Nutraceutical Potential of Djulis (Chenopodium formosanum) Hull: Phytochemicals, Antioxidant Activity, and Liver Protection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Extraction and Identification of Hulls and Kernels from Three Djulis Strains
2.2. Determination of Total Phenolic Contents, Total Flavonoid Contents, and Antioxidant Activity
2.2.1. Total Phenolic Contents
2.2.2. Total Flavonoid Contents
2.2.3. 1,1-Diphenyl-2-picrylhydrazyl (DPPH) Radical Scavenging Activity
2.3. Animals and Treatments
2.4. Histopathology Analysis
2.5. Measurement of Antioxidant Enzyme Activity and Lipid Peroxidative Index in the Liver
2.6. Enzyme-Linked Immunosorbent Assay (ELISA)
2.7. Western Blot
2.8. Statistical Analysis
3. Results and Discussion
3.1. Differences in Composition among the Three Strains of Djulis Hull and Kernel Crude Extracts
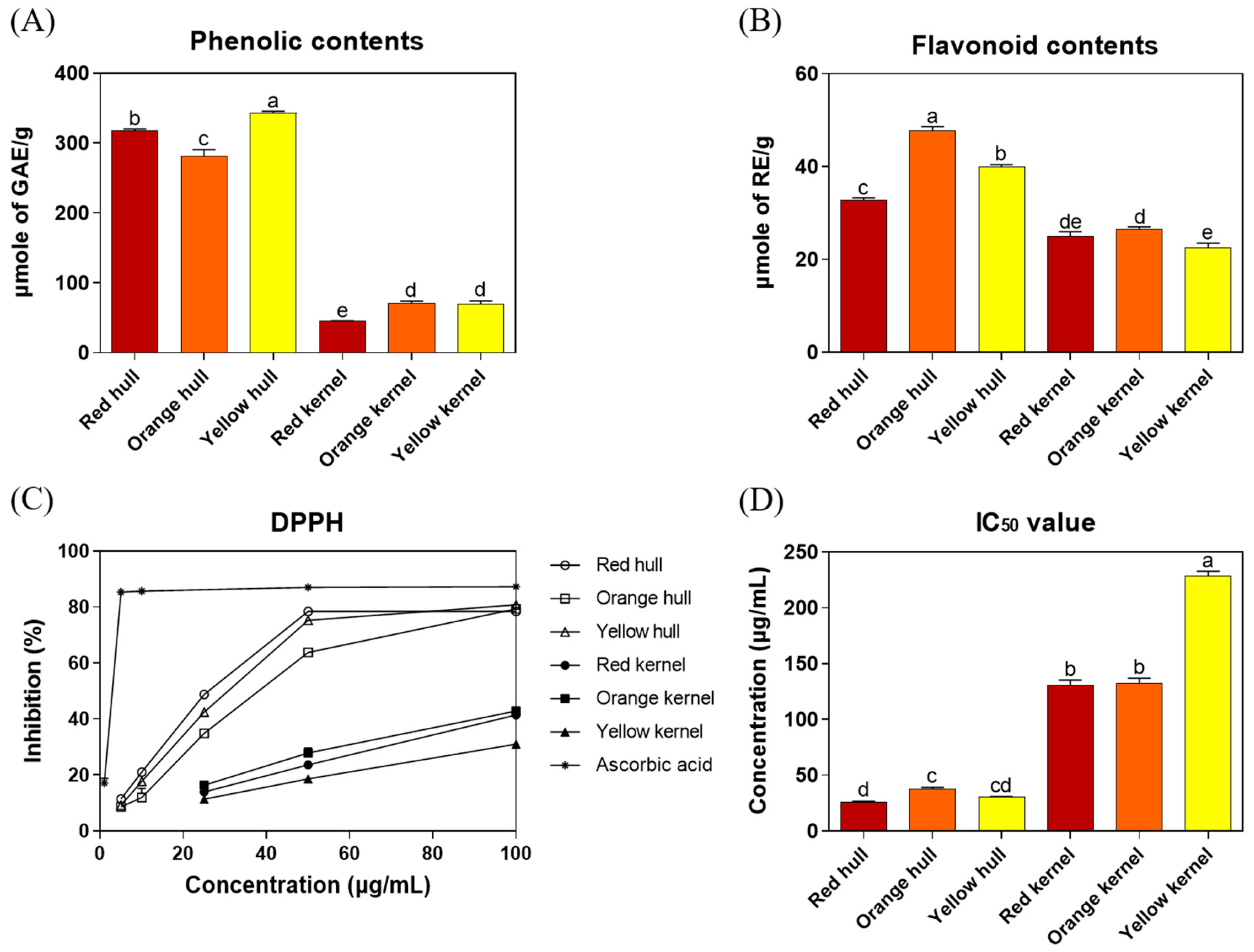
3.2. The Total Phenolic and Flavonoid Contents and Antioxidant Activity in Three Strains of Djulis Hull and Kernel Crude Extracts
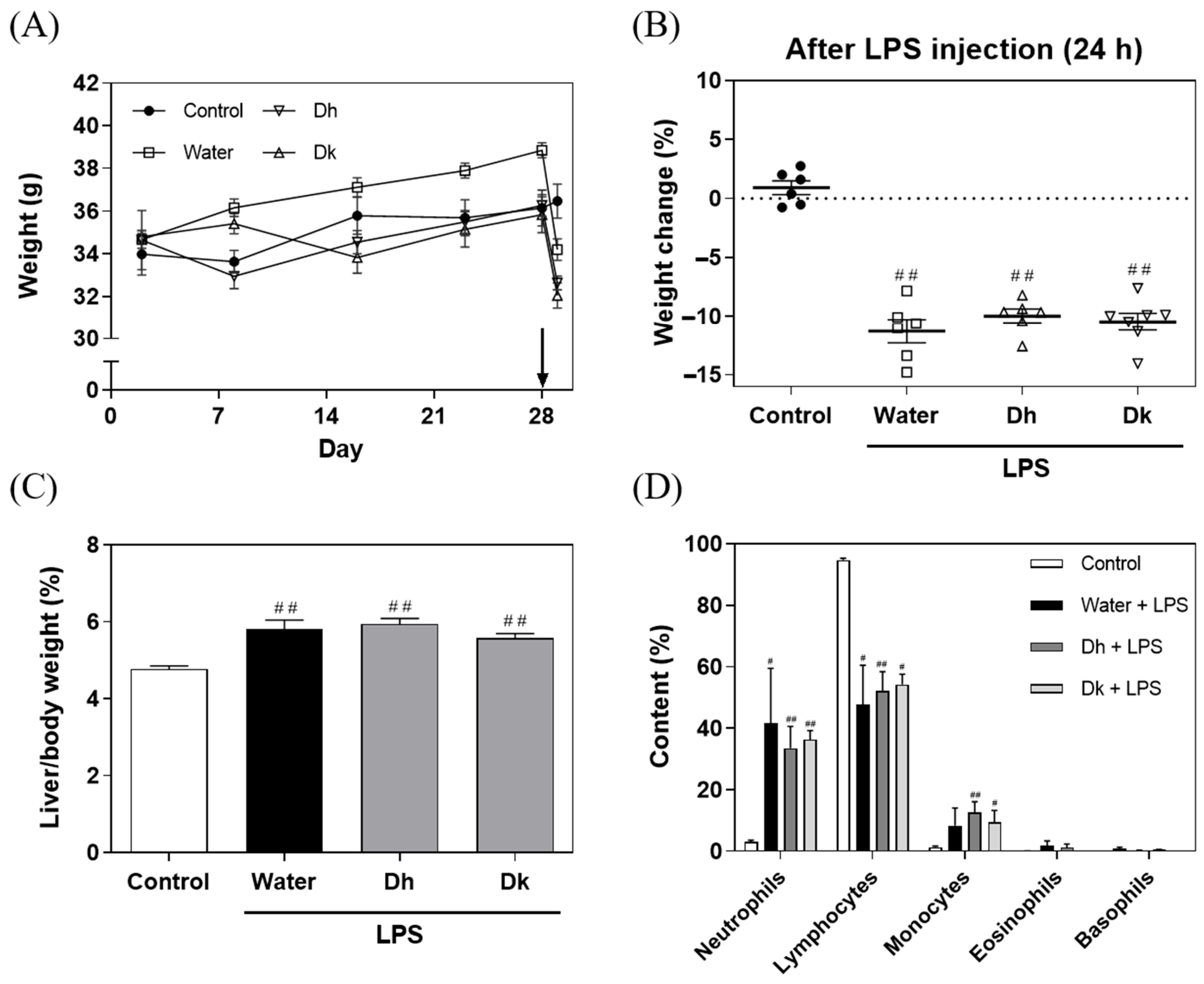
3.3. Effects of Djulis Hull and Kernel Crude Extracts on Food Intake, Water Consumption, Body Weight, and White Blood Cell Classification in LPS-Induced ALI in Mice
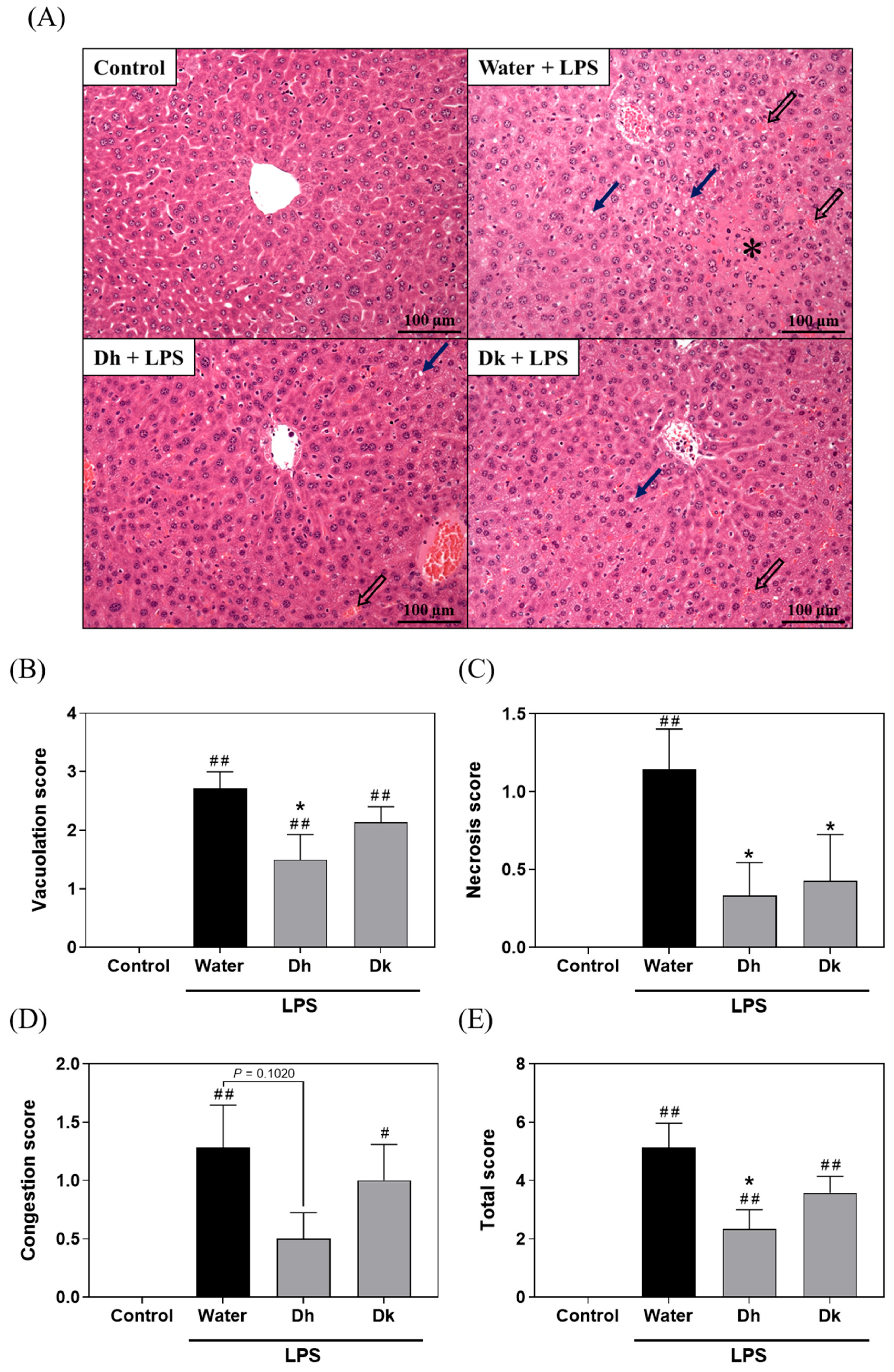
3.4. Djulis Hull and Kernel Crude Extracts Alleviate Liver Histopathological Changes in LPS-Induced ALI in Mice
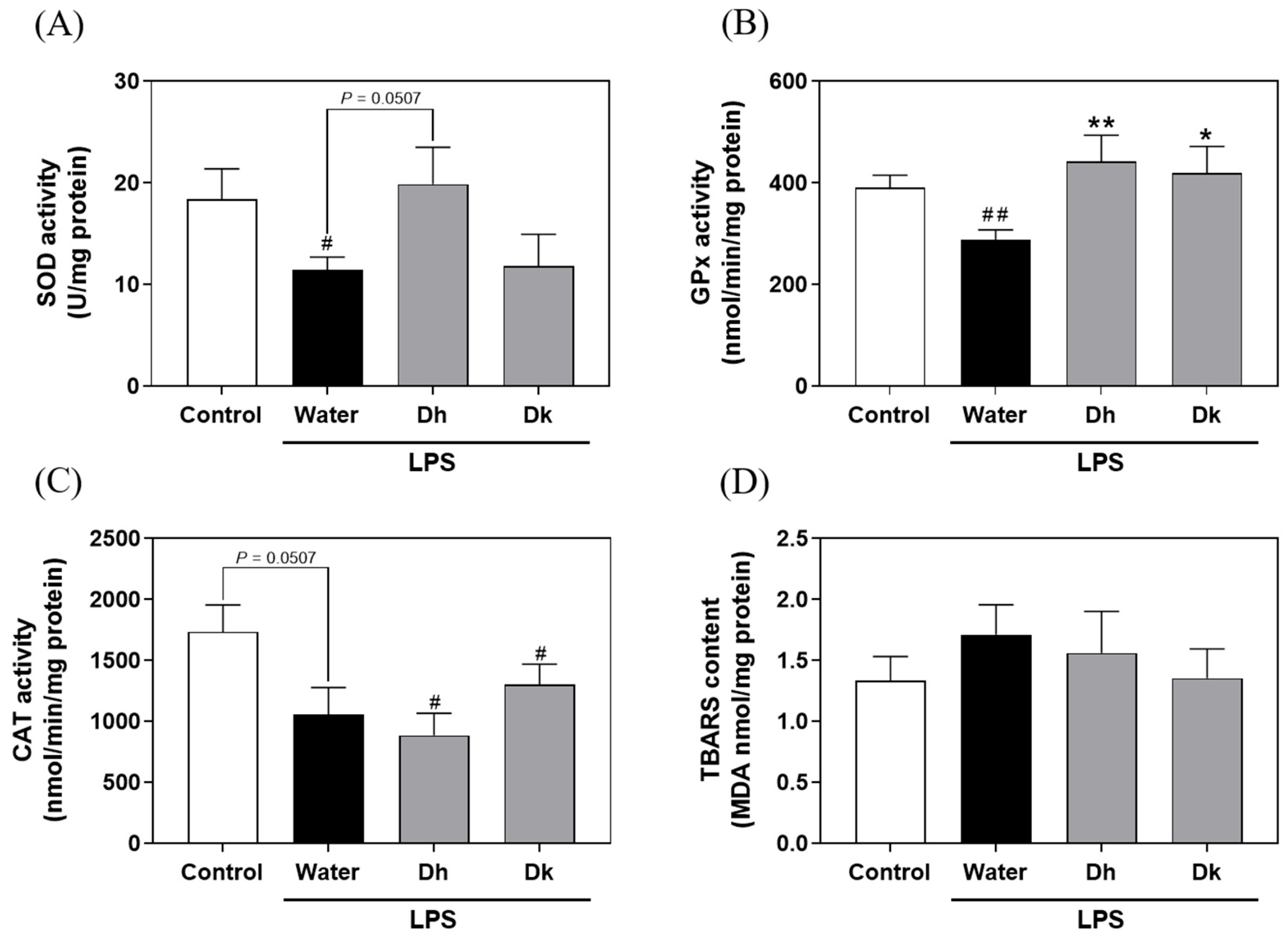
3.5. Evaluation of Djulis Hull and Kernel Crude Extracts on Antioxidant Enzymes and Lipid Peroxidation in the Liver of LPS-Induced ALI in Mice
3.6. Evaluation of Djulis Hull and Kernel Crude Extracts on Pro-Inflammatory and Anti-Inflammatory Cytokine Levels in Mouse Plasma and Liver Tissue of LPS-Induced ALI in Mice
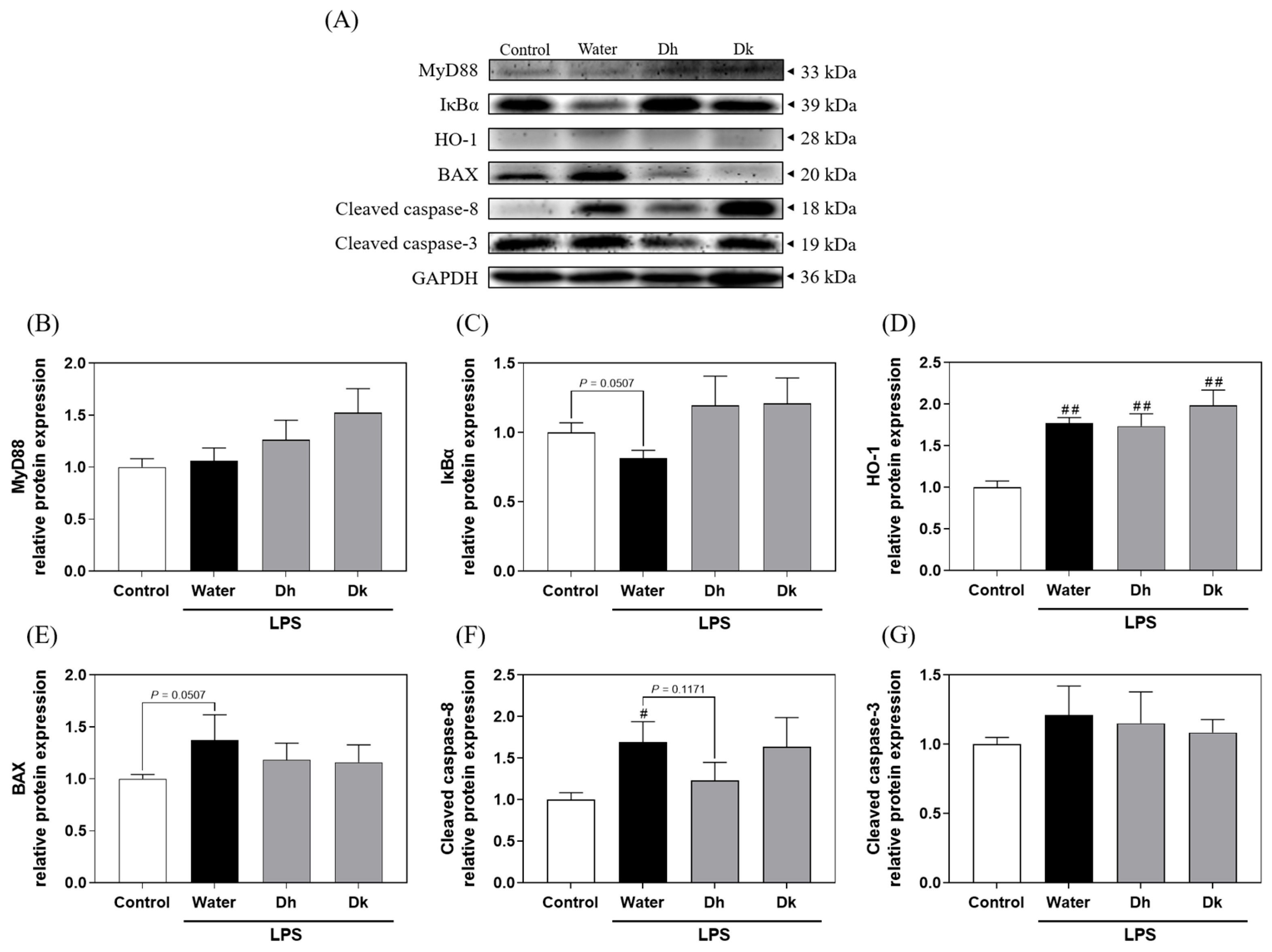
3.7. Mechanistic Insights into the Prevention Effects of Djulis Hull and Kernel Crude Extracts on ALI in Mice
