Lianweng Granules Alleviate Intestinal Barrier Damage via the IL-6/STAT3/PI3K/AKT Signaling Pathway with Dampness-Heat Syndrome Diarrhea
Abstract
:1. Introduction
2. Materials and Methods
2.1. Medicine
2.2. Collection of LWG-Containing Serum and UHPLC-MS/MS Analysis
2.3. Evaluation of Differential Components
2.4. Network Pharmacology Analysis
2.4.1. Collection of Relevant Targets for LWG and DHSD
2.4.2. Construction of Protein–Protein Interaction Network (PPI)
2.4.3. Construction of “DHSD-LWG-Compounds-Core Targets”
2.4.4. Enrichment Analysis
2.5. Molecular Docking
2.6. Animal Model
2.7. Cell Culture
2.8. Cell Viability Assay and Cell Transfection
2.9. Determination of ROS, CAT, and SOD Levels
2.10. Evaluation of DAI and Histopathological Observations
2.11. Biochemical Analysis
2.12. Western Blot
2.13. RT-qPCR
2.14. Transmission Electron Microscopy (TEM) Study and TdT-Mediated dUTP-Biotin Nick End Labeling (TUNEL) Staining
2.15. Immunofluorescence
2.16. CETSA
2.17. DARTS
2.18. Statistical Analysis
3. Result
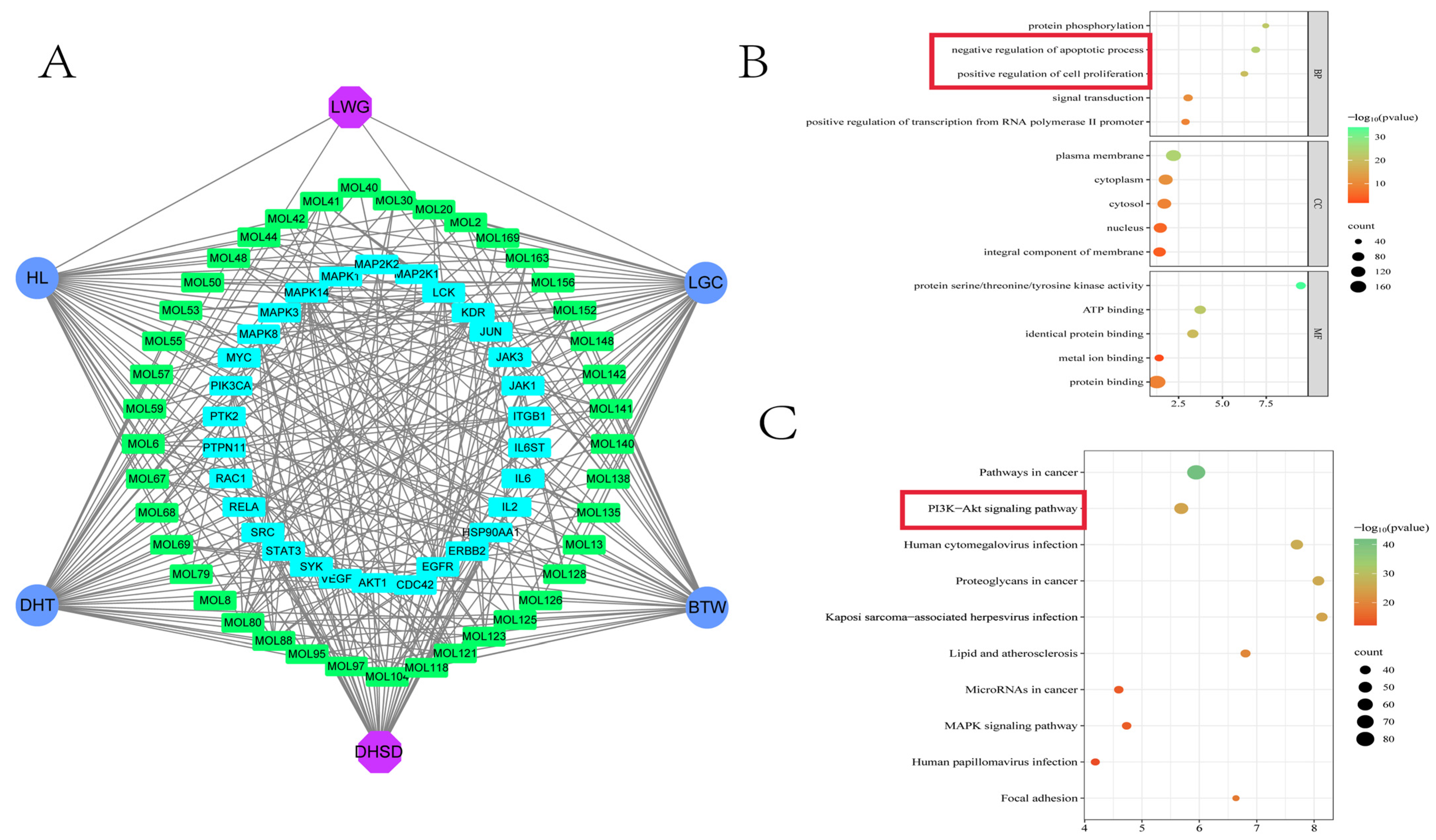
3.1. Identification of Blood Components and Network Pharmacology Predicts Key Targets
3.2. The Enrichment Results Showed That the PI3K/AKT Pathway Was Involved in the Pharmacological Mechanism of LWG
3.3. Molecular Docking
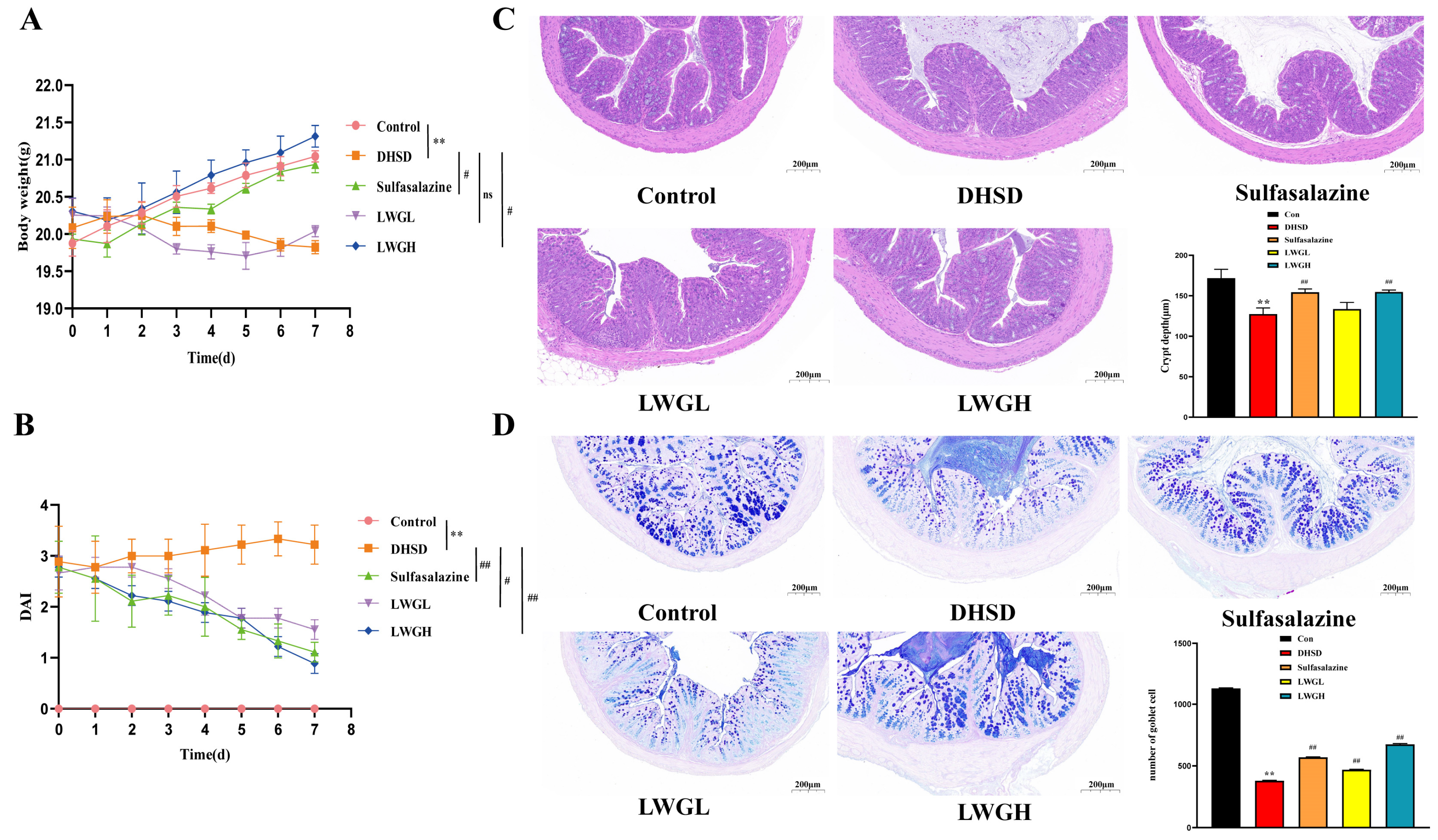
3.4. LWG Restores Intestinal Morphology
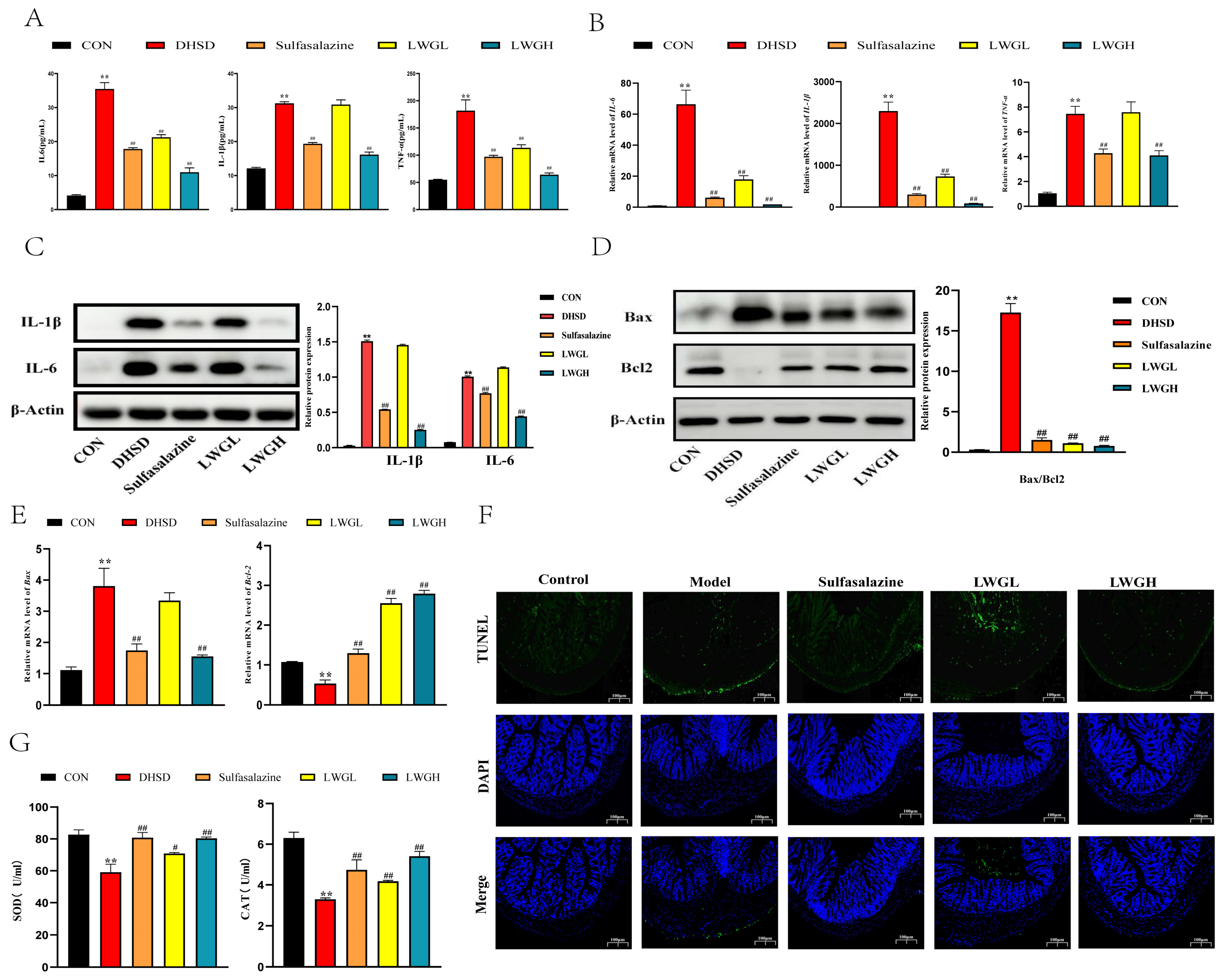
3.5. LWG Alleviates Inflammation, Oxidative Stress, and Apoptosis
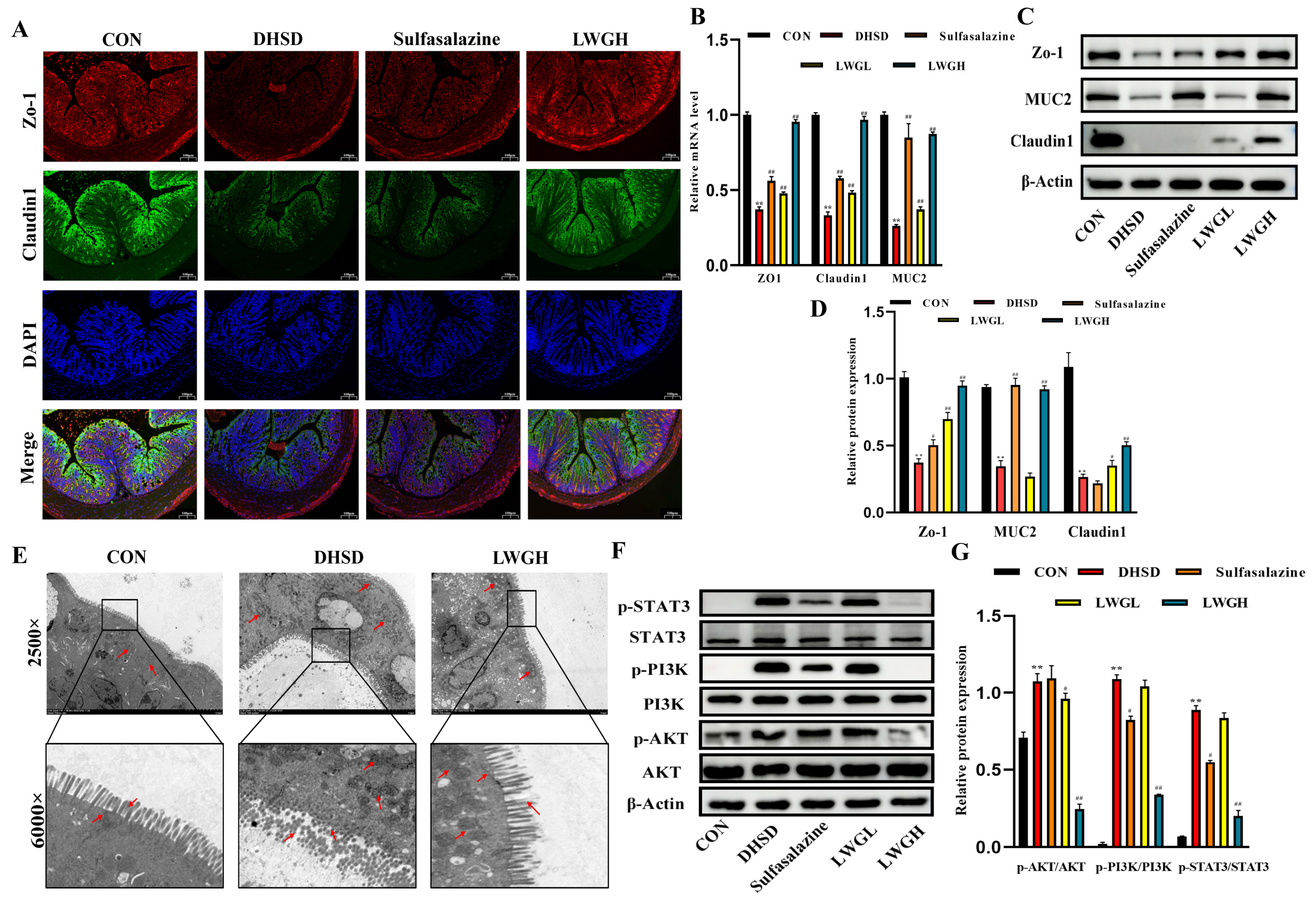
3.6. LWG Repairs Barrier Function and Inhibits STAT3/PI3K/AKT Activation in DHSD Mice
3.7. LWG Protects the LPS-Induced Cellular Injury Model and Targets STAT3
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AB-PAS | Alcian blue-periodic acid-Schiff |
| BBR | Berberine |
| BCA | Bicinchoninic Acid Assay |
| BP | Biological process |
| BTW | Pulsatilliae radix |
| CAT | Catalase |
| CC | Cellular component |
| CCK-8 | Cell Counting Kit-8 |
| CETSA | Cellular thermal shift assay |
| DAI | Disease activity index |
| DAPI | 4′,6-diamidino-2-phenylindole |
| DARTS | Drug affinity responsive target stability |
| DCFH-DA | 2′,7′-Dichlorodihydrofluorescein diacetate |
| DHSD | Dampness-heat syndrome diarrhea |
| DHT | Rhubarb charcoal |
| FITC | Fluorescein 5-isothiocyanate |
| ECL | Enhanced chemiluminescence |
| GO | Gene Ontology |
| Hacc | Hydrogen bond acceptors |
| Hdon | Hydrogen bond donors |
| H&E | Hematoxylin eosin |
| HL | Coptidis rhizoma |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| LGC | Geranium wilfordii maxim |
| LWG | Lian weng granules |
| MCC | Maximum cluster centrality |
| MF | Molecular function |
| MLOGP | The lipid–water partition coefficient |
| M/W | The relative molecular mass |
| OMIM | Online Mendelian Inheritance in Man |
| PBS | Phosphate buffer saline |
| PBST | Phosphate buffered saline with tween 20 |
