Blends of Organic Acids Are Weaponizing the Host iNOS and Nitric Oxide to Reduce Infection of Piscirickettsia salmonis in vitro
Abstract
:1. Introduction
2. Materials and Methods
2.1. Piscirickettsia salmonis, CHSE-214 Cell Lines and Organic Acid Blend
2.2. Minimum Inhibitory (MIC) and Minimum Bactericidal (MBC) Concentration against P. salmonis
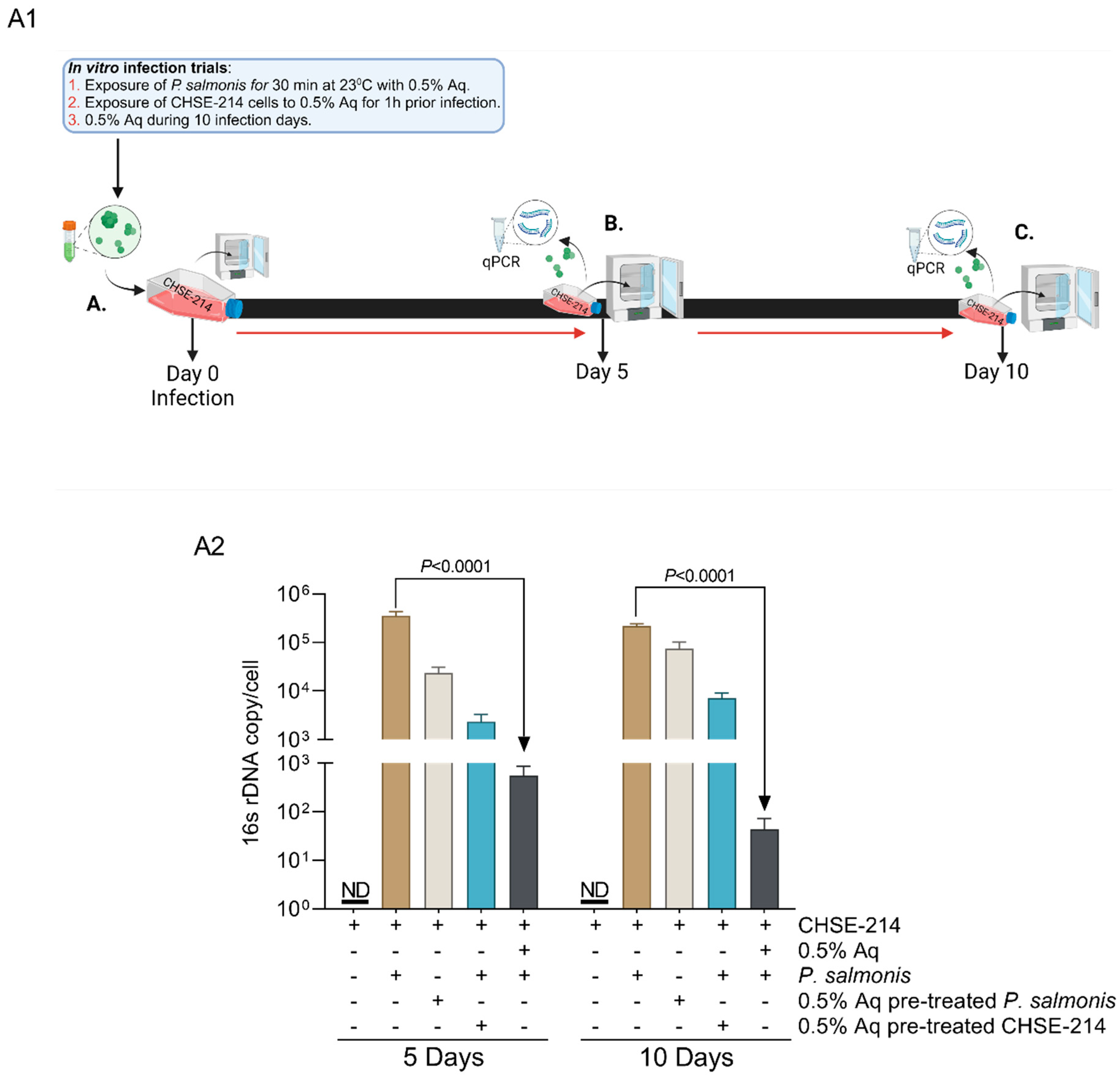
2.3. In Vitro Infection Assay and Intracellular Bacterial Quantification
2.4. Quantification of Antimicrobial and Anti-Inflammatory Activity in CHSE-214 Cells Infected with P. salmonis in the Presence of 0.5% Aq
2.5. Determination of Aq Cytotoxicity and and LDH Release
2.6. Nitric Oxide (NO) Measurement in Infected CHSE-214 Cells Treated with Aq
2.7. P. salmonis Lipid Peroxidation (TBARS Assay)
2.8. Statistical Analysis
3. Results
3.1. Minimum Inhibitory Concentration (MIC), Minimum Bactericidal Concentration (MBC), and the In Vitro Cytotoxic Effect of Aq
3.2. In Vitro, the Organic Acid Blend Prevents P. salmonis Infection in CHSE-214 Cells
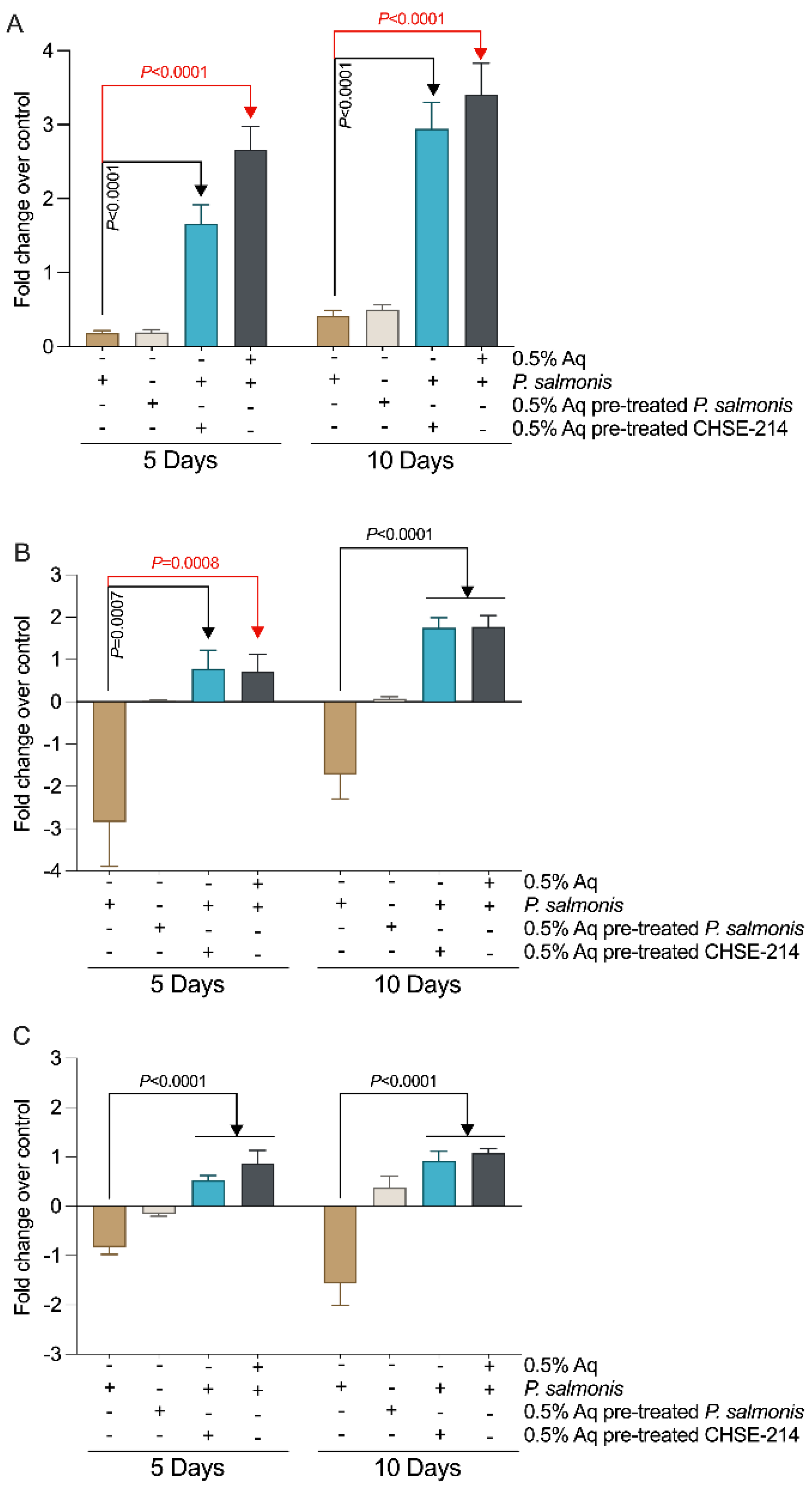
3.3. The Effect of Aq on Immune Gene Expression in P. salmonis-Infected CHSE-214 Cells
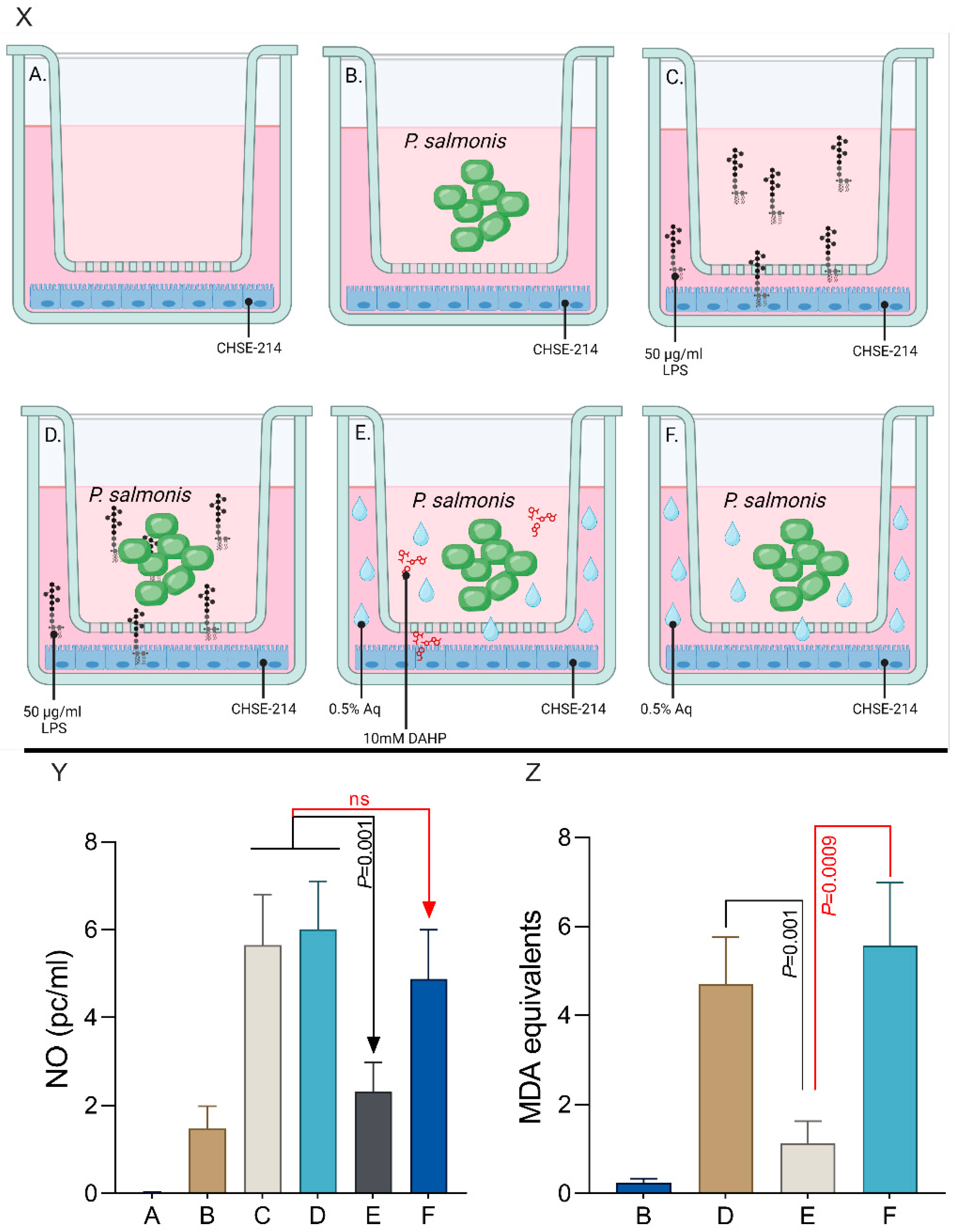
3.4. Nitric Oxide (NO) Levels in P. salmonis-Infected CHSE-214 Cells and the Impact of Aq
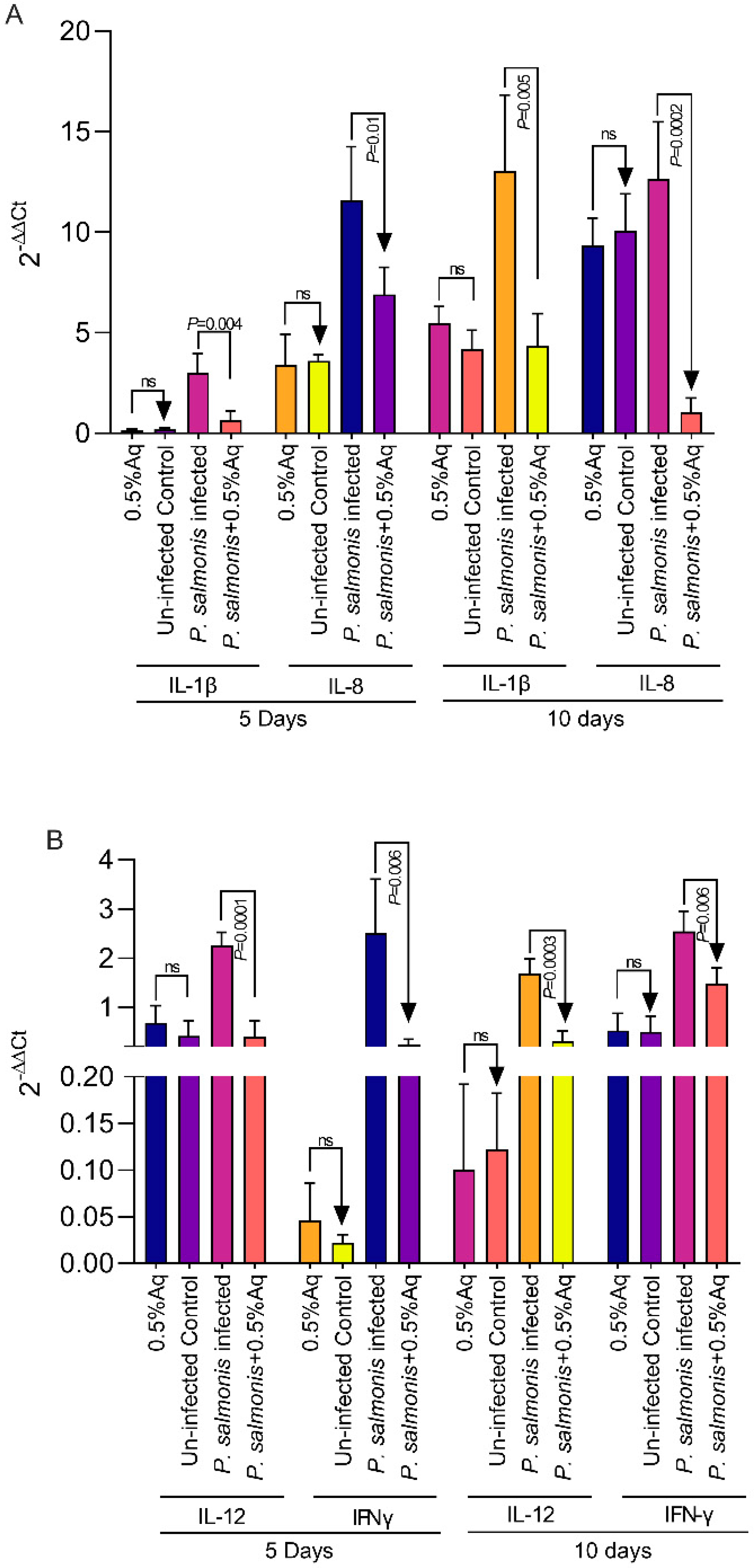
3.5. The Effect of 0.5% Aq on the Pro-Inflammatory Cytokine Levels’ Expression in P. salmonis-Infected CHSE-214 Cells
3.6. The Impact of CHSE-214-Released Nitric Oxide on P. salmonis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xue, X.; Caballero-Solares, A.; Hall, J.R.; Umasuthan, N.; Kumar, S.; Jakob, E.; Skugor, S.; Hawes, C.; Santander, J.; Taylor, R.G.; et al. Transcriptome Profiling of Atlantic Salmon (Salmo salar) Parr With Higher and Lower Pathogen Loads Following Piscirickettsia salmonis Infection. Front. Immunol. 2021, 12, 789465. [Google Scholar] [CrossRef] [PubMed]
- Caruffo, M.; Mandakovic, D.; Mejías, M.; Chávez-Báez, I.; Salgado, P.; Ortiz, D.; Montt, L.; Pérez-Valenzuela, J.; Vera-Tamargo, F.; Yánez, J.M.; et al. Pharmacological iron-chelation as an assisted nutritional immunity strategy against Piscirickettsia salmonis infection. Vet. Res. 2020, 51, 134. [Google Scholar] [CrossRef] [PubMed]