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bi, J.; Zhang, J.; Ke, M.; Wang, T.; Wang, M.; Liu, W.; Du, Z.; Ren, Y.; Zhang, S.; Wu, Z.; et al. HSF2BP protects against acute liver injury by regulating HSF2/HSP70/MAPK signaling in mice. Cell Death Dis. 2022, 13, 830. [Google Scholar] [CrossRef] [PubMed]
- O’Grady, J.G. Acute liver failure. Postgrad. Med. J. 2005, 81, 148–154. [Google Scholar] [CrossRef]
- Stravitz, R.T.; Lee, W.M. Acute liver failure. Lancet 2019, 394, 869–881. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Liu, P.; Chen, Z.L.; Zhang, S.J.; Wang, Y.Q.; Cai, X.; Luo, L.; Zhou, X.; Zhao, L. Emodin attenuates lipopolysaccharide-induced acute liver injury via inhibiting the TLR4 signaling pathway in vitro and in vivo. Front. Pharmacol. 2018, 9, 962. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Meng, Y.; Bo, L.; Wang, C.; Bian, J.; Deng, X. Sophocarpine attenuates LPS-induced liver injury and improves survival of mice through suppressing oxidative stress, inflammation, and apoptosis. Mediat. Inflamm. 2018, 2018, 5871431. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.F.; Fan, C.C.; Yang, M.J.; Dong, M.L.; Bucala, R.; Pei, Z.H.; Zhang, Y.M.; Ren, J. CD74 knockout protects against LPS-induced myocardial contractile dysfunction through AMPK-Skp2-SUV39H1-mediated demethylation of BCLB. Br. J. Pharmacol. 2020, 177, 1881–1897. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.N.; Yan, K.; Cui, Y.L.; Fan, G.W.; Wang, Y.F. Protective effect of Salvia miltiorrhiza and Carthamus tinctorius extract against lipopolysaccharide-induced liver injury. World J. Gastroenterol. 2015, 21, 9079–9092. [Google Scholar] [CrossRef] [PubMed]
- Ingawale, D.K.; Mandlik, S.K.; Naik, S.R. Models of hepatotoxicity and the underlying cellular, biochemical and immunological mechanism(s): A critical discussion. Environ. Toxicol. Pharmacol. 2014, 37, 118–133. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Koduru, B.; Carlisle, C.; Akhter, H.; Liu, R.M.; Schroder, K.; Brandes, R.P.; Ojcius, D.M. NADPH oxidase 4 modulates hepatic responses to lipopolysaccharide mediated by Toll-like receptor-4. Sci. Rep. 2017, 7, 14346. [Google Scholar] [CrossRef]
