Cytotoxic Oxidative Stress Effects of Neutrophil Extracellular Traps’ Components on Cattle Spermatozoa
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Declaration, Site, and Reagents
2.2. Sperm Selection
2.3. PMN Isolation
2.4. DNA Staining
2.5. Sperm Treatments
2.6. Lipoperoxidation
2.7. Intracellular O2·− Production
2.8. Mitochondrial O2·− Production
2.9. Flow Cytometry Analysis
2.10. Statistical Analysis
3. Results
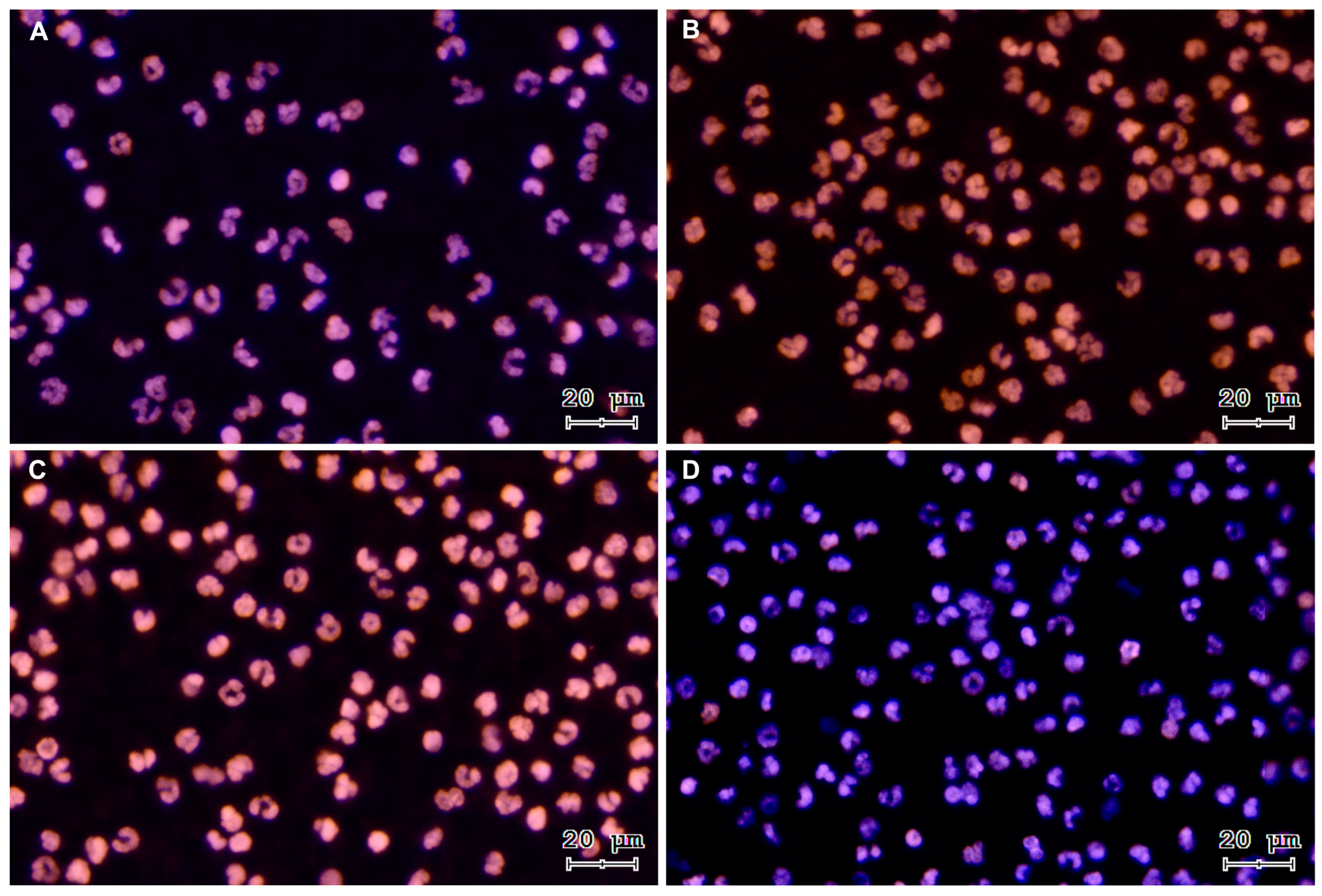
3.1. Sperm-Triggered NETs and NETs Phenotypes
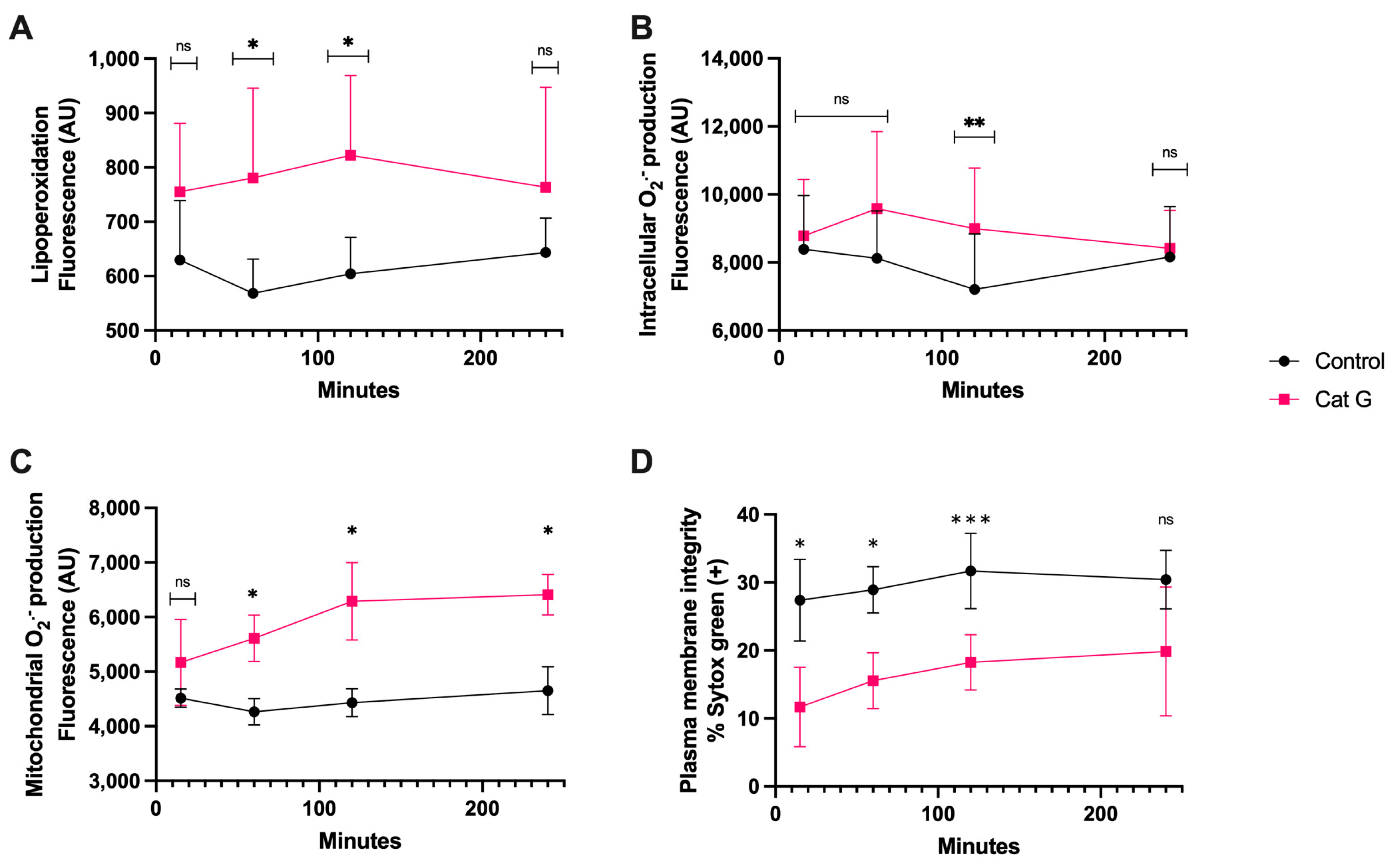
3.2. CatG Treatment
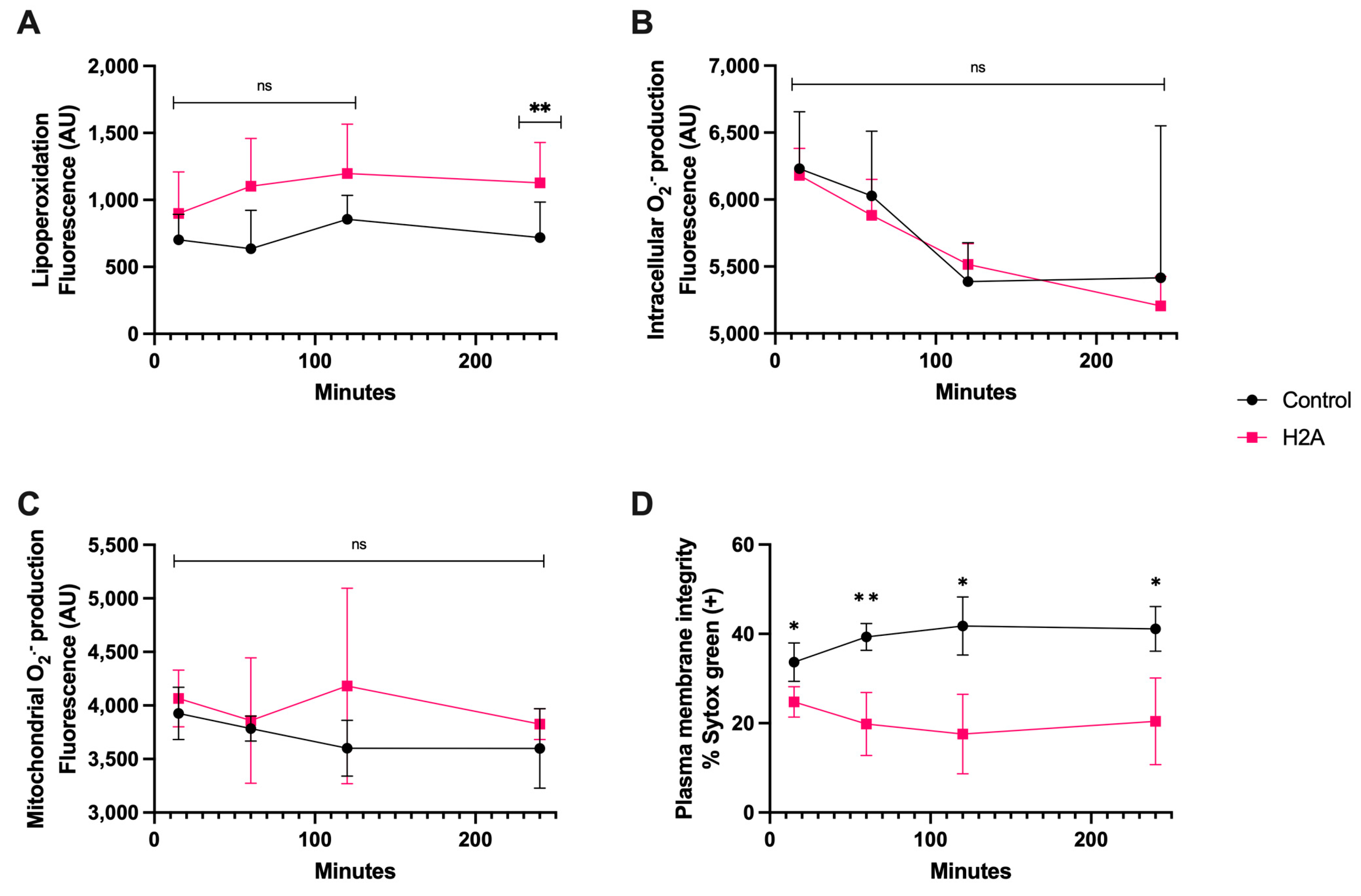
3.3. H2A Treatment
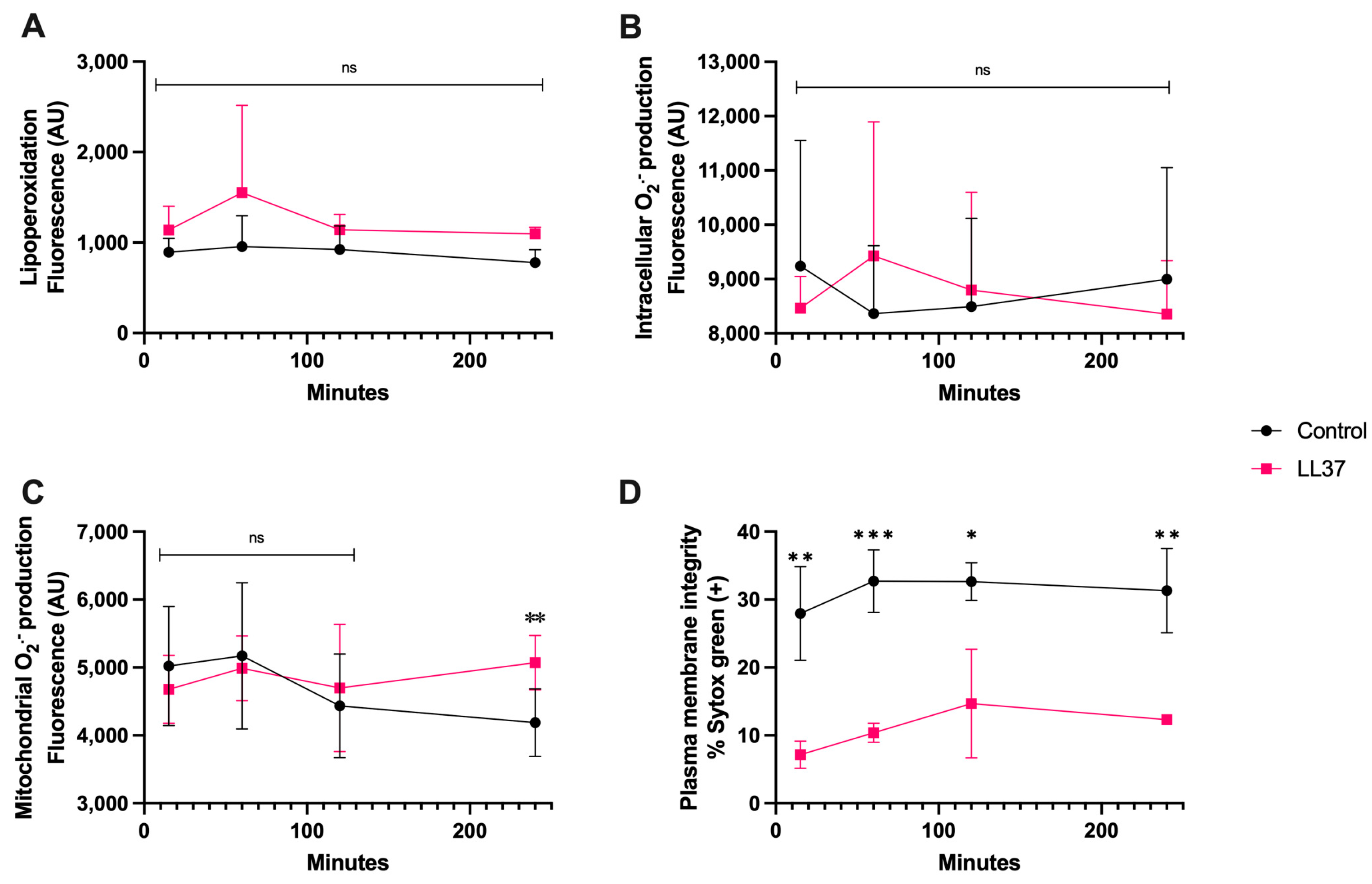
3.4. LL-37 Treatment
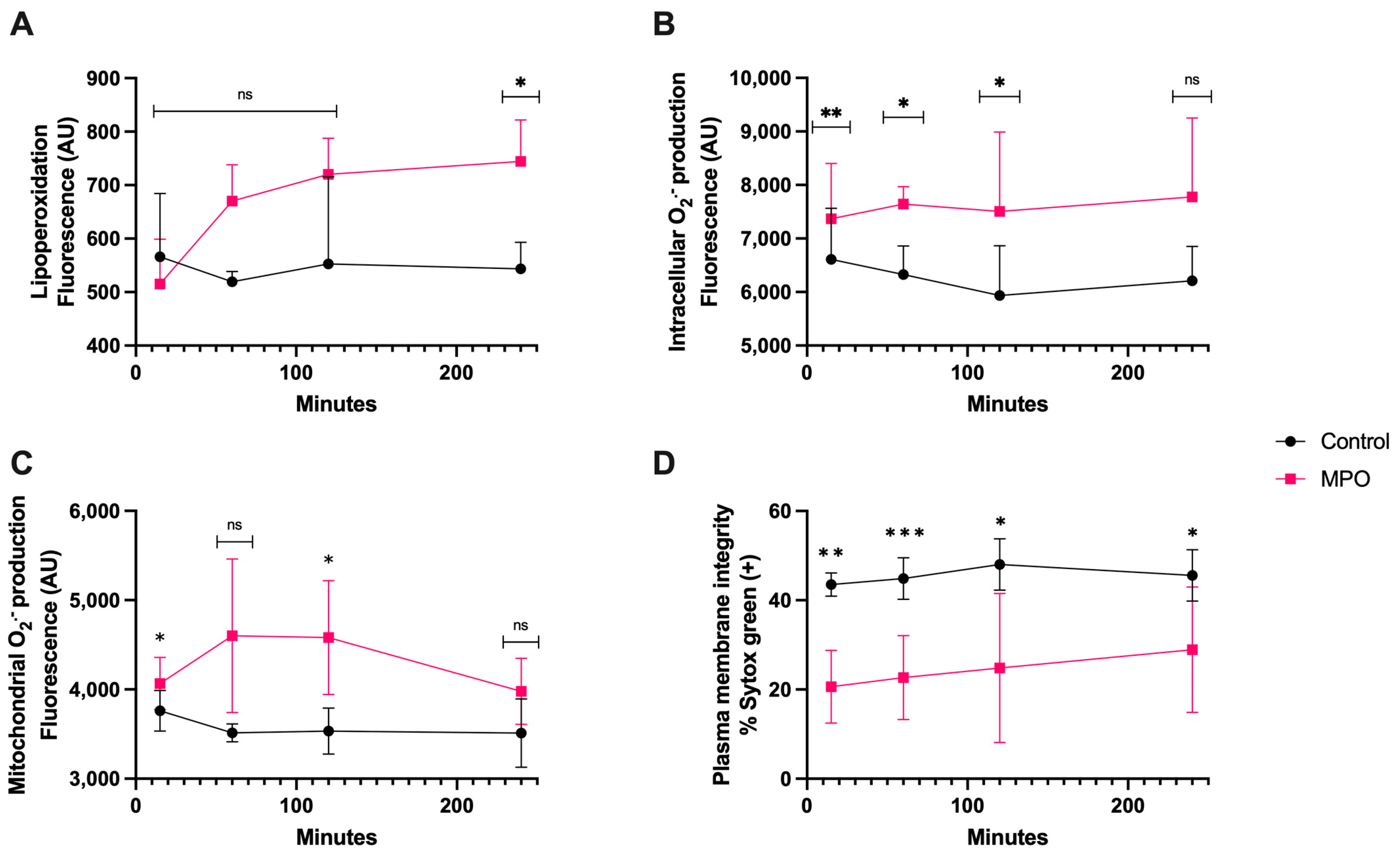
3.5. MPO Treatment
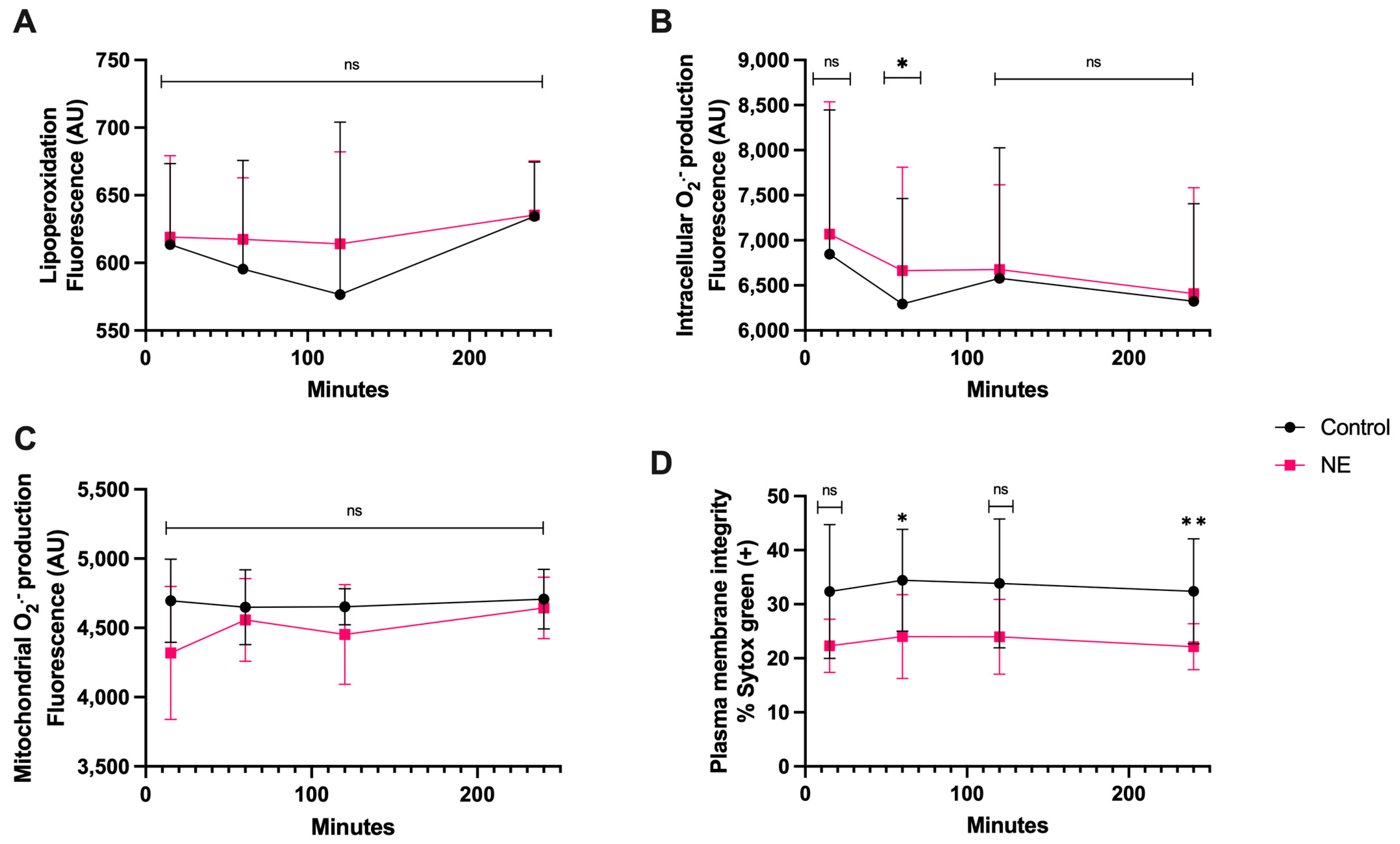
3.6. NE Treatment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Koppers, A.J.; De Iuliis, G.N.; Finnie, J.M.; McLaughlin, E.A.; Aitken, R.J. Significance of Mitochondrial Reactive Oxygen Species in the Generation of Oxidative Stress in Spermatozoa. J. Clin. Endocrinol. Metab. 2008, 93, 3199–3207. [Google Scholar] [CrossRef]
- Sies, H. Oxidative stress: A concept in redox biology and medicine. Redox Biol. 2015, 4, 180–183. [Google Scholar] [CrossRef] [PubMed]
- Sies, H. Strategies of antioxidant defense. Eur. J. Biochem. 1993, 215, 213–219. [Google Scholar] [CrossRef]
- Du Plessis, S.S.; Agarwal, A.; Halabi, J.; Tvrda, E. Contemporary evidence on the physiological role of reactive oxygen species in human sperm function. J. Assist. Reprod. Genet. 2015, 32, 509–520. [Google Scholar] [CrossRef] [PubMed]
- Athayde, K.S.; Cocuzza, M.; Agarwal, A.; Krajcir, N.; Lucon, A.M.; Srougi, M.; Hallak, J. Development of Normal Reference Values for Seminal Reactive Oxygen Species and Their Correlation With Leukocytes and Semen Parameters in a Fertile Population. J. Androl. 2007, 28, 613–620. [Google Scholar] [CrossRef]
- Kurkowska, W.; Bogacz, A.; Janiszewska, M.; Gabryś, E.; Tiszler, M.; Bellanti, F.; Kasperczyk, S.; Machoń-Grecka, A.; Dobrakowski, M.; Kasperczyk, A. Oxidative Stress is Associated with Reduced Sperm Motility in Normal Semen. Am. J. Men’s Health 2020, 14, 1557988320939731. [Google Scholar] [CrossRef]