- Rozas-Serri, M. Why Does Piscirickettsia salmonis Break the Immunological Paradigm in Farmed Salmon? Biological Context to Understand the Relative Control of Piscirickettsiosis. Front. Immunol. 2022, 13, 856896. [Google Scholar] [CrossRef] [PubMed]
- Yáñez, J.M.; Yoshida, G.M.; Parra, Á.; Correa, K.; Barría, A.; Bassini, L.N.; Christensen, K.A.; López, M.E.; Carvalheiro, R.; Lhorente, J.P.; et al. Comparative Genomic Analysis of Three Salmonid Species Identifies Functional Candidate Genes Involved in Resistance to the Intracellular Bacterium Piscirickettsia salmonis. Front. Genet. 2019, 10, 665. [Google Scholar] [CrossRef] [PubMed]
- Cortés, H.; Castillo-Ruiz, M.; Cañon-Jones, H.; Schlotterbeck, T.; San Martín, R.; Padilla, L. In Vivo Efficacy of Purified Quillaja Saponin Extracts in Protecting against Piscirickettsia salmonis Infections in Atlantic Salmon (Salmo salar). Animals 2023, 13, 2845. [Google Scholar] [CrossRef] [PubMed]
- Laurin, E.; Gardner, I.A.; Peña, A.; Rozas-Serri, M.; Gayosa, J.; Neumann Heise, J.; Mardones, F.O. Bayesian estimation of diagnostic sensitivity and specificity of a qPCR and a bacteriological culture method for Piscirickettsia salmonis in farmed Atlantic salmon (Salmo salar L.) in Chile. J. Fish. Dis. 2020, 43, 1167–1175. [Google Scholar] [CrossRef]
- Godoy, M.; Coca, Y.; Suárez, R.; Montes de Oca, M.; Bledsoe, J.W.; Burbulis, I.; Caro, D.; Pontigo, J.P.; Maracaja-Coutinho, V.; Arias-Carrasco, R.; et al. Salmo salar Skin and Gill Microbiome during Piscirickettsia salmonis Infection. Animals 2024, 14, 97. [Google Scholar] [CrossRef] [PubMed]
- Avendaño-Herrera, R. Salmon aquaculture, Piscirickettsia salmonis virulence, and one health: Dealing with harmful synergies between heavy antimicrobial use and piscine and human health comment on Cabello and Godfrey (2019). Aquaculture 2021, 532, 736062. [Google Scholar] [CrossRef]