- Strnad, P.; Tacke, F.; Koch, A.; Trautwein, C. Liver—Guardian, modifier and target of sepsis. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 55–66. [Google Scholar] [CrossRef]
- Kuzmich, N.N.; Sivak, K.V.; Chubarev, V.N.; Porozov, Y.B.; Savateeva-Lyubimova, T.N.; Peri, F. TLR4 signaling pathway modulators as potential therapeutics in inflammation and sepsis. Vaccines 2017, 5, 34. [Google Scholar] [CrossRef] [PubMed]
- Loboda, A.; Damulewicz, M.; Pyza, E.; Jozkowicz, A.; Dulak, J. Role of Nrf2/HO-1 system in development, oxidative stress response and diseases: An evolutionarily conserved mechanism. Cell. Mol. Life Sci. 2016, 73, 3221–3247. [Google Scholar] [CrossRef] [PubMed]
- Vince, J.E.; De Nardo, D.; Gao, W.; Vince, A.J.; Hall, C.; McArthur, K.; Simpson, D.; Vijayaraj, S.; Lindqvist, L.M.; Bouillet, P.; et al. The mitochondrial apoptotic effectors BAX/BAK activate caspase-3 and -7 to trigger NLRP3 inflammasome and caspase-8 driven IL-1beta activation. Cell Rep. 2018, 25, 2339–2353 e2334. [Google Scholar] [CrossRef]
- Singh, R.; Letai, A.; Sarosiek, K. Regulation of apoptosis in health and disease: The balancing act of BCL-2 family proteins. Nat. Rev. Mol. Cell Biol. 2019, 20, 175–193. [Google Scholar] [CrossRef]
- Li, P.H.; Chan, Y.J.; Lu, W.C.; Huang, D.W.; Chang, T.C.; Chang, W.H.; Nie, X.B.; Jiang, C.X.; Zhang, X.L. Bioresource utilization of Djulis (Chenopodium formosanum) biomass as natural antioxidants. Sustainability 2020, 12, 5926. [Google Scholar] [CrossRef]
- Chen, S.Y.; Chu, C.C.; Chyau, C.C.; Yang, J.W.; Duh, P.D. Djulis (Chenopodium formosanum) and its bioactive compounds affect vasodilation, angiotensin converting enzyme activity, and hypertension. Food Biosci. 2019, 32, 100469. [Google Scholar] [CrossRef]
- Chu, C.C.; Chen, S.Y.; Chyau, C.C.; Fu, Z.H.; Liu, C.C.; Duh, P.D. Protective effect of Djulis (Chenopodium formosanum) and its bioactive compounds against carbon tetrachloride-induced liver injury. J. Funct. Foods 2016, 26, 585–597. [Google Scholar] [CrossRef]
