Valproic Acid Causes Redox-Regulated Post-Translational Protein Modifications That Are Dependent upon P19 Cellular Differentiation States
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Neuronal Differentiation
2.3. Cell Treatment
2.4. Redox Couple Chromatography
2.5. RNA-Sequencing
2.6. Differential Gene Expression Analysis and Clustering
2.7. Redox Immunoblotting
2.8. Confocal Microscopy
2.9. Statistical Analyses
2.10. Graphic Design
3. Results
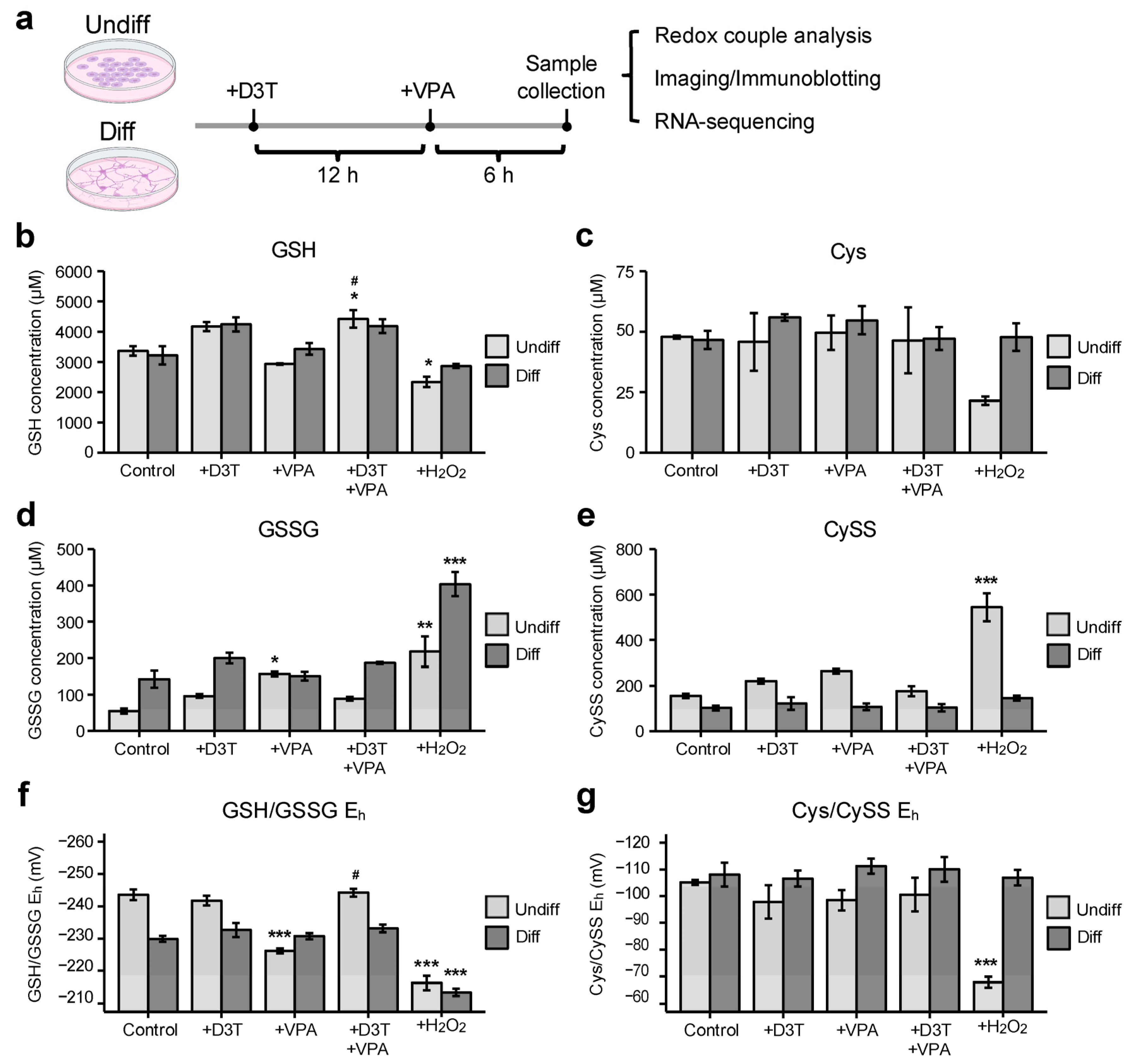
3.1. Valproic Acid (VPA) Alters the Glutathione Redox Couple in Undifferentiated Cells
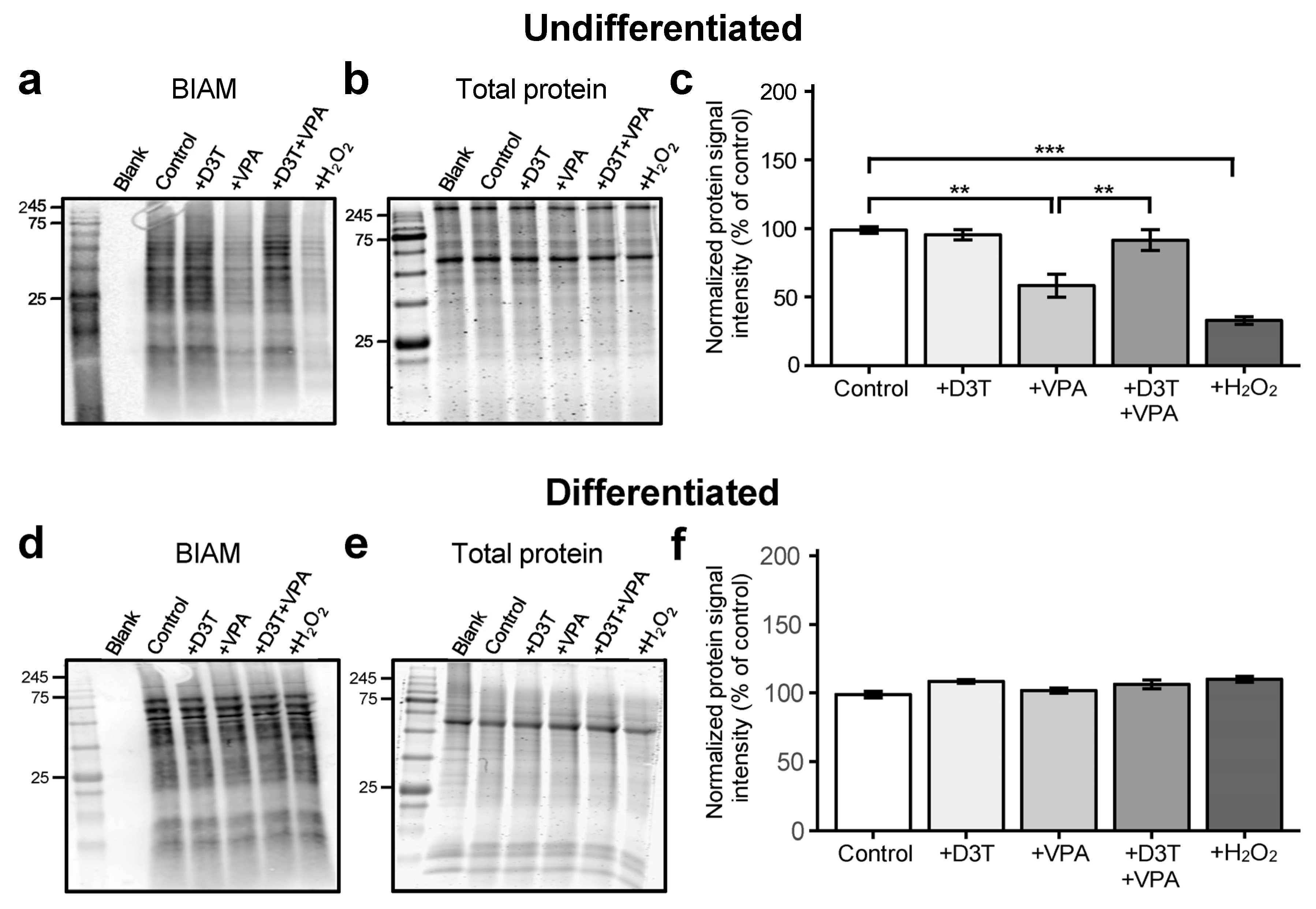
3.2. Valproic Acid Increases Protein Oxidation in Undifferentiated Cells but Not in Differentiated Neurons
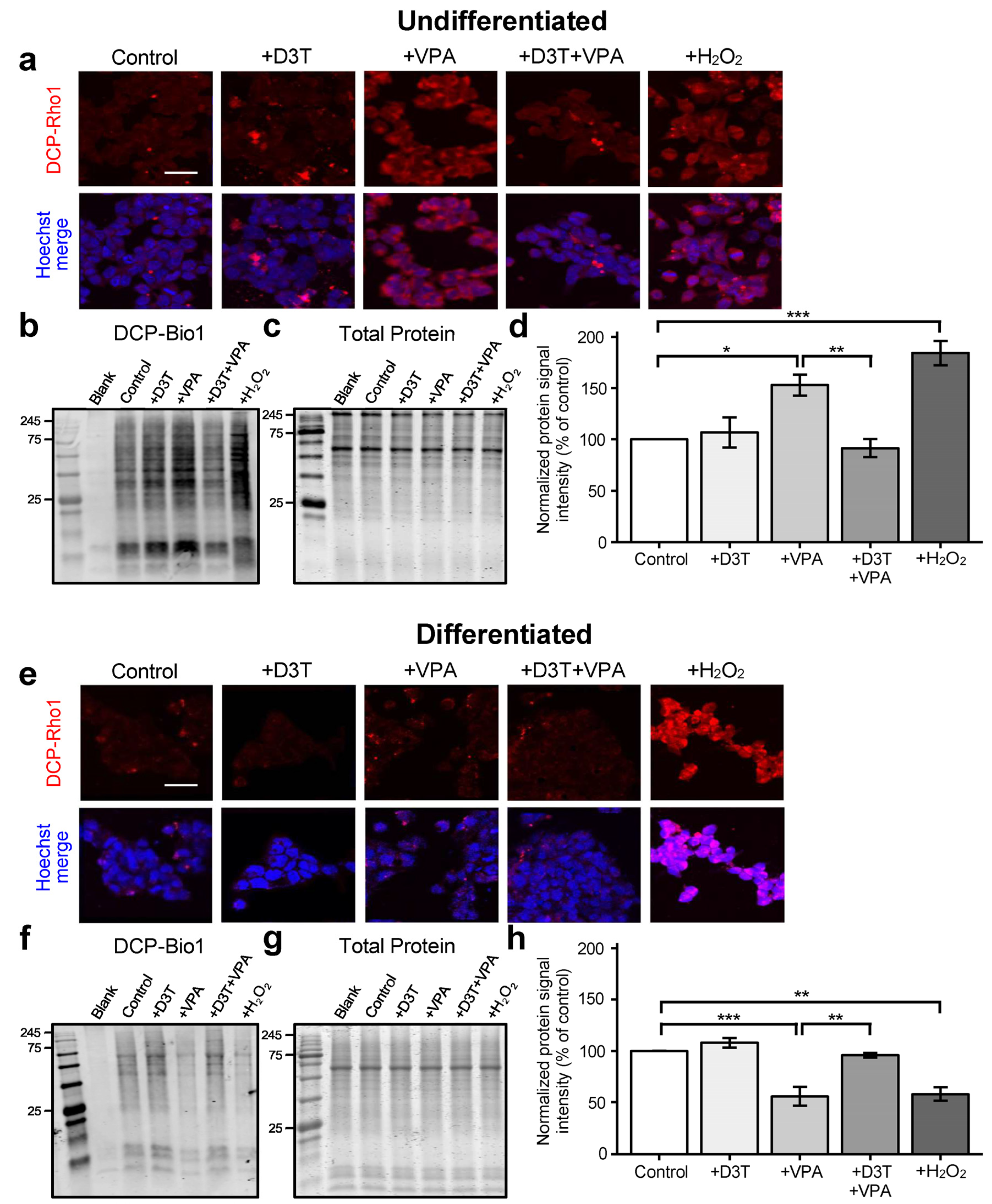
3.3. Sulfenic Acid Formation Is Increased in Undifferentiated Cells but Decreased in Neurons Treated with VPA
3.4. Protein S-Glutathionylation Is Unchanged in Undifferentiated Cells but Increased in Differentiated Neurons following VPA Exposure
3.5. Nuclear Factor Erythroid 2-Related Factor 2 (NRF2) Activation Protects Neurodevelopmental Transcription Pathways from VPA Exposure in Undifferentiated Cells
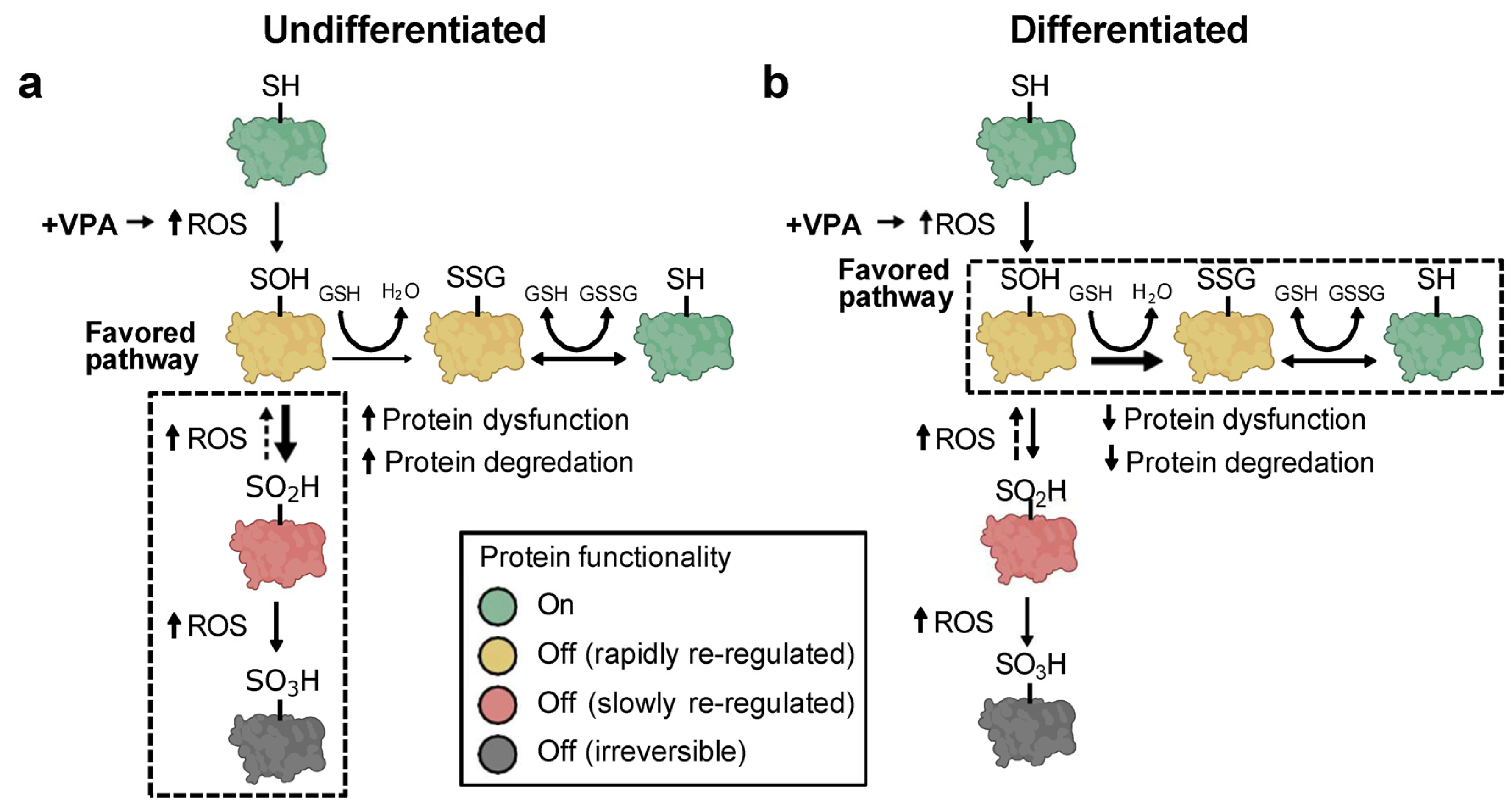
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BIAM | Biotinylated iodoacetamide |
| BioGEE | Biotinylated glutathione ethyl ester |
| Cys | Cysteine |
| CySS | Cystine |
| D3T | 3H-1,2-dithiole-3-thione |
| DCP-Bio1 | 3-(2,4-dioxo cyclohexyl)propyl biotin |
| DCP-Rho1 | Rhodamine B [4-[3-(2,4-dioxocyclohexyl)propyl]carbamate] |
| piperazine amide | |
| Diff | Differentiated |
| Eh | Redox potential |
| FVS | Fetal valproate syndrome |
| GSH | Glutathione |
| GSSG | Glutathione disulfide |
| H2O2 | Hydrogen peroxide |
| IAM | Iodoacetamide |
| NRF2 | Nuclear factor erythroid 2-related factor 2 |
| oxPTM | Oxidative post-translational modification |
| Redox | Reduction-oxidation |
| ROS | Reactive oxygen species |
| Trx1ox | Oxidized thioredoxin 1 |
| Trx1red | Reduced thioredoxin 1 |
| Undiff | Undifferentiated |
| VPA | Valproic acid |
References
- Rahman, M.; Awosika, A.O.; Nguyen, H. Valproic Acid. [Updated 2023 Aug 17]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. Available online: https://www.ncbi.nlm.nih.gov/books/NBK559112/ (accessed on 1 April 2024).
- Ardinger, H.H.; Atkin, J.F.; Blackston, R.D.; Elsas, L.J.; Clarren, S.K.; Livingstone, S.; Flannery, D.B.; Pellock, J.M.; Harrod, M.J.; Lammer, E.J.; et al. Verification of the fetal valproate syndrome phenotype. Am. J. Med Genet. 1988, 29, 171–185. [Google Scholar] [CrossRef] [PubMed]
