The Coming Age of Antisense Oligos for the Treatment of Hepatic Ischemia/Reperfusion (IRI) and Other Liver Disorders: Role of Oxidative Stress and Potential Antioxidant Effect
Abstract
1. Introduction
2. OxS in Liver Ischemia-Reperfusion Injury
3. OxS in Other Liver Diseases
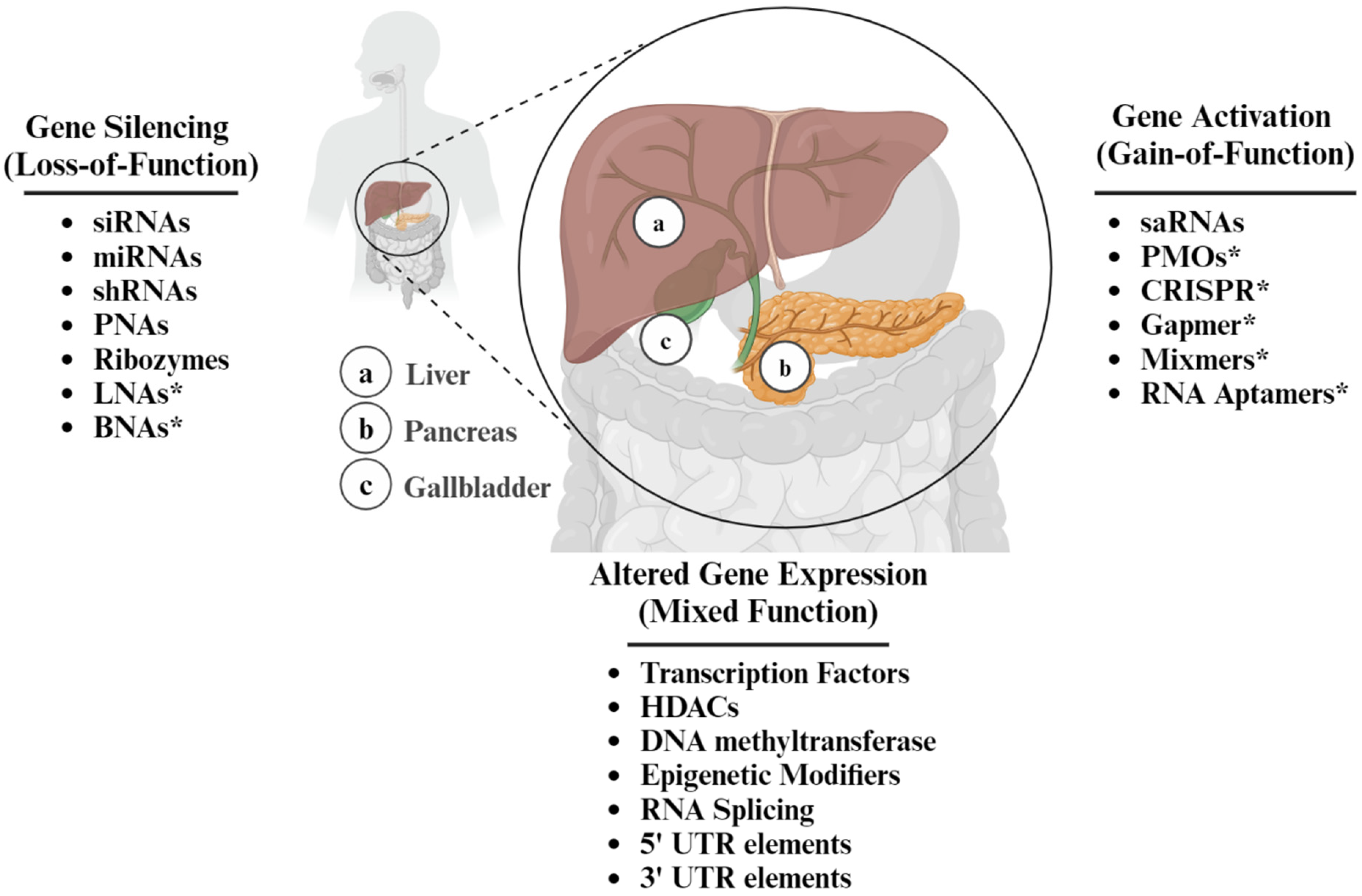
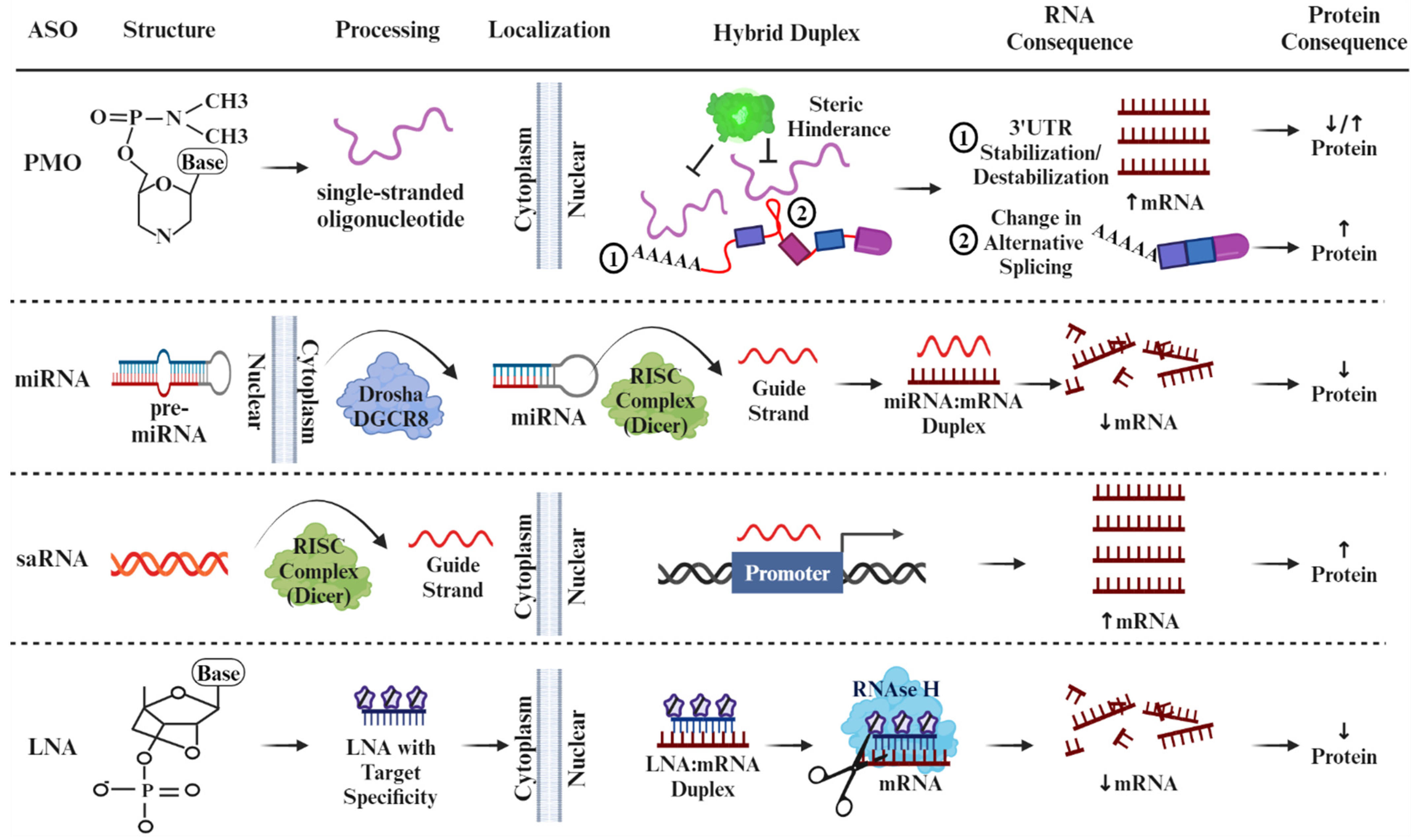
4. The Duality of ASOs as a Therapeutic Intervention
5. Modulation of Signaling Pathways Involved in Liver IRI by ASO
5.1. ASOs Targeting Liver Hepatocytes
5.2. ASOs Targeting Liver Kupffer Cells
5.3. ASOs Targeting Other Liver Signaling Pathways
5.4. ASOs That Activate Gene Expression in Liver Pathology
6. ASOs in Recent Clinical Studies
7. Challenges, Future Directions, and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Endale, H.T.; Tesfaye, W.; Mengstie, T.A. ROS induced lipid peroxidation and their role in ferroptosis. Front. Cell Dev. Biol. 2023, 11, 1226044. [Google Scholar] [CrossRef] [PubMed]
- Jaeschke, H. Reactive oxygen and mechanisms of inflammatory liver injury: Present concepts. J. Gastroenterol. Hepatol. 2011, 26 (Suppl. 1), 173–179. [Google Scholar] [CrossRef] [PubMed]
- Sepulveda-Crespo, D.; Resino, S.; Martinez, I. Strategies Targeting the Innate Immune Response for the Treatment of Hepatitis C Virus-Associated Liver Fibrosis. Drugs 2021, 81, 419–443. [Google Scholar] [CrossRef] [PubMed]
- Allameh, A.; Niayesh-Mehr, R.; Aliarab, A.; Sebastiani, G.; Pantopoulos, K. Oxidative Stress in Liver Pathophysiology and Disease. Antioxidants 2023, 12, 1653. [Google Scholar] [CrossRef] [PubMed]
- Yahoo, N.; Dudek, M.; Knolle, P.; Heikenwälder, M. Role of immune responses in the development of NAFLD-associated liver cancer and prospects for therapeutic modulation. J. Hepatol. 2023, 79, 538–551. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhang, B. RNA therapeutics: Updates and future potential. Sci. China Life Sci. 2022, 66, 12–30. [Google Scholar] [CrossRef] [PubMed]
- Bennett, C.F. Therapeutic Antisense Oligonucleotides Are Coming of Age. Annu. Rev. Med. 2019, 70, 307–321. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, T.R.; Taufiq, T.; Ishida, K.; Islam, A.; Kasahara, Y.; Osawa, T.; Obika, S. Synthesis and biophysical properties of tetravalent PEG-conjugated antisense oligonucleotide. Bioorganic Med. Chem. 2023, 78, 117149. [Google Scholar] [CrossRef]
- Cascone, T.; Kar, G.; Spicer, J.D.; García-Campelo, R.; Weder, W.; Daniel, D.B.; Spigel, D.R.; Hussein, M.; Mazieres, J.; Oliveira, J.; et al. Neoadjuvant Durvalumab Alone or Combined with Novel Immuno-Oncology Agents in Resectable Lung Cancer: The Phase II NeoCOAST Platform Trial. Cancer Discov. 2023, 13, 2394–2411. [Google Scholar] [CrossRef]
- Besse, B.; Pons-Tostivint, E.; Park, K.; Hartl, S.; Forde, P.M.; Hochmair, M.J.; Awad, M.M.; Thomas, M.; Goss, G.; Wheatley-Price, P.; et al. Biomarker-directed targeted therapy plus durvalumab in advanced non-small-cell lung cancer: A phase 2 umbrella trial. Nat. Med. 2024, 30, 716–729. [Google Scholar] [CrossRef]
- Han, K.; Ito, H.; Elston, R.; Cremer, J.; Hood, S.; Paff, M.; Theodore, D. Comparison of Pharmacokinetics of the GalNAc-Conjugated Antisense Oligonucleotide GSK3389404 in Participants with Chronic Hepatitis B Infection across the Asia-Pacific Region. Antimicrob. Agents Chemother. 2023, 67, e0090022. [Google Scholar] [CrossRef] [PubMed]
- Karwatowska-Prokopczuk, E.; Lesogor, A.; Yan, J.-H.; Hurh, E.; Hoenlinger, A.; Margolskee, A.; Xia, S.; Tsimikas, S. Efficacy and safety of pelacarsen in lowering Lp(a) in healthy Japanese subjects. J. Clin. Lipidol. 2022, 17, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Cremer, J.; Elston, R.; Campbell, F.M.; Kendrick, S.; Paff, M.; Quinn, G.; Theodore, D. B-Clear Phase 2b Study Design: Establishing the Efficacy and Safety of Bepirovirsen in Patients with Chronic Hepatitis B Virus Infection. Adv. Ther. 2023, 40, 4101–4110. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.E.; Terra, S.G.; Liu, J. A population pharmacokinetic and pharmacokinetic-pharmacodynamic analysis of vupanorsen from phase I and phase II studies. CPT Pharmacomet. Syst. Pharmacol. 2023, 12, 988–1000. [Google Scholar] [CrossRef] [PubMed]
- Fukuhara, K.; Furihata, K.; Matsuoka, N.; Itamura, R.; Ramos, V.; Hagi, T.; Kalluru, H.; Bramson, C.; Terra, S.G.; Liu, J. A multi-purpose Japanese phase I study in the global development of vupanorsen: Randomized, placebo-controlled, single-ascending dose study in adults. Clin. Transl. Sci. 2023, 16, 886–897. [Google Scholar] [CrossRef] [PubMed]
- Baker, B.F.; Xia, S.; Partridge, W.; Kwoh, T.J.; Tsimikas, S.; Bhanot, S.; Geary, R.S. Integrated Assessment of Phase 2 Data on Gal-NAc(3)-Conjugated 2′-O-Methoxyethyl-Modified Antisense Oligonucleotides. Nucleic Acid Ther. 2023, 33, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Baker, B.F.; Xia, S.; Partridge, W.; Engelhardt, J.A.; Tsimikas, S.; Crooke, S.T.; Bhanot, S.; Geary, R.S. Safety and Tolerability of GalNAc3-Conjugated Antisense Drugs Compared to the Same-Sequence 2′-O-Methoxyethyl-Modified Antisense Drugs: Results from an Integrated Assessment of Phase 1 Clinical Trial Data. Nucleic Acid Ther. 2024, 34, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Petersen, R.S.; Bordone, L.; Riedl, M.A.; Tachdjian, R.; Craig, T.J.; Lumry, W.R.; Manning, M.E.; Bernstein, J.A.; Raasch, J.; Zuraw, B.L.; et al. A phase 2 open-label extension study of prekallikrein inhibition with donidalorsen for hereditary angioedema. Allergy 2023, 79, 724–734. [Google Scholar] [CrossRef] [PubMed]
