Persulfidome of Sweet Pepper Fruits during Ripening: The Case Study of Leucine Aminopeptidase That Is Positively Modulated by H2S
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Dimedone Switch Method and Proteomics
2.3. Functional Analysis
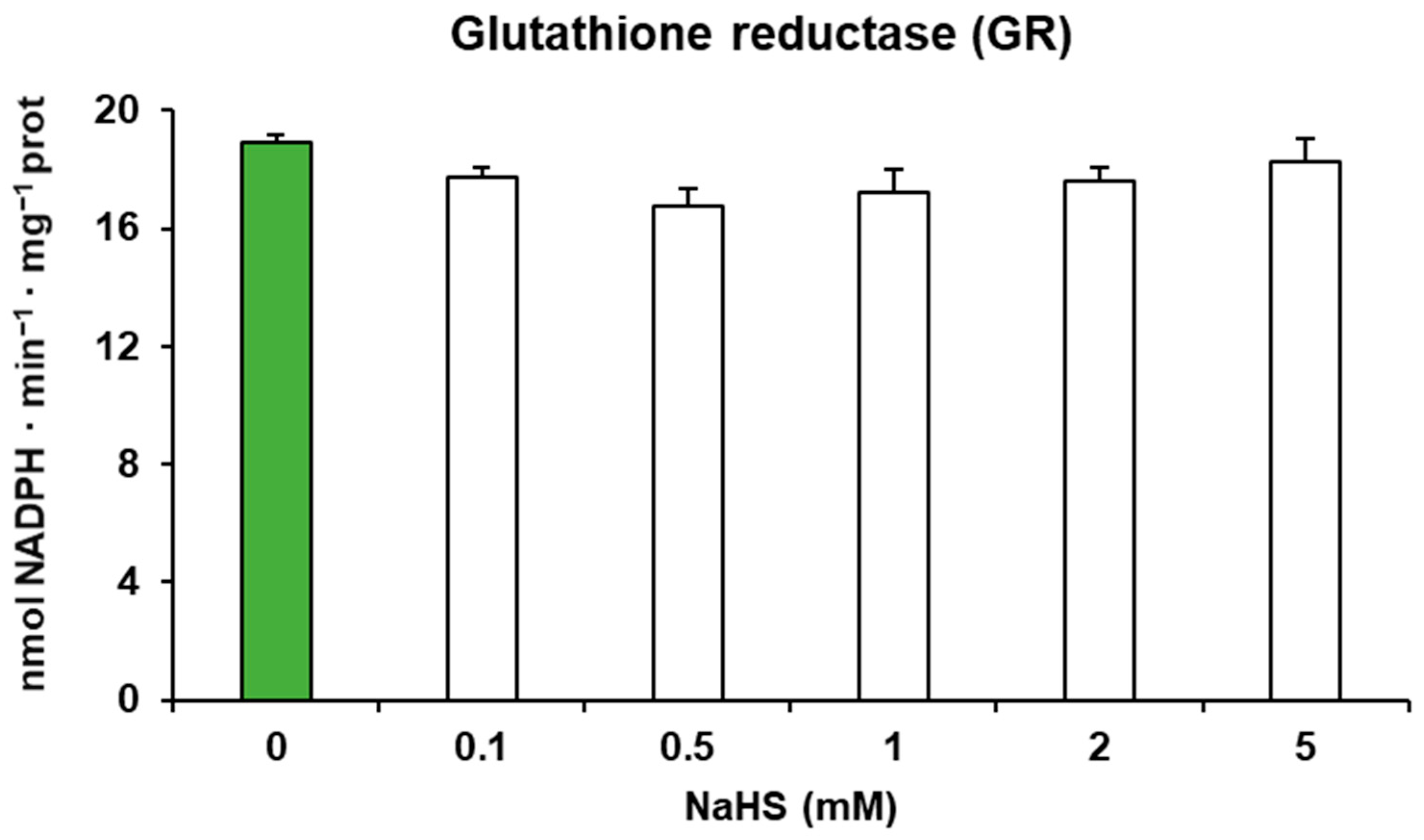
2.4. Fruit Extracts and Leucine Aminopeptidase (LAP) and Glutathione Reductase (GR) Activities
2.5. In Vitro Treatment of Pepper Samples
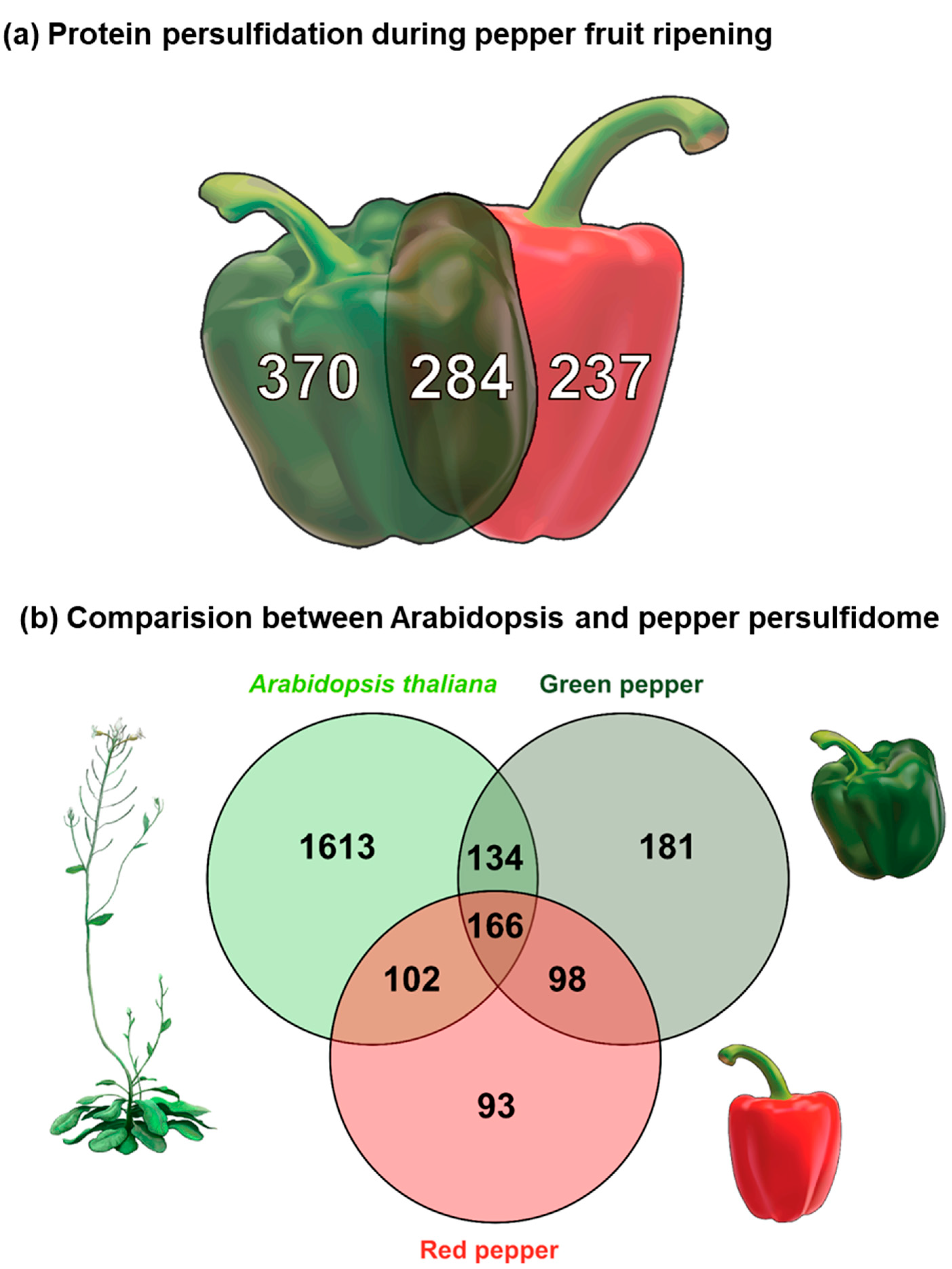
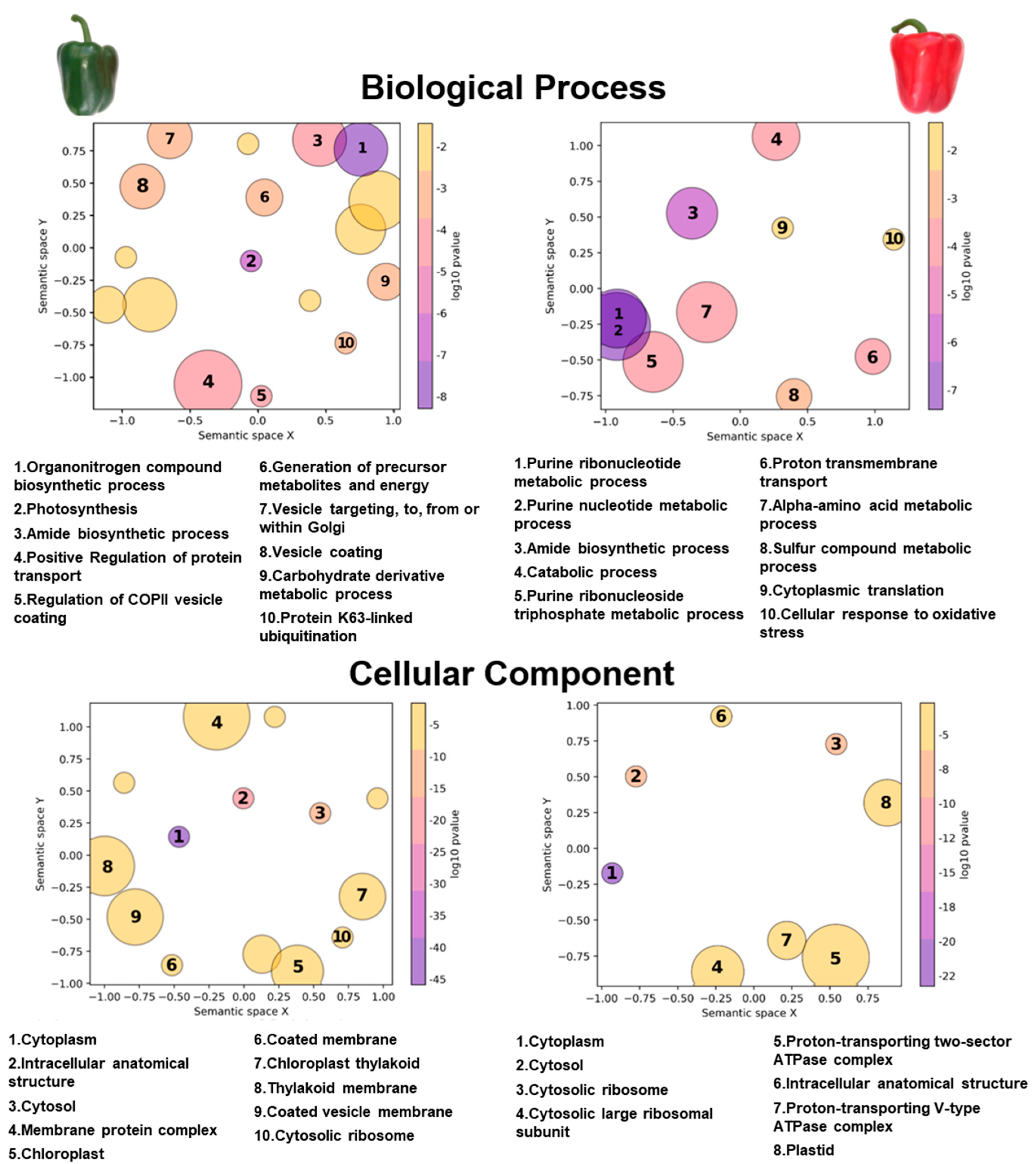
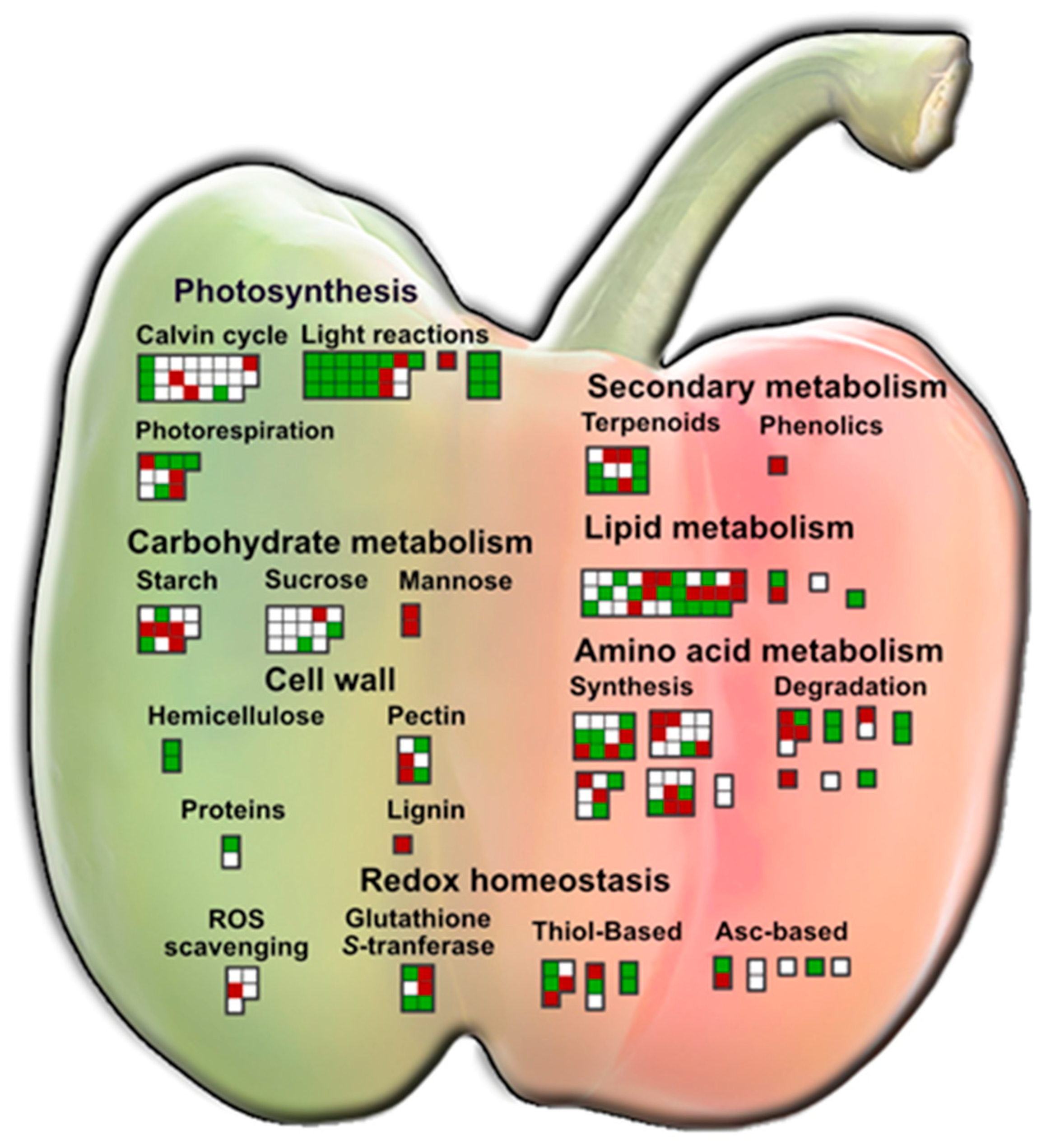
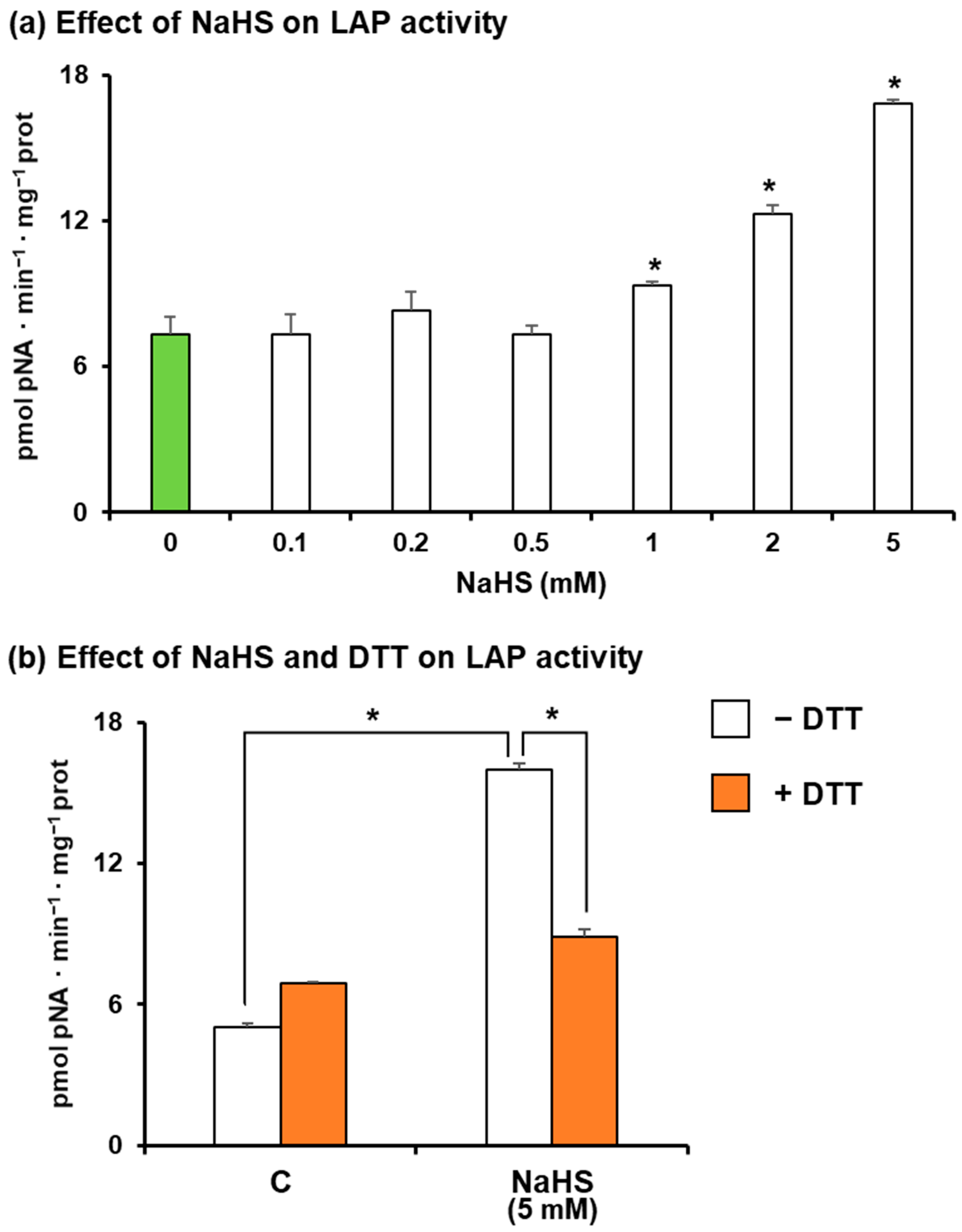
3. Results
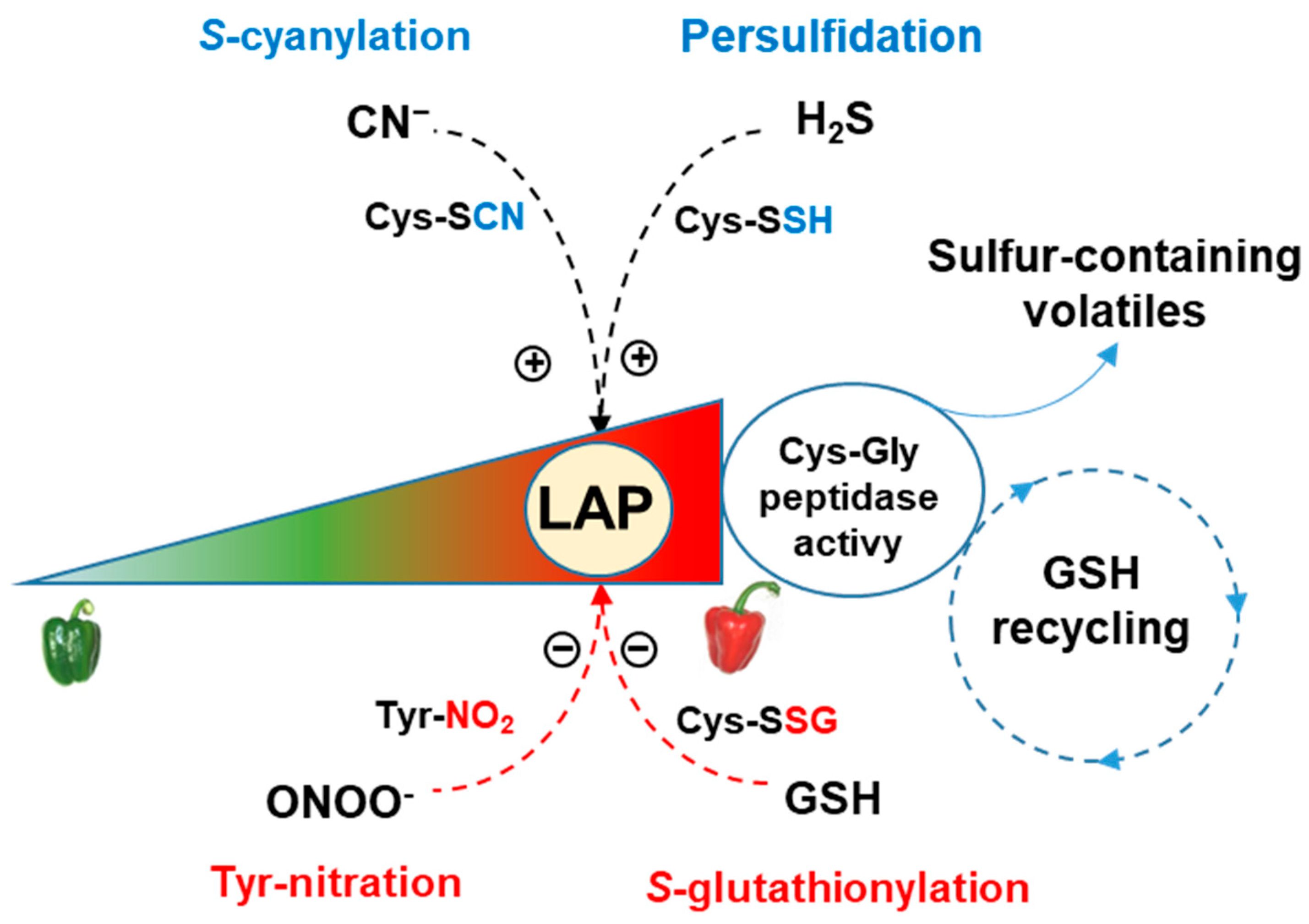
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abe, K.; Kimura, H. The possible role of hydrogen sulfide as an endogenous neuromodulator. J. Neurosci. 1996, 16, 1066–1071. [Google Scholar] [CrossRef]
- Kimura, H. Signaling by hydrogen sulfide (H2S) and polysulfides (H2Sn) in the central nervous system. Neurochem. Int. 2019, 126, 118–125. [Google Scholar] [CrossRef]
- Szabo, C. Novel Regulatory Roles of Hydrogen Sulfide in Health and Disease. Biomolecules 2022, 12, 1372. [Google Scholar] [CrossRef]
- Yamasaki, H.; Cohen, M.F. Biological consilience of hydrogen sulfide and nitric oxide in plants: Gases of primordial earth linking plant, microbial and animal physiologies. Nitric Oxide 2016, 55–56, 91–100. [Google Scholar] [CrossRef]
- Iciek, M.B.; Bilska-Wilkosz, A.; Kozdrowicki, M.; Górny, M. Reactive Sulfur Species in Human Diseases. Antioxid. Redox Signal. 2023, 39, 1000–1023. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, M.; Zhou, H.; Zhao, D.; Gotor, C.; Romero, L.C.; Shen, J.; Ge, Z.; Zhang, Z.; Shen, W.; et al. Hydrogen sulfide, a signaling molecule in plant stress responses. J. Integr. Plant Biol. 2021, 63, 146–160. [Google Scholar] [CrossRef]
- Khan, M.S.S.; Islam, F.; Ye, Y.; Ashline, M.; Wang, D.; Zhao, B.; Fu, Z.Q.; Chen, J. The Interplay between Hydrogen Sulfide and Phytohormone Signaling Pathways under Challenging Environments. Int. J. Mol. Sci. 2022, 23, 4272. [Google Scholar] [CrossRef]
- Jurado-Flores, A.; Aroca, A.; Romero, L.C.; Gotor, C. Sulfide promotes tolerance to drought through protein persulfidation in Ar-abidopsis. J. Exp. Bot. 2023, 74, 4654–4669. [Google Scholar] [CrossRef]
- Huang, J.; Xie, Y. Hydrogen Sulfide Signaling in Plants. Antioxid. Redox Signal. 2023, 39, 40–58. [Google Scholar] [CrossRef]
- Filipovic, M.R.; Zivanovic, J.; Alvarez, B.; Banerjee, R. Chemical biology of H2S signaling through persulfidation. Chem. Rev. 2018, 118, 1253–1337. [Google Scholar] [CrossRef]
- Cuevasanta, E.; Lange, M.; Bonanata, J.; Coitiño, E.L.; Ferrer-Sueta, G.; Filipovic, M.R.; Alvarez, B. Reaction of Hydrogen Sulfide with Disulfide and Sulfenic Acid to Form the Strongly Nucleophilic Persulfide. J. Biol. Chem. 2015, 290, 26866–26880. [Google Scholar] [CrossRef]
- Sawa, T.; Motohashi, H.; Ihara, H.; Akaike, T. Enzymatic Regulation and Biological Functions of Reactive Cysteine Persulfides and Polysulfides. Biomolecules 2020, 10, 1245. [Google Scholar] [CrossRef]
- Ogata, S.; Matsunaga, T.; Jung, M.; Barayeu, U.; Morita, M.; Akaike, T. Persulfide Biosynthesis Conserved Evolutionarily in All Or-ganisms. Antioxid. Redox Signal. 2023, 39, 983–999. [Google Scholar] [CrossRef]
- Abidin, Q.H.Z.; Ida, T.; Morita, M.; Matsunaga, T.; Nishimura, A.; Jung, M.; Hassan, N.; Takata, T.; Ishii, I.; Kruger, W.; et al. Synthesis of Sulfides and Persulfides Is Not Impeded by Disruption of Three Canonical Enzymes in Sulfur Metabolism. Antioxidants 2023, 12, 868. [Google Scholar] [CrossRef]
- Kasamatsu, S.; Kinno, A.; Hishiyama, J.-I.; Akaike, T.; Ihara, H. Development of methods for quantitative determination of the total and reactive polysulfides: Reactive polysulfide profiling in vegetables. Food Chem. 2023, 413, 135610. [Google Scholar] [CrossRef]
- Benchoam, D.; Semelak, J.A.; Cuevasanta, E.; Mastrogiovanni, M.; Grassano, J.S.; Ferrer-Sueta, G.; Zeida, A.; Trujillo, M.; Möller, M.N.; Estrin, D.A.; et al. Acidity and nucleophilic reactivity of glutathione persulfide. J. Biol. Chem. 2020, 295, 15466–15481. [Google Scholar] [CrossRef]