- Santibañez, N.; Vega, M.; Pérez, T.; Yáñez, A.; González-Stegmaier, R.; Figueroa, J.; Enríquez, R.; Oliver, C.; Romero, A. Biofilm Produced In Vitro by Piscirickettsia salmonis Generates Differential Cytotoxicity Levels and Expression Patterns of Immune Genes in the Atlantic Salmon Cell Line SHK-1. Microorganisms 2020, 8, 1609. [Google Scholar] [CrossRef]
- Ghiselli, F.; Giovagnoni, G.; Felici, M.; Tugnoli, B.; Piva, A.; Grilli, E. A mixture of organic acids and thymol protects primary chicken intestinal epithelial cells from Clostridium perfringens infection in vitro. Poult. Sci. 2022, 101, 102101. [Google Scholar] [CrossRef]
- Welsby, I.; Detienne, S.; N’Kuli, F.; Thomas, S.; Wouters, S.; Bechtold, V.; De Wit, D.; Gineste, R.; Reinheckel, T.; Elouahabi, A.; et al. Lysosome-Dependent Activation of Human Dendritic Cells by the Vaccine Adjuvant QS-21. Front. Immunol. 2017, 7, 663. [Google Scholar] [CrossRef] [PubMed]
- Balta, I.; Marcu, A.; Linton, M.; Kelly, C.; Stef, L.; Pet, I.; Ward, P.; Pircalabioru, G.G.; Chifiriuc, C.; Gundogdu, O.; et al. The in vitro and in vivo anti-virulent effect of organic acid mixtures against Eimeria tenella and Eimeria bovis. Sci. Rep. 2021, 11, 16202. [Google Scholar] [CrossRef] [PubMed]
- Balta, I.; McCleery, D.; David, S.R.F.; Pet, E.; Stef, D.; Iancu, T.; Pet, I.; Stef, L.; Corcionivoschi, N. The mechanistic role of natural antimicrobials in preventing Staphylococcus aureus invasion of MAC-T cells using an in vitro mastitis model. Ir. Vet. J. 2024, 77, 3. [Google Scholar] [CrossRef] [PubMed]
- Pinkerton, L.; Linton, M.; Kelly, C.; Ward, P.; Gradisteanu Pircalabioru, G.; Pet, I. Attenuation of vibrio parahaemolyticus virulence factors by a mixture of natural antimicrobials. Microorganisms 2019, 7, 679. [Google Scholar] [CrossRef] [PubMed]
- Cortés, M.P.; Mendoza, S.N.; Travisany, D.; Gaete, A.; Siegel, A.; Cambiazo, V.; Maass, A. Analysis of Piscirickettsia salmonis Metabolism Using Genome-Scale Reconstruction, Modeling, and Testing. Front. Microbiol. 2017, 8, 300912. [Google Scholar] [CrossRef]
- Balta, I.; Marcu, A.; Linton, M.; Kelly, C.; Gundogdu, O.; Stef, L.; Pet, I.; Ward, P.; Deshaies, M.; Callaway, T.; et al. Mixtures of natural antimicrobials can reduce Campylobacter jejuni, Salmonella enterica and Clostridium perfringens infections and cellular inflammatory response in MDCK cells. Gut Pathog. 2021, 13, 37. [Google Scholar] [CrossRef] [PubMed]
