Exploring Chemical Composition, Antioxidant, Enzyme Inhibitory and Cytotoxic Properties of Glaucium acutidentatum Hausskn. & Bornm. from Turkey Flora: A Novel Source of Bioactive Agents to Design Functional Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Extraction
2.3. Assay for Total Phenolic and Flavonoid Contents
2.4. Liquid Chromatography—Mass Spectrometry Analysis
2.5. Antioxidant Tests
2.6. Enzyme Inhibitory Tests
2.7. Cytotoxic Evaluation
2.8. Molecular Modeling
2.9. Statistical Analysis
3. Results and Discussion
3.1. Total Phenolic (TPC) and Flavonoids (TFC) Contents
3.2. Chemical Profile of Extracts
| No | Tentative Identification | Rt (min) | Molecular Formula | Precursor Ion (m/z) | Fragment Ions (m/z) | HAE-M | HAE-W | MAC-M | MAC-W | INF-W | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Organic acids | |||||||||||
| 1. | Malic acid | 1.85 | C4H6O5 | 133.0008 a | 115.0037; 89.0245; 72.9956; 71.0158 | √ | √ | √ | √ | √ | [58] |
| 2. | Maleic acid | 2.18 | C4H4O4 | 115.0058 a | 73.0305; 71.0154; 87.0103 | √ | √ | √ | √ | Fragmentation; PubChem | |
| 3. | Citric acid | 2.26 | C6H8O7 | 191.0230 a | 111.0067; 87.0079; 57.0341 | √ | √ | √ | √ | [58] | |
| 4. | Succinic acid | 2.86 | C4H6O4 | 117.0207 a | 99.0101; 73.0310; 55.0211 | √ | Fragmentation; PubChem | ||||
| 5. | Fumaric acid | 3.45 | C4H4O4 | 115.0040 a | 99.0088; 73.0298 | √ | Fragmentation; Kegg | ||||
| 6. | Isopropylmalic acid | 13.62 | C7H12O5 | 175.0633 a | 115.0390; 113.0615; 85.0655 | √ | √ | √ | √ | √ | PubChem |
| Phenolic acids | |||||||||||
| 7. | Dihydroxybenzoic acid hexoside | 12.20 | C13H15O9 | 315.1090 a | 153.0562; 135.0455; 123.0453; 109.0289 | √ | √ | √ | Fragmentation; PubChem | ||
| 8. | beta-D-Glucosyl-2-coumarate | 16.26 | C15H18O8 | 325.0969 a | 163.0376; 119.0502 | √ | √ | √ | Fragmentation; PubChem | ||
| 9. | 3-p-Coumaroylquinic acid | 16.71 | C16H18O8 | 337.0932 a | 191.0548; 163.0397; 119.0500 | √ | √ | √ | √ | Fragmentation; PubChem | |
| 10. | Ferulic acid derivative | 19.58 | — | 551.1859 a | 193.0503; 178.0268; 149.0609; 134.0356 | √ | √ | √ | √ | √ | Fragmentation; PubChem |
| 11. | 2-Feruoyl-isocitric acid | 21.16 | C17H20O9 | 367.1090 a | 193.0490; 173.0453; 155.0343; 134.0366; 111.0448 | √ | √ | √ | √ | √ | [48] |
| 12. | Caffeoylmalic acid (=Phaselic acid) | 21.62 | C13H12O8 | 295.0545 a | 179.0336; 135.0429; 134.0179; 133.0137; 115.0040 | √ | √ | [47] | |||
| 13. | Malic acid p-coumarate | 24.52 | C13H12O7 | 279.0558 a | 163.0393; 133.0138; 119.0497 | √ | √ | √ | √ | √ | Fragmentation; PubChem |
| 14. | Feruloylmalic acid | 25.27 | C14H14O8 | 309.0650 a | 193.0511; 178.0270; 149.0609; 134.0371; 115.0049 | √ | √ | √ | √ | √ | Fragmentation |
| Alkaloids | |||||||||||
| 15. | Lotusine | 13.24 | C19H23NO3 | 314.1759 b | 269.1086; 237.0828; 175.0684; 143.0417; 121.0571; 107.0421 | √ | √ | √ | √ | √ | [56] |
| 16. | Isoboldine or boldine | 14.02 | C19H21NO4 | 328.1537 b | 297.0995; 282.0759; 265.0728; 251.0570 | √ | √ | √ | √ | √ | [11] |
| 17. | N-methylcoclaurine | 14.79 | C18H21NO3 | 300.1579 b | 269.1145; 237.0897; 175.0734; 137.0555; 107.0467 | √ | √ | √ | [50,55] | ||
| 18. | Reticuline derivative | 15.16 | C20H25NO4 | 344.1866 b | 299.1262; 267.1000; 192.1006; 175.0745; 137.0587 | √ | √ | √ | √ | √ | Fragmentation |
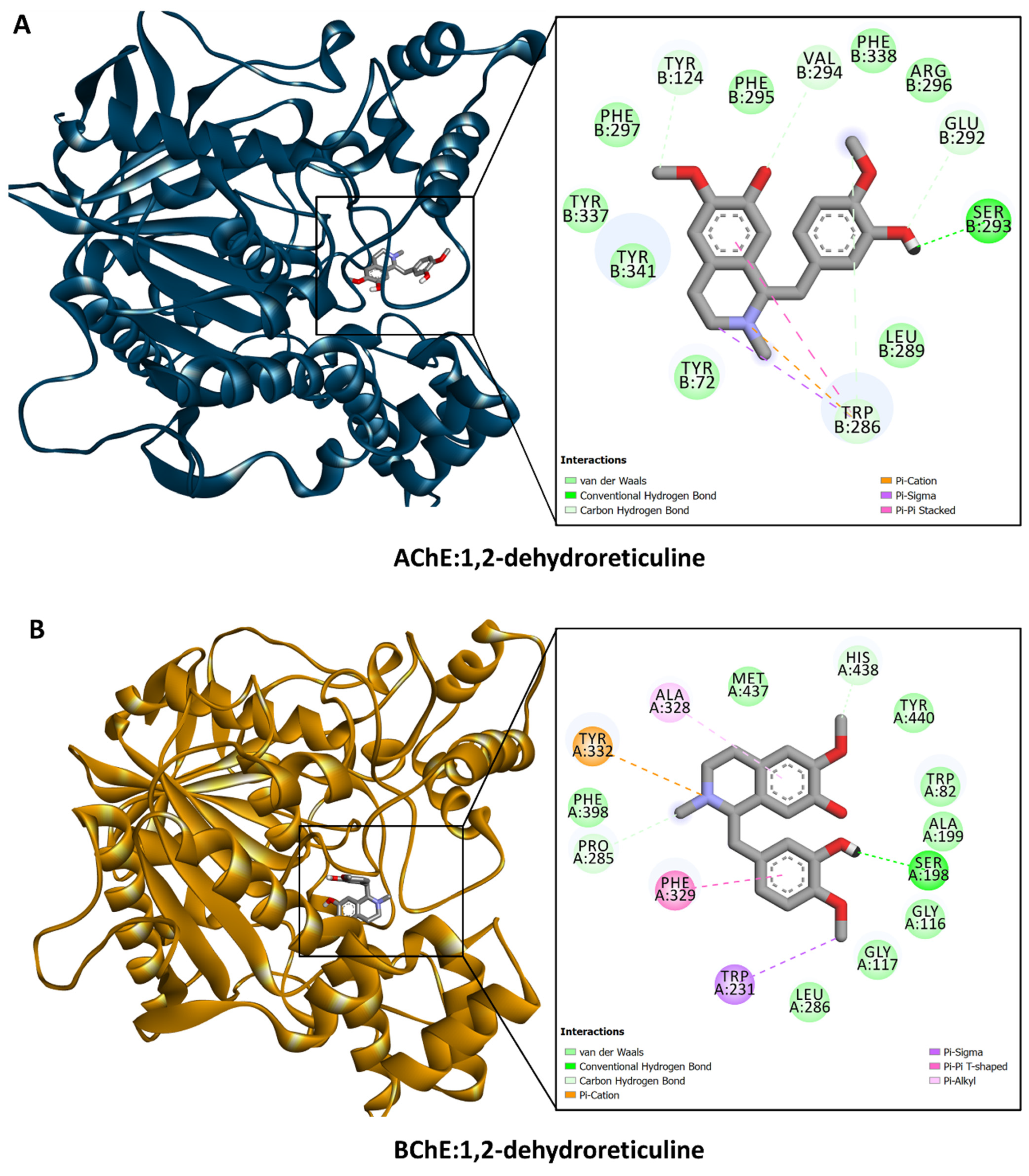
| 19. | 1,2-dehydroreticuline | 15.52 | C19H21NO4 | 328.1534 b | 297.1105; 265.0840; 192.1003; 175.0735; 137.0576 | √ | √ | √ | √ | √ | Fragmentation; PubChem |
| 20. | Reticuline | 16.11 | C19H23NO4 | 330.1710 b | 299.1275; 267.1012; 192.1013; 175.0745; 137.0594 | √ | √ | √ | √ | √ | Fragmentation; PubChem [52] |
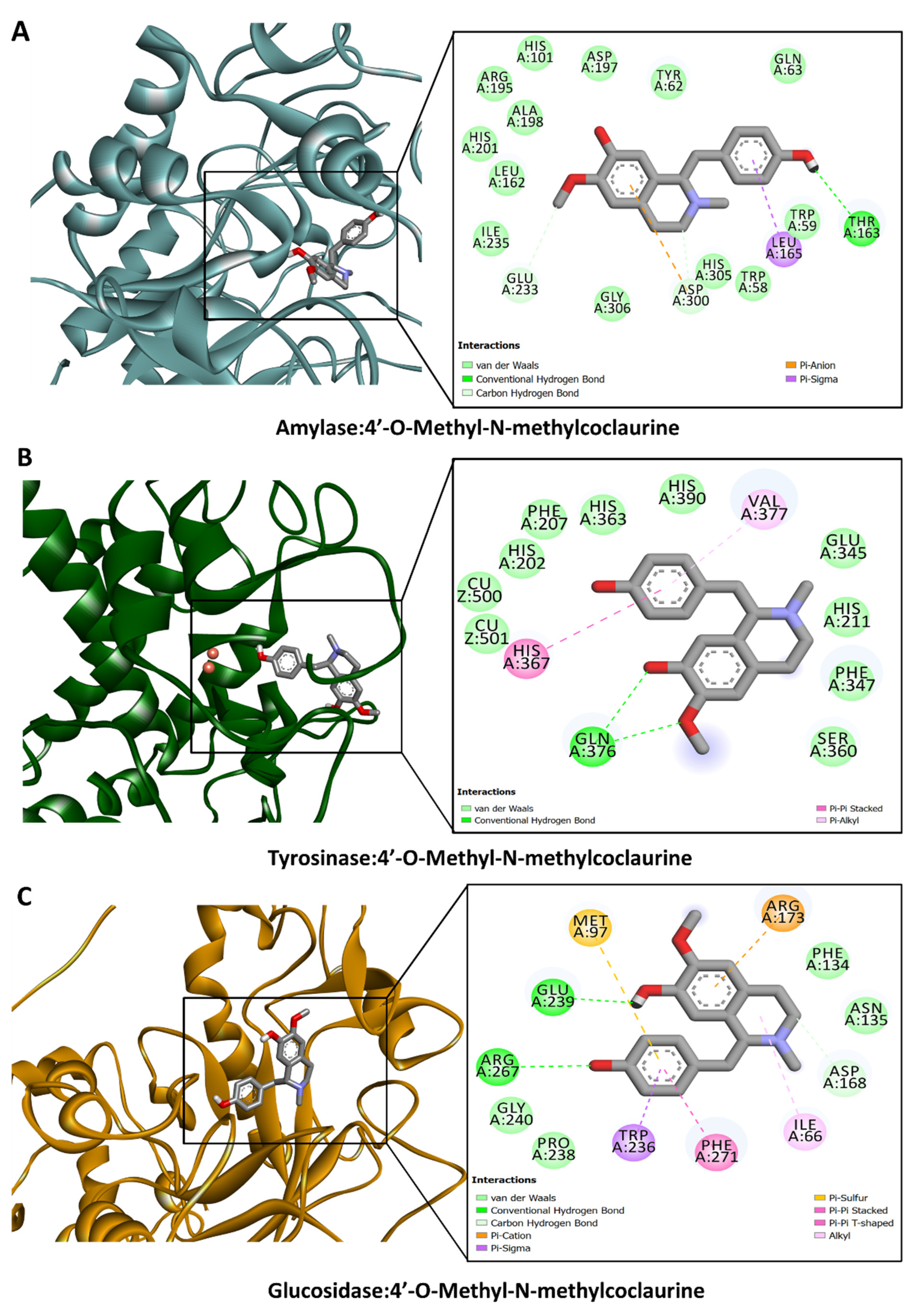
| 21. | 4′-O-Methyl-N-methylcoclaurine | 16.21 | C19H23NO3 | 314.1759 b | 299.1129; 269.1153; 175.0767; 137.0590; 107.0485 | √ | √ | √ | [53,55] | ||
| 22. | Isoboldine or boldine | 16.86 | C19H21NO4 | 328.1542 b | 297.1116; 282.0873; 265.0857; 251.0570 | √ | √ | √ | √ | √ | Fragmentation; [11] |
| 23. | Isocorydine | 17.53 | C20H23NO4 | 342.1715 b | 311.1266; 280.1064; 279.0997; 206.1163; 189.0746 | √ | √ | √ | √ | √ | [57] |
| 24. | Laudanine | 17.77 | C20H25NO4 | 344.1827 b | 313.1390; 281.1077; 206.1152; 189.0875; 137.0575 | √ | √ | √ | √ | Fragmentation; [52] | |