- Thornton, C.A.; Moxley, R.T.; Eichinger, K.; Heatwole, C.; Mignon, L.; Arnold, W.D.; Ashizawa, T.; Day, J.W.; Dent, G.; Tanner, M.K.; et al. Antisense oligonucleotide targeting DMPK in patients with myotonic dystrophy type 1: A multicentre, randomised, dose-escalation, placebo-controlled, phase 1/2a trial. Lancet Neurol. 2023, 22, 218–228. [Google Scholar] [CrossRef]
- Woodcock, I.R.; Tachas, G.; Desem, N.; Houweling, P.J.; Kean, M.; Emmanuel, J.; Kennedy, R.; Carroll, K.; de Valle, K.; Adams, J.; et al. A phase 2 open-label study of the safety and efficacy of weekly dosing of ATL1102 in patients with non-ambulatory Duchenne muscular dystrophy and pharmacology in mdx mice. PLoS ONE 2024, 19, e0294847. [Google Scholar] [CrossRef]
- Mummery, C.J.; Börjesson-Hanson, A.; Blackburn, D.J.; Vijverberg, E.G.; De Deyn, P.P.; Ducharme, S.; Jonsson, M.; Schneider, A.; Rinne, J.O.; Ludolph, A.C.; et al. Tau-targeting antisense oligonucleotide MAPT(Rx) in mild Alzheimer’s disease: A phase 1b, randomized, placebo-controlled trial. Nat. Med. 2023, 29, 1437–1447. [Google Scholar] [CrossRef] [PubMed]
- Edwards, A.L.; Collins, J.A.; Junge, C.; Kordasiewicz, H.; Mignon, L.; Wu, S.; Li, Y.; Lin, L.; DuBois, J.; Hutchison, R.M.; et al. Exploratory Tau Biomarker Results From a Multiple Ascending-Dose Study of BIIB080 in Alzheimer Disease: A Randomized Clinical Trial. JAMA Neurol. 2023, 80, 1344–1352. [Google Scholar] [CrossRef] [PubMed]
- Yarlas, A.; Lovley, A.; Brown, D.; Vera-Llonch, M.; Khella, S.; Karam, C. The impact of inotersen on Neuropathy Impairment Score in patients with hereditary transthyretin amyloidosis with polyneuropathy. BMC Neurol. 2023, 23, 108. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Du, C.; Xu, C.; Wang, Q.; Wang, Z.; Zhu, Q.; Lv, X.; Zhang, L.; Li, J.; Huang, C.; et al. Verbenalin attenuates hepatic damage and mitochondrial dysfunction in alcohol-associated steatohepatitis by regulating MDMX/PPARα-mediated ferroptosis. J. Ethnopharmacol. 2023, 307, 116227. [Google Scholar] [CrossRef] [PubMed]
- Machado, I.F.; Miranda, R.G.; Dorta, D.J.; Rolo, A.P.; Palmeira, C.M. Targeting Oxidative Stress with Polyphenols to Fight Liver Diseases. Antioxidants 2023, 12, 1212. [Google Scholar] [CrossRef] [PubMed]
- Dery, K.J.; Yao, S.; Cheng, B.; Kupiec-Weglinski, J.W. New therapeutic concepts against ischemia-reperfusion injury in organ transplantation. Expert Rev. Clin. Immunol. 2023, 19, 1205–1224. [Google Scholar] [CrossRef]
- Zhong, Z.; Lemasters, J.J.; Thurman, R.G. Role of purines and xanthine oxidase in reperfusion injury in perfused rat liver. J. Pharmacol. Exp. Ther. 1989, 250, 470–475. [Google Scholar] [PubMed]
- Corpas, F.J.; Barroso, J.B. Nitro-oxidative stress vs oxidative or nitrosative stress in higher plants. New Phytol. 2013, 199, 633–635. [Google Scholar] [CrossRef] [PubMed]
- Samuvel, D.J.; Krishnasamy, Y.; Li, L.; Lemasters, J.J.; Chou, C.J.; Zhong, Z. LP342, a novel histone deacetylase inhibitor, decreases nitro-oxidative stress, mitochondrial dysfunction and hepatic ischaemia/reperfusion injury in mice. RPS Pharm. Pharmacol. Rep. 2023, 2, rqad013. [Google Scholar] [CrossRef]
- Dery, K.J.; Kupiec-Weglinski, J.W. New insights into ischemia-reperfusion injury signaling pathways in organ transplantation. Curr. Opin. Organ Transplant. 2022, 27, 424–433. [Google Scholar] [CrossRef]
- Lee, D.D.; Croome, K.P.; Shalev, J.A.; Musto, K.R.; Sharma, M.; Keaveny, A.P.; Taner, C.B. Early allograft dysfunction after liver trans-plantation: An intermediate outcome measure for targeted improvements. Ann. Hepatol. 2016, 15, 53–60. [Google Scholar] [CrossRef]
- Fuentes-Valenzuela, E.; Tejedor-Tejada, J.; García-Pajares, F.; Rubiales, B.M.; Nájera-Muñoz, R.; Maroto-Martín, C.; Sánchez-Delgado, L.; Alonso-Martín, C.; Álvarez, C.A.; Sánchez-Antolín, G. Postreperfusion Liver Biopsy as Predictor of Early Graft Dysfunction and Survival After Orthotopic Liver Transplantation. J. Clin. Exp. Hepatol. 2022, 12, 1133–1141. [Google Scholar] [CrossRef]
- Wilson, E.A.; Weinberg, D.L.; Patel, G.P. Intraoperative Anesthetic Strategies to Mitigate Early Allograft Dysfunction After Or-thotopic Liver Transplantation: A Narrative Review. Anesth. Analg. 2024, 10.1213. [Google Scholar] [CrossRef]
- Xie, M.; He, Z.; Bin, B.; Wen, N.; Wu, J.; Cai, X.; Sun, X. Bulk and single-cell RNA sequencing analysis with 101 machine learning combinations reveal neutrophil extracellular trap involvement in hepatic ischemia-reperfusion injury and early allograft dysfunction. Int. Immunopharmacol. 2024, 131, 111874. [Google Scholar] [CrossRef]
- Kupiec-Weglinski, J.W.; Zhai, Y.; Coito, A.J.; Petrowsky, H.; Hong, J.C.; Busuttil, R.W. Chapter 105—Ischemia-Reperfusion Injury in Liver Transplantation. In Transplantation of the Liver, 3rd ed.; Busuttil, R.W., Klintmalm, G.B.G., Eds.; W.B. Saunders: Philadelphia, PA, USA, 2015; pp. 1438–1451. [Google Scholar]
