Dopamine D2 Receptor Activation Blocks GluA2/ROS Positive Feedback Loop to Alienate Chronic-Migraine-Associated Pain Sensitization
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Materials
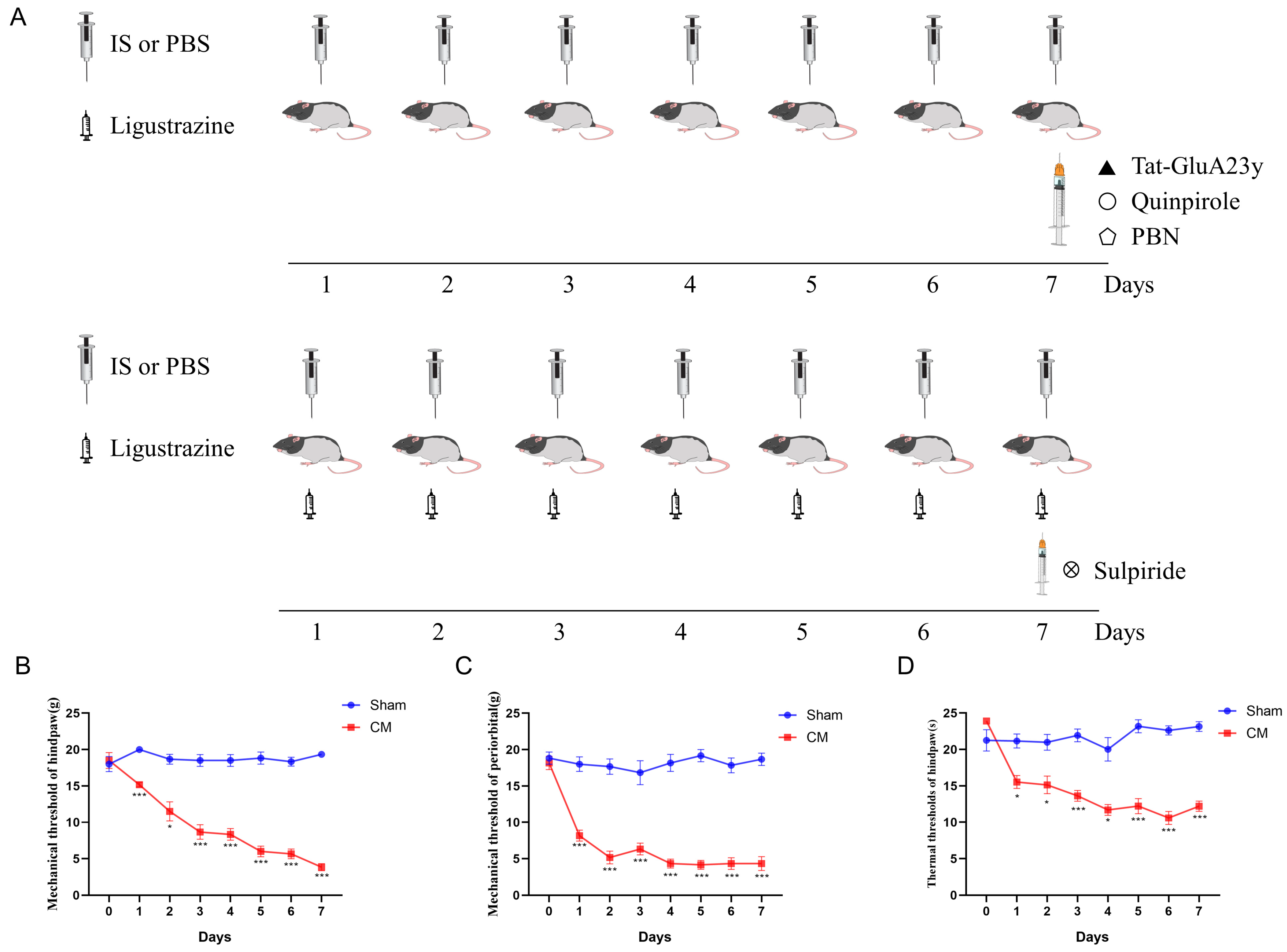
2.3. Surgery and Chronic Migraine Models
2.4. Pain Threshold Test
2.5. Drug Administration
2.6. Protein Extraction and Western Blot
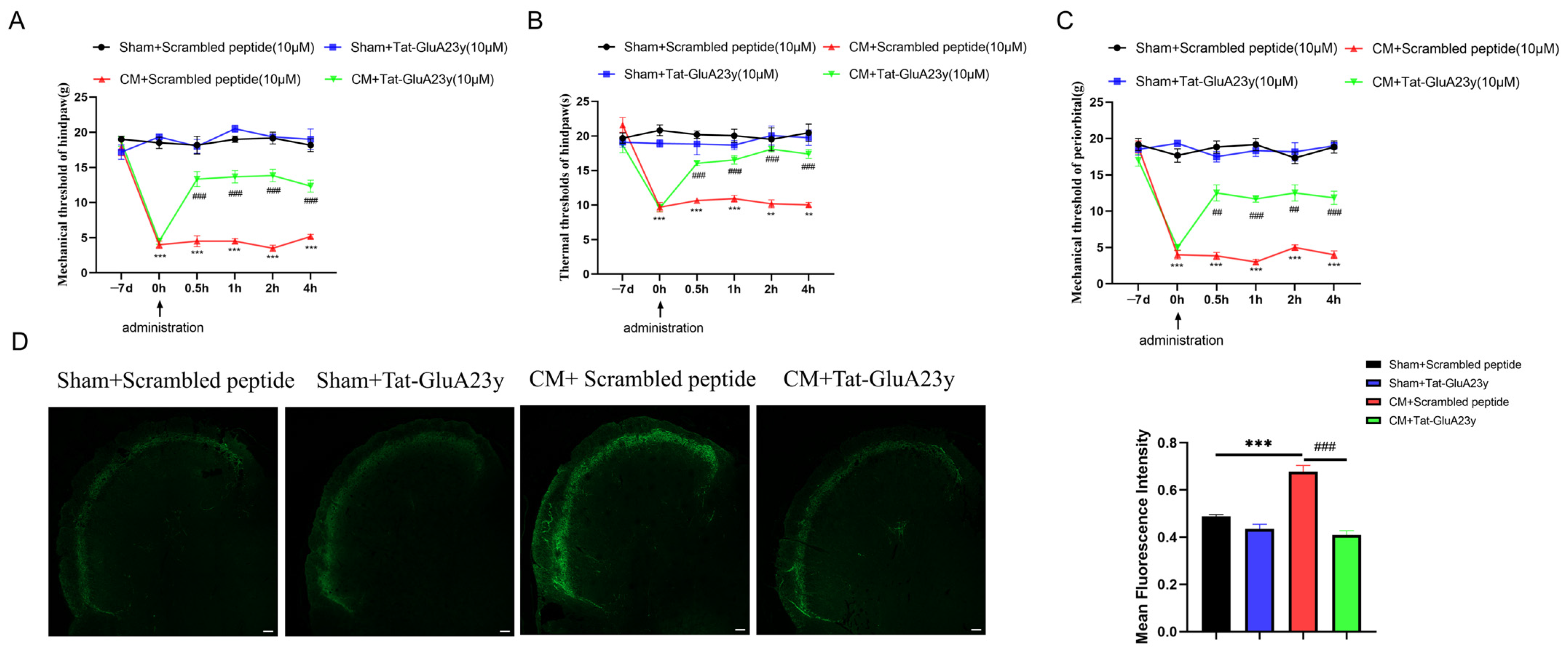
2.7. Immunofluorescence Staining
2.8. Primary Neuron Culture
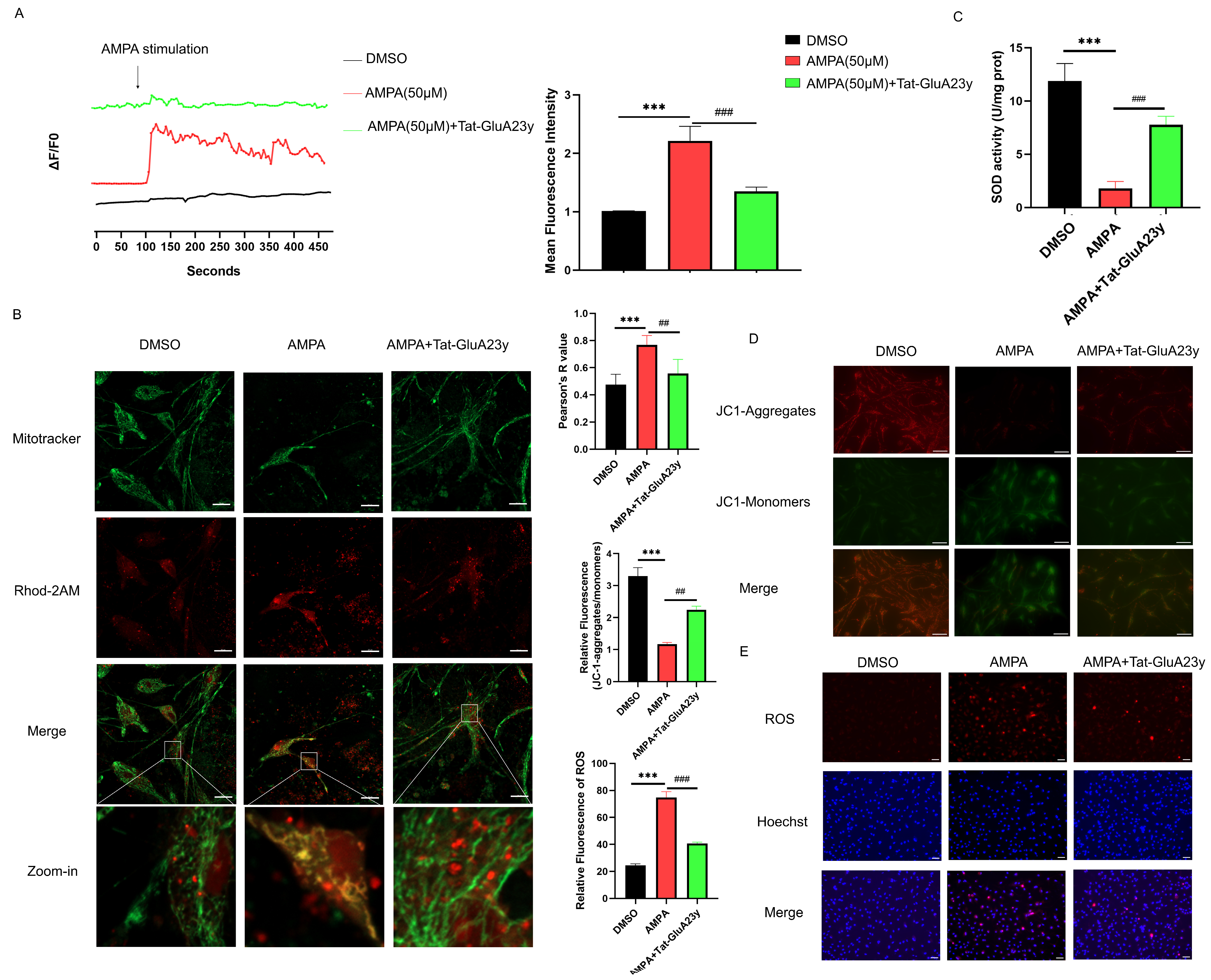
2.9. Monitoring of Ca2+ Concentration
2.10. Mitochondrial Ca2+ Detection
2.11. JC-1 and ROS Staining
2.12. Molecular Docking
2.13. Statistical Analysis
3. Results
3.1. Pharmacological Blockade of GluA2 Endocytosis Alleviated Chronic Migraine
3.2. GluA2 Endocytosis Inhibition Reduced Mitochondrial Calcium Overload and ROS Production
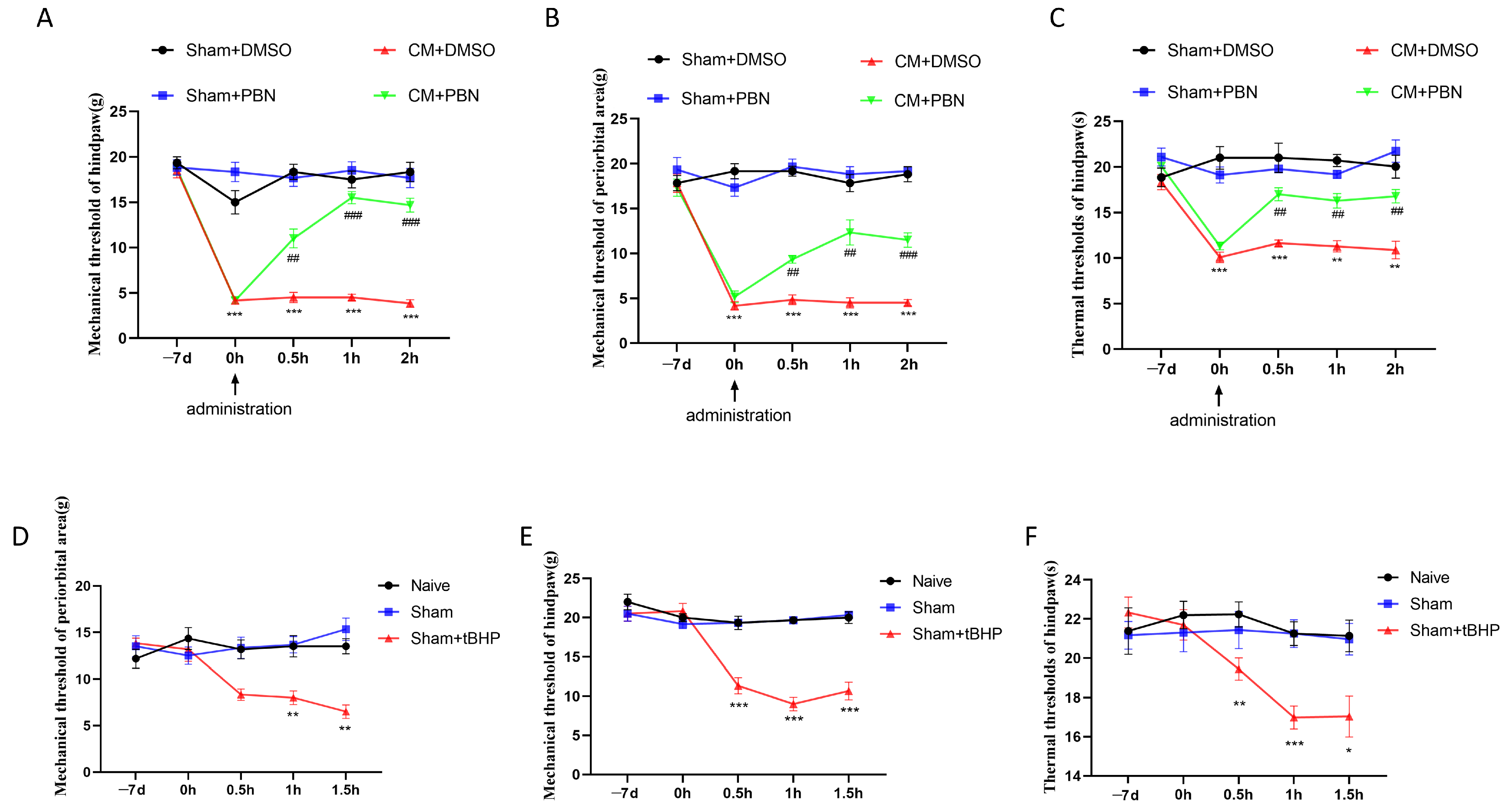
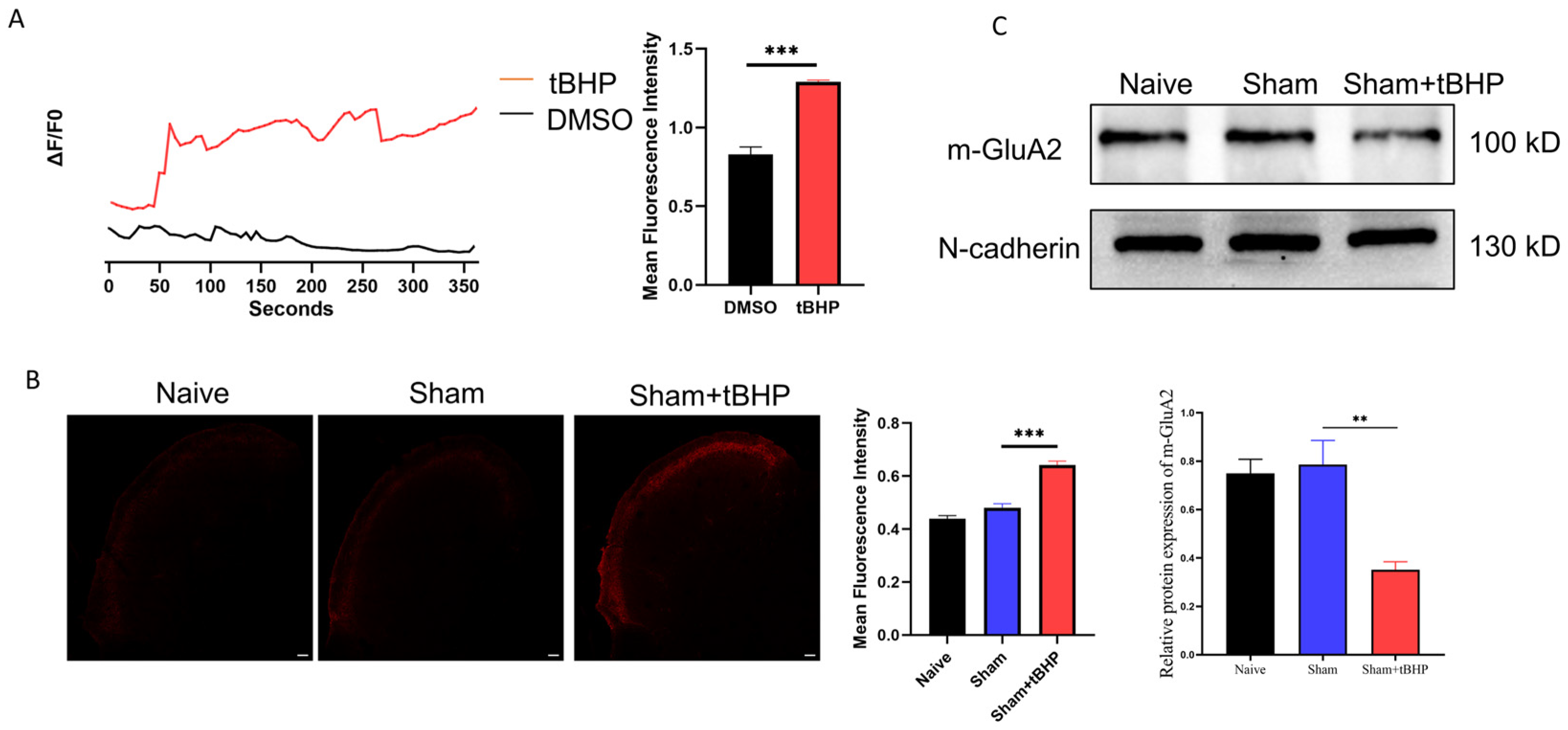
3.3. ROS Regulated Migraine-like Allodynia and GluA2 Trafficking
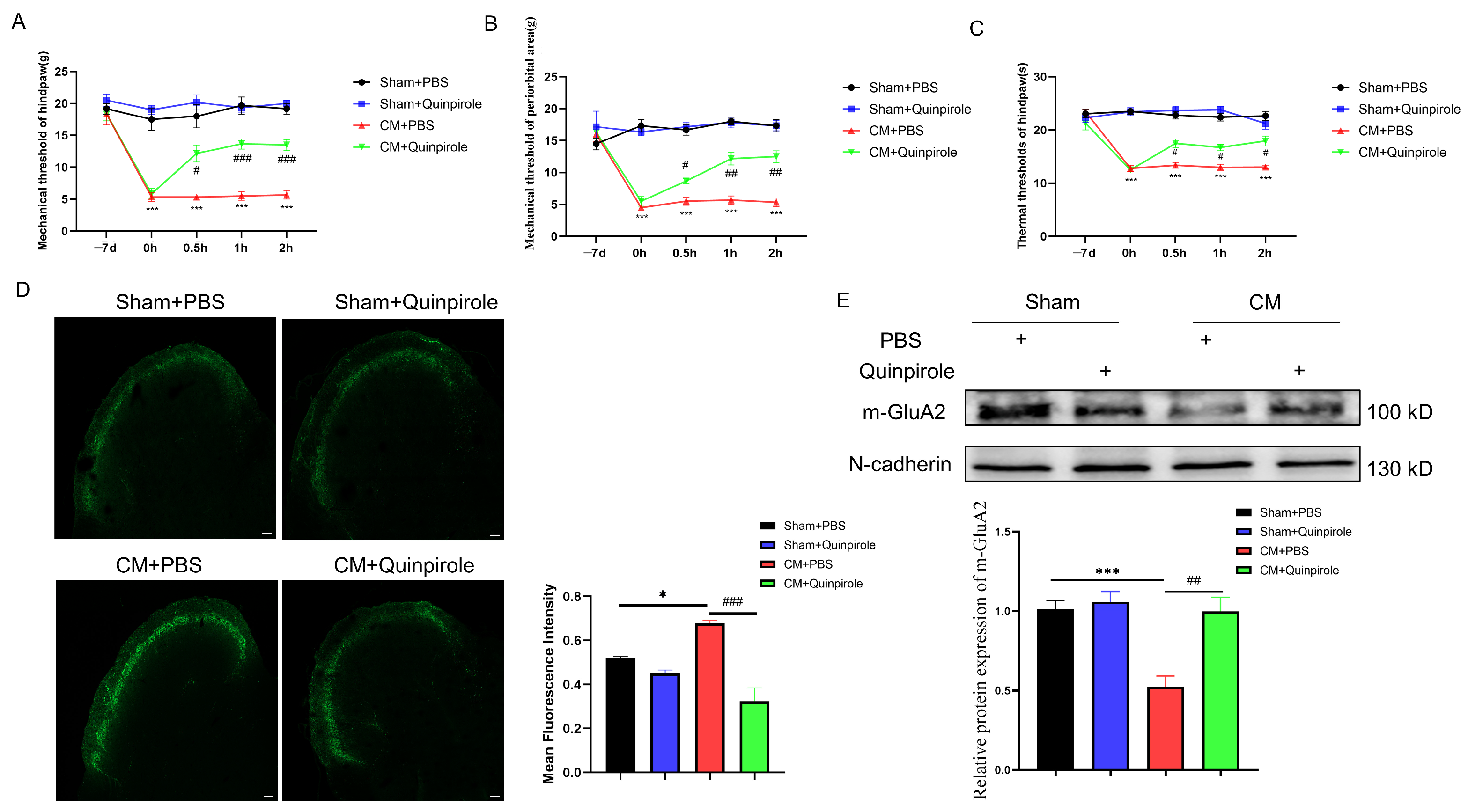
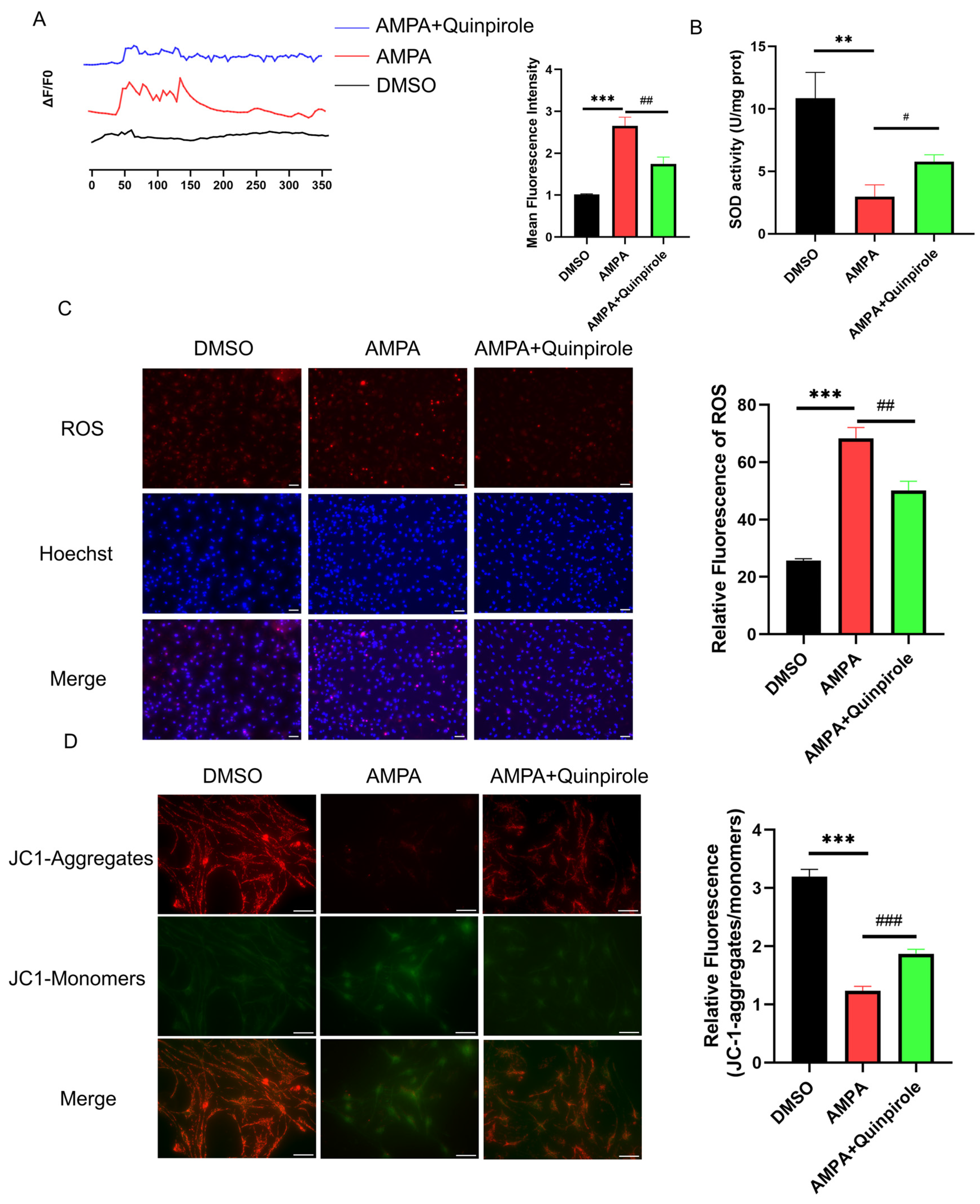
3.4. D2R Activation Intercepted the GluA2/ROS Feedback Loop
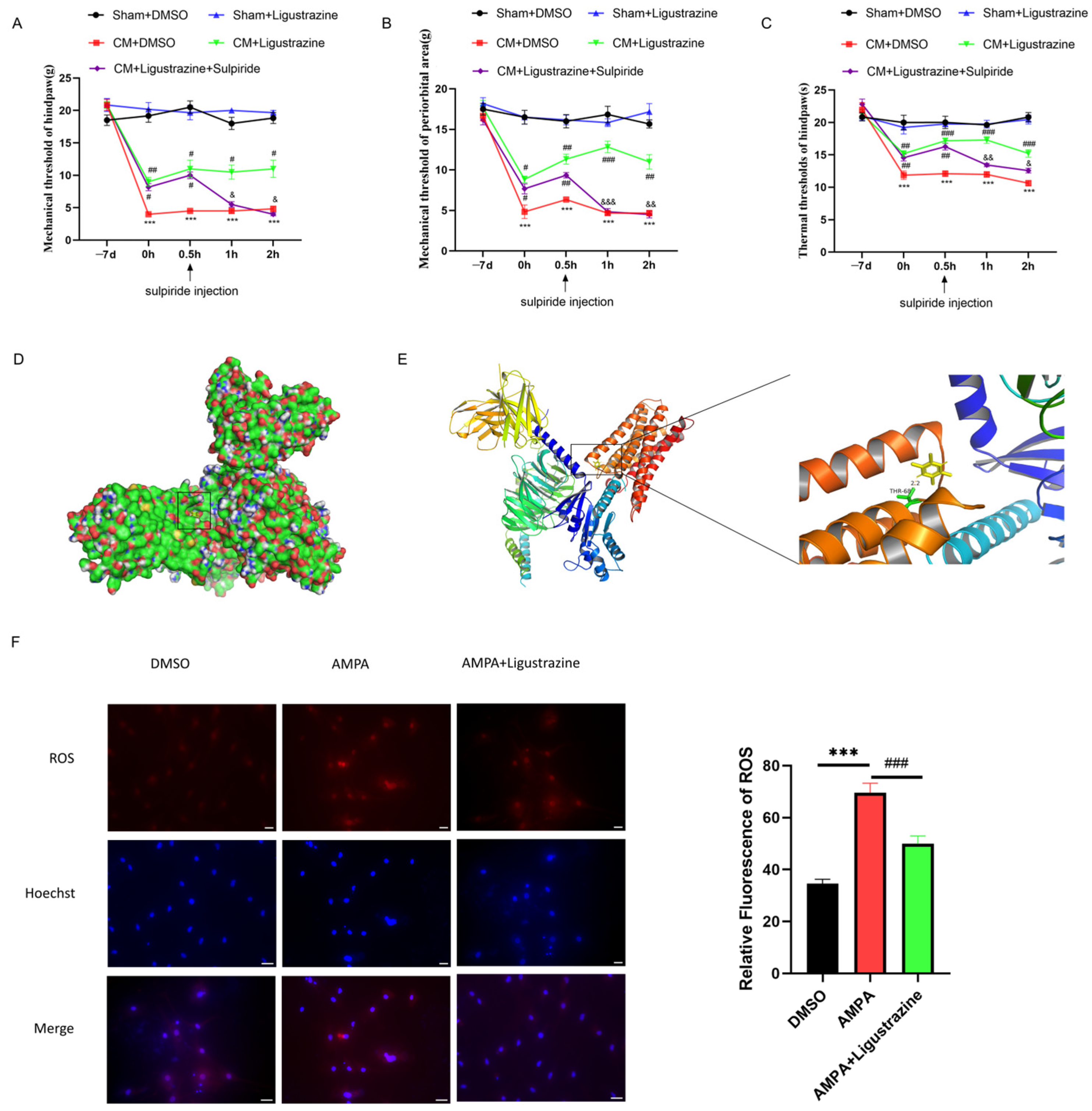
3.5. D2R May Be a Molecular Target of Ligustrazine for Treating CM
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AMPARs | α-Amino-3-hydroxy-5-methyl-4-isoxazole propionic acid receptors |
| CM | Chronic migraine |
| CNS | Central Nervous System |
| D2R | Dopamine D2 receptor |
| D3R | Dopamine D3 receptor |
| IS | Inflammatory soup |
| LCH | Ligusticum chuanxiong hort |
| PFA | Paraformaldehyde |
| ROS | Reactive oxygen species |
| TNC | Trigeminal nucleus caudalis |
References
- Schwedt, T.J. Chronic migraine. BMJ 2014, 348, g1416. [Google Scholar] [CrossRef] [PubMed]
- Su, M.; Yu, S. Chronic migraine: A process of dysmodulation and sensitization. Mol. Pain 2018, 14, 697. [Google Scholar] [CrossRef] [PubMed]
- Iyengar, S.; Johnson, K.W.; Ossipov, M.H.; Aurora, S.K. CGRP and the Trigeminal System in Migraine. Headache J. Head Face Pain 2019, 59, 659–681. [Google Scholar] [CrossRef] [PubMed]