| 25. | Reticuline isomer | 18.29 | C19H23NO4 | 330.1710 b | 299.1230; 267.0935; 192.1000; 175.0736; 137.0588 | √ | √ | Fragmentation; [52] | |||
| 26. | Corydine (=glaucentrin) | 18.45 | C20H23NO4 | 342.1675 b | 311.1258; 280.1037; 279.0994; 189.0890 | √ | √ | √ | √ | √ | [57] |
| 27. | 3-Hydroxyglaucine | 18.68 | C21H25NO5 | 372.1832 b | 354.1682; 323.1275; 308.1061 | √ | √ | √ | √ | √ | PubChem |
| 28. | Protopine | 18.79 | C20H19NO5 | 354.1327 b | 336.1223; 275.0678; 206.0782; 189.0762; 188.0687; 149.0592 | √ | √ | √ | √ | √ | PubChem; [57] |
| 29. | α-Allocryptopine | 19.34 | C21H23NO5 | 370.1624 b | 352.1520; 306.0887; 290.0906; 206.0783; 189.0751; 188.0706; 181.0828; 165.0883 | √ | √ | √ | √ | √ | [57] |
| 30. | Glaucine syn. Boldine dimethyl ether | 20.83 | C21H25NO4 | 356.1879 b | 325.1371; 310.1138; 295.1033; 294.1188; 279.0962; 251.1011 | √ | √ | √ | √ | √ | PubChem; Fragmentation |
| 31. | Cataline | 22.45 | C21H25NO5 | 372.1797 b | 355.1753; 341.1370; 325.1424; 312.1342 | √ | √ | √ | √ | √ | [11,45] |
| 32. | Corunnine (=glauvine) | 30.19 | C20H18NO5 | 352.1203 b | 337.0920; 336.0836; 322.0688; 307.0775; 306.0744; 294.1212; 279.1000; 251.1025 | √ | √ | √ | √ | √ | [51] |
| 33. | Glaucine isomer (e.g., takatonin) | 32.06 | C21H25NO4 | 356.1879 b | 325.1421; 310.1176; 295.1043; 294.1230; 279.0991; 251.1047 | √ | √ | √ | √ | √ | Fragmentation; PubChem |
| Coumarins | |||||||||||
| 34. | Dihydroxycoumarin-hexoside | 15.49 | C15H16O9 | 339.0710 a | 177.0194 | √ | √ | Fragmentation; PubChem | |||
| 35. | Dihydroxycoumarin | 21.50 | C9H6O4 | 177.0216 a | 159.8905; 133.0291; 105.0344 | √ | √ | Fragmentation; PubChem | |||
| Flavonoids | |||||||||||
| 36. | Rutoside | 23.15 | C27H30O16 | 609.1636 a | 301.0132; 300.0227; 271.0230; 255.0163; 178.9974; 151.0001 | √ | √ | √ | √ | [47] | |
| 37. | Tetrahydroxymethoxyflavone O-rutinoside (Isorhamnetin 3-O-rutinoside) | 24.12 | C28H32O16 | 623.1696 a | 315.0464; 314.0414; 300.0198; 299.0166; 271.0204; 243.0299; 151.0022 | √ | √ | Fragmentation; PubChem | |||
| 38. | Isoquercitrin | 24.27 | C21H20O12 | 463.0666 a | 301.0368; 300.0295; 271.0262; 255.0310; 178.9994; 151.0041 | √ | √ | [47] | |||
| 39. | Tetrahydroxyflavone-7-O-hexoside (Kaempferol-7-O-hexoside) | 25.08 | C21H20O11 | 447.0915 a | 285.0390; 284.0326; 255.0282; 227.0343; 151.0023 | √ | √ | Fragmentation; PubChem | |||
| 40. | Tetrahydroxyflavone-3-O-hexoside-pentoside (Kaempferol-3-O-hexoside-pentoside) | 25.37 | C27H30O15 | 593.1599 a | 285.0408; 255.0309; 227.0366; 151.0021 | √ | √ | Fragmentation; PubChem | |||
| 41. | Pentahydroxyflavone-3-O-rhamnoside (Quercetin-3-O-rhamnoside isomer) | 25.77 | C27H30O16 | 609.1543 a | 301.0343; 300.0268; 271.0230; 255.0306; 178.9971; 151.0054 | √ | √ | Fragmentation; PubChem | |||
| 42. | Isorhamnetin-3-O-hexoside | 25.92 | C22H22O12 | 477.1025 a | 314.0428; 299.0217; 271.0269; 151.0017 | √ | √ | [59] | |||
| 43. | Quercetin | 30.63 | C23H20O12 | 301.0396 a | 178.9975; 151.0023 | √ | [47] | ||||
| 44. | Isorhamnetin | 34.58 | C16H12O7 | 315.0553 a | 300.0290; 271.0196; 151.0034 | √ | [59] | ||||
| Isoprenoids | |||||||||||
| 45. | Norisoprenoid glucoside | 15.52 | C19H34O9 | 451.2207 c | 405.2145; 225.1456; 179.0584; 167.1064 | √ | √ | √ | √ | PubChem | |
| Fatty acids | |||||||||||
| 46. | 4,10-dimethyl-9-[3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxydodeca-2,4,6-trienedioic acid | 25.58 | C20H30O10 | 429.1831 a | 249.1131; 205.1232 | √ | √ | √ | √ | √ | PubChem |
| 47. | Fatty acid | 33.73 | C18H34O5 | 329.2386 a | 229.1441; 211.1336; 171.1014 | √ | √ | √ | √ | √ | PubChem |
| 48. | Fatty acid | 46.86 | C18H30O3 | 293.2158 a | 275.2018; 224.1411; 195.1386; 171.1016 | √ | √ | √ | √ | √ | PubChem |
| 49. | Glyceryl linolenate | 51.61 | C21H36O4 | 353.2712 b | 335.2530; 261.2168; 243.2060 | √ | PubChem | ||||
| 50. | 2-hydroxy-6-[(8Z,11Z)-pentadeca-8,11,14-trienyl]benzoic acid | 51.80 | C22H30O3 | 241.2119 a | 297.2194; 229.1177; 159.0807; 106.0422 | √ | PubChem | ||||
| 51. | Linoleic acid amide = 9,12-Octadecadienamide | 52.36 | C18H33NO | 280.2652 b | 263.2340; 245.2235 | √ | √ | √ | √ | PubChem | |
