Dietary Protease Supplementation Improved Growth Performance and Nutrients Digestion via Modulating Intestine Barrier, Immunological Response, and Microbiota Composition in Weaned Piglets
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design and Processing
2.2. Sample Collection
2.3. Chemical Analyses
2.4. Intestinal Morphology
2.5. Blood Profile Measurements
2.6. Antioxidant Properties
2.7. Western Blotting Analysis
2.8. Real-Time Quantitative PCR
2.9. 16S rRNA Sequencing
2.10. Statistical Analysis
3. Results
3.1. Dietary Protease Improved Growth Performance of Weaned Piglets
3.2. Dietary Protease Increased the Nutrient Digestion in Weaned Piglets
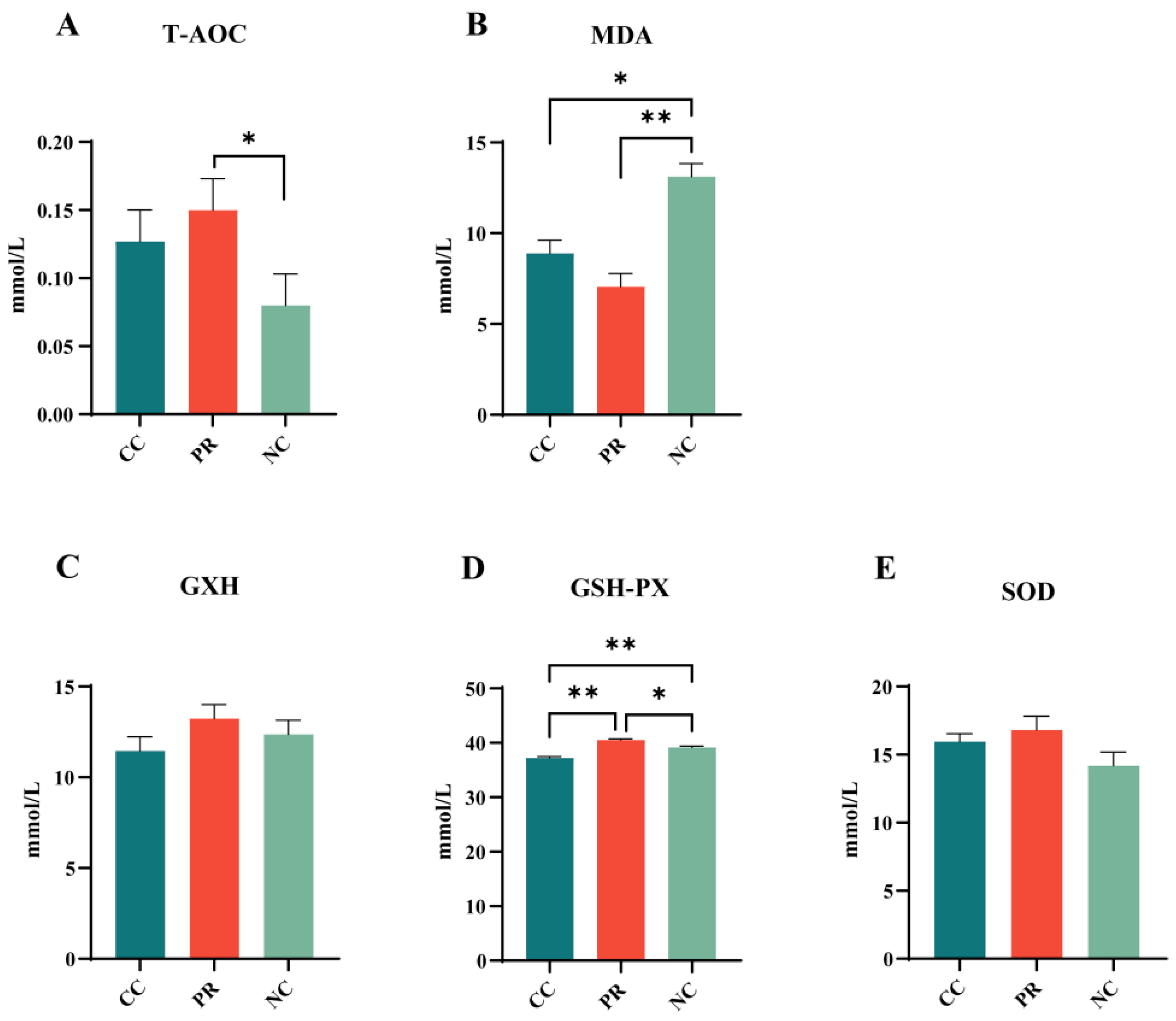
3.3. Dietary Protease Improved Plasma Antioxidant Levels in Weaned Piglets
3.4. Dietary Protease Reduced Blood Urea Nitrogen and Plasma Inflammation Indicators in Weaned Piglets
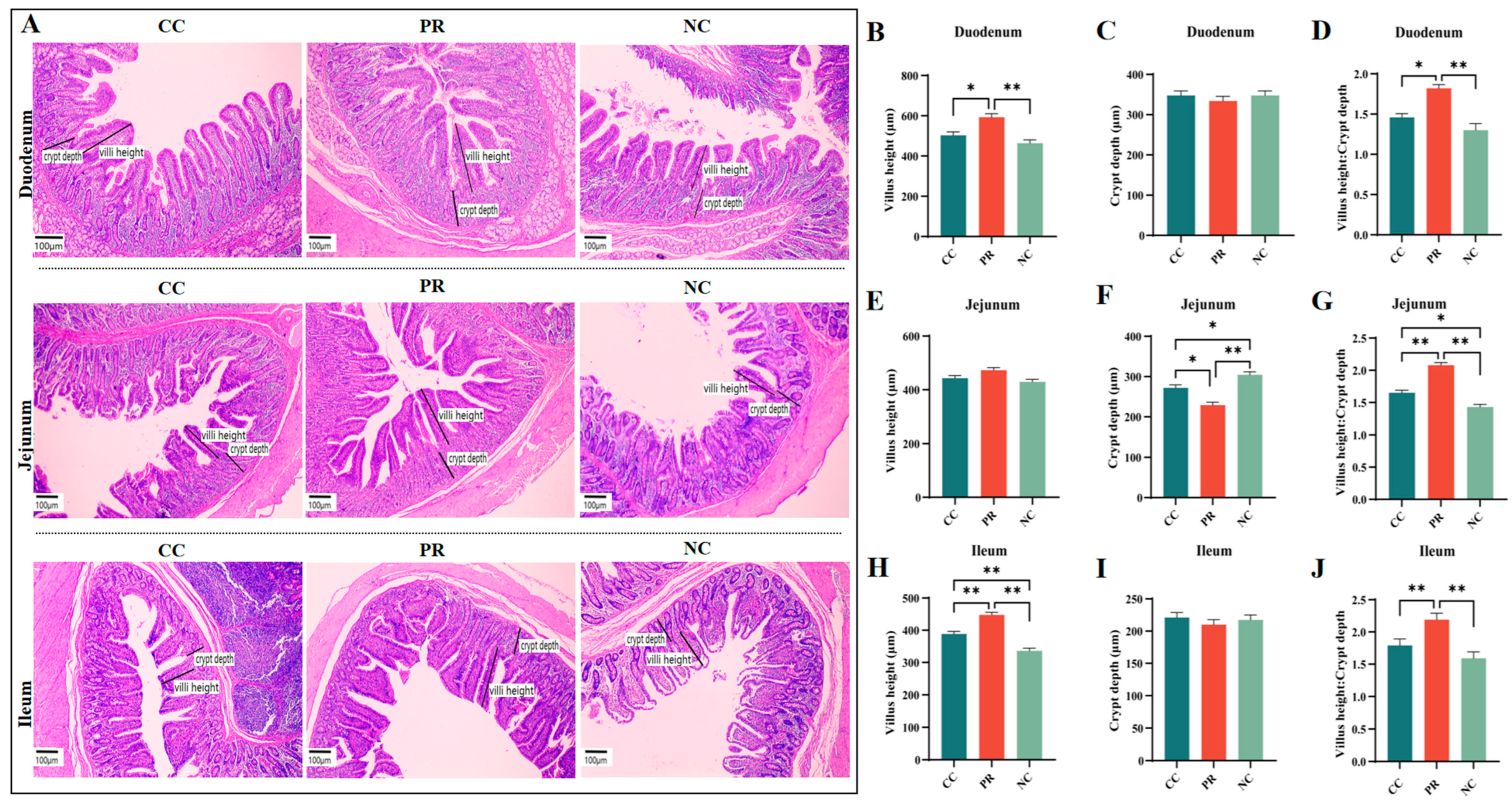
3.5. Dietary Protease Improved Intestinal Morphology of Weaned Piglets
3.6. Dietary Protease Improved Intestinal Function and Barrier Integrity in Weaned Piglets
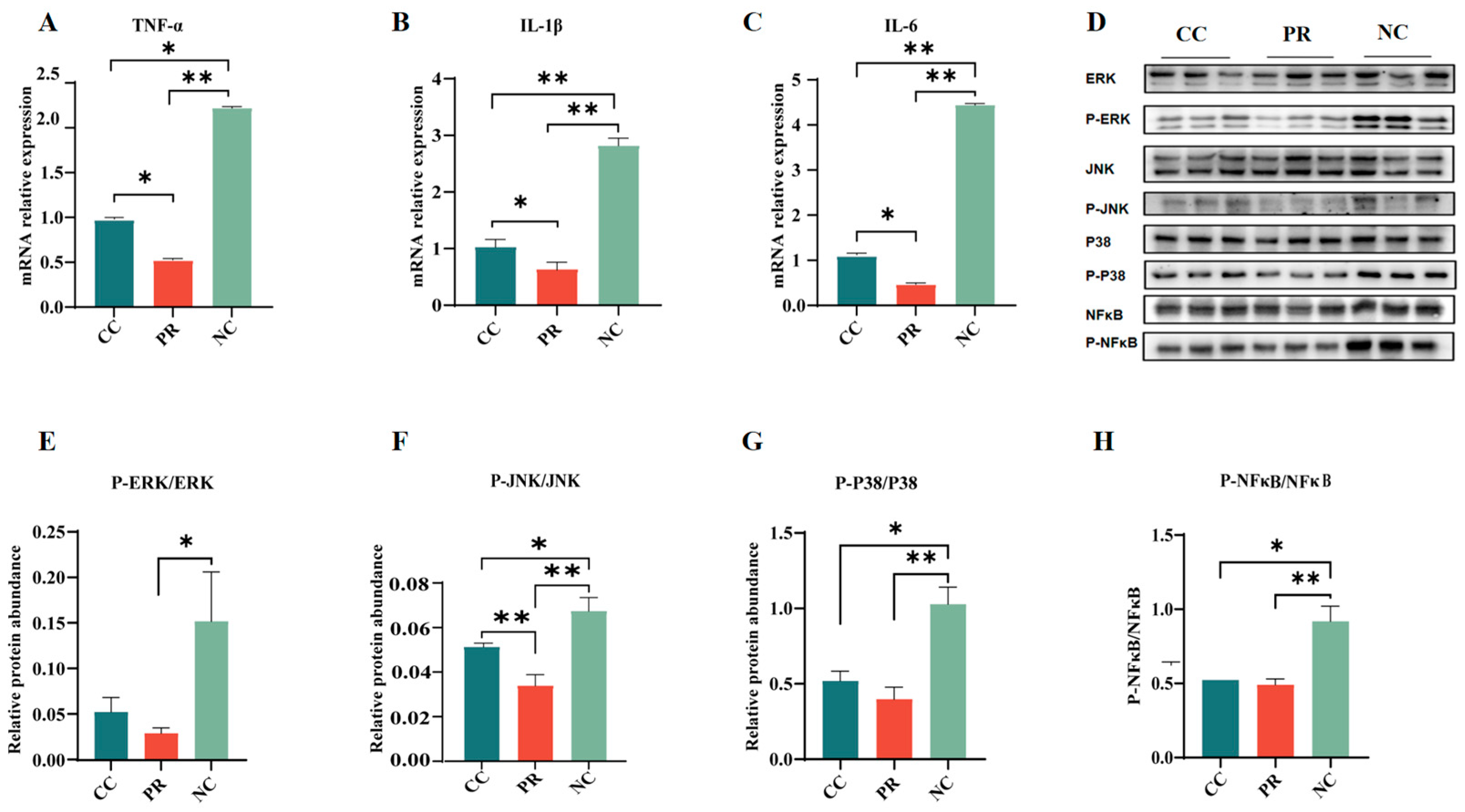
3.7. Dietary Protease Reduced Inflammation Response via Inhibiting the MAPK Signaling Pathway in Piglet Jejunum
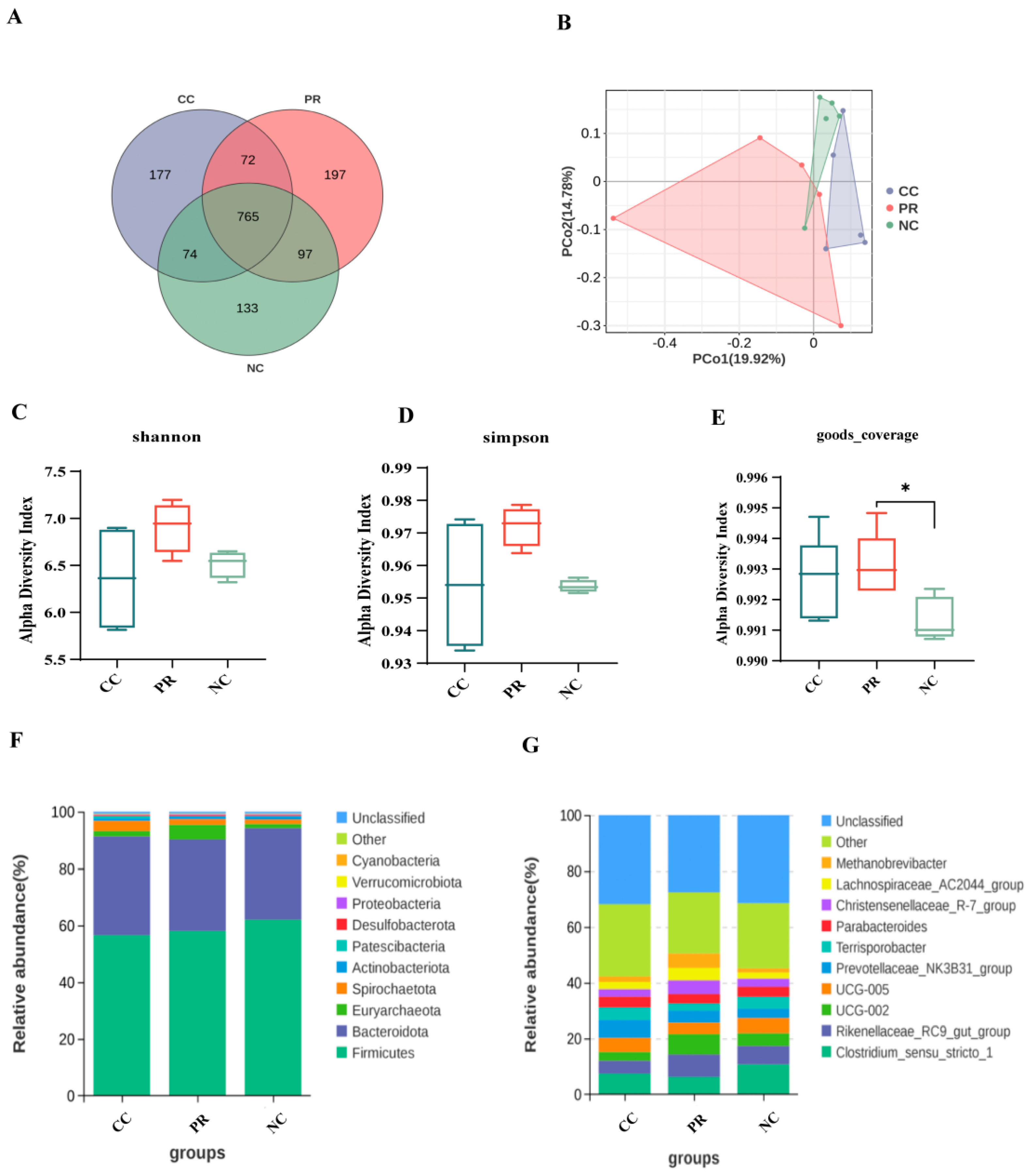
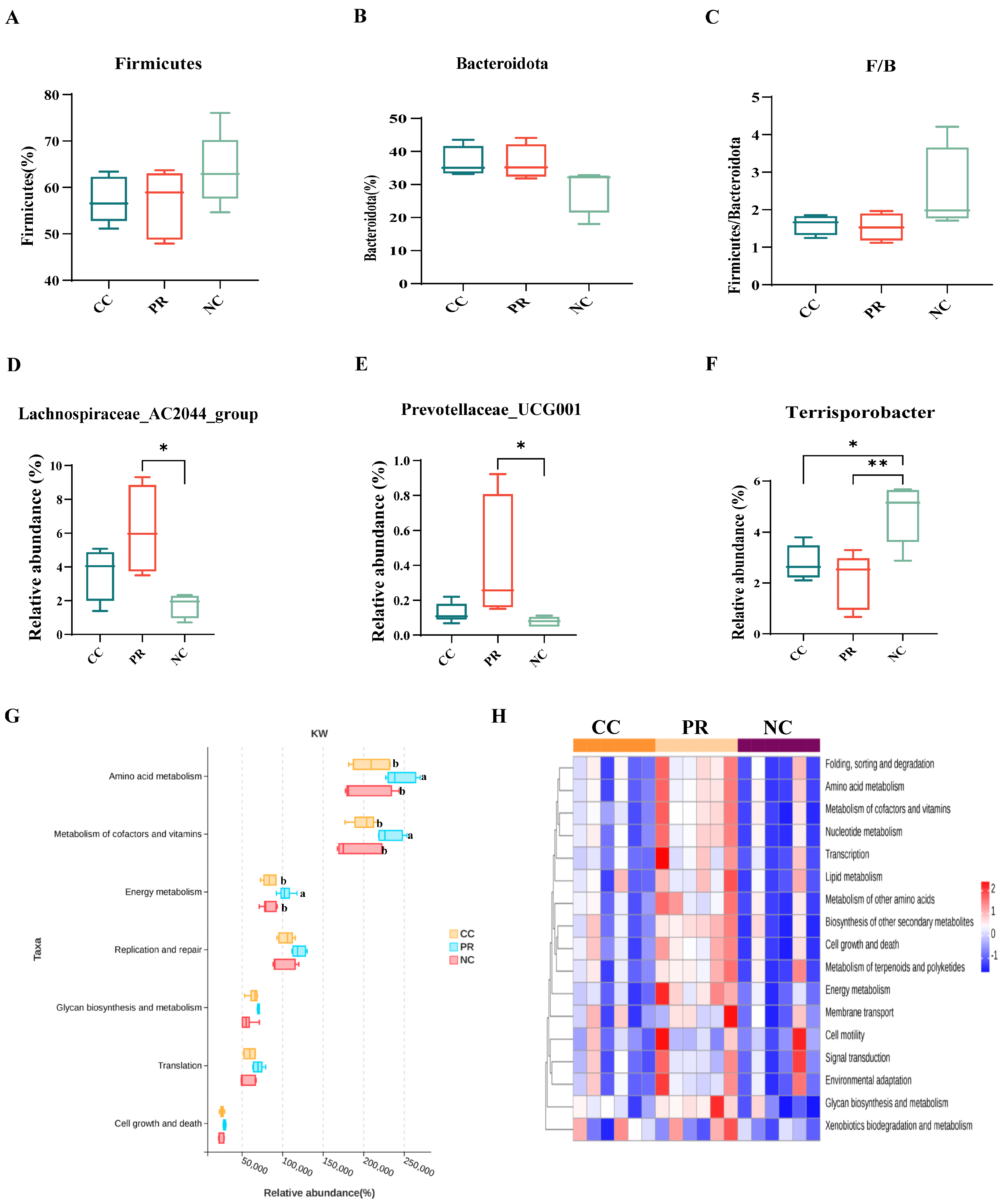
3.8. Dietary Protease Improved Intestinal Microbiota Composition of Weaned Piglets
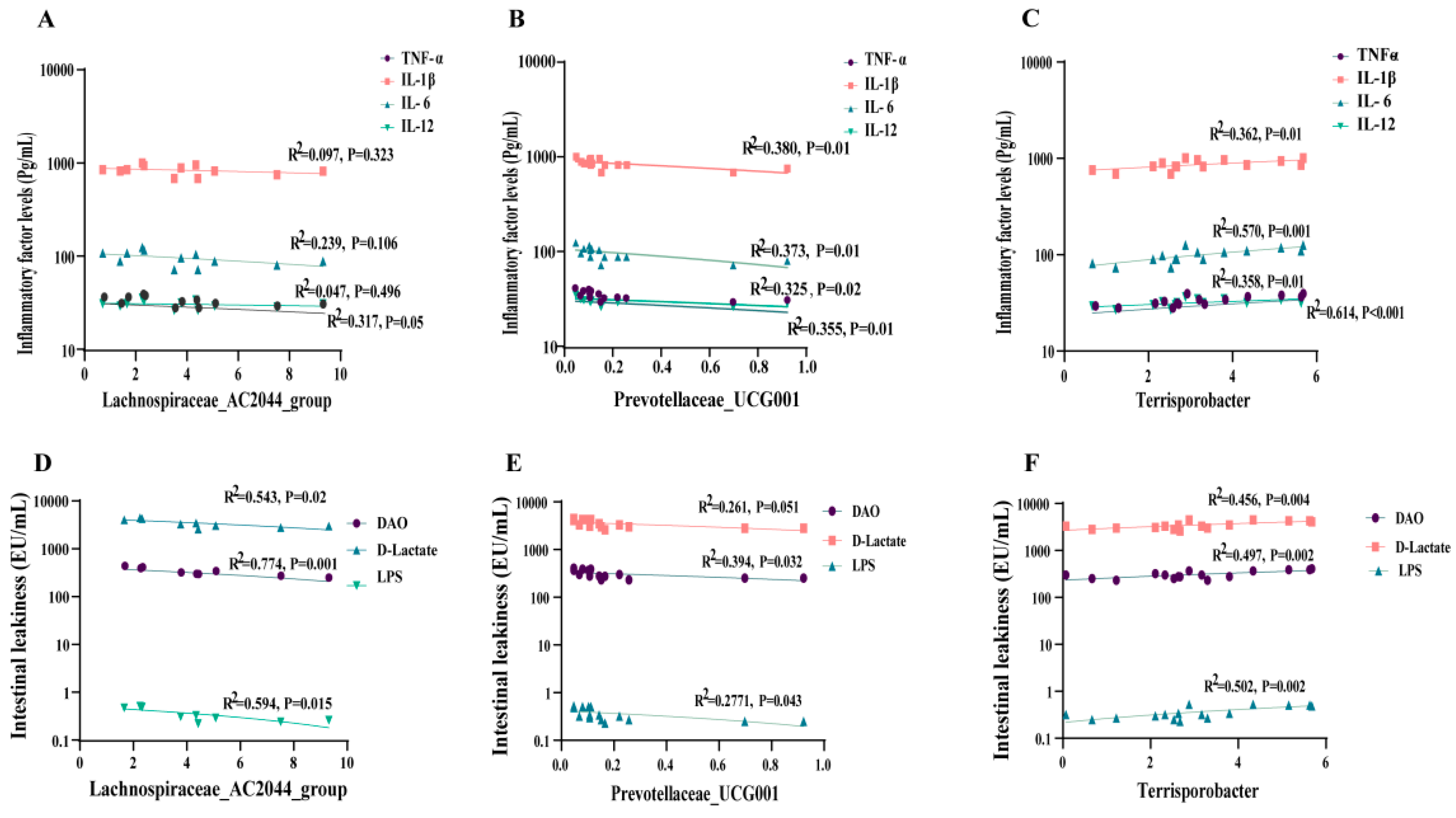
3.9. Correlation between Intestinal Microbiota, Plasma Inflammation, Intestinal Permeability, and Growth Performance of Weaned Piglets
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Tactacan, G.B.; Cho, S.Y.; Cho, J.H.; Kim, I.H. Performance Responses, Nutrient Digestibility, Blood Characteristics, and Measures of Gastrointestinal Health in Weanling Pigs Fed Protease Enzyme. Asian-Australas. J. Anim. Sci. 2016, 29, 998–1003. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Lee, K.Y.; Kim, I.H. Effects of dietary protected organic acids on growth performance, nutrient digestibility, fecal microflora, diarrhea score, and fecal gas emission in weanling pigs. Can. J. Anim. Sci. 2019, 99, 514–520. [Google Scholar] [CrossRef]
- Xiang, X.D.; Deng, Z.C.; Wang, Y.W.; Sun, H.; Wang, L.; Han, Y.M.; Wu, Y.Y.; Liu, J.G.; Sun, L.H. Organic Acids Improve Growth Performance with Potential Regulation of Redox Homeostasis, Immunity, and Microflora in Intestines of Weaned Piglets. Antioxidants 2021, 10, 1665. [Google Scholar] [CrossRef] [PubMed]
- Suiryanrayna, M.V.; Ramana, J.V. A review of the effects of dietary organic acids fed to swine. J. Anim. Sci. Biotechnol. 2015, 6, 45. [Google Scholar] [CrossRef]