- Ferroni, P.; Barbanti, P.; Della-Morte, D.; Palmirotta, R.; Jirillo, E.; Guadagni, F. Redox Mechanisms in Migraine: Novel Therapeutics and Dietary Interventions. Antioxidants Redox Signal. 2018, 28, 1144–1183. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Li, R.; Tang, W.; Ma, Z.; Miao, S.; Li, C.; Yang, C.; Li, B.; Wang, T.; Gong, Z.; et al. Proteomics profiling reveals mitochondrial damage in the thalamus in a mouse model of chronic migraine. J. Headache Pain 2023, 24, 122. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Ma, Y.-Y. Calcium Permeable-AMPA Receptors and Excitotoxicity in Neurological Disorders. Front. Neural Circuits 2021, 15, 711564. [Google Scholar] [CrossRef]
- Currò, D.; Navarra, P.; Samengo, I.; Martire, M. P2X7 receptors exert a permissive effect on the activation of presynaptic AMPA receptors in rat trigeminal caudal nucleus glutamatergic nerve terminals. J. Headache Pain 2020, 21, 83. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, J.; Chen, X.; Su, S.; Li, X.; Xu, X.; Yu, X.; Ke, C.; Zhu, X. Neuroligin1 Contributes to Neuropathic Pain by Promoting Phosphorylation of Cofilin in Excitatory Neurons. Front. Mol. Neurosci. 2021, 14, 533. [Google Scholar] [CrossRef]
- Cui, W.; Li, Y.; Wang, Z.; Song, C.; Yu, Y.; Wang, G.; Li, J.; Wang, C.; Zhang, L. Spinal caspase-6 regulates AMPA receptor trafficking and dendritic spine plasticity through netrin-1 in postoperative pain after orthopedic surgery for tibial fracture in mice. Pain 2020, 162, 124–134. [Google Scholar] [CrossRef]
- Greger, I.H.; Watson, J.F.; Cull-Candy, S.G. Structural and Functional Architecture of AMPA-Type Glutamate Receptors and Their Auxiliary Proteins. Neuron 2017, 94, 713–730. [Google Scholar] [CrossRef]
- Traynelis, S.F.; Wollmuth, L.P.; McBain, C.J.; Menniti, F.S.; Vance, K.M.; Ogden, K.K.; Hansen, K.B.; Yuan, H.; Myers, S.J.; Dingledine, R. Glutamate Receptor Ion Channels: Structure, Regulation, and Function. Pharmacol. Rev. 2010, 62, 405–496. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Lei, M.; Wen, Q.; Zhang, D.; Qin, G.; Zhou, J.; Chen, L. Dopamine receptor D2 regulates GLUA1-containing AMPA receptor trafficking and central sensitization through the PI3K signaling pathway in a male rat model of chronic migraine. J. Headache Pain 2022, 23, 98. [Google Scholar] [CrossRef]
