Targeted Metabolomics Highlights Dramatic Antioxidant Depletion, Increased Oxidative/Nitrosative Stress and Altered Purine and Pyrimidine Concentrations in Serum of Primary Myelofibrosis Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients’ Recruitment and Criteria for Inclusion in the Study
2.2. Blood Sampling and Serum Processing for the HPLC Analysis of Metabolites
2.3. Analysis of Purines, Pyrimidines, Antioxidants, and Oxidative/Nitrosative Stress Biomarkers by HPLC
2.4. Statistical Analysis
3. Results
3.1. Clinical Features of PMF Patients
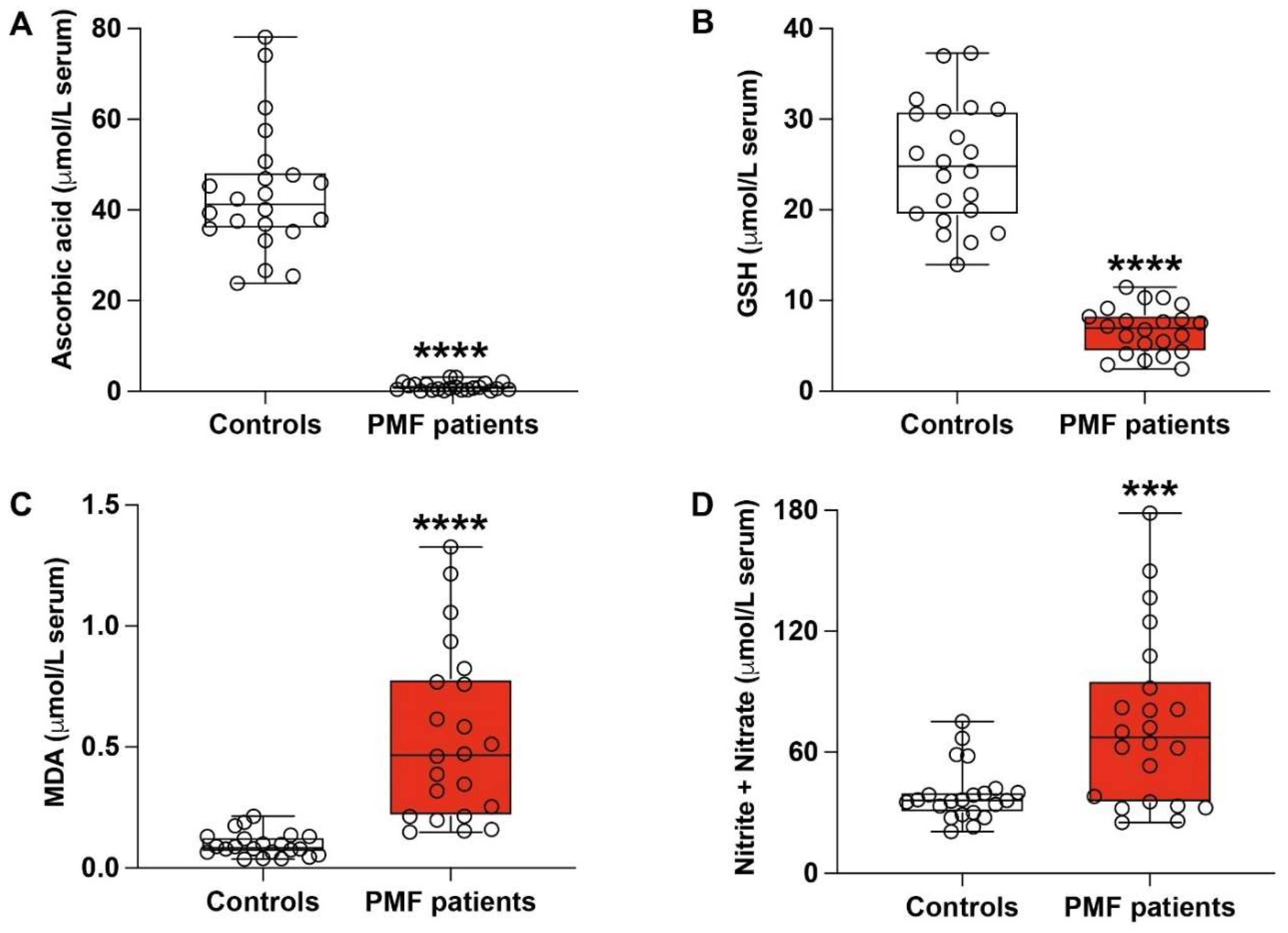
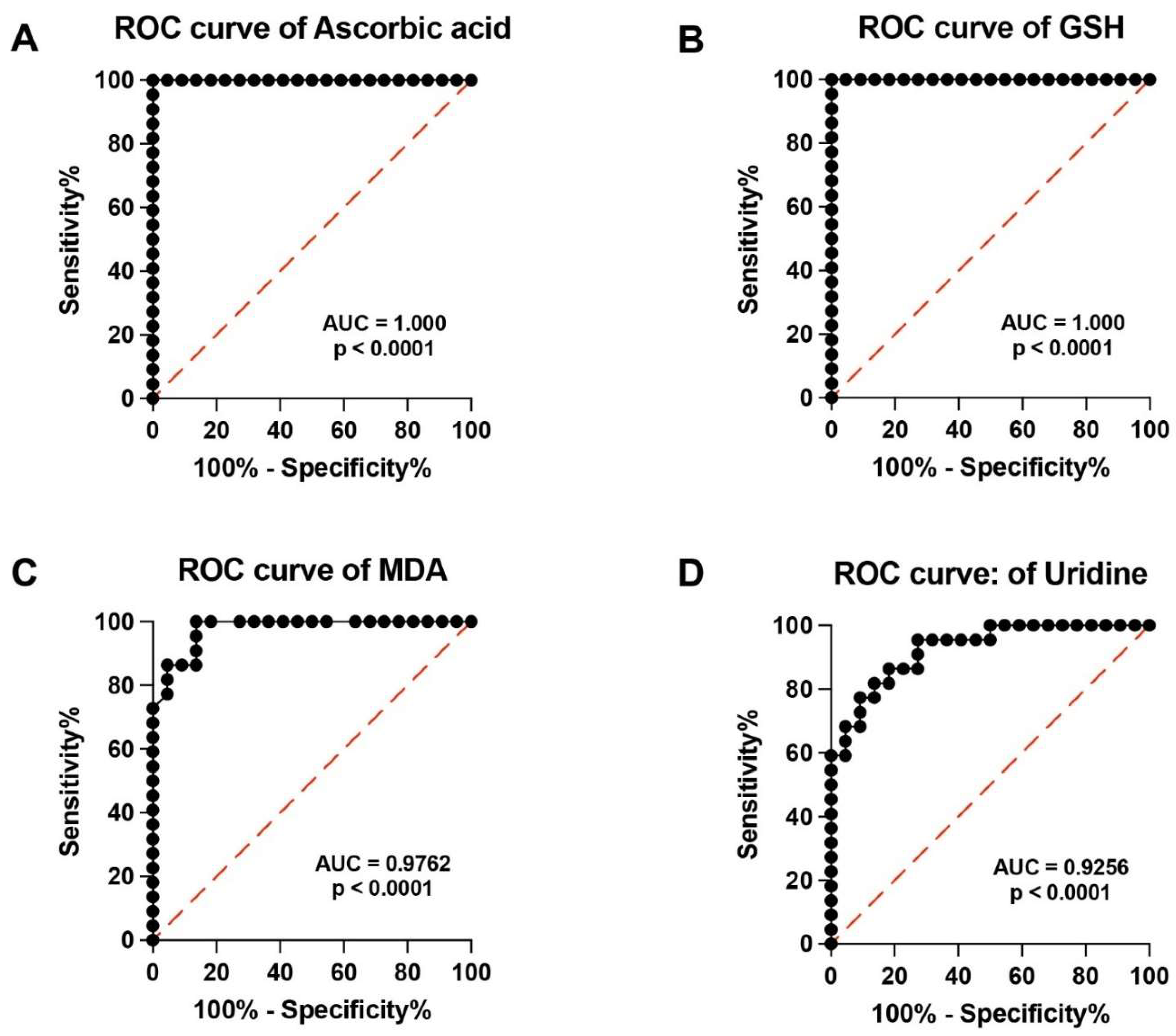
3.2. PMF Patients Have Dramatic Depletion of Serum Ascorbic Acid and Concomitant Marked Biochemical Evidence of Sustained Oxidative/Nitrosative Stress
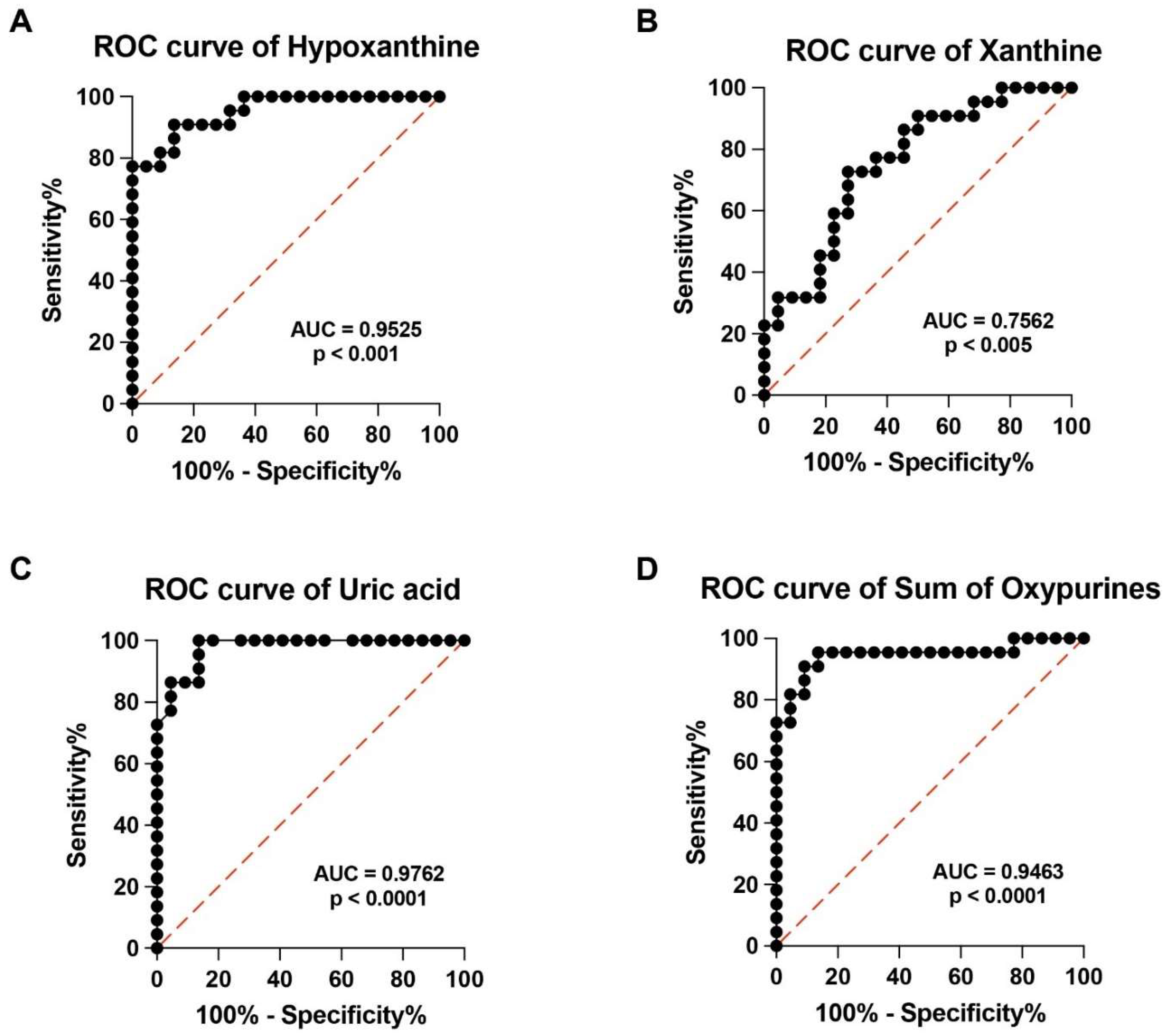
3.3. PMF Patients Have Altered Serum Profile of Purines, Pyrimidines and Creatinine
3.4. ROC Curves of Specific Metabolites Allow Clustering PMF Patients and Controls into Two Distinct Groups on the Basis of Their Respective Serum Metabolic Profiles
3.5. Correlation between Targeted Metabolomics and PMF Characteristics
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ghosh, K.; Shome, D.K.; Kulkarni, B.; Ghosh, M.K.; Ghosh, K. Fibrosis and bone marrow: Understanding causation and pathobiology. J. Transl. Med. 2023, 21, 703. [Google Scholar] [CrossRef] [PubMed]
- Spampinato, M.; Giallongo, C.; Romano, A.; Longhitano, L.; La Spina, E.; Avola, R.; Scandura, G.; Dulcamare, I.; Bramanti, V.; Di Rosa, M.; et al. Focus on Osteosclerotic Progression in Primary Myelofibrosis. Biomolecules 2021, 11, 122. [Google Scholar] [CrossRef] [PubMed]
- Hong, J. Prognostication in myeloproliferative neoplasms, including mutational abnormalities. Blood Res. 2023, 58, S37–S45. [Google Scholar] [CrossRef] [PubMed]
- Vannucchi, A.M.; Lasho, T.L.; Guglielmelli, P.; Biamonte, F.; Pardanani, A.; Pereira, A.; Finke, C.; Score, J.; Gangat, N.; Mannarelli, C.; et al. Mutations and prognosis in primary myelofibrosis. Leukemia 2013, 27, 1861–1869. [Google Scholar] [CrossRef] [PubMed]
- Waksal, J.A.; Mascarenhas, J. Novel Therapies in Myelofibrosis: Beyond JAK Inhibitors. Curr. Hematol. Malig. Rep. 2022, 17, 140–154. [Google Scholar] [CrossRef] [PubMed]
