Optimizing Cellulase—Limosilactobacillus fermentum ZC529 Synergy Fermentation for Preserving Macadamia integrifolia Pericarp’s Potential Use as Antioxidants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Optimization Design of the Fermentation Process
2.2.1. Experimental Design for Single-Factor
2.2.2. Experimental Design for Optimization
2.3. Determination of Total Antioxidant Capacity, Total Phenolic Content, and Flavonoid Content
2.4. Feeding and Treatment of Flies
2.5. Statistical Analysis
3. Results
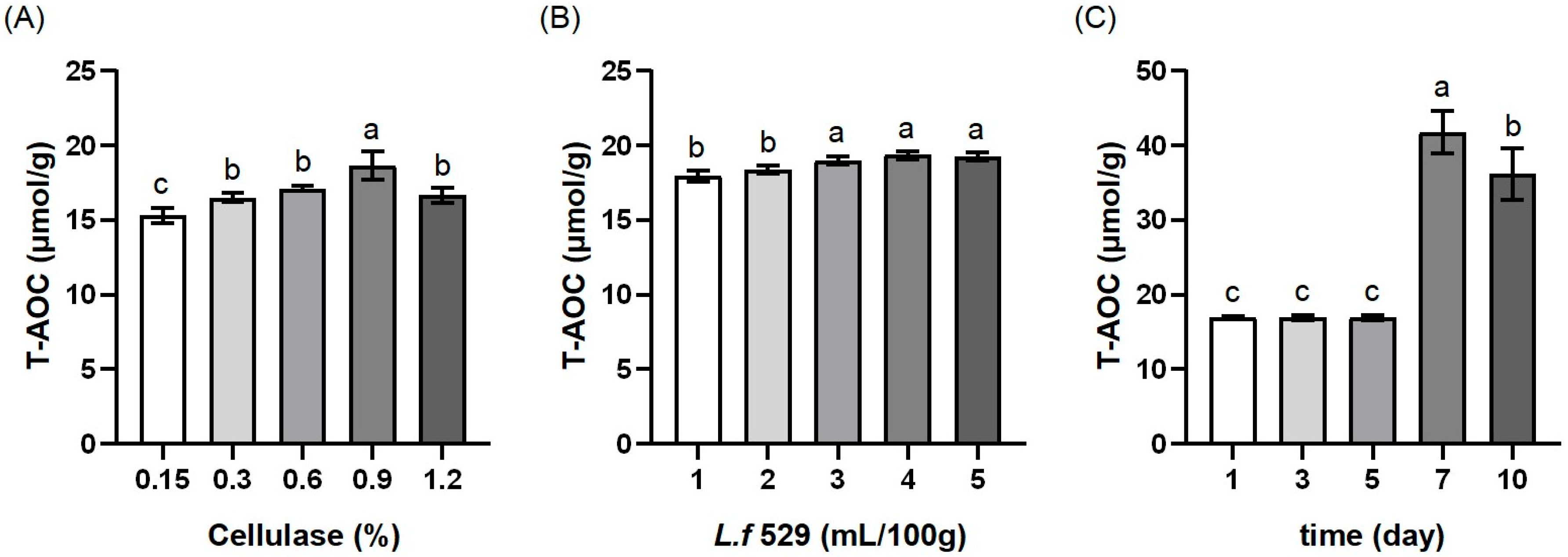
3.1. The Results of the Single-Factor Experiments
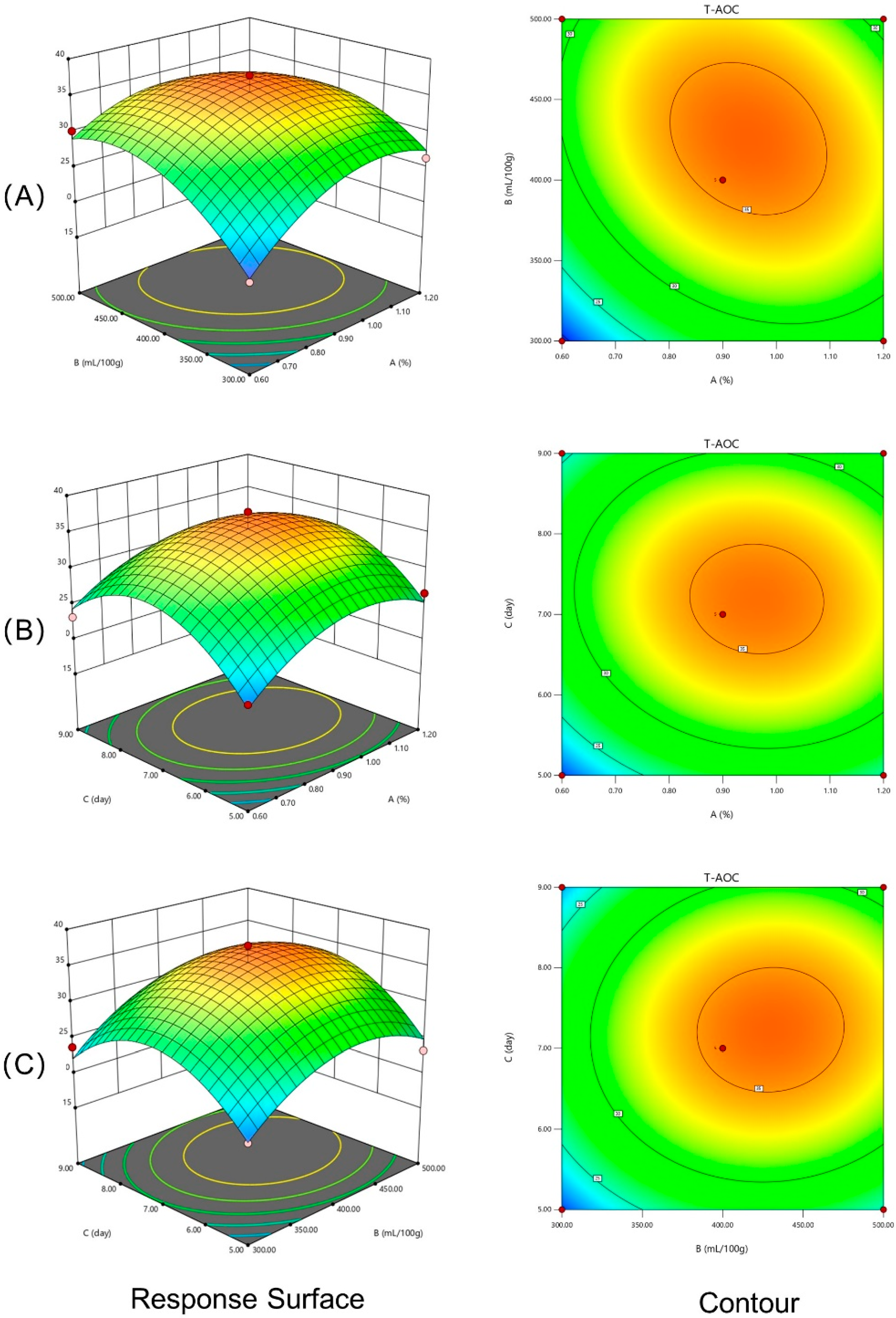
3.2. Modeling of the MIP Fermentation Process
3.3. Interaction Effects of Different Experimental Factors on Response Variables
3.4. The Antioxidant Composition of Fermented MIP under the Optimum Conditions
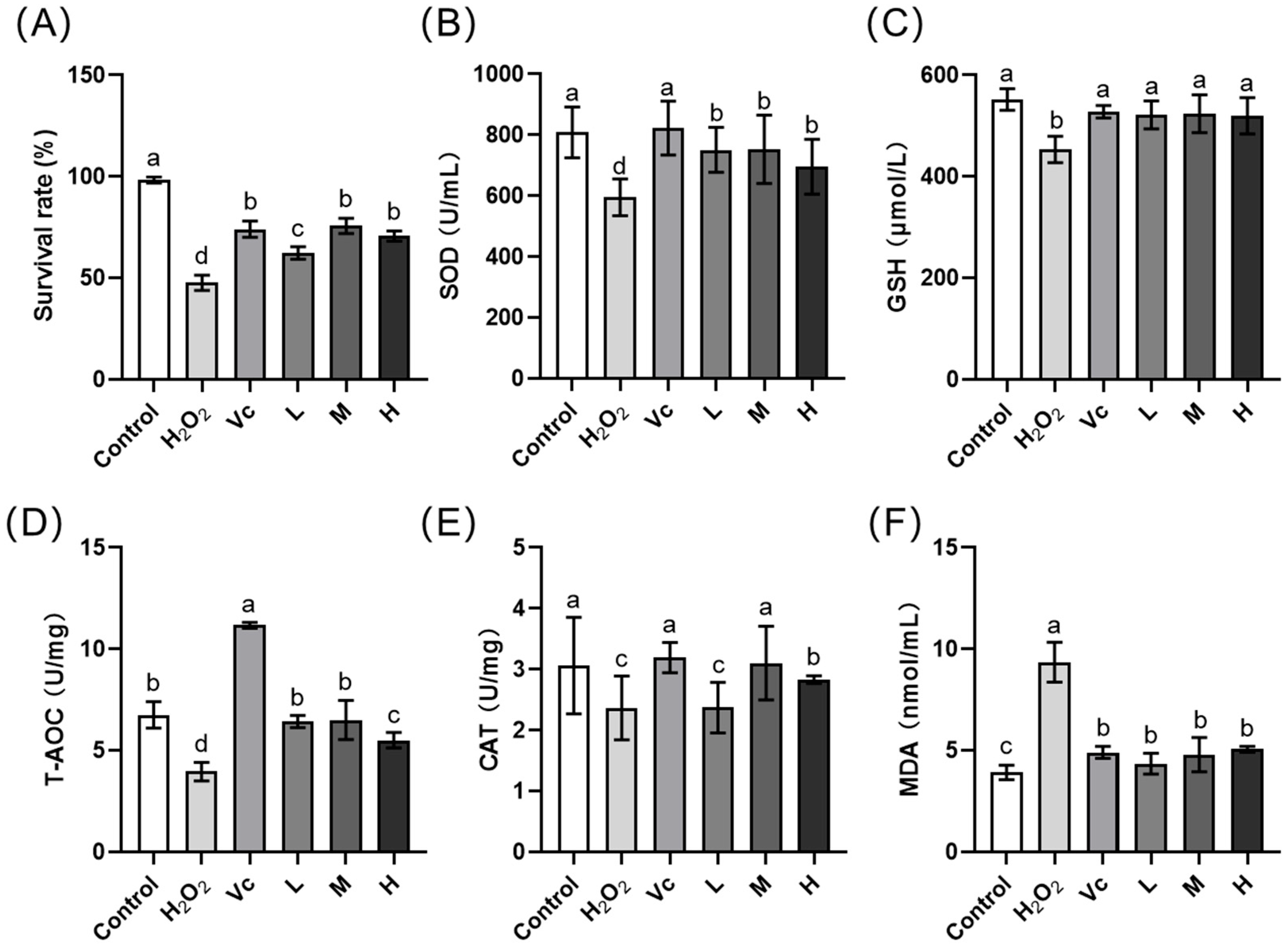
3.5. In Vivo Irritation Properties of the Optimal Fermentation MIP Extract
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huang, H.; Wang, Y.; Li, B.; Zhang, C.; Tang, X.; Tan, B.; Yin, Y.; Zhang, C.; Jiang, Q. Screening of Macadamia Green Peel Fermentation Strain and Improvement of Its Feeding Value after Fermentation. Chin. J. Anim. Nutr. 2024, 36, 1292–1302. [Google Scholar] [CrossRef]
- Wechsler, A.; Ramírez, A.; Crosky, A.; Zaharia, M.; Jones, H.; Ballerini, A.; Núñez, M.; Sahajwalla, V. Physical properties of furniture panels from macadamia shells. In Proceedings of the 18th International Conferences on Composite Materials 2011, Jeju Island, Republic of Korea, 21–26 August 2011. [Google Scholar]
