Polyphenolic Profiling and Antioxidant Activity in Berry Extracts of Pyracantha Wild Varieties from the Mediterranean Region
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Apparatus
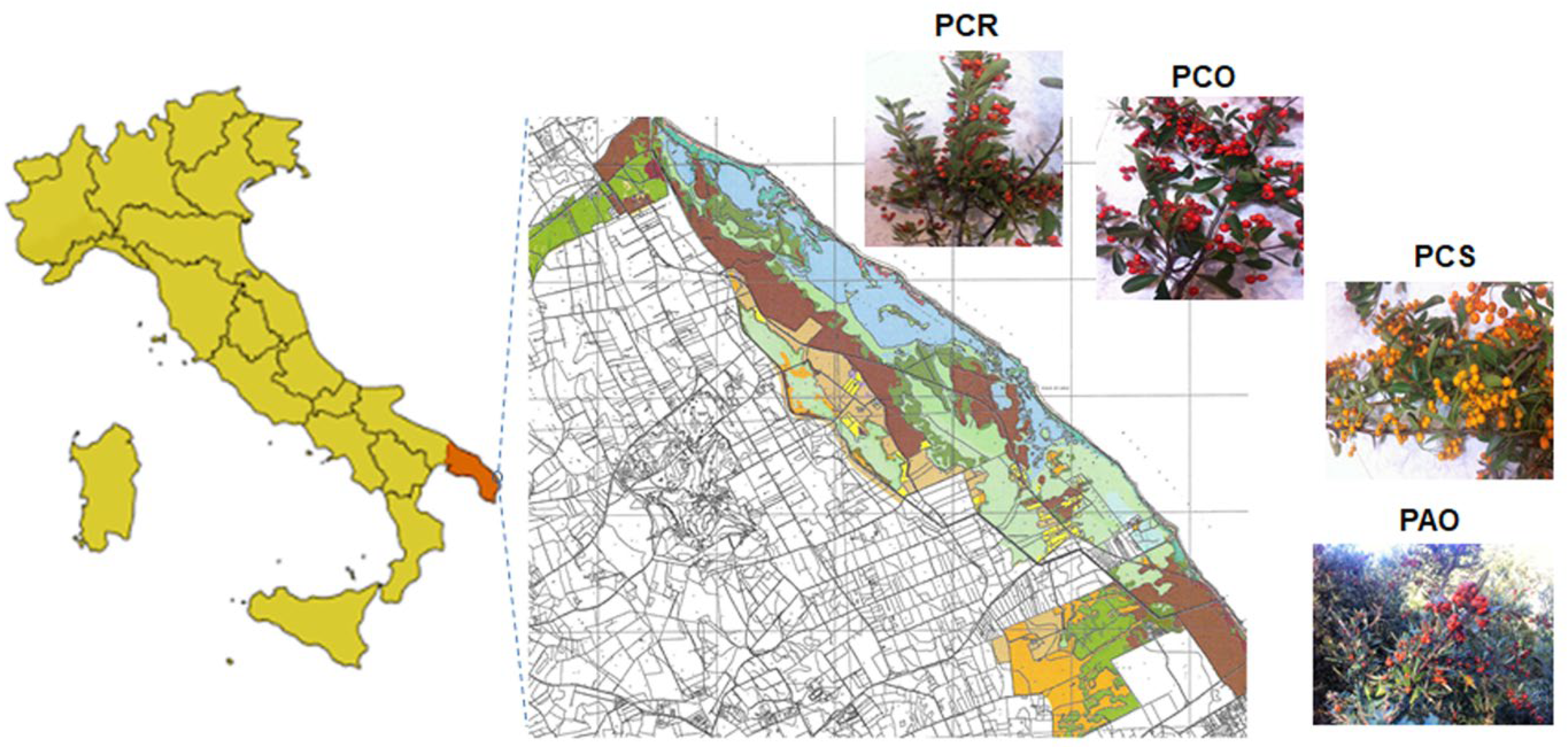
2.3. Pyracantha Berries Samples
2.4. Preparation of Phenolic Extracts
2.5. Total Phenolic Content Studies
2.6. Assay of Antioxidant Activity
2.7. HPLC Analysis for Polyphenols’ Characterization
2.8. Quantification of Polyphenols in the Pyracantha Extracts
2.9. Anthocyanins’ Extraction and Purification
2.10. Anthocyanins’ Separation and Characterization
2.11. Statistical Analysis
3. Results
3.1. Extraction of Polyphenols in Pyracantha Varieties
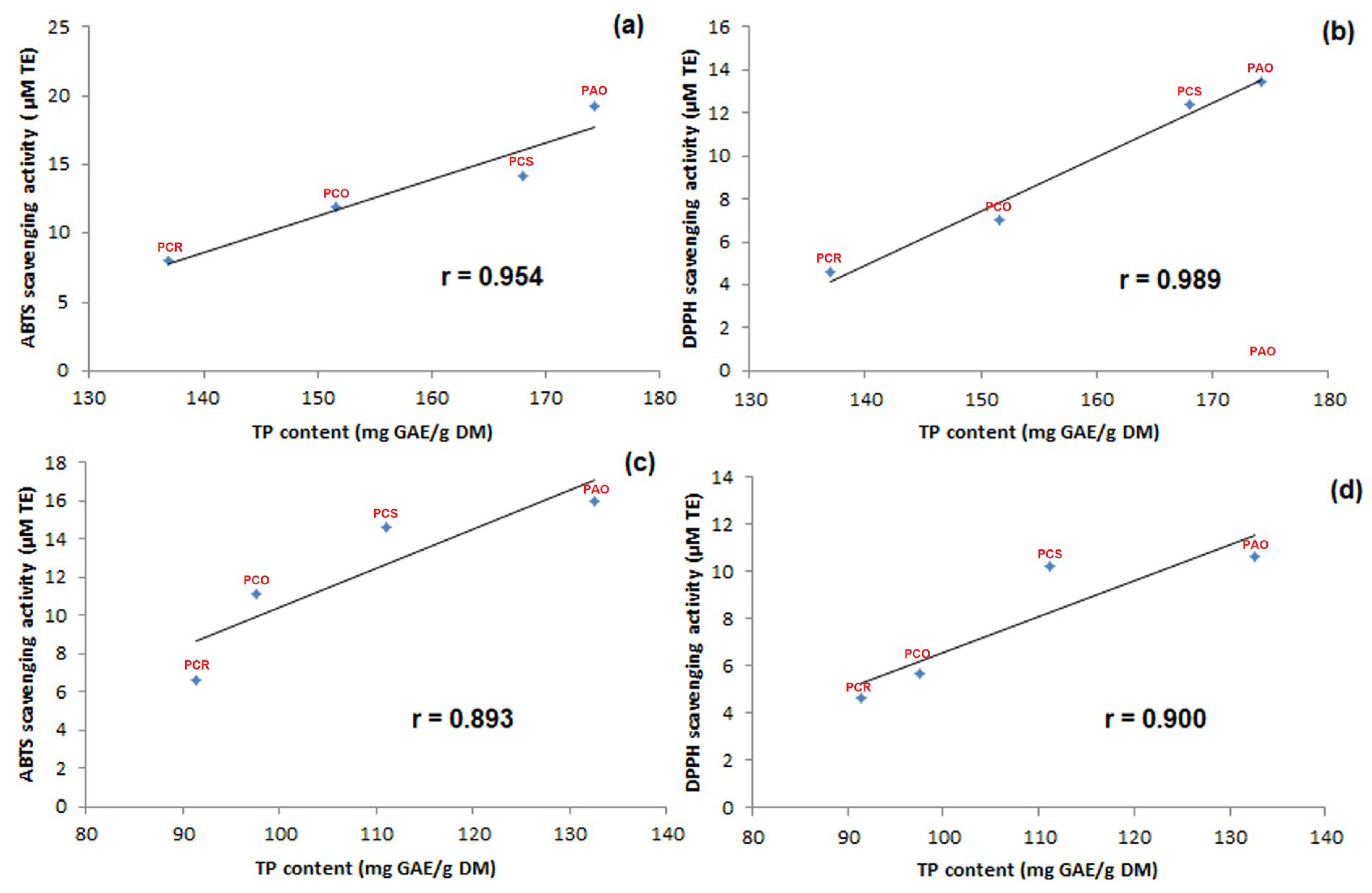
3.2. In Vitro Assay of Total Antioxidants
3.3. HPLC Profiling of Phenolic Compounds in MeOH Extracts
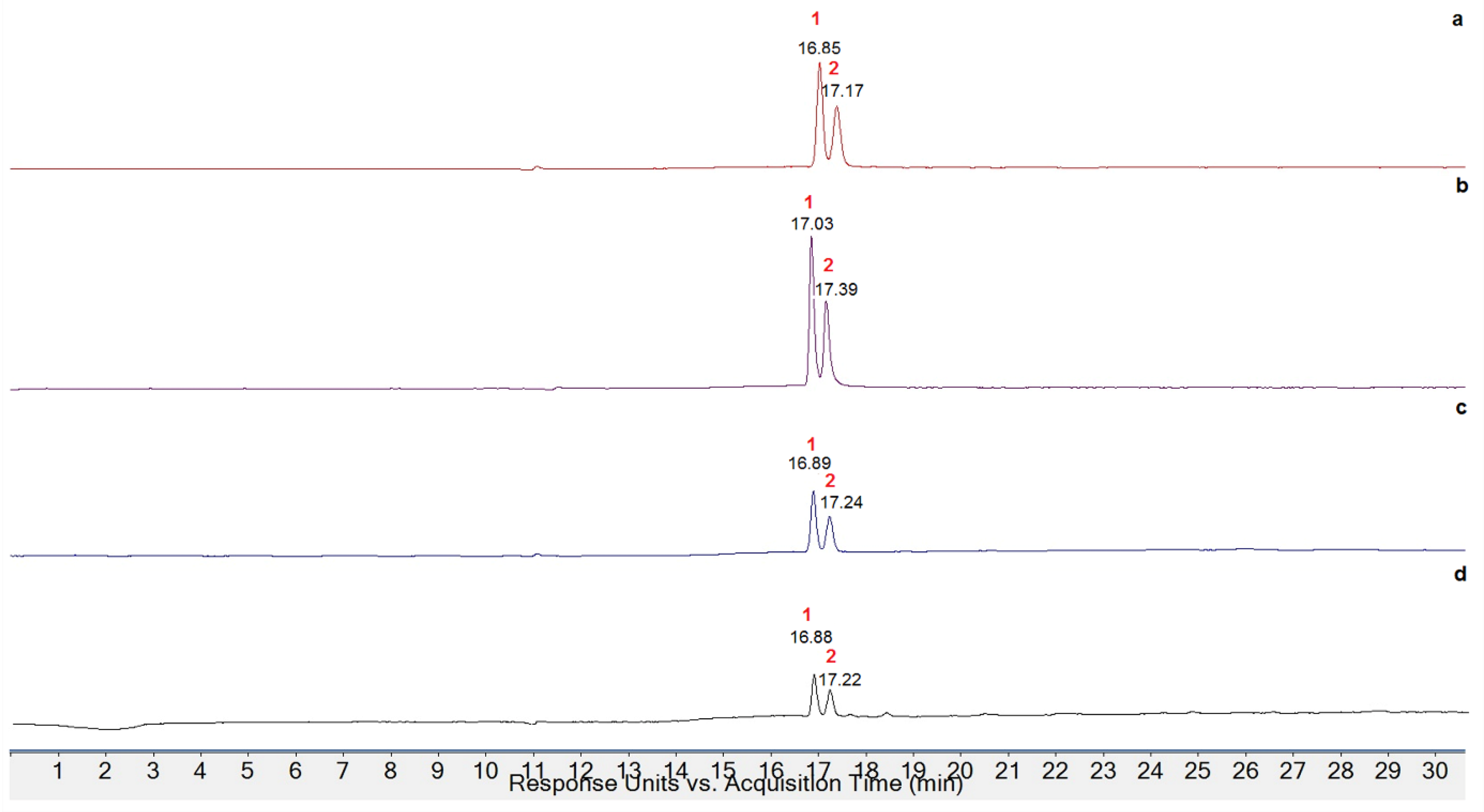
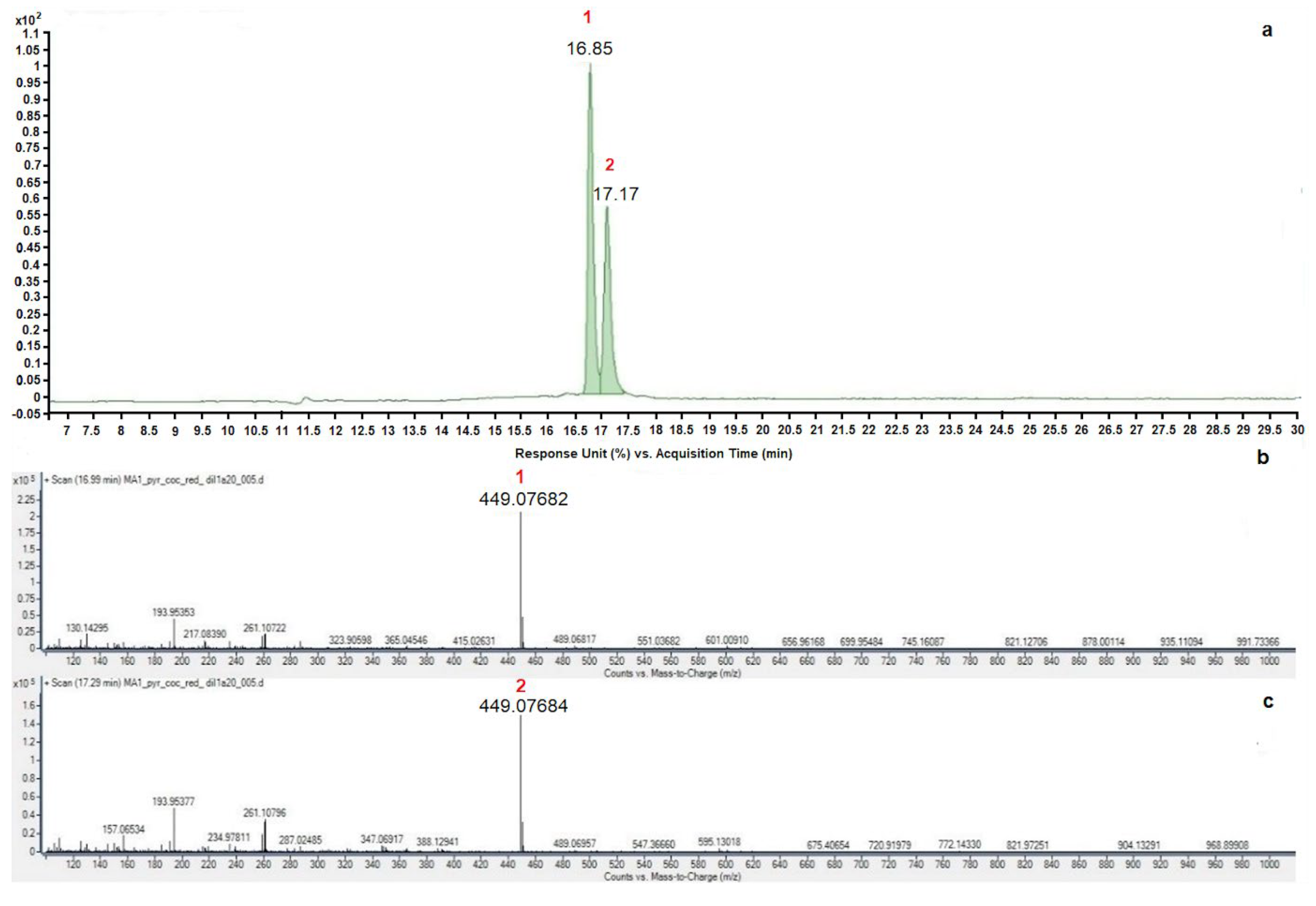
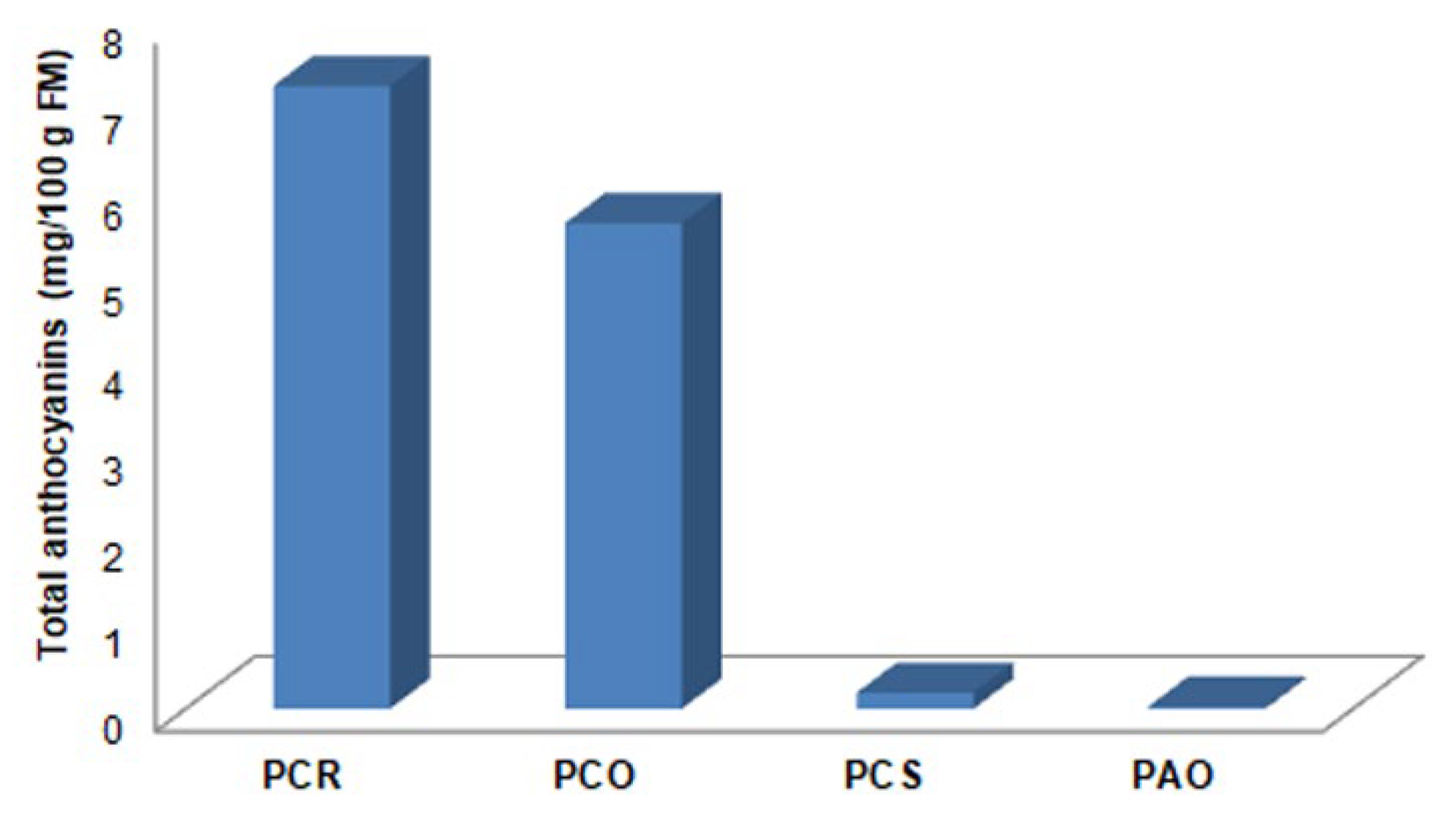
3.4. Extraction, Quantification, and Identification of Anthocyanins
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Atrahimovich, D.; Avni, D.; Khatib, S. Flavonoids-macromolecules interactions in human diseases with focus on alzheimer, atherosclerosis and cancer. Antioxidants 2021, 10, 423. [Google Scholar] [CrossRef]