- Longhitano, L.; Li Volti, G.; Giallongo, C.; Spampinato, M.; Barbagallo, I.; Di Rosa, M.; Romano, A.; Avola, R.; Tibullo, D.; Palumbo, G.A. The Role of Inflammation and Inflammasome in Myeloproliferative Disease. J. Clin. Med. 2020, 9, 2334. [Google Scholar] [CrossRef] [PubMed]
- Allegra, A.; Pioggia, G.; Tonacci, A.; Casciaro, M.; Musolino, C.; Gangemi, S. Synergic Crosstalk between Inflammation, Oxidative Stress, and Genomic Alterations in BCR-ABL-Negative Myeloproliferative Neoplasm. Antioxidants 2020, 9, 1037. [Google Scholar] [CrossRef] [PubMed]
- Hole, P.S.; Darley, R.L.; Tonks, A. Do reactive oxygen species play a role in myeloid leukemias? Blood 2011, 117, 5816–5826. [Google Scholar] [CrossRef] [PubMed]
- Pietras, E.M. Inflammation: A key regulator of hematopoietic stem cell fate in health and disease. Blood 2017, 130, 1693–1698. [Google Scholar] [CrossRef]
- La Spina, E.; Giallongo, S.; Giallongo, C.; Vicario, N.; Duminuco, A.; Parenti, R.; Giuffrida, R.; Longhitano, L.; Li Volti, G.; Cambria, D.; et al. Mesenchymal stromal cells in tumor microenvironment remodeling of BCR-ABL negative myeloproliferative diseases. Front. Oncol. 2023, 3, 1141610. [Google Scholar] [CrossRef]
- Tefferi, A. Primary myelofibrosis: 2013 Update on diagnosis, risk-stratification, and management. Am. J. Hematol. 2013, 88, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Hasselbalch, H.C. Chronic inflammation as a promotor of mutagenesis in essential thrombocythemia, polycythemia vera and myelofibrosis. A human inflammation model for cancer development? Leuk. Res. 2013, 37, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Calle, N.; Pascual, M.; Ordonez, R.; Eneriz, E.S.J.; Kulis, M.; Miranda, E.; Guruceaga, E.; Segura, V.; Larrayoz, M.J.; Bellosillo, B.; et al. Epigenomic profiling of myelofibrosis reveals widespread DNA methylation changes in enhancer elements and ZFP36L1 as a potential tumor suppressor gene that is epigenetically regulated. Haematologica 2019, 104, 1572–1579. [Google Scholar] [CrossRef] [PubMed]
- Fourouclas, N.; Li, J.; Gilby, D.C.; Campbell, P.J.; Beer, P.A.; Boyd, E.M.; Goodeve, A.C.; Bareford, D.; Harrison, C.N.; Reilly, J.T.; et al. Methylation of the suppressor of cytokine signaling 3 gene (SOCS3) in myeloproliferative disorders. Haematologica 2008, 93, 1635–1644. [Google Scholar] [CrossRef] [PubMed]