- Chen, Z.; Tian, R.; She, Z.; Cai, J.; Li, H. Role of oxidative stress in the pathogenesis of nonalcoholic fatty liver disease. Free Radic. Biol. Med. 2020, 152, 116–141. [Google Scholar] [CrossRef]
- Nd, A.M. Non-Alcoholic Fatty Liver Disease, an Overview. Integr. Med. 2019, 18, 42–49. [Google Scholar]
- Seen, S. Chronic liver disease and oxidative stress—A narrative review. Expert Rev. Gastroenterol. Hepatol. 2021, 15, 1021–1035. [Google Scholar] [CrossRef]
- Arroyave-Ospina, J.C.; Wu, Z.; Geng, Y.; Moshage, H. Role of Oxidative Stress in the Pathogenesis of Non-Alcoholic Fatty Liver Disease: Implications for Prevention and Therapy. Antioxidants 2021, 10, 174. [Google Scholar] [CrossRef]
- Abe, Y.; Hines, I.N.; Zibari, G.; Pavlick, K.; Gray, L.; Kitagawa, Y.; Grisham, M.B. Mouse model of liver ischemia and reperfusion injury: Method for studying reactive oxygen and nitrogen metabolites in vivo. Free. Radic. Biol. Med. 2009, 46, 1–7. [Google Scholar] [CrossRef]
- Al-Asmari, A.; Khan, A.; Al-Masri, N. Mitigation of 5-fluorouracil–induced liver damage in rats by vitamin C via targeting redox–sensitive transcription factors. Hum. Exp. Toxicol. 2016, 35, 1203–1213. [Google Scholar] [CrossRef]
- Barreby, E.; Strunz, B.; Nock, S.; Naudet, L.; Shen, J.X.; Johansson, H.; Sönnerborg, I.; Ma, J.; Urgard, E.; Pallett, L.J.; et al. Human resident liver myeloid cells protect against metabolic stress in obesity. Nat. Metab. 2023, 5, 1188–1203. [Google Scholar] [CrossRef]
- Song, W.; Yan, X.; Zhai, Y.; Ren, J.; Wu, T.; Guo, H.; Song, Y.; Li, X.; Guo, Y. Probiotics attenuate valproate-induced liver steatosis and oxidative stress in mice. PLoS ONE 2023, 18, e0294363. [Google Scholar] [CrossRef]
- Ala, M.; Jafari, R.M.; Nematian, H.; Ganjedanesh, M.R.; Dehpour, A.R. Sodium Valproate Improves Skin Flap Survival via Gamma-Aminobutyric Acid and Histone Deacetylase Inhibitory System. J. Surg. Res. 2020, 246, 519–526. [Google Scholar] [CrossRef]
- Lee, T.J.; Zanello, A.F.; Morrison, T.R.; Ricci, L.A.; Melloni, R.H.J. Valproate selectively suppresses adolescent anabolic/androgenic steroid-induced aggressive behavior: Implications for a role of hypothalamic γ-aminobutyric acid neural signaling. Behav. Pharmacol. 2021, 32, 295–307. [Google Scholar] [CrossRef]
- Dixon, S.J.; Olzmann, J.A. The cell biology of ferroptosis. Nat. Rev. Mol. Cell Biol. 2024, 25, 424–442. [Google Scholar] [CrossRef]
- Su, W.; Gao, W.; Zhang, R.; Wang, Q.; Li, L.; Bu, Q.; Xu, Z.; Liu, Z.; Wang, M.; Zhu, Y.; et al. TAK1 deficiency promotes liver injury and tumorigenesis via ferroptosis and macrophage cGAS-STING signalling. JHEP Rep. 2023, 5, 100695. [Google Scholar] [CrossRef]
- Thakur, S.; Daley, B.; Gaskins, K.; Vasko, V.V.; Boufraqech, M.; Patel, D.; Sourbier, C.; Reece, J.; Cheng, S.Y.; Kebebew, E.; et al. Metformin Targets Mitochondrial Glyc-erophosphate Dehydrogenase to Control Rate of Oxidative Phosphorylation and Growth of Thyroid Cancer In Vitro and In Vivo. Clin. Cancer Res. 2018, 24, 4030–4043. [Google Scholar] [CrossRef]
- Madsen, K.S.; Kähler, P.; Kähler, L.K.; Madsbad, S.; Gnesin, F.; Metzendorf, M.-I.; Richter, B.; Hemmingsen, B. Metformin and second- or third-generation sulphonylurea combination therapy for adults with type 2 diabetes mellitus. Cochrane Database Syst. Rev. 2019, 4, CD012368. [Google Scholar] [CrossRef]
- Piao, C.; Wang, Y.; Lu, X.; Liu, T.; Ma, Y.; Li, Y.; Zhang, J.; Wang, H. Met-Exo attenuates mitochondrial dysfunction after hepatic ische-mia-reperfusion injury in rats by modulating AMPK/SIRT1 signaling pathway. Free Radic. Biol. Med. 2024, 213, 430–442. [Google Scholar] [CrossRef]
- Rubio-Ruiz, M.E.; Guarner-Lans, V.; Cano-Martínez, A.; Díaz-Díaz, E.; Manzano-Pech, L.; Gamas-Magaña, A.; Castrejón-Tellez, V.; Tapia-Cortina, C.; Pérez-Torres, I. Resveratrol and Quercetin Administration Improves Antioxidant DEFENSES and reduces Fatty Liver in Metabolic Syndrome Rats. Molecules 2019, 24, 1297. [Google Scholar] [CrossRef]
- Finkel, R.S.; Mercuri, E.; Darras, B.T.; Connolly, A.M.; Kuntz, N.L.; Kirschner, J.; Chiriboga, C.A.; Saito, K.; Servais, L.; Tizzano, E.; et al. Nusinersen versus Sham Control in Infantile-Onset Spinal Muscular Atrophy. N. Engl. J. Med. 2017, 377, 1723–1732. [Google Scholar] [CrossRef]
- Cuchel, M.; Bloedon, L.T.; Szapary, P.O.; Kolansky, D.M.; Wolfe, M.L.; Sarkis, A.; Millar, J.S.; Ikewaki, K.; Siegelman, E.S.; Gregg, R.E.; et al. Inhibition of Microsomal Triglyceride Transfer Protein in Familial Hypercholesterolemia. N. Engl. J. Med. 2007, 356, 148–156. [Google Scholar] [CrossRef]
- Marafini, I.; Monteleone, G. Therapeutic Oligonucleotides for Patients with Inflammatory Bowel Diseases. Biol. Targets Ther. 2020, 14, 47–51. [Google Scholar] [CrossRef]
- Shi, J.; Chen, M.; Ouyang, L.; Wang, Q.; Guo, Y.; Huang, L.; Jiang, S. miR-142-5p and miR-130a-3p regulate pulmonary macrophage polarization and asthma airway remodeling. Immunol. Cell Biol. 2020, 98, 715–725. [Google Scholar] [CrossRef]