- Tarozzi, A. Oxidative stress in neurodegenerative diseases: From preclinical studies to clinical applications. J. Clin. Med. 2020, 9, 1223. [Google Scholar] [CrossRef] [PubMed]
- Niemann, B.; Rohrbach, S.; Miller, M.R.; Newby, D.E.; Fuster, V.; Kovacic, J.C. Oxidative stress and cardiovascular risk: Obesity, diabetes, smoking, and pollution. Part 3 of a 3-Part Series. J. Am. Coll. Cardiol. 2017, 70, 230–251. [Google Scholar] [CrossRef] [PubMed]
- Rekatsina, M.; Paladini, A.; Piroli, A.; Zis, P.; Pergolizzi, J.V.; Varrassi, G. Pathophysiology and therapeutic perspectives of oxidative stress and neurodegenerative diseases: A narrative review. Adv. Ther. 2020, 37, 113–139. [Google Scholar] [CrossRef] [PubMed]
- Malcangi, G.; Patano, A.; Ciocia, A.M.; Netti, A.; Viapiano, F.; Palumbo, I.; Trilli, I.; Guglielmo, M.; Inchingolo, A.D.; Dipalma, G.; et al. Benefits of natural antioxidants on oral health. Antioxidants 2023, 12, 1309. [Google Scholar] [CrossRef] [PubMed]
- Phaniendra, A.; Jestadi, D.B. Free radicals: Properties, sources, targets, and their implication in various diseases. Indian J. Clin. Biochem. 2015, 30, 11–26. [Google Scholar] [CrossRef] [PubMed]
- Unsal, V.; Dakiran, T.; Çiçek, M.; Kölükçü, E. The role of natural antioxidants against reactive oxygen species produced by cadmium toxicity: A review. Adv. Pharm. Bull. 2020, 10, 184–202. [Google Scholar] [CrossRef] [PubMed]