- Bogani, C.; Ponziani, V.; Guglielmelli, P.; Desterke, C.; Rosti, V.; Bosi, A.; Le Bousse-Kerdiles, M.C.; Barosi, G.; Vannucchi, A.M. Myeloproliferative Disorders Research C: Hypermethylation of CXCR4 promoter in CD34+ cells from patients with primary myelofibrosis. Stem Cells 2008, 26, 1920–1930. [Google Scholar] [CrossRef] [PubMed]
- Hasselbalch, H.C. Perspectives on chronic inflammation in essential thrombocythemia, polycythemia vera, and myelofibrosis: Is chronic inflammation a trigger and driver of clonal evolution and development of accelerated atherosclerosis and second cancer? Blood 2012, 119, 3219–3225. [Google Scholar] [CrossRef] [PubMed]
- Genovese, E.; Mirabile, M.; Rontauroli, S.; Sartini, S.; Fantini, S.; Tavernari, L.; Maccaferri, M.; Guglielmelli, P.; Bianchi, E.; Parenti, S.; et al. On Behalf Of The MynervaMYeloidNEoplasmsResearch Venture Airc. The Response to Oxidative Damage Correlates with Driver Mutations and Clinical Outcome in Patients with Myelofibrosis. Antioxidants 2022, 11, 113. [Google Scholar] [CrossRef] [PubMed]
- Duminuco, A.; Torre, E.; Palumbo, G.A.; Harrison, C. A Journey Through JAK Inhibitors for the Treatment of Myeloproliferative Diseases. Curr. Hematol. Malig. Rep. 2023, 18, 176–189. [Google Scholar] [CrossRef] [PubMed]
- Tefferi, A.; Pardanani, A.; Gangat, N. Momelotinib expands the therapeutic armamentarium for myelofibrosis: Impact on hierarchy of treatment choices. Am. J. Hematol. 2024, 99, 300–308. [Google Scholar] [CrossRef]
- Yaylali, Y.T.; Yilmaz, S.; Akgun-Cagliyan, G.; Kilic, O.; Kaya, E.; Senol, H.; Ozen, F. Association of Disease Subtype and Duration with Echocardiographic Evidence of Pulmonary Hypertension in Myeloproliferative Neoplasm. Med. Princ. Pract. 2020, 29, 486–491. [Google Scholar] [CrossRef]
- Lucijanic, M.; Krecak, I.; Galusic, D.; Sedinic, M.; Holik, H.; Perisa, V.; MoricPeric, M.; Zekanovic, I.; Stoos-Veic, T.; Pejsa, V.; et al. Higher serum uric acid is associated with higher risks of thrombosis and death in patients with primary myelofibrosis. Wien. Klin. Wochenschr. 