| 52. | Linolenic acid (9Z,12Z,15Z)-octadeca-9,12,15-trienoic acid | 52.71 | C18H30O2 | 279.2336 b | 109.1001; 95.0849; 81.0697; 67.0544;55.0547 | √ | √ | PubChem | |||
| 53. | Hexadecanamide | 53.46 | C16H33NO | 256.2652 b | 102.0903; 88.0751; 74.0598; 57.0703 | √ | √ | √ | √ | √ | PubChem |
| 54. | Oleamide | 53.701 | C18H35NO | 282.2809 b | 265.2488; 248.2385 | √ | √ | √ | √ | PubChem | |
| 55. | Linolenyl alcohol | 54.01 | C18H32O | 265.2546 b | 247.2389 | √ | PubChem |
3.3. Antioxidant Activity
3.4. Enzyme Inhibition Activity
3.5. Cytotoxic Effects
3.6. Molecular Docking
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tavakkoli, Z.; Assadi, M. A taxonomic revision of the genus Glaucium (Papaveraceae) in Iran. Acta Bot. Croat. 2019, 78, 57–65. [Google Scholar] [CrossRef]
- Aykurt, C.; Yıldız, K.; Özçandır, A.; Mungan, F.; Deniz, G. Glaucium alakirensis (Papaveraceae), a new species from Southern Anatolia, Turkey. Phytotaxa 2017, 295, 255–262. [Google Scholar] [CrossRef]
- Al-Qura’n, S. Ethnopharmacological survey of wild medicinal plants in Showbak, Jordan. J. Ethnopharmacol. 2009, 123, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Kocanci, F.; Hamamcioglu, B.; Aslım, B. The anti-AChE and anti-proliferative Activities of Glaucium acutidentatum and Glaucium corniculatum Alkaloid Extracts. J. Appl. Pharm. Sci. 2017, 7, 191–200. [Google Scholar]
- Zheng, S.; Zheng, S. Bocconoline from Glaucium fimbrilligerum Boiss. Induces Apoptosis of Human Breast Cancer MCF-7 Cells via Mitochondria-Dependent Pathway. Lat. Am. J. Pharm. 2020, 39, 2022–2028. [Google Scholar]
- Morteza-Semnani, K.; Saeedi, M.; Mahdavi, M.R. Antibacterial studies on extracts of three species of Glaucium. from Iran. Pharm. Biol. 2005, 43, 234–236. [Google Scholar] [CrossRef]
- Kusman Saygi, T.; Tan, N.; Alim Toraman, G.Ö.; Gurer, C.U.; Tugay, O.; Topcu, G. Isoquinoline alkaloids isolated from Glaucium corniculatum var. corniculatum and Glaucium grandiflorum subsp. refractum var. torquatum with bioactivity studies. Pharm. Biol. 2023, 61, 907–917. [Google Scholar] [CrossRef]
- Darya, G.H.; Nowroozi-Asl, A.; Khoshvaghti, A.; Musavi, S.M. Effect of hydro-alcoholic extract of yellow horned poppy (Glaucium flavum) on serum concentration of glucose and lipid profile and weight changes in alloxan induced diabetic rats. Sci. J. Kurd. Univ. Med. Sci. 2019, 24, 45–55. [Google Scholar] [CrossRef]
- Shamma, M.; Slusarchyk, W.A. The aporphine alkaloids. Chem. Rev. 1964, 64, 59–79. [Google Scholar] [CrossRef]
- Park, C.Y.; Lee, K.-Y.; Gul, K.; Rahman, M.S.; Kim, A.-N.; Chun, J.; Kim, H.-J.; Choi, S.-G. Phenolics and antioxidant activity of aqueous turmeric extracts as affected by heating temperature and time. LWT 2019, 105, 149–155. [Google Scholar] [CrossRef]
- Akaberi, T.; Shourgashti, K.; Emami, S.A.; Akaberi, M. Phytochemistry and pharmacology of alkaloids from Glaucium spp. Phytochemistry 2021, 191, 112923. [Google Scholar] [CrossRef] [PubMed]
- Bozkurt, B.; Ulkar, D.; Nurlu, N.; Kaya, G.I.; Unver-Somer, N. Chemical profile, acetylcholinesterase, butyrylcholinesterase, and prolyl oligopeptidase inhibitory activity of Glaucium corniculatum subsp. Refractum. Braz. J. Pharm. Sci. 2022, 58, e20464. [Google Scholar] [CrossRef]
- Erbay, M.Ş.; Anıl, S.; Melikoğlu, G. Plants used as painkiller in traditional treatment in Turkey-II Headache. Marmara Pharm. J. 2018, 22, p29. [Google Scholar] [CrossRef]
- Hayta, S.; Polat, R.; Selvi, S. Traditional uses of medicinal plants in Elazığ (Turkey). J. Ethnopharmacol. 2014, 154, 613–623. [Google Scholar] [CrossRef] [PubMed]
- Sargin, S.A. Ethnobotanical survey of medicinal plants in Bozyazı district of Mersin, Turkey. J. Ethnopharmacol. 2015, 173, 105–126. [Google Scholar] [CrossRef] [PubMed]
- Gözler, T. Alkaloids of Turkish Glaucium Species. Planta Medica 1982, 46, 179–180. [Google Scholar] [CrossRef] [PubMed]
- Dolanbay, S.N.; Kocanci, F.G.; Aslim, B. Neuroprotective effects of allocryptopine-rich alkaloid extracts against oxidative stress-induced neuronal damage. Biomed. Pharmacother. 2021, 140, 111690. [Google Scholar]
- Sari, A. The constituents of the aerial parts of Glaucium grandiflorum var. grandiflorum. ACTA Pharm. Sci. 2001, 43, 89–92. [Google Scholar]
