Vitamin C Alleviates the Negative Effects of Heat Stress on Reproductive Processes by Regulating Amino Acid Metabolism in Granulosa Cells
Abstract
1. Introduction
2. Methods
2.1. Granulosa Cells Culture and Treatment
2.2. Cell Growth Assay
2.3. Reactive Oxygen Species
2.4. Apoptosis Measurement
2.5. Hormone Measurements
2.6. Cell Cycle Analysis
2.7. Western Blot
2.8. Statistical Analysis
2.9. LC-MS/MS Analysis
2.10. Metabolome Analysis
3. Results
3.1. Physical Parameters of GCs
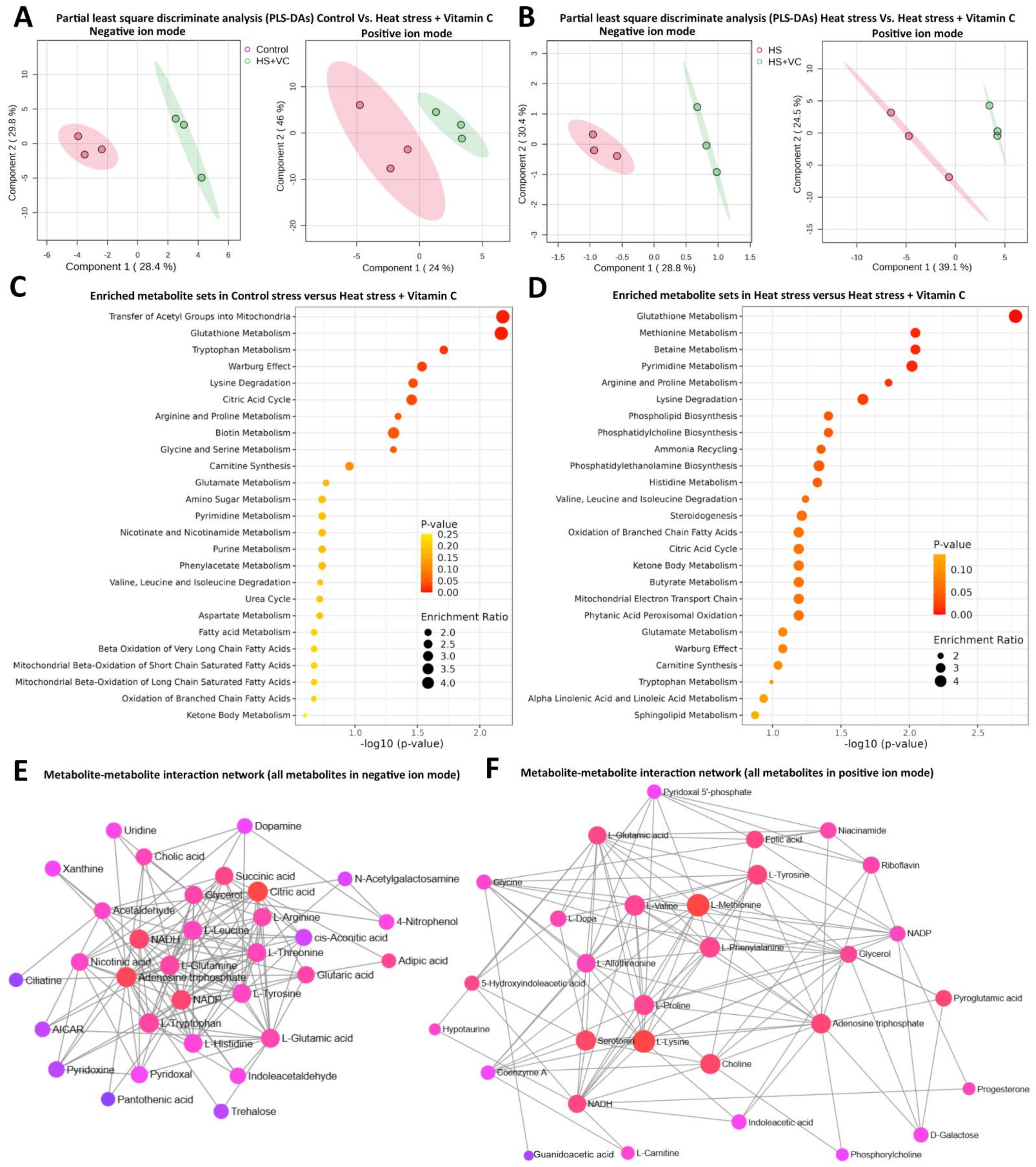
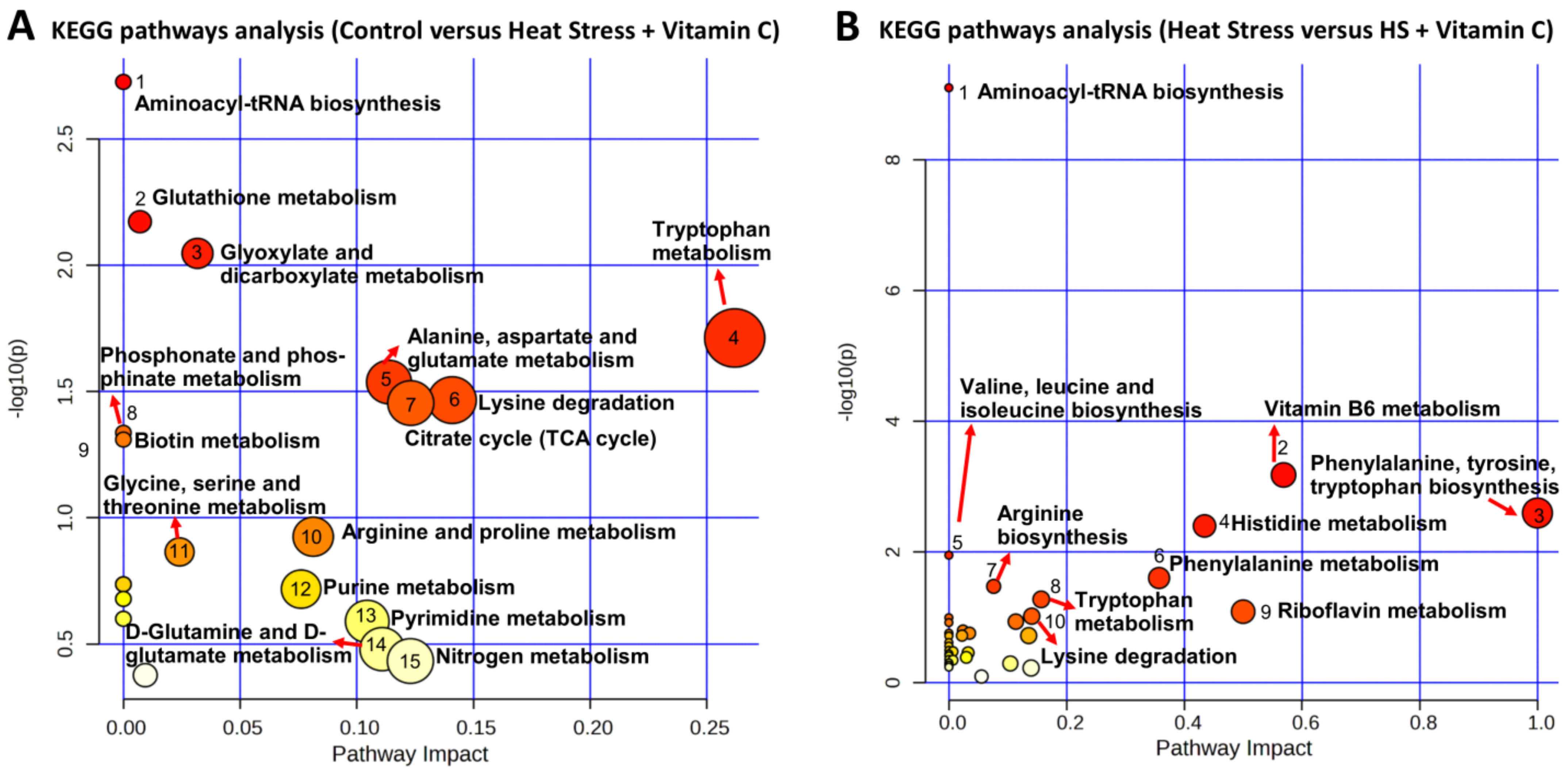
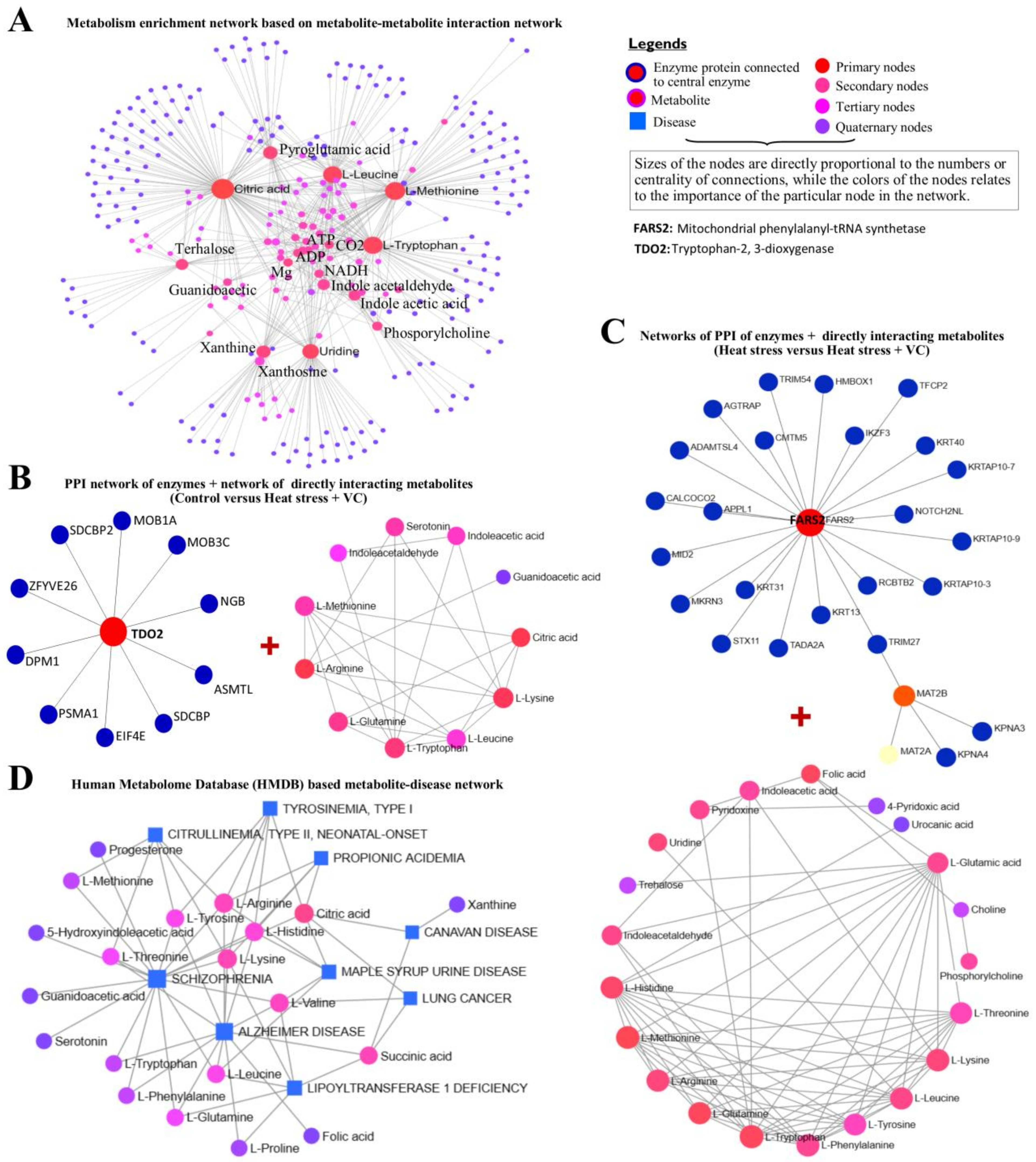
3.2. Metabolome Analysis
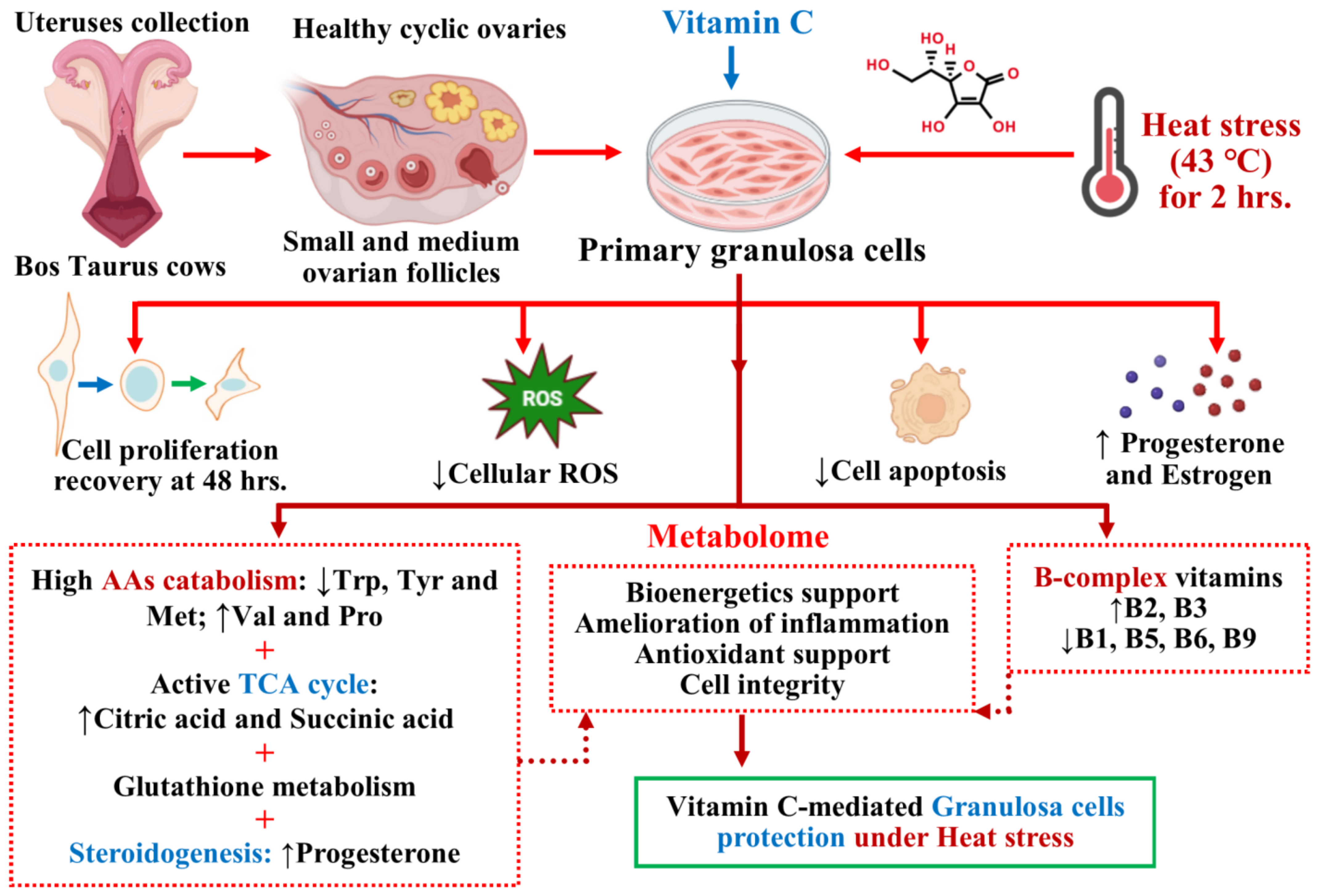
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Asseng, S.; Spänkuch, D.; Hernandez-Ochoa, I.M.; Laporta, J. The upper temperature thresholds of life. Lancet Planet. Health 2021, 5, E378–E385. [Google Scholar] [CrossRef]
- Boni, R. Heat stress, a serious threat to reproductive function in animals and humans. Mol. Reprod. Dev. 2019, 86, 1307–1323. [Google Scholar] [CrossRef] [PubMed]
- Hansen, P.J. Effects of heat stress on mammalian reproduction. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 3341–3350. [Google Scholar] [CrossRef]
- Dado-Senn, B.; Laporta, J.; Dahl, G.E. Carry over effects of late-gestational heat stress on dairy cattle progeny. Theriogenology 2020, 154, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Roth, Z. Effect of Heat Stress on Reproduction in Dairy Cows: Insights into the Cellular and Molecular Responses of the Oocyte. Annu. Rev. Anim. Biosci. 2017, 5, 151–170. [Google Scholar] [CrossRef] [PubMed]
- Tellam, R.L.; Lemay, D.G.; Van Tassell, C.P.; Lewin, H.A.; Worley, K.C.; Elsik, C.G. Unlocking the bovine genome. BMC Genom. 2009, 10, 193. [Google Scholar] [CrossRef] [PubMed]
- Hamernik, D.L. Farm Animals are Important Biomedical Models. Anim. Front. 2019, 9, 3–5. [Google Scholar] [CrossRef]
- Abedal-Majed, M.A.; Cupp, A.S. Livestock animals to study infertility in women. Anim. Front. 2019, 9, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Matsui, T. Vitamin C nutrition in cattle. Asian-Australas. J. Anim. Sci. 2012, 25, 597–605. [Google Scholar] [CrossRef]
- Kim, J.H.; Mamuad, L.L.; Yang, C.J.; Kim, S.H.; Ha, J.K.; Lee, W.S.; Cho, K.K.; Lee, S.S. Hemato-biochemical and cortisol profile of Holstein growing-calves supplemented with vitamin C during summer season. Asian-Australas. J. Anim. Sci. 2012, 25, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Ellulu, M.S.; Rahmat, A.; Patimah, I.; Khaza’Ai, H.; Abed, Y. Effect of vitamin C on inflammation and metabolic markers in hypertensive and/or diabetic obese adults: A randomized controlled trial. Drug Des. Devel. Ther. 2015, 9, 3405–3412. [Google Scholar] [CrossRef] [PubMed]
- Abdollahifar, M.A.; Azad, N.; Sajadi, E.; Mofarahe, Z.S.; Zare, F.; Moradi, A.; Rezaee, F.; Gholamin, M.; Abdi, S. Vitamin C restores ovarian follicular reservation in a mouse model of aging. Anat. Cell Biol. 2019, 52, 196. [Google Scholar] [CrossRef] [PubMed]