- Rhouma, M.; Fairbrother, J.M.; Beaudry, F.; Letellier, A. Post weaning diarrhea in pigs: Risk factors and non-colistin-based control strategies. Acta Vet. Scand. 2017, 59, 31. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Hao, Y.; Piao, X.S.; Ma, X.; Wu, G.Y.; Qiao, S.Y.; Li, D.F.; Wang, J.J. Soybean-derived β-conglycinin affects proteome expression in pig intestinal cells in vivo and in vitro. J. Anim. Sci. 2011, 89, 743–753. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Lee, J.W.; Cowieson, A.J.; Pappenberger, G.; Woyengo, T.A. Soybean meal allergenic protein degradation and gut health of piglets fed protease-supplemented diets. J. Anim. Sci. 2020, 98, skaa308. [Google Scholar] [CrossRef] [PubMed]
- Zuo, J.; Ling, B.; Long, L.; Li, T.; Lahaye, L.; Yang, C.; Feng, D. Effect of dietary supplementation with protease on growth performance, nutrient digestibility, intestinal morphology, digestive enzymes and gene expression of weaned piglets. Anim. Nutr. 2015, 1, 276–282. [Google Scholar] [CrossRef]
- Wang, D.; Zeng, Z.K.; Piao, X.S.; Li, P.F.; Xue, L.F.; Zhang, Q.; Han, X.; Zhang, H.Y.; Dong, B.; Kim, S.W. Effects of keratinase supplementation of corn-soybean meal based diets on apparent ileal amino acid digestibility in growing pigs and serum amino acids, cytokines, immunoglobulin levels and loin muscle area in nursery pigs. Arch. Anim. Nutr. 2011, 65, 290–302. [Google Scholar] [CrossRef]
- Min, Y.; Choi, Y.; Kim, Y.; Jeong, Y.; Kim, D.; Kim, J.; Jung, H.; Song, M. Effects of protease supplementation on growth performance, blood constituents, and carcass characteristics of growing-finishing pigs. J. Anim. Sci. Technol. 2019, 61, 234–238. [Google Scholar] [CrossRef]
- Lemme, A.; Ravindran, V.; Bryden, W.L. Heal digestibility of amino acids in feed ingredients for broilers. Worlds Poult. Sci. J. 2004, 60, 423–437. [Google Scholar] [CrossRef]
- Choe, J.; Kim, K.S.; Kim, H.B.; Park, S.; Kim, J.; Kim, S.; Kim, B.; Cho, S.H.; Cho, J.Y.; Park, I.H.; et al. Effects of protease on growth performance and carcass characteristics of growingfinishing pigs. S. Afr. J. Anim. Sci. 2017, 47, 697–703. [Google Scholar] [CrossRef][Green Version]
- Schokker, D.; Zhang, J.; Zhang, L.L.; Vastenhouw, S.A.; Heilig, H.G.; Smidt, H.; Rebel, J.M.; Smits, M.A. Early-life environmental variation affects intestinal microbiota and immune development in new-born piglets. PLoS ONE 2014, 9, e100040. [Google Scholar] [CrossRef]
- Han, G.G.; Lee, J.-Y.; Jin, G.-D.; Park, J.; Choi, Y.H.; Chae, B.J.; Kim, E.B.; Choi, Y.-J. Evaluating the association between body weight and the intestinal microbiota of weaned piglets via 16S rRNA sequencing. Appl. Microbiol. Biotechnol. 2017, 101, 5903–5911. [Google Scholar] [CrossRef] [PubMed]
- Mulder, I.E.; Schmidt, B.; Stokes, C.R.; Lewis, M.; Bailey, M.; Aminov, R.I.; Prosser, J.I.; Gill, B.P.; Pluske, J.R.; Mayer, C.D.; et al. Environmentally-acquired bacteria influence microbial diversity and natural innate immune responses at gut surfaces. BMC Biol. 2009, 7, 79. [Google Scholar] [CrossRef] [PubMed]