- Aroca, A.; Benito, J.M.; Gotor, C.; Romero, L.C. Persulfidation proteome reveals the regulation of protein function by hydrogen sulfide in diverse biological processes in Arabidopsis. J. Exp. Bot. 2017, 68, 4915–4927. [Google Scholar] [CrossRef]
- Corpas, F.J.; González-Gordo, S.; Muñoz-Vargas, M.A.; Rodríguez-Ruiz, M.; Palma, J.M. The Modus operandi of hydrogen sulfide(H2S)-dependent protein persulfidation in higher plants. Antioxidants 2021, 10, 1686. [Google Scholar] [CrossRef]
- Tripodi, P.; Kumar, S. The Capsicum Crop: An Introduction. In The Capsicum Genome. Compendium of Plant Genomes; Ramchiary, N., Kole, C., Eds.; Springer: Cham, Switzerland, 2019. [Google Scholar] [CrossRef]
- Baenas, N.; Belovi’c, N.; Ilicb, N.; Moreno, D.A.; García-Viguera, C. Industrial use of pepper (Capsicum annum L.) derived products: Technological benefits and biological advantages. Food Chem. 2019, 274, 872–885. [Google Scholar] [CrossRef]
- Materska, M.; Perucka, I. Antioxidant activity of the main phenolic compounds isolated from hot pepper fruit (Capsicum annuum L.). J. Agric. Food Chem. 2005, 53, 1750–1756. [Google Scholar] [CrossRef]
- Howard, L.R.; Wildman, R.E.C. Antioxidant vitamin and phytochemical content of fresh and processed pepper fruit (Capsicum annuum). In Handbook of Nutraceuticals and Functional Foods; Wildman, R.E.C., Ed.; CRC Press: Boca Raton, FL, USA, 2007; pp. 165–191. [Google Scholar]
- Wahyuni, Y.; Ballester, A.-R.; Sudarmonowati, E.; Bino, R.J.; Bovy, A.G. Secondary metabolites of Capsicum species and their importance in the human diet. J. Nat. Prod. 2013, 76, 783–793. [Google Scholar] [CrossRef]
- Hamed, M.; Kalita, D.; Bartolo, M.E.; Jayanty, S.S. Capsaicinoids, Polyphenols and Antioxidant Activities of Capsicum annuum: Comparative Study of the Effect of Ripening Stage and Cooking Methods. Antioxidants 2019, 8, 364. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, F.; Wang, Y.; Ma, X.; Shen, Y.; Wang, X.; Yang, H.; Zhang, W.; Lakshmanan, P.; Hu, Y.; et al. Physiological and metabolomic analysis reveals maturity stage-dependent nitrogen regulation of vitamin C content in pepper fruit. Front. Plant Sci. 2023, 13, 1049785. [Google Scholar] [CrossRef]
- González-Gordo, S.; Bautista, R.; Claros, M.G.; Cañas, A.; Palma, J.M.; Corpas, F.J. Nitric oxide-dependent regulation of sweet pepper fruit ripening. J. Exp. Bot. 2019, 70, 4557–4570. [Google Scholar] [CrossRef]
- González-Gordo, S.; Muñoz-Vargas, M.A.; Palma, J.M.; Corpas, F.J. Class III peroxidases (POD) in pepper (Capsicum annuum L.): Genome-wide identification and regulation during nitric oxide (NO)-influenced fruit ripening. Antioxidants 2023, 12, 1013. [Google Scholar] [CrossRef]
- González-Gordo, S.; Rodríguez-Ruiz, M.; López-Jaramillo, J.; Muñoz-Vargas, M.A.; Palma, J.M.; Corpas, F.J. Nitric Oxide (NO) differ-entially modulates the ascorbate peroxidase (APX) isozymes of sweet pepper (Capsicum annuum L.) fruits. Antioxidants 2022, 11, 765. [Google Scholar] [CrossRef]
- Muñoz-Vargas, M.A.; González-Gordo, S.; Taboada, J.; Palma, J.M.; Corpas, F.J. In silico RNAseq and biochemical analyses of glucose-6-phosphate dehydrogenase (G6PDH) from sweet pepper fruits: Involvement of nitric oxide (NO) in ripening and modulation. Plants 2023, 12, 3408. [Google Scholar] [CrossRef]
- Muñoz-Vargas, M.A.; López-Jaramillo, J.; González-Gordo, S.; Paradela, A.; Palma, J.M.; Corpas, F.J. H2S-generating cytosolic L-cysteine desulfhydrase and mitochondrial D-cysteine desulfhydrase from sweet pepper (Capsicum annuum L.) are regulated during fruit ripening and by nitric oxide. Antioxid. Redox Signal. 2023, 39, 2–18. [Google Scholar] [CrossRef]
- Muñoz-Vargas, M.A.; Taboada, J.; González-Gordo, S.; Palma, J.M.; Corpas, F.J. Characterization of leucine aminopeptidase (LAP) activity in sweet pepper fruits during ripening and its inhibition by nitration and reducing events. Plant Cell Rep. 2024, 43, 92. [Google Scholar] [CrossRef]