| PI3K | Phosphoinositide 3-Kinase |
| PPI | Protein-protein interaction network |
| Rbon | The number of rotatable bonds |
| SDS-PAGE | Sodium dodecyl sulfate–polyacrylamide gel electrophoresis |
| SiSTAT3 | STAT3-specific small interfering RNA |
| STAT3 | Signal transducer and activator of transcription-3 |
| SOD | Superoxide dismutase |
| TCM | Traditional Chinese medicine |
| TEM | Transmission electron microscopy |
| TTD | Therapeutic target database |
| TUNEL | TdT-mediated dUTP-biotin nick end labeling |
| TPSA | Topological polar surface area |
| UHPLC-MS/MS | Ultra-high performance liquid chromatography-tandem mass spectrometry |
| ZO-1 | Tight junction protein ZO-1. |
References
- Tang, Y.; Forsyth, C.B.; Keshavarzian, A. New molecular insights into inflammatory bowel disease-induced diarrhea. Expert Rev. Gastroenterol. Hepatol. 2011, 5, 615–625. [Google Scholar] [CrossRef] [PubMed]
- Lazzerini, M.; Ronfani, L. Oral zinc for treating diarrhoea in children. Cochrane Database Syst. Rev. 2012, 12, CD005436. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Cui, Y.; Xu, B.; Wang, Y.; Lv, F.; Li, Z.; Li, H.; Chen, X.; Peng, X.; Chen, Y.; et al. Main active components of Jiawei Gegen Qinlian decoction protects against ulcerative colitis under different dietary environments in a gut microbiota-dependent manner. Pharmacol. Res. 2021, 170, 105694. [Google Scholar] [CrossRef] [PubMed]
- Hua, Y.-L.; Ma, Q.; Zhang, X.-S.; Jia, Y.-Q.; Peng, X.-T.; Yao, W.-L.; Ji, P.; Hu, J.-J.; Wei, Y.-M. Pulsatilla Decoction Can Treat the Dampness-Heat Diarrhea Rat Model by Regulating Glycerinphospholipid Metabolism Based Lipidomics Approach. Front. Pharmacol. 2020, 11, 197. [Google Scholar] [CrossRef] [PubMed]
- Xie, Q.; Li, H.; Ma, R.; Ren, M.; Li, Y.; Li, J.; Chen, H.; Chen, Z.; Gong, D.; Wang, J. Effect of Coptis chinensis franch and Magnolia officinalis on intestinal flora and intestinal barrier in a TNBS-induced ulcerative colitis rats model. Phytomedicine 2022, 97, 153927. [Google Scholar] [CrossRef] [PubMed]
- Teschke, R.; Wolff, A.; Frenzel, C.; Eickhoff, A.; Schulze, J. Herbal traditional Chinese medicine and its evidence base in gastrointestinal disorders. World J. Gastroenterol. 2015, 21, 4466–4490. [Google Scholar] [CrossRef] [PubMed]
- Lin, A.X.; Chan, G.; Hu, Y.; Ouyang, D.; Ung, C.O.L.; Shi, L.; Hu, H. Internationalization of traditional Chinese medicine: Current international market, internationalization challenges and prospective suggestions. Chin. Med. 2018, 13, 9. [Google Scholar] [CrossRef] [PubMed]
- Yan, G.-L.; Sun, H.; Zhang, A.-H.; Han, Y.; Wang, P.; Wu, X.-H.; Meng, X.-C.; Wang, X.-J. Progress of serum pharmacochemistry of traditional Chinese medicine and further development of its theory and method. Zhongguo Zhong Yao Za Zhi = Zhongguo Zhongyao Zazhi = China J. Chin. Mater. Medica 2015, 40, 3406–3412. [Google Scholar]