- Ma, N.; Tian, Y.A.; Wu, Y.; Ma, X. Contributions of the Interaction Between Dietary Protein and Gut Microbiota to Intestinal Health. Curr. Protein Pept. Sc. 2017, 18, 795–808. [Google Scholar] [CrossRef] [PubMed]
- Holmes, A.J.; Chew, Y.V.; Colakoglu, F.; Cliff, J.B.; Klaassens, E.; Read, M.N.; Solon-Biet, S.M.; McMahon, A.C.; Cogger, V.C.; Ruohonen, K.; et al. Diet-Microbiome Interactions in Health Are Controlled by Intestinal Nitrogen Source Constraints. Cell Metab. 2017, 25, 140–151. [Google Scholar] [CrossRef] [PubMed]
- Farhadi, A.; Banan, A.; Fields, J.; Keshavarzian, A. Intestinal barrier: An interface between health and disease. J. Gastroenterol. Hepatol. 2003, 18, 479–497. [Google Scholar] [CrossRef] [PubMed]
- Landy, J.; Ronde, E.; English, N.; Clark, S.K.; Hart, A.L.; Knight, S.C.; Ciclitira, P.J.; Al-Hassi, H.O. Tight junctions in inflammatory bowel diseases and inflammatory bowel disease associated colorectal cancer. World J. Gastroenterol. 2016, 22, 3117–3126. [Google Scholar] [CrossRef]
- Awawdeh, M.S.; Obeidat, B.S. Effect of supplemental exogenous enzymes on performance of finishing Awassi lambs fed olive cake-containing diets. Livest. Sci. 2011, 138, 20–24. [Google Scholar] [CrossRef]
- Yi, J.Q.; Piao, X.S.; Li, Z.C.; Zhang, H.Y.; Chen, Y.; Li, Q.Y.; Liu, J.D.; Zhang, Q.; Ru, Y.J.; Dong, B. The Effects of Enzyme Complex on Performance, Intestinal Health and Nutrient Digestibility of Weaned Pigs. Asian Australas. J. Anim. 2013, 26, 1181–1188. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhang, G.G.; Yang, Z.B.; Wang, Y.; Yang, W.R.; Zhou, H.J. Effects of dietary supplementation of multi-enzyme on growth performance, nutrient digestibility, small intestinal digestive enzyme activities, and large intestinal selected microbiota in weanling pigs. J. Anim. Sci. 2014, 92, 2063–2069. [Google Scholar] [CrossRef]
- Lange, C. New NRC (2012) Nutrient Requirements of Swine; National Research Council: Washington, DC, USA, 2014. [Google Scholar]
- Wan, J.; Jiang, F.; Xu, Q.S.; Chen, D.W.; Yu, B.; Huang, Z.Q.; Mao, X.B.; Yu, J.; He, J. New insights into the role of chitosan oligosaccharide in enhancing growth performance, antioxidant capacity, immunity and intestinal development of weaned pigs. RSC Adv. 2017, 7, 9669–9679. [Google Scholar] [CrossRef]
- Moeser, A.J.; Pohl, C.S.; Rajput, M. Weaning stress and gastrointestinal barrier development: Implications for lifelong gut health in pigs. Anim. Nutr. 2017, 3, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Lallès, J.P.; Boudry, G.; Favier, C.; Le Floc’h, N.; Lurona, I.; Montagne, L.; Oswald, I.P.; Pié, S.; Piel, C.; Sève, B. Gut function and dysfunction in young pigs:: Physiology. Anim. Res. 2004, 53, 301–316. [Google Scholar] [CrossRef]
- Wang, Y.B.; Xie, Q.H.; Sun, S.; Huang, B.J.; Zhang, Y.; Xu, Y.; Zhang, S.M.; Xiang, H.Y. Probiotics-fermented Massa Medicata Fermentata ameliorates weaning stress in piglets related to improving intestinal homeostasis. Appl. Microbiol. Biotechnol. 2018, 102, 10713–10727. [Google Scholar] [CrossRef]