- Liu, T.-Y.; Cheng, Y.; Qin, X.-Y.; Yu, L.-C. Pharmacologically inhibiting GluR2 internalization alleviates neuropathic pain. Neurosci. Bull. 2015, 31, 611–616. [Google Scholar] [CrossRef] [PubMed]
- Doser, R.L.; Amberg, G.C.; Hoerndli, F.J. Reactive Oxygen Species Modulate Activity-Dependent AMPA Receptor Transport in C. elegans. J. Neurosci. 2020, 40, 7405–7420. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.Z.; Chung, J.M.; Chung, K.; Kang, M.-G. Reactive oxygen species (ROS) modulate AMPA receptor phosphorylation and cell-surface localization in concert with pain-related behavior. Pain 2012, 153, 1905–1915. [Google Scholar] [CrossRef] [PubMed]
- Missale, C.; Nash, S.R.; Robinson, S.W.; Jaber, M.; Caron, M.G.; Wishart, D.S.; Anselmi, L.; Toti, L.; Bove, C.; Prakash, Y.S.; et al. Dopamine Receptors: From Structure to Function. Physiol. Rev. 1998, 78, 189–225. [Google Scholar] [CrossRef] [PubMed]
- Beaulieu, J.-M.; Gainetdinov, R.R. The Physiology, Signaling, and Pharmacology of Dopamine Receptors. Pharmacol. Rev. 2011, 63, 182–217. [Google Scholar] [CrossRef]
- Liu, S.; Shu, H.; Crawford, J.; Ma, Y.; Li, C.; Tao, F. Optogenetic Activation of Dopamine Receptor D1 and D2 Neurons in Anterior Cingulate Cortex Differentially Modulates Trigeminal Neuropathic Pain. Mol. Neurobiol. 2020, 57, 4060–4068. [Google Scholar] [CrossRef] [PubMed]
- Zou, S.; Li, L.; Pei, L.; Vukusic, B.; Van Tol, H.H.M.; Lee, F.J.S.; Wan, Q.; Liu, F. Protein-Protein Coupling/Uncoupling Enables Dopamine D2Receptor Regulation of AMPA Receptor-Mediated Excitotoxicity. J. Neurosci. 2005, 25, 4385–4395. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Li, S.; Guo, Z.; Chen, Z.; Zhang, X.; Xu, C.; Chen, J.; Wei, C. Dopamine receptor D2 inhibition alleviates diabetic hepatic stellate cells fibrosis by regulating the TGF-β1/Smads and NFκB pathways. Clin. Exp. Pharmacol. Physiol. 2021, 48, 370–380. [Google Scholar] [CrossRef]
- Fu, G.; Fan, X.; Liang, X.; Wei, J.; Jia, M.; Liu, S.; Shen, W.; Zhang, Y. An Overview of Systematic Reviews of Chinese Herbal Medicine in the Treatment of Migraines. Front. Pharmacol. 2022, 13, 924994. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Ni, N.; Hong, Y.; Lin, X.; Feng, Y.; Shen, L. Progress in Traditional Chinese Medicine for the Treatment of Migraine. Am. J. Chin. Med. 2020, 48, 1731–1748. [Google Scholar] [CrossRef] [PubMed]