- Alasalvar, C.; Shahidi, F. Natural antioxidants in tree nuts. Eur. J. Lipid Sci. Technol. 2009, 111, 1056–1062. [Google Scholar] [CrossRef]
- Zhang, M.; Yang, W.; Zeng, H.; Zou, M.; Luo, L.; Zhang, H.; Lu, C. Analysis of the Main Functional Components in Pericarp of Macadamia. Chin. J. Trop. Agric. 2011, 31, 73–75. [Google Scholar] [CrossRef]
- Zhang, M.; Ma, F.; Du, L.; Li, Y.; Huang, H.; Tu, X.; Chen, M. Ultrasonic Assisted Extraction and Purification of Total Phenols from Macadamia Green Peel. Chin. J. Trop. Crops 2021, 42, 2043–2051. [Google Scholar] [CrossRef]
- Zhang, M.; Shuai, X.; Du, L.; Tu, X. Optimization of extraction and antioxidant activity of polyphenols from macadamia green peel. Sci. Technol. Food Ind. 2017, 38, 195–199. [Google Scholar] [CrossRef]
- Liu, Q.; Ye, L.; Huang, W.; Huang, X. Determination of 4 Polyphenols in Macadamia Green Husk by High Performance Liquid Chromatography. Chin. J. Trop. Agric. 2016, 36, 106–111. [Google Scholar]
- Shi, R.; Xiong, Z.; Ke, J.; Liu, S.; Shi, Z.; Kan, H. Reparation Method and Lotion of Macadamia Green Skin Extract. Chinese Patent CN102727412A, 8 May 2013. [Google Scholar]
- Dailey, A.; Vuong, Q.V. Optimization of aqueous extraction conditions for recovery of phenolic content and antioxidant properties from Macadamia (Macadamia tetraphylla) skin waste. Antioxidants 2015, 4, 699–718. [Google Scholar] [CrossRef] [PubMed]
