In Vitro Biological Activities of Hesperidin-Related Compounds with Different Solubility
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Analysis of HTL
2.3. Determination of Partition Coefficient
2.4. Determination of Antioxidant Capacity
2.4.1. DPPH Assay
2.4.2. β-Carotene Bleaching Assay
2.5. Determination of Cytotoxicity and Effects on Inflammatory Cytokines
2.5.1. Measurement of Cell Viability
2.5.2. Measurement of Inflammatory Mediator and Cytokines
2.6. Determination of Antimicrobial Capacity
2.6.1. Microorganisms and Culture Conditions
2.6.2. Minimum Inhibitory Concentration (MIC)
2.7. Statistical Analyses
3. Results
3.1. Instrumantal Analysis of HTL
3.2. Partition Coefficient of HTL, HT, HD, and HDG
3.3. Antioxidant Capacity of HTL, HT, HD, and HDG
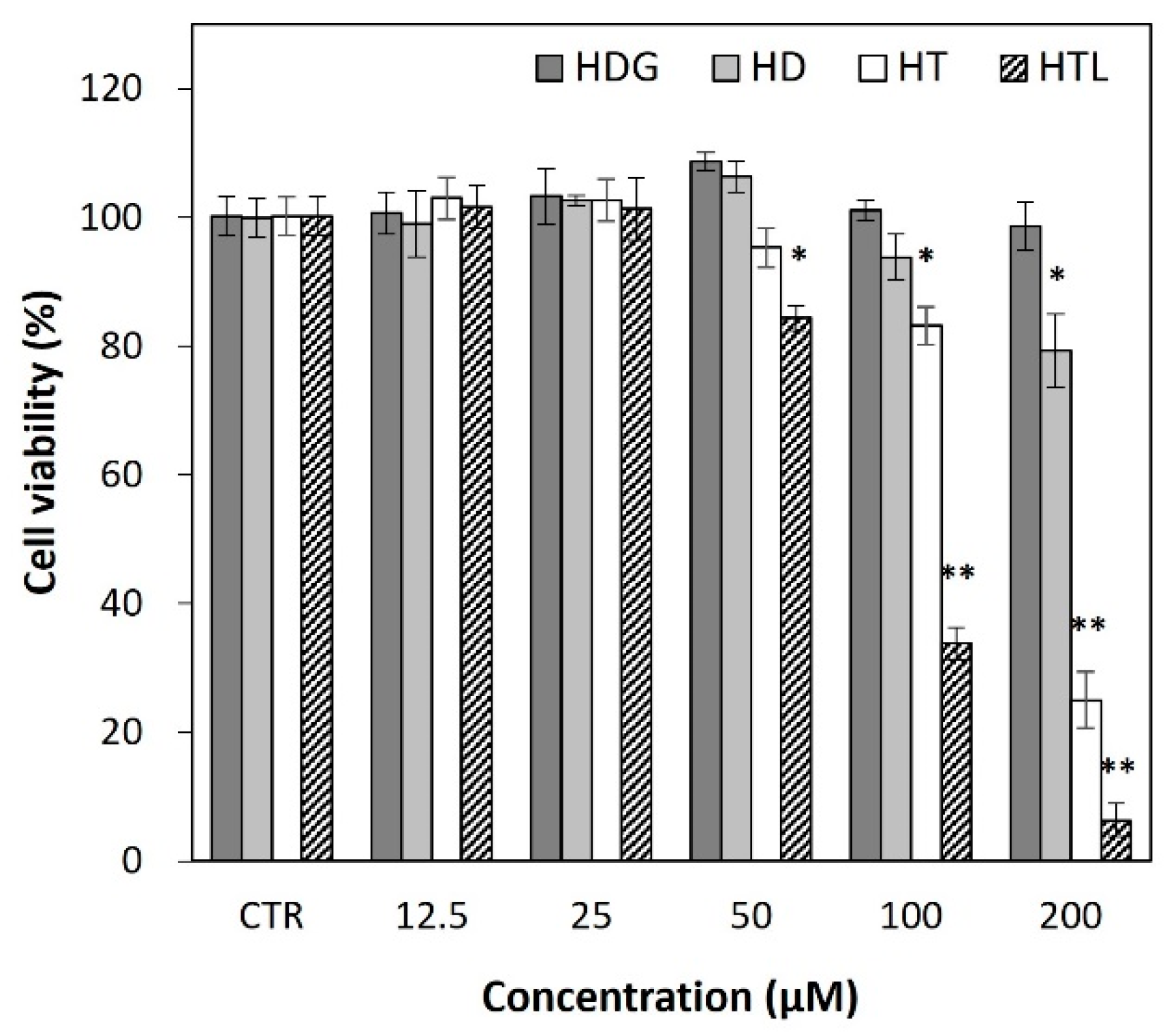
3.4. Cytotoxicity of HTL, HT, HD, and HDG
3.5. Inhibitory Effects of HTL, HT, HD, and HDG on Inflammatory Mediators and Cytokines
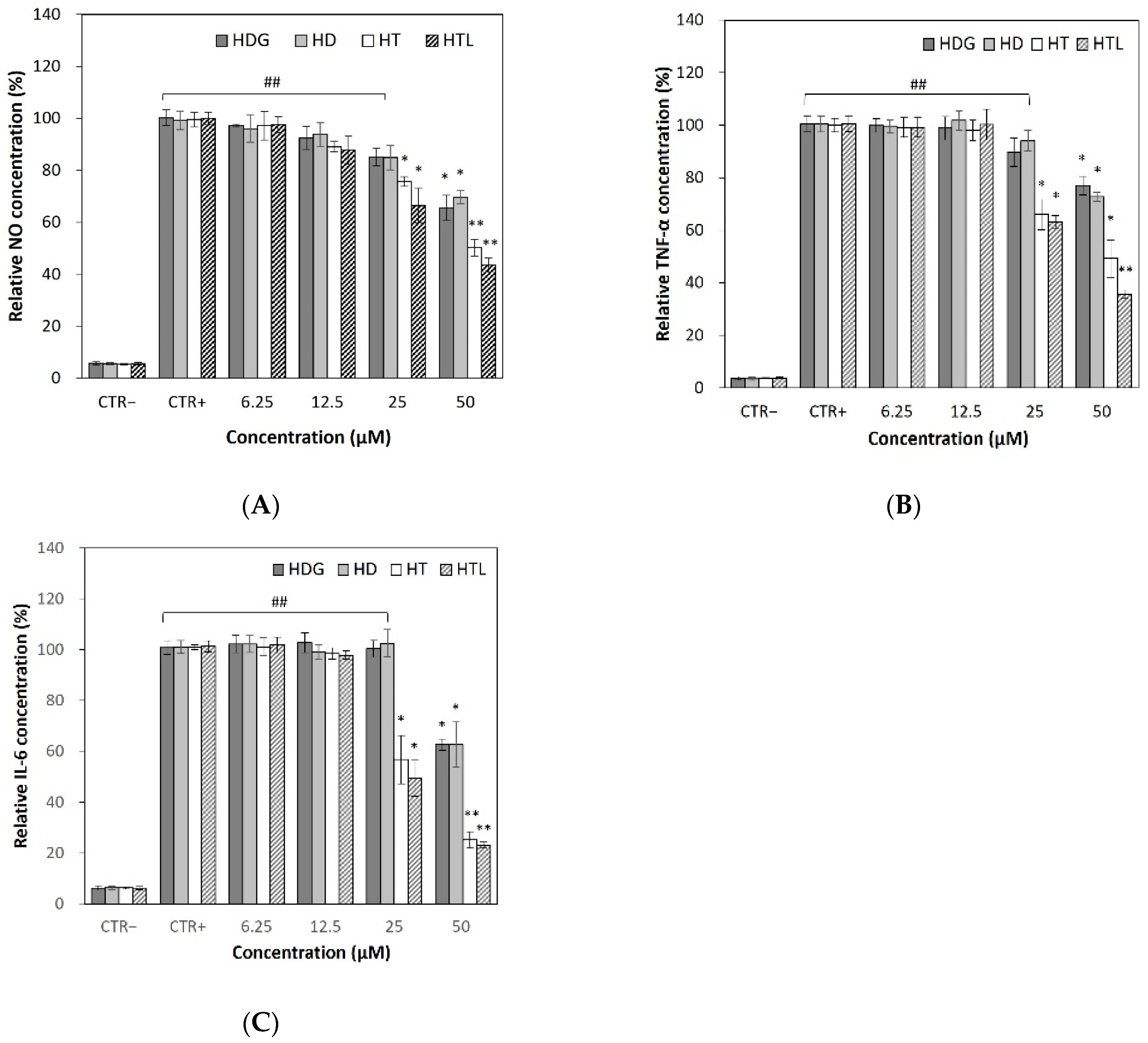
3.5.1. Effects on Cellular NO Levels
3.5.2. Effects on Cellular TNF-α and IL-6 Production
3.6. Antimicrobial Capacity of HTL, HT, HD, and HDG on Skin-Resident Microorganisms
Effects on Microbial Growth
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Beecher, G.R. Overview of dietary flavonoids: Nomenclature, occurrence and intake. J. Nutr. 2003, 133, 3248S–3254S. [Google Scholar] [CrossRef] [PubMed]
- Németh, K.; Plumb, G.W.; Berrin, J.-G.; Juge, N.; Jacob, R.; Naim, H.Y.; Williamson, G.; Swallow, D.M.; Kroon, P.A. Deglycosylation by small intestinal epithelial cell β-glycosidases is a critical step in the absorption and metabolism of dietary flavonoid glycosides in humans. Eur. J. Nutr. 2003, 42, 29–42. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, H.; Ikoma, Y.; Sugiura, M.; Yano, M.; Hasegawa, Y. Identification and quantification of the conjugated metabolites derived from orally administered hesperidin in rat plasma. J. Agric. Food Chem. 2004, 52, 6653–6659. [Google Scholar] [CrossRef] [PubMed]
- Cho, J. Antioxidant and neuroprotective effects of hesperidin and its aglycone hesperetin. Arch. Pharmacal. Res. 2006, 29, 699–706. [Google Scholar] [CrossRef] [PubMed]