- Xiong, H.; Li, N.; Zhao, L.; Li, Z.; Yu, Y.; Cui, X.; Liu, Q.; Zhao, C. Integrated Serum Pharmacochemistry, Metabolomics, and Network Pharmacology to Reveal the Material Basis and Mechanism of Danggui Shaoyao San in the Treatment of Primary Dysmenorrhea. Front. Pharmacol. 2022, 13, 942955. [Google Scholar] [CrossRef]
- Martini, E.; Krug, S.M.; Siegmund, B.; Neurath, M.F.; Becker, C. Mend Your Fences The Epithelial Barrier and its Relationship With Mucosal Immunity in Inflammatory Bowel Disease. Cell. Mol. Gastroenterol. Hepatol. 2017, 4, 33–46. [Google Scholar] [CrossRef]
- Yao, W.; Yang, C.; Wen, Y.; Zhang, W.; Zhang, X.; Ma, Q.; Ji, P.; Hua, Y.; Wei, Y. Treatment effects and mechanisms of Yujin Powder on rat model of large intestine dampness-heat syndrome. J. Ethnopharmacol. 2017, 202, 265–280. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.E.; O’Keefe, R.A.; Grandis, J.R. Targeting the IL-6/JAK/STAT3 signalling axis in cancer. Nat. Rev. Clin. Oncol. 2018, 15, 234–248. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Liang, Q.; Yang, Q.; Dai, W.; Xiao, Y.; Pan, H.; Zhang, Z.; Liu, L.; Li, X. Hexahydrocurcumin from Zingiberis rhizoma attenuates lipopolysaccharide-induced acute pneumonia through JAK1/STAT3 signaling pathway. Phytomedicine 2024, 122, 155141. [Google Scholar] [CrossRef] [PubMed]
- Xia, S.; Chen, L.; Li, Z.; Li, Y.; Zhou, Y.; Sun, S.; Su, Y.; Xu, X.; Shao, J.; Zhang, Z.; et al. Qingchang Wenzhong Decoction reduce ulcerative colitis in mice by inhibiting Th17 lymphocyte differentiation. Phytomedicine 2022, 107, 154460. [Google Scholar] [CrossRef] [PubMed]
- Ahuja, A.; Kim, E.; Sung, G.-H.; Cho, J.Y. STAT3 Differentially Regulates TLR4-Mediated Inflammatory Responses in Early or Late Phases. Int. J. Mol. Sci. 2020, 21, 7675. [Google Scholar] [CrossRef] [PubMed]
- Bito, T.; Sumita, N.; Ashida, M.; Budiyanto, A.; Ueda, M.; Ichihashi, M.; Tokura, Y.; Nishigori, C. Inhibition of Epidermal Growth Factor Receptor and PI3K/Akt Signaling Suppresses Cell Proliferation and Survival through Regulation of Stat3 Activation in Human Cutaneous Squamous Cell Carcinoma. J. Ski. Cancer 2011, 2011, 874571. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Cui, Y.; Ouyang, H.; Zhu, W.; Feng, Y.; Yao, M.; Yang, S. Radix Pueraria lobata polysaccharide relieved DSS-induced ulcerative colitis through modulating PI3K signaling. J. Funct. Foods 2023, 104, 105514. [Google Scholar] [CrossRef]
- Coskun, M.; Salem, M.; Pedersen, J.; Nielsen, O.H. Involvement of JAK/STAT signaling in the pathogenesis of inflammatory bowel disease. Pharmacol. Res. 2013, 76, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Hua, Y.; Chen, W.; Zheng, H.; Wu, H.; Qin, S.; Huang, S. Therapeutic targets and pharmacological mechanisms of Coptidis Rhizoma against ulcerative colitis: Findings of system pharmacology and bioinformatics analysis. Front. Pharmacol. 2022, 13, 1037856. [Google Scholar] [CrossRef]
