Probiotic Characteristics and Anti-Inflammatory Effects of Limosilactobacillus fermentum 664 Isolated from Chinese Fermented Pickles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Isolation and Preservation of Strains
2.2. Bacterial Strain and Cultivation Conditions
2.3. Acid Resistance Test
2.4. Bile Salt Tolerance Test
2.5. Cell Surface Hydrophobicity Measurement
2.6. Adhesion to HT-29 Intestinal Cells
2.7. Antimicrobial Activity
2.8. Bacterial Suspension Preparation
2.9. Inflammatory Cytokines Measurement
2.10. Western Blot Analysis
2.11. Measurement of ROS
2.12. Whole-Genome Sequencing
2.13. Statistical Analysis
3. Results
3.1. Acid Resistance and Bile Salt Tolerance
3.2. Hydrophobicity and Adhesion Abilities
3.3. Antimicrobial Activity
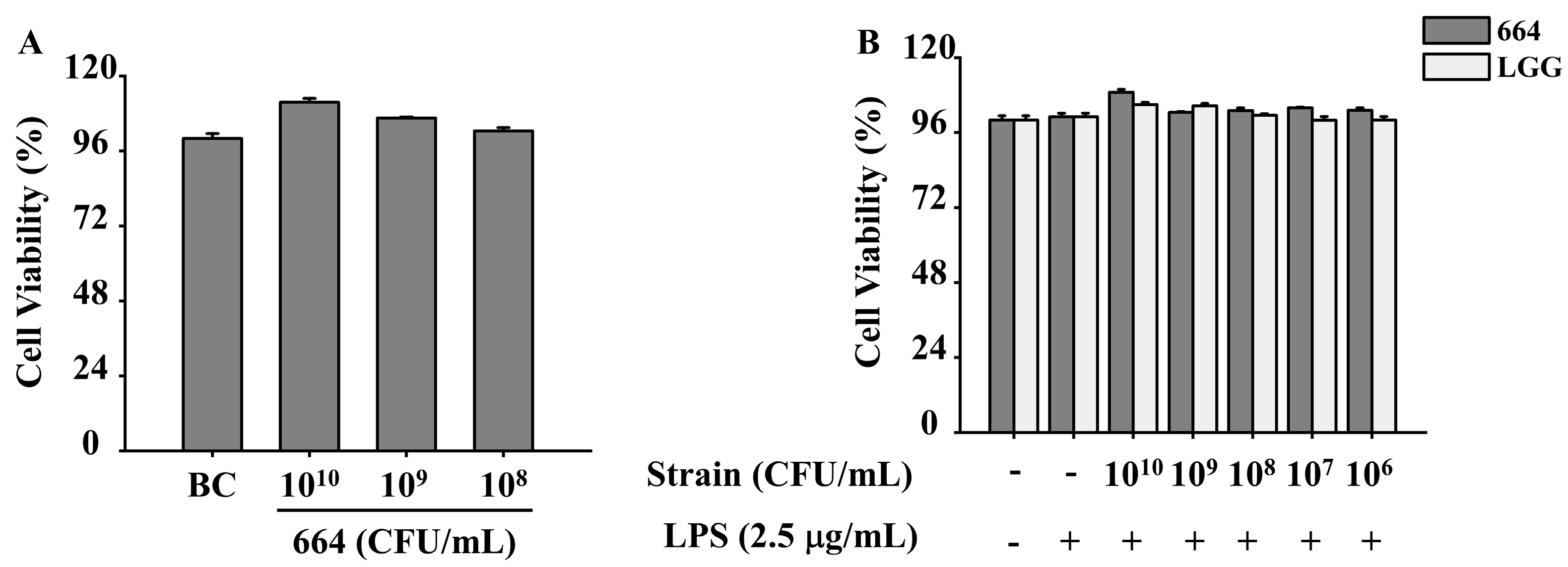
3.4. Inhibition of Pro-Inflammatory Cytokines Release in RAW264.7 Cells by L. fermentum 664
3.5. Inhibition of NF-κB Activation and IκB-α Phosphorylation in RAW264.7 Cells by L. fermentum 664
3.6. Suppression of COX-2 Expression by L. fermentum 664
3.7. Inhibition of MAPK Activation in RAW264.7 Cells by L. fermentum 664
3.8. Suppression of ROS and HO-1 Expression by L. fermentum 664
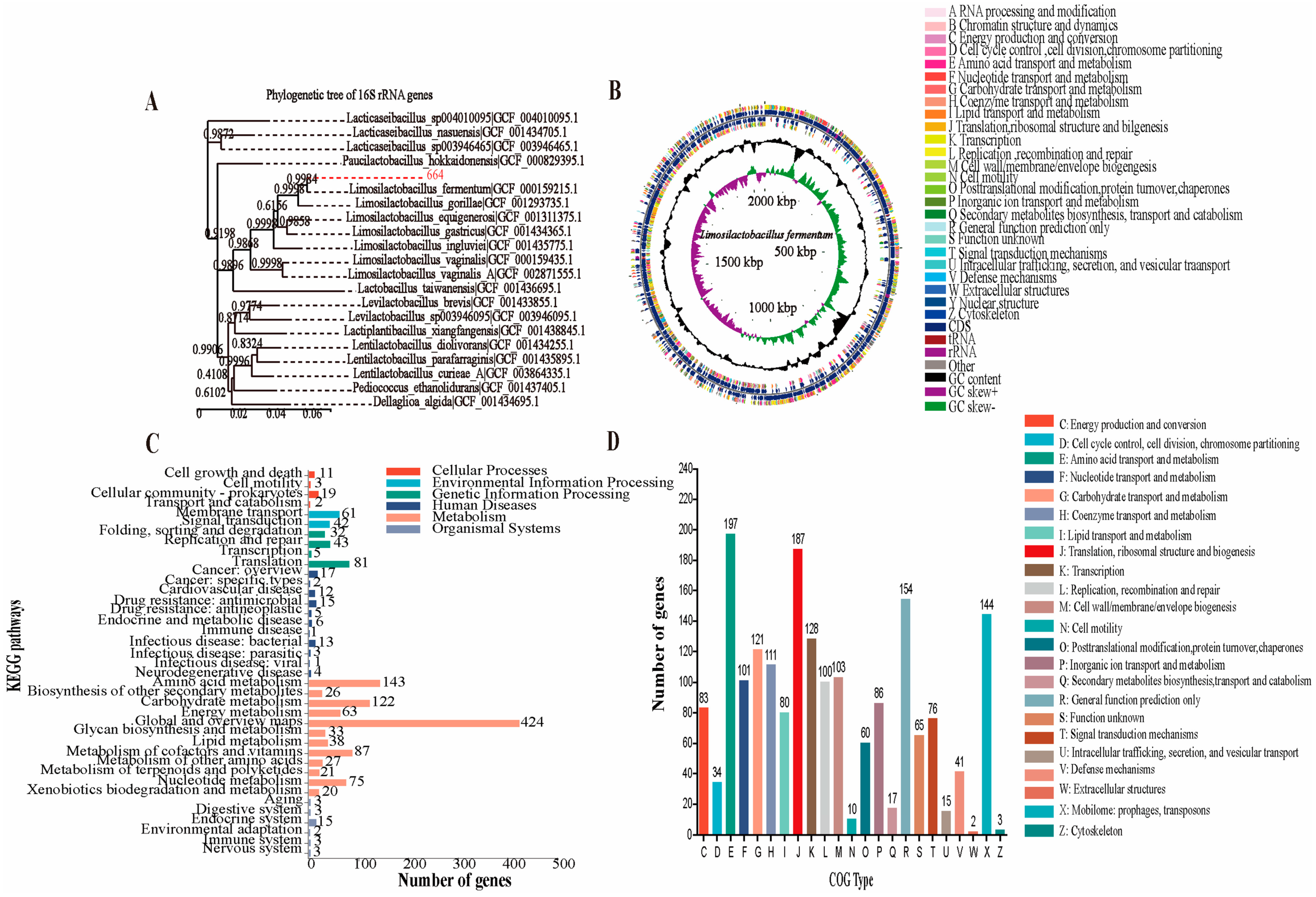
3.9. Analysis of the Genome Properties, Antibiotic Resistance and Reducing Oxidative Stress Potential of L. fermentum 664
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
References
- Zhou, Y.; Gong, W.; Xu, C.; Zhu, Z.; Peng, Y.; Xie, C. Probiotic assessment and antioxidant characterization of Lactobacillus plantarum GXL94 isolated from fermented chili. Front. Microbiol. 2022, 13, 997940. [Google Scholar] [CrossRef] [PubMed]
- Anjum, N.; Maqsood, S.; Masud, T.; Ahmad, A.; Sohail, A.; Momin, A. Lactobacillus acidophilus: Characterization of the species and application in food production. Crit. Rev. Food Sci. Nutr. 2014, 54, 1241–1251. [Google Scholar] [CrossRef]
- Chae, S.A.; Ramakrishnan, S.R.; Kim, T.; Kim, S.-R.; Bang, W.Y.; Jeong, C.-R.; Yang, J.; Kim, S.-J. Anti-inflammatory and anti-pathogenic potential of Lacticaseibacillus rhamnosus IDCC 3201 isolated from feces of breast-fed infants. Microb. Pathog. 2022, 173, 105857. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yang, J.; Zhang, C.; Chi, H.; Zhang, C.; Zhang, J.; Li, T.; Liu, L.; Li, A. Effects of Lactobacillus fermentum HY01 on the quality characteristics and storage stability of yak yogurt. J. Dairy Sci. 2022, 105, 2025–2037. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, H.C.; de Sousa Melo, D.; Ramos, C.L.; Dias, D.R.; Schwan, R.F. Probiotic properties of lactobacilli and their ability to inhibit the adhesion of enteropathogenic bacteria to Caco-2 and HT-29 cells. Probiotics Antimicrob. Proteins 2021, 13, 102–112. [Google Scholar] [CrossRef] [PubMed]