- De Iuliis, G.N.; Thomson, L.K.; Mitchell, L.A.; Finnie, J.M.; Koppers, A.J.; Hedges, A.; Nixon, B.; Aitken, R.J. DNA Damage in Human Spermatozoa Is Highly Correlated with the Efficiency of Chromatin Remodeling and the Formation of 8-Hydroxy-2′-Deoxyguanosine, a Marker of Oxidative Stress. Biol. Reprod. 2009, 81, 517–524. [Google Scholar] [CrossRef] [PubMed]
- Aitken, R.J.; De Iuliis, G.N. On the possible origins of DNA damage in human spermatozoa. Mol. Hum. Reprod. 2009, 16, 3–13. [Google Scholar] [CrossRef]
- Bittner, L.; Wyck, S.; Herrera, C.; Siuda, M.; Wrenzycki, C.; van Loon, B.; Bollwein, H. Negative effects of oxidative stress in bovine spermatozoa on in vitro development and DNA integrity of embryos. Reprod. Fertil. Dev. 2018, 30, 1359–1368. [Google Scholar] [CrossRef]
- Aitken, R.J.; Clarkson, J.S. Cellular basis of defective sperm function and its association with the genesis of reactive oxygen species by human spermatozoa. Reproduction 1987, 81, 459–469. [Google Scholar] [CrossRef]
- Aitken, R.J.; Gibb, Z.; Baker, M.A.; Drevet, J.; Gharagozloo, P. Causes and consequences of oxidative stress in spermatozoa. Reprod. Fertil. Dev. 2015, 28, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Villegas, J.; Schulz, M.; Soto, L.; Iglesias, T.; Miska, W.; Sánchez, R. Influence of reactive oxygen species produced by activated leukocytes at the level of apoptosis in mature human spermatozoa. Fertil. Steril. 2005, 83, 808–810. [Google Scholar] [CrossRef] [PubMed]
- Mortaz, E.; Alipoor, S.D.; Adcock, I.M.; Mumby, S.; Koenderman, L. Update on Neutrophil Function in Severe Inflammation. Front. Immunol. 2018, 9, 2171. [Google Scholar] [CrossRef] [PubMed]
- Brinkmann, V.; Reichard, U.; Goosmann, C.; Fauler, B.; Uhlemann, Y.; Weiss, D.S.; Weinrauch, Y.; Zychlinsky, A. Neutrophil Extracellular Traps Kill Bacteria. Science 2004, 303, 1532–1535. [Google Scholar] [CrossRef] [PubMed]
- Urban, C.F.; Reichard, U.; Brinkmann, V.; Zychlinsky, A. Neutrophil extracellular traps capture and kill Candida albicans yeast and hyphal forms. Cell. Microbiol. 2006, 8, 668–676. [Google Scholar] [CrossRef] [PubMed]
