in Postmenopausal Osteoporosis: A Systematic Review and Meta-Analyses. Dis. Markers 2016, 2016, 7067984. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Zhang, J.; Li, J.; Qin, R.; Chen, J.; Wang, R.; Goltzman, D.; Miao, D. Inhibition of Nrf2 Degradation Alleviates Age-Related Osteoporosis Induced by 1,25-Dihydroxyvitamin D Deficiency. Free Radic. Biol. Med. 2022, 178, 246–261. [Google Scholar] [CrossRef] [PubMed]
- Bollag, A.E.; Guo, T.; Ding, K.-H.; Choudhary, V.; Chen, X.; Zhong, Q.; Xu, J.; Yu, K.; Awad, M.E.; Elsalanty, M.; et al. Monomethylfumarate Protects against Ovariectomy-Related Changes in Body Composition. J. Endocrinol. 2019, 243, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Chen, X.; Chen, A.C.; Shi, Q.; Pan, G.; Pei, M.; Yang, H.; Liu, T.; He, F. Melatonin Restores the Osteoporosis-Impaired Osteogenic Potential of Bone Marrow Mesenchymal Stem Cells by Preserving SIRT1-Mediated Intracellular Antioxidant Properties. Free Radic. Biol. Med. 2020, 146, 92–106. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Wang, X.; Pan, Y.-J.; Shi, X.-F.; Yang, L.; Lou, Y.-L. Orientin Suppresses Osteoclastogenesis and Ameliorates Ovariectomy-Induced Osteoporosis via Suppressing ROS Production. Food Sci. Nutr. 2023, 11, 5582–5595. [Google Scholar] [CrossRef] [PubMed]
- Weinstein, R.S.; Roberson, P.K.; Manolagas, S.C. Giant Osteoclast Formation and Long-Term Oral Bisphosphonate Therapy. N. Engl. J. Med. 2009, 360, 53–62. [Google Scholar] [CrossRef]
- Lauritzen, D.B.; Balena, R.; Shea, M.; Seedor, J.G.; Markatos, A.; Le, H.M.; Toolan, B.C.; Myers, E.R.; Rodan, G.A.; Hayes, W.C. Effects of Combined Prostaglandin and Alendronate Treatment on the Histomorphometry and Biomechanical Properties of Bone in Ovariectomized Rats. J. Bone Miner. Res. 1993, 8, 871–879. [Google Scholar] [CrossRef]
- McClung, M.R.; Wasnich, R.D.; Hosking, D.J.; Christiansen, C.; Ravn, P.; Wu, M.; Mantz, A.M.; Yates, J.; Ross, P.D.; Santora, A.C.; et al. Prevention of Postmenopausal Bone Loss: Six-Year Results from the Early Postmenopausal Intervention Cohort Study. J. Clin. Endocrinol. Metab. 2004, 89, 4879–4885. [Google Scholar] [CrossRef] [PubMed]
- Rodan, G.A.; Martin, T.J. Therapeutic Approaches to Bone Diseases. Science 2000, 289, 1508–1514. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-H.; Park, E.-K.; Koo, D.-W.; Lee, S.; Lee, S.-H.; Kim, G.-T.; Lee, S.-G. Compliance and Persistence with Oral Bisphosphonates for the Treatment of Osteoporosis in Female Patients with Rheumatoid Arthritis. BMC Musculoskelet. Disord. 2017, 18, 152. [Google Scholar] [CrossRef] [PubMed]
- Kocer, G.; Naziroglu, M.; Celik, O.; Onal, L.; Ozcelik, D.; Kocer, M.; Sonmez, T.T. Basic Fibroblast Growth Factor Attenuates Bisphosphonate-Induced Oxidative Injury but Decreases Zinc and Copper Levels in Oral Epithelium of Rat. Biol. Trace Elem. Res. 2013, 153, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Karabulut, A.B.; Gui, M.; Karabulut, E.; Kiran, T.R.; Ocak, S.G.; Otlu, O. Oxidant and Antioxidant Activity in Rabbit Livers Treated With Zoledronic Acid. Transplant. Proc. 2010, 42, 3820–3822. [Google Scholar] [CrossRef] [PubMed]