- Adeola, O.; Cowieson, A.J. BOARD-INVITED REVIEW: Opportunities and challenges in using exogenous enzymes to improve nonruminant animal production. J. Anim. Sci. 2011, 89, 3189–3218. [Google Scholar] [CrossRef]
- Cowieson, A.J.; Roos, F.F. Toward optimal value creation through the application of exogenous mono-component protease in the diets of non-ruminants. Anim. Feed. Sci. Technol. 2016, 221, 331–340. [Google Scholar] [CrossRef]
- O’Shea, C.J.; Mc Alpine, P.O.; Solan, P.; Curran, T.; Varley, P.F.; Walsh, A.M.; Doherty, J.V.O. The effect of protease and xylanase enzymes on growth performance, nutrient digestibility, and manure odour in grower-finisher pigs. Anim. Feed. Sci. Technol. 2014, 189, 88–97. [Google Scholar] [CrossRef]
- Nesci, A.; Carnuccio, C.; Ruggieri, V.; D’Alessandro, A.; Di Giorgio, A.; Santoro, L.; Gasbarrini, A.; Santoliquido, A.; Ponziani, F.R. Gut Microbiota and Cardiovascular Disease: Evidence on the Metabolic and Inflammatory Background of a Complex Relationship. Int. J. Mol. Sci. 2023, 24, 9087. [Google Scholar] [CrossRef]
- Wang, X.C.; Geng, F.F.; Wu, J.J.; Kou, Y.A.; Xu, S.L.; Sun, Z.K.; Feng, S.B.; Ma, L.Y.; Luo, Y. Effects of β-conglycinin on growth performance, immunoglobulins and intestinal mucosal morphology in piglets. Arch. Anim. Nutr. 2014, 68, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Friedman, M.; Brandon, D.L. Nutritional and health benefits of soy proteins. J. Agric. Food Chem. 2001, 49, 1069–1086. [Google Scholar] [CrossRef] [PubMed]
- Lauridsen, C. From oxidative stress to inflammation: Redox balance and immune system. Poult. Sci. 2019, 98, 4240–4246. [Google Scholar] [CrossRef] [PubMed]
- Li, S.-S.; Wu, C.-Z.; Zhang, B.-W.; Qiu, L.; Chen, W.; Yuan, Y.-H.; Liu, X.-C.; Li, C.-J.; Li, L.-J. Nerve growth factor protects salivary glands from irradiation-induced damage. Life Sci. 2021, 265, 118748. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Jacobson, K.; Schaller, M.D. MAP kinases and cell migration. J. Cell Sci. 2004, 117, 4619–4628. [Google Scholar] [CrossRef] [PubMed]
- Lodolce, J.P.; Kolodziej, L.; Chang, J.; Schneider, J.R.; Messer, J.S.; Turner, J.R.; Boone, D.L. Regulation of Intestinal Permeability and Epithelial Cell Tight Junctions by the Ubiquitin-Editing Enzyme TNFAIP3. Gastroenterology 2010, 138, S425. [Google Scholar] [CrossRef]
- Turner, J.R. Molecular basis of epithelial barrier regulation—From basic mechanisms to clinical application. Am. J. Pathol. 2006, 169, 1901–1909. [Google Scholar] [CrossRef] [PubMed]
- Ling, K.H.; Wan, M.L.; El-Nezami, H.; Wang, M. Protective Capacity of Resveratrol, a Natural Polyphenolic Compound, against Deoxynivalenol-Induced Intestinal Barrier Dysfunction and Bacterial Translocation. Chem. Res. Toxicol. 2016, 29, 823–833. [Google Scholar] [CrossRef] [PubMed]