- Henriquez, M.; Gonzalez, E.; Marshall, S.H.; Henriquez, V.; Gomez, F.A.; Martinez, I.; Altamirano, C. A novel liquid medium for the efficient growth of the salmonid pathogen Piscirickettsia salmonis and optimization of culture conditions. PLoS ONE 2013, 8, e71830. [Google Scholar] [CrossRef]
- Perez-Stuardo, D.; Morales-Reyes, J.; Tapia, S.; Ahumada, D.E.; Espinoza, A.; Soto-Herrera, V.; Brianson, B.; Ibaceta, V.; Sandino, A.M.; Spencer, E.; et al. Non-lysosomal Activation in Macrophages of Atlantic Salmon (Salmo salar) after Infection with Piscirickettsia salmonis. Front. Immunol. 2019, 10, 434. [Google Scholar] [CrossRef]
- Estevez, R.A.; Mostazo, M.G.C.; Rodriguez, E.; Espinoza, J.C.; Kuznar, J.; Jonsson, Z.O.; Guethmundsson, G.H.; Maier, V.H. Inducers of salmon innate immunity: An in vitro and in vivo approach. Fish Shellfish Immunol. 2018, 72, 247–258. [Google Scholar] [CrossRef]
- Karatas, S.; Mikalsen, J.; Steinum, T.M.; Taksdal, T.; Bordevik, M.; Colquhoun, D.J. Real time PCR detection of Piscirickettsia salmonis from formalin-fixed paraffin-embedded tissues. J. Fish. Dis. 2008, 31, 747–753. [Google Scholar] [CrossRef]
- Mangmool, S.; Limpichai, C.; Han, K.K.; Reutrakul, V.; Anantachoke, N. Anti-Inflammatory Effects of Mitrephora sirikitiae Leaf Extract and Isolated Lignans in RAW 264.7 Cells. Molecules 2022, 27, 3313. [Google Scholar] [CrossRef] [PubMed]
- Kolodziejski, P.J.; Koo, J.S.; Eissa, N.T. Regulation of inducible nitric oxide synthase by rapid cellular turnover and cotranslational down-regulation by dimerization inhibitors. Proc. Natl. Acad. Sci. USA 2004, 101, 18141–18146. [Google Scholar] [CrossRef] [PubMed]
- Maier, V.H.; Schmitt, C.N.; Gudmundsdottir, S.; Gudmundsson, G.H. Bacterial DNA indicated as an important inducer of fish cathelicidins. Mol. Immunol. 2008, 45, 2352–2358. [Google Scholar] [CrossRef] [PubMed]