- Robert, E.; Guibaud, P. Maternal valproic acid and congenital neural tube defects. Lancet 1982, 320, 937. [Google Scholar] [CrossRef] [PubMed]
- Christensen, J.; Grønborg, T.K.; Sørensen, M.J.; Schendel, D.; Parner, E.T.; Pedersen, L.H.; Vestergaard, M. Prenatal valproate exposure and risk of autism spectrum disorders and childhood autism. JAMA 2013, 309, 1696–1703. [Google Scholar] [CrossRef] [PubMed]
- Kurvits, K.; Laius, O.; Uusküla, M.; Haldre, S.; Rakitin, A. Valproic acid prescription trends among females of childbearing age in Estonia: A 14-year nationwide prescription database study. Seizure 2020, 76, 28–31. [Google Scholar] [CrossRef]
- Adedinsewo, D.A.; Thurman, D.J.; Luo, Y.; Williamson, R.S.; Odewole, O.A.; Oakley, G.P. Valproate prescriptions for nonepilepsy disorders in reproductive-age women. Birth Defects Res. Part A Clin. Mol. Teratol. 2013, 97, 403–408. [Google Scholar] [CrossRef] [PubMed]
- Karanti, A.; Bobeck, C.; Osterman, M.; Kardell, M.; Tidemalm, D.; Runeson, B.; Lichtenstein, P.; Landén, M. Gender differences in the treatment of patients with bipolar disorder: A study of 7354 patients. J. Affect. Disord. 2015, 174, 303–309. [Google Scholar] [CrossRef]
- Krämer, O.H.; Zhu, P.; Ostendorff, H.P.; Golebiewski, M.; Tiefenbach, J.; Peters, M.A.; Brill, B.; Groner, B.; Bach, I.; Heinzel, T.; et al. The histone deacetylase inhibitor valproic acid selectively induces proteasomal degradation of HDAC2. EMBO J. 2003, 22, 3411–3420. [Google Scholar] [CrossRef]
- Jang, E.-H.; Lee, J.-H.; Kim, S.-A. Acute Valproate Exposure Induces Mitochondrial Biogenesis and Autophagy with FOXO3a Modulation in SH-SY5Y Cells. Cells 2021, 10, 2522. [Google Scholar] [CrossRef]
- Chuang, Y.-F.; Yang, H.-Y.; Ko, T.-L.; Hsu, Y.-F.; Sheu, J.-R.; Ou, G.; Hsu, M.-J. Valproic acid suppresses lipopolysaccharide-induced cyclooxygenase-2 expression via MKP-1 in murine brain microvascular endothelial cells. Biochem. Pharmacol. 2014, 88, 372–383. [Google Scholar] [CrossRef]
- Phiel, C.J.; Zhang, F.; Huang, E.Y.; Guenther, M.G.; Lazar, M.A.; Klein, P.S. Histone Deacetylase Is a Direct Target of Valproic Acid, a Potent Anticonvulsant, Mood Stabilizer, and Teratogen. J. Biol. Chem. 2001, 276, 36734–36741. [Google Scholar] [CrossRef]