- Chu, C.C.; Chen, S.Y.; Chyau, C.C.; Wu, Y.C.; Chu, H.L.; Duh, P.D. Anticancer activity and mediation of apoptosis in hepatoma carcinoma cells induced by djulis and its bioactive compounds. J. Funct. Foods 2020, 75, 104225. [Google Scholar] [CrossRef]
- Hong, Y.H.; Huang, Y.L.; Liu, Y.C.; Tsai, P.J. Djulis (Chenopodium formosanum Koidz.) water extract and its bioactive components ameliorate dermal damage in UVB-irradiated skin models. BioMed Res. Int. 2016, 2016, 7368797. [Google Scholar] [CrossRef]
- Lin, T.A.; Ke, B.J.; Cheng, C.S.; Wang, J.J.; Wei, B.L.; Lee, C.L. Red quinoa bran extracts protects against carbon tetrachloride-induced liver injury and fibrosis in mice via activation of antioxidative enzyme systems and blocking TGF-beta1 pathway. Nutrients 2019, 11, 395. [Google Scholar] [CrossRef]
- Chyau, C.C.; Chu, C.C.; Chen, S.Y.; Duh, P.D. The inhibitory effects of Djulis (Chenopodium formosanum) and its bioactive compounds on adipogenesis in 3T3-L1 adipocytes. Molecules 2018, 23, 1780. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.W.; Cheng, M.C.; Chen, B.Y.; Wang, C.Y. Effects of high pressure extraction on the extraction yield, phenolic compounds, antioxidant and anti-tyrosinase activity of Djulis hull. J. Food Sci. Technol. 2019, 56, 4016–4024. [Google Scholar] [CrossRef] [PubMed]
- Tung, Y.T.; Zeng, J.L.; Ho, S.T.; Xu, J.W.; Li, S.; Wu, J.H. Anti-NAFLD effect of Djulis hull and its major compound, rutin, in mice with high-fat diet (HFD)-Induced obesity. Antioxidants 2021, 10, 1694. [Google Scholar] [CrossRef]
- Kujala, T.S.; Loponen, J.M.; Klika, K.D.; Pihlaja, K. Phenolics and betacyanins in red beetroot (Beta vulgaris) root: Distribution and effect of cold storage on the content of total phenolics and three individual compounds. J. Agric. Food Chem. 2000, 48, 5338–5342. [Google Scholar] [CrossRef]