- Tierney, K.; Cai, Y. Assisted reproductive technology use in the United States: A population assessment. Fertil. Steril. 2019, 112, 1136–1143.E4. [Google Scholar] [CrossRef] [PubMed]
- Auclair, S.; Uzbekov, R.; Elis, S.; Sanchez, L.; Kireev, I.; Lardic, L.; Dalbies-Tran, R.; Uzbekova, S. Absence of cumulus cells during in vitro maturation affects lipid metabolism in bovine oocytes. Am. J. Physiol.—Endocrinol. Metab. 2013, 304, E599–E613. [Google Scholar] [CrossRef] [PubMed]
- Moradi, A.; Ghasemian, F.; Mashayekhi, F. The reaggregation of normal granulosa-cumulus cells and mouse oocytes with polycystic ovarian syndrome in vitro: An experimental study. Int. J. Reprod. Biomed. 2021, 19, 987. [Google Scholar] [CrossRef] [PubMed]
- Sammad, A.; Luo, H.; Hu, L.; Zhu, H.; Wang, Y. Transcriptome Reveals Granulosa Cells Coping through Redox, Inflammatory and Metabolic Mechanisms under Acute Heat Stress. Cells 2022, 11, 1443. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Khan, M.Z.; Dou, J.; Umer, S.; Xu, H.; Sammad, A.; Zhu, H.B.; Wang, Y. RNAi-Mediated Silencing of Catalase Gene Promotes Apoptosis and Impairs Proliferation of Bovine Granulosa Cells under Heat Stress. Animals 2020, 10, 1060. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Sammad, A.; Hu, L.; Luo, H.; Abbas, Z.; Umer, S.; Zhao, S.; Xu, Q.; Khan, A.; Wang, Y.; Zhu, H.; et al. Investigation of Metabolome Underlying the Biological Mechanisms of Acute Heat Stressed Granulosa Cells. Int. J. Mol. Sci. 2022, 23, 2146. [Google Scholar] [CrossRef] [PubMed]
- Tsugawa, H.; Cajka, T.; Kind, T.; Ma, Y.; Higgins, B.; Ikeda, K.; Kanazawa, M.; Vandergheynst, J.; Fiehn, O.; Arita, M. MS-DIAL: Data-independent MS/MS deconvolution for comprehensive metabolome analysis. Nat. Methods 2015, 12, 523–526. [Google Scholar] [CrossRef] [PubMed]
- Pang, Z.; Chong, J.; Zhou, G.; De Lima Morais, D.A.; Chang, L.; Barrette, M.; Gauthier, C.; Jacques, P.É.; Li, S.; Xia, J. MetaboAnalyst 5.0: Narrowing the gap between raw spectra and functional insights. Nucleic Acids Res. 2021, 49, W388–W396. [Google Scholar] [CrossRef]
- Jewison, T.; Su, Y.; Disfany, F.M.; Liang, Y.; Knox, C.; MacIejewski, A.; Poelzer, J.; Huynh, J.; Zhou, Y.; Arndt, D.; et al. SMPDB 2.0: Big improvements to the small molecule pathway database. Nucleic Acids Res. 2014, 42, D478–D484. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Furumichi, M.; Sato, Y.; Kawashima, M.; Ishiguro-Watanabe, M. KEGG for taxonomy-based analysis of pathways and genomes. Nucleic Acids Res. 2023, 51, D587–D592. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Santos, A.; Von Mering, C.; Jensen, L.J.; Bork, P.; Kuhn, M. STITCH 5: Augmenting protein-chemical interaction networks with tissue and affinity data. Nucleic Acids Res. 2016, 44, D380–D384. [Google Scholar] [CrossRef] [PubMed]
- Hinshaw, S.J.; Lee, A.H.Y.; Gill, E.E.; Hancock, R.E.W. MetaBridge: Enabling network-based integrative analysis via direct protein interactors of metabolites. Bioinformatics 2018, 34, 3225–3227. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Soufan, O.; Ewald, J.; Hancock, R.E.W.; Basu, N.; Xia, J. NetworkAnalyst 3.0: A visual analytics platform for comprehensive gene expression profiling and meta-analysis. Nucleic Acids Res. 2019, 47, W234–W241. [Google Scholar] [CrossRef] [PubMed]