- Hansen, J.M.; Lucas, S.M.; Ramos, C.D.; Green, E.J.; Nuttall, D.J.; Clark, D.S.; Marchant, E.D.; Hancock, C.R.; Piorczynski, T.B. Valproic acid promotes SOD2 acetylation: A potential mechanism of valproic acid-induced oxidative stress in developing systems. Free. Radic. Res. 2021, 55, 1130–1144. [Google Scholar] [CrossRef]
- Hussein, A.M.; Awadalla, A.; Abbas, K.M.; Sakr, H.F.; Elghaba, R.; Othman, G.; Mokhtar, N.; Helal, G.M. Chronic valproic acid administration enhances oxidative stress, upregulates IL6 and downregulates Nrf2, Glut1 and Glut4 in rat’s liver and brain. NeuroReport 2021, 32, 840–850. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.K.H.; Abbott, F.S. Oxidative Stress as a Mechanism of Valproic Acid-Associated Hepatotoxicity. Drug Metab. Rev. 2006, 38, 627–639. [Google Scholar] [CrossRef] [PubMed]
- Uzel, G.; Oylumlu, E.; Durmus, L.; Ciraci, C. Duality of Valproic Acid Effects on Inflammation, Oxidative Stress and Autophagy in Human Eosinophilic Cells. Int. J. Mol. Sci. 2023, 24, 13446. [Google Scholar] [CrossRef] [PubMed]
- Cengiz, M.; Yüksel, A.; Seven, M. The effects of carbamazepine and valproic acid on the erythrocyte glutathione, glutathione peroxidase, superoxide dismutase and serum lipid peroxidation in epileptic children. Pharmacol. Res. 2000, 41, 423–425. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Ballesteros, C.; Pita-Calandre, E.; Sánchez-González, Y.; Rodríguez-López, C.M.; Agil, A. Lipid peroxidation in adult epileptic patients treated with valproic acid. Rev. Neurol. 2004, 38, 101–106. [Google Scholar] [PubMed]
- Tung, E.W.Y.; Winn, L.M. Valproic acid increases formation of reactive oxygen species and induces apoptosis in Postimplantation embryos: A role for oxidative stress in valproic acid-induced neural tube defects. Mol. Pharmacol. 2011, 80, 979–987. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.; Chen, K.; Lin, P.; Peng, C.; Peng, R.Y. Resveratrol and vitamin E rescue valproic acid-induced teratogenicity: The mechanism of action. Clin. Exp. Pharmacol. Physiol. 2014, 41, 210–219. [Google Scholar] [CrossRef]
- Lapehn, S.; Piorczynski, T.B.; Hansen, J.M.; Harris, C. Spatiotemporal evaluation of the mouse embryonic redox environment and histiotrophic nutrition following treatment with valproic acid and 1,2-dithiole-3-thione during early organogenesis. Reprod. Toxicol. 2021, 101, 81–92. [Google Scholar] [CrossRef]
- Defoort, E.N.; Kim, P.M.; Winn, L.M. Valproic acid increases conservative homologous recombination frequency and reactive oxygen species formation: A potential mechanism for valproic acid-induced neural tube defects. Mol. Pharmacol. 2006, 69, 1304–1310. [Google Scholar] [CrossRef]