2022, 134, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Lazzarino, G.; Raatikainen, P.; Nuutinen, M.; Nissinen, J.; Tavazzi, B.; Di Pierro, D.; Giardina, B.; Peuhkurinen, K. Myocardial release of malondialdehyde and purine compounds during coronary bypass surgery. Circulation 1994, 90, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Lazzarino, G.; Mangione, R.; Belli, A.; Di Pietro, V.; Nagy, Z.; Barnes, N.M.; Bruce, L.; Ropero, B.M.; Persson, L.I.; Manca, B.; et al. ILB® Attenuates Clinical Symptoms and Serum Biomarkers of Oxidative/Nitrosative Stress and Mitochondrial Dysfunction in Patients with Amyotrophic Lateral Sclerosis. J. Pers. Med. 2021, 11, 794. [Google Scholar] [CrossRef] [PubMed]
- Lazzarino, G.; Amorini, A.M.; Petzold, A.; Gasperini, C.; Ruggieri, S.; Quartuccio, M.E.; Lazzarino, G.; Di Stasio, E.; Tavazzi, B. Serum Compounds of Energy Metabolism Impairment Are Related to Disability, Disease Course and Neuroimaging in Multiple Sclerosis. Mol. Neurobiol. 2017, 54, 7520–7533. [Google Scholar] [CrossRef] [PubMed]
- Arber, D.A.; Orazi, A.; Hasserjian, R.P.; Borowitz, M.J.; Calvo, K.R.; Kvasnicka, H.M.; Wang, S.A.; Bagg, A.; Barbui, T.; Branford, S.; et al. International Consensus Classification of Myeloid Neoplasms and Acute Leukemias: Integrating morphologic, clinical, and genomic data. Blood 2022, 140, 1200–1228. [Google Scholar] [CrossRef] [PubMed]
- Tavazzi, B.; Lazzarino, G.; Leone, P.; Amorini, A.M.; Bellia, F.; Janson, C.G.; Di Pietro, V.; Ceccarelli, L.; Donzelli, S.; Francis, J.S.; et al. Simultaneous high performance liquid chromatographic separation of purines, pyrimidines, N-acetylated amino acids, and dicarboxylic acids for the chemical diagnosis of inborn errors of metabolism. Clin. Biochem. 2005, 38, 997–1008. [Google Scholar] [CrossRef] [PubMed]
- Koyuncu, M.B.; Ilgan, M.; Basir, H.; Tombak, A.; Ucar, M.A.; Koseci, T.; Akdeniz, A.; Tiftik, E.N.; Erel, O. Ruxolitinib Reduces Oxidative Stress in Patients with Primary Myelofibrosis: A Multicenter Study. Cureus 2022, 14, e20929. [Google Scholar] [CrossRef] [PubMed]
- Djikic, D.; Markovic, D.; Bogdanovic, A.; Mitrovic-Ajtic, O.; Suboticki, T.; Diklic, M.; Beleslin-Cokic, B.; Bjelica, S.; Kovacic, M.; Cokic, V.P. Oxidative and nitrosative stress in myeloproliferative neoplasms: The impact on the AKT/mTOR signaling pathway. J. BUON 2018, 23, 1481–1491. [Google Scholar] [PubMed]
- Fu, J.; Wu, Z.; Liu, J.; Wu, T. Vitamin C: A stem cell promoter in cancer metastasis and immunotherapy. Biomed. Pharmacother. 2020, 131, 110588. [Google Scholar] [CrossRef]
- Mikirova, N.A.; Ichim, T.E.; Riordan, N.H. Anti-angiogenic effect of high doses of ascorbic acid. J. Transl. Med. 2008, 6, 50. [Google Scholar] [CrossRef]