- Ozsoy, N.; Yilmaz-Ozden, T.; Aksoy-Sagirli, P.; Şahin, H.; Sarı, A. Antioxidant, Anti-acetylcholinesterase, Anti-inflammatory and DNA Protection Activities of Glaucium grandiflorum var. grandiflorum. Iran. J. Pharm. Res. IJPR 2018, 17, 677. [Google Scholar]
- Slinkard, K.; Singleton, V.L. Total phenol analysis: Automation and comparison with manual methods. Am. J. Enol. Vitic. 1977, 28, 49–55. [Google Scholar] [CrossRef]
- Arvouet-Grand, A.; Vennat, B.; Pourrat, A.; Legret, P. Standardization of propolis extract and identification of principal constituents. J. De Pharm. De Belg. 1994, 49, 462–468. [Google Scholar]
- Bakar, K.; Nilofar; Mohamed, A.; Świątek, Ł.; Hryć, B.; Sieniawska, E.; Rajtar, B.; Ferrante, C.; Menghini, L.; Zengin, G. Evaluating Phytochemical Profiles, Cytotoxicity, Antiviral Activity, Antioxidant Potential, and Enzyme Inhibition of Vepris boiviniana Extracts. Molecules 2023, 28, 7531. [Google Scholar] [CrossRef] [PubMed]
- Kirby, A.J.; Schmidt, R.J. The antioxidant activity of Chinese herbs for eczema and of placebo herbs—I. J. Ethnopharmacol. 1997, 56, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free. Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Apak, R.; Güçlü, K.; Özyürek, M.; Karademir, S.E. Novel total antioxidant capacity index for dietary polyphenols and vitamins C and E, using their cupric ion reducing capability in the presence of neocuproine: CUPRAC method. J. Agric. Food Chem. 2004, 52, 7970–7981. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Prieto, P.; Pineda, M.; Aguilar, M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: Specific application to the determination of vitamin E. Anal. Biochem. 1999, 269, 337–341. [Google Scholar] [CrossRef]
- Dinis, T.C.; Madeira, V.M.; Almeida, L.M. Action of phenolic derivatives (acetaminophen, salicylate, and 5-aminosalicylate) as inhibitors of membrane lipid peroxidation and as peroxyl radical scavengers. Arch. Biochem. Biophys. 1994, 315, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Šafašík, I. Rapid Detection of Alpha-Amylase Inhibitors. J. Enzym. Inhib. 1990, 3, 245–247. [Google Scholar] [CrossRef]
- Ting, L.; Zhang, X.-D.; Song, Y.-W.; Liu, J.-W. A microplate-based screening method for alpha-glucosidase inhibitors. Chin. J. Clin. Pharmacol. Ther. 2005, 10, 1128. [Google Scholar]
- Ellman, G.L.; Courtney, K.D.; Andres, V., Jr.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Masuda, T.; Yamashita, D.; Takeda, Y.; Yonemori, S. Screening for tyrosinase inhibitors among extracts of seashore plants and identification of potent inhibitors from Garcinia subelliptica. Biosci. Biotechnol. Biochem. 2005, 69, 197–201. [Google Scholar] [CrossRef]
- Świątek, Ł.; Sieniawska, E.; Sinan, K.I.; Zengin, G.; Boguszewska, A.; Hryć, B.; Bene, K.; Polz-Dacewicz, M.; Dall’Acqua, S. Chemical Characterization of Different Extracts of Justicia secunda Vahl and Determination of Their Anti-Oxidant, Anti-Enzymatic, Anti-Viral, and Cytotoxic Properties. Antioxidants 2023, 12, 509. [Google Scholar] [CrossRef] [PubMed]
- Kurumbail, R.G.; Stevens, A.M.; Gierse, J.K.; McDonald, J.J.; Stegeman, R.A.; Pak, J.Y.; Gildehaus, D.; Iyashiro, J.M.; Penning, T.D.; Seibert, K.; et al. Structural basis for selective inhibition of cyclooxygenase-2 by anti-inflammatory agents. Nature 1996, 384, 644–648. [Google Scholar] [CrossRef] [PubMed]
- Maurus, R.; Begum, A.; Williams, L.K.; Fredriksen, J.R.; Zhang, R.; Withers, S.G.; Brayer, G.D. Alternative Catalytic Anions Differentially Modulate Human α-Amylase Activity and Specificity. Biochemistry 2008, 47, 3332–3344. [Google Scholar] [CrossRef] [PubMed]
- Gerlits, O.; Ho, K.-Y.; Cheng, X.; Blumenthal, D.; Taylor, P.; Kovalevsky, A.; Radić, Z. A new crystal form of human acetylcholinesterase for exploratory room-temperature crystallography studies. Chem.-Biol. Interact. 2019, 309, 108698. [Google Scholar] [CrossRef] [PubMed]
- Rosenberry, T.; Brazzolotto, X.; Macdonald, I.; Wandhammer, M.; Trovaslet-Leroy, M.; Darvesh, S.; Nachon, F. Comparison of the Binding of Reversible Inhibitors to Human Butyrylcholinesterase and Acetylcholinesterase: A Crystallographic, Kinetic and Calorimetric Study. Molecules 2017, 22, 2098. [Google Scholar] [CrossRef] [PubMed]