- Piorczynski, T.B.; Larsen, M.W.; Lee, S.J.; Hansen, J.M. NRF2 activation protects against valproic acid-induced disruption of neurogenesis in P19 cells. Differentiation 2022, 123, 18–29. [Google Scholar] [CrossRef]
- Piorczynski, T.B.; Lapehn, S.; Ringer, K.P.; Allen, S.A.; Johnson, G.A.; Call, K.; Lucas, S.M.; Harris, C.; Hansen, J.M. NRF2 activation inhibits valproic acid-induced neural tube defects in mice. Neurotoxicology Teratol. 2022, 89, 107039. [Google Scholar] [CrossRef]
- Hansen, J.M.; Harris, C. Glutathione during embryonic development. Biochim. et Biophys. Acta (BBA)—Gen. Subj. 2015, 1850, 1527–1542. [Google Scholar] [CrossRef]
- Schafer, F.Q.; Buettner, G.R. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free. Radic. Biol. Med. 2001, 30, 1191–1212. [Google Scholar] [CrossRef]
- Davies, B.M.; Katayama, J.K.; Monsivais, J.E.; Adams, J.R.; Dilts, M.E.; Eberting, A.L.; Hansen, J.M. Real-time analysis of dynamic compartmentalized GSH redox shifts and H2O2 availability in undifferentiated and differentiated cells. Biochim. et Biophys. Acta (BBA)—Gen. Subj. 2023, 1867, 130321. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.P. Radical-free biology of oxidative stress. Am. J. Physiol. Cell Physiol. 2008, 295, C849–C868. [Google Scholar] [CrossRef]
- Hansen, J.M.; Jones, D.P.; Harris, C. The Redox Theory of Development. Antioxid. Redox Signal. 2020, 32, 715–740. [Google Scholar] [CrossRef] [PubMed]
- Leszczyński, P.; Śmiech, M.; Teeli, A.S.; Zołocińska, A.; Słysz, A.; Pojda, Z.; Pierzchała, M.; Taniguchi, H. Neurogenesis Using P19 Embryonal Carcinoma Cells. J. Vis. Exp. 2019, 146, e58225. [Google Scholar] [CrossRef] [PubMed]
- Sargent, C.Y.; Berguig, G.Y.; McDevitt, T.C. Cardiomyogenic differentiation of embryoid bodies is promoted by rotary orbital suspension culture. Tissue Eng. Part A 2009, 15, 331–342. [Google Scholar] [CrossRef]
- Jones, D.P.; Carlson, J.L.; Mody, V.C.; Cai, J.; Lynn, M.J.; Sternberg, P. Redox state of glutathione in human plasma. Free. Radic. Biol. Med. 2000, 28, 625–635. [Google Scholar] [CrossRef]
- Harris, C.; Hansen, J.M. Oxidative stress, thiols, and redox profiles. Methods Mol. Biol. 2012, 889, 325–346. [Google Scholar] [CrossRef] [PubMed]
- Andrews, S. FastQC: A Quality Control Tool for High Throughput Sequence Data [Online]. Available online: https://www.bioinformatics.babraham.ac.uk/projects/fastqc/ (accessed on 1 March 2024).