- Aroca, Á.; Serna, A.; Gotor, C.; Romero, L.C. S-sulfhydration: A cysteine posttranslational modification in plant systems. Plant Physiol. 2015, 168, 334–342. [Google Scholar] [CrossRef]
- Aroca, A.; Yruela, I.; Gotor, C.; Bassham, D.C. Persulfidation of ATG18a regulates autophagy under ER stress in Arabidopsis. Proc. Natl. Acad. Sci. USA 2021, 118, e2023604118. [Google Scholar] [CrossRef]
- Matamoros, M.A.; Romero, L.C.; Tian, T.; Román, Á.; Duanmu, D.; Becana, M. Persulfidation of plant and bacteroid proteins is involved in legume nodule development and senescence. J. Exp. Bot. 2023, 75, 3009–3025. [Google Scholar] [CrossRef]
- Aroca, A.; Jurado-Flores, A.; Filipovic, M.R.; Gotor, C.; Romero, L.C. Detection of protein persulfidation in plants by the dimedone switch method. Methods Enzymol. 2022, 676, 385–402. [Google Scholar]
- Kolberg, L.; Raudvere, U.; Kuzmin, I.; Adler, P.; Vilo, J.; Peterson, H. g:Profiler—Interoperable web service for functional enrichment analysis and gene identifier mapping (2023 update). Nucleic Acids Res. 2023, 51, W207–W212. [Google Scholar] [CrossRef]
- Reijnders, M.J.M.F.; Waterhouse, R.M. Summary Visualizations of Gene Ontology Terms With GO-Figure! Front. Bioinform. 2021, 1, 638255. [Google Scholar] [CrossRef]
- Schwacke, R.; Ponce-Soto, G.Y.; Krause, K.; Bolger, A.M.; Arsova, B.; Hallab, A.; Gruden, K.; Stitt, M.; Bolger, M.E.; Usadel, B. MapMan4: A Refined Protein Classification and Annotation Framework Applicable to Multi-Omics Data Analysis. Mol. Plant 2019, 12, 879–892. [Google Scholar] [CrossRef]
- Bolger, M.; Schwacke, R.; Usadel, B. MapMan Visualization of RNA-Seq Data Using Mercator4 Functional Annotations. Methods Mol. Biol. 2021, 2354, 195–212. [Google Scholar]
- Thimm, O.; Bläsing, O.; Gibon, Y.; Nagel, A.; Meyer, S.; Krüger, P.; Selbig, J.; Müller, L.A.; Rhee, S.Y.; Stitt, M. MAPMAN: A user-driven tool to display genomics data sets onto diagrams of metabolic pathways and other biological processes. Plant J. 2004, 37, 914–939. [Google Scholar] [CrossRef]
- Usadel, B.; Poree, F.; Nagel, A.; Lohse, M.; Czedik-Eysenberg, A.; Stitt, M. A guide to using MapMan to visualize and compare Omics data in plants: A case study in the crop species, Maize. Plant Cell Environ. 2009, 32, 1211–1229. [Google Scholar] [CrossRef]
- Camacho, C.; Coulouris, G.; Avagyan, V.; Ma, N.; Papadopoulos, J.; Bealer, K.; Madden, T.L. BLAST+: Architecture and applications. BMC Bioinform. 2009, 10, 421. [Google Scholar] [CrossRef]
- Corpas, F.J.; Palma, J.M.; Del Río, L.A. Evidence for the presence of proteolytic activity in peroxisomes. Eur. J. Cell Biol. 1993, 61, 81–85. [Google Scholar]
- Tuppy, H.; Wiesbauer, U.; Wintersberger, E. Amino acid-p-nitroanilide as a substrate for aminopeptidases and other proteolytic enzymes. Hoppe Seylers Z Physiol. Chem. 1962, 329, 278–288. [Google Scholar] [CrossRef]
- Edwards, E.A.; Rawsthorne, S.; Mullineaux, P.M. Subcellular distribution of multiple forms of glutathione reductase in leaves of pea (Pisum sativum L.). Planta 1990, 180, 278–284. [Google Scholar] [CrossRef]
- Fang, T.; Cao, Z.; Li, J.; Shen, W.; Huang, L. Auxin-induced hydrogen sulfide generation is involved in lateral root formation in tomato. Plant Physiol. Biochem. 2014, 76, 44–51. [Google Scholar] [CrossRef]
- Fang, H.; Liu, R.; Yu, Z.; Shao, Y.; Wu, G.; Pei, Y. Gasotransmitter H2S accelerates seed germination via activating AOX mediated cyanide-resistant respiration pathway. Plant Physiol. Biochem. 2022, 190, 193–202. [Google Scholar] [CrossRef]
- Liu, Q.; Zhou, Y.; Li, H.; Liu, R.; Wang, W.; Wu, W.; Yang, N.; Wang, S. Osmotic stress-triggered stomatal closure requires Phospholipase Dδ and hydrogen sulfide in Arabidopsis thaliana. Biochem. Biophys. Res. Commun. 2020, 534, 914–920. [Google Scholar] [CrossRef]
- Dai, J.; Wen, D.; Li, H.; Yang, J.; Rao, X.; Yang, Y.; Yang, J.; Yang, C.; Yu, J. Effect of hydrogen sulfide (H2S) on the growth and development of tobacco seedlings in absence of stress. BMC Plant Biol. 2024, 24, 162. [Google Scholar] [CrossRef]