- Feng, F.; Xu, D.-Q.; Yue, S.-J.; Chen, Y.-Y.; Tang, Y.-P. Neuroprotection by tetramethylpyrazine and its synthesized analogues for central nervous system diseases: A review. Mol. Biol. Rep. 2024, 51, 159. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Zhao, S.; Guo, Z.; Zhu, Y.; Zhang, S.; Li, D.; Shu, G. Tetramethylpyrazine Antagonizes the Subchronic Cadmium Exposure-Induced Oxidative Damage in Mouse Livers via the Nrf2/HO-1 Pathway. Molecules 2024, 29, 1434. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.; Zheng, C.; Cao, J.; Luo, F.; Li, H.; Hu, S.; Lee, S.M.; Yang, X.; Zhang, G.; Zhang, Z.; et al. Tetramethylpyrazine nitrone exerts neuroprotection via activation of PGC-1α/Nrf2 pathway in Parkinson’s disease models. J. Adv. Res. 2023. [Google Scholar] [CrossRef] [PubMed]
- Adamson, A.; Buck, S.A.; Freyberg, Z.; De Miranda, B.R. Sex Differences in Dopaminergic Vulnerability to Environmental Toxicants—Implications for Parkinson’s Disease. Curr. Environ. Heal. Rep. 2022, 9, 563–573. [Google Scholar] [CrossRef] [PubMed]
- De la Luz-Cuellar, Y.E.; Coffeen, U.; Mercado, F.; Granados-Soto, V. Spinal dopaminergic D1-and D2-like receptors have a sex-dependent effect in an experimental model of fibromyalgia. Eur. J. Pharmacol. 2023, 948, 175696. [Google Scholar] [CrossRef]
- Suzuki, K.; Suzuki, S.; Shiina, T.; Kobayashi, S.; Hirata, K. Central Sensitization in Migraine: A Narrative Review. J. Pain Res. 2022, ume 15, 2673–2682. [Google Scholar] [CrossRef]
- Lai, T.-H.; Protsenko, E.; Cheng, Y.-C.; Loggia, M.L.; Coppola, G.; Chen, W.-T. Neural Plasticity in Common Forms of Chronic Headaches. Neural Plast. 2015, 2015, 205985. [Google Scholar] [CrossRef]
- Citri, A.; Malenka, R.C. Synaptic Plasticity: Multiple Forms, Functions, and Mechanisms. Neuropsychopharmacology 2008, 33, 18–41. [Google Scholar] [CrossRef]
- Ji, R.-R.; Nackley, A.; Huh, Y.; Terrando, N.; Maixner, W. Neuroinflammation and Central Sensitization in Chronic and Widespread Pain. Anesthesiology 2018, 129, 343–366. [Google Scholar] [CrossRef]