- Haidari, F.; Keshavarz, S.A.; Rashidi, M.R.; Shahi, M.M. Orange juice and hesperetin supplementation to hyperuricemic rats alter oxidative stress markers and xanthine oxidoreductase activity. J. Clin. Biochem. Nutr. 2009, 45, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Hirata, A.; Murakami, Y.; Shoji, M.; Kadoma, Y.; Fujisawa, S. Kinetics of radical-scavenging activity of hesperetin and hesperidin and their inhibitory activity on COX-2 expression. Anticancer Res. 2005, 25, 3367–3374. [Google Scholar]
- Hao, Y.; Wei, Z.; Wang, Z.; Li, G.; Yao, Y.; Dun, B. Biotransformation of flavonoids improves antimicrobial and anti-breast cancer activities in vitro. Foods 2021, 10, 2367. [Google Scholar] [CrossRef]
- Olas, B. A review of in vitro studies of the anti-platelet potential of citrus fruit flavonoids. Food Chem. Toxicol. 2021, 150, 112090. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.J.; Huynh, T.K.; Yang, C.S.; Hu, D.W.; Shen, Y.C.; Tu, C.Y.; Wu, Y.C.; Tang, C.H.; Huang, W.C.; Chen, Y. Hesperidin is a potential inhibitor against SARS-CoV-2 infection. Nutrients 2021, 13, 2800. [Google Scholar] [CrossRef]
- Zalpoor, H.; Bakhtiyari, M.; Shapourian, H.; Puria, R.; Chanour, T.; Mohsen, N. Hesperetin as an anti-SARS-CoV-2 agent can inhibit COVID-19-associated cancer progression by suppressing intracellular signaling pathways. Inflammopharmacology 2022, 30, 1533–1539. [Google Scholar] [CrossRef]
- Man, G.; Mauro, T.M.; Zhai, Y.; Kim, P.L.; Cheung, C.; Hupe, M.; Crumrine, D.; Elias, P.M.; Man, M.Q. Topical hesperidin enhances epidermal function in an aged murine model. J. Investig. Dermatol. 2015, 135, 1184–1187. [Google Scholar] [CrossRef] [PubMed]
- Man, M.Q.; Yang, B.; Elias, P.M. Benefits of hesperidin for cutaneous functions. Evid. Based Complement. Altern. Med. 2019, 2019, 266307. [Google Scholar] [CrossRef] [PubMed]
- Cao, R.; Zhao, Y.; Zhou, Z.; Zhao, X. Enhancement of the water solubility and antioxidant activity of hesperidin by chitooligosaccharide. J. Sci. Food Agric. 2018, 98, 2422–2427. [Google Scholar] [CrossRef] [PubMed]
- Wdowiak, K.; Rosiak, N.; Tykarska, E.; Zarowski, M.; Płazínska, A.; Płazínski, W.; Cielecka-Piontek, J. Amorphous inclusion complexes: Molecular interactions of hesperidin and hesperetin with HP-B-CD and their biological effects. Int. J. Mol. Sci. 2022, 23, 4000. [Google Scholar] [CrossRef] [PubMed]
- Slámová, K.; Kapešová, J.; Valentová, K. “Sweet flavonoids”: Glycosidase-catalyzed modifications. Int. J. Mol. Sci. 2018, 19, 2126. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, M.; Suzuki, A.; Hase, T. Short-term effects of glucosyl hesperidin and hesperetin on blood pressure and vascular endothelial function in spontaneously hypertensive rats. J. Nutr. Sci. Vitaminol. 2008, 54, 95–98. [Google Scholar] [CrossRef] [PubMed]