- Rolland, T.; Taşan, M.; Charloteaux, B.; Pevzner, S.J.; Zhong, Q.; Sahni, N.; Yi, S.; Lemmens, I.; Fontanillo, C.; Mosca, R.; et al. A proteome-scale map of the human interactome network. Cell 2014, 159, 1212–1226. [Google Scholar] [CrossRef]
- Wishart, D.S.; Guo, A.C.; Oler, E.; Wang, F.; Anjum, A.; Peters, H.; Dizon, R.; Sayeeda, Z.; Tian, S.; Lee, B.L.; et al. HMDB 5.0: The Human Metabolome Database for 2022. Nucleic Acids Res. 2022, 50, D622–D631. [Google Scholar] [CrossRef] [PubMed]
- Jahromi, B.N.; Mosallanezhad, Z.; Matloob, N.; Davari, M.; Ghobadifar, M.A. The potential role of granulosa cells in the maturation rate of immature human oocytes and embryo development: A co-culture study. Clin. Exp. Reprod. Med. 2015, 42, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Diaz, F.J.; Wigglesworth, K.; Eppig, J.J. Oocytes determine cumulus cell lineage in mouse ovarian follicles. J. Cell Sci. 2007, 120, 1330–1340. [Google Scholar] [CrossRef]
- Fu, Y.; He, C.-J.; Ji, P.-Y.; Zhuo, Z.-Y.; Tian, X.-Z.; Wang, F.; Tan, D.-X.; Liu, G.-S. Effects of Melatonin on the Proliferation and Apoptosis of Sheep Granulosa Cells under Thermal Stress. Int. J. Mol. Sci. 2014, 15, 21090–21104. [Google Scholar] [CrossRef] [PubMed]
- Saadeldin, I.M.; Swelum, A.A.A.; Elsafadi, M.; Mahmood, A.; Osama, A.; Shikshaky, H.; Alfayez, M.; Alowaimer, A.N.; Magdeldin, S. Thermotolerance and plasticity of camel somatic cells exposed to acute and chronic heat stress. J. Adv. Res. 2020, 22, 105–118. [Google Scholar] [CrossRef] [PubMed]
- Yin, B.; Tang, S.; Sun, J.; Zhang, X.; Xu, J.; Di, L.; Li, Z.; Hu, Y.; Bao, E. Vitamin C and sodium bicarbonate enhance the antioxidant ability of H9C2 cells and induce HSPs to relieve heat stress. Cell Stress Chaperones 2018, 23, 735–748. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Yin, B.; Tang, S.; Zhang, X.; Xu, J.; Bao, E. Vitamin C mitigates heat damage by reducing oxidative stress, inducing HSP expression in TM4 Sertoli cells. Mol. Reprod. Dev. 2019, 86, 673–685. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.L.; Zhang, J.; Ping, Z.G.; Wang, C.Q.; Sun, Y.F.; Chen, L.; Li, X.Y.; Li, C.J.; Zhu, X.L.; Liu, Z.; et al. Relationship between Apoptosis and Proliferation in Granulosa and Theca Cells of Cystic Follicles in Sows. Reprod. Domest. Anim. 2012, 47, 601–608. [Google Scholar] [CrossRef]
- Sirotkin, A.V. Effect of two types of stress (heat shock/high temperature and malnutrition/serum deprivation) on porcine ovarian cell functions and their response to hormones. J. Exp. Biol. 2010, 213, 2125–2130. [Google Scholar] [CrossRef] [PubMed]
- Scott, M.; Bonnefin, P.; Vieyra, D.; Boisvert, F.M.; Young, D.; Bazett-Jones, D.P.; Riabowol, K. UV-induced binding of ING1 to PCNA regulates the induction of apoptosis. J. Cell Sci. 2001, 114, 3455–3462. [Google Scholar] [CrossRef] [PubMed]