- Sanchez de Medina, F.; Romero-Calvo, I.; Mascaraque, C.; Martinez-Augustin, O. Intestinal Inflammation and Mucosal Barrier Function. Inflamm. Bowel Dis. 2014, 20, 2394–2404. [Google Scholar] [CrossRef]
- Montagne, L.; Pluske, J.R.; Hampson, D.J. A review of interactions between dietary fibre and the intestinal mucosa, and their consequences on digestive health in young non-ruminant animals. Anim. Feed. Sci. Technol. 2003, 108, 95–117. [Google Scholar] [CrossRef]
- Liao, P.; Liao, M.; Li, L.; Tan, B.; Yin, Y. Effect of deoxynivalenol on apoptosis, barrier function, and expression levels of genes involved in nutrient transport, mitochondrial biogenesis and function in IPEC-J2 cells. Toxicol. Res. 2017, 6, 866–877. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Okyere, S.K.; Gao, P.; Wen, J.; Cao, S.; Wang, Y.; Deng, J.; Hu, Y. Ageratina adenophora Disrupts the Intestinal Structure and Immune Barrier Integrity in Rats. Toxins 2021, 13, 651. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, W.; Xin, S.; Wu, S.; Peng, C.; Ding, H.; Feng, S.; Zhao, C.; Wu, J.; Wang, X. Soybean glycinin and beta-conglycinin damage the intestinal barrier by triggering oxidative stress and inflammatory response in weaned piglets. Eur. J. Nutr. 2023, 62, 2841–2854. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Liu, D.; Han, R.; Zhang, X.; Zhang, S.; Qin, G. Soybean allergen glycinin induced the destruction of the mechanical barrier function in IPEC-J2. Food Agric. Immunol. 2015, 26, 601–609. [Google Scholar] [CrossRef]
- Duarte, M.E.; Zhou, F.X.; Dutra, W.M., Jr.; Kim, S.W. Dietary supplementation of xylanase and protease on growth performance, digesta viscosity, nutrient digestibility, immune and oxidative stress status, and gut health of newly weaned pigs. Anim. Nutr. 2019, 5, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Piao, X.S.; Zeng, Z.K.; Lu, T.; Zhang, Q.; Li, P.F.; Xue, L.F.; Kim, S.W. Effects of Keratinase on Performance, Nutrient Utilization, Intestinal Morphology, Intestinal Ecology and Inflammatory Response of Weaned Piglets Fed Diets with Different Levels of Crude Protein. Asian Australas. J. Anim. 2011, 24, 1718–1728. [Google Scholar] [CrossRef]
- Heo, J.M.; Opapeju, F.O.; Pluske, J.R.; Kim, J.C.; Hampson, D.J.; Nyachoti, C.M. Gastrointestinal health and function in weaned pigs: A review of feeding strategies to control post-weaning diarrhoea without using in-feed antimicrobial compounds. J. Anim. Physiol. Anim. Nutr. 2013, 97, 207–237. [Google Scholar] [CrossRef] [PubMed]
- Roselli, M.; Pieper, R.; Rogel-Gaillard, C.; de Vries, H.; Bailey, M.; Smidt, H.; Lauridsen, C. Immunomodulating effects of probiotics for microbiota modulation, gut health and disease resistance in pigs. Anim. Feed. Sci. Technol. 2017, 233, 104–119. [Google Scholar] [CrossRef]