- Su, C.; D’amour, J.; Lee, M.; Lin, H.-Y.; Manders, T.; Xu, D.; Eberle, S.E.; Goffer, Y.; Zou, A.H.; Rahman, M.; et al. Persistent pain alters AMPA receptor subunit levels in the nucleus accumbens. Mol. Brain 2015, 8, 46. [Google Scholar] [CrossRef]
- Rebola, N.; Srikumar, B.N.; Mulle, C. Activity-dependent synaptic plasticity of NMDA receptors. J. Physiol. 2010, 588, 93–99. [Google Scholar] [CrossRef]
- Ishida, M.; Mitsui, T.; Izawa, M.; Arita, J. Activation of D2 dopamine receptors inhibits estrogen response element-mediated estrogen receptor transactivation in rat pituitary lactotrophs. Mol. Cell. Endocrinol. 2013, 375, 58–67. [Google Scholar] [CrossRef]
- Sengupta, A.; Sarkar, D.K. Estrogen inhibits D2S receptor-regulated Gi3 and Gs protein interactions to stimulate prolactin production and cell proliferation in lactotropic cells. J. Endocrinol. 2012, 214, 67–78. [Google Scholar] [CrossRef]
- Al Sweidi, S.; Morissette, M.; Rouillard, C.; Di Paolo, T. Estrogen receptors and lesion-induced response of striatal dopamine receptors. Neuroscience 2013, 236, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Hardt, O.; Nader, K.; Wang, Y.-T. GluA2-dependent AMPA receptor endocytosis and the decay of early and late long-term potentiation: Possible mechanisms for forgetting of short- and long-term memories. Philos. Trans. R. Soc. B Biol. Sci. 2014, 369, 20130141. [Google Scholar] [CrossRef]
- Jiang, H.; Liu, J.-P.; Xi, K.; Liu, L.-Y.; Kong, L.-Y.; Cai, J.; Cai, S.-Q.; Han, X.-Y.; Song, J.-G.; Yang, X.-M.; et al. Contribution of AMPA Receptor-Mediated LTD in LA/BLA-CeA Pathway to Comorbid Aversive and Depressive Symptoms in Neuropathic Pain. J. Neurosci. 2021, 41, 7278–7299. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.-B.; Zhang, M.-M.; Cheng, L.-F.; Shi, J.; Lu, J.-S.; Zhuo, M. Long-term upregulation of cortical glutamatergic AMPA receptors in a mouse model of chronic visceral pain. Mol. Brain 2015, 8, 76. [Google Scholar] [CrossRef]
- Delgado, J.Y.; Selvin, P.R. A Revised View on the Role of Surface AMPAR Mobility in Tuning Synaptic Transmission: Limitations, Tools, and Alternative Views. Front. Synaptic Neurosci. 2018, 10, 21. [Google Scholar] [CrossRef]