- Schonbrunn, E.; Betzi, S.; Alam, R.; Martin, M.P.; Becker, A.; Han, H.; Francis, R.; Chakrasali, R.; Jakkaraj, S.; Kazi, A.; et al. Development of Highly Potent and Selective Diaminothiazole Inhibitors of Cyclin-Dependent Kinases. J. Med. Chem. 2013, 56, 3768–3782. [Google Scholar] [CrossRef]
- Omer, H.A.A.; Caprioli, G.; Abouelenein, D.; Mustafa, A.M.; Uba, A.I.; Ak, G.; Ozturk, R.B.; Zengin, G.; Yagi, S. Phenolic Profile, Antioxidant and Enzyme Inhibitory Activities of Leaves from Two Cassia and Two Senna Species. Molecules 2022, 27, 5590. [Google Scholar] [CrossRef]
- Martínez-Rosell, G.; Giorgino, T.; De Fabritiis, G. PlayMolecule ProteinPrepare: A Web Application for Protein Preparation for Molecular Dynamics Simulations. J. Chem. Inf. Model. 2017, 57, 1511–1516. [Google Scholar] [CrossRef]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera?A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef] [PubMed]
- Sinan, K.I.; Zengin, G.; Uba, A.I.; Caprioli, G.; Angeloni, S.; Vittori, S.; Jugreet, S.; Etienne, O.K.; Shariati, M.A.; Mahomoodally, M.F. Chemical characterization and biological abilities of Anthocleista djalonensis collected from two locations of Ivory Coast. eFood 2023, 4, e100. [Google Scholar] [CrossRef]
- Bibi, N.; Shah, M.H.; Khan, N.; Al-Hashimi, A.; Elshikh, M.S.; Iqbal, A.; Ahmad, S.; Abbasi, A.M. Variations in total phenolic, total flavonoid contents, and free radicals’ scavenging potential of onion varieties planted under diverse environmental conditions. Plants 2022, 11, 950. [Google Scholar] [CrossRef] [PubMed]
- Bournine, L.; Bensalem, S.; Wauters, J.-N.; Iguer-Ouada, M.; Maiza-Benabdesselam, F.; Bedjou, F.; Castronovo, V.; Bellahcène, A.; Tits, M.; Frédérich, M. Identification and quantification of the main active anticancer alkaloids from the root of Glaucium flavum. Int. J. Mol. Sci. 2013, 14, 23533–23544. [Google Scholar] [CrossRef] [PubMed]
- Nikolova, M.T.; Berkov, S.H.; Doycheva, I.V.; Stoyanov, S.S.; Stanilova, M.I. GC/MS based metabolite profiling of five populations of Glaucium flavum (Ranunculales: Papaveraceae) from the Black Sea coast of Bulgaria. Acta Zool Bulg. 2018, 11, 91–94. [Google Scholar]
- Wojtanowski, K.K.; Mroczek, T. Detection, identification and structural elucidation of flavonoids using liquid chromatography coupled to mass spectrometry. Curr. Org. Chem. 2020, 24, 104–112. [Google Scholar] [CrossRef]
- Maciejewska-Turska, M.; Zgórka, G. In-depth phytochemical and biological studies on potential AChE inhibitors in red and zigzag clover dry extracts using reversed–phase liquid chromatography (RP-LC) coupled with photodiode array (PDA) and electron spray ionization-quadrupole/time of flight-mass spectrometric (ESI-QToF/MS-MS) detection and thin-layer chromatography-bioautography. Food Chem. 2022, 375, 131846. [Google Scholar] [PubMed]
- Masike, K.; Mhlongo, M.I.; Mudau, S.P.; Nobela, O.; Ncube, E.N.; Tugizimana, F.; George, M.J.; Madala, N.E. Highlighting mass spectrometric fragmentation differences and similarities between hydroxycinnamoyl-quinic acids and hydroxycinnamoyl-isocitric acids. Chem. Cent. J. 2017, 11, 29. [Google Scholar] [CrossRef]
- Sun, M.; Liu, J.; Lin, C.; Miao, L.; Lin, L. Alkaloid profiling of the traditional Chinese medicine Rhizoma corydalis using high performance liquid chromatography-tandem quadrupole time-of-flight mass spectrometry. Acta Pharm. Sin. B 2014, 4, 208–216. [Google Scholar] [CrossRef]
- Zuo, Z.; Zheng, Y.; Liang, Z.; Liu, Y.; Tang, Q.; Liu, X.; Zhao, Z.; Zeng, J. Tissue-specific metabolite profiling of benzylisoquinoline alkaloids in the root of Macleaya cordata by combining laser microdissection with ultra-high-performance liquid chromatography/tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2017, 31, 397–410. [Google Scholar] [CrossRef]
- Ribas, I.; Sueiras, J.; Castedo, L. Corunnine and pontevedrine, two new aporphine alkaloids from Glaucium flavum Cr. var. Vestitum. Tetrahedron Lett. 1971, 12, 3093–3096. [Google Scholar] [CrossRef]
- Han, X.; Lamshöft, M.; Grobe, N.; Ren, X.; Fist, A.J.; Kutchan, T.M.; Spiteller, M.; Zenk, M.H. The biosynthesis of papaverine proceeds via (S)-reticuline. Phytochemistry 2010, 71, 1305–1312. [Google Scholar] [CrossRef] [PubMed]