- Azagra-Boronat, I.; Massot-Cladera, M.; Knipping, K.; Garssen, J.; Ben Amor, K.; Knol, J.; Franch, À.; Castell, M.; Rodríguez-Lagunas, M.J.; Pérez-Cano, F.J. Strain-specific probiotic properties of bifidobacteria and lactobacilli for the prevention of diarrhea caused by rotavirus in a preclinical model. Nutrients 2020, 12, 498. [Google Scholar] [CrossRef]
- Kostelac, D.; Gerić, M.; Gajski, G.; Frece, J. Probiotic bacteria isolated from fermented meat displays high antioxidant and anti-inflammatory potential. Mutagenesis 2023, 38, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Kumari, M.; Haranahalli Nataraj, B.; Prasad, W.G.; Ali, S.A.; Behare, P.V. Multi-Faceted bioactivity assessment of an exopolysaccharide from Limosilactobacillus fermentum NCDC400: Antioxidant, antibacterial, and immunomodulatory proficiencies. Foods 2023, 12, 3595. [Google Scholar] [CrossRef] [PubMed]
- Shin, M.J.; Lee, C.S.; Kim, S.H. Screening for lactic acid bacterial strains as probiotics exhibiting anti-inflammatory and antioxidative characteristic via immune modulation in HaCaT cell. Probiotics Antimicrob. Proteins 2023, 15, 1665–1680. [Google Scholar] [CrossRef]
- Xu, X.; Wu, B.; Zhao, W.; Lao, F.; Chen, F.; Liao, X.; Wu, J. Shifts in autochthonous microbial diversity and volatile metabolites during the fermentation of chili pepper (Capsicum frutescens L.). Food Chem. 2021, 335, 127512. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.; Tan, F.; Mu, J.; Yi, R.; Zhou, X.; Zhao, X. Anti-obesity effects of Lactobacillus fermentum CQPC05 isolated from Sichuan pickle in high-fat diet-induced obese mice through PPAR-a signaling pathway. Microorganisms 2019, 7, 194. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Huang, S.-Y.; Deng, Q.; Li, G.; Su, G.; Liu, J.; Wang, H.-M.D. Extraction and characterization of phenolic compounds with antioxidant and antimicrobial activities from pickled radish. Food Chem. Toxicol. 2020, 136, 111050. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Tang, H.; Mei, Y.; Chen, J.; Wang, X.; Liu, B.; Cai, Y.; Zhao, N.; Yang, M.; Li, H. Effects of endogenous capsaicin stress and fermentation time on the microbial succession and flavor compounds of chili paste (a Chinese fermented chili pepper). Food Res. Int. 2023, 168, 112763. [Google Scholar] [CrossRef] [PubMed]