- Zhang, Y.-Y.; Liu, F.; Fang, Z.-H.; Li, Y.-L.; Liao, H.-L.; Song, Q.-X.; Zhou, C.; Shen, J.-F. Differential roles of NMDAR subunits 2A and 2B in mediating peripheral and central sensitization contributing to orofacial neuropathic pain. Brain, Behav. Immun. 2022, 106, 129–146. [Google Scholar] [CrossRef] [PubMed]
- Harding, E.K.; Zamponi, G.W. Central and peripheral contributions of T-type calcium channels in pain. Mol. Brain 2022, 15, 39. [Google Scholar] [CrossRef] [PubMed]
- Walkon, L.L.; Strubbe-Rivera, J.O.; Bazil, J.N. Calcium Overload and Mitochondrial Metabolism. Biomolecules 2022, 12, 1891. [Google Scholar] [CrossRef] [PubMed]
- Sidlauskaite, E.; Gibson, J.W.; Megson, I.L.; Whitfield, P.D.; Tovmasyan, A.; Batinic-Haberle, I.; Murphy, M.P.; Moult, P.R.; Cobley, J.N. Mitochondrial ROS cause motor deficits induced by synaptic inactivity: Implications for synapse pruning. Redox Biol. 2018, 16, 344–351. [Google Scholar] [CrossRef] [PubMed]
- Anggono, V.; Huganir, R.L. Regulation of AMPA receptor trafficking and synaptic plasticity. Curr. Opin. Neurobiol. 2012, 22, 461–469. [Google Scholar] [CrossRef]
- Hanley, J.G. Regulation of AMPAR expression by microRNAs. Neuropharmacology 2021, 197, 108723. [Google Scholar] [CrossRef]
- Widagdo, J.; Guntupalli, S.; Jang, S.E.; Anggono, V. Regulation of AMPA Receptor Trafficking by Protein Ubiquitination. Front. Mol. Neurosci. 2017, 10, 347. [Google Scholar] [CrossRef]
- Kamalova, A.; Nakagawa, T. AMPA receptor structure and auxiliary subunits. J. Physiol. 2020, 599, 453–469. [Google Scholar] [CrossRef]
- Neve, K.A.; Seamans, J.K.; Trantham-Davidson, H. Dopamine Receptor Signaling. J. Recept. Signal Transduct. 2004, 24, 165–205. [Google Scholar] [CrossRef]
- Kaushik, P.; Ali, M.; Tabassum, H.; Parvez, S. Post-ischemic administration of dopamine D2 receptor agonist reduces cell death by activating mitochondrial pathway following ischemic stroke. Life Sci. 2020, 261, 118349. [Google Scholar] [CrossRef]