- Muñoz Caro, T.; Hermosilla, C.; Silva, L.M.R.; Cortes, H.; Taubert, A. Neutrophil Extracellular Traps as Innate Immune Reaction against the Emerging Apicomplexan Parasite Besnoitia besnoiti. PLoS ONE 2014, 9, e91415. [Google Scholar] [CrossRef]
- Kaplan, M.J.; Radic, M. Neutrophil Extracellular Traps: Double-Edged Swords of Innate Immunity. J. Immunol. 2012, 189, 2689–2695. [Google Scholar] [CrossRef] [PubMed]
- Silk, E.; Zhao, H.; Weng, H.; Ma, D. The role of extracellular histone in organ injury. Cell. Death Dis. 2017, 8, e2812. [Google Scholar] [CrossRef] [PubMed]
- Papayannopoulos, V.; Zychlinsky, A. NETs: A new strategy for using old weapons. Trends Immunol. 2009, 30, 513–521. [Google Scholar] [CrossRef]
- Marin-Esteban, V.; Turbica, I.; Dufour, G.; Semiramoth, N.; Gleizes, A.; Gorges, R.; Beau, I.; Servin, A.L.; Lievin-Le Moal, V.; Sandré, C.; et al. Afa/Dr Diffusely Adhering Escherichia coli Strain C1845 Induces Neutrophil Extracellular Traps That Kill Bacteria and Damage Human Enterocyte-Like Cells. Infec. Immun. 2012, 80, 1891–1899. [Google Scholar] [CrossRef]
- Saffarzadeh, M.; Juenemann, C.; Queisser, M.A.; Lochnit, G.; Barreto, G.; Galuska, S.P.; Lohmeyer, J.; Preissner, K.T. Neutrophil Extracellular Traps Directly Induce Epithelial and Endothelial Cell Death: A Predominant Role of Histones. PLoS ONE 2012, 7, e32366. [Google Scholar] [CrossRef] [PubMed]
- Donis-Maturano, L.; Sánchez-Torres, L.E.; Cerbulo-Vázquez, A.; Chacón-Salinas, R.; García-Romo, G.S.; Orozco-Uribe, M.C.; Yam-Puc, J.C.; González-Jiménez, M.A.; Paredes-Vivas, Y.L.; Calderón-Amador, J.; et al. Prolonged exposure to neutrophil extracellular traps can induce mitochondrial damage in macrophages and dendritic cells. SpringerPlus 2015, 4, 161. [Google Scholar] [CrossRef] [PubMed]
- Butt, B.M.; Senger, P.L.; Widders, P.R. Neutrophil migration into the bovine uterine lumen following intrauterine inoculation with killed Haemophilus somnus. J. Reprod. Fertil. 1991, 93, 341–345. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Li, J.C.; Yamaguchi, S.; Funahashi, H. Boar seminal plasma or hen’s egg yolk decrease the in-vitro chemotactic and phagocytotic activities of neutrophils when co-incubated with boar or bull sperm. Theriogenology 2012, 77, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Zambrano, F.; Carrau, T.; Gärtner, U.; Seipp, A.; Taubert, A.; Felmer, R.; Sanchez, R.; Hermosilla, C. Leukocytes coincubated with human sperm trigger classic neutrophil extracellular traps formation, reducing sperm motility. Fertil. Steril. 2016, 106, 1053–1060.e1051. [Google Scholar] [CrossRef] [PubMed]