- Shahbazi, R.; Sharifzad, F.; Bagheri, R.; Alsadi, N.; Yasavoli-Sharahi, H.; Matar, C. Anti-inflammatory and immunomodulatory properties of fermented plant foods. Nutrients 2021, 13, 1516. [Google Scholar] [CrossRef] [PubMed]
- Dick, S.A.; Epelman, S. Chronic heart failure and inflammation: What do we really know? Circ. Res. 2016, 119, 159–176. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xiong, Z.; Li, C.; Liu, D.; Li, X.; Xu, J.; Chen, N.; Wang, X.; Li, Q.; Li, Y. Multiple beneficial effects of aloesone from Aloe vera on LPS-Induced RAW264. 7 Cells, Including the Inhibition of Oxidative Stress, Inflammation, M1 Polarization, and Apoptosis. Molecules 2023, 28, 1617. [Google Scholar] [CrossRef] [PubMed]
- Nishida, K.; Otsu, K. Inflammation and metabolic cardiomyopathy. Cardiovasc. Res. 2017, 113, 389–398. [Google Scholar] [CrossRef]
- Seo, K.; Yang, J.H.; Kim, S.C.; Ku, S.K.; Ki, S.H.; Shin, S.M. The antioxidant effects of isorhamnetin contribute to inhibit COX-2 expression in response to inflammation: A potential role of HO-1. Inflammation 2014, 37, 712–722. [Google Scholar] [CrossRef]
- Falcicchia, C.; Tozzi, F.; Arancio, O.; Watterson, D.M.; Origlia, N. Involvement of p38 MAPK in synaptic function and dysfunction. Int. J. Mol. Sci. 2020, 21, 5624. [Google Scholar] [CrossRef]
- Marafini, I.; Sedda, S.; Dinallo, V.; Monteleone, G. Inflammatory cytokines: From discoveries to therapies in IBD. Expert Opin Biol. Ther. 2019, 19, 1207–1217. [Google Scholar] [CrossRef]
- Panelli, S.; Epis, S.; Cococcioni, L.; Perini, M.; Paroni, M.; Bandi, C.; Drago, L.; Zuccotti, G.V. Inflammatory bowel diseases, the hygiene hypothesis and the other side of the microbiota: Parasites and fungi. Pharmacol. Res. 2020, 159, 104962. [Google Scholar] [CrossRef] [PubMed]
- Caruso, R.; Lo, B.C.; Núñez, G. Host–microbiota interactions in inflammatory bowel disease. Nat. Rev. Immunol. 2020, 20, 411–426. [Google Scholar] [CrossRef] [PubMed]
- Huligere, S.S.; Chandana Kumari, V.; Alqadi, T.; Kumar, S.; Cull, C.A.; Amachawadi, R.G.; Ramu, R. Isolation and characterization of lactic acid bacteria with potential probiotic activity and further investigation of their activity by a-amylase and a-glucosidase inhibitions of fermented batters. Front. Microbiol. 2023, 13, 1042263. [Google Scholar] [CrossRef] [PubMed]