- Liu, L.; Zhao, C.; Fu, X.; Tan, X.; Wang, Y.; Peng, X.; Wu, T.; Wang, X.; Wang, L. Investigation of Synergetic Antioxidant Effects Among Four Herbal Extracts. J. Nanosci. Nanotechnol. 2016, 16, 7220–7224. [Google Scholar] [CrossRef]
- Tan, Q.; He, Q.; Peng, Z.; Zeng, X.; Liu, Y.; Li, D.; Wang, S.; Wang, J. Topical rhubarb charcoal-crosslinked chitosan/silk fibroin sponge scaffold for the repair of diabetic ulcers improves hepatic lipid deposition in db/db mice via the AMPK signalling pathway. Lipids Health Dis. 2024, 23, 52. [Google Scholar] [CrossRef] [PubMed]
- Kong, C.; Pang, X.; Su, Z.; Liu, Y. Botany, ethnopharmacology, phytochemistry and pharmacology of Erodii Herba Geranii Herba-An review. J. Ethnopharmacol. 2023, 302, 115858. [Google Scholar] [CrossRef] [PubMed]
- Ning, L.; Ye, N.; Ye, B.; Miao, Z.; Cao, T.; Lu, W.; Xu, D.; Tan, C.; Xu, Y.; Yan, J. Qingre Xingyu recipe exerts inhibiting effects on ulcerative colitis development by inhibiting TNFα/NLRP3/Caspase-1/IL-1β pathway and macrophage M1 polarization. Cell Death Discov. 2023, 9, 84. [Google Scholar] [CrossRef] [PubMed]
- Li, M.-Z.; Dai, X.-Y.; Zhao, Y.-X.; Li, X.-W.; Zhao, Y.; Li, J.-L. Lycopene Attenuates Di(2-ethylhexyl) Phthalate-Induced Mitochondrial Damage and Inflammation in Kidney via cGAS-STING Signaling. J. Agric. Food Chem. 2023, 71, 569–579. [Google Scholar] [CrossRef] [PubMed]
- Raimondo, S.; Urzi, O.; Meraviglia, S.; Di Simone, M.; Corsale, A.M.; Ganji, N.R.; Piccionello, A.P.; Polito, G.; Presti, E.L.; Dieli, F.; et al. Anti-inflammatory properties of lemon-derived extracellular vesicles are achieved through the inhibition of ERK/NF-κB signalling pathways. J. Cell. Mol. Med. 2022, 26, 4195–4209. [Google Scholar] [CrossRef] [PubMed]
- Almezgagi, M.; Tahir, M.S.; Zhang, Y.; Gamah, M.; Gao, X.; Zhu, Q.; Zhang, H.; Zhang, W. Evaluation of diacerein efficacy on colitis as anti-inflammatory and anti-oxidant and its role in enhancing of colon barrier in mice. Acta Pol. Pharm. 2023, 80, 649–659. [Google Scholar] [CrossRef]
- Reza, A.S.M.A.; Rashid, M.M.; Islam, M.S.; Hossen, A.; Abu Ahmed, A.; Haque, A.; Nasrin, M.S.; Uddin, N.; Khan, J.; Rahman, A. Polyphenolics of purple devil fruits potentiate anti-inflammatory action by regulating the expression of inflammatory cytokines and apoptotic signaling molecules evident in extensive and combined experimental models. J. Funct. Foods 2023, 106, 105610. [Google Scholar] [CrossRef]
- Salem, M.B.; El-Lakkany, N.M.; el-Din, S.H.S.; Hammam, O.A.; Samir, S. Diosmin alleviates ulcerative colitis in mice by increasing Akkermansia muciniphila abundance, improving intestinal barrier function, and modulating the NF-κB and Nrf2 pathways. Heliyon 2024, 10, e27527. [Google Scholar] [CrossRef] [PubMed]