- Travaglini, S.; Gurnari, C.; Antonelli, S.; Silvestrini, G.; Noguera, N.I.; Ottone, T.; Voso, M.T. The Anti-Leukemia Effect of Ascorbic Acid: From the Pro-Oxidant Potential to the Epigenetic Role in Acute Myeloid Leukemia. Front. Cell Dev. Biol. 2022, 10, 930205. [Google Scholar] [CrossRef] [PubMed]
- Agathocleous, M.; Meacham, C.E.; Burgess, R.J.; Piskounova, E.; Zhao, Z.; Crane, G.M.; Cowin, B.L.; Bruner, E.; Murphy, M.M.; Chen, W.; et al. Ascorbate regulates haematopoietic stem cell function and leukaemogenesis. Nature 2017, 549, 476–481. [Google Scholar] [CrossRef]
- Blaszczak, W.; Barczak, W.; Masternak, J.; Kopczynski, P.; Zhitkovich, A.; Rubis, B. Vitamin C as a Modulator of the Response to Cancer Therapy. Molecules 2019, 24, 453. [Google Scholar] [CrossRef]
- Yun, J.; Mullarky, E.; Lu, C.; Bosch, K.N.; Kavalier, A.; Rivera, K.; Roper, J.; Chio, I.I.; Giannopoulou, E.G.; Rago, C.; et al. Vitamin C selectively kills KRAS and BRAF mutant colorectal cancer cells by targeting GAPDH. Science 2015, 350, 1391–1396. [Google Scholar] [CrossRef] [PubMed]
- Fischer, A.P.; Miles, S.L. Ascorbic acid, but not dehydroascorbic acid increases intracellular vitamin C content to decrease Hypoxia Inducible Factor-1 alpha activity and reduce malignant potential in human melanoma. Biomed. Pharmacother. 2017, 86, 502–513. [Google Scholar] [CrossRef]
- Cimmino, L.; Neel, B.G.; Aifantis, I. Vitamin C in Stem Cell Reprogramming and Cancer. Trends Cell Biol. 2018, 28, 698–708. [Google Scholar] [CrossRef] [PubMed]
- Ohkura, N.; Yoshiba, K.; Yoshiba, N.; Edanami, N.; Ohshima, H.; Takenaka, S.; Noiri, Y. SVCT2-GLUT1-mediated ascorbic acid transport pathway in rat dental pulp and its effects during wound healing. Sci. Rep. 2023, 13, 1251. [Google Scholar] [CrossRef]
- Koyuncu, M.B.; Cavusoglu, C.; Basir, H.; Ilgan, M.; Ucar, M.A.; Akdeniz, A.; Tombak, A.; Tiftik, E.N.; Temel, G.O.; Neselioglu, S.; et al. Thiol/Disulfide Balance in Older Patients with BCR-ABL Negative Myeloproliferative Neoplasms. Clin. Lab. 2021, 67, 2700. [Google Scholar] [CrossRef]
- Gok, F.; Ekin, S.; Karaman, E.; Erten, R.; Yildiz, D.; Bakir, A. Total Sialic Acid, Antioxidant Enzyme Activities, Trace Elements, and Vitamin Status before and after Surgery in Women with Uterine Myoma and Endometrial Cancer. Reprod. Sci. 2023, 30, 2743–2757. [Google Scholar] [CrossRef]