- De Souza, V.T.; de Franco, É.P.; de Araújo, M.E.; Messias, M.C.F.; Priviero, F.B.M.; Alexabder, C.H.; Sawaya, A.C.H.F.; de Oliveira Carvalho, P. Characterization of the antioxidant activity of aglycone and glycosylated derivatives of hesperetin: An in vitro and in vivo study. J. Mol. Recognit. 2016, 29, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Chebil, L.; Humeau, C.; Falcimaigne, A.; Engasser, J.M.; Ghoul, M. Enzymatic acylation of flavonoids. Process Biochem. 2006, 41, 2237–2251. [Google Scholar] [CrossRef]
- de Araújo, M.E.; Franco, Y.E.; Messias, M.C.; Longato, G.B.; Pamphile, J.A.; Carvalho, P.O. Biocatalytic synthesis of flavonoid esters by lipases and their biological benefits. Planta Med. 2017, 83, 7–22. [Google Scholar] [CrossRef]
- Mellou, F.; Loutrari, H.; Stamatis, H.; Roussos, C.; Kolisis, F.N. Enzymatic esterification of flavonoids with unsaturated fatty acids: Effect of the novel esters on vascular endothelial growth factor release from K562 cells. Process Biochem. 2006, 41, 2029–2034. [Google Scholar] [CrossRef]
- Li, X.; Zhao, Y.; Lai, X.; Nong, J.; Zhao, G.; Xiao, X. One-pot biocatalytic synthesis and antioxidant activities of highly lipophilic naringin derivatives by using bi-functional whole-cells. Food Res. Int. 2020, 136, 109291. [Google Scholar] [CrossRef]
- Choi, S.S.; Lee, S.H.; Lee, K.A. A comparative study of hesperetin, hesperidin and hesperidin glucoside: Antioxidant, anti-Inflammatory, and antibacterial activities in vitro. Antioxidants 2022, 11, 1618. [Google Scholar] [CrossRef] [PubMed]
- Makuch, E.; Nowak, A.; Günther, A.; Pełech, R.; Kucharski, L.; Duchnik, W.; Klimowicz, A. Enhancement of the antioxidant and skin permeation properties of eugenol by the esterification of eugenol to new derivatives. AMB Express 2020, 10, 187. [Google Scholar] [CrossRef]
- Schroeder, P.; Klotz, L.O.; Sies, H. Amphiphilic properties of (-)-epicatechin and their significance for protection of cells against peroxynitrite. Biochem. Biophys. Res. Commun. 2003, 307, 69–73. [Google Scholar] [CrossRef]
- Ratha, P.; Jhon, D.Y. Increase of rutin, quercetin, and antioxidant activity during germinated buckwheat (Fagopyrum esculentum Moench) fermentation. Ferment. Technol. 2017, 6, 147. [Google Scholar]