- Habibi, P.; Ostad, S.N.; Heydari, A.; Aliebrahimi, S.; Montazeri, V.; Foroushani, A.R.; Monazzam, M.R.; Ghazi-Khansari, M.; Golbabaei, F. Effect of heat stress on DNA damage: A systematic literature review. Int. J. Biometeorol. 2022, 66, 2147–2158. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Dou, J.; Wang, Y.; Jiang, X.; Khan, M.Z.; Luo, H.; Usman, T.; Zhu, H. Evaluation of heat stress effects on cellular and transcriptional adaptation of bovine granulosa cells. J. Anim. Sci. Biotechnol. 2020, 11, 25. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yang, C.; Elsheikh, N.A.H.; Li, C.; Yang, F.; Wang, G.; Li, L. HO-1 reduces heat stress-induced apoptosis in bovine granulosa cells by suppressing oxidative stress. Aging 2019, 11, 5535–5547. [Google Scholar] [CrossRef] [PubMed]
- Azad, M.A.K.; Kikusato, M.; Sudo, S.; Amo, T.; Toyomizu, M. Time course of ROS production in skeletal muscle mitochondria from chronic heat-exposed broiler chicken. Comp. Biochem. Physiol.—A Mol. Integr. Physiol. 2010, 157, 266–271. [Google Scholar] [CrossRef] [PubMed]
- Gu, Z.T.; Li, L.; Wu, F.; Zhao, P.; Yang, H.; Liu, Y.S.; Geng, Y.; Zhao, M.; Su, L. Heat stress induced apoptosis is triggered by transcription-independent p53, Ca2+ dyshomeostasis and the subsequent Bax mitochondrial translocation. Sci. Rep. 2015, 5, 11497. [Google Scholar] [CrossRef] [PubMed]
- Hou, C.H.; Lin, F.L.; Hou, S.M.; Liu, J.F. Hyperthermia induces apoptosis through endoplasmic reticulum and reactive oxygen species in human Osteosarcoma cells. Int. J. Mol. Sci. 2014, 15, 17380–17395. [Google Scholar] [CrossRef] [PubMed]
- Alemu, T.W.; Pandey, H.O.; Salilew Wondim, D.; Gebremedhn, S.; Neuhof, C.; Tholen, E.; Holker, M.; Schellander, K.; Tesfaye, D. Oxidative and endoplasmic reticulum stress defense mechanisms of bovine granulosa cells exposed to heat stress. Theriogenology 2018, 110, 130–141. [Google Scholar] [CrossRef] [PubMed]
- Villeneuve, N.F.; Sun, Z.; Chen, W.; Zhang, D.D. Nrf2 and p21 regulate the fine balance between life and death by controlling ROS levels. Cell Cycle 2009, 8, 3255–3256. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, S.; Summers, C.M.; Pearce, S.C.; Gabler, N.K.; Valentine, R.J.; Baumgard, L.H.; Rhoads, R.P.; Selsby, J.T. Short-term heat stress altered metabolism and insulin signaling in skeletal muscle. J. Anim. Sci. 2018, 96, 154–167. [Google Scholar] [CrossRef] [PubMed]
- Hambright, H.G.; Meng, P.; Kumar, A.P.; Ghosh, R. Inhibition of PI3K/AKT/mTOR axis disrupts oxidative stress-mediated survival of melanoma cells. Oncotarget 2015, 6, 7195–7208. [Google Scholar] [CrossRef] [PubMed]
- Reddy, N.M.; Potteti, H.R.; Vegiraju, S.; Chen, H.J.; Tamatam, C.M.; Reddy, S.P. PI3K-AKT Signaling via Nrf2 Protects against Hyperoxia-Induced Acute Lung Injury, but Promotes Inflammation Post-Injury Independent of Nrf2 in Mice. PLoS ONE 2015, 10, e0129676. [Google Scholar] [CrossRef] [PubMed]
- Cantó, C.; Auwerx, J. PGC-1α, SIRT1 and AMPK, an energy sensing network that controls energy expenditure. Curr. Opin. Lipidol. 2009, 20, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Cecchino, G.N.; Pacheco, A.; García-Velasco, J.A. Reproductive senescence and energetic metabolism of human luteinized granulosa cells: Is it all about ATP? A prospective cohort and critical view. Gynecol. Endocrinol. 2021, 37, 523–527. [Google Scholar] [CrossRef] [PubMed]