- Sheng, H.; Nakamura, K.; Kanno, T.; Sasaki, K.; Niwano, Y. Bactericidal Effect of Photolysis of H2O2 in Combination with Sonolysis of Water via Hydroxyl Radical Generation. PLoS ONE 2015, 10, e0132445. [Google Scholar] [CrossRef] [PubMed]
- Levipan, H.A.; Irgang, R.; Opazo, L.F.; Araya-León, H.; Avendaño-Herrera, R. Collective behavior and virulence arsenal of the fish pathogen Piscirickettsia salmonis in the biofilm realm. Front. Cell. Infect. Microbiol. 2022, 12, 1067514. [Google Scholar] [CrossRef]
- Santibáñez, N.; Vega, M.; Pérez, T.; Enriquez, R.; Escalona, C.E.; Oliver, C.; Romero, A. In vitro effects of phytogenic feed additive on Piscirickettsia salmonis growth and biofilm formation. J. Fish. Dis. 2024. early view. [Google Scholar] [CrossRef]
- Levipan, H.A.; Irgang, R.; Yáñez, A.; Avendaño-Herrera, R. Improved understanding of biofilm development by Piscirickettsia salmonis reveals potential risks for the persistence and dissemination of piscirickettsiosis. Sci. Rep. 2020, 10, 12224. [Google Scholar] [CrossRef]
- Schairer, D.O.; Chouake, J.S.; Nosanchuk, J.D.; Friedman, A.J. The potential of nitric oxide releasing therapies as antimicrobial agents. Virulence 2012, 3, 271–279. [Google Scholar] [CrossRef]
- Butucel, E.; Balta, I.; McCleery, D.; Marcu, A.; Stef, D.; Pet, I.; Callaway, T.; Stef, L.; Corcionivoschi, N. The Prebiotic Effect of an Organic Acid Mixture on Faecalibacterium prausnitzii Metabolism and Its Anti-Pathogenic Role against Vibrio parahaemolyticus in Shrimp. Biology 2022, 12, 57. [Google Scholar] [CrossRef]
- Korhonen, R.; Korpela, R.; Saxelin, M.; Mäki, M.; Kankaanranta, H.; Moilanen, E. Induction of nitric oxide synthesis by probiotic Lactobacillus rhamnosus GG in J774 macrophages and human T84 intestinal epithelial cells. Inflammation 2001, 25, 223–232. [Google Scholar] [CrossRef]
- Bunduruș, I.A.; Balta, I.; Butucel, E.; Callaway, T.; Popescu, C.A.; Iancu, T.; Pet, I.; Stef, L.; Corcionivoschi, N. Natural Antimicrobials Block the Host NF-κB Pathway and Reduce Enterocytozoon hepatopenaei Infection Both In Vitro and In Vivo. Pharmaceutics 2023, 15, 1994. [Google Scholar] [CrossRef]