- Mateo-Otero, Y.; Zambrano, F.; Catalán, J.; Sánchez, R.; Yeste, M.; Miro, J.; Fernandez-Fuertes, B. Seminal plasma, and not sperm, induces time and concentration-dependent neutrophil extracellular trap release in donkeys. Equine Vet. J. 2022, 54, 415–426. [Google Scholar] [CrossRef] [PubMed]
- Zambrano, F.; Namuncura, C.; Uribe, P.; Schulz, M.; Pezo, F.; Burgos, R.A.; Taubert, A.; Hermosilla, C.; Sanchez, R. Swine spermatozoa trigger aggregated neutrophil extracellular traps leading to adverse effects on sperm function. J. Reprod. Immunol. 2021, 146, 103339. [Google Scholar] [CrossRef]
- Alghamdi, A.S.; Lovaas, B.J.; Bird, S.L.; Lamb, G.C.; Rendahl, A.K.; Taube, P.C.; Foster, D.N. Species-specific interaction of seminal plasma on sperm–neutrophil binding. Anim. Reprod. Sci. 2009, 114, 331–344. [Google Scholar] [CrossRef] [PubMed]
- Moya, C.; Rivera-Concha, R.; Pezo, F.; Uribe, P.; Schulz, M.; Sánchez, R.; Hermosilla, C.; Taubert, A.; Gärtner, U.; Zambrano, F. Adverse Effects of Single Neutrophil Extracellular Trap-Derived Components on Bovine Sperm Function. Animals 2022, 12, 1308. [Google Scholar] [CrossRef]
- Yánez-Ortiz, I.; Catalán, J.; Mateo-Otero, Y.; Dordas-Perpinyà, M.; Gacem, S.; Yeste, N.; Bassols, A.; Yeste, M.; Miró, J. Extracellular Reactive Oxygen Species (ROS) Production in Fresh Donkey Sperm Exposed to Reductive Stress, Oxidative Stress and NETosis. Antioxidants 2021, 10, 1367. [Google Scholar] [CrossRef]
- Bavister, B.D.; Yanagimachi, R. The Effects of Sperm Extracts and Energy Sources on the Motility and Acrosome Reaction of Hamster Spermatozoa in vitro. Biol. Reprod. 1977, 16, 228–237. [Google Scholar] [CrossRef] [PubMed]
- Parrish, J.J.; Susko-Parrish, J.L.; First, N.L. Effect of heparin and chondroitin sulfate on the acrosome reaction and fertility of bovine sperm in vitro. Theriogenology 1985, 24, 537–549. [Google Scholar] [CrossRef] [PubMed]