- Ma, Z.; Yang, M.; Foda, M.F.; Zhang, K.; Li, S.; Liang, H.; Zhao, Y.; Han, H. Polarization of Tumor-Associated Macrophages Promoted by Vitamin C-Loaded Liposomes for Cancer Immunotherapy. ACS Nano 2022, 16, 17389–17401. [Google Scholar] [CrossRef]
- Morris, G.; Gevezova, M.; Sarafian, V.; Maes, M. Redox regulation of the immune response. Cell. Mol. Immunol. 2022, 19, 1079–1101. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.C.; Sindhu, H.; Chen, C.; Kundra, A.; Kafeel, M.I.; Wong, C.; Lichter, S. Immune derangements in patients with myelofibrosis: The role of Treg, Th17, and sIL2Ralpha. PLoS ONE 2015, 10, e0116723. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Liu, J.; Li, Z.; Xiong, H.; Zhang, Y.; Song, Y.; Lai, J. Expression profile analysis reveals hub genes that are associated with immune system dysregulation in primary myelofibrosis. Hematology 2021, 26, 478–490. [Google Scholar] [CrossRef]
- Lewis, C.M.; Pegrum, G.D. Immune complexes in myeloproliferative disorders. Lancet 1977, 2, 1151–1153. [Google Scholar] [CrossRef]
- Nesci, S.; Lenaz, G. Impaired Mitochondrial Bioenergetics under Pathological Conditions. Life 2022, 12, 205. [Google Scholar] [CrossRef]
- Domański, L.; Safranow, K.; Ostrowski, M.; Pawlik, A.; Olszewska, M.; Dutkiewicz, G.; Ciechanowski, K. Oxypurine and purine nucleoside concentrations in renal vein of allograft are potential markers of energy status of renal tissue. Arch. Med. Res. 2007, 38, 240–246. [Google Scholar] [CrossRef]
- Battelli, M.G.; Polito, L.; Bolognesi, A. Xanthine oxidoreductase in atherosclerosis pathogenesis: Not only oxidative stress. Atherosclerosis 2014, 237, 562–567. [Google Scholar] [CrossRef] [PubMed]
- Maiuolo, J.; Oppedisano, F.; Gratteri, S.; Muscoli, C.; Mollace, V. Regulation of uric acid metabolism and excretion. Int. J. Cardiol. 2015, 213, 8–14. [Google Scholar] [CrossRef]
- Burakowski, S.; Smoleński, R.T.; Bellwon, J.; Kubasik, A.; Ciećwierz, D.; Rynkiewicz, A. Exercise stress test and comparison of ST change with cardiac nucleotide catabolite production in patients with coronary artery disease. Cardiol. J. 2007, 14, 573–579. [Google Scholar] [PubMed]
- Harkness, R.A. Hypoxanthine, xanthine and uridine in body fluids, indicators of ATP depletion. J. Chromatogr. B 1988, 429, 255–278. [Google Scholar] [CrossRef]