- Ma, T.; Xu, S.; Wang, Y.; Zhang, L.; Liu, Z.; Liu, D.; Jin, Z.; Pei, Y. Exogenous hydrogen sulphide promotes plant flowering through the Arabidopsis splicing factor AtU2AF65a. Plant Cell Environ. 2024, 47, 1782–1796. [Google Scholar] [CrossRef]
- Younis, A.A.; Mansour, M.M.F. Hydrogen sulfide priming enhanced salinity tolerance in sunflower by modulating ion hemostasis, cellular redox balance, and gene expression. BMC Plant Biol. 2023, 23, 525. [Google Scholar] [CrossRef]
- Wang, J.; Dou, J.; Yue, Z.; Wang, J.; Chen, T.; Li, J.; Dai, H.; Dou, T.; Yu, J.; Liu, Z. Effect of hydrogen sulfide on cabbage photosynthesis under black rot stress. Plant Physiol. Biochem. 2024, 208, 108453. [Google Scholar] [CrossRef]
- Chen, J.; Shang, Y.-T.; Wang, W.-H.; Chen, X.-Y.; He, E.-M.; Zheng, H.-L.; Shangguan, Z. Hydrogen sulfide-mediated polyamines and sugar changes are involved in hydrogen sulfide-induced drought tolerance in spinacia oleracea seedlings. Front. Plant Sci. 2016, 7, 1173. [Google Scholar] [CrossRef]
- Liu, R.; Lal, R. Effects of low-level aqueous hydrogen sulfide and other sulfur species on lettuce (Lactuca sativa) seed germination. Commun. Soil Sci. Plant Anal. 2015, 46, 576–587. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, C.; Cao, B.; Xu, K. A hydrogen sulfide application can alleviate the toxic effects of cadmium on ginger (Zingiber officinale Roscoe). Environ. Sci. Pollut. Res. 2022, 29, 68422–68431. [Google Scholar] [CrossRef]
- Ali, S.; Nawaz, A.; Naz, S.; Ejaz, S.; Maqbool, M.; Siddiqui, M.H.; Kalaji, H.M.; Wróbel, J.; Telesiński, A.; Auriga, A. Hydrogen sulfide mitigates chilling injury of postharvest banana fruits by regulating γ-aminobutyric acid shunt pathway and ascorbate–glutathione cycle. Front. Plant Sci. 2022, 13, 941246. [Google Scholar] [CrossRef]
- Li, D.; Limwachiranon, J.; Li, L.; Du, R.; Luo, Z. Involvement of energy metabolism to chilling tolerance induced by hydrogen sulfide in cold-stored banana fruit. Food Chem. 2016, 208, 272–278. [Google Scholar] [CrossRef]
- Ge, Y.; Hu, K.D.; Wang, S.S.; Hu, L.Y.; Chen, X.Y.; Li, Y.H.; Yang, Y.; Yang, F.; Zhang, H. Hydrogen sulfide alleviates postharvest ripening and senescence of banana by antagonizing the effect of ethylene. PLoS ONE 2017, 12, e018011. [Google Scholar] [CrossRef]
- Hu, L.-Y.; Hu, S.-L.; Wu, J.; Li, Y.-H.; Zheng, J.-L.; Wei, Z.-J.; Liu, J.; Wang, H.-L.; Liu, Y.-S.; Zhang, H. Hydrogen sulfide prolongs postharvest shelf life of strawberry and plays an antioxidative role in fruits. J. Agric. Food Chem. 2012, 60, 8684–8693. [Google Scholar] [CrossRef]
- Zhang, C.; Shi, J.Y.; Zhu, L.P.; Li, C.L.; Wang, Q.G. Cooperative effects of hydrogen sulfide and nitric oxide on delaying softening and decay of strawberry. Int. J. Agric. Biol. Eng. 2014, 7, 114–122. [Google Scholar]
- Yu, M.; Chen, Y.; Zhu, Q.; Wu, X.; Jiang, S.; Wei, Y.; Ye, J.; Xu, F.; Shao, X. Hydrogen sulfide mediated methyl jasmonate -induced cold resistance in peach fruit. Postharvest Biol. Technol. 2024, 210, 112779. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhu, D.; Zhao, L.; Luo, Y.; Li, J.; Xie, B.; Liu, Y.; Bao, Y.; Wu, Z.; Zheng, Y.; et al. Hydrogen sulfide retards fruit softening and prevents flesh browning in cold-stored peaches by regulating cell wall-modifying enzymes, phenolic, and proline metabolism. Postharvest Biol. Technol. 2024, 207, 112620. [Google Scholar] [CrossRef]
- Deng, H.; Wang, B.; Liu, Y.; Ma, L.; Zong, Y.; Prusky, D.; Bi, Y. Sodium hydrosulfide induces resistance against Penicillium expansum in apples by regulating hydrogen peroxide and nitric oxide activation of phenylpropanoid metabolism. Front. Microbiol. 2021, 12, 720372. [Google Scholar] [CrossRef]
- Yang, C.-T.; Devarie-Baez, N.O.; Hamsath, A.; Fu, X.-D.; Xian, M. S-Persulfidation: Chemistry, Chemical Biology, and Significance in Health and Disease. Antioxid. Redox Signal. 2020, 33, 1092–1114. [Google Scholar] [CrossRef]
- Luo, S.; Kong, C.; Ye, D.; Liu, X.; Wang, Y.; Meng, G.; Han, Y.; Xie, L.; Ji, Y. Protein Persulfidation: Recent Progress and Future Directions. Antioxid. Redox Signal. 2023, 39, 829–852. [Google Scholar] [CrossRef]