- Roth, J.A.; Kaeberle, M.L. Isolation of neutrophils and eosinophils from the peripheral blood of cattle and comparison of their functional activities. J. Immunol. Methods 1981, 45, 153–164. [Google Scholar] [CrossRef] [PubMed]
- Conejeros, I.; Gibson, A.J.; Werling, D.; Muñoz-Caro, T.; Hermosilla, C.; Taubert, A.; Burgos, R.A. Effect of the synthetic Toll-like receptor ligands LPS, Pam3CSK4, HKLM and FSL-1 in the function of bovine polymorphonuclear neutrophils. Dev. Comp. Immunol. 2015, 52, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Concha, R.; Moya, C.; León, M.; Uribe, P.; Schulz, M.; Prado, A.; Taubert, A.; Hermosilla, C.; Sánchez, R.; Zambrano, F. Effect of different sperm populations on neutrophils extracellular traps (NETs) formation in cattle. Res. Vet. Sci. 2023, 164, 105028. [Google Scholar] [CrossRef] [PubMed]
- Ayad, B.; Omolaoye, T.S.; Louw, N.; Ramsunder, Y.; Skosana, B.T.; Oyeipo, P.I.; Du Plessis, S.S. Oxidative Stress and Male Infertility: Evidence From a Research Perspective. Front. Reprod. Health 2022, 4, 822257. [Google Scholar] [CrossRef] [PubMed]
- Sordillo, L.M.; Aitken, S.L. Impact of oxidative stress on the health and immune function of dairy cattle. Vet. Immunol. Immunopathol. 2009, 128, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Pintus, E.; Ros-Santaella, J.L. Impact of Oxidative Stress on Male Reproduction in Domestic and Wild Animals. Antioxidants 2021, 10, 1154. [Google Scholar] [CrossRef] [PubMed]
- Fichtner, T.; Kotarski, F.; Gärtner, U.; Conejeros, I.; Hermosilla, C.; Wrenzycki, C.; Taubert, A. Bovine sperm samples induce different NET phenotypes in a NADPH oxidase-, PAD4-, and Ca-dependent process. Biol. Reprod. 2020, 102, 902–914. [Google Scholar] [CrossRef]
- Hakkim, A.; Fuchs, T.A.; Martinez, N.E.; Hess, S.; Prinz, H.; Zychlinsky, A.; Waldmann, H. Activation of the Raf-MEK-ERK pathway is required for neutrophil extracellular trap formation. Nat. Chem. Biol. 2011, 7, 75–77. [Google Scholar] [CrossRef]
- Katherine, J.B.; Jodie, L.S.; Lisa, G.W.; Rodney, J.S.; Peter, G.G. Systemic upregulation of neutrophil α-defensins and serine proteases in neutrophilic asthma. Thorax 2011, 66, 942. [Google Scholar] [CrossRef]