- Nagpal, R.; Kumar, M.; Yadav, A.K.; Hemalatha, R.; Yadav, H.; Marotta, F.; Yamashiro, Y. Gut microbiota in health and disease: An overview focused on metabolic inflammation. Benef. Microbes 2016, 7, 181–194. [Google Scholar] [CrossRef]
- De Filippo, C.; Cavalieri, D.; Di Paola, M.; Ramazzotti, M.; Poullet, J.B.; Massart, S.; Collini, S.; Pieraccini, G.; Lionetti, P. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc. Natl. Acad. Sci. USA 2010, 107, 14691–14696. [Google Scholar] [CrossRef]
- Cronin, P.; Joyce, S.A.; O’Toole, P.W.; O’Connor, E.M. Dietary Fibre Modulates the Gut Microbiota. Nutrients 2021, 13, 1655. [Google Scholar] [CrossRef]
- Frese, S.A.; Parker, K.; Calvert, C.C.; Mills, D.A. Diet shapes the gut microbiome of pigs during nursing and weaning. Microbiome 2015, 3, 28. [Google Scholar] [CrossRef]
- Kim, H.B.; Isaacson, R.E. The pig gut microbial diversity: Understanding the pig gut microbial ecology through the next generation high throughput sequencing. Vet. Microbiol. 2015, 177, 242–251. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, R.; Ingerslev, H.-C.; Sturek, M.; Alloosh, M.; Cirera, S.; Christoffersen, B.O.; Moesgaard, S.G.; Larsen, N.; Boye, M. Characterisation of Gut Microbiota in Ossabaw and Gottingen Minipigs as Models of Obesity and Metabolic Syndrome. PLoS ONE 2013, 8, e56612. [Google Scholar] [CrossRef]
- Magrone, T.; Jirillo, E. The Interplay between the Gut Immune System and Microbiota in Health and Disease: Nutraceutical Intervention for Restoring Intestinal Homeostasis. Curr. Pharm. Des. 2013, 19, 1329–1342. [Google Scholar] [PubMed]
- Pellegrini, S.; Sordi, V.; Bolla, A.M.; Saita, D.; Ferrarese, R.; Canducci, F.; Clementi, M.; Invernizzi, F.; Mariani, A.; Bonfanti, R.; et al. Duodenal Mucosa of Patients With Type 1 Diabetes Shows Distinctive Inflammatory Profile and Microbiota. J. Clin. Endocrinol. Metab. 2017, 102, 1468–1477. [Google Scholar] [CrossRef] [PubMed]
- Ley, R.E.; Turnbaugh, P.J.; Klein, S.; Gordon, J.I. Microbial ecology—Human gut microbes associated with obesity. Nature 2006, 444, 1022–1023. [Google Scholar] [CrossRef]
- Biddle, A.; Stewart, L.; Blanchard, J.; Leschine, S. Untangling the Genetic Basis of Fibrolytic Specialization by Lachnospiraceae and Ruminococcaceae in Diverse Gut Communities. Diversity 2013, 5, 627–640. [Google Scholar] [CrossRef]
- Shahir, M.H.; Rahimi, R.; Taheri, H.R.; Heidariniya, A.; Baradaran, N.; Asadi Kermani, Z. Effect of protein source and protease addition on performance, blood metabolites and nutrient digestibility of turkeys fed on low-protein diets from 28 to 55 d post hatch. Br. Poult. Sci. 2016, 57, 390–396. [Google Scholar] [CrossRef]



| Ingredients | Content, % | Nutrition Composition 3 | Content |
|---|---|---|---|
| Corn | 27.8 | DE, kcal/kg | 3.45 |
| Soybean meal | 12 | ME, kcal/kg | 3.21 |
| Broken rice | 20 | NE, kcal/kg | 2.53 |
| Five-sugar peptide 1 | 2.5 | CP, % | 18.48 |