- Petito-da-Silva, T.I.; Villardi Junior, F.M.; Penna-de-Carvalho, A.; Mandarim-De-Lacerda, C.A.; Souza-Mello, V.; Barbosa-Da-Silva, S. An Intestinal FXR Agonist Mitigates Dysbiosis, Intestinal Tight Junctions, and Inflammation in High-Fat Diet-Fed Mice. Mol. Nutr. Food Res. 2024, 68, 2300148. [Google Scholar] [CrossRef]
- Zhi, D.; Zhang, M.; Lin, J.; Liu, P.; Duan, M. GPR120 Ameliorates Apoptosis and Inhibits the Production of Inflammatory Cytokines in Renal Tubular Epithelial Cells. Inflammation 2021, 44, 493–505. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Qiao, B.; Li, X.; Peng, M.; Hui, H.; Tan, Z. Alteration of intestinal mucosal microbiota in mice with Chinese dampness-heat syndrom diarrhea by improper diet combined with high temperature and humidity environments. Front. Cell. Infect. Microbiol. 2023, 12, 1096202. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.-C.; Zheng, L.; Zhang, Y.-L.; Chen, X.; Chen, D.-L.; Tang, Z.-P. Effects of Jianpi Qingchang decoction on the quality of life of patients with ulcerative colitis A randomized controlled trial. Medicine 2017, 96, e6651. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, B.-G.; Su, Y.-H.; Zhao, R.-X.; Song, P.; Li, H.; Cui, X.-H.; Gao, H.-M.; Zhai, R.-X.; Fu, X.-J.; et al. Potential activity of Traditional Chinese Medicine against Ulcerative colitis: A review. J. Ethnopharmacol. 2022, 289, 115084. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, Z.-Y.; Zheng, J.-H.; Li, S. TCM network pharmacology: A new trend towards combining computational, experimental and clinical approaches. Chin. J. Nat. Med. 2021, 19, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, X.; Shi, Y.; Chen, T.; Xu, Z.; Wang, P.; Yu, M.; Chen, W.; Li, B.; Jing, Z.; et al. ETCM v2.0: An update with comprehensive resource and rich annotations for traditional Chinese medicine. Acta Pharm. Sin. B 2023, 13, 2559–2571. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Su, H.; Chen, H.; Tang, X.; Li, W.; Huang, A.; Fang, G.; Chen, Q.; Luo, Y.; Pang, Y. Integrated serum pharmacochemistry and network pharmacology to explore the mechanism of Yi-Shan-Hong formula in alleviating chronic liver injury. Phytomedicine Int. J. Phytother. Phytopharm. 2024, 128, 155439. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.; Liu, L.; Zhou, W.; Yang, C.; Mai, G.; Li, H.; Chen, Y. Gut microbiota-derived butyrate regulates gut mucus barrier repair by activating the macrophage/WNT/ERK signaling pathway. Clin. Sci. 2022, 136, 291–307. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Zhang, T.; Wang, Y.Y.; Yuan, W.; Li, L.; Li, J.; Yang, Y.Y.; Wu, D.M.; Xu, Y. Isofraxidin attenuates dextran sulfate sodium-induced ulcerative colitis through inhibiting pyroptosis by upregulating Nrf2 and reducing reactive oxidative species. Int. Immunopharmacol. 2024, 128, 111570. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, Y.; Lu, J.; Wu, J. Effects of Traditional Chinese Medicine on Serum Cytokines for the Dampness-heat Syndrome of Ulcerative Colitis: A Systematic Review and Meta-analysis. Altern. Ther. Health Med. 2023, 29, 386–395. [Google Scholar]