- Brehm, A.; Geraghty, P.; Campos, M.; Garcia-Arcos, I.; Dabo, A.J.; Gaffney, A.; Eden, E.; Jiang, X.-C.; D’Armiento, J.; Foronjy, R. Cathepsin G degradation of phospholipid transfer protein (PLTP) augments pulmonary inflammation. FASEB J. 2014, 28, 2318–2331. [Google Scholar] [CrossRef]
- Amaral, A.; Fernandes, C.; Morazzo, S.; Rebordão, M.R.; Szóstek-Mioduchowska, A.; Lukasik, K.; Gawronska-Kozak, B.; Telo da Gama, L.; Skarzynski, D.J.; Ferreira-Dias, G. The Inhibition of Cathepsin G on Endometrial Explants With Endometrosis in the Mare. Front. Vet. Sci. 2020, 7, 582211. [Google Scholar] [CrossRef]
- Rebordão, M.R.; Amaral, A.; Fernandes, C.; Silva, E.; Lukasik, K.; Szóstek-Mioduchowska, A.; Pinto-Bravo, P.; Galvão, A.; Skarzynski, D.J.; Ferreira-Dias, G. Enzymes Present in Neutrophil Extracellular Traps May Stimulate the Fibrogenic PGF2α Pathway in the Mare Endometrium. Animals 2021, 11, 2615. [Google Scholar] [CrossRef]
- Lai, H.-J.; Doan, H.T.; Lin, E.Y.; Chiu, Y.-L.; Cheng, Y.-K.; Lin, Y.-H.; Chiang, H.-S. Histones of Neutrophil Extracellular Traps Directly Disrupt the Permeability and Integrity of the Intestinal Epithelial Barrier. Inflamm. Bowel Dis. 2023, 29, 783–797. [Google Scholar] [CrossRef]
- Jung, J.; Lee, L.E.; Kim, H.; Kim, J.E.; Jang, S.H.; Roh, J.S.; Lee, B.; Robinson, W.H.; Sohn, D.H.; Pyun, J.-C.; et al. Extracellular histones aggravate autoimmune arthritis by lytic cell death. Front. Immunol. 2022, 13, 961197. [Google Scholar] [CrossRef]
- Ligi, D.; Giglio, R.V.; Henry, B.M.; Lippi, G.; Ciaccio, M.; Plebani, M.; Mannello, F. What is the impact of circulating histones in COVID-19: A systematic review. Clin. Chem. Lab. Med. 2022, 60, 1506–1517. [Google Scholar] [CrossRef]
- Singh, A.; Verma, S.; Modak, S.B.; Chaturvedi, M.M.; Purohit, J.S. Extra-nuclear histones: Origin, significance and perspectives. Mol. Cell. Biochem. 2022, 477, 507–524. [Google Scholar] [CrossRef]
- Ulfig, A.; Leichert, L.I. The effects of neutrophil-generated hypochlorous acid and other hypohalous acids on host and pathogens. Cell. Mol. Life Sci. 2021, 78, 385–414. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Chen, H.; Chen, X.; Guo, C. The Roles of Neutrophil-Derived Myeloperoxidase (MPO) in Diseases: The New Progress. Antioxidants 2024, 13, 132. [Google Scholar] [CrossRef] [PubMed]