- Hu, P.-L.; Yuan, Y.-H.; Yue, T.-L.; Guo, C.-F. A new method for the in vitro determination of the bile tolerance of potentially probiotic lactobacilli. Appl. Microbiol. Biotechnol. 2018, 102, 1903–1910. [Google Scholar] [CrossRef]
- Darmastuti, A.; Hasan, P.N.; Wikandari, R.; Utami, T.; Rahayu, E.S.; Suroto, D.A. Adhesion properties of Lactobacillus plantarum Dad-13 and Lactobacillus plantarum Mut-7 on Sprague Dawley rat intestine. Microorganisms 2021, 9, 2336. [Google Scholar] [CrossRef] [PubMed]
- Collado, M.C.; Meriluoto, J.; Salminen, S. Adhesion and aggregation properties of probiotic and pathogen strains. Eur. Food Res. Technol. 2008, 226, 1065–1073. [Google Scholar] [CrossRef]
- Vasudha, M.; Prashantkumar, C.S.; Bellurkar, M.; Kaveeshwar, V.; Gayathri, D. Probiotic potential of b-galactosidase-producing lactic acid bacteria from fermented milk and their molecular characterization. Biomed. Rep. 2023, 18, 23. [Google Scholar] [CrossRef]
- Barzegar, H.; Alizadeh Behbahani, B.; Falah, F. Safety, probiotic properties, antimicrobial activity, and technological performance of Lactobacillus strains isolated from Iranian raw milk cheeses. Food Sci. Nutr. 2021, 9, 4094–4107. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zou, L.; Li, T.; Lai, D.; Wu, Y.; Qin, S. Mogroside V inhibits LPS-induced COX-2 expression/ROS production and overexpression of HO-1 by blocking phosphorylation of AKT1 in RAW264. 7 cells. Acta Biochim. Biophys. Sin. 2019, 51, 365–374. [Google Scholar] [CrossRef]
- Cui, J.; Jia, J. Natural COX-2 inhibitors as promising anti-inflammatory agents: An update. Curr. Med. Chem. 2021, 28, 3622–3646. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Xu, W.; Xu, X.; Lu, W.; Zhao, J.; Zhang, H.; Chen, W. Effects of Limosilactobacillus fermentum CCFM1139 on experimental periodontitis in rats. Food Funct. 2021, 12, 4670–4678. [Google Scholar] [CrossRef]
- Cristofori, F.; Dargenio, V.N.; Dargenio, C.; Miniello, V.L.; Barone, M.; Francavilla, R. Anti-inflammatory and immunomodulatory effects of probiotics in gut inflammation: A door to the body. Front. Immunol. 2021, 12, 578386. [Google Scholar] [CrossRef]