- Bray, N.L.; Pimentel, H.; Melsted, P.; Pachter, L. Near-optimal probabilistic RNA-seq quantification. Nat. Biotechnol. 2016, 34, 525–527. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Soneson, C.; Hickey, P.F.; Johnson, L.K.; Pierce, N.T.; Shepherd, L.; Morgan, M.; Patro, R. Tximeta: Reference sequence checksums for provenance identification in RNA-seq. PLOS Comput. Biol. 2020, 16, e1007664. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Abu-Jamous, B.; Kelly, S. Clust: Automatic extraction of optimal co-expressed gene clusters from gene expression data. Genome Biol. 2018, 19, 172. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Wang, L.-G.; Han, Y.; He, Q.-Y. clusterProfiler: An R package for comparing biological themes among gene clusters. OMICS J. Integr. Biol. 2012, 16, 284–287. [Google Scholar] [CrossRef] [PubMed]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis, 2nd ed.; Springer: Cham, Switzerland, 2016. [Google Scholar]
- Palsamy, P.; Bidasee, K.R.; Shinohara, T. Valproic acid suppresses Nrf2/Keap1 dependent antioxidant protection through induction of endoplasmic reticulum stress and Keap1 promoter DNA demethylation in human lens epithelial cells. Exp. Eye Res. 2014, 121, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.I.; Choi, C.; Shin, S.-W.; Son, A.; Lee, G.-H.; Kim, S.-Y.; Park, H.C. Valproic Acid Sensitizes Hepatocellular Carcinoma Cells to Proton Therapy by Suppressing NRF2 Activation. Sci. Rep. 2017, 7, 14986. [Google Scholar] [CrossRef]
- Li, X.; Gluth, A.; Zhang, T.; Qian, W. Thiol redox proteomics: Characterization of thiol-based post-translational modifications. Proteomics 2023, 23, e2200194. [Google Scholar] [CrossRef]
- Liu, H.; Colavitti, R.; Rovira, I.I.; Finkel, T. Redox-dependent transcriptional regulation. Circ. Res. 2005, 97, 967–974. [Google Scholar] [CrossRef]
- Kemp, M.; Go, Y.-M.; Jones, D.P. Nonequilibrium thermodynamics of thiol/disulfide redox systems: A perspective on redox systems biology. Free. Radic. Biol. Med. 2008, 44, 921–937. [Google Scholar] [CrossRef] [PubMed]
- Hansen, J.M.; Harris, C. Redox control of teratogenesis. Reprod. Toxicol. 2013, 35, 165–179. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.P.; Liang, Y. Measuring the poise of thiol/disulfide couples in vivo. Free. Radic. Biol. Med. 2009, 47, 1329–1338. [Google Scholar] [CrossRef] [PubMed]