- Villalobos-Escobedo, F.S.; Jijón-Lorenzo, R.; Avalos-Fuentes, J.A.; Paz-Bermúdez, F.; Recillas-Morales, S.; Rojas, I.C.; Leyva-Gómez, G.; Cortés, H.; Florán, B. Dopamine D3 receptor modulates D2 receptor effects on cAMP and GABA release at striatopallidal terminals-Modulation by the Ca2+-Calmodulin-CaMKII system. Eur. J. Neurosci. 2023, 59, 1441–1459. [Google Scholar] [CrossRef] [PubMed]
- DaSilva, A.F.; Nascimento, T.D.; Jassar, H.; Heffernan, J.; Toback, R.L.; Lucas, S.; DosSantos, M.F.; Bellile, E.L.; Boonstra, P.S.; Taylor, J.M.; et al. Dopamine D2/D3 imbalance during migraine attack and allodynia in vivo. Neurology 2017, 88, 1634–1641. [Google Scholar] [CrossRef] [PubMed]
- Seeman, P.; Schaus, J.M. Dopamine receptors labelled by [3H] quinpirole. Eur. J. Pharmacol. 1991, 203, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Xing, B.; Chu, Z.; Liu, F.; Lei, G.; Zhu, L.; Gao, Y.; Chen, T.; Dang, Y. Dopamine D3 receptor knockout mice exhibit abnormal nociception in a sex-different manner. J. Neurosci. Res. 2016, 95, 1438–1445. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Clemens, S.; Sawchuk, M.; Hochman, S. Expression and distribution of all dopamine receptor subtypes (D1–D5) in the mouse lumbar spinal cord: A real-time polymerase chain reaction and non-autoradiographic in situ hybridization study. Neuroscience 2007, 149, 885–897. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Pan, C.-L.; Wang, C.-Y.; Liu, B.-Q.; Han, Y.; Hu, L.; Liu, L.; Yang, Y.; Qu, J.-W.; Liu, W.-T. Selective suppression of the JNK-MMP2/9 signal pathway by tetramethylpyrazine attenuates neuropathic pain in rats. J. Neuroinflammation 2017, 14, 174. [Google Scholar] [CrossRef] [PubMed]
- Rao, Y.; Li, J.; Qiao, R.; Luo, J.; Liu, Y. Synergistic effects of tetramethylpyrazine and astragaloside IV on spinal cord injury via alteration of astrocyte A1/A2 polarization through the Sirt1-NF-κB pathway. Int. Immunopharmacol. 2024, 131, 111686. [Google Scholar] [CrossRef]
- Gao, T.; Li, T.; Jiang, W.; Fan, W.; Xu, X.-J.; Zhao, X.; Yin, Z.; Guo, H.; Wang, L.; Gao, J.; et al. Antinociceptive Effects of Sinomenine Combined with Ligustrazine or Paracetamol in Animal Models of Incisional and Inflammatory Pain. Front. Physiol. 2021, 11, 3769. [Google Scholar] [CrossRef]
| Component | Manufacturer | Concentration |
|---|---|---|