- Rupa, P.; Mine, Y. Recent advances in the role of probiotics in human inflammation and gut health. J. Agric. Food Chem. 2012, 60, 8249–8256. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Lai, S.; Zhou, Z.; Yang, J.; Liu, H.; Zhong, Z.; Fu, H.; Ren, Z.; Shen, L.; Cao, S. Screening and evaluation of lactic acid bacteria with probiotic potential from local Holstein raw milk. Front. Microbiol. 2022, 13, 918774. [Google Scholar] [CrossRef]
- Ayyash, M.M.; Abdalla, A.K.; AlKalbani, N.S.; Baig, M.A.; Turner, M.S.; Liu, S.-Q.; Shah, N.P. Invited review: Characterization of new probiotics from dairy and nondairy products—Insights into acid tolerance, bile metabolism and tolerance, and adhesion capability. J. Dairy Sci. 2021, 104, 8363–8379. [Google Scholar] [CrossRef]
- Han, S.; Lu, Y.; Xie, J.; Fei, Y.; Zheng, G.; Wang, Z.; Liu, J.; Lv, L.; Ling, Z.; Berglund, B. Probiotic gastrointestinal transit and colonization after oral administration: A long journey. Front. Cell. Infect. Microbiol. 2021, 11, 609722. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Pan, L.-L.; Sun, J. Novel probiotic lactic acid bacteria were identified from healthy infant feces and exhibited anti-inflammatory capacities. Antioxidants 2022, 11, 1246. [Google Scholar] [CrossRef]
- Chouraddi, R.; Kumar, S.; Kumar, B.; Bhatia, M.; Varada, V.V.; Tyagi, N.; Mallapa, R.H. Techno-functional characterization of fecal lactobacilli isolates of Bos indicus calves for probiotic properties. Vet Res. Commun. 2023, 47, 1285–1302. [Google Scholar] [CrossRef]
- Shokryazdan, P.; Sieo, C.C.; Kalavathy, R.; Liang, J.B.; Alitheen, N.B.; Faseleh Jahromi, M.; Ho, Y.W. Probiotic potential of Lactobacillus strains with antimicrobial activity against some human pathogenic strains. Biomed. Res. Int. 2014, 2014, 927268. [Google Scholar] [CrossRef]
- Tulumoğlu, Ş.; Kaya, H.İ.; Şimşek, Ö. Probiotic characteristics of Lactobacillus fermentum strains isolated from tulum cheese. Anaerobe 2014, 30, 120–125. [Google Scholar] [PubMed]
- Šušković, J.; Kos, B.; Beganović, J.; Leboš Pavunc, A.; Habjanič, K.; Matošić, S. Antimicrobial activity–the most important property of probiotic and starter lactic acid bacteria. Food Technol. Biotechnol. 2010, 48, 296–307. [Google Scholar]
- Fang, Y.; Yang, L.; He, J. Plantanone C attenuates LPS-stimulated inflammation by inhibiting NF-kB/iNOS/COX-2/MAPKs/Akt pathways in RAW 264.7 macrophages. Biomed. Pharmacother. 2021, 143, 112104. [Google Scholar] [CrossRef]