- Menéndez-Perdomo, I.M.; Facchini, P.J. Benzylisoquinoline alkaloids biosynthesis in sacred lotus. Molecules 2018, 23, 2899. [Google Scholar] [CrossRef] [PubMed]
- Vadhel, A.; Bashir, S.; Mir, A.H.; Girdhar, M.; Kumar, D.; Kumar, A.; Mohan, A.; Malik, T.; Mohan, A. Opium alkaloids, biosynthesis, pharmacology and association with cancer occurrence. Open Biol. 2023, 13, 220355. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Jiao, Q.; Wang, C.; Jin, R.; Zhou, Y.; Shi, S.; Huang, Z.; Li, M.; Qin, X.; Chen, S. Establishment of holistic quality control methods for Nelumbinis Folium containing alkaloids and flavonoids with simple HPLC conditions. J. Chromatogr. Sci. 2022, 60, 871–879. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Yang, R.; Guan, Z.; Chen, A.; Li, W. Ultra-performance LC separation and quadrupole time-of-flight MS identification of major alkaloids in Plumula Nelumbinis. Phytochem. Anal. 2014, 25, 485–494. [Google Scholar] [CrossRef] [PubMed]
- Barakat, S.; Feras, Q.A.; Al Hammouri, M. Dereplication Study on Glaucium aleppicum Boiss. in Jordan. Orient. J. Chem. 2016, 32, 1815–1822. [Google Scholar] [CrossRef]
- Świątek, Ł.; Sieniawska, E.; Sinan, K.I.; Zengin, G.; Uba, A.I.; Bene, K.; Maciejewska-Turska, M.; Rajtar, B.; Polz-Dacewicz, M.; Aktumsek, A. Bridging the Chemical Profiles and Biological Effects of Spathodea campanulat a Extracts: A New Contribution on the Road from Natural Treasure to Pharmacy Shelves. Molecules 2022, 27, 4694. [Google Scholar] [CrossRef] [PubMed]
- Wolfender, J.-L.; Waridel, P.; Ndjoko, K.; Hobby, K.; Major, H.; Hostettmann, K. Evaluation of Q-TOF-MS/MS and multiple stage IT-MSn for the dereplication of flavonoids and related compounds in crude plant extracts. Analusis 2000, 28, 895–906. [Google Scholar] [CrossRef]
- Flieger, J.; Flieger, W.; Baj, J.; Maciejewski, R. Antioxidants: Classification, natural sources, activity/capacity measurements, and usefulness for the synthesis of nanoparticles. Materials 2021, 14, 4135. [Google Scholar] [CrossRef]
- Dall’Acqua, S.; Kumar, G.; Sinan, K.I.; Sut, S.; Ferrarese, I.; Mahomoodally, M.F.; Seebaluck-Sandoram, R.; Etienne, O.K.; Zengin, G. An insight into Cochlospermum planchonii extracts obtained by traditional and green extraction methods: Relation between chemical compositions and biological properties by multivariate analysis. Ind. Crops Prod. 2020, 147, 112226. [Google Scholar] [CrossRef]
- Li, D.; Rui, Y.-X.; Guo, S.-D.; Luan, F.; Liu, R.; Zeng, N. Ferulic acid: A review of its pharmacology, pharmacokinetics and derivatives. Life Sci. 2021, 284, 119921. [Google Scholar] [CrossRef]
- Marques, C.; Sotiles, A.R.; Farias, F.O.; Oliveira, G.; Mitterer-Daltoe, M.L.; Masson, M.L. Full physicochemical characterization of malic acid: Emphasis in the potential as food ingredient and application in pectin gels. Arab. J. Chem. 2020, 13, 9118–9129. [Google Scholar] [CrossRef]
- Akaranta, O.; Akaho, A. Synergic effect of Citric Acid and Red Onion skin extract on the Oxidative stability of Vegetable Oil. J. Appl. Sci. Environ. Manag. 2012, 16. [Google Scholar]
- O’Brien, P.; Carrasco-Pozo, C.; Speisky, H. Boldine and its antioxidant or health-promoting properties. Chem.-Biol. Interact. 2006, 159, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Pei, K.; Ou, J.; Huang, J.; Ou, S. p-Coumaric acid and its conjugates: Dietary sources, pharmacokinetic properties and biological activities. J. Sci. Food Agric. 2016, 96, 2952–2962. [Google Scholar] [CrossRef]
- Ayele, D.T.; Akele, M.; Melese, A. Analysis of total phenolic contents, flavonoids, antioxidant and antibacterial activities of Croton macrostachyus root extracts. BMC Chem. 2022, 16, 30. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, Y.; Ren, X.; Zhang, X.; Wu, Z.; Liu, L. The positive correlation of antioxidant activity and prebiotic effect about oat phenolic compounds. Food Chem. 2023, 402, 134231. [Google Scholar] [CrossRef] [PubMed]
- Gong, G.; Guan, Y.-Y.; Zhang, Z.-L.; Rahman, K.; Wang, S.-J.; Zhou, S.; Luan, X.; Zhang, H. Isorhamnetin: A review of pharmacological effects. Biomed. Pharmacother. 2020, 128, 110301. [Google Scholar] [CrossRef]