- Sarikurkcu, C.; Uren, M.C.; Tepe, B.; Cengiz, M.; Kocak, M.S. Phenolic content, enzyme inhibitory and antioxidative activity potentials of Phlomis nissolii and P. pungens var. pungens. Ind. Crop. Prod. 2014, 62, 333–340. [Google Scholar] [CrossRef]
- Yilmaz, B.S.; Altun, M.L.; Orhan, I.E.; Ergene, B.; Citoglu, G.S. Enzyme inhibitory and antioxidant activities of Viburnum tinus L. relevant to its neuroprotective potential. Food Chem. 2013, 141, 582–588. [Google Scholar] [CrossRef] [PubMed]
- Varzaru, I.; Oancea, A.G.; Vlaicu, P.A.; Saracila, M.; Untea, A.E. Exploring the antioxidant potential of blackberry and raspberry leaves: Phytochemical analysis, scavenging activity, and in vitro polyphenol bioaccessibility. Antioxidants 2023, 12, 2125. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, A.; Madrones, C.; Vega, M.; Dorau, C.; von Baer, D. Polyphenols and antioxidant activity of Calafate (Berberis microphylla) fruits and other native berries from southern Chile. J. Agric. Food Chem. 2010, 58, 6081–6089. [Google Scholar] [CrossRef]
- Swallah, M.S.; Sun, H.; Affoh, R.; Fu, H.; Yu, H. Antioxidant potential overviews of secondary metabolites (polyphenols) in fruits. Int. J. Food Sci. 2020, 2020, 9081686. [Google Scholar] [CrossRef] [PubMed]