- Fang, W.; Wen, X.; Meng, Q.; Liu, L.; Xie, J.; Everaert, N.; Zhang, H. Running Head: Heat Affects Cholesterol and Bile Acid Alterations in Cholesterol and Bile Acids Metabolism in Large White Pigs during Short-Term Heat Exposure. Animals 2020, 10, 359. [Google Scholar] [CrossRef] [PubMed]
- Faheem, M.S.; Dessouki, S.M.; Abdel- Rahman, F.E.S.; Ghanem, N. Physiological and molecular aspects of heat-treated cultured granulosa cells of Egyptian buffalo (Bubalus bubalis). Anim. Reprod. Sci. 2021, 224, 106665. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, V.S.; Tomonaga, S.; Ikegami, T.; Erwan, E.; Ito, K.; Cockrem, J.F.; Furuse, M. Oxidative damage and brain concentrations of free amino acid in chicks exposed to high ambient temperature. Comp. Biochem. Physiol.—A Mol. Integr. Physiol. 2014, 169, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, V.S. Heat stress biomarker amino acids and neuropeptide afford thermotolerance in chicks. J. Poult. Sci. 2019, 56, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Maeda, E.; Kimura, S.; Yamada, M.; Tashiro, M.; Ohashi, T. Enhanced gap junction intercellular communication inhibits catabolic and pro-inflammatory responses in tenocytes against heat stress. J. Cell Commun. Signal. 2017, 11, 369–380. [Google Scholar] [CrossRef] [PubMed]
- Sammad, A.; Wang, Y.J.; Umer, S.; Lirong, H.; Khan, I.; Khan, A.; Ahmad, B.; Wang, Y. Nutritional Physiology and Biochemistry of Dairy Cattle under the Influence of Heat Stress: Consequences and Opportunities. Animals 2020, 10, 793. [Google Scholar] [CrossRef] [PubMed]
- Ito, K.; Erwan, E.; Nagasawa, M.; Furuse, M.; Chowdhury, V.S. Changes in free amino acid concentrations in the blood, brain and muscle of heat-exposed chicks. Br. Poult. Sci. 2014, 55, 644–652. [Google Scholar] [CrossRef] [PubMed]
- Haq, S.; Grondin, J.A.; Khan, W.I. Tryptophan-derived serotonin-kynurenine balance in immune activation and intestinal inflammation. FASEB J. 2021, 35, e21888. [Google Scholar] [CrossRef] [PubMed]
- Munn, D.H.; Mellor, A.L. Indoleamine 2,3 dioxygenase and metabolic control of immune responses. Trends Immunol. 2013, 34, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Paudel, S.; Wu, G.; Wang, X. Amino Acids in Cell Signaling: Regulation and Function. In Advances in Experimental Medicine and Biology; Springer: Cham, Switzerland, 2021; Volume 1332. [Google Scholar]
- Zheng, X.; Zhu, Y.; Zhao, Z.; Chu, Y.; Yang, W. The role of amino acid metabolism in inflammatory bowel disease and other inflammatory diseases. Front. Immunol. 2023, 14, 1284133. [Google Scholar] [CrossRef] [PubMed]
- Tram, N.K.; McLean, R.M.; Swindle-Reilly, K.E. Glutathione Improves the Antioxidant Activity of Vitamin C in Human Lens and Retinal Epithelial Cells: Implications for Vitreous Substitutes. Curr. Eye Res. 2021, 46, 470–481. [Google Scholar] [CrossRef] [PubMed]