- Xu, D.; Hu, M.-J.; Wang, Y.-Q.; Cui, Y.-L. Antioxidant activities of quercetin and its complexes for medicinal application. Molecules 2019, 24, 1123. [Google Scholar] [CrossRef]
- Lee, H.S.; Cho, H.J.; Yu, R.; Lee, K.W.; Chun, H.S.; Park, J.H.Y. Mechanisms underlying apoptosis-inducing effects of Kaempferol in HT-29 human colon cancer cells. Int. J. Mol. Sci. 2014, 15, 2722–2737. [Google Scholar] [CrossRef] [PubMed]
- Todorov, L.; Saso, L.; Kostova, I. Antioxidant Activity of Coumarins and Their Metal Complexes. Pharmaceuticals 2023, 16, 651. [Google Scholar] [CrossRef] [PubMed]
- Losada-Barreiro, S.; Sezgin-Bayindir, Z.; Paiva-Martins, F.; Bravo-Díaz, C. Biochemistry of antioxidants: Mechanisms and pharmaceutical applications. Biomedicines 2022, 10, 3051. [Google Scholar] [CrossRef] [PubMed]
- Ademosun, A.O.; Oboh, G.; Bello, F.; Ayeni, P.O. Antioxidative properties and effect of quercetin and its glycosylated form (Rutin) on acetylcholinesterase and butyrylcholinesterase activities. J. Evid. -Based Complement. Altern. Med. 2016, 21, NP11–NP17. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.K.; Lee, K.T.; Baek, N.-I.; Kim, S.-H.; Park, H.W.; Lim, J.P.; Shin, T.Y.; Eom, D.O.; Yang, J.H.; Eun, J.S. Acetylcholinesterase inhibitors from the aerial parts of Corydalis speciosa. Arch. Pharmacal Res. 2004, 27, 1127–1131. [Google Scholar] [CrossRef]
- Kim, S.R.; Hwang, S.Y.; Jang, Y.P.; Park, M.J.; Markelonis, G.J.; Oh, T.H.; Kim, Y.C. Protopine from Corydalis ternata has anticholinesterase and antiamnesic activities. Planta Medica 1999, 65, 218–221. [Google Scholar] [CrossRef] [PubMed]
- El-Nashar, H.A.; El-Din, M.I.G.; Hritcu, L.; Eldahshan, O.A. Insights on the inhibitory power of flavonoids on tyrosinase activity: A survey from 2016 to 2021. Molecules 2021, 26, 7546. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Su, A.; Yuan, S.; Zhao, H.; Tan, S.; Hu, C.; Deng, H.; Guo, Y. Evaluation of total flavonoids, myricetin, and quercetin from Hovenia dulcis Thunb. as inhibitors of α-amylase and α-glucosidase. Plant Foods Hum. Nutr. 2016, 71, 444–449. [Google Scholar] [CrossRef] [PubMed]
- Geran, R.S.; Greenberg, N.H.; Macdonald, M.M.; Schumacher, A.M.; Abbott, B.J. Protocols for Screening Chemical Agents and Natural Products against Animal Tumors and Other Biological Systems. Cancer Chemother. Rep. 1972, 13, 1–87. [Google Scholar]
- Canga, I.; Vita, P.; Oliveira, A.I.; Castro, M.A.; Pinho, C. In Vitro Cytotoxic Activity of African Plants: A Review. Molecules 2022, 27, 4989. [Google Scholar] [CrossRef]
- Suffness, M.; Pezzuto, J.M.; Hostettmann, K. Methods in plant biochemistry: Assays for bioactivity. In Methods in Plant Biochemistry, 6th ed.; Hostettmann, K., Ed.; Academic Press: London, UK, 1990; pp. 33–71. [Google Scholar]
- Campoccia, D.; Ravaioli, S.; Santi, S.; Mariani, V.; Santarcangelo, C.; De Filippis, A.; Montanaro, L.; Arciola, C.R.; Daglia, M. Exploring the anticancer effects of standardized extracts of poplar-type propolis: In vitro cytotoxicity toward cancer and normal cell lines. Biomed. Pharmacother. 2021, 141, 111895. [Google Scholar] [CrossRef] [PubMed]
- Chandan, P.; Dev, A.; Ezhilarasan, D.; Harini, K.S.; Panigrahi, C.; Arora, D.; Devaraj, E.; Karthik, S.H. Boldine Treatment Induces Cytotoxicity in Human Colorectal Carcinoma and Osteosarcoma Cells. Cureus 2023, 15, e48126. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Zhang, Q.; Liao, L.; Li, Q.; Qu, H.; Wang, X.; Zhou, Y.; Zhang, G.; Sun, M.; Zhang, K. Isocorydine Exerts Anticancer Activity by Disrupting the Energy Metabolism and Filamentous Actin Structures of Oral Squamous Carcinoma Cells. Curr. Issues Mol. Biol. 2024, 46, 650–662. [Google Scholar] [CrossRef] [PubMed]
- Konda, Y.; Imai, Y.; Hojo, H.; Endo, T.; Nozoe, S. Suppression of tumor cell growth and mitogen response by aporphine alkaloids, dicentrine, glaucine, corydine, and apomorphine. J. Pharmacobio-Dyn. 1990, 13, 426–431. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Kong, L.; Cao, Y.; Yan, L. Identification and quantification, metabolism and pharmacokinetics, pharmacological activities, and botanical preparations of protopine: A review. Molecules 2021, 27, 215. [Google Scholar] [CrossRef] [PubMed]