- Blois, M.S. Antioxidant determinations by the use of a stable free radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Wanasundara, U.; Amarowicz, R.; Shahidi, F. Isolation and identification of an antioxidative component in canola meal. J. Agric. Food Chem. 1994, 42, 1285–1290. [Google Scholar] [CrossRef]
- Hidalgo, M.E.; Fernández, E.; Quilhot, W.; Lissi, E. Antioxidant activity of depsides and depsidones. Phytochemistry 1994, 37, 1585–1587. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Diaz Iglesias, Y.; Wilms, T.; Vanbever, R.; Van Bambeke, F. Activity of Antibiotics against Staphylococcus aureus in an In Vitro Model of Biofilms in the Context of Cystic Fibrosis: Influence of the Culture Medium. Antimicrob. Agents Chemother. 2019, 63, e00602-19. [Google Scholar] [CrossRef]
- Park, E.J.; Oh, J.H. Antimicrobial activities of Korean mugwort (Artemisia iwayomogi and Artemisia princeps) extracts against Staphylococcus aureus and Cutibacterium acnes. Food Sci. Preserv. 2019, 26, 381–390. [Google Scholar] [CrossRef]
- Rodriguez-Tudela, J.L.; Martinez-Suárez, J.V. Defining conditions for microbroth antifungal susceptibility tests: Influence of RPMI and RPMI-2% glucose on the selection of endpoint criteria. J. Antimicrob. Chemother. 1995, 35, 739–749. [Google Scholar] [CrossRef] [PubMed]
- Leong, C.; Buttafuoco, A.; Glatz, M.; Bosshard, P.P. Antifungal susceptibility testing of Malassezia spp. with an optimized colorimetric broth microdilution method. J. Clin. Microbiol. 2017, 55, 1883–1893. [Google Scholar] [CrossRef] [PubMed]
- Wiegand, I.; Hilpert, K.; Hancock, R. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substance. Nat. Protoc. 2008, 3, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Elshikh, M.; Ahmed, S.; Funston, S.; Dunlop, P.; McGaw, M.; Marchant, R.; Banat, I.M. Resazurin-based 96-well plate microdilution method for the determination of minimum inhibitory concentration of biosurfactants. Biotechnol. Lett. 2016, 38, 1015–1019. [Google Scholar] [CrossRef] [PubMed]
- Seo, C.S.; Lim, H.S.; Ha, H.; Jin, S.E.; Shin, H.K. Quantitative analysis and anti-inflammatory effects of Gleditsia sinensis thorns in RAW 264.7 macrophages and HaCaT keratinocytes. Mol. Med. Rep. 2015, 12, 4773–4781. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, L.E.; Belgi, G.; Parslew, R.; McLoughlin, L.; Clough, G.F.; Friedmann, P.S. Ultraviolet-B-induced erythema is mediated by nitric oxide and prostaglandin E2 in combination. J. Investig. Dermatol. 2001, 117, 880–885. [Google Scholar] [CrossRef] [PubMed]