- Guo, G.; Hu, X.; Fu, J.; Ma, S.; Xu, R.; Huang, K.; Peng, Z.; He, X.; Zou, J. Determination and Correlation Analysis of Functional Components and Antioxidant Activity of Successive Solvent Extracts from Macadamia Green Husk. Food Sci. 2020, 42, 74–82. [Google Scholar] [CrossRef]
- Somwongin, S.; Sirilun, S.; Chantawannakul, P.; Anuchapreeda, S.; Yawootti, A.; Chaiyana, W. Ultrasound-assisted green extraction methods: An approach for cosmeceutical compounds isolation from Macadamia integrifolia pericarp. Ultrason. Sonochem 2023, 92, 106266. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.; Zulfiqar, F.; Raza, A.; Mohsin, S.M.; Mahmud, J.A.; Fujita, M.; Fotopoulos, V. Reactive Oxygen Species and Antioxidant Defense in Plants under Abiotic Stress: Revisiting the Crucial Role of a Universal Defense Regulator. Antioxidants 2020, 9, 681. [Google Scholar] [CrossRef]
- Dailey, A.; Vuong, Q.V. Optimisation of ultrasonic conditions as an advanced extraction technique for recovery of phenolic compounds and antioxidant activity from macadamia (Macadamia tetraphylla) skin waste. Technologies 2015, 3, 302–320. [Google Scholar] [CrossRef]
- Bae, J.S.; Su, S. Macadamia nut shell-derived carbon composites for post combustion CO2 capture. Int. J. Greenh. Gas. Control 2013, 19, 174–182. [Google Scholar] [CrossRef]