- Jang, B.C.; Paik, J.H.; Kim, S.P.; Bae, J.H.; Mun, K.C.; Song, D.K.; Cho, C.H.; Shin, D.H.; Kwon, T.K.; Park, J.W.; et al. Catalase induces the expression of inducible nitric oxide synthase through activation of NF-kappaB and PI3K signaling pathway in Raw 264.7 cells. Biochem. Pharmacol. 2004, 68, 2167–2176. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.A.; Song, H.Y.; Ju, S.M.; Lee, S.J.; Kwon, H.-J.; Eum, W.S.; Jang, S.H.; Choi, S.Y.; Park, J. Differential regulation of inducible nitric oxide synthase and cyclooxygenase-2 expression by superoxide dismutase in lipopolysaccharide stimulated RAW 264.7 cells. Exp. Mol. Med. 2009, 41, 629–637. [Google Scholar] [CrossRef] [PubMed]
- Hall, J.R.; Rouillard, K.R.; Suchyta, D.J.; Brown, M.D.; Ahonen, M.J.R.; Schoenfisc, M.H. Mode of nitric oxide delivery affects antibacterial action. ACS Biomater. Sci. Eng. 2020, 6, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.L.; Ganopolsky, J.G.; Labbe, A.; Wahl, C.; Prakash, S. Antimicrobial properties of nitric oxide and its application in antimicrobial formulations and medical devices. Appl. Microbiol. Biotechnol. 2010, 88, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Cowley, S.C.; Myltseva, S.V.; Nano, F.E. Phase variation in Francisella tularensis affecting intracellular growth, lipopolysaccharide antigenicity and nitric oxide production. Mol. Microbiol. 1996, 20, 867–874. [Google Scholar] [CrossRef] [PubMed]
- Katsube, T.; Tsuji, H.; Onoda, M. Nitric oxide attenuates hydrogen peroxide-induced barrier disruption and protein tyrosine phosphorylation in monolayers of intestinal epithelial cell. Biochim. Biophys. Acta 2007, 1773, 794–803. [Google Scholar] [CrossRef] [PubMed]
- Oliver, C.; Coronado, J.L.; Martínez, D.; Kashulin-Bekkelund, A.; Lagos, L.X.; Ciani, E.; Sanhueza-Oyarzún, C.; Mancilla-Nova, A.; Enríquez, R.; Winther-Larsen, H.C.; et al. Outer membrane vesicles from Piscirickettsia salmonis induce the expression of inflammatory genes and production of IgM in Atlantic salmon Salmo salar. Fish Shellfish Immunol. 2023, 139, 108887. [Google Scholar] [CrossRef] [PubMed]
- Cinelli, M.A.; Do, H.T.; Miley, G.P.; Silverman, R.B. Inducible nitric oxide synthase: Regulation, structure, and inhibition. Med. Res. Rev. 2020, 40, 158–189. [Google Scholar] [CrossRef] [PubMed]