- Faure, A.; Werder, J.; Nystrom, L. Reactive oxygen species responsible for beta-glucan degradation. Food Chem. 2013, 141, 589–596. [Google Scholar] [CrossRef] [PubMed]
- Keser, S. Antiradical activities and phytochemical compounds of firethorn (Pyracantha coccinea) fruit extracts. Nat. Prod. Res. 2014, 28, 1789–1794. [Google Scholar] [CrossRef] [PubMed]
- Javan, A.J.; Javan, M.J. Electronic structure of some thymol derivatives correlated with the radical scavenging activity: Theoretical study. Food Chem. 2014, 165, 451–459. [Google Scholar] [CrossRef] [PubMed]
- Lourenço, S.C.; Moldão-Martins, M.; Alves, V.D. Antioxidants of natural plant origins: From sources to food industry applications. Molecules 2019, 24, 4132. [Google Scholar] [CrossRef] [PubMed]
- Lapornik, B.; Prosek, M.; Golc, W.A. Comparison of extracts prepared from plant by-products using different solvents and extraction time. J. Food Eng. 2005, 71, 214–222. [Google Scholar] [CrossRef]
- Pignatti, S. Pyracantha coccinea M. J. Roemer. In Flora d’Italia; Edagricole: Bologna, Italy, 1982; Volume 1, p. 610. [Google Scholar]
- Wang, H.; Ye, Y.H.; Wang, H.H.; Liu, J.; Liu, Y.J.; Jiang, B.W. HPLC-QTOF-MS/MS profiling, antioxidant, and α-glucosidase inhibitory activities of Pyracantha fortuneana fruit extracts. J. Food Biochem. 2019, 43, e12821. [Google Scholar]