- Habib, Z.A. Bisphosphonates in the Treatment of Osteoporosis: A Review of Skeletal Safety Concerns. Expert Rev. Endocrinol. Metab. 2017, 12, 59–71. [Google Scholar] [CrossRef] [PubMed]
- Yoon, R.S.; Hwang, J.S.; Beebe, K.S. Long-Term Bisphosphonate Usage and Subtrochanteric Insufficiency Fractures: A Cause for Concern? J. Bone Jt. Surg.-Br. Vol. 2011, 93, 1289–1295. [Google Scholar] [CrossRef]
- Rachner, T.D.; Khosla, S.; Hofbauer, L.C. Osteoporosis: Now and the Future. Lancet 2011, 377, 1276–1287. [Google Scholar] [CrossRef] [PubMed]
- Dell, R.M.; Adams, A.L.; Greene, D.F.; Funahashi, T.T.; Silverman, S.L.; Eisemon, E.O.; Zhou, H.; Burchette, R.J.; Ott, S.M. Incidence of Atypical Nontraumatic Diaphyseal Fractures of the Femur. J. Bone Miner. Res. 2012, 27, 2544–2550. [Google Scholar] [CrossRef]
- Nicolatou-Galitis, O.; Schiødt, M.; Mendes, R.A.; Ripamonti, C.; Hope, S.; Drudge-Coates, L.; Niepel, D.; Van den Wyngaert, T. Medication-Related Osteonecrosis of the Jaw: Definition and Best Practice for Prevention, Diagnosis, and Treatment. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2019, 127, 117–135. [Google Scholar] [CrossRef]
- Oh, Y.; Yamamoto, K.; Hashimoto, J.; Fujita, K.; Yoshii, T.; Fukushima, K.; Kurosa, Y.; Wakabayashi, Y.; Kitagawa, M.; Okawa, A. Biological Activity Is Not Suppressed in Mid-Shaft Stress Fracture of the Bowed Femoral Shaft Unlike in “Typical” Atypical Subtrochanteric Femoral Fracture: A Proposed Theory of Atypical Femoral Fracture Subtypes. Bone 2020, 137, 115453. [Google Scholar] [CrossRef]
- Taniguchi, N.; Osaki, M.; Onuma, K.; Ishikawa, M.; Ryoke, K.; Kodani, I.; Okada, F. Bisphosphonate-Induced Reactive Oxygen Species Inhibit Proliferation and Migration of Oral Fibroblasts: A Pathogenesis of Bisphosphonate-Related Osteonecrosis of the Jaw. J. Periodontol. 2020, 91, 947–955. [Google Scholar] [CrossRef] [PubMed]
- Bagan, J.; Saez, G.T.; Tormos, M.C.; Gavalda-Esteve, C.; Bagan, L.; Leopoldo-Rodado, M.; Calvo, J.; Camps, C. Oxidative Stress in Bisphosphonate-Related Osteonecrosis of the Jaws. J. Oral Pathol. Med. 2014, 43, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Murphy, T.H.; Miyamoto, M.; Sastre, A.; Schnaar, R.L.; Coyle, J.T. Glutamate toxicity in a neuronal cell-line involves inhibition of cystine transport leading to oxidative stress. Neuron 1989, 2, 1547–1558. [Google Scholar] [CrossRef]
- Kelsey, N.A.; Wilkins, H.M.; Linseman, D.A. Nutraceutical Antioxidants as Novel Neuroprotective Agents. Molecules 2010, 15, 7792–7814. [Google Scholar] [CrossRef] [PubMed]
- Moinova, H.R.; Mulcahy, R.T. Up-Regulation of the Human γ-Glutamylcysteine Synthetase Regulatory Subunit Gene Involves Binding of Nrf-2 to an Electrophile Responsive Element. Biochem. Biophys. Res. Commun. 1999, 261, 661–668. [Google Scholar] [CrossRef]
- Wang, Q.; Chuikov, S.; Taitano, S.; Wu, Q.; Rastogi, A.; Tuck, S.J.; Corey, J.M.; Lundy, S.K.; Mao-Draayer, Y. Dimethyl Fumarate Protects Neural Stem/Progenitor Cells and Neurons from Oxidative Damage through Nrf2-ERK1/2 MAPK Pathway. Int. J. Mol. Sci. 2015, 16, 13885–13907. [Google Scholar] [CrossRef]
- Kensler, T.W.; Wakabayash, N.; Biswal, S. Cell Survival Responses to Environmental Stresses via the Keap1-Nrf2-ARE Pathway. Annu. Rev. Pharmacol. Toxicol. 2007, 47, 89–116. [Google Scholar] [CrossRef]
- Ibanez, L.; Ferrandiz, M.L.; Brines, R.; Guede, D.; Cuadrado, A.; Alcaraz, M.J. Effects of Nrf2 Deficiency on Bone Microarchitecture in an Experimental Model of Osteoporosis. Oxidative Med. Cell. Longev. 2014, 2014, 726590. [Google Scholar] [CrossRef]
- Sun, X.; Xie, Z.; Hu, B.; Zhang, B.; Ma, Y.; Pan, X.; Huang, H.; Wang, J.; Zhao, X.; Jie, Z.; et al. The Nrf2 Activator RTA-408 Attenuates Osteoclastogenesis by Inhibiting STING Dependent NF-Κb Signaling. Redox Biol. 2020, 28, 101309. [Google Scholar] [CrossRef]
- Horev-Azaria, L.; Eliav, S.; Izigov, N.; Pri-Chen, S.; Mirelman, D.; Miron, T.; Rabinkov, A.; Wilchek, M.; Jacob-Hirsch, J.; Amariglio, N.; et al. Allicin Up-Regulates Cellular Glutathione Level in Vascular Endothelial Cells. Eur. J. Nutr. 2009, 48, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Izigov, N.; Farzam, N.; Savion, N. S-Allylmercapto-N-Acetylcysteine up-Regulates Cellular Glutathione and Protects Vascular Endothelial Cells from Oxidative Stress. Free Radic. Biol. Med. 2011, 50, 1131–1139. [Google Scholar] [CrossRef]
- Savion, N.; Izigov, N.; Morein, M.; Pri-Chen, S.; Kotev-Emeth, S. S-Allylmercapto-N-Acetylcysteine (ASSNAC) Protects Cultured Nerve Cells from Oxidative Stress and Attenuates Experimental Autoimmune Encephalomyelitis. Neurosci. Lett. 2014, 583, 108–113. [Google Scholar] [CrossRef]
- Savion, N.; Levine, A.; Kotev-Emeth, S.; Abu-Shach, U.B.; Broday, L. S-Allylmercapto-N-Acetylcysteine Protects against Oxidative Stress and Extends Lifespan in Caenorhabditis Elegans. PLoS ONE 2018, 13, e0194780. [Google Scholar] [CrossRef]
- Savion, N.; Dahamshi, S.; Morein, M.; Kotev-Emeth, S. S-Allylmercapro-N-Acetylcysteine Attenuates the Oxidation-Induced Lens Opacification and Retinal Pigment Epithelial Cell Death in Vitro. Antioxidants 2019, 8, 25. [Google Scholar] [CrossRef]
- Abu-Kheit, R.; Kotev-Emeth, S.; Hiram-Bab, S.; Gabet, Y.; Savion, N. S-Allylmercapto-N-Acetylcysteine Protects Bone Cells from Oxidation and Improves Femur Microarchitecture in Healthy and Diabetic Mice. Exp. Biol. Med. 2022, 247, 1489–1500. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Ye, W.; Liu, Y.; Niu, D.; Zhao, X.; Li, G.; Qu, Y.; Zhao, Z. S-Allylmercapto-N-Acetylcysteine Ameliorates Pulmonary Fibrosis in Mice via Nrf2 Pathway Activation and NF-κB, TGF-Β1/Smad2/3 Pathway Suppression. Biomed. Pharmacother. 2023, 157, 114018. [Google Scholar] [CrossRef]
- Watkins, M.P.; Norris, J.Y.; Grimston, S.K.; Zhang, X.; Phipps, R.J.; Ebetino, F.H.; Civitelli, R. Bisphosphonates Improve Trabecular Bone Mass and Normalize Cortical Thickness in Ovariectomized, Osteoblast Connexin43 Deficient Mice. Bone 2012, 51, 787–794. [Google Scholar] [CrossRef] [PubMed]
- Liron, T.; Raphael, B.; Hiram-Bab, S.; Bab, I.A.; Gabet, Y. Bone Loss in C57BL/6J-OlaHsd Mice, a Substrain of C57BL/6J Carrying Mutated Alpha-Synuclein and Multimerin-1 Genes. J. Cell. Physiol. 2018, 233, 371–377. [Google Scholar] [CrossRef]
- Bouxsein, M.L.; Boyd, S.K.; Christiansen, B.A.; Guldberg, R.E.; Jepsen, K.J.; Müller, R. Guidelines for Assessment of Bone Microstructure in Rodents Using Micro–Computed Tomography. J. Bone Miner. Res. 2010, 25, 1468–1486. [Google Scholar] [CrossRef]
- Gabet, Y.; Baniwal, S.K.; Leclerc, N.; Shi, Y.; Kohn-Gabet, A.E.; Cogan, J.; Dixon, A.; Bachar, M.; Guo, L.; Turman, J.E.J.; et al. Krox20/EGR2 Deficiency Accelerates Cell Growth and Differentiation in the Monocytic Lineage and Decreases Bone Mass. Blood 2010, 116, 3964–3971. [Google Scholar] [CrossRef] [PubMed]
- Anderson, M.E. Determination of Glutathione and Glutathione Disulfide in Biological Samples. Methods Enzymol. 1985, 113, 548–555. [Google Scholar] [PubMed]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein Measurement with the Folin Phenol Reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Draper, H.H.; Hadley, M. Malondialdehyde Determination as Index of Lipid-Peroxidation. Methods Enzymol. 1990, 186, 421–431. [Google Scholar] [PubMed]
- Oh, Y.; Ahn, C.-B.; Cho, W.H.; Yoon, N.Y.; Je, J.-Y. Anti-Osteoporotic Effects of Antioxidant Peptides PIISVYWK and FSVVPSPK from Mytilus Edulis on Ovariectomized Mice. Antioxidants 2020, 9, 866. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Shi, X.; Xie, J.; Weng, S.-J.; Xie, Z.-J.; Tang, J.-H.; Yan, D.-Y.; Wang, B.-Z.; Fang, K.-H.; Hong, C.-X.; et al. Apelin-13 Induces Mitophagy in Bone Marrow Mesenchymal Stem Cells to Suppress Intracellular Oxidative Stress and Ameliorate Osteoporosis by Activation of AMPK Signaling Pathway. Free Radic. Biol. Med. 2021, 163, 356–368. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-W.; Lin, H.-C.; Wang, B.Y.-H.; Wang, A.Y.-F.; Shin, R.L.-Y.; Cheung, S.Y.L.; Lee, O.K.-S. Ginkgolide B Monotherapy Reverses Osteoporosis by Regulating Oxidative Stress-Mediated Bone Homeostasis. Free Radic. Biol. Med. 2021, 168, 234–246. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Lee, S.-H.; Song, S.-J.; Kim, W.H.; Song, E.-S.; Lee, J.-C.; Lee, S.-J.; Han, D.-W.; Lee, J.-H. Protective Effects of Melon Extracts on Bone Strength, Mineralization, and Metabolism in Rats with Ovariectomy-Induced Osteoporosis. Antioxidants 2019, 8, 306. [Google Scholar] [CrossRef] [PubMed]
- Ozturk, S.; Cuneyit, I.; Altuntas, F.; Karagur, E.R.; Donmez, A.C.; Ocak, M.; Unal, M.; Sarikanat, M.; Donmez, B.O. Resveratrol Prevents Ovariectomy-Induced Bone Quality Deterioration by Improving the Microarchitectural and Biophysicochemical Properties of Bone. J. Bone Miner. Metab. 2023, 41, 443–456. [Google Scholar] [CrossRef]
- Xu, Y.; Song, D.; Lin, X.; Peng, H.; Su, Y.; Liang, J.; Hai, N.; Zhao, J.; Liu, Q. Corylifol A Protects against Ovariectomized-Induced Bone Loss and Attenuates RANKL-Induced Osteoclastogenesis via ROS Reduction, ERK Inhibition, and NFATc1 Activation. Free Radic. Biol. Med. 2023, 196, 121–132. [Google Scholar] [CrossRef]
- Rao, L.G.; Rao, A.V. Oxidative Stress and Antioxidants in the Risk of Osteoporosis—Role of the Antioxidants Lycopene and Polyphenols. In Topics in Osteoporosis; Flores, M.V., Ed.; IntechOpen: Rijeka, Croatia, 2013. [Google Scholar]
- Rissanen, J.P.; Suominen, M.I.; Peng, Z.; Morko, J.; Rasi, S.; Risteli, J.; Halleen, J.M. Short-Term Changes in Serum PINP Predict Long-Term Changes in Trabecular Bone in the Rat Ovariectomy Model. Calcif. Tissue Int. 2008, 82, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Nojiri, H.; Saita, Y.; Morikawa, D.; Kobayashi, K.; Tsuda, C.; Miyazaki, T.; Saito, M.; Marumo, K.; Yonezawa, I.; Kaneko, K.; et al. Cytoplasmic Superoxide Causes Bone Fragility Owing to Low-Turnover Osteoporosis and Impaired Collagen Cross-Linking. J. Bone Miner. Res. 2011, 26, 2682–2694. [Google Scholar] [CrossRef] [PubMed]
- Basu, S.; Michaelsson, K.; Olofsson, H.; Johansson, S.; Melhus, H. Association between Oxidative Stress and Bone Mineral Density. Biochem. Biophys. Res. Commun. 2001, 288, 275–279. [Google Scholar] [CrossRef] [PubMed]
- Cervellati, C.; Bonaccorsi, G.; Cremonini, E.; Bergamini, C.M.; Patella, A.; Castaldini, C.; Ferrazzini, S.; Capatti, A.; Picarelli, V.; Pansini, F.S.; et al. Bone Mass Density Selectively Correlates with Serum Markers of Oxidative Damage in Post-Menopausal Women. Clin. Chem. Lab. Med. 2013, 51, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Lean, J.M.; Davies, J.T.; Fuller, K.; Jagger, C.J.; Kirstein, B.; Partington, G.A.; Urry, Z.L.; Chambers, T.J. A Crucial Role for Thiol Antioxidants in Estrogen-Deficiency Bone Loss. J. Clin. Investig. 2003, 112, 915–923. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Shi, Z.-G.; Yu, Y.-S.; Hu, J.; Wang, R.; Luan, Z.-P.; Guo, D.-H. Protection against Osteoporosis by Statins Is Linked to a Reduction of Oxidative Stress and Restoration of Nitric Oxide Formation in Aged and Ovariectomized Rats. Eur. J. Pharmacol. 2012, 674, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Du, J.; Sun, J.; Li, M. Parathyroid Hormone-Related Protein Inhibits Nitrogen-Containing Bisphosphonate-Induced Apoptosis of Human Periodontal Ligament Fibroblasts by Activating MKP1 Phosphatase. Bioengineered 2021, 12, 1997–2006. [Google Scholar] [CrossRef]
- Tamaoka, J.; Takaoka, K.; Hattori, H.; Ueta, M.; Maeda, H.; Yamamura, M.; Yamanegi, K.; Noguchi, K.; Kishimoto, H. Osteonecrosis of the Jaws Caused by Bisphosphonate Treatment and Oxidative Stress in Mice. Exp. Ther. Med. 2019, 17, 1440–1448. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bleichman, I.; Hiram-Bab, S.; Gabet, Y.; Savion, N. S-Allylmercapto-N-Acetylcysteine (ASSNAC) Attenuates Osteoporosis in Ovariectomized (OVX) Mice. Antioxidants 2024, 13, 474. https://doi.org/10.3390/antiox13040474
Bleichman I, Hiram-Bab S, Gabet Y, Savion N. S-Allylmercapto-N-Acetylcysteine (ASSNAC) Attenuates Osteoporosis in Ovariectomized (OVX) Mice. Antioxidants. 2024; 13(4):474. https://doi.org/10.3390/antiox13040474
Chicago/Turabian StyleBleichman, Itay, Sahar Hiram-Bab, Yankel Gabet, and Naphtali Savion. 2024. "S-Allylmercapto-N-Acetylcysteine (ASSNAC) Attenuates Osteoporosis in Ovariectomized (OVX) Mice" Antioxidants 13, no. 4: 474. https://doi.org/10.3390/antiox13040474
APA StyleBleichman, I., Hiram-Bab, S., Gabet, Y., & Savion, N. (2024). S-Allylmercapto-N-Acetylcysteine (ASSNAC) Attenuates Osteoporosis in Ovariectomized (OVX) Mice. Antioxidants, 13(4), 474. https://doi.org/10.3390/antiox13040474