- Akram, M. Citric Acid Cycle and Role of its Intermediates in Metabolism. Cell Biochem. Biophys. 2013, 68, 475–478. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Salam, O.M.E.; Youness, E.R.; Mohammed, N.A.; Morsy, S.M.Y.; Omara, E.A.; Sleem, A.A. Citric acid effects on brain and liver oxidative stress in lipopolysaccharide-treated mice. J. Med. Food 2014, 17, 588–598. [Google Scholar] [CrossRef] [PubMed]
- Elbaz, A.M.; Ibrahim, N.S.; Shehata, A.M.; Mohamed, N.G.; Abdel-Moneim, A.M.E. Impact of multi-strain probiotic, citric acid, garlic powder or their combinations on performance, ileal histomorphometry, microbial enumeration and humoral immunity of broiler chickens. Trop. Anim. Health Prod. 2021, 53, 115. [Google Scholar] [CrossRef] [PubMed]
- Current, J.Z.; Mentler, M.; Whitaker, B.D. Linoleic and linolenic acids reduce the effects of heat stress–induced damage in pig oocytes during maturation in vitro. Vitr. Cell. Dev. Biol.—Anim. 2022, 58, 599–609. [Google Scholar] [CrossRef]
- Bennett, M.J.; Sheng, F.; Saada, A. Biochemical assays of TCA cycle and β-oxidation metabolites. In Methods in Cell Biology; Elsevier: Amsterdam, The Netherlands, 2020; Volume 155. [Google Scholar]
- Chan, M.K.; Tsang, T.M.; Harris, L.W.; Guest, P.C.; Holmes, E.; Bahn, S. Evidence for disease and antipsychotic medication effects in post-mortem brain from schizophrenia patients. Mol. Psychiatry 2011, 16, 1189–1202. [Google Scholar] [CrossRef] [PubMed]
- Lyon, P.; Strippoli, V.; Fang, B.; Cimmino, L. B vitamins and one-carbon metabolism: Implications in human health and disease. Nutrients 2020, 12, 2867. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Zhang, L.; Li, J.; Xing, T.; Jiang, Y.; Gao, F. Heat stress alters muscle protein and amino acid metabolism and accelerates liver gluconeogenesis for energy supply in broilers. Poult. Sci. 2021, 100, 215–223. [Google Scholar] [CrossRef]
- Ridgway, N.D.; McLeod, R.S. Biochemistry of Lipids, Lipoproteins and Membranes, 6th ed.; Elsevier: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Gonçalves, A.C.; Portari, G.V. The B-complex vitamins related to energy metabolism and their role in exercise performance: A narrative review. Sci. Sport. 2021, 36, 433–440. [Google Scholar] [CrossRef]


| Metabolites (Positive Ion-Mode) | C vs. HS | C vs. HS + VC | HS vs. HS + VC | |||
|---|---|---|---|---|---|---|
| Log2(FC) VIP | Log2(FC) VIP | Log2(FC) VIP | ||||
| (-)-Riboflavin | −0.404 | 1.489 | 0.007 | 0.287 | 0.421 | 1.145 |
| 1-Amino-1-cyclopentanecarboxylic acid | 0.015 | 0.850 | 0.226 | 1.713 | 0.210 | 0.860 |
| 1-Methylhistamine | −0.054 | 0.970 | 0.090 | 0.624 | 0.148 | 1.554 |
| 3,4-Dihydrocoumarin | 0.037 | 0.964 * | 0.034 | 0.479 | 0.006 | 0.274 |
| 3-Formylindole | 0.031 | 0.754 | 0.166 | 0.853 | 0.150 | 0.588 |
| 4-Acetamidoantipyrine | −0.423 | 1.454 | 0.101 | 0.724 | 0.517 | 1.559 * |
| 4-Hydroxybenzoylcholine | 0.208 | 0.485 | 0.004 | 0.499 | −0.188 | 1.406 |
| 5-Hydroxyindole-3-acetic acid | 0.076 | 0.688 | 0.093 | 0.586 | 0.041 | 0.145 |
| Aspartame | −0.038 | 1.093 * | −0.138 | 0.570 | −0.087 | 0.568 |
| beta-Guanidinopropionic acid | −0.199 | 1.361 * | −0.301 | 1.395 | −0.099 | 0.445 |
| Choline | 1.113 | 1.588 * | 0.201 | 0.584 | −0.895 | 1.528 * |
| D-(+)-Galactosamine | 0.323 | 0.023 * | −0.099 | 0.235 | −0.421 | 1.666 |
| D-(+)-Glucosamine | 0.155 | 0.459 | 0.095 | 0.707 | −0.071 | 0.062 |
| DOPA | 0.137 | 0.579 | −0.344 | 1.305 | −0.463 | 0.789 |
| Folic Acid | −0.429 | 1.295 | 0.077 | 0.503 | 0.490 | 1.235 |
| Galactose | 0.177 | 0.497 * | 0.032 | 0.663 | −0.148 | 0.663 |
| Gentiobiose | 0.632 | 0.281 | −0.393 | 1.974 | −0.999 | 1.251 |
| Glycocyamine | −0.304 | 1.501 * | −0.340 | 1.493 | −0.033 | 0.190 |
| Hexylamine | 0.030 | 0.988 * | 0.015 | 0.426 | −0.003 | 0.232 |
| Hypotaurine | −0.494 | 0.873 | 0.362 | 0.632 | 0.819 | 0.854 |
| Indoleacetic acid | −0.092 | 0.955 | 0.263 | 1.181 | 0.355 | 1.422 |
| L-2-Aminoadipic acid | −0.303 | 1.230 | 0.319 | 1.164 | 0.622 | 1.763 * |
| L-5-Oxoproline | 0.107 | 0.760 | −0.551 | 2.141 * | −0.642 | 1.769 * |
| L-allo-Threonine | 0.164 | 0.728 * | 0.182 | 1.703 | 0.027 | 0.328 |
| L-Carnitine | −0.358 | 1.389 | −0.497 | 1.310 | −0.116 | 0.498 |
| Levetiracetam | −0.386 | 1.136 | 0.136 | 0.610 | 0.521 | 1.022 |
| Lysine | 0.344 | 0.067 | 0.098 | 1.456 | −0.231 | 0.636 |
| Methionine | 0.097 | 0.632 | −0.353 | 1.692 | −0.431 | 1.074 |
| Mycophenolic acid | 0.167 | 0.615 | 0.146 | 1.164 * | −0.016 | 1.190 |
| Niacinamide | −0.008 | 0.814 | 0.278 | 0.981 | 0.283 | 0.418 |
| Phenanthridine | 0.249 | 0.313 | 0.023 | 0.366 | −0.223 | 1.143 |
| Phenylalanine | 0.055 | 0.939 * | 0.027 | 0.489 | −0.017 | 0.099 |
| Phosphocholine | −0.890 | 0.832 | 0.350 | 1.043 | 1.174 | 1.282 |
| Progesterone | −1.281 | 1.747 | −0.493 | 0.281 | 0.769 | 1.471 |
| Proline | −0.218 | 1.323 * | 0.058 | 0.589 | 0.278 | 1.401 |
| Pyrimidinol | 0.261 | 0.140 | 0.046 | 1.401 | −0.196 | 0.605 |
| Salsolinol | −0.066 | 1.101 * | −0.183 | 0.524 | −0.106 | 0.594 |
| Serotonin | 0.169 | 0.515 | 0.182 | 1.108 | 0.014 | 0.072 |
| Thiamine | −0.127 | 1.134 | −0.179 | 0.525 | −0.035 | 0.071 |
| Tyrosine | 0.150 | 0.567 | −0.065 | 0.127 | −0.204 | 0.722 |
| Urocanic acid | −0.588 | 1.351 | 0.383 | 1.011 | 0.957 | 1.569 * |
| Valine | −0.190 | 1.086 | 0.042 | 0.281 | 0.244 | 0.616 |
| Metabolites (Negative Ion-Mode) | C vs. HS | C vs. HS + VC | HS vs. HS + VC | |||
|---|---|---|---|---|---|---|
| Log2(FC) | VIP | Log2(FC) | VIP | Log2(FC) | VIP | |
| (-)-Citramalic acid | −0.127 | 0.712 | 0.228 | 1.102 | 0.348 | 1.561 |
| 16-Hydroxyhexadecanoic acid | −0.367 | 1.523 * | −0.236 | 1.848 * | 0.123 | 1.019 * |
| 2-Hydroxyisobutyric acid | −0.048 | 0.289 | 0.488 | 1.439 | 0.528 | 1.902 |
| 2-Hydroxyisocaproic acid | −0.058 | 0.316 | −0.219 | 1.243 | −0.168 | 0.927 |
| 3-Hydroxy-3-methylglutaric acid | 0.197 | 1.097 * | 0.148 | 1.443 | −0.058 | 0.495 |
| 3-Indoxyl sulfate | −0.139 | 0.866 * | −0.195 | 1.641 * | −0.064 | 0.695 |
| 4-Nitrophenol | 0.160 | 0.845 | 0.186 | 1.332 | 0.016 | 0.096 |
| AICAR | −0.287 | 1.169 | 0.057 | 0.432 | 0.333 | 1.634 * |
| Cholic acid | −0.585 | 1.558 | −0.216 | 0.561 | 0.362 | 1.310 |
| Ciliatine | −1.324 | 2.741 * | −1.097 | 1.672 * | 0.217 | 0.953 |
| cis-Aconitic acid | −0.255 | 0.875 | −0.010 | 0.009 | 0.237 | 1.115 |
| Citric acid | 0.359 | 1.333 | 0.505 | 1.592 * | 0.139 | 0.756 |
| D-(−)-Quinic acid | −0.198 | 0.944 | −0.177 | 0.894 | 0.010 | 0.039 |