- Fico, G.; Bilia, A.R.; Morelli, I.; Tomè, F. Flavonoid distribution in Pyracantha coccinea plants at different growth phases. Biochem. Syst. Ecol. 2000, 28, 673–678. [Google Scholar] [CrossRef] [PubMed]
- Kowaleuki, Z.; Mrugasiewicz, M. Neue flavanone heteroside in Crataegus phenophyrum. Planta Med. 1971, 19, 311–313. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Li, J.; Liu, H.; Qi, Y.; Subbiah, V.; Sharifi-Rad, J.; Setzer, W.N.; Hafiz, A.R. Suleria. Pyracantha as a promising functional food: A comprehensive review on bioactive characteristics, pharmacological activity, and industrial applications. Food Front. 2023, 4, 1720–1736. [Google Scholar] [CrossRef]
- Chauhan, A.; Banerjee, R. Evaluation of traditional uses, phytochemical constituents, therapeutic uses and future prospects of Pyracantha genus: A systematic review. Nat. Prod. Res. 2024, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Zhou, G.X.; Kurihara, H.; Ye, W.C.; Yao, X.S. Biphenyl glycosides from the fruit of Pyracantha fortuneana. J. Nat. Prod. 2006, 69, 1022–1024. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Zhou, G.X.; Kurihara, H.; Ye, W.C.; Yao, X.S. Fortuneanosides G-L, dibenzofuran glycosides from the fruit of Pyracantha fortuneana. Chem. Pharm. Bull. 2008, 56, 439–442. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; He, X.J.; Zhou, G.X.; Kurihara, H.; Ye, W.C.; Yao, X.S. Acylphloroglucinol glycosides from the fruits of Pyracantha fortuneana. J. Asian Nat. Prod. Res. 2008, 10, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.F.; Li, S.; Li, S.J.; Song, G.H.; Yu, L.J.; Zhang, H. Extraction optimization approach to improve accessibility of functional fraction based on combination of total polyphenol, chromatographic profiling and antioxidant activity evaluation: Pyracantha fortuneana fruit as an example. J. Funct. Foods 2013, 5, 715–728. [Google Scholar] [CrossRef]
- Sarikurkcu, C.; Tepe, B. Biological activity and phytochemistry of firethorn (Pyracantha coccinea M. J. Roemer). J. Funct. Foods 2015, 19, 669–675. [Google Scholar] [CrossRef]
- Şahin, S.; Aybastier, Ő.; Demir, C. Optimization of ultrasonic-assisted extraction of quercetin and cyanidin from Pyracantha coccinea and their scavenging effect on free radicals. J. Food Biochem. 2016, 40, 472–479. [Google Scholar] [CrossRef]
- Dewanto, V.; Wu, X.; Adom, K.K.; Liu, R.H. Thermal processing enhances the nutritional value of tomatoes by increasing total antioxidant activity. J. Agric. Food Chem. 2002, 50, 3010–3014. [Google Scholar] [CrossRef] [PubMed]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free-radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Longo, L.; Vasapollo, G. Extraction and identification of anthocyanins from Smilax aspera L. berries. Food Chem. 2006, 94, 226–231. [Google Scholar] [CrossRef]