- Weiss, S.J. Tissue Destruction by Neutrophils. N. Engl. J. Med. 1989, 320, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Ehrens, A.; Rüdiger, N.; Heepmann, L.; Linnemann, L.; Hartmann, W.; Hübner, M.P.; Breloer, M. Eosinophils and Neutrophils Eliminate Migrating Strongyloides ratti Larvae at the Site of Infection in the Context of Extracellular DNA Trap Formation. Front. Immunol. 2021, 12, 715766. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Wang, Y.; Zhang, X.; Wang, X.; Gong, P.; Li, J.; Taubert, A.; Hermosilla, C.; Zhang, X.; Yang, Z. Bovine macrophage-derived extracellular traps act as early effectors against the abortive parasite Neospora caninum. Vet. Parasitol. 2018, 258, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Zapponi, K.C.S.; Orsi, F.A.; Cunha, J.L.R.; de Brito, I.R.; Romano, A.V.C.; Bittar, L.F.; De Paula, E.V.; Penteado, C.F.; Montalvão, S.; Annichino-Bizzacchi, J.M. Neutrophil activation and circulating neutrophil extracellular traps are increased in venous thromboembolism patients for at least one year after the clinical event. J. Thromb. Thrombolysis 2022, 53, 30–42. [Google Scholar] [CrossRef] [PubMed]
- Tanphaichitr, N.; Srakaew, N.; Alonzi, R.; Kiattiburut, W.; Kongmanas, K.; Zhi, R.; Li, W.; Baker, M.; Wang, G.; Hickling, D. Potential Use of Antimicrobial Peptides as Vaginal Spermicides/Microbicides. Pharmaceuticals 2016, 9, 13. [Google Scholar] [CrossRef] [PubMed]
- Srakaew, N.; Young, C.D.; Sae-wu, A.; Xu, H.; Quesnel, K.L.; di Brisco, R.; Kongmanas, K.; Fongmoon, D.; Hommalai, G.; Weerachatyanukul, W.; et al. Antimicrobial host defence peptide, LL-37, as a potential vaginal contraceptive. Hum. Reprod. 2014, 29, 683–696. [Google Scholar] [CrossRef] [PubMed]
- Villanueva, E.; Yalavarthi, S.; Berthier, C.C.; Hodgin, J.B.; Khandpur, R.; Lin, A.M.; Rubin, C.J.; Zhao, W.; Olsen, S.H.; Klinker, M.; et al. Netting Neutrophils Induce Endothelial Damage, Infiltrate Tissues, and Expose Immunostimulatory Molecules in Systemic Lupus Erythematosus. J. Immunol. 2011, 187, 538–552. [Google Scholar] [CrossRef] [PubMed]
- Korkmaz, B.; Horwitz, M.S.; Jenne, D.E.; Gauthier, F. Neutrophil Elastase, Proteinase 3, and Cathepsin G as Therapeutic Targets in Human Diseases. Pharmacol. Rev. 2010, 62, 726–759. [Google Scholar] [CrossRef] [PubMed]