- Vignane, T.; Filipovic, M.R. Emerging Chemical Biology of Protein Persulfidation. Antioxid. Redox Signal. 2023, 39, 19–39. [Google Scholar] [CrossRef]
- Gao, X.H.; Krokowski, D.; Guan, B.J.; Bederman, I.; Majumder, M.; Parisien, M.; Diatchenko, L.; Kabil, O.; Willard, B.; Banerjee, R.; et al. Quantitative H2S-mediated protein sulfhydration reveals metabolic reprogramming during the integrated stress response. Elife 2015, 4, e10067. [Google Scholar] [CrossRef]
- Wu, Q.; Zhao, B.; Weng, Y.; Shan, Y.; Li, X.; Hu, Y.; Liang, Z.; Yuan, H.; Zhang, L.; Zhang, Y. Site-Specific Quantification of Persulfidome by Combining an Isotope-Coded Affinity Tag with Strong Cation-Exchange-Based Fractionation. Anal. Chem. 2019, 91, 14860–14864. [Google Scholar] [CrossRef]
- Zivanovic, J.; Kouroussis, E.; Kohl, J.B.; Adhikari, B.; Bursac, B.; Schott-Roux, S.; Petrovic, D.; Miljkovic, J.L.; Thomas-Lopez, D.; Jung, Y.; et al. Selective Persulfide Detection Reveals Evolutionarily Conserved Antiaging Effects of S-Sulfhydration. Cell Metab. 2019, 30, 1152–1170.e13. [Google Scholar] [CrossRef]
- Fu, L.; Liu, K.; He, J.; Tian, C.; Yu, X.; Yang, J. Direct Proteomic Mapping of Cysteine Persulfidation. Antioxid. Redox Signal. 2020, 33, 1061–1076. [Google Scholar] [CrossRef]
- Bithi, N.; Link, C.; Henderson, Y.O.; Kim, S.; Yang, J.; Li, L.; Wang, R.; Willard, B.; Hine, C. Dietary restriction transforms the mammalian protein persulfidome in a tissue-specific and cystathionine γ-lyase-dependent manner. Nat. Commun. 2021, 12, 1745. [Google Scholar] [CrossRef]
- Bai, J.; Jiao, F.; Salmeron, A.G.; Xu, S.; Xian, M.; Huang, L.; Chen, D.-B. Mapping Pregnancy-dependent Sulfhydrome Unfolds Diverse Functions of Protein Sulfhydration in Human Uterine Artery. Endocrinology 2023, 164, bqad107. [Google Scholar] [CrossRef]
- García-Calderón, M.; Vignane, T.; Filipovic, M.R.; Ruiz, M.T.; Romero, L.C.; Márquez, A.J.; Gotor, C.; Aroca, A. Persulfidation protects from oxidative stress under nonphotorespiratory conditions in Arabidopsis. New Phytol. 2023, 238, 1431–1445. [Google Scholar] [CrossRef]
- Jurado-Flores, A.; Gotor, C.; Romero, L.C. Proteome Dynamics of Persulfidation in Leaf Tissue under Light/Dark Conditions and Carbon Deprivation. Antioxidants 2023, 12, 789. [Google Scholar] [CrossRef]
- Jurado-Flores, A.; Romero, L.C.; Gotor, C. Label-free quantitative proteomic analysis of nitrogen starvation in arabidopsis root reveals new aspects of H2S signaling by protein persulfidation. Antioxidants 2021, 10, 508. [Google Scholar] [CrossRef]
- Chen, J.; Wu, F.-H.; Wang, W.-H.; Zheng, C.-J.; Lin, G.-H.; Dong, X.-J.; He, J.-X.; Pei, Z.-M.; Zheng, H.-L. Hydrogen sulphide enhances photosynthesis through promoting chloroplast biogenesis, photosynthetic enzyme expression, and thiol redox modification in Spinacia oleracea seedlings. J. Exp. Bot. 2011, 62, 4481–4493. [Google Scholar] [CrossRef]
- Chen, S.; Jia, H.; Wang, X.; Shi, C.; Wang, X.; Ma, P.; Wang, J.; Ren, M.; Li, J. Hydrogen sulfide positively regulates abscisic acid signaling through persulfidation of SnRK2.6 in guard cells. Mol. Plant 2020, 13, 732–744. [Google Scholar] [CrossRef]
- Li, J.; Shi, C.; Wang, X.; Liu, C.; Ding, X.; Ma, P.; Wang, X.; Jia, H. Hydrogen sulfide regulates the activity of antioxidant enzymes through persulfidation and improves the resistance of tomato seedling to Copper Oxide nanoparticles (CuO NPs)-induced oxidative stress. Plant Physiol. Biochem. 2020, 156, 257–266. [Google Scholar] [CrossRef]
- Shen, J.; Zhang, J.; Zhou, M.; Zhou, H.; Cui, B.; Gotor, C.; Romero, L.C.; Fu, L.; Yang, J.; Foyer, C.H.; et al. Persulfidation-based modification of cysteine desulfhydrase and the NADPH oxidase RBOHD controls guard cell abscisic acid signaling. Plant Cell 2020, 32, 1000–1017. [Google Scholar] [CrossRef]
- Zhou, M.; Zhang, J.; Shen, J.; Zhou, H.; Zhao, D.; Gotor, C.; Romero, L.C.; Fu, L.; Li, Z.; Yang, J.; et al. Hydrogen sulfide-linked persulfidation of ABI4 controls ABA responses through the transactivation of MAPKKK18 in Arabidopsis. Mol. Plants 2021, 14, 921–936. [Google Scholar] [CrossRef]
- Du, X.; Jin, Z.; Liu, Z.; Liu, D.; Zhang, L.; Ma, X.; Yang, G.; Liu, S.; Guo, Y.; Pei, Y. H2S persulfidated and increased kinase activity of MPK4 to response cold stress in Arabidopsis. Front. Mol. Biosci. 2021, 8, 635470. [Google Scholar] [CrossRef]
- Chen, S.; Wang, X.; Jia, H.; Li, F.; Ma, Y.; Liesche, J.; Liao, M.; Ding, X.; Liu, C.; Chen, Y.; et al. Persulfidation-induced structural change in SnRK2.6 establishes intramolecular interaction between phosphorylation and persulfidation. Mol. Plant 2021, 14, 1814–1830. [Google Scholar] [CrossRef]
- Zhou, H.; Zhou, Y.; Zhang, F.; Guan, W.; Su, Y.; Yuan, X.; Xie, Y. Persulfidation of nitrate reductase 2 is involved in L-cysteine desulfhydrase-regulated rice drought tolerance. Int. J. Mol. Sci. 2021, 22, 12119. [Google Scholar] [CrossRef]
- Jia, H.; Chen, S.; Liu, D.; Liesche, J.; Shi, C.; Wang, J.; Ren, M.; Wang, X.; Yang, J.; Shi, W.; et al. Ethylene-induced hydrogen sulfide negatively regulates ethylene biosynthesis by persulfidation of ACO in tomato under osmotic stress. Front. Plant Sci. 2018, 9, 1517. [Google Scholar] [CrossRef]
- Laureano-Marín, A.M.; Aroca, A.; Perez-Perez, M.E.; Yruela, I.; Jurado-Flores, A.; Moreno, I.; Crespo, J.L.; Romero, L.C.; Gotor, C. Abscisic acid-triggered persulfidation of cysteine protease ATG4 mediates regulation of autophagy by sulfide. Plant Cell 2020, 32, 3902–3920. [Google Scholar] [CrossRef]
- Muñoz-Vargas, M.A.; González-Gordo, S.; Cañas, A.; López-Jaramillo, J.; Palma, J.M.; Corpas, F.J. Endogenous hydrogen sulfide (H2S) is up-regulated during sweet pepper (Capsicum annuum L.) fruit ripening. In vitro analysis shows that NADP-dependent isocitrate dehydrogenase (ICDH) activity is inhibited by H2S and NO. Nitric Oxide 2018, 81, 36–45. [Google Scholar] [CrossRef]
- Muñoz-Vargas, M.A.; González-Gordo, S.; Palma, J.M.; Corpas, F.J. Inhibition of NADP-malic enzyme activity by H2S and NO in sweet pepper (Capsicum annuum L.) fruits. Physiol. Plant. 2020, 168, 278–288. [Google Scholar] [CrossRef]
- Corpas, F.J.; Barroso, J.B.; González-Gordo, S.; Muñoz-Vargas, M.A.; Palma, J.M. Hydrogen sulfide: A novel component in Arabidopsis peroxisomes which triggers catalase inhibition. J. Integr. Plant Biol. 2019, 61, 871–883. [Google Scholar] [CrossRef]
- Ma, X.; Zhang, L.; Pei, Z.; Zhang, L.; Liu, Z.; Liu, D.; Hao, X.; Jin, Z.; Pei, Y. Hydrogen sulfide promotes flowering in heading Chinese cabbage by S-sulfhydration of BraFLCs. Hortic. Res. 2021, 8, 19. [Google Scholar] [CrossRef]
- González-Gordo, S.; López-Jaramillo, J.; Palma, J.M.; Corpas, F.J. Soybean (Glycine max L.) Lipoxygenase 1 (LOX 1) Is Modulated by Nitric Oxide and Hydrogen Sulfide: An In Vitro Approach. Int. J. Mol. Sci. 2023, 24, 8001. [Google Scholar] [CrossRef]
- Wang, X.; Shi, C.; Hu, Y.; Ma, Y.; Yi, Y.; Jia, H.; Li, F.; Sun, H.; Li, T.; Wang, X.; et al. Persulfidation maintains cytosolic G6PDs activity through changing tetrameric structure and competing cysteine sulfur oxidation under salt stress in Arabidopsis and tomato. New Phytol. 2023, 240, 626–643. [Google Scholar] [CrossRef]
- Ma, Y.; Li, F.; Yi, Y.; Wang, X.; Li, T.; Wang, X.; Sun, H.; Li, L.; Ren, M.; Han, S.; et al. Hydrogen sulfide improves salt tolerance through persulfidation of PMA1 in Arabidopsis. Plant Cell Rep. 2023, 42, 1265–1277. [Google Scholar] [CrossRef]
- Sun, C.; Yao, G.-F.; Li, L.-X.; Li, T.-T.; Zhao, Y.-Q.; Hu, K.-D.; Zhang, C.; Zhang, H. E3 ligase BRG3 persulfidation delays tomato ripening by reducing ubiquitination of the repressor WRKY71. Plant Physiol. 2023, 192, 616–632. [Google Scholar] [CrossRef]
- Zhang, M.; Hu, K.; Ma, L.; Geng, M.; Zhang, C.; Yao, G.; Zhang, H. Persulfidation and phosphor-ylation of transcription factor SlWRKY6 differentially regulate tomato fruit ripening. Plant Physiol. 2024, kiae271. [Google Scholar] [CrossRef]
- Yao, G.; Gou, S.; Zhong, T.; Wei, S.; An, X.; Sun, H.; Sun, C.; Hu, K.; Zhang, H. Persulfidation of transcription factor MYB10 inhibits an-thocyanin synthesis in red-skinned pear. Plant Physiol. 2023, 192, 2185–2202. [Google Scholar] [CrossRef]