- Muflihah, Y.M.; Gollavelli, G.; Ling, Y.C. Correlation study of antioxidant activity with phenolic and flavonoid compounds in 12 indonesian indigenous herbs. Antioxidants 2021, 10, 1530. [Google Scholar] [CrossRef] [PubMed]


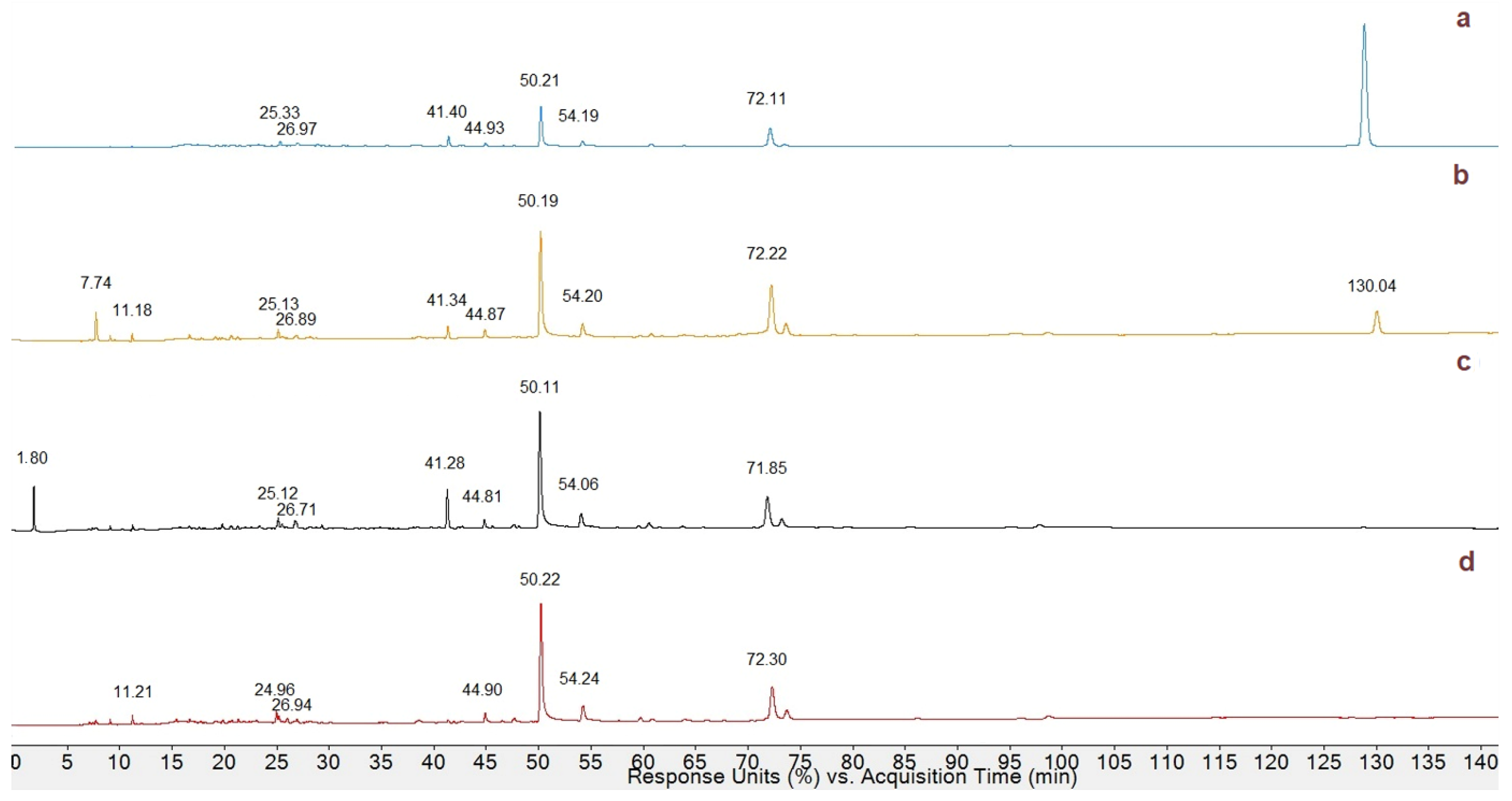

| Peak | tR | ʎmax | MW | M-H+ or M-Na+ (m/z) | Proposed Compound | Classification |
|---|---|---|---|---|---|---|
| 1 | 25.33 | 280 | 610 | 611.17238 | Rutin | Flavonol |
| 2 | 26.97 | 280 | 464 | 465.09546 | Quercetine hexose | Flavonol |
| 3 | 50.21 | 280 | 596 | 597.48036 | Neoeriocitrin | Flavanone |
| 4 | 72.11 | 280 | 578 | 579.32239 | Procyanidin B | Tannin |
| 5 | 128.84 | 280 | 390 | 413.25839 | Resveratrol glucoside | Stilbenoid |
| PCR | PCO | PCS | PAO | PCR | PCO | PCS | PAO | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Retention Time (min) | MW (M-H+, M-Na+) | (mg/100 g DM) a | ||||||||
| 1 | 25.13 | 25.12 | 24.96 | 25.33 | 610 (611, 633) | Rutin | 2.13 | 2.27 | 2.48 | 3.50 |
| 2 | 26.89 | 26.71 | 26.94 | 26.97 | 464 (465) | Quercetin-hexose | 1.33 | 2.81 | 1.40 | 3.40 |
| 3 | 50.19 | 50.11 | 50.22 | 50.21 | 596 (597) | Neoeriocitrin | 29.46 | 30.29 | 30.78 | 33.27 |