- Kashani, K.; Rosner, M.H.; Ostermann, M. Creatinine: From physiology to clinical application. Eur. J. Intern. Med. 2020, 72, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.R.; Won, S.J.; Fabian, C.; Kang, M.G.; Szardenings, M.; Shin, M.G. Mitochondrial DNA aberrations and pathophysiological implications in hematopoietic diseases, chronic inflammatory diseases, and cancers. Ann. Lab. Med. 2015, 35, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Squadrito, G.L.; Cueto, R.; Splenser, A.E.; Valavanidis, A.; Zhang, H.; Uppu, R.M.; Pryor, W.A. Reaction of uric acid with peroxynitrite and implications for the mechanism of neuroprotection by uric acid. Arch. Biochem. Biophys. 2000, 376, 333–337. [Google Scholar] [CrossRef] [PubMed]
- Yeum, K.J.; Russell, R.M.; Krinsky, N.I.; Aldini, G. Biomarkers of antioxidant capacity in the hydrophilic and lipophilic compartments of human plasma. Arch. Biochem. Biophys. 2004, 430, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Di Pietro, V.; Lazzarino, G.; Amorini, A.M.; Tavazzi, B.; D’Urso, S.; Longo, S.; Vagnozzi, R.; Signoretti, S.; Clementi, E.; Giardina, B.; et al. Neuroglobin expression and oxidant/antioxidant balance after gradedtraumatic brain injury in the rat. Free Radic. Biol. Med. 2014, 69, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Kadowaki, D.; Sakaguchi, S.; Miyamoto, Y.; Taguchi, K.; Muraya, N.; Narita, Y.; Sato, K.; Chuang, V.T.; Maruyama, T.; Otagiri, M.; et al. Direct radical scavenging activity of benzbromarone provides beneficial antioxidant properties for hyperuricemia treatment. Biol. Pharm. Bull. 2015, 38, 487–492. [Google Scholar] [CrossRef]
- Maffioli, M.; Mora, B.; Ball, S.; Iurlo, A.; Elli, E.M.; Finazzi, M.C.; Polverelli, N.; Rumi, E.; Caramella, M.; Carraro, M.C.; et al. A prognostic model to predict survival after 6 months of ruxolitinib in patients with myelofibrosis. Blood Adv. 2022, 6, 1855–1864. [Google Scholar] [CrossRef]


| Patients | Healthy Controls | |
|---|---|---|
| Median Years of Age [Range] | 68 [41–76] | 63 [31–75] |
| Sex | 15 M (68%), 7 F (32%) | 13 M (59%), 9 (41%) |
| Blood count | ||
| 10.5 [6.3–17.2] | 13.8 [11.9–15.6] |
| 9.8 [1.3–149.1] | 6.1 [4.3–9.6] |
| 7.2 [1.3–127] | 3.8 [2.4–5.6] |
| 1.7 [0.4–12.2] | 3.2 [0.9–5.2] |
| 0.6 [0.2–1.6] | 0.5 [0.2–1.4] |