- Quettier-Deleu, C.; Gressier, B.; Vasseur, J.; Dine, T.; Brunet, C.; Luyckx, M.; Cazin, M.; Cazin, J.C.; Bailleul, F.; Trotin, F. Phenolic compounds and antioxidant activities of buckwheat (Fagopyrum esculentum Moench) hulls and flour. J. Ethnopharmacol. 2000, 72, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Gyamfi, M.A.; Yonamine, M.; Aniya, Y. Free-radical scavenging action of medicinal herbs from Ghana: Thonningia sanguinea on experimentally induced liver injuries. Gen. Pharmacol. 1999, 32, 661–667. [Google Scholar] [CrossRef]
- Guo, X.X.; Qiu, J.; Qian, Y.Z. 6-Shogaol mitigates sepsis-associated hepatic injury through transcriptional regulation. Nutrients 2021, 13, 3427. [Google Scholar] [CrossRef]
- Guo, X.X.; Zhang, Y.D.; Wang, T.C.; Wang, X.L.; Xu, Y.Y.; Wang, Y.; Qiu, J. Ginger and 6-gingerol prevent lipopolysaccharide-induced intestinal barrier damage and liver injury in mice. J. Sci. Food Agric. 2022, 102, 1066–1075. [Google Scholar] [CrossRef]
- Lim, J.Y.; Lee, J.H.; Yun, D.H.; Lee, Y.M.; Kim, D.K. Inhibitory effects of nodakenin on inflammation and cell death in lipopolysaccharide-induced liver injury mice. Phytomedicine 2021, 81, 153411. [Google Scholar] [CrossRef]
- Chen, S.H.; Chu, C.C.; Lin, Y.C.; Duh, P.D. Djulis (Chenopodium formosanum) and its bioactive compounds for management of hyperlipidemia and hyperglycemia in high-fat diet-fed mice. Food Nutr. Res. 2019, 7, 452–457. [Google Scholar] [CrossRef]
- Chen, S.Y.; Chu, C.C.; Chyau, C.C.; Fu, Z.H.; Duh, P.D. Effect of water extract of Djulis (Chenopodium formosaneum) and its bioactive compounds on alcohol-induced liver damage in rats. Int. J. Food Sci. Nutr. 2018, 5, 55–63. [Google Scholar]
- Chyau, C.C.; Chu, C.C.; Chen, S.Y.; Duh, P.D. Djulis (Chenopodiun formosaneum) and its bioactive compounds protect against oxidative stress in human HepG2 cells. J. Funct. Foods 2015, 18, 159–170. [Google Scholar] [CrossRef]
- Hsu, B.Y.; Lin, S.W.; Inbaraj, B.S.; Chen, B.H. Simultaneous determination of phenolic acids and flavonoids in Chenopodium formosanum Koidz. (djulis) by HPLC-DAD-ESI-MS/MS. J. Pharm. Biomed. Anal. 2017, 132, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Zhang, M.; Wang, T.; Cai, M.; Qian, F.; Sun, Y.; Wang, Y. Green tea polyphenols prevent lipopolysaccharide-induced inflammatory liver injury in mice by inhibiting NLRP3 inflammasome activation. Food Funct. 2019, 10, 3898–3908. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Gao, Q.; Zhao, G.; Kan, Z.; Wang, X.; Wang, H.; Huang, J.; Wang, T.; Qian, F.; Ho, C.T.; et al. Protective effect and mechanism of theanine on lipopolysaccharide-induced inflammation and acute liver injury in mice. J. Agric. Food Chem. 2018, 66, 7674–7683. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Ding, Y.; Zeng, T.; Zhao, X.; Zhang, C. Hepatoprotective effect of diallyl trisulfide against lipopolysaccharide and D-galactosamine induced acute liver failure in mice via suppressing inflammation and apoptosis. Toxicol. Res. 2022, 11, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.A.; Ke, B.J.; Cheng, S.C.; Lee, C.L. Red quinoa bran extract prevented alcoholic fatty liver disease via increasing antioxidative system and repressing fatty acid synthesis factors in mice fed alcohol liquid diet. Molecules 2021, 26, 6973. [Google Scholar] [CrossRef]
- Tung, Y.T.; Zeng, J.L.; Ho, S.T.; Xu, J.W.; Lin, I.H.; Wu, J.H. Djulis hull improves insulin resistance and modulates the gut microbiota in high-fat diet (HFD)-induced hyperglycaemia. Antioxidants 2022, 11, 45. [Google Scholar] [CrossRef]
- Schwab, C.L.; Fan, R.; Zheng, Q.; Myers, L.P.; Hebert, P.; Pruett, S.B. Modeling and predicting stress-induced immunosuppression in mice using blood parameters. Toxicol. Sci. 2005, 83, 101–113. [Google Scholar] [CrossRef]
- Brown, D.; Libby, S.; Moreland, S.; McCoy, M.; Brabb, T.; Stepanek, A.; Fang, F.; Detweiler, C. Salmonella enterica causes more severe inflammatory disease in C57/BL6 Nramp1G169 mice than Sv129S6 mice. Vet. Pathol. 2013, 50, 867–876. [Google Scholar] [CrossRef]