| Flour | 7.5 | Lys, % | 1.35 |
| Soybean oil | 1 | Met, % | 0.50 |
| Whey powder | 2.5 | Met + Cys, % | 0.79 |
| Glucose | 2.5 | Thr, % | 0.89 |
| Fermented soybean meal | 5 | Ash, % | 5.24 |
| Extruded soybean | 5 | Ca, % | 0.78 |
| Fish meal | 4 | TP, % | 0.59 |
| White sugar | 5 | EP, % | 0.43 |
| Mold inhibitor | 0.2 | EE, % | 4.08 |
| Premix 2 | 5 | Salt, % | 0.71 |
| Total | 100 | Cu, ppm | 59.10 |
| Fe, ppm | 172.00 | ||
| Zn, ppm | 1500.00 | ||
| Mn, ppm | 36.07 |
| Protein | Product Name | Code No. | Company |
|---|---|---|---|
| P38 | p38 MAPK Monoclonal antibody | 8690s | Proteintech (1:3000, 8690S, Cell Signaling Technology, Boston, MA, USA) |
| P-P38 | Phospho-p38 MAPK (Thr180/Tyr182) (3D7) Rabbit mAb (Biotinylated) #4092 | 4511S | Cellsignaling (1:3000, 4511S, Cell Signaling Technology, Boston, MA, USA) |
| JNK | SAPK/JNK Antibody #9252 | 9252S | Cellsignaling (1:1000, 9252S, Cell Signaling Technology, Boston, MA, USA) |
| P-JNK | Phospho-SAPK/JNK (Thr183/Tyr185) (81E11) Rabbit mAb #4668 | 4668S | Cellsignaling (1:1000, 4688S, Cell Signaling Technology, Boston, MA, USA) |
| ERK | p44/42 MAPK (Erk1/2) Antibody #9102 | 9102S | Cellsignaling (1:3000, 9102S, Cell Signaling Technology, Boston, MA, USA) |
| P-ERK | Phospho-p44/42 MAPK (Erk1/2) (Thr202/Tyr204) Antibody #9101 | 9101S | Cellsignaling (1:3000, 9101S, Cell Signaling Technology, Boston, MA, USA) |
| NF-κB | NF-κB p65 Polyclonal antibody | 10745-1-AP | Proteintech (1:1000, 10745-1-AP, Proteintech, Wuhan, China) |
| P-NF-κB | Phospho-NF-κB p65 (Ser536) (93H1) Rabbit mAb #3033 | 3033S | Cellsignaling (1:1000, 3033S, Cell Signaling Technology, Boston, MA, USA) |
| ZO-1 | ZO-1 Polyclonal antibody | 21773-1-AP | Proteintech (1:1000, 21773-1-AP, Proteintech, Wuhan, China) |
| Occlaudin | Occludin Polyclonal antibody | 27260-1-AP | Proteintech (1:1000, 27260-1-AP, Proteintech, Wuhan, China) |
| Claudin-1 | Claudin 1 Polyclonal antibody | ab129119 | Proteintech (1:1000, ab129119, Abcam, Cambridge, UK) |
| secondary antibody | Goat Anti-Rabbit IgG H&L | 511203 | Zenbio |
| Gene Name | Gene Accession | Primer Sequences (5′→3′) |
|---|---|---|
| TNF-α | NM_214022.1 | F: GCCCTTCCACCAACGTTTTC |
| R: CAAGGGCTCTTGATGGCAGA | ||
| IL-1β | NM_214055.1 | F: ATTCAGGGACCCTACCCTCTC |
| R: ATCACTTCCTTGGCGGGTTC | ||
| IL-6 | NM_214399.1 | F: ACAAAGCCACCACCCCTAAC |
| R: CGTGGACGGCATCAATCTCA | ||
| β-actin | XM_021086047.1 | F: GATCTGGCACCACACCTTCTACAAC |
| R: RTCATCTTCTCACGGTTGGCTTTGG |
| Item | CC | PR | NC | SEM | p-Value |
|---|---|---|---|---|---|
| Body weight, kg | |||||
| Day 1 | 11.77 | 11.78 | 11.76 | 0.11 | 0.998 |
| Day 28 | 26.66 | 27.02 | 25.35 | 0.40 | 0.213 |
| Day 1 to 28 | |||||
| ADFI, g | 947 | 910 | 881 | 13.51 | 0.131 |
| ADG, g | 552 | 565 | 503 | 11.77 | 0.072 |
| F/G, g/g | 1.72 a | 1.62 b | 1.75 a | 0.02 | 0.008 |
| Diarrhea rate, % | 4.14 a | 2.38 b | 5.12 a | 0.33 | 0.051 |