- Chakravortty, D.; Hensel, M. Inducible nitric oxide synthase and control of intracellular bacterial pathogens. Microbes Infect. 2003, 5, 621–627. [Google Scholar] [CrossRef]
- Campos-Perez, J.J.; Ward, M.; Grabowski, P.S.; Ellis, A.E.; Secombes, C.J. The gills are an important site of iNOS expression in rainbow trout Oncorhynchus mykiss after challenge with the gram-positive pathogen Renibacterium salmoninarum. Immunology 2000, 99, 153–161. [Google Scholar] [CrossRef]
- Alvarez, C.A.; Guzman, F.; Cardenas, C.; Marshall, S.H.; Mercado, L. Antimicrobial activity of trout hepcidin. Fish Shellfish Immunol. 2014, 41, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Douglas, S.E.; Gallant, J.W.; Liebscher, R.S.; Dacanay, A.; Tsoi, S.C. Identification and expression analysis of hepcidin-like antimicrobial peptides in bony fish. Dev. Comp. Immunol. 2003, 27, 589–601. [Google Scholar] [CrossRef]
- Sharma, J.N.; Al-Omran, A.; Parvathy, S.S. Role of nitric oxide in inflammatory diseases. Inflammopharmacology 2007, 15, 252–259. [Google Scholar] [CrossRef]
- Tripathi, P.; Tripathi, P.; Kashyap, L.; Singh, V. The role of nitric oxide in inflammatory reactions. FEMS Immunol. Med. Microbiol. 2007, 51, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.P.; Niedbala, W.; Wei, X.Q.; Xu, D.; Feng, G.J.; Robinson, J.H.; Lam, C.; Liew, F.Y. Nitric oxide regulates Th1 cell development through the inhibition of IL-12 synthesis by macrophages. Eur. J. Immunol. 1998, 28, 4062–4070. [Google Scholar] [CrossRef]
- Villarete, L.H.; Remick, D.G. Nitric oxide regulation of interleukin-8 gene expression. Shock 1997, 7, 29–35. [Google Scholar] [CrossRef]
- Sansonetti, P.J.; Arondel, J.; Huerre, M.; Harada, A.; Matsushima, K. Interleukin-8 controls bacterial transepithelial translocation at the cost of epithelial destruction in experimental shigellosis. Infect. Immun. 1999, 67, 1471–1480. [Google Scholar] [CrossRef]
- Corcionivoschi, N.; Alvarez, L.A.; Sharp, T.H.; Strengert, M.; Alemka, A.; Mantell, J. Mucosal reactive oxygen species decrease virulence by disrupting Campylobacter jejuni phosphotyrosine signaling. Cell Host Microbe 2012, 12, 47–59. [Google Scholar] [CrossRef]