- Nkabyo, Y.S.; Ziegler, T.R.; Gu, L.H.; Watson, W.H.; Jones, D.P.; Mannery, Y.O.; Hao, L.; Shyntum, Y.; Anderson, C.L.; Iyer, S.S. Glutathione and thioredoxin redox during differentiation in human colon epithelial (Caco-2) cells. Am. J. Physiol. Gastrointest. Liver Physiol. 2002, 283, G1352–G1359. [Google Scholar] [CrossRef] [PubMed]
- Imhoff, B.R.; Hansen, J.M. Differential redox potential profiles during adipogenesis and osteogenesis. Cell. Mol. Biol. Lett. 2011, 16, 149–161. [Google Scholar] [CrossRef] [PubMed]
- Choe, H.; Hansen, J.M.; Harris, C. Spatial and temporal ontogenies of glutathione peroxidase and glutathione disulfide reductase during development of the prenatal rat. J. Biochem. Mol. Toxicol. 2001, 15, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Go, Y.-M.; Jones, D.P. The redox proteome. J. Biol. Chem. 2013, 288, 26512–26520. [Google Scholar] [CrossRef] [PubMed]
- Seres, T.; Ravichandran, V.; Moriguchi, T.; Rokutan, K.; A Thomas, J.; Johnston, R.B. Protein S-thiolation and dethiolation during the respiratory burst in human monocytes. A reversible post-translational modification with potential for buffering the effects of oxidant stress. J. Immunol. 1996, 156, 1973–1980. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, A.; Taketa, K.; Kosaka, K. Glutathione-dependent interconversion of microheterogeneous forms of glucose-6-Phosphate Dehydrogenase in Rat Liver. J. Biochem. 1972, 72, 695–701. [Google Scholar] [CrossRef]
- Watson, W.H.; Chen, Y.; Jones, D.P. Redox state of glutathione and thioredoxin in differentiation and apoptosis. BioFactors 2003, 17, 307–314. [Google Scholar] [CrossRef]
- Gupta, V.; Carroll, K.S. Sulfenic acid chemistry, detection and cellular lifetime. Biochim. et Biophys. Acta (BBA)—Gen. Subj. 2014, 1840, 847–875. [Google Scholar] [CrossRef]
- Xiong, Y.; Uys, J.D.; Tew, K.D.; Townsend, D.M. S-Glutathionylation: From molecular mechanisms to health outcomes. Antioxid. Redox Signal. 2011, 15, 233–270. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; An, C.; Gao, Y.; Leak, R.K.; Chen, J.; Zhang, F. Emerging roles of Nrf2 and phase II antioxidant enzymes in neuroprotection. Prog Neurobiol. 2013, 100, 30–47. [Google Scholar]
- Poole, L.B.; Karplus, P.A.; Claiborne, A. protein sulfenic acids in redox signaling. Annu. Rev. Pharmacol. Toxicol. 2004, 44, 325–347. [Google Scholar] [CrossRef] [PubMed]
- Gallogly, M.M.; Mieyal, J.J. Mechanisms of reversible protein glutathionylation in redox signaling and oxidative stress. Curr. Opin. Pharmacol. 2007, 7, 381–391. [Google Scholar] [CrossRef] [PubMed]