| Item | CC | PR | NC | SEM | p-Value |
|---|---|---|---|---|---|
| Crude protein (%) | 82.00 a | 84.39 b | 78.77 c | 0.513 | 0.007 |
| Gross energy (%) | 88.50 a | 89.70 ab | 84.16 b | 0.216 | 0.002 |
| EAA | |||||
| Thr (%) | 82.23 ab | 86.54 b | 81.36 a | 0.930 | 0.018 |
| Val (%) | 81.13 a | 83.86 ab | 78.98 b | 0.758 | 0.021 |
| Met (%) | 82.44 b | 88.24 a | 77.07 c | 1.766 | 0.001 |
| Ile (%) | 83.44 | 84.58 | 81.32 | 0.398 | 0.414 |
| Leu (%) | 85.16 | 85.83 | 84.44 | 0.357 | 0.320 |
| Phe (%) | 83.17 a | 85.65 b | 82.54 a | 0.860 | 0.050 |
| His (%) | 78.36 a | 82.84 b | 77.43 ab | 0.232 | 0.048 |
| Lys (%) | 85.29 a | 88.52 b | 84.78 ab | 0.340 | 0.035 |
| Arg (%) | 89.07 a | 91.14 b | 86.49 b | 0.179 | 0.029 |
| NEAA | |||||
| Tau (%) | 87.23 a | 92.64 b | 83.06 c | 0.130 | 0.026 |
| Asp (%) | 87.08 | 88.29 | 86.74 | 0.366 | 0.201 |
| Ser (%) | 86.14 a | 86.78 a | 84.83 b | 0.359 | 0.047 |
| Glu (%) | 89.11 | 89.81 | 88.67 | 0.225 | 0.088 |
| Gly (%) | 83.17 | 83.43 | 82.07 | 0.304 | 0.149 |
| Ala (%) | 82.33 | 82.54 | 81.22 | 0.425 | 0.590 |
| Cys (%) | 81.22 a | 82.77 b | 83.03 c | 0.400 | 0.125 |
| Tyr (%) | 85.76 a | 84.28 a | 81.49 b | 0.682 | 0.004 |
| His (%) | 78.36 | 77.84 | 77.43 | 0.232 | 0.288 |
| Pro (%) | 86.88 | 86.71 | 85.85 | 0.256 | 0.227 |
| TAA (%) | 86.08 | 86.70 | 85.59 | 0.290 | 0.337 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, T.; Ma, W.; Wang, J.; Wei, Y.; Wang, Y.; Luo, Z.; Zhang, Y.; Zeng, X.; Guan, W.; Shao, D.; et al. Dietary Protease Supplementation Improved Growth Performance and Nutrients Digestion via Modulating Intestine Barrier, Immunological Response, and Microbiota Composition in Weaned Piglets. Antioxidants 2024, 13, 816. https://doi.org/10.3390/antiox13070816
Liu T, Ma W, Wang J, Wei Y, Wang Y, Luo Z, Zhang Y, Zeng X, Guan W, Shao D, et al. Dietary Protease Supplementation Improved Growth Performance and Nutrients Digestion via Modulating Intestine Barrier, Immunological Response, and Microbiota Composition in Weaned Piglets. Antioxidants. 2024; 13(7):816. https://doi.org/10.3390/antiox13070816
Chicago/Turabian StyleLiu, Tao, Wen Ma, Jun Wang, Yulong Wei, Yibo Wang, Zheng Luo, Ying Zhang, Xiangfang Zeng, Wutai Guan, Dan Shao, and et al. 2024. "Dietary Protease Supplementation Improved Growth Performance and Nutrients Digestion via Modulating Intestine Barrier, Immunological Response, and Microbiota Composition in Weaned Piglets" Antioxidants 13, no. 7: 816. https://doi.org/10.3390/antiox13070816
APA StyleLiu, T., Ma, W., Wang, J., Wei, Y., Wang, Y., Luo, Z., Zhang, Y., Zeng, X., Guan, W., Shao, D., & Chen, F. (2024). Dietary Protease Supplementation Improved Growth Performance and Nutrients Digestion via Modulating Intestine Barrier, Immunological Response, and Microbiota Composition in Weaned Piglets. Antioxidants, 13(7), 816. https://doi.org/10.3390/antiox13070816