- Thilakarathna, S.H.; Rupasinghe, H.P.V. Flavonoid bioavailability and attempts for bioavailability enhancement. Nutrients 2013, 5, 3367–3387. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Liu, H.; Yang, J.; Gupta, V.K.; Jiang, Y. New insights on bioactivities and biosynthesis of flavonoid glycosides. Trends Food Sci. Technol. 2018, 79, 116–124. [Google Scholar] [CrossRef]
- Xiao, J. Dietary flavonoid aglycones and their glycosides: Which show better biological significance? Crit. Rev. Food Sci. Nutr. 2017, 57, 1874–1905. [Google Scholar] [CrossRef]
- Lesjak, M.; Beara, I.; Simin, N.; Pintác, D.; Majkíc, T.; Bekvalac, K.; Ořcíc, D.; Mimica-Dukíc, N. Antioxidant and anti-inflammatory activities of quercetin and its derivatives. J. Funct. Foods 2018, 40, 68–75. [Google Scholar] [CrossRef]
- Ávila-Gálvez, M.Á.; Giménez-Bastida, J.A.; González-Sarrías, A.; Espín, J.C. New insights into the metabolism of the flavanones eriocitrin and hesperidin: A comparative human pharmacokinetic study. Antioxidants 2021, 10, 435. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Hassan, Y.I.; Liu, R.; Mats, L.; Yang, C.; Liu, C.; Tsao, R. Molecular mechanisms underlying the absorption of aglycone and glycosidic flavonoids in a Caco-2 BBe1 cell model. ACS Omega 2020, 6, 10782–10793. [Google Scholar] [CrossRef] [PubMed]
- Bellocco, A.; Barreca, D.; Laganà, G.; Leuzzi, U.; Tellone, E.; Ficarra, S.; Kotyk, A.; Galtieri, A. Influence of L-rhamnosyl-D-glucosyl derivatives on properties and biological interaction of flavonoids. Mol. Cell. Biochem. 2009, 321, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.-S.; Park, H.-R.; Lee, K.-A. A comparative sudy of rutin and rutin glycoside: Antioxidant activity, anti-inflammatory effect, effect on platelet aggregation and blood coagulation. Antioxidants 2021, 10, 1696. [Google Scholar] [CrossRef]
- Yamada, M.; Mitsuzumi, H.; Tsuzaki, Y.; Miwa, Y.; Chaen, H.; Yamamoto, I. Antioxidant activity of glycosylated vitamin P and its suppressive effect on oxidative stress in hyperlipidemic mice. Jpn. Soc. Nutr. Food Sci. 2003, 56, 355–363. [Google Scholar] [CrossRef]