- Geary, R.S.; Norris, D.; Yu, R.; Bennett, C.F. Pharmacokinetics, biodistribution and cell uptake of antisense oligonucleotides. Adv. Drug Deliv. Rev. 2015, 87, 46–51. [Google Scholar] [CrossRef]
- Johansson, H.E.; Belsham, G.J.; Sproat, B.S.; Hentze, M.W. Target-specific arrest of mRNA translation by antisense 2’-O-alkyloligoribonucleotides. Nucleic Acids Res. 1994, 22, 4591–4598. [Google Scholar] [CrossRef]
- Miller, C.M.; Harris, E.N. Antisense Oligonucleotides: Treatment Strategies and Cellular Internalization. RNA Dis. 2016, 3, e1393. [Google Scholar] [CrossRef]
- Gardin, A.; Remih, K.; Gonzales, E.; Andersson, E.R.; Strnad, P. Modern therapeutic approaches to liver-related disorders. J. Hepatol. 2022, 76, 1392–1409. [Google Scholar] [CrossRef]
- Dias, N.; Stein, C.A. Antisense oligonucleotides: Basic concepts and mechanisms. Mol. Cancer Ther. 2002, 1, 347–355. [Google Scholar]
- Gagliardi, M.; Ashizawa, A.T. The Challenges and Strategies of Antisense Oligonucleotide Drug Delivery. Biomedicines 2021, 9, 433. [Google Scholar] [CrossRef]
- Kim, J.; Hu, C.; Moufawad El Achkar, C.; Black, L.E.; Douville, J.; Larson, A.; Pendergast, M.K.; Goldkind, S.F.; Lee, E.A.; Kuniholm, A.; et al. Patient-Customized Oligonucleotide Therapy for a Rare Genetic Disease. N. Engl. J. Med. 2019, 381, 1644–1652. [Google Scholar] [CrossRef] [PubMed]
- Bartolucci, D.; Pession, A.; Hrelia, P.; Tonelli, R. Precision Anti-Cancer Medicines by Oligonucleotide Therapeutics in Clinical Research Targeting Undruggable Proteins and Non-Coding RNAs. Pharmaceutics 2022, 14, 1453. [Google Scholar] [CrossRef]
- Ait Benichou, S.; Jauvin, D.; de Serres-Bérard, T.; Pierre, M.; Ling, K.K.; Bennett, C.F.; Rigo, F.; Gourdon, G.; Chahine, M.; Puymirat, J. Antisense oligonucleotides as a potential treatment for brain deficits observed in myotonic dystrophy type 1. Gene Ther. 2022, 29, 698–709. [Google Scholar] [CrossRef]
- Østergaard, M.E.; Jackson, M.; Low, A.; EChappell, A.; GLee, R.; Peralta, R.Q.; Yu, J.; Kinberger, G.A.; Dan, A.; Carty, R.; et al. Conjugation of hydrophobic moieties enhances potency of antisense oligonucleotides in the muscle of rodents and non-human primates. Nucleic Acids Res. 2019, 47, 6045–6058. [Google Scholar] [CrossRef]
- Juliano, R.L. Chemical Manipulation of the Endosome Trafficking Machinery: Implications for Oligonucleotide Delivery. Biomedicines 2021, 9, 512. [Google Scholar] [CrossRef]
- Wang, S.; Sun, H.; Tanowitz, M.; Liang, X.-H.; Crooke, S.T. Intra-endosomal trafficking mediated by lysobisphosphatidic acid con-tributes to intracellular release of phosphorothioate-modified antisense oligonucleotides. Nucleic Acids Res. 2017, 45, 5309–5322. [Google Scholar] [CrossRef]
- Gilbert, J.; Baker, S.D.; Bowling, M.K.; Grochow, L.; Figg, W.D.; Zabelina, Y.; Donehower, R.C.; A Carducci, M. A phase I dose escalation and bioavailability study of oral sodium phenylbutyrate in patients with refractory solid tumor malignancies. Clin. Cancer Res. 2001, 7, 2292–2300. [Google Scholar] [PubMed]
- Nissim-Rafinia, M.; Aviram, M.; Randell, S.H.; Shushi, L.; Ozeri, E.; Chiba-Falek, O.; Eidelman, O.; Pollard, H.B.; Yankaskas, J.R.; Kerem, B. Restoration of the cystic fibrosis transmembrane conductance regulator function by splicing modulation. Embo Rep. 2004, 5, 1071–1077. [Google Scholar] [CrossRef] [PubMed]
- Zamecnik, P.C.; Stephenson, M.L. Inhibition of Rous sarcoma virus replication and cell transformation by a specific oligode-oxynucleotide. Proc. Natl. Acad. Sci. USA 1978, 75, 280–284. [Google Scholar] [CrossRef]
- Debjyoti, K.; Lindsey, K.; Tianhao, Z.; Burcin, E.; Vik, M.; Amelia, S.; Konstantina, K.; Lixian, C.; Ludovica, C.; Nan, W.; et al. p16 INK4A drives nonalcoholic fatty liver disease phenotypes in high fat diet fed mice through biliary E2F1/FOXO1/IGF-1 signaling. Hepatology 2023, 78, 243–257. [Google Scholar]
- van Riet, S.; Julien, A.; Atanasov, A.; Nordling, Å.; Ingelman-Sundberg, M. The role of sinusoidal endothelial cells and TIMP1 in the regulation of fibrosis in a novel human liver 3D NASH model. Hepatol. Commun. 2024, 8, e0374. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wang, H.; He, T.; Ma, B.; Chen, G.; Tzeng, C. Knockdown of Yap attenuates TAA-induced hepatic fibrosis by interaction with hedgehog signals. J. Cell Commun. Signal. 2023, 17, 1335–1354. [Google Scholar] [CrossRef] [PubMed]
- Dugbartey, G.J. Cellular and molecular mechanisms of cell damage and cell death in ischemia–reperfusion injury in organ transplantation. Mol. Biol. Rep. 2024, 51, 473. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Kageyama, S.; Kaldas, F.M.; Hirao, H.; Ito, T.; Kadono, K.; Dery, K.J.; Kojima, H.; Gjertson, D.W.; Sosa, R.A.; et al. Hepatic CEACAM1 expression indicates donor liver quality and prevents early transplantation injury. J. Clin. Investig. 2020, 130, 2689–2704. [Google Scholar] [CrossRef] [PubMed]
- Jastrząb, A.; Skrzydlewska, E. Thioredoxin-dependent system. Application of inhibitors. J. Enzym. Inhib. Med. Chem. 2021, 36, 362–371. [Google Scholar] [CrossRef] [PubMed]
- Tong, H.; Wang, L.; Zhang, K.; Shi, J.; Wu, Y.; Bao, Y.; Wang, C. S100A6 Activates Kupffer Cells via the p-P38 and p-JNK Pathways to Induce Inflammation, Mononuclear/macrophage Infiltration Sterile Liver Injury in Mice. Inflammation 2022, 46, 534–554. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Li, J.; Jiang, C.; Yuan, T.; Ma, H. Cellular communication network factor 1 (CCN1) knockdown exerts a protective effect for hepatic ischemia/reperfusion injury by deactivating the MEK/ERK pathway. Clin. Res. Hepatol. Gastroenterol. 2021, 45, 101737. [Google Scholar] [CrossRef] [PubMed]
- Bonaccorsi-Riani, E.M.; Gillooly, A.R.; Iesari, S.; Brüggenwirth, I.M.; Ferguson, C.M.B.; Komuta, M.; Xhema, D.B.; Daumerie, A.B.; Maistriaux, L.; Leuvenink, H.; et al. Delivering siRNA Compounds During HOPE to Modulate Organ Function: A Proof-of-concept Study in a Rat Liver Transplant Model. Transplantation 2022, 106, 1565–1576. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhang, J.; Xia, L.; Wang, L.; Li, H.; Liu, H.; Zhou, J.; Feng, Z.; Jin, H.; Yang, J.; et al. β-Arrestin-2 attenuates hepatic ischemia-reperfusion injury by activating PI3K/Akt signaling. Aging 2020, 13, 2251–2263. [Google Scholar] [CrossRef]
- Fujii, T.; Duarte, S.; Lee, E.; Ke, B.; Busuttil, R.W.; Coito, A.J. Tissue Inhibitor of Metalloproteinase 3 Deficiency Disrupts the Hepatocyte E-Cadherin/β-Catenin Complex and Induces Cell Death in Liver Ischemia/Reperfusion Injury. Liver Transplant. 2019, 26, 113–126. [Google Scholar] [CrossRef]
- Wang, T.; Fang, Y.; Zhang, X.; Yang, Y.; Jin, L.; Li, Z.; Miao, Y.; Zeng, Z.; Huang, H. Heme Oxygenase-1 Alleviates Ischemia-Reperfusion Injury by Inhibiting Hepatocyte Pyroptosis after Liver Transplantation in Rats. Front. Biosci. 2023, 28, 275. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.; Caruthers, M.H. Synthesis of Backbone-Modified Morpholino Oligonucleotides Using Phosphoramidite Chemistry. Molecules 2023, 28, 5380. [Google Scholar] [CrossRef] [PubMed]
- Dierolf, J.G.; Hunter, H.L.M.; Watson, A.J.; Betts, D.H. Modulation of PKM1/2 Levels by Steric Blocking Morpholinos Alters the Metabolic and Pluripotent State of Murine Pluripotent Stem Cells. Stem Cells Dev. 2022, 31, 278–295. [Google Scholar] [CrossRef] [PubMed]
- Dery, K.J.; Kojima, H.; Kageyama, S.; Kadono, K.; Hirao, H.; Cheng, B.; Zhai, Y.; Farmer, D.G.; Kaldas, F.M.; Yuan, X.; et al. Alternative splicing of CEACAM1 by hypox-ia-inducible factor–1α enhances tolerance to hepatic ischemia in mice and humans. Sci. Transl. Med. 2023, 15, eadf2059. [Google Scholar] [CrossRef] [PubMed]
- Götz, L.; Rueckschloss, U.; Balk, G.; Pfeiffer, V.; Ergün, S.; Kleefeldt, F. The role of carcinoembryonic antigen-related cell adhesion molecule 1 in cancer. Front. Immunol. 2023, 14, 1295232. [Google Scholar] [CrossRef] [PubMed]
- Dery, K.J.; Gaur, S.; Gencheva, M.; Yen, Y.; Shively, J.E.; Gaur, R.K. Mechanistic control of carcinoembryonic antigen-related cell adhesion molecule-1 (CEACAM1) splice isoforms by the heterogeneous nuclear ribonuclear proteins hnRNP L, hnRNP A1, and hnRNP M. J. Biol. Chem. 2011, 286, 16039–16051. [Google Scholar] [CrossRef] [PubMed]
- Dery, K.J.; Kujawski, M.; Grunert, D.; Wu, X.; Ngyuen, T.; Cheung, C.; Yim, J.H.; Shively, J.E. IRF-1 regulates alternative mRNA splicing of carcinoembryonic antigen-related cell adhesion molecule 1 (CEACAM1) in breast epithelial cells generating an immuno-receptor tyrosine-based inhibition motif (ITIM) containing isoform. Mol. Cancer 2014, 13, 64. [Google Scholar] [CrossRef] [PubMed]
- Dery, K.J.; Silver, C.; Yang, L.; Shively, J.E. Interferon regulatory factor 1 and a variant of heterogeneous nuclear ribonucleoprotein L coordinately silence the gene for adhesion protein CEACAM1. J. Biol. Chem. 2018, 293, 9277–9291. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, O.; Yokota, T. Pharmacology and toxicology of eteplirsen and SRP-5051 for DMD exon 51 skipping: An update. Arch. Toxicol. 2021, 96, 1–9. [Google Scholar] [CrossRef]
- Xin, Y.; Zhao, L.; Peng, R. HIF-1 signaling: An emerging mechanism for mitochondrial dynamics. J. Physiol. Biochem. 2023, 79, 489–500. [Google Scholar] [CrossRef]
- Wu, X.Y.; Chen, Y.J.; Liu, C.A.; Gong, J.H.; Xu, X.S. STING Induces Liver Ischemia-Reperfusion Injury by Promoting Calci-um-Dependent Caspase 1-GSDMD Processing in Macrophages. Oxid. Med. Cell Longev. 2022, 2022, 8123157. [Google Scholar] [PubMed]
- Kong, E.; Zhang, Y.; Geng, X.; Zhao, Y.; Yue, W.; Feng, X. Inhibition of Sirt3 activates the cGAS-STING pathway to aggravate hepatocyte damage in hepatic ischemia-reperfusion injury mice. Int. Immunopharmacol. 2024, 128, 111474. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.Z.; Cao, Z.R.; Zheng, W.X.; Zhao, M.J.; Gong, J.H.; Chen, C.; Wu, Z.J.; Tao, R. HSP110 aggravates ischemia-reperfusion injury after liver transplantation by promoting NF-κB pathway. Hepatobiliary Pancreat. Dis. Int. 2023, 23, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, P.; Song, X.; Cui, H.; Shen, W. PPARγ Mediates Protective Effect against Hepatic Ischemia/Reperfusion Injury via NF-κB Pathway. J. Investig. Surg. 2022, 35, 1648–1659. [Google Scholar] [CrossRef]
- Zhuang, L.; Ding, W.; Zhang, Q.; Ding, W.; Xu, X.; Yu, X.; Xi, D. TGR5 Attenuated Liver Ischemia-Reperfusion Injury by Activating the Keap1-Nrf2 Signaling Pathway in Mice. Inflammation 2020, 44, 859–872. [Google Scholar] [CrossRef]
- Baird, L.; Yamamoto, M. The molecular mechanisms regulating the KEAP1-NRF2 pathway. Mol. Cell. Biol. 2020, 40, e00099-20. [Google Scholar] [CrossRef]
- Zhang, M.M.; Bahal, R.; Rasmussen, T.P.; Manautou, J.E.; Zhong, X.B. The growth of siRNA-based therapeutics: Updated clinical studies. Biochem. Pharmacol. 2021, 189, 114432. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Fu, J. GDF15 as a key disease target and biomarker: Linking chronic lung diseases and ageing. Mol. Cell. Biochem. 2023, 479, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Baek, S.J.; Kim, J.-S.; Nixon, J.B.; DiAugustine, R.P.; Eling, T.E. Expression of NAG-1, a transforming growth factor-β superfamily member, by troglitazone requires the early growth response gene EGR-1. J. Biol. Chem. 2004, 279, 6883–6892. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, C.; Chen, J.; Sang, T.; Peng, H.; Lin, X.; Zhao, Q.; Chen, S.; Eling, T.; Wang, X. Overexpression of NAG-1/GDF15 prevents hepatic steatosis through inhibiting oxidative stress-mediated dsDNA release and AIM2 inflammasome activation. Redox Biol. 2022, 52, 102322. [Google Scholar] [CrossRef]
- Huang, J.; Huang, H.; Wang, Y.; Xu, B.; Lin, M.; Han, S.; Yuan, Y.; Wang, Y.; Shuai, X. Retinol-binding protein-hijacking nanopolyplex delivering siRNA to cytoplasm of hepatic stellate cell for liver fibrosis alleviation. Biomaterials 2023, 299, 122134. [Google Scholar] [CrossRef] [PubMed]
- Latorre, J.; Díaz-Trelles, R.; Comas, F.; Gavaldà-Navarro, A.; Milbank, E.; Dragano, N.; Morón-Ros, S.; Mukthavaram, R.; Ortega, F.; Castells-Nobau, A.; et al. Downregulation of hepatic lipopolysaccharide binding protein improves lipogenesis-induced liver lipid accumulation. Mol. Ther. Nucleic Acids 2022, 29, 599–613. [Google Scholar] [CrossRef]
- Ariffianto, A.; Deng, L.; Harada, S.; Liang, Y.; Matsui, C.; Abe, T.; Shoji, I. Transcription Factor JunB Suppresses Hepatitis C Virus Replication. Kobe J. Med. Sci. 2023, 69, E86–E95. [Google Scholar]
- Ly, H.H.; Daniel, S.; Soriano, S.K.V.; Kis, Z.; Blakney, A.K. Optimization of Lipid Nanoparticles for saRNA Expression and Cellular Activation Using a Design-of-Experiment Approach. Mol. Pharm. 2022, 19, 1892–1905. [Google Scholar] [CrossRef] [PubMed]
- Roth, C.; Kilpinen, H.; Kurian, M.A.; Barral, S. Histone lysine methyltransferase-related neurodevelopmental disorders: Current knowledge and saRNA future therapies. Front. Cell Dev. Biol. 2023, 11, 1090046. [Google Scholar] [CrossRef]
- Fu, J.; Dong, H.; Wu, J.; Jin, Y. Emerging Progress of RNA-Based Antitumor Therapeutics. Int. J. Biol. Sci. 2023, 19, 3159–3183. [Google Scholar] [CrossRef]
- Reebye, V.; Sætrom, P.; Mintz, P.J.; Rossi, J.J.; Kasahara, N.; Nteliopoulos, G.; Nicholls, J.; Haoudi, A.; Gordon, M.; Habib, N.A. A Short-activating RNA Oligonucleotide Targeting the Islet β-cell Transcriptional Factor MafA in CD34+ Cells. Mol. Ther. Nucleic Acids 2013, 2, e97. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.P.; Sinigaglia, L.; Gomez, V.; Nicholls, J.; Habib, N.A. RNA Activation—A Novel Approach to Therapeutically Upregulate Gene Transcription. Molecules 2021, 26, 6530. [Google Scholar] [CrossRef]
- Zhao, X.; Reebye, V.; Hitchen, P.; Fan, J.; Jiang, H.; Sætrom, P.; Rossi, J.; Habib, N.A.; Huang, K.-W. Mechanisms involved in the activation of C/EBPα by small activating RNA in hepatocellular carcinoma. Oncogene 2019, 38, 3446–3457. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, Y.; Wang, Y.; Chao, Y.; Zhang, J.; Jia, Y.; Tie, J.; Hu, D. Regulation of SIRT1 and Its Roles in Inflammation. Front. Immunol. 2022, 13, 831168. [Google Scholar] [CrossRef]
- Wada, F.; Yamamoto, T.; Kobayashi, T.; Tachibana, K.; Ito, K.R.; Hamasaki, M.; Kayaba, Y.; Terada, C.; Yamayoshi, A.; Obika, S.; et al. Drug discovery and development scheme for liver-targeting bridged nucleic acid antisense oligonucleotides. Mol. Ther. Nucleic Acids 2021, 26, 957–969. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, T.; Hirakawa, Y.; Yamairi, F.; Kurita, T.; Murahashi, K.; Nishimura, H.; Iwazaki, N.; Yasuhara, H.; Tateoka, T.; Ohta, T.; et al. Altered Biodistribution and Hepatic Safety Profile of a Gapmer Antisense Oligonucleotide Bearing Guanidine-Bridged Nucleic Acids. Nucleic Acid Ther. 2022, 32, 177–184. [Google Scholar] [CrossRef]
- Kusznir, E.-A.; Hau, J.-C.; Portmann, M.; Reinhart, A.-G.; Falivene, F.; Bastien, J.; Worm, J.; Ross, A.; Lauer, M.; Ringler, P.; et al. Propensities of Fatty Acid-Modified ASOs: Self-Assembly vs Albumin Binding. Bioconjugate Chem. 2023, 34, 866–879. [Google Scholar] [CrossRef]
- Grünweller, A.; Hartmann, R.K. Locked nucleic acid oligonucleotides: The next generation of antisense agents? BioDrugs 2007, 21, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Kamali, M.J.; Salehi, M.; Fatemi, S.; Moradi, F.; Khoshghiafeh, A.; Ahmadifard, M. Locked nucleic acid (LNA): A modern approach to cancer diagnosis and treatment. Exp. Cell Res. 2023, 423, 113442. [Google Scholar] [CrossRef] [PubMed]