- Pandey, P.; Khan, F.; Qari, H.A.; Oves, M. Rutin (Bioflavonoid) as cell signaling pathway modulator: Prospects in treatment and chemoprevention. Pharmaceuticals 2021, 14, 1069. [Google Scholar] [CrossRef]
- Bao, X.; Li, W.; Jia, R.; Meng, D.; Zhang, H.; Xia, L. Molecular mechanism of ferulic acid and its derivatives in tumor progression. Pharmacol. Rep. 2023, 75, 891–906. [Google Scholar] [CrossRef]




| Extract | TPC (mg GAE/g) | TFC (mg RE/g) |
|---|---|---|
| HAE-M | 53.22 ± 0.10 a | 20.30 ± 0.25 b |
| HAE-W | 23.10 ± 0.16 e | 2.10 ± 0.14 c |
| MAC-M | 36.49 ± 0.05 b | 30.28 ± 0.51 a |
| MAC-W | 23.81 ± 0.16 d | 1.32 ± 0.47 c |
| INF-W | 30.88 ± 0.43 c | 1.94 ± 0.38 c |
| Extracts | DPPH (mg TE/g) | ABTS (mg TE/g) | CUPRAC (mg TE/g) | FRAP (mg TE/g) | Chelating (mg EDTAE/g) | PBD (mmol TE/g) |
|---|---|---|---|---|---|---|
| HAE-M | 41.42 ± 0.62 a | 77.00 ± 2.01 c | 113.55 ± 6.44 a | 74.52 ± 4.74 a | 15.42 ± 0.33 b | 1.20 ± 0.17 a |
| HAE-W | 8.94 ± 0.32 d | 89.52 ± 0.43 b | 60.96 ± 1.65 d | 65.54 ± 3.15 b | 19.81 ± 0.05 a | 0.03 ± 0.01 d |
| MAC-M | 33.20 ± 0.27 b | 66.45 ± 5.79 d | 104.07 ± 1.04 b | 60.58 ± 0.40 b | 12.79 ± 0.29 c | 1.00 ± 0.12 b |
| MAC-W | 0.86 ± 0.03 e | 91.70 ± 0.89 b | 61.78 ± 0.35 d | 42.60 ± 1.03 c | na | 0.06 ± 0.01 d |
| INF-W | 19.48 ± 0.48 c | 103.59 ± 1.49 a | 85.76 ± 0.47 c | 65.13 ± 2.05 b | na | 0.40 ± 0.03 c |
| Extracts | AchE (mg ALAE/g) | BChE (mg GALAE/g) | Tyrosinase (mg KAE/g) | Amylase (mmol ACAE/g) |
|---|---|---|---|---|
| HAE-M | 2.55 ± 0.10 a | 1.45 ± 0.10 b | 25.15 ± 1.00 a | 0.51 ± 0.02 a |
| HAE-W | 1.25 ± 0.09 c | 1.09 ± 0.03 c | na | 0.09 ± 0.01 d |
| MAC-M | 2.07 ± 0.11 b | 3.76 ± 0.31 a | 26.79 ± 2.36 a | 0.45 ± 0.01 b |
| MAC-W | 0.65 ± 0.06 d | na | na | 0.30 ± 0.01 c |
| INF-W | 0.53 ± 0.05 d | na | na | 0.09 ± 0.01 d |
| Glaucium acutidentatum | VERO | FaDu | AGS | RKO | |||
|---|---|---|---|---|---|---|---|
| CC50 | CC50 | SI | CC50 | SI | CC50 | SI | |
| HAE-M | 371.95 ± 22.69 | 229.60 ± 28.08 | 1.62 | 317.50 ± 8.84 | 1.17 | 270.45 ± 12.29 | 1.38 |
| HAE-W | 157.73 ± 7.12 | 90.95 ± 9.17 | 1.73 | 387.17 ± 16.2 | 0.41 | 447.87 ± 15.97 | 0.35 |
| MAC-M | 591.60 ± 21.45 | 274.65 ± 11.70 | 2.15 | 337.25 ± 6.72 | 1.75 | 294.70 ± 16.78 | 2.01 |
| MAC-W | 225.70 ± 20.95 | 24.98 ± 4.78 | 9.04 | 266.20 ± 17.88 | 0.85 | 506.73 ± 32.68 | 0.45 |
| INF-W | 332.97 ± 32.17 | 112.13 ± 11.75 | 2.97 | 929.40 ± 69.93 | 0.36 | 1191.50 ± 129.40 | 0.28 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yagi, S.; Zengin, G.; Uba, A.I.; Maciejewska-Turska, M.; Sieniawska, E.; Świątek, Ł.; Rajtar, B.; Bahşi, M.; Guler, O.; Dall’Acqua, S.; et al. Exploring Chemical Composition, Antioxidant, Enzyme Inhibitory and Cytotoxic Properties of Glaucium acutidentatum Hausskn. & Bornm. from Turkey Flora: A Novel Source of Bioactive Agents to Design Functional Applications. Antioxidants 2024, 13, 643. https://doi.org/10.3390/antiox13060643
Yagi S, Zengin G, Uba AI, Maciejewska-Turska M, Sieniawska E, Świątek Ł, Rajtar B, Bahşi M, Guler O, Dall’Acqua S, et al. Exploring Chemical Composition, Antioxidant, Enzyme Inhibitory and Cytotoxic Properties of Glaucium acutidentatum Hausskn. & Bornm. from Turkey Flora: A Novel Source of Bioactive Agents to Design Functional Applications. Antioxidants. 2024; 13(6):643. https://doi.org/10.3390/antiox13060643
Chicago/Turabian StyleYagi, Sakina, Gokhan Zengin, Abdullahi Ibrahim Uba, Magdalena Maciejewska-Turska, Elwira Sieniawska, Łukasz Świątek, Barbara Rajtar, Muammer Bahşi, Osman Guler, Stefano Dall’Acqua, and et al. 2024. "Exploring Chemical Composition, Antioxidant, Enzyme Inhibitory and Cytotoxic Properties of Glaucium acutidentatum Hausskn. & Bornm. from Turkey Flora: A Novel Source of Bioactive Agents to Design Functional Applications" Antioxidants 13, no. 6: 643. https://doi.org/10.3390/antiox13060643
APA StyleYagi, S., Zengin, G., Uba, A. I., Maciejewska-Turska, M., Sieniawska, E., Świątek, Ł., Rajtar, B., Bahşi, M., Guler, O., Dall’Acqua, S., & Polz-Dacewicz, M. (2024). Exploring Chemical Composition, Antioxidant, Enzyme Inhibitory and Cytotoxic Properties of Glaucium acutidentatum Hausskn. & Bornm. from Turkey Flora: A Novel Source of Bioactive Agents to Design Functional Applications. Antioxidants, 13(6), 643. https://doi.org/10.3390/antiox13060643