| Bradykinin | Sigma-Aldrich, St. Louis, MO, USA | 1 mM |
| Histamine | Sigma-Aldrich, St. Louis, MO, USA | 1 mM |
| Serotonin | Sigma-Aldrich, St. Louis, MO, USA | 1 mM |
| Prostaglandin E2 | Sigma-Aldrich, St. Louis, MO, USA | 0.1 mM |
| Antibody | Manufacturer | Dilution |
|---|---|---|
| Anti-GluA2 | Abcam, Cambridge, UK | 1:1000 |
| Proteintech, Wuhan, China | 1:1500 | |
| Anti-N-cadherin | PTM Biolabs, Hangzhou, China | 1:1000 |
| GAPDH | ZEN-BIOSCIENCE, Chengdu, China | 1:8000 |
| Anti-D2R | Proteintech, Rosemont, IL, USA | 1:1000 |
| Anti-CGRP (For IF) | Santa Cruz, Santa Cruz, CA, USA | 1:400 |
| Goat anti-rabbit IgG | ZEN-BIOSCIENCE, Chengdu, China | 1:5000 |
| Goat anti-mouse IgG | ZEN-BIOSCIENCE, Chengdu, China | 1:5000 |
| Alexa Fluor 488 anti-mouse IgG | Beyotime, Haimen, China | 1:400 |
| Cy3-labeled goat anti-mouse IgG | Beyotime, Haimen, China | 1:400 |
| Binding Energy (kcal/mol) |
|---|
| −4.24 |
| −4.23 |
| −4.08 |
| −3.96 |
| −3.91 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, W.; Zhang, X.; Lei, M.; Zhang, D.; Qin, G.; Zhou, J.; Ji, L.; Chen, L. Dopamine D2 Receptor Activation Blocks GluA2/ROS Positive Feedback Loop to Alienate Chronic-Migraine-Associated Pain Sensitization. Antioxidants 2024, 13, 725. https://doi.org/10.3390/antiox13060725
Zhang W, Zhang X, Lei M, Zhang D, Qin G, Zhou J, Ji L, Chen L. Dopamine D2 Receptor Activation Blocks GluA2/ROS Positive Feedback Loop to Alienate Chronic-Migraine-Associated Pain Sensitization. Antioxidants. 2024; 13(6):725. https://doi.org/10.3390/antiox13060725
Chicago/Turabian StyleZhang, Wei, Xiaoyan Zhang, Ming Lei, Dunke Zhang, Guangcheng Qin, Jiying Zhou, Lichun Ji, and Lixue Chen. 2024. "Dopamine D2 Receptor Activation Blocks GluA2/ROS Positive Feedback Loop to Alienate Chronic-Migraine-Associated Pain Sensitization" Antioxidants 13, no. 6: 725. https://doi.org/10.3390/antiox13060725
APA StyleZhang, W., Zhang, X., Lei, M., Zhang, D., Qin, G., Zhou, J., Ji, L., & Chen, L. (2024). Dopamine D2 Receptor Activation Blocks GluA2/ROS Positive Feedback Loop to Alienate Chronic-Migraine-Associated Pain Sensitization. Antioxidants, 13(6), 725. https://doi.org/10.3390/antiox13060725