- Srirangam, R.; Hippalgaonkar, K.; Majumdar, S. Intravitreal kinetics of hesperidin, hesperetin, and hesperidin G: Effect of dose and physicochemical properties. J. Pharm. Sci. 2012, 101, 1631–1638. [Google Scholar] [CrossRef] [PubMed]
- Danihelová, M.; Viskupičová, J.; Šturdík, E. Lipophilization of flavonoids for their food, therapeutic and cosmetic applications. Acta Chim. Slovaca 2012, 5, 59–69. [Google Scholar] [CrossRef]
- Cao, G.; Sofic, E.; Prior, R.L. Antioxidant and prooxidant behavior of flavonoids: Structure—Activity relationships. Free Radic. Biol. Med. 1997, 22, 749–760. [Google Scholar] [CrossRef]
- Procházková, D.; Boušová, I.; Wilhelmová, N. Antioxidant and prooxidant properties of flavonoids. Fitoterapia 2011, 82, 513–523. [Google Scholar] [CrossRef]
- Kim, J.H.; Park, S.H.; Beak, E.J.; Han, C.H.; Kang, N.J. Anti-oxidant and anti-inflammatory effects of rutin and its metabolites. Curr. Res. Agric. Life Sci. 2013, 31, 165–169. [Google Scholar]
- Laskin, D.L.; Pendino, K.J. Macrophages and inflammatory mediators in tissue injury. Annu. Rev. Pharmacol. Toxicol. 1995, 35, 655–677. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Song, J.; Zhang, M.; Wang, H.; Zhang, Y.; Suo, H. Comparison of in vitro and in vivo antioxidant activities of six flavonoids with similar structures. Antioxidants 2020, 9, 732. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, M.; Sasaki, N.; Saga, K.; Kaneko, T. Cytotoxicity of flavonoids toward cultured normal human cells. Biol. Pharm. Bull. 2005, 28, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Ramos, S. Effects of dietary flavonoids on apoptotic pathways related to cancer chemoprevention. J. Nutr. Biochem. 2007, 18, 427–442. [Google Scholar] [CrossRef] [PubMed]
- Yadegarynia, S.; Pham, A.; Ng, A.; Nguyen, D.; Lialiutska, T.; Bortolazzo, A.; Sivryuk, V.; Bremer, M.; White, J.B. Profiling flavonoid cytotoxicity in human breast cancer cell lines: Determination of structure-function relationships. Nat. Prod. Commun. 2012, 7, 1295–1304. [Google Scholar] [CrossRef] [PubMed]
- Hendrich, A.B. Flavonoid-membrane interactions: Possible consequences for biological effects of some polyphenolic compounds. Acta Pharmacol. Sin. 2006, 27, 27–40. [Google Scholar] [CrossRef] [PubMed]
- Oztanir, M.N.; Ciftci, O.; Cetin, A.; Aladag, M.A. Hesperidin attenuates oxidative and neuronal damage caused by global cerebral ischemia/reperfusion in a C57BL/J6 mouse model. Neurol. Sci. 2014, 35, 1393–1399. [Google Scholar] [CrossRef] [PubMed]