- Oh, N.S.; Joung, J.Y.; Lee, J.Y.; Kim, Y. Probiotic and anti-inflammatory potential of Lactobacillus rhamnosus 4B15 and Lactobacillus gasseri 4M13 isolated from infant feces. PLoS ONE 2018, 13, e0192021. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.U. An overview of inflammation: Mechanism and consequences. Front. Biol. 2011, 6, 274–281. [Google Scholar] [CrossRef]
- Kim, H.; Lee, Y.-S.; Yu, H.-Y.; Kwon, M.; Kim, K.-K.; In, G.; Hong, S.-K.; Kim, S.-K. Anti-inflammatory effects of Limosilactobacillus fermentum KGC1601 isolated from panax ginseng and its probiotic characteristics. Foods 2022, 11, 1707. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.-C. NF-kB signaling in inflammation. Signal Transduct. Target Ther. 2017, 2, 1–9. [Google Scholar]
- Morgan, M.J.; Liu, Z.-G. Crosstalk of reactive oxygen species and NF-kB signaling. Cell Res. 2011, 21, 103–115. [Google Scholar] [CrossRef]
- Capece, D.; Verzella, D.; Flati, I.; Arboretto, P.; Cornice, J.; Franzoso, G. NF-kB: Blending metabolism, immunity, and inflammation. Trends Immunol. 2022, 43, 757–775. [Google Scholar] [CrossRef]
- Ambati, G.G.; Jachak, S.M. Natural product inhibitors of cyclooxygenase (COX) enzyme: A review on current status and future perspectives. Curr. Med. Chem. 2021, 28, 1877–1905. [Google Scholar] [CrossRef]
- Lim, S.-M.; Jang, H.; Jang, S.-E.; Han, M.; Kim, D.-H. Lactobacillus fermentum IM12 attenuates inflammation in mice by inhibiting NF-kB-STAT3 signalling pathway. Benef. Microbes 2017, 8, 407–419. [Google Scholar] [CrossRef]
- Lee, J.-M.; Hwang, K.-T.; Jun, W.-J.; Park, C.-S.; Lee, M.-Y. Anti-inflammatory Effect of Lactic Acid Bacteria: Inhibition of Cyclooxygenase-2 by Suppressing Nuclear Factor-kB in RAW264. 7 Macrophage Cells. J. Microbiol. Biotechnol. 2008, 18, 1683–1688. [Google Scholar]
- Kaminska, B. MAPK signalling pathways as molecular targets for anti-inflammatory therapy—From molecular mechanisms to therapeutic benefits. Biochim. Biophys. Acta 2005, 1754, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Johnson, G.L.; Lapadat, R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science 2002, 298, 1911–1912. [Google Scholar] [CrossRef] [PubMed]
- Khanna, S.; Bishnoi, M.; Kondepudi, K.K.; Shukla, G. Isolation, characterization and anti-inflammatory mechanism of probiotics in lipopolysaccharide-stimulated RAW 264.7 macrophages. World J. Microbiol. Biotechnol. 2020, 36, 74. [Google Scholar] [CrossRef] [PubMed]
- Son, Y.; Cheong, Y.-K.; Kim, N.-H.; Chung, H.-T.; Kang, D.G.; Pae, H.-O. Mitogen-activated protein kinases and reactive oxygen species: How can ROS activate MAPK pathways? J. Signal Transduct. 2011, 2011, 792639. [Google Scholar] [CrossRef] [PubMed]