- Brown, D.E.; McCoy, M.W.; Pilonieta, M.C.; Nix, R.N.; Detweiler, C.S. Chronic murine typhoid fever Is a natural model of secondary hemophagocytic lymphohistiocytosis. PLoS ONE 2010, 5, e9441. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, G.C.; Lopez, M.F.; Gomez, S.A.; Ramos, M.V.; Bentancor, L.V.; Fernandez-Brando, R.J.; Landoni, V.I.; Dran, G.I.; Meiss, R.; Isturiz, M.A.; et al. Relevance of neutrophils in the murine model of haemolytic uraemic syndrome: Mechanisms involved in Shiga toxin type 2-induced neutrophilia. Clin. Exp. Immunol. 2006, 146, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Bi, Y.; Wang, R.; Shen, B.; Zhang, Y.; Yang, H.; Wang, X.; Liu, H.; Lu, Y.; Han, F. Kinase AKT1 negatively controls neutrophil recruitment and function in mice. J. Immunol. 2013, 191, 2680–2690. [Google Scholar] [CrossRef] [PubMed]
- O’Connell, K.E.; Mikkola, A.M.; Stepanek, A.M.; Vernet, A.; Hall, C.D.; Sun, C.C.; Yildirim, E.; Staropoli, J.F.; Lee, J.T.; Brown, D.E. Practical murine hematopathology: A comparative review and implications for research. Comp. Med. 2015, 65, 96–113. [Google Scholar] [PubMed]
- Kamalakkannan, N.; Prince, P.S.M. Rutin improves the antioxidant status in streptozotocin-induced diabetic rat tissues. Mol. Cell Biochem. 2006, 293, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tang, C.; Zhang, H. Hepatoprotective effects of kaempferol 3-O-rutinoside and kaempferol 3-O-glucoside from Carthamus tinctorius L. on CCl4-induced oxidative liver injury in mice. J. Food Drug Anal. 2015, 23, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.C.; Chen, S.Y.; Chyau, C.C.; Wang, S.C.; Chu, H.L.; Duh, P.D. Djulis (Chenopodium formosanum) and its bioactive compounds protect human lung epithelial A549 Cells from oxidative injury induced by particulate matter via Nrf2 signaling pathway. Molecules 2021, 27, 253. [Google Scholar] [CrossRef] [PubMed]