- Branchford, B.R.; Carpenter, S.L. The Role of Inflammation in Venous Thromboembolism. Front. Pediatr. 2018, 6, 142. [Google Scholar] [CrossRef]
- Donato, F.; de Gomes, M.G.; Goes, A.T.R.; Filho, C.B.; Del Fabbro, L.; Antunes, M.S.; Souza, L.C.; Boeira, S.P.; Jesse, C.R. Hesperidin exerts antidepressant-like effects in acute and chronic treatments in mice: Possible role of l-arginine-NO-cGMP pathway and BDNF levels. Brain Res. Bull. 2014, 104, 19–26. [Google Scholar] [CrossRef]
- Medzhitov, R. Origin and physiological roles of inflammation. Nature 2008, 454, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Wang, L.; Walzem, R.L.; Miller, E.G.; Pike, L.M.; Patil, B.S. Antioxidant activity of citrus limonoids, flavonoids, and coumarins. J. Agric. Food Chem. 2005, 53, 2009–2014. [Google Scholar] [CrossRef] [PubMed]
- Duda-Chodak, A. The inhibitory effect of polyphenols on human gut microbiota. J. Physiol. Pharmacol. 2012, 63, 497–503. [Google Scholar] [PubMed]
- Trivedi, P.P.; Tripathi, D.N.; Jena, G.B. Hesperetin protects testicular toxicity of doxorubicin in rat: Role of NFkappaB, p38 and caspase-3. Food Chem. Toxicol. 2011, 49, 838–847. [Google Scholar] [CrossRef] [PubMed]
- Iranshahi, M.; Rezaee, R.; Parhiz, H.; Roohbakhsh, A.; Soltani, F. Protective effects of flavonoids against microbes and toxins: The cases of hesperidin and hesperetin. Life Sci. 2015, 137, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Yuan, G.; Guan, Y.; Yi, H.; Lai, S.; Sun, Y.; Cao, S. Antibacterial activity and mechanism of plant flavonoids to gram-positive bacteria predicted from their lipophilicities. Sci. Rep. 2021, 11, 10471. [Google Scholar] [CrossRef] [PubMed]
- Han, S.S.; You, I.J. Studies on antimicrobial activities and safety of natural naringin in Korea. Korean J. Mycol. 1988, 16, 33–40. [Google Scholar]
- Jia, Y.; Guan, Z.; Liu, C.; Huang, M.; Li, J.; Feng, J.; Shen, B.; Yang, G. Staphylococcus aureus β-hemolysin causes skin inflammation by acting as an agonist of epidermal growth factor receptor. Microbiol. Spectr. 2024, 12, e02227-23. [Google Scholar] [CrossRef]
- Borrel, V.; Gannesen, A.V.; Barreau, M.; Gaviard, C.; Duclairoir-Poc, C.; Hardouin, J.; Konto-Ghiorghi, Y.; Lefeuvre, L.; Feuilloley, M.G.J. Adaptation of acneic and non acneic strains of Cutibacterium acnes to sebum-like environment. Microbiologyopen 2019, 8, e00841. [Google Scholar] [CrossRef]