- Chelombitko, M. Role of reactive oxygen species in inflammation: A minireview. Moscow Univ. Biol. Sci. Bull. 2018, 73, 199–202. [Google Scholar] [CrossRef]
- Forrester, S.J.; Kikuchi, D.S.; Hernandes, M.S.; Xu, Q.; Griendling, K.K. Reactive oxygen species in metabolic and inflammatory signaling. Circ. Res. 2018, 122, 877–902. [Google Scholar] [CrossRef]
- Rizwan, S.; ReddySekhar, P.; MalikAsrar, B. Reactive oxygen species in inflammation and tissue injury. Antioxid. Redox. Signal 2014, 20, 1126–1167. [Google Scholar]
- Kaufmann, K.B.; Gothwal, M.; Schallner, N.; Ulbrich, F.; Rücker, H.; Amslinger, S.; Goebel, U. The anti-inflammatory effects of E-a-(p-methoxyphenyl)-2’, 3, 4, 4’-tetramethoxychalcone are mediated via HO-1 induction. Int. Immunopharmacol. 2016, 35, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Campbell, N.K.; Fitzgerald, H.K.; Dunne, A. Regulation of inflammation by the antioxidant haem oxygenase 1. Nat. Rev. Immunol. 2021, 21, 411–425. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.; Yan, X.; Xiao, J.; Chen, Y.; Chen, M.; Jin, J.; Bai, Y.; Wang, Q.; Liao, Z.; Chen, Q. Isolation, identification, and whole genome sequence analysis of the alginate-degrading bacterium Cobetia sp. cqz5-12. Sci. Rep. 2020, 10, 10920. [Google Scholar] [CrossRef]
- Peng, X.; Ed-Dra, A.; Yue, M. Whole genome sequencing for the risk assessment of probiotic lactic acid bacteria. Crit. Rev. Food Sci. Nutr. 2023, 63, 11244–11262. [Google Scholar] [CrossRef] [PubMed]
- Jose, N.M.; Bunt, C.R.; Hussain, M.A. Implications of antibiotic resistance in probiotics. Food Rev. Int. 2015, 31, 52–62. [Google Scholar] [CrossRef]
- Kanehisa, M.; Furumichi, M.; Tanabe, M.; Sato, Y.; Morishima, K. KEGG: New perspectives on genomes, pathways, diseases and drugs. Nucleic Acids Res. 2017, 45, D353–D361. [Google Scholar] [CrossRef] [PubMed]
- Borisov, V.; Nastasi, M.R.; Forte, E. Cytochrome bd as Antioxidant Redox Enzyme. J. Mol. Biol. 2023, 57, 1077–1084. [Google Scholar] [CrossRef]
- Jung, K.-A.; Kwak, M.-K. The Nrf2 system as a potential target for the development of indirect antioxidants. Molecules 2010, 15, 7266–7291. [Google Scholar] [CrossRef] [PubMed]
- Galperin, M.Y.; Kristensen, D.M.; Makarova, K.S.; Wolf, Y.I.; Koonin, E.V. Microbial genome analysis: The COG approach. Briefings Bioinf. 2019, 20, 1063–1070. [Google Scholar] [CrossRef] [PubMed]