| D-(+)-Pantothenic acid | −0.225 | 0.948 | −0.104 | 0.685 | 0.113 | 0.596 |
| D-(+)-Trehalose | −0.341 | 0.938 | −0.256 | 0.607 | 0.072 | 0.055 |
| DL-3-(4-Hydroxyphenyl)lactic acid | −0.383 | 1.293 | 0.052 | 0.020 | 0.428 | 1.412 |
| gamma-Linolenic acid | −0.107 | 0.247 | 0.348 | 0.921 | 0.442 | 1.592 |
| Glutamine | 0.159 | 0.903 | 0.147 | 1.226 | −0.022 | 0.200 |
| Glutaric acid | −0.102 | 0.707 | −0.021 | 0.640 | 0.072 | 0.700 |
| Indole-3-acetaldehyde | 0.357 | 1.338 | 0.387 | 1.160 | 0.021 | 0.023 |
| Indole-3-carboxyaldehyde | 0.095 | 0.301 | 0.702 | 1.295 | 0.598 | 1.781 |
| L-(−)-Mandelic acid | −0.322 | 1.394 * | −0.042 | 1.788 * | 0.041 | 0.380 |
| L-(+)-Arginine | 0.261 | 0.984 | 0.221 | 0.977 | −0.050 | 0.257 |
| L-Histidine | 0.111 | 0.511 | 0.154 | 0.646 | 0.035 | 0.113 |
| L-Iditol | −0.104 | 0.722 | −0.104 | 1.374 | −0.008 | 0.090 |
| L-Leucine | 0.298 | 1.272 * | 0.075 | 0.584 | −0.231 | 1.349 * |
| L-Norvaline | 0.114 | 0.577 | −0.114 | 0.638 | −0.238 | 1.272 |
| L-Tryptophan | −0.042 | 0.282 | −0.270 | 1.763 * | −0.238 | 1.366 * |
| L-Tyrosine | 0.230 | 1.005 | −0.003 | 0.024 | −0.240 | 1.241 |
| Nicotinic acid | 0.100 | 0.532 | 0.044 | 0.617 | −0.065 | 0.453 |
| N-Tigloylglycine | −0.091 | 0.452 | −0.176 | 1.332 | −0.092 | 0.375 |
| Pyridoxal | 0.168 | 0.704 | 0.097 | 0.665 | −0.081 | 0.382 |
| Pyridoxine | 0.331 | 1.094 | −0.037 | 0.266 | −0.380 | 1.407 |
| Sebacic acid | 0.179 | 0.974 | 0.120 | 1.212 | −0.069 | 0.577 |
| Succinic acid | −0.259 | 1.162 * | 0.331 | 0.993 | 0.582 | 1.985 |
| Threonine | 0.169 | 0.878 | 0.083 | 0.668 | −0.095 | 0.640 |
| Uridine | −0.913 | 2.182 * | −0.302 | 0.756 | 0.602 | 2.153 * |
| Xanthine | 0.324 | 1.231 | −0.198 | 0.891 | −0.450 | 1.819 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sammad, A.; Ahmed, T.; Ullah, K.; Hu, L.; Luo, H.; Alphayo Kambey, P.; Faisal, S.; Zhu, H.; Li, Y.; Wang, Y. Vitamin C Alleviates the Negative Effects of Heat Stress on Reproductive Processes by Regulating Amino Acid Metabolism in Granulosa Cells. Antioxidants 2024, 13, 653. https://doi.org/10.3390/antiox13060653
Sammad A, Ahmed T, Ullah K, Hu L, Luo H, Alphayo Kambey P, Faisal S, Zhu H, Li Y, Wang Y. Vitamin C Alleviates the Negative Effects of Heat Stress on Reproductive Processes by Regulating Amino Acid Metabolism in Granulosa Cells. Antioxidants. 2024; 13(6):653. https://doi.org/10.3390/antiox13060653
Chicago/Turabian StyleSammad, Abdul, Tanveer Ahmed, Khair Ullah, Lirong Hu, Hanpeng Luo, Piniel Alphayo Kambey, Shah Faisal, Huabin Zhu, Yinxiong Li, and Yachun Wang. 2024. "Vitamin C Alleviates the Negative Effects of Heat Stress on Reproductive Processes by Regulating Amino Acid Metabolism in Granulosa Cells" Antioxidants 13, no. 6: 653. https://doi.org/10.3390/antiox13060653
APA StyleSammad, A., Ahmed, T., Ullah, K., Hu, L., Luo, H., Alphayo Kambey, P., Faisal, S., Zhu, H., Li, Y., & Wang, Y. (2024). Vitamin C Alleviates the Negative Effects of Heat Stress on Reproductive Processes by Regulating Amino Acid Metabolism in Granulosa Cells. Antioxidants, 13(6), 653. https://doi.org/10.3390/antiox13060653