- Corpas, F.J.; González-Gordo, S.; Rodríguez-Ruiz, M.; Muñoz-Vargas, M.A.; Palma, J.M. Thiol-based oxidative posttranslational modifications (oxiPTMs) of plant proteins. Plant Cell Physiol. 2022, 63, 889–900. [Google Scholar] [CrossRef]
- Couto, N.; Wood, J.; Barber, J. The role of glutathione reductase and related enzymes on cellular redox homoeostasis network. Free Radic. Biol. Med. 2016, 95, 27–42. [Google Scholar] [CrossRef]
- Dorion, S.; Ouellet, J.C.; Rivoal, J. Glutathione Metabolism in Plants under Stress: Beyond Reactive Oxygen Species Detoxification. Metabolites 2021, 11, 641. [Google Scholar] [CrossRef]
- Broniowska, K.A.; Diers, A.R.; Hogg, N. S-nitrosoglutathione. Biochim. Biophys. Acta 2013, 1830, 3173–3181. [Google Scholar] [CrossRef]
- Saini, D.; Bapatla, R.B.; Vemula, C.K.; Gahir, S.; Bharath, P.; Gupta, K.J.; Raghavendra, A.S. Moderate modulation by S-nitrosoglutathione of photorespiratory enzymes in pea (Pisum sativum) leaves, compared to the strong effects of high light. Protoplasma 2023, 261, 43–51. [Google Scholar] [CrossRef]
- Pallavicini, C.; Peruffo, A.D.B.; Santoro, M. Isolation and partial characterization of grape aminopeptidase. J. Agric. Food Chem. 1981, 29, 1216–1220. [Google Scholar] [CrossRef]
- Casano, L.M.; Desimone, M.; Trippi, V.S. Proteolytic Activity at Alkaline pH in Oat Leaves, Isolation of an Aminopeptidase. Plant Physiol. 1989, 91, 1414–1418. [Google Scholar] [CrossRef]
- Matsui, M.; Fowler, J.H.; Walling, L.L. Leucine aminopeptidases: Diversity in structure and function. Biol. Chem. 2006, 387, 1535–1544. [Google Scholar] [CrossRef]
- Oszywa, B.; Makowski, M.; Pawełczak, M. Purification and partial characterization of aminopeptidase from barley (Hordeum vulgare L.) seeds. Plant Physiol. Biochem. 2013, 65, 75–80. [Google Scholar] [CrossRef]
- Herbers, K.; Willmitzer, L.; Prat, S. Functional analysis of a leucine aminopeptidase from Solanum tuberosum L. Planta 1994, 194, 230–240. [Google Scholar] [CrossRef]
- Kang, H.; Hahn, T.; Chung, I.; Park, J. Characterization of an Aminopeptidase from Grapes. Int. J. Plant Sci. 1999, 160, 299–306. [Google Scholar] [CrossRef]
- Desimone, M.; Krüger, M.; Wessel, T.; Wehofsky, M.; Hoffmann, R.; Wagner, E. Purification and characterization of an aminopeptidase from the chloroplast stroma of barley leaves by chromatographic and electrophoretic methods. J. Chromatogr. B Biomed. Sci. Appl. 2000, 737, 285–293. [Google Scholar] [CrossRef]
- Narváez-Vásquez, J.; Tu, C.-J.; Park, S.-Y.; Walling, L.L. Targeting and localization of wound-inducible leucine aminopeptidase A in tomato leaves. Planta 2007, 227, 341–351. [Google Scholar] [CrossRef]
- Tu, C.-J.; Park, S.-Y.; Walling, L.L. Isolation and characterization of the neutral leucine aminopeptidase (LapN) of tomato. Plant Physiol. 2003, 132, 243–255. [Google Scholar] [CrossRef]
- Kolehmainen, L.; Mikola, J. Partial purification and enzymatic properties of an aminopeptidase from barley. Arch. Biochem. Biophys. 1971, 145, 633–642. [Google Scholar] [CrossRef]
- Gupta, V.K.; Pawar, V.S. Leucine Aminopeptidase activity in tall and dwarf cultivars of rice at successive stages of development. Ann. Bot. 1974, 38, 205–208. [Google Scholar] [CrossRef]
- Murphy, A.; Peer, W.A.; Taiz, L. Regulation of auxin transport by aminopeptidases and endogenous flavonoids. Planta 2000, 211, 315–324. [Google Scholar] [CrossRef]
- Mahagamasekera, M.G.P.; Leung, D.W.M. Development of leucine aminopeptidase activity during daylily flower growth and senescence. Acta Physiol. Plant. 2001, 23, 181–186. [Google Scholar] [CrossRef]
- Gu, Y.-Q.; Chao, W.S.; Walling, L.L. Localization and post-translational processing of the wound-induced leucine aminopeptidase proteins of tomato. J. Biol. Chem. 1996, 271, 25880–25887. [Google Scholar] [CrossRef]
- Gu, Y.Q.; Walling, L.L. Specificity of the wound-induced leucine aminopeptidase LAP-A) of tomato: Activity on dipeptide and tripeptide substrates. Eur. J. Biochem. 2000, 267, 1178–1187. [Google Scholar] [CrossRef]