- Chon, H.; Choi, B.; Jeong, G.; Lee, E.; Lee, S. Suppression of proinflammatory cytokine production by specific metabolites of Lactobacillus plantarum 10hk2 via inhibiting NF-kB and p38 MAPK expressions. Comp. Immunol. Microbiol. Infect. Dis. 2010, 33, e41–e49. [Google Scholar] [CrossRef] [PubMed]
- Mechoud, M.A.; Mateos, M.V.; de Valdez, G.F.; Villena, J.; Salvador, G.A.; Rodriguez, A.V. Lactobacillus reuteri CRL1098 soluble factors modulate tumor necrosis factor alpha production in peripheral blood mononuclear cells: Involvement of lipid rafts. Int. Immunopharmacol. 2012, 14, 446–453. [Google Scholar] [CrossRef] [PubMed]




| Characteristics | Rate (%) * |
|---|---|
| Acid resistance | 107 ± 5 |
| Bile salt tolerance | 109 ± 4 |
| Hydrophobicity | 54.21 ± 5 |
| Adhesion | 89.53 ± 6.65 |
| Pathogen | Inhibitory Zone Diameter (mm) * |
|---|---|
| S. aureus | 20.70 ± 0.29 a |
| E. coli | 19.36 ± 0.07 c |
| S. enterica | 20.27 ± 0.01 b |
| Genomic Characteristics | Explanation |
|---|---|
| Identification | Limosilactobacillus fermentum |
| Genome size (bp) | 2,060,326 |
| GC contents (%) | 51.72 |
| CDS | 2006 |
| CDS/total genes (%) | 85.7 |
| No. of rRNA genes | 15 |
| No. of tRNA genes | 58 |
| Gene ID | KO ID | KO Name | KO Description |
|---|---|---|---|
| gene0309 | K00425 | cydA | cytochrome bd ubiquinol oxidase subunit I |
| gene0310 | K00426 | cydB | cytochrome bd ubiquinol oxidase subunit II |
| gene0759 | K00355 | NQO1 | NAD(P)H dehydrogenase quinone 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hao, H.; Nie, Z.; Wu, Y.; Liu, Z.; Luo, F.; Deng, F.; Zhao, L. Probiotic Characteristics and Anti-Inflammatory Effects of Limosilactobacillus fermentum 664 Isolated from Chinese Fermented Pickles. Antioxidants 2024, 13, 703. https://doi.org/10.3390/antiox13060703
Hao H, Nie Z, Wu Y, Liu Z, Luo F, Deng F, Zhao L. Probiotic Characteristics and Anti-Inflammatory Effects of Limosilactobacillus fermentum 664 Isolated from Chinese Fermented Pickles. Antioxidants. 2024; 13(6):703. https://doi.org/10.3390/antiox13060703
Chicago/Turabian StyleHao, Huichao, Ziyu Nie, Yanyang Wu, Zhiwei Liu, Fenglian Luo, Fangming Deng, and Lingyan Zhao. 2024. "Probiotic Characteristics and Anti-Inflammatory Effects of Limosilactobacillus fermentum 664 Isolated from Chinese Fermented Pickles" Antioxidants 13, no. 6: 703. https://doi.org/10.3390/antiox13060703