- Benedict, A.L.; Mountney, A.; Hurtado, A.; Bryan, K.E.; Schnaar, R.L.; Dinkova-Kostova, A.T.; Talalay, P. Neuroprotective effects of sulforaphane after contusive spinal cord injury. J. Neurotrauma 2012, 29, 2576–2586. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, K.; Kume, T.; Muto, C.; Takada-Takatori, Y.; Izumi, Y.; Sugimoto, H.; Akaike, A. Glutathione biosynthesis via activation of the nuclear factor e2–related factor 2 (nrf2)—antioxidant-response element (are) pathway is essential for neuroprotective effects of sulforaphane and 6-(methylsulfinyl) hexyl isothiocyanate. J. Pharmacol. Sci. 2011, 115, 320–328. [Google Scholar] [CrossRef] [PubMed]
- Danielewicz, H.; Myszczyszyn, G.; Dębińska, A.; Myszkal, A.; Boznański, A.; Hirnle, L. Diet in pregnancy—More than food. Eur. J. Pediatr. 2017, 176, 1573–1579. [Google Scholar] [CrossRef] [PubMed]
- Jergil, M.; Kultima, K.; Gustafson, A.-L.; Dencker, L.; Stigson, M. Valproic acid–induced deregulation in vitro of genes associated in vivo with neural tube defects. Toxicol. Sci. 2009, 108, 132–148. [Google Scholar] [CrossRef]
- Hainaut, P.; Milner, J. Redox modulation of p53 conformation and sequence-specific DNA binding in vitro. Cancer Res. 1993, 53, 4469–4473. [Google Scholar]
- Galter, D.; Mihm, S.; Dröge, W. Distinct effects of glutathione disulphide on the nuclear transcription factors κB and the activator protein-1. Eur. J. Biochem. 1994, 221, 639–648. [Google Scholar] [CrossRef] [PubMed]
- Toledano, M.B.; Leonard, W.J. Modulation of transcription factor NF-kappa B binding activity by oxidation-reduction in vitro. Proc. Natl. Acad. Sci. USA 1991, 88, 4328–4332. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Oberley, L.W. Redox regulation of transcriptional activators. Free. Radic. Biol. Med. 1996, 21, 335–348. [Google Scholar] [CrossRef] [PubMed]
- McBurney, M.W. P19 embryonal carcinoma cells. Int. J. Dev. Biol. 1993, 37, 135–140. [Google Scholar] [PubMed]
- Monzo, H.J.; Park, T.I.; Montgomery, J.M.; Faull, R.L.; Dragunow, M.; Curtis, M.A. A method for generating high-yield enriched neuronal cultures from P19 embryonal carcinoma cells. J. Neurosci. Methods 2012, 204, 87–103. [Google Scholar] [CrossRef]