- Zorn, B.; Ihan, A.; Kopitar, A.N.; Kolbezen, M.; Sesek-Briski, A.; Meden-Vrtovec, H. Changes in sperm apoptotic markers as related to seminal leukocytes and elastase. Reprod. Biomed. Online 2010, 21, 84–92. [Google Scholar] [CrossRef]
- Wang, Q.; Que, C.; Chen, G. Effects of leukocyte elastase in semen on sperm quality. Medicine 2022, 101, e31111. [Google Scholar] [CrossRef]
- Tirpák, F.; Halo, M.; Tomka, M.; Slanina, T.; Tokárová, K.; Błaszczyk-Altman, M.; Dianová, L.; Ivanič, P.; Kirchner, R.; Greń, A.; et al. Sperm Quality Affected by Naturally Occurring Chemical Elements in Bull Seminal Plasma. Antioxidants 2022, 11, 1796. [Google Scholar] [CrossRef]
- Fichtner, T.; Kotarski, F.; Hermosilla, C.; Taubert, A.; Wrenzycki, C. Semen extender and seminal plasma alter the extent of neutrophil extracellular traps (NET) formation in cattle. Theriogenology 2021, 160, 72–80. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rivera-Concha, R.; León, M.; Prado-Sanhueza, A.; Sánchez, R.; Taubert, A.; Hermosilla, C.; Uribe, P.; Zambrano, F. Cytotoxic Oxidative Stress Effects of Neutrophil Extracellular Traps’ Components on Cattle Spermatozoa. Antioxidants 2024, 13, 733. https://doi.org/10.3390/antiox13060733
Rivera-Concha R, León M, Prado-Sanhueza A, Sánchez R, Taubert A, Hermosilla C, Uribe P, Zambrano F. Cytotoxic Oxidative Stress Effects of Neutrophil Extracellular Traps’ Components on Cattle Spermatozoa. Antioxidants. 2024; 13(6):733. https://doi.org/10.3390/antiox13060733
Chicago/Turabian StyleRivera-Concha, Rodrigo, Marion León, Aurora Prado-Sanhueza, Raúl Sánchez, Anja Taubert, Carlos Hermosilla, Pamela Uribe, and Fabiola Zambrano. 2024. "Cytotoxic Oxidative Stress Effects of Neutrophil Extracellular Traps’ Components on Cattle Spermatozoa" Antioxidants 13, no. 6: 733. https://doi.org/10.3390/antiox13060733
APA StyleRivera-Concha, R., León, M., Prado-Sanhueza, A., Sánchez, R., Taubert, A., Hermosilla, C., Uribe, P., & Zambrano, F. (2024). Cytotoxic Oxidative Stress Effects of Neutrophil Extracellular Traps’ Components on Cattle Spermatozoa. Antioxidants, 13(6), 733. https://doi.org/10.3390/antiox13060733