- Celik, M.O.; Labuz, D.; Keye, J.; Glauben, R.; Machelska, H. IL-4 induces M2 macrophages to produce sustained analgesia via opioids. JCI Insight 2020, 5, e133093. [Google Scholar] [CrossRef]
- Wang, C.; Ma, C.; Gong, L.; Guo, Y.; Fu, K.; Zhang, Y.; Zhou, H.; Li, Y. Macrophage polarization and its role in liver disease. Front. Immunol. 2021, 12, 803037. [Google Scholar] [CrossRef]
- Jia, Y.N.; Lu, H.P.; Peng, Y.L.; Zhang, B.S.; Gong, X.B.; Su, J.; Zhou, Y.; Pan, M.H.; Xu, L. Oxyresveratrol prevents lipopolysaccharide/D-galactosamine-induced acute liver injury in mice. Int. Immunopharmacol. 2018, 56, 105–112. [Google Scholar] [CrossRef]
- Peng, Z.; Gong, X.; Yang, Y.; Huang, L.; Zhang, Q.; Zhang, P.; Wan, R.; Zhang, B. Hepatoprotective effect of quercetin against LPS/d-GalN induced acute liver injury in mice by inhibiting the IKK/NF-kappaB and MAPK signal pathways. Int. Immunopharmacol. 2017, 52, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.-C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Lin, L.; Zhang, Z.; Zhang, H.; Hu, H. Targeting NF-kappaB pathway for the therapy of diseases: Mechanism and clinical study. Signal Transduct. Target. Ther. 2020, 5, 209. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Y.-C.; Tung, C.-L.; Ho, S.-T.; Li, W.-S.; Li, S.; Tung, Y.-T.; Wu, J.-H. Nutraceutical Potential of Djulis (Chenopodium formosanum) Hull: Phytochemicals, Antioxidant Activity, and Liver Protection. Antioxidants 2024, 13, 721. https://doi.org/10.3390/antiox13060721
Huang Y-C, Tung C-L, Ho S-T, Li W-S, Li S, Tung Y-T, Wu J-H. Nutraceutical Potential of Djulis (Chenopodium formosanum) Hull: Phytochemicals, Antioxidant Activity, and Liver Protection. Antioxidants. 2024; 13(6):721. https://doi.org/10.3390/antiox13060721
Chicago/Turabian StyleHuang, Yu-Chen, Chun-Liang Tung, Shang-Tse Ho, Wei-Sung Li, Shiming Li, Yu-Tang Tung, and Jyh-Horng Wu. 2024. "Nutraceutical Potential of Djulis (Chenopodium formosanum) Hull: Phytochemicals, Antioxidant Activity, and Liver Protection" Antioxidants 13, no. 6: 721. https://doi.org/10.3390/antiox13060721