- Nakayama, Y.; Wada, A.; Inoue, R.; Terasawa, K.; Kimura, I.; Nakamura, N.; Kurosaka, A. A rapid and efficient method for neuronal induction of the P19 embryonic carcinoma cell line. J. Neurosci. Methods 2014, 227, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Hansen, J.M. Oxidative stress as a mechanism of teratogenesis. Birth Defects Res. Part C Embryo Today Rev. 2006, 78, 293–307. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Keston, A.S.; Brandt, R. The Fluorometric Analysis of Ultramicro Quantities of Hydrogen Peroxide. Anal. Biochem. 1965, 11, 1–5. [Google Scholar] [CrossRef]
- Alam, J.; Stewart, D.; Touchard, C.; Boinapally, S.; Choi, A.M.K.; Cook, J.L. Nrf2, a Cap’n’Collar transcription factor, regulates induction of the heme oxygenase-1 gene. J. Biol. Chem. 1999, 274, 26071–26078. [Google Scholar] [CrossRef]
- Venugopal, R.; Jaiswal, A.K. Nrf1 and Nrf2 positively and c-Fos and Fra1 negatively regulate the human antioxidant response element-mediated expression of NAD(P)H:quinone oxidoreductase 1 gene. Proc. Natl. Acad. Sci. USA 1996, 93, 14960–14965. [Google Scholar] [CrossRef] [PubMed]
- Wild, A.C.; Moinova, H.R.; Mulcahy, R.T. Regulation of γ-Glutamylcysteine synthetase subunit gene expression by the transcription factor Nrf2. J. Biol. Chem. 1999, 274, 33627–33636. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piorczynski, T.B.; Calixto, J.; Henry, H.C.; England, K.; Cowley, S.; Hansen, J.M.; Hill, J.T.; Hansen, J.M. Valproic Acid Causes Redox-Regulated Post-Translational Protein Modifications That Are Dependent upon P19 Cellular Differentiation States. Antioxidants 2024, 13, 560. https://doi.org/10.3390/antiox13050560
Piorczynski TB, Calixto J, Henry HC, England K, Cowley S, Hansen JM, Hill JT, Hansen JM. Valproic Acid Causes Redox-Regulated Post-Translational Protein Modifications That Are Dependent upon P19 Cellular Differentiation States. Antioxidants. 2024; 13(5):560. https://doi.org/10.3390/antiox13050560
Chicago/Turabian StylePiorczynski, Ted B., Jouber Calixto, Haley C. Henry, Kelli England, Susannah Cowley, Jackson M. Hansen, Jonathon T. Hill, and Jason M. Hansen. 2024. "Valproic Acid Causes Redox-Regulated Post-Translational Protein Modifications That Are Dependent upon P19 Cellular Differentiation States" Antioxidants 13, no. 5: 560. https://doi.org/10.3390/antiox13050560
APA StylePiorczynski, T. B., Calixto, J., Henry, H. C., England, K., Cowley, S., Hansen, J. M., Hill, J. T., & Hansen, J. M. (2024). Valproic Acid Causes Redox-Regulated Post-Translational Protein Modifications That Are Dependent upon P19 Cellular Differentiation States. Antioxidants, 13(5), 560. https://doi.org/10.3390/antiox13050560