| Gene | Primer | Reference |
|---|---|---|
| Cath2 | F: atgggaaacgaatgatgtgc R: cggtcagtgttgagggtatt | [19] |
| Hepcidin 1 | F: gcttctgctgcaaattctgagg R: gtacaagattgaggttgtgcag | |
| iNOS | F: aacgagagccaacaggtgtc R: ggtgcagcatgtctttgaga | |
| IFNγ | F—ttcaggagacccagaaacactac R—taatgaactcggacagagccttc | AY795563.1 |
| IL-8 | F—gcaacagcggtcaggagatt R—tggaatgattccccttcttca | HM162835.1 |
| IL-12 | F—ctgaatgaggtggactggtatg R—atcgtcctgttcctccg | XM_014205516.1 |
| IL-1β | F—caagctgcctcagggtct R—cggcaccctttaacctctcc | NM_001123582.1 |
| 16S rRNA | F—agggagactgccggtgata R—actacgaggcgctttctca | [20] |
| GADPH | F—ttccacggcacagtcaag R—actcagcaccagcatcac | [16] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Corcionivoschi, N.; Balta, I.; McCleery, D.; Pet, I.; Iancu, T.; Julean, C.; Marcu, A.; Stef, L.; Morariu, S. Blends of Organic Acids Are Weaponizing the Host iNOS and Nitric Oxide to Reduce Infection of Piscirickettsia salmonis in vitro. Antioxidants 2024, 13, 542. https://doi.org/10.3390/antiox13050542
Corcionivoschi N, Balta I, McCleery D, Pet I, Iancu T, Julean C, Marcu A, Stef L, Morariu S. Blends of Organic Acids Are Weaponizing the Host iNOS and Nitric Oxide to Reduce Infection of Piscirickettsia salmonis in vitro. Antioxidants. 2024; 13(5):542. https://doi.org/10.3390/antiox13050542
Chicago/Turabian StyleCorcionivoschi, Nicolae, Igori Balta, David McCleery, Ioan Pet, Tiberiu Iancu, Calin Julean, Adela Marcu, Lavinia Stef, and Sorin Morariu. 2024. "Blends of Organic Acids Are Weaponizing the Host iNOS and Nitric Oxide to Reduce Infection of Piscirickettsia salmonis in vitro" Antioxidants 13, no. 5: 542. https://doi.org/10.3390/antiox13050542
APA StyleCorcionivoschi, N., Balta, I., McCleery, D., Pet, I., Iancu, T., Julean, C., Marcu, A., Stef, L., & Morariu, S. (2024). Blends of Organic Acids Are Weaponizing the Host iNOS and Nitric Oxide to Reduce Infection of Piscirickettsia salmonis in vitro. Antioxidants, 13(5), 542. https://doi.org/10.3390/antiox13050542