- Bayat, E.; Moosavi-Nasab, M.; Fazaeli, M.; Majdinasab, M.; Mirzapour-Kouhdasht, A.; Garcia-Vaquero, M. Wheat Germ Fermentation with Saccharomyces cerevisiae and Lactobacillus plantarum: Process Optimization for Enhanced Composition and Antioxidant Properties In Vitro. Foods 2022, 11, 1125. [Google Scholar] [CrossRef]
- Vivek, K.; Mishra, S.; Pradhan, R.C.; Jayabalan, R. Effect of probiotification with Lactobacillus plantarum MCC 2974 on quality of Sohiong juice. Lwt-Food Sci. Technol. 2019, 108, 55–60. [Google Scholar] [CrossRef]
- Li, S.; Jin, Z.; Hu, D.; Yang, W.; Yan, Y.; Nie, X.; Lin, J.; Zhang, Q.; Gai, D.; Ji, Y.; et al. Effect of solid-state fermentation with Lactobacillus casei on the nutritional value, isoflavones, phenolic acids and antioxidant activity of whole soybean flour. Lwt-Food Sci. Technol. 2020, 125, 109264. [Google Scholar] [CrossRef]
- Kuo, H.C.; Kwong, H.K.; Chen, H.Y.; Hsu, H.Y.; Yu, S.H.; Hsieh, C.W.; Lin, H.W.; Chu, Y.L.; Cheng, K.C. Enhanced antioxidant activity of Chenopodium formosanum Koidz. by lactic acid bacteria: Optimization of fermentation conditions. PLoS ONE 2021, 16, e0249250. [Google Scholar] [CrossRef]
- Kumari, M.; Haranahalli Nataraj, B.; Prasad, W.G.; Ali, S.A.; Behare, P.V. Multi-Faceted Bioactivity Assessment of an Exopolysaccharide from Limosilactobacillus fermentum NCDC400: Antioxidant, Antibacterial, and Immunomodulatory Proficiencies. Foods 2023, 12, 3595. [Google Scholar] [CrossRef]
- Paulino do Nascimento, L.C.; Lacerda, D.C.; Ferreira, D.J.S.; de Souza, E.L.; de Brito Alves, J.L. