Corilagin Inhibits Neutrophil Extracellular Trap Formation and Protects against Hydrochloric Acid/Lipopolysaccharide-Induced Acute Lung Injury in Mice by Suppressing the STAT3 and NOX2 Signaling Pathways
Abstract
:1. Introduction
2. Materials and Methods
2.1. Human Neutrophil Preparation
2.2. Immunofluorescence Staining for Human Neutrophils
2.3. Animals
2.4. Experimental Protocols
2.5. Histology and Immunohistochemistry
2.6. Expression of Neutrophil Extracellular Traps (NETs)
2.7. Measurement of TNF-α and IL-6 Levels in Lung Tissue
2.8. Measurement of Tissue MDA and GSH Levels
2.9. Western Blotting
2.10. Statistical Analysis
3. Results
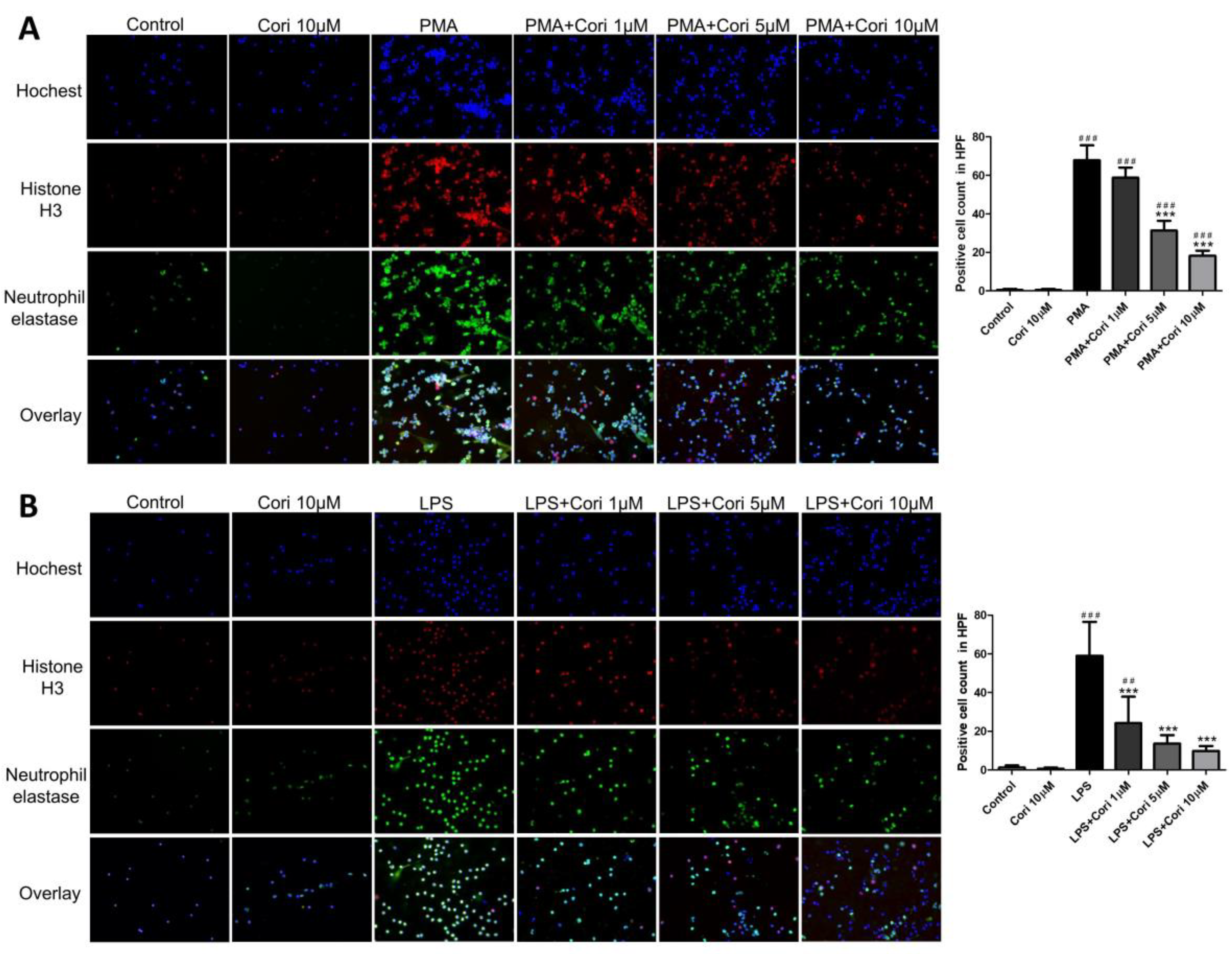
3.1. Effects of Corilagin on the Formation of NETs in Activated Human Neutrophils
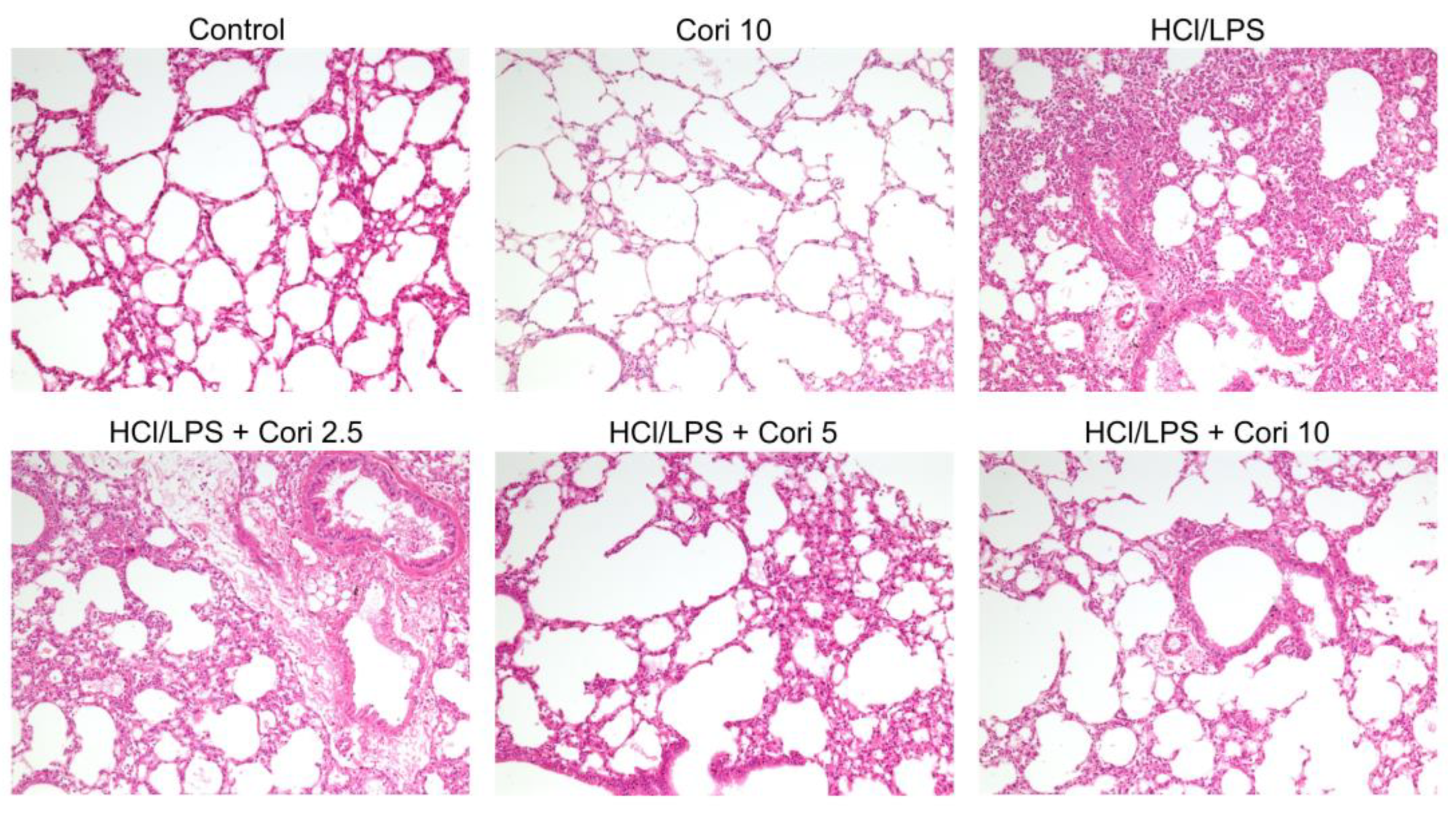
3.2. Effects of Corilagin on Histological Changes in the Lung following HCl/LPS-Induced Lung Injury
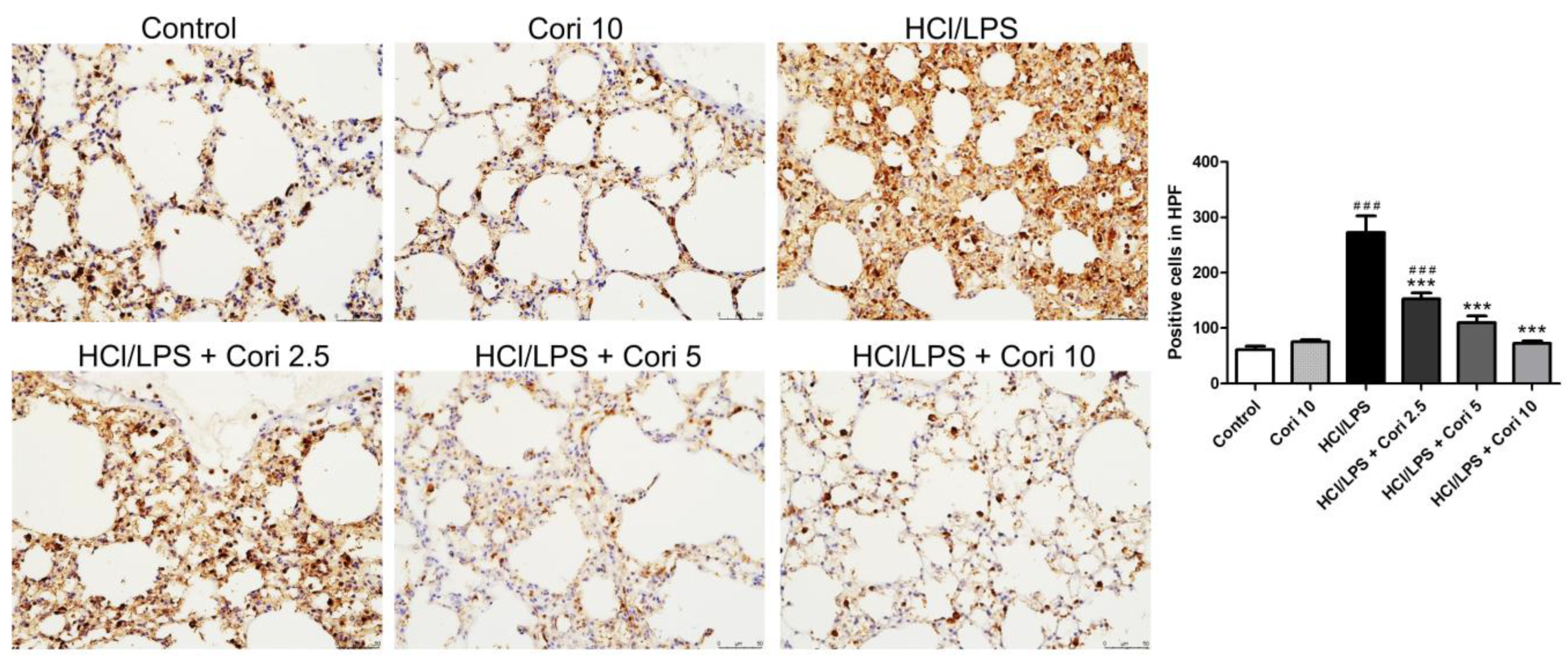
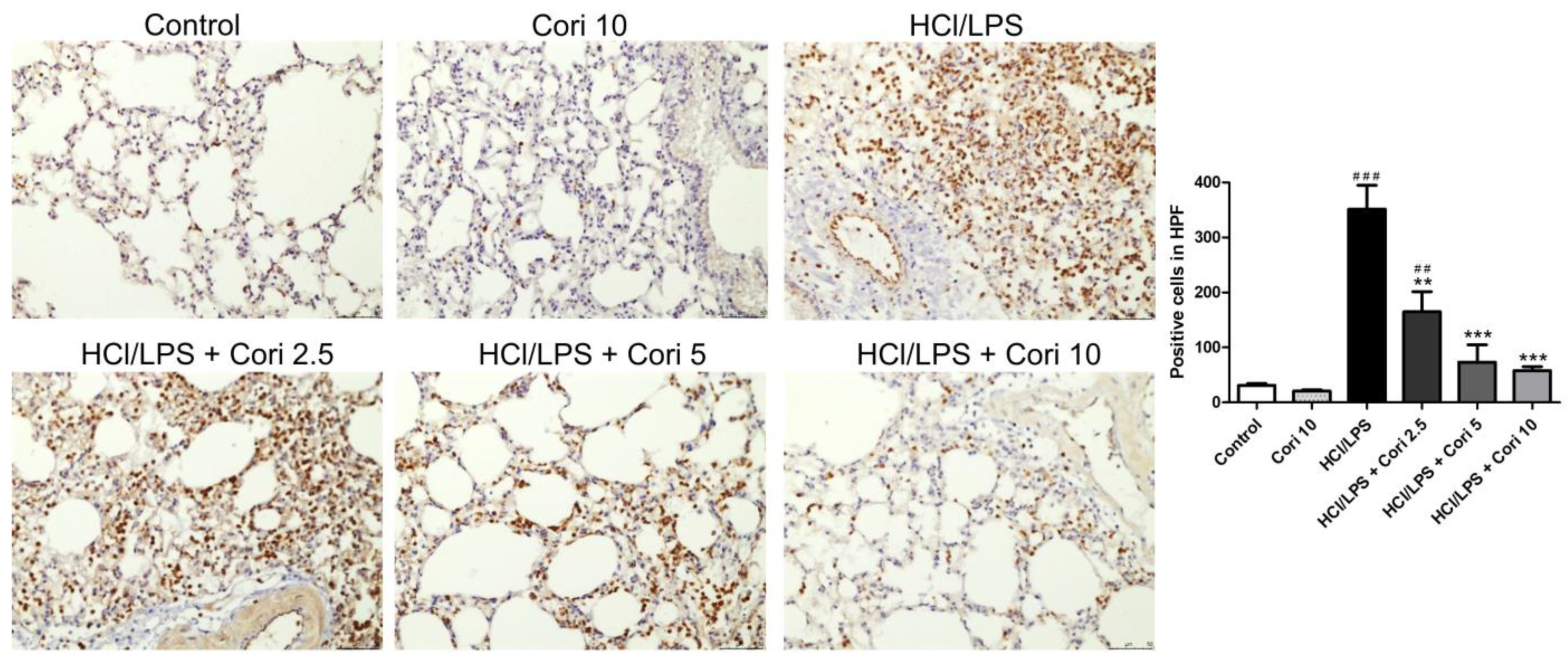
3.3. Effects of Corilagin on the Infiltration of Neutrophils and Macrophages in HCl/LPS-Induced Lung Injury
3.4. Effects of Corilagin on Pneumonic TNF-α and IL-6 Levels in HCl/LPS-Induced Lung Injury
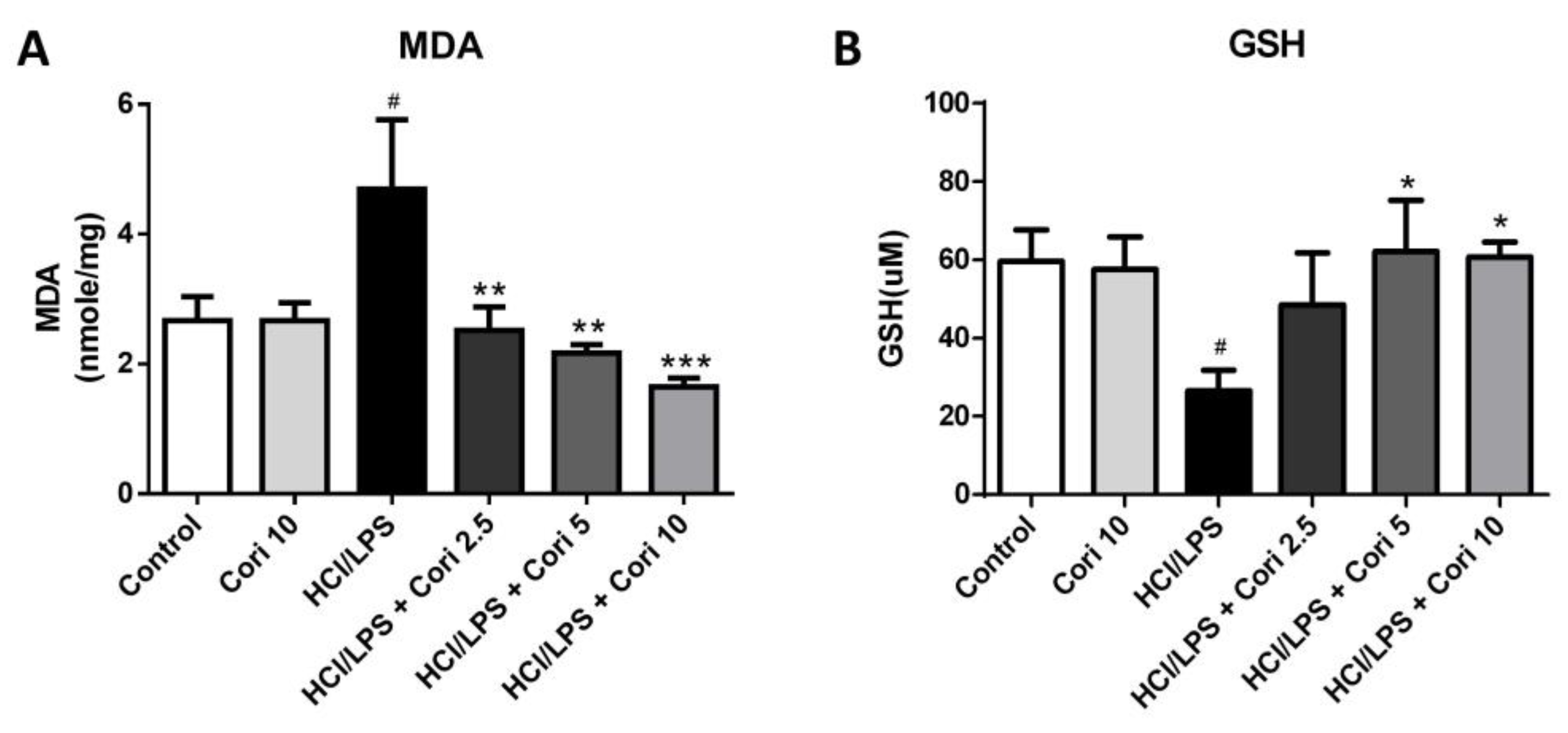
3.5. Effects of Corilagin on Pneumonic MDA and GSH Levels in HCl/LPS-Induced Lung Injury
3.6. Effects of Corilagin on Pneumonic Neutrophil Elastase Expression in HCl/LPS-Induced Lung Injury
3.7. Effects of Corilagin on the Formation of NETs in HCl/LPS-Induced Lung Injury
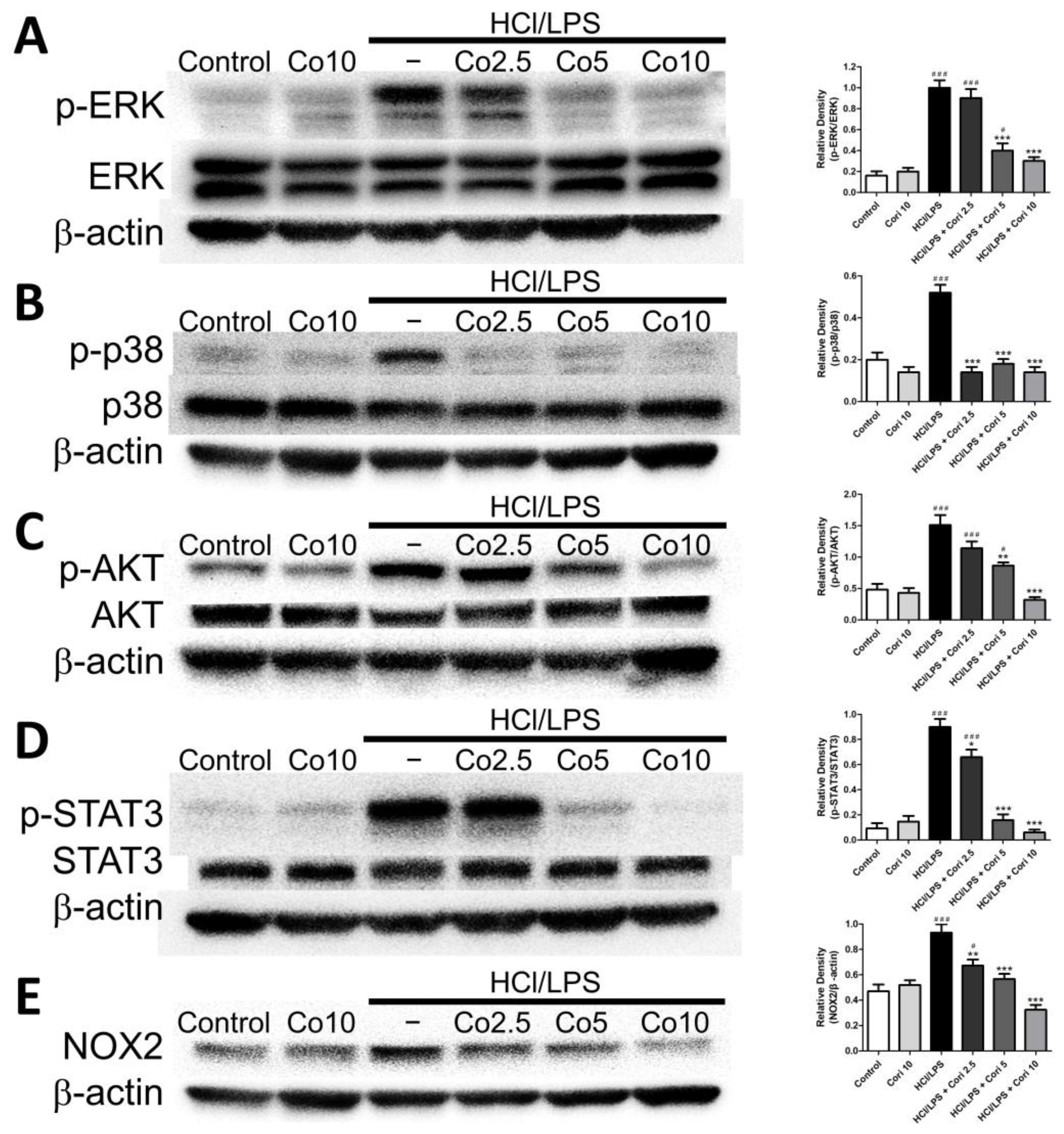
3.8. Effects of Corilagin on Pneumonic ERK, p38, AKT, STAT3, and NOX2 Expression in HCl/LPS-Induced Lung Injury
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Matuschak, G.M.; Lechner, A.J. Acute lung injury and the acute respiratory distress syndrome: Pathophysiology and treatment. Mo. Med. 2010, 107, 252–258. [Google Scholar] [PubMed]
- Zhu, W.; Zhang, Y.; Wang, Y. Immunotherapy strategies and prospects for acute lung injury: Focus on immune cells and cytokines. Front. Pharmacol. 2022, 13, 1103309. [Google Scholar] [CrossRef] [PubMed]
- Vassiliou, A.G.; Kotanidou, A.; Dimopoulou, I.; Orfanos, S.E. Endothelial Damage in Acute Respiratory Distress Syndrome. Int. J. Mol. Sci. 2020, 21, 8793. [Google Scholar] [CrossRef] [PubMed]
- DiSilvio, B.; Young, M.; Gordon, A.; Malik, K.; Singh, A.; Cheema, T. Complications and Outcomes of Acute Respiratory Distress Syndrome. Crit. Care Nurs. Q. 2019, 42, 349–361. [Google Scholar] [CrossRef] [PubMed]
- Chimenti, L.; Morales-Quinteros, L.; Puig, F.; Camprubi-Rimblas, M.; Guillamat-Prats, R.; Gomez, M.N.; Tijero, J.; Blanch, L.; Matute-Bello, G.; Artigas, A. Comparison of direct and indirect models of early induced acute lung injury. Intensive Care Med. Exp. 2020, 8, 62. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Yang, Q.; Xu, J.; Qian, G.; Liu, Y. Effects of HMGB1 on PMN apoptosis during LPS-induced acute lung injury. Exp. Mol. Pathol. 2008, 85, 214–222. [Google Scholar] [CrossRef] [PubMed]
- El-Shahat, R.A.; El-Demerdash, R.S.; El Sherbini, E.S.; Saad, E.A. HCl-induced acute lung injury: A study of the curative role of mesenchymal stem/stromal cells and cobalt protoporphyrin. J. Genet. Eng. Biotechnol. 2021, 19, 41. [Google Scholar] [CrossRef]
- Malech, H.L.; Deleo, F.R.; Quinn, M.T. The role of neutrophils in the immune system: An overview. Methods Mol. Biol. 2014, 1124, 3–10. [Google Scholar] [CrossRef]
- Czaikoski, P.G.; Mota, J.M.; Nascimento, D.C.; Sonego, F.; Castanheira, F.V.; Melo, P.H.; Scortegagna, G.T.; Silva, R.L.; Barroso-Sousa, R.; Souto, F.O.; et al. Neutrophil Extracellular Traps Induce Organ Damage during Experimental and Clinical Sepsis. PLoS ONE 2016, 11, e0148142. [Google Scholar] [CrossRef]
- Mutua, V.; Gershwin, L.J. A Review of Neutrophil Extracellular Traps (NETs) in Disease: Potential Anti-NETs Therapeutics. Clin. Rev. Allergy Immunol. 2021, 61, 194–211. [Google Scholar] [CrossRef]
- Edwards, N.J.; Hwang, C.; Marini, S.; Pagani, C.A.; Spreadborough, P.J.; Rowe, C.J.; Yu, P.; Mei, A.; Visser, N.; Li, S.; et al. The role of neutrophil extracellular traps and TLR signaling in skeletal muscle ischemia reperfusion injury. FASEB J. 2020, 34, 15753–15770. [Google Scholar] [CrossRef]
- Zhang, F.; Li, Y.; Wu, J.; Zhang, J.; Cao, P.; Sun, Z.; Wang, W. The role of extracellular traps in ischemia reperfusion injury. Front. Immunol. 2022, 13, 1022380. [Google Scholar] [CrossRef]
- Yu, S.; Liu, J.; Yan, N. Endothelial Dysfunction Induced by Extracellular Neutrophil Traps Plays Important Role in the Occurrence and Treatment of Extracellular Neutrophil Traps-Related Disease. Int. J. Mol. Sci. 2022, 23, 5626. [Google Scholar] [CrossRef]
- Block, H.; Rossaint, J.; Zarbock, A. The Fatal Circle of NETs and NET-Associated DAMPs Contributing to Organ Dysfunction. Cells 2022, 11, 1919. [Google Scholar] [CrossRef]
- Li, X.; Deng, Y.; Zheng, Z.; Huang, W.; Chen, L.; Tong, Q.; Ming, Y. Corilagin, a promising medicinal herbal agent. Biomed. Pharmacother. 2018, 99, 43–50. [Google Scholar] [CrossRef]
- Liu, F.C.; Yu, H.P.; Chou, A.H.; Lee, H.C.; Liao, C.C. Corilagin reduces acetaminophen-induced hepatotoxicity through MAPK and NF-kappaB signaling pathway in a mouse model. Am. J. Transl. Res. 2020, 12, 5597–5607. [Google Scholar]
- Tong, F.; Zhang, J.; Liu, L.; Gao, X.; Cai, Q.; Wei, C.; Dong, J.; Hu, Y.; Wu, G.; Dong, X. Corilagin Attenuates Radiation-Induced Brain Injury in Mice. Mol. Neurobiol. 2016, 53, 6982–6996. [Google Scholar] [CrossRef]
- Wang, Z.; Guo, Q.Y.; Zhang, X.J.; Li, X.; Li, W.T.; Ma, X.T.; Ma, L.J. Corilagin attenuates aerosol bleomycin-induced experimental lung injury. Int. J. Mol. Sci. 2014, 15, 9762–9779. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.C.; Liao, C.C.; Lee, H.C.; Chou, A.H.; Yu, H.P. Effects of Corilagin on Lipopolysaccharide-Induced Acute Lung Injury via Regulation of NADPH Oxidase 2 and ERK/NF-kappaB Signaling Pathways in a Mouse Model. Biology 2022, 11, 1058. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.C.; Fessler, M.B. Regulatory mechanisms of neutrophil migration from the circulation to the airspace. Cell Mol. Life Sci. 2021, 78, 4095–4124. [Google Scholar] [CrossRef]
- Klesney-Tait, J.; Keck, K.; Li, X.; Gilfillan, S.; Otero, K.; Baruah, S.; Meyerholz, D.K.; Varga, S.M.; Knudson, C.J.; Moninger, T.O.; et al. Transepithelial migration of neutrophils into the lung requires TREM-1. J. Clin. Investig. 2013, 123, 138–149. [Google Scholar] [CrossRef]
- Johnson, E.R.; Matthay, M.A. Acute lung injury: Epidemiology, pathogenesis, and treatment. J. Aerosol Med. Pulm. Drug Deliv. 2010, 23, 243–252. [Google Scholar] [CrossRef]
- Arango Duque, G.; Descoteaux, A. Macrophage cytokines: Involvement in immunity and infectious diseases. Front. Immunol. 2014, 5, 491. [Google Scholar] [CrossRef]
- Matthay, M.A.; Zemans, R.L. The acute respiratory distress syndrome: Pathogenesis and treatment. Annu. Rev. Pathol. 2011, 6, 147–163. [Google Scholar] [CrossRef]
- Matute-Bello, G.; Frevert, C.W.; Martin, T.R. Animal models of acute lung injury. Am. J. Physiol. Lung Cell Mol. Physiol. 2008, 295, L379–L399. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, J.; Ling, Z.; Zhang, J.; Zeng, Y.; Wang, K.; Zhang, Y.; Nong, L.; Sang, L.; Xu, Y.; et al. A diagnostic model for sepsis-induced acute lung injury using a consensus machine learning approach and its therapeutic implications. J. Transl. Med. 2023, 21, 620. [Google Scholar] [CrossRef]
- Yu, P.J.; Li, J.R.; Zhu, Z.G.; Kong, H.Y.; Jin, H.; Zhang, J.Y.; Tian, Y.X.; Li, Z.H.; Wu, X.Y.; Zhang, J.J.; et al. Praeruptorin D and E attenuate lipopolysaccharide/hydrochloric acid induced acute lung injury in mice. Eur. J. Pharmacol. 2013, 710, 39–48. [Google Scholar] [CrossRef]
- Kraus, R.F.; Gruber, M.A. Neutrophils-From Bone Marrow to First-Line Defense of the Innate Immune System. Front. Immunol. 2021, 12, 767175. [Google Scholar] [CrossRef]
- Vorobjeva, N.V.; Chernyak, B.V. NETosis: Molecular Mechanisms, Role in Physiology and Pathology. Biochemistry 2020, 85, 1178–1190. [Google Scholar] [CrossRef]
- Papayannopoulos, V. Neutrophil extracellular traps in immunity and disease. Nat. Rev. Immunol. 2018, 18, 134–147. [Google Scholar] [CrossRef]
- Papayannopoulos, V.; Metzler, K.D.; Hakkim, A.; Zychlinsky, A. Neutrophil elastase and myeloperoxidase regulate the formation of neutrophil extracellular traps. J. Cell Biol. 2010, 191, 677–691. [Google Scholar] [CrossRef]
- Scozzi, D.; Liao, F.; Krupnick, A.S.; Kreisel, D.; Gelman, A.E. The role of neutrophil extracellular traps in acute lung injury. Front. Immunol. 2022, 13, 953195. [Google Scholar] [CrossRef]
- Zou, S.; Jie, H.; Han, X.; Wang, J. The role of neutrophil extracellular traps in sepsis and sepsis-related acute lung injury. Int. Immunopharmacol. 2023, 124, 110436. [Google Scholar] [CrossRef]
- Liu, C.P.; Liu, J.X.; Gu, J.; Liu, F.; Li, J.H.; Bin, Y.; Yuan, Z.; Jie, L.; Wu, S.H.; Wu, Q.H.; et al. Combination Effect of Three Main Constituents from Sarcandra glabra Inhibits Oxidative Stress in the Mice Following Acute Lung Injury: A Role of MAPK-NF-kappaB Pathway. Front. Pharmacol. 2020, 11, 580064. [Google Scholar] [CrossRef]
- Li, R.; Zou, X.; Huang, H.; Yu, Y.; Zhang, H.; Liu, P.; Pan, S.; Ouyang, Y.; Shang, Y. HMGB1/PI3K/Akt/mTOR Signaling Participates in the Pathological Process of Acute Lung Injury by Regulating the Maturation and Function of Dendritic Cells. Front. Immunol. 2020, 11, 1104. [Google Scholar] [CrossRef]
- Gao, H.; Guo, R.F.; Speyer, C.L.; Reuben, J.; Neff, T.A.; Hoesel, L.M.; Riedemann, N.C.; McClintock, S.D.; Sarma, J.V.; Van Rooijen, N.; et al. Stat3 activation in acute lung injury. J. Immunol. 2004, 172, 7703–7712. [Google Scholar] [CrossRef]
- Liang, Y.; Luo, J.; Yang, N.; Wang, S.; Ye, M.; Pan, G. Activation of the IL-1beta/KLF2/HSPH1 pathway promotes STAT3 phosphorylation in alveolar macrophages during LPS-induced acute lung injury. Biosci. Rep. 2020, 40, BSR20193572. [Google Scholar] [CrossRef]
- Zhao, J.; Yu, H.; Liu, Y.; Gibson, S.A.; Yan, Z.; Xu, X.; Gaggar, A.; Li, P.K.; Li, C.; Wei, S.; et al. Protective effect of suppressing STAT3 activity in LPS-induced acute lung injury. Am. J. Physiol. Lung Cell Mol. Physiol. 2016, 311, L868–L880. [Google Scholar] [CrossRef]
- Zhang, H.; Sha, J.; Feng, X.; Hu, X.; Chen, Y.; Li, B.; Fan, H. Dexmedetomidine ameliorates LPS induced acute lung injury via GSK-3beta/STAT3-NF-kappaB signaling pathway in rats. Int. Immunopharmacol. 2019, 74, 105717. [Google Scholar] [CrossRef]
- Chen, I.C.; Wang, S.C.; Chen, Y.T.; Tseng, H.H.; Liu, P.L.; Lin, T.C.; Wu, H.E.; Chen, Y.R.; Tseng, Y.H.; Hsu, J.H.; et al. Corylin Ameliorates LPS-Induced Acute Lung Injury via Suppressing the MAPKs and IL-6/STAT3 Signaling Pathways. Pharmaceuticals 2021, 14, 1046. [Google Scholar] [CrossRef]
- Marimuthu, M.K.; Moorthy, A.; Ramasamy, T. Diallyl Disulfide Attenuates STAT3 and NF-kappaB Pathway Through PPAR-gamma Activation in Cerulein-Induced Acute Pancreatitis and Associated Lung Injury in Mice. Inflammation 2022, 45, 45–58. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.L.; Xie, J.; Liu, Q.; Wang, Y.; Li, H.R.; Yu, C.M.; Li, P.; Deng, X.M.; Bian, J.J.; Wang, J.F. PD-L1 promotes GSDMD-mediated NET release by maintaining the transcriptional activity of Stat3 in sepsis-associated encephalopathy. Int. J. Biol. Sci. 2023, 19, 1413–1429. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.C.; Lee, H.C.; Liao, C.C.; Chou, A.H.; Yu, H.P. Role of NADPH Oxidase-Derived ROS-Mediated IL-6/STAT3 and MAPK/NF-kappaB Signaling Pathways in Protective Effect of Corilagin against Acetaminophen-Induced Liver Injury in Mice. Biology 2023, 12, 334. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.C.; Yu, H.P.; Chen, P.J.; Yang, H.W.; Chang, S.H.; Tzeng, C.C.; Cheng, W.J.; Chen, Y.R.; Chen, Y.L.; Hwang, T.L. A novel NOX2 inhibitor attenuates human neutrophil oxidative stress and ameliorates inflammatory arthritis in mice. Redox Biol. 2019, 26, 101273. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Szczepaniak, W.S.; Shiva, S.; Liu, H.; Wang, Y.; Wang, L.; Wang, Y.; Kelley, E.E.; Chen, A.F.; Gladwin, M.T.; et al. Nox2-dependent glutathionylation of endothelial NOS leads to uncoupled superoxide production and endothelial barrier dysfunction in acute lung injury. Am. J. Physiol. Lung Cell Mol. Physiol. 2014, 307, L987–L997. [Google Scholar] [CrossRef] [PubMed]
- Fisher, A.B.; Dodia, C.; Chatterjee, S.; Feinstein, S.I. A Peptide Inhibitor of NADPH Oxidase (NOX2) Activation Markedly Decreases Mouse Lung Injury and Mortality Following Administration of Lipopolysaccharide (LPS). Int. J. Mol. Sci. 2019, 20, 2395. [Google Scholar] [CrossRef] [PubMed]
- Domer, D.; Walther, T.; Moller, S.; Behnen, M.; Laskay, T. Neutrophil Extracellular Traps Activate Proinflammatory Functions of Human Neutrophils. Front. Immunol. 2021, 12, 636954. [Google Scholar] [CrossRef]
- Hook, J.S.; Cao, M.; Potera, R.M.; Alsmadi, N.Z.; Schmidtke, D.W.; Moreland, J.G. Nox2 Regulates Platelet Activation and NET Formation in the Lung. Front. Immunol. 2019, 10, 1472. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, F.-C.; Yu, H.-P.; Liao, C.-C.; Chou, A.-H.; Lee, H.-C. Corilagin Inhibits Neutrophil Extracellular Trap Formation and Protects against Hydrochloric Acid/Lipopolysaccharide-Induced Acute Lung Injury in Mice by Suppressing the STAT3 and NOX2 Signaling Pathways. Antioxidants 2024, 13, 491. https://doi.org/10.3390/antiox13040491
Liu F-C, Yu H-P, Liao C-C, Chou A-H, Lee H-C. Corilagin Inhibits Neutrophil Extracellular Trap Formation and Protects against Hydrochloric Acid/Lipopolysaccharide-Induced Acute Lung Injury in Mice by Suppressing the STAT3 and NOX2 Signaling Pathways. Antioxidants. 2024; 13(4):491. https://doi.org/10.3390/antiox13040491
Chicago/Turabian StyleLiu, Fu-Chao, Huang-Ping Yu, Chia-Chih Liao, An-Hsun Chou, and Hung-Chen Lee. 2024. "Corilagin Inhibits Neutrophil Extracellular Trap Formation and Protects against Hydrochloric Acid/Lipopolysaccharide-Induced Acute Lung Injury in Mice by Suppressing the STAT3 and NOX2 Signaling Pathways" Antioxidants 13, no. 4: 491. https://doi.org/10.3390/antiox13040491
APA StyleLiu, F.-C., Yu, H.-P., Liao, C.-C., Chou, A.-H., & Lee, H.-C. (2024). Corilagin Inhibits Neutrophil Extracellular Trap Formation and Protects against Hydrochloric Acid/Lipopolysaccharide-Induced Acute Lung Injury in Mice by Suppressing the STAT3 and NOX2 Signaling Pathways. Antioxidants, 13(4), 491. https://doi.org/10.3390/antiox13040491