| 347 [70–1014] | 239 [146–401] |
| Blast > 5% | 2 (9.1%) | - |
| BM fibrosis | - | |
| 3 (13.6%) | |
| 10 (45.5%) | |
| 6 (27.3%) | |
| 3 (13.6%) | |
| Driver mutations | - | |
| 14 (63.71%) | |
| 3 (13.6%) | |
| 0 | |
| 3 (13.6%) | |
| 2 (9.1%) | |
| IPSS/MYSEC-PM | - | |
| 2 (9.1%) | |
| 5 (22.7%) | |
| 10 (45.5%) | |
| 5 (22.7%) |
| Hyp | Xan | UA | Ura | Uri | Creat | AA | GSH | MDA | Nitrite + Nitrate | |
|---|---|---|---|---|---|---|---|---|---|---|
| Age, <67 years (n = 11) vs. >68 years (n = 11) | 0.024 | 0.654 | 0.47 | 0.81 | 0.705 | 0.863 | 0.282 | 0.31 | 0.467 | 0.557 |
| Sex, M (n = 15) vs. F (n = 7) | 0.235 | >0.999 | 0.34 | 0.424 | 0.97 | 0.004 | 0.968 | 0.733 | 0.97 | 0.842 |
| BM fibrosis, 0–1 (n = 13) vs. 2–3 (n = 9) | 0.916 | 0.595 | 0.645 | 0.651 | 0.029 | >0.999 | 0.86 | 0.704 | 0.185 | 0.743 |
| Risk class, IPSS Low-Int-1 (n = 7) vs. Int-2-High (n = 15) | 0.287 | 0.361 | 0.443 | 0.037 | 0.535 | 0.856 | 0.804 | 0.332 | 0.11 | 0.016 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mangione, R.; Giallongo, C.; Duminuco, A.; La Spina, E.; Longhitano, L.; Giallongo, S.; Tibullo, D.; Lazzarino, G.; Saab, M.W.; Sbriglione, A.; et al. Targeted Metabolomics Highlights Dramatic Antioxidant Depletion, Increased Oxidative/Nitrosative Stress and Altered Purine and Pyrimidine Concentrations in Serum of Primary Myelofibrosis Patients. Antioxidants 2024, 13, 490. https://doi.org/10.3390/antiox13040490
Mangione R, Giallongo C, Duminuco A, La Spina E, Longhitano L, Giallongo S, Tibullo D, Lazzarino G, Saab MW, Sbriglione A, et al. Targeted Metabolomics Highlights Dramatic Antioxidant Depletion, Increased Oxidative/Nitrosative Stress and Altered Purine and Pyrimidine Concentrations in Serum of Primary Myelofibrosis Patients. Antioxidants. 2024; 13(4):490. https://doi.org/10.3390/antiox13040490
Chicago/Turabian StyleMangione, Renata, Cesarina Giallongo, Andrea Duminuco, Enrico La Spina, Lucia Longhitano, Sebastiano Giallongo, Daniele Tibullo, Giuseppe Lazzarino, Miriam Wissam Saab, Arianna Sbriglione, and et al. 2024. "Targeted Metabolomics Highlights Dramatic Antioxidant Depletion, Increased Oxidative/Nitrosative Stress and Altered Purine and Pyrimidine Concentrations in Serum of Primary Myelofibrosis Patients" Antioxidants 13, no. 4: 490. https://doi.org/10.3390/antiox13040490