- Horie, S.; Suzuki, Y.; Yamamoto, T.; Obika, S.; Mohri, K.; Kiyota, C.; Ren, Q.; Warashina, S.; Wada, Y.; Watanabe, Y.; et al. Novel strategy of liver cancer treatment with modified antisense oligonucleotides targeting human vasohibin-2. Cancer Sci. 2023, 114, 3740–3749. [Google Scholar] [CrossRef] [PubMed]
- Aguti, S.; Marrosu, E.; Muntoni, F.; Zhou, H. Gapmer Antisense Oligonucleotides to Selectively Suppress the Mutant Allele in COL6A Genes in Dominant Ullrich Congenital Muscular Dystrophy. Methods Mol. Biol. 2020, 2176, 221–230. [Google Scholar] [PubMed]
- Komaki, H.; Nagata, T.; Saito, T.; Masuda, S.; Takeshita, E.; Sasaki, M.; Tachimori, H.; Nakamura, H.; Aoki, Y.; Takeda, S.I. Systemic administration of the antisense oligonucleotide NS-065/NCNP-01 for skipping of exon 53 in patients with Duchenne muscular dystrophy. Sci. Transl. Med. 2018, 10, eaan0713. [Google Scholar] [CrossRef] [PubMed]
- Ohmura, T.; Saeki, S.; Ogiwara, K.; Tobita, K.; Ling, Y.; Torii, S. Pharmacological and clinical profile of spinal muscular atrophy (SMA) therapeutic drug nusinersen (Spinraza®). Folia Pharmacol. Jpn. 2018, 152, 147–159. [Google Scholar] [CrossRef]
- Tanaka, A.; Sata, M.; Okada, Y.; Teragawa, H.; Eguchi, K.; Shimabukuro, M.; Taguchi, I.; Matsunaga, K.; Kanzaki, Y.; Yoshida, H.; et al. Effect of ipragliflozin on carotid inti-ma-media thickness in patients with type 2 diabetes: A multicenter, randomized, controlled trial. Eur. Heart J. -Cardiovasc. Pharmacother. 2023, 9, 165–172. [Google Scholar] [CrossRef]
- Yang, Q.; Humphreys, S.C.; Lade, J.M.; Li, A.P. Prolonged cultured human hepatocytes as an in vitro experimental system for the evaluation of potency and duration of activity of RNA therapeutics: Demonstration of prolonged duration of gene silencing effects of a GalNAc-conjugated human hypoxanthine phosphoribosyl transferase (HPRT1) siRNA. Biochem. Pharmacol. 2021, 189, 114374. [Google Scholar]
- Prakash, T.P.; Graham, M.J.; Yu, J.; Carty, R.; Low, A.; Chappell, A.; Schmidt, K.; Zhao, C.; Aghajan, M.; Murray, H.F.; et al. Targeted delivery of antisense oligonucleotides to hepatocytes using triantennary N-acetyl galactosamine improves potency 10-fold in mice. Nucleic Acids Res. 2014, 42, 8796–8807. [Google Scholar] [CrossRef] [PubMed]
- Yeang, C.; Karwatowska-Prokopczuk, E.; Su, F.; Dinh, B.; Xia, S.; Witztum, J.L.; Tsimikas, S. Effect of Pelacarsen on Lipoprotein(a) Cholesterol and Corrected Low-Density Lipoprotein Cholesterol. J. Am. Coll. Cardiol. 2022, 79, 1035–1046. [Google Scholar] [CrossRef] [PubMed]
- Wei, T.; Cho, L. Recent lipoprotein(a) trials. Curr. Opin. Lipidol. 2022, 33, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Yuen, M.-F.; Heo, J.; Jang, J.-W.; Yoon, J.-H.; Kweon, Y.-O.; Park, S.-J.; Tami, Y.; You, S.; Yates, P.; Tao, Y.; et al. Safety, tolerability and antiviral activity of the antisense oligonucleotide bepirovirsen in patients with chronic hepatitis B: A phase 2 randomized controlled trial. Nat. Med. 2021, 27, 1725–1734. [Google Scholar] [CrossRef] [PubMed]
- Yuen, M.-F.; Lim, S.-G.; Plesniak, R.; Tsuji, K.; Janssen, H.L.; Pojoga, C.; Gadano, A.; Popescu, C.P.; Stepanova, T.; Asselah, T.; et al. Efficacy and Safety of Bepirovirsen in Chronic Hepatitis B Infection. N. Engl. J. Med. 2022, 387, 1957–1968. [Google Scholar] [CrossRef] [PubMed]
- Muiños-Bühl, A.; Rombo, R.; Ling, K.K.; Zilio, E.; Rigo, F.; Bennett, C.F.; Wirth, B. Long-Term SMN- and Ncald-ASO Combinatorial Therapy in SMA Mice and NCALD-ASO Treatment in hiPSC-Derived Motor Neurons Show Protective Effects. Int. J. Mol. Sci. 2023, 24, 4198. [Google Scholar] [CrossRef]
- Muinos-Buehl, A.; Rombo, R.; Janzen, E.; Ling, K.K.; Hupperich, K.; Rigo, F.; Bennett, C.F.; Wirth, B. Combinatorial ASO-mediated therapy with low dose SMN and the protective modifier Chp1 is not sufficient to ameliorate SMA pathology hallmarks. Neurobiol. Dis. 2022, 171, 105795. [Google Scholar] [CrossRef]
- de la Hoz, R.; Diban, N.; Berciano, M.T.; Emeterio, C.S.; Urtiaga, A.; Lafarga, M.; Rodríguez-Rey, J.C.; Tapia, O. Coaxial Synthesis of PEI-Based Nanocarriers of Encapsulated RNA-Therapeutics to Specifically Target Muscle Cells. Biomolecules 2022, 12, 1012. [Google Scholar] [CrossRef]
- Lau, A.W.; Chou, M.M. The adaptor complex AP-2 regulates post-endocytic trafficking through the non-clathrin Arf6-dependent endocytic pathway. J. Cell Sci. 2008, 121, 4008–4017. [Google Scholar] [CrossRef]
- Moya-Alvarado, G.; Guerra, M.V.; Tiburcio, R.; Bravo, E.; Bronfman, F.C. The Rab11-regulated endocytic pathway and BDNF/TrkB signaling: Roles in plasticity changes and neurodegenerative diseases. Neurobiol. Dis. 2022, 171, 105796. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.; Fan, Q.; Zou, X. Antisense oligonucleotide is a promising intervention for liver diseases. Front. Pharmacol. 2022, 13, 1061842. [Google Scholar] [CrossRef] [PubMed]
- Kay, E.; Stulz, R.; Becquart, C.; Lovric, J.; Tängemo, C.; Thomen, A.; Baždarević, D.; Najafinobar, N.; Dahlén, A.; Pielach, A.; et al. NanoSIMS Imaging Reveals the Impact of Ligand-ASO Conjugate Stability on ASO Subcellular Distribution. Pharmaceutics 2022, 14, 463. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.-H.; Sun, H.; Nichols, J.G.; Allen, N.; Wang, S.; Vickers, T.A.; Shen, W.; Hsu, C.-W.; Crooke, S.T. COPII vesicles can affect the activity of antisense oligonucleotides by facilitating the release of oligonucleotides from endocytic pathways. Nucleic Acids Res. 2018, 46, 10225–10245. [Google Scholar] [CrossRef]