- Liu, C.; Zeng, Y.; Wen, Y.; Huang, X.; Liu, Y. Natural Products Modulate Cell Apoptosis: A Promising Way for the Treatment of Ulcerative Colitis. Front. Pharmacol. 2022, 13, 806148. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-S.; Xia, T.; Luo, Z.-Y.; Wu, Y.-Y.; Hu, Y.-N.; Chen, F.-L.; Tang, Q.-F.; Tan, X.-M. Network pharmacology and pharmacokinetics integrated strategy to investigate the pharmacological mechanism of Xianglian pill on ulcerative colitis. Phytomedicine 2021, 82, 153458. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, Q.; Xiong, B.; Chen, H.; Wang, X.; Zhang, D. Discoidin domain receptor 1(DDR1) promote intestinal barrier disruption in Ulcerative Colitis through tight junction proteins degradation and epithelium apoptosis. Pharmacol. Res. 2022, 183, 106368. [Google Scholar] [CrossRef] [PubMed]
- Yin, S.J.; Yang, H.F.; Tao, Y.; Wei, S.M.; Li, L.H.; Liu, M.J.; Li, J.G. Artesunate ameliorates DSS-induced ulcerative colitis by protecting intestinal barrier and inhibiting inflammatory response. Inflammation 2020, 43, 765–776. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Vilar, S.; Tatonetti, N.P. High-Throughput Methods for Combinatorial Drug Discovery. Sci. Transl. Med. 2013, 5, 205rv1. [Google Scholar] [CrossRef]
- Cheng, F.; Kovacs, I.A.; Barabasi, A.-L. Network-based prediction of drug combinations. Nat. Commun. 2019, 10, 1197. [Google Scholar] [CrossRef]




| NO. | NAME | TPSA | M/W | Rbon | Hacc | Hdon | MLogP |
|---|---|---|---|---|---|---|---|
| MOL1 | N3,N4-Dimethyl-L-arginine | 99.74 | 202.25 | 7 | 4 | 4 | −2.52 |
| MOL2 | Prolylleucine | 95.94 | 362.42 | 10 | 5 | 2 | 1.5 |
| MOL3 | 3-Hydroxysebacic acid | 94.83 | 218.25 | 9 | 5 | 3 | 0.72 |
| MOL5 | Prednisolone | 94.83 | 360.44 | 2 | 5 | 3 | 1.3 |
| MOL6 | Quillaic acid | 94.83 | 486.68 | 2 | 5 | 3 | 4.04 |
| MOL7 | Aloe-emodin | 94.83 | 270.24 | 1 | 5 | 3 | 0.1 |
| MOL8 | Emodin | 94.83 | 270.24 | 0 | 5 | 3 | 0.36 |
| MOL9 | N-Formylmethionine | 91.7 | 177.22 | 6 | 3 | 2 | 0.25 |
| MOL11 | Telocinobufagin | 90.9 | 402.53 | 1 | 5 | 3 | 2.75 |
| MOL13 | Xanthurenic acid | 90.39 | 205.17 | 1 | 4 | 3 | −0.15 |
| MOL20 | 4-Hydroxyhippuric acid | 86.63 | 195.17 | 4 | 4 | 3 | 0.28 |
| MOL23 | DL-Tryptophan | 79.11 | 204.23 | 3 | 3 | 3 | −1.66 |
| MOL25 | Deoxycholic acid | 77.76 | 392.57 | 4 | 4 | 3 | 3.88 |
| MOL29 | 2-Mercaptobenzothiazole | 76.12 | 167.25 | 0 | 0 | 1 | 1.42 |
| MOL30 | Sakuranetin | 75.99 | 286.28 | 2 | 5 | 2 | 0.96 |
| MOL32 | 3-tert-Butyladipic acid | 74.6 | 202.25 | 6 | 4 | 2 | 1.55 |
| MOL36 | Itaconic acid | 74.6 | 130.1 | 3 | 4 | 2 | −0.23 |
| MOL37 | Terephthalic acid | 74.6 | 166.13 | 2 | 4 | 2 | 1.2 |
| MOL40 | Dantron | 74.6 | 240.21 | 0 | 4 | 2 | 0.67 |
| MOL41 | Rubiadin | 74.6 | 254.24 | 0 | 4 | 2 | 0.92 |
| MOL42 | 5-Hydroxyindole-3-acetic acid | 73.32 | 191.18 | 2 | 3 | 3 | 0.53 |
| MOL44 | Bufalin | 70.67 | 386.52 | 1 | 4 | 2 | 3.58 |
| MOL48 | N8-Acetylspermidine | 67.15 | 187.28 | 9 | 3 | 3 | 0.14 |
| MOL49 | Senkyunolide H | 66.76 | 224.25 | 2 | 4 | 2 | 0.83 |
| MOL50 | Isovanillic acid | 66.76 | 168.15 | 2 | 4 | 2 | 0.74 |
| MOL51 | 5-Methoxysalicylic acid | 66.76 | 168.15 | 2 | 4 | 2 | 0.74 |
| MOL53 | Hexanoylglycine | 66.4 | 173.21 | 7 | 3 | 2 | 0.53 |
| MOL55 | Cinnamoylglycine | 66.4 | 205.21 | 5 | 3 | 2 | 1.08 |
| MOL57 | 3-Methylhippuric acid | 66.4 | 193.2 | 4 | 3 | 2 | 1.14 |
| MOL58 | N-Tigloylglycine | 66.4 | 157.17 | 4 | 3 | 2 | 0.1 |
| MOL59 | 4-Methylhippuric acid | 66.4 | 193.2 | 4 | 3 | 2 | 1.14 |
| MOL62 | Uracil | 65.72 | 112.09 | 0 | 2 | 2 | −0.8 |
| MOL63 | Xylenesulfonate | 65.58 | 185.22 | 1 | 3 | 0 | 1.78 |
| MOL65 | Spermidine | 64.07 | 145.25 | 7 | 3 | 3 | 0.08 |
| MOL67 | Monobutyl phthalate | 63.6 | 222.24 | 6 | 4 | 1 | 2.39 |
| MOL68 | 2-Furyl(5-hydroxy-1-Benzofuran-3-yl)methanone | 63.58 | 228.2 | 2 | 4 | 1 | 0.37 |
| MOL69 | Levetiracetam | 63.4 | 170.21 | 3 | 2 | 1 | −0.27 |
| MOL71 | L-Isoleucine | 63.32 | 131.17 | 3 | 3 | 2 | −1.82 |
| MOL79 | Estriol | 60.69 | 288.38 | 0 | 3 | 3 | 2.65 |
| MOL80 | (+)-Magnoflorine | 58.92 | 342.41 | 2 | 4 | 2 | −1.71 |
| MOL81 | Creatinine | 58.69 | 113.12 | 0 | 2 | 1 | −0.53 |
| MOL82 | 3-Hydroxydecanoic acid | 57.53 | 188.26 | 8 | 3 | 2 | 1.7 |
| MOL85 | 3-Hydroxybutyric acid | 57.53 | 104.1 | 2 | 3 | 2 | −0.39 |
| MOL87 | 4-Hydroxybenzoic acid | 57.53 | 138.12 | 1 | 3 | 2 | 0.99 |
| MOL88 | Hecogenin | 55.76 | 430.62 | 0 | 4 | 1 | 4.09 |
| MOL89 | Dodecamethylcyclohexasiloxane | 55.38 | 444.92 | 0 | 6 | 0 | −1.28 |
| MOL95 | Melatonin | 54.12 | 232.28 | 5 | 2 | 2 | 1.86 |
| MOL96 | Indole-3-carboxylic acid | 53.09 | 161.16 | 1 | 2 | 2 | 1.08 |
| MOL97 | Dibutyl phthalate | 52.6 | 278.34 | 10 | 4 | 0 | 3.43 |
| MOL100 | 5-Phenylnicotinic acid | 50.19 | 199.21 | 2 | 3 | 1 | 0.5 |
| MOL103 | Cyclo(leucylprolyl) | 49.41 | 210.27 | 2 | 2 | 1 | 0.64 |
| MOL104 | N-Acetyltyramine | 49.33 | 179.22 | 4 | 2 | 2 | 1.27 |
| MOL113 | 3,5-Dimethyl-4- Methoxybenzoic acid | 46.53 | 180.2 | 2 | 3 | 1 | 1.94 |
| MOL114 | Vanillin | 46.53 | 152.15 | 2 | 3 | 1 | 0.51 |
| MOL115 | N,N′-Dicyclohexylurea | 46.33 | 224.34 | 3 | 1 | 1 | 2.56 |
| MOL117 | 2-Methyl-6-phenylpyrimidin-4-ol | 45.75 | 186.21 | 1 | 2 | 1 | 1.46 |
| MOL118 | Primobolan | 43.37 | 344.49 | 2 | 3 | 0 | 4.1 |
| MOL121 | Berberine | 40.8 | 336.36 | 2 | 4 | 0 | 2.19 |
| MOL122 | Fraxinellone | 39.44 | 232.28 | 1 | 3 | 0 | 2.08 |
| MOL123 | 7-Methoxy-4-methylcoumarin | 39.44 | 190.2 | 1 | 3 | 0 | 1.63 |
| MOL125 | Parthenolide | 38.83 | 248.32 | 0 | 3 | 0 | 2.47 |
| MOL126 | Arglabin | 38.83 | 246.3 | 0 | 3 | 0 | 2.47 |