| 4 | 72.22 | 71.85 | 72.30 | 72.11 | 578 (579) | Procyanidin B | 20.80 | 12.47 | 14.05 | 23.65 |
| 5 | 130.04 | - | - | 128.84 | 390 (391, 413) | Resveratrol glucoside | 10.69 | - | - | 184.89 |
| Sample | Peak (Retention Time) | Concentration (mg/100 g FM) a |
|---|---|---|
| PCR | 1 (16.85 min) | 4.21 |
| 2 (17.17 min) | 3.05 | |
| PCO | 1 (16.93 min) | 3.25 |
| 2 (17.39 min) | 2.41 | |
| PCS | 1 (16.89 min) | 0.11 |
| 2 (17.24 min) | 0.08 | |
| PAO | 1 (16.88 min) | 0.02 |
| 2 (17.22 min) | 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Del Sole, R.; Montefusco, M.A.; Filippini, R.; Mergola, L. Polyphenolic Profiling and Antioxidant Activity in Berry Extracts of Pyracantha Wild Varieties from the Mediterranean Region. Antioxidants 2024, 13, 765. https://doi.org/10.3390/antiox13070765
Del Sole R, Montefusco MA, Filippini R, Mergola L. Polyphenolic Profiling and Antioxidant Activity in Berry Extracts of Pyracantha Wild Varieties from the Mediterranean Region. Antioxidants. 2024; 13(7):765. https://doi.org/10.3390/antiox13070765
Chicago/Turabian StyleDel Sole, Roberta, Maria Assunta Montefusco, Raffaella Filippini, and Lucia Mergola. 2024. "Polyphenolic Profiling and Antioxidant Activity in Berry Extracts of Pyracantha Wild Varieties from the Mediterranean Region" Antioxidants 13, no. 7: 765. https://doi.org/10.3390/antiox13070765