- Ochaba, J.; Powers, A.F.; Tremble, K.A.; Greenlee, S.; Post, N.M.; Matson, J.E.; MacLeod, A.R.; Guo, S.; Aghajan, M. A novel and translational role for autophagy in antisense oligonucleotide trafficking and activity. Nucleic Acids Res. 2019, 47, 11284–11303. [Google Scholar] [CrossRef]


| Disease | Therapy | Study Design Phase, N, Year | Novelty | Outcome | Source |
|---|---|---|---|---|---|
| Cancer | |||||
| NSCLC * | Danvatirsen αSTAT3 ASO | CT *, P2 *, 83, 2023 | Combination regimen using PD-(L)-1 inhibition with other agents, ASOs | Preliminary efficacy signals showed no major pathologic response (MPR) as the primary endpoint | [9] |
| NSCLC | CT, P2, 268, 2024 | Combination therapy with Danvatirsen did not improve immunosuppressive TME * targeting | [10] | ||
| Liver | |||||
| CHB * | GSK3389404 | RCT, 64, P2, 2023 | GalNac targeting to parenchymal hepatocytes | Safety study showing that plasma pharmacokinetics from any Asia-Pacific population may be used to guide ASO dose selection | [11] |
| Lp(a) * | Pelacarsen | RCT, 29, P1, 2023 | Safety and efficacy in Japanese populations studied | No clinically relevant abnormalities were detected | [12] |
| HBV * | Bepirovirsen GSK3228836 | RCT, 440 P2b, 2023 | Efficacy and safety study | Assessment of hepatitis B surface antigen and DNA seroclearance levels may be discontinued in the presence and absence of background nucleos(t)ide analogue therapy | [13] |
| Dyslipidemia | Vupanorsen (PF-07285557) | CT, P1/2, 451, 2023 | 2nd generation ligand-conjugated 2′O-methoxyethyl modified angiopoietin-like 3 | ANGPTL3 target reduction of 75% achieved with a 320-mg dose of Vupanorsen per month | [14] |
| Dyslipidemia | CT, P1, 6, 2023 | Vupanorsen reduces triglycerides, lipids, and apolipoproteins. | [15] | ||
| No Disease | GalNAc3 *-conjugated 2′MOE * ASOs | CT, P2, 195, 2023 | Safety and Efficacy Study | No class effect was identified, ASOs were well-tolerated in all doses tested compared to controls | [16] |
| No Disease | CT, P1, 195, 2024 | GalNac targeting to parenchymal hepatocytes | Safety and tolerability observed in GalNac-conjugated than unmodified ASOs | [17] | |
| Immune | |||||
| HAE * | Donidalorsen ligand-conjugated antisense LICA | CT, P2, 20, 2024 | Targets the prekallikrein (PKK) pathway | No adverse effects with Donidalorsen. Showing durable efficacy with 96% less HAE attacks | [18] |
| Muscular | |||||
| MDT1 * | Baliforsen (ISIS 598769) | CT, P1/2, 20, 2023 | ASO targeting DM1 protein kinase (DMPK) mRNA | Baliforsen was well-tolerated but below levels predicted to achieve substantial target reduction—more studies will be needed | [19] |
| DMD * | ATL1102 | CT, P2, 9, 2024 | 2′MOE gapmer antisense oligonucleotide to the CD49d alpha subunit of VLA-4 | Multiple muscle disease progression parameters assessed show stabilization and safety in non-ambulant boys with DMD | [20] |
| Nervous | |||||
| AD * | MAPTRx | CT, P1b, 46, 2023 | Inhibition of MAPT expression with a Tau-targeting ASO | Reduction in CSF total tau concentration with 50% mean reduction 24 weeks following last dose | [21] |
| AD * | BIIB080 | RCT, P1b, 46, 2023 | a MAPT-targeting antisense oligonucleotide | BIIB080 reduced tau biomarkers in study subjects with mild AD | [22] |
| ATTRv * | Inotersen | CT, P3, 172, 2023 | Inhibits the production of transthyretin (TTR) protein | Inotersen led to lower muscle weakness measures following 65 weeks treatment as compared to placebo controls | [23] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, S.; Kasargod, A.; Chiu, R.; Torgerson, T.R.; Kupiec-Weglinski, J.W.; Dery, K.J. The Coming Age of Antisense Oligos for the Treatment of Hepatic Ischemia/Reperfusion (IRI) and Other Liver Disorders: Role of Oxidative Stress and Potential Antioxidant Effect. Antioxidants 2024, 13, 678. https://doi.org/10.3390/antiox13060678
Yao S, Kasargod A, Chiu R, Torgerson TR, Kupiec-Weglinski JW, Dery KJ. The Coming Age of Antisense Oligos for the Treatment of Hepatic Ischemia/Reperfusion (IRI) and Other Liver Disorders: Role of Oxidative Stress and Potential Antioxidant Effect. Antioxidants. 2024; 13(6):678. https://doi.org/10.3390/antiox13060678
Chicago/Turabian StyleYao, Siyuan, Aanchal Kasargod, Richard Chiu, Taylor R. Torgerson, Jerzy W. Kupiec-Weglinski, and Kenneth J. Dery. 2024. "The Coming Age of Antisense Oligos for the Treatment of Hepatic Ischemia/Reperfusion (IRI) and Other Liver Disorders: Role of Oxidative Stress and Potential Antioxidant Effect" Antioxidants 13, no. 6: 678. https://doi.org/10.3390/antiox13060678
APA StyleYao, S., Kasargod, A., Chiu, R., Torgerson, T. R., Kupiec-Weglinski, J. W., & Dery, K. J. (2024). The Coming Age of Antisense Oligos for the Treatment of Hepatic Ischemia/Reperfusion (IRI) and Other Liver Disorders: Role of Oxidative Stress and Potential Antioxidant Effect. Antioxidants, 13(6), 678. https://doi.org/10.3390/antiox13060678