- Nicholas-Haizelden, K.; Murphy, B.; Hoptroff, M.; Horsburgh, M.J. Bioprospecting the Skin Microbiome: Advances in Therapeutics and Personal Care Products. Microorganisms 2023, 11, 1899. [Google Scholar] [CrossRef]
- Echeverría, J.; Opazo, J.; Mendoza, L.; Urzúa, A.; Wilkens, M. Structure-activity and lipophilicity relationships of selected antibacterial natural flavones and flavanones of chilean flora. Molecules 2017, 22, 608. [Google Scholar] [CrossRef] [PubMed]
- Parhiz, H.; Roohbakhsh, A.; Soltani, F.; Rezaee, R.; Iranshahi, M. Antioxidant and anti-inflammatory properties of the citrus flavonoids hesperidin and hesperetin: An updated review of their molecular mechanisms and experimental models. Phytother. Res. 2015, 29, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Ioannou, I.; Barboza, E.; Willig, G.; Marié, T.; Texeira, A.; Darme, P.; Renault, J.-H.; Allais, F. Implementation of an enzyme membrane reactor to intensify the α-O-glycosylation of resveratrol using cyclodextrins. Pharmaceuticals 2021, 14, 319. [Google Scholar] [CrossRef] [PubMed]
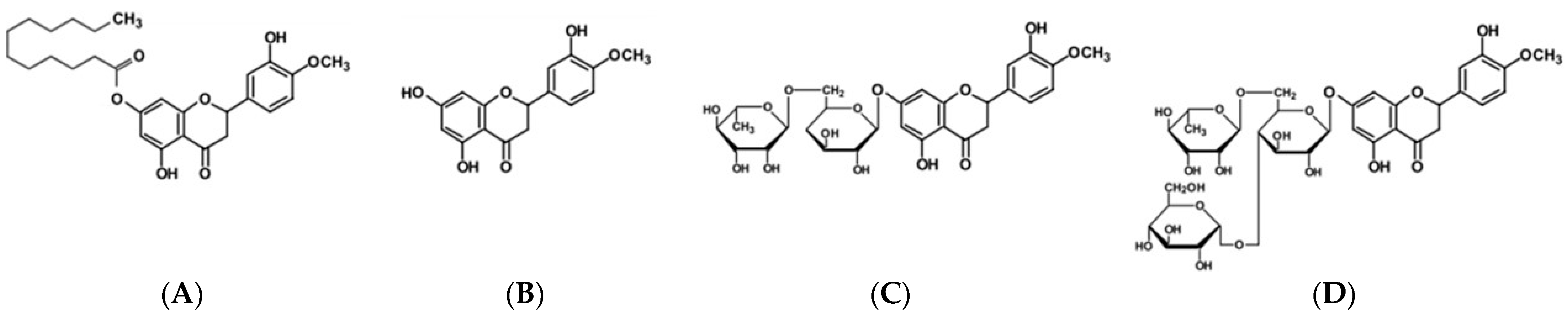



| Compound | Log Po/w |
|---|---|
| Hesperetin laurate Hesperetin | 7.08 ± 0.06 a 2.59 ± 0.04 b |
| Hesperidin Hesperidin glucoside | 2.13 ± 0.03 b −3.45 ± 0.06 c |
| Microorganism | MIC Value (μg/mL) | |||
|---|---|---|---|---|
| Hesperetin Laurate | Hesperetin | Hesperidin | Hesperidin Glucoside | |
| Staphylococcus aureus | 2000 | 2000 | ND | ND |
| Cutibacterium acnes | 1000 | 1000 | ND | ND |
| Candida albicans | 250 | 500 | 2000 | 2000 |
| Malassezia furfur | 1000 | ND | ND | ND |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, H.-J.; Lee, S.-H.; Hong, S.-K.; Gil, B.-I.; Lee, K.-A. In Vitro Biological Activities of Hesperidin-Related Compounds with Different Solubility. Antioxidants 2024, 13, 727. https://doi.org/10.3390/antiox13060727
Lee H-J, Lee S-H, Hong S-K, Gil B-I, Lee K-A. In Vitro Biological Activities of Hesperidin-Related Compounds with Different Solubility. Antioxidants. 2024; 13(6):727. https://doi.org/10.3390/antiox13060727
Chicago/Turabian StyleLee, Hyo-Jun, Sun-Hyung Lee, Sun-Ki Hong, Bog-Im Gil, and Kyung-Ae Lee. 2024. "In Vitro Biological Activities of Hesperidin-Related Compounds with Different Solubility" Antioxidants 13, no. 6: 727. https://doi.org/10.3390/antiox13060727