- Scranton, M.A.; Yee, A.; Park, S.-Y.; Walling, L.L. Plant leucine aminopeptidases moonlight as molecular chaperones to alleviate stress-induced damage. J. Biol. Chem. 2012, 287, 18408–18417. [Google Scholar] [CrossRef]
- Pautot, V.; Holzer, F.M.; Chaufaux, J.; Walling, L.L. The induction of tomato leucine aminopeptidase genes LapA after Pseudomonas syringae pv. tomato infection is primarily a wound response triggered by coronatine. Mol. Plant Microbe Interact. 2001, 14, 214–224. [Google Scholar]
- Panpetch, P.; Sirikantaramas, S. Fruit ripening-associated leucylaminopeptidase with cysteinylglycine dipeptidase activity from durian suggests its involvement in glutathione recycling. BMC Plant Biol. 2021, 21, 69. [Google Scholar] [CrossRef]
- Naef, R.; Velluz, A.; Jaquier, A. New volatile sulfur-containing constituents in a simultaneous distillation-extraction extract of red bell peppers (Capsicum annuum). J. Agric. Food Chem. 2007, 56, 517–527. [Google Scholar] [CrossRef]
- Du, X.; Song, M.; Rouseff, R. Identification of new strawberry sulfur volatiles and changes during maturation. J. Agric. Food Chem. 2011, 59, 1293–1300. [Google Scholar] [CrossRef]
- Del Corso, A.; Vilardo, P.G.; Cappiello, M.; Cecconi, I.; Dal Monte, M.; Barsacchi, D.; Mura, U. Physiological thiols as promoters of glutathione oxidation and modifying agents in protein S-thiolation. Arch. Biochem. Biophys. 2002, 397, 392–398. [Google Scholar] [CrossRef]
- Ito, T.; Ohkama-Ohtsu, N. Degradation of glutathione and glutathione conjugates in plants. J. Exp. Bot. 2023, 74, 3313–3327. [Google Scholar] [CrossRef]
- Kumar, S.; Kaur, A.; Chattopadhyay, B.; Bachhawat, A.K. Defining the cytosolic pathway of glutathione degradation in Arabidopsis thaliana: Role of the ChaC/GCG family of γ-glutamyl cyclotransferases as glutathione-degrading enzymes and AtLAP1 as the Cys-Gly peptidase. Biochem. J. 2015, 468, 73–85. [Google Scholar] [CrossRef]
- Guerra, D.; Ballard, K.; Truebridge, I.; Vierling, E. S-Nitrosation of Conserved Cysteines Modulates Activity and Stability of S-Nitrosoglutathione Reductase (GSNOR). Biochemistry 2016, 55, 2452–2464. [Google Scholar] [CrossRef]
- Mata-Pérez, C.; Padilla, M.N.; Sánchez-Calvo, B.; Begara-Morales, J.C.; Valderrama, R.; Chaki, M.; Aranda-Caño, L.; Moreno-González, D.; Molina-Díaz, A.; Barroso, J.B. Endogenous Biosynthesis of S-Nitrosoglutathione from Nitro-Fatty Acids in Plants. Front. Plant Sci. 2020, 11, 962. [Google Scholar] [CrossRef]
- Starkenmann, C.; Niclass, Y. New cysteine-S-conjugate precursors of volatile sulfur compounds in bell peppers (Capsicum annuum L. cultivar). J. Agric. Food Chem. 2011, 59, 3358–3365. [Google Scholar] [CrossRef]
- Cannon, R.J.; Ho, C.-T. Volatile sulfur compounds in tropical fruits. J. Food Drug Anal. 2018, 26, 445–468. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muñoz-Vargas, M.A.; González-Gordo, S.; Aroca, A.; Romero, L.C.; Gotor, C.; Palma, J.M.; Corpas, F.J. Persulfidome of Sweet Pepper Fruits during Ripening: The Case Study of Leucine Aminopeptidase That Is Positively Modulated by H2S. Antioxidants 2024, 13, 719. https://doi.org/10.3390/antiox13060719
Muñoz-Vargas MA, González-Gordo S, Aroca A, Romero LC, Gotor C, Palma JM, Corpas FJ. Persulfidome of Sweet Pepper Fruits during Ripening: The Case Study of Leucine Aminopeptidase That Is Positively Modulated by H2S. Antioxidants. 2024; 13(6):719. https://doi.org/10.3390/antiox13060719
Chicago/Turabian StyleMuñoz-Vargas, María A., Salvador González-Gordo, Angeles Aroca, Luis C. Romero, Cecilia Gotor, José M. Palma, and Francisco J. Corpas. 2024. "Persulfidome of Sweet Pepper Fruits during Ripening: The Case Study of Leucine Aminopeptidase That Is Positively Modulated by H2S" Antioxidants 13, no. 6: 719. https://doi.org/10.3390/antiox13060719
APA StyleMuñoz-Vargas, M. A., González-Gordo, S., Aroca, A., Romero, L. C., Gotor, C., Palma, J. M., & Corpas, F. J. (2024). Persulfidome of Sweet Pepper Fruits during Ripening: The Case Study of Leucine Aminopeptidase That Is Positively Modulated by H2S. Antioxidants, 13(6), 719. https://doi.org/10.3390/antiox13060719