| MOL127 | 4-Propylbenzoic acid | 37.3 | 164.2 | 3 | 2 | 1 | 2.55 |
| MOL128 | Altrenogest | 37.3 | 310.43 | 2 | 2 | 1 | 3.77 |
| MOL129 | 2-Norbornaneacetic acid | 37.3 | 154.21 | 2 | 2 | 1 | 1.88 |
| MOL130 | Cyclopentylacetic acid | 37.3 | 128.17 | 2 | 2 | 1 | 1.23 |
| MOL132 | Trichloroacetic acid | 37.3 | 163.39 | 1 | 2 | 1 | 0.89 |
| MOL133 | 2-Methylbenzoic acid | 37.3 | 136.15 | 1 | 2 | 1 | −2.16 |
| MOL134 | Benzoic acid | 37.3 | 122.12 | 1 | 2 | 1 | 1.6 |
| MOL135 | Nandrolone | 37.3 | 274.4 | 0 | 2 | 1 | 3.36 |
| MOL138 | 4′-(Imidazol-1-yl)acetophenone | 34.89 | 186.21 | 2 | 2 | 0 | 0.96 |
| MOL139 | 2,6-Di-tert-butyl-1,4-benzoquinone | 34.14 | 220.31 | 2 | 2 | 0 | 2.19 |
| MOL140 | Phthaldialdehyde | 34.14 | 134.13 | 2 | 2 | 0 | 0.77 |
| MOL141 | Progesterone | 34.14 | 314.46 | 1 | 2 | 0 | 3.95 |
| MOL142 | 3,3′,5,5′-Tetramethyldiphenoquinone | 34.14 | 240.3 | 0 | 2 | 0 | 2.45 |
| MOL144 | Perillartine | 32.59 | 165.23 | 2 | 2 | 1 | 1.98 |
| MOL145 | Curcumol | 29.46 | 236.35 | 1 | 2 | 1 | 3.15 |
| MOL147 | N,N-Dimethyldecylamine N-oxide | 29.43 | 201.35 | 9 | 1 | 0 | 1.46 |
| MOL148 | 2-Oxindole | 29.1 | 133.15 | 0 | 1 | 1 | 1.13 |
| MOL150 | Norharman | 28.68 | 168.19 | 0 | 1 | 1 | 1.62 |
| MOL151 | Acetyl-β-methylcholine | 26.3 | 195.69 | 4 | 2 | 0 | −1.67 |
| MOL152 | Nabumetone | 26.3 | 228.29 | 4 | 2 | 0 | 2.9 |
| MOL153 | Isobornyl methacrylate | 26.3 | 222.32 | 3 | 2 | 0 | 3.19 |
| MOL155 | Isoalantolactone | 26.3 | 232.32 | 0 | 2 | 0 | 3.35 |
| MOL156 | Dehydrocostus lactone | 26.3 | 230.3 | 0 | 2 | 0 | 3.26 |
| MOL157 | Clareolide | 26.3 | 250.38 | 0 | 2 | 0 | 3.8 |
| MOL163 | Spiroxamine | 21.7 | 297.48 | 6 | 3 | 0 | 3.14 |
| MOL166 | Acetophenone | 17.07 | 120.15 | 1 | 1 | 0 | 1.78 |
| MOL167 | 2,4-Dimethylbenzaldehyde | 17.07 | 134.18 | 1 | 1 | 0 | 2.1 |
| MOL168 | Diphenylamine | 12.03 | 169.22 | 2 | 0 | 1 | 3.34 |
| MOL169 | Tributylamine | 3.24 | 185.35 | 9 | 1 | 0 | 3.41 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lv, J.; Fu, Y.; Ga, Y.; Han, C.; Fan, Y.; Wei, Y.; Hao, S.; Hao, Z. Lianweng Granules Alleviate Intestinal Barrier Damage via the IL-6/STAT3/PI3K/AKT Signaling Pathway with Dampness-Heat Syndrome Diarrhea. Antioxidants 2024, 13, 661. https://doi.org/10.3390/antiox13060661
Lv J, Fu Y, Ga Y, Han C, Fan Y, Wei Y, Hao S, Hao Z. Lianweng Granules Alleviate Intestinal Barrier Damage via the IL-6/STAT3/PI3K/AKT Signaling Pathway with Dampness-Heat Syndrome Diarrhea. Antioxidants. 2024; 13(6):661. https://doi.org/10.3390/antiox13060661
Chicago/Turabian StyleLv, Jianyu, Yuchen Fu, Yu Ga, Chao Han, Yimeng Fan, Yuanyuan Wei, Sijia Hao, and Zhihui Hao. 2024. "Lianweng Granules Alleviate Intestinal Barrier Damage via the IL-6/STAT3/PI3K/AKT Signaling Pathway with Dampness-Heat Syndrome Diarrhea" Antioxidants 13, no. 6: 661. https://doi.org/10.3390/antiox13060661







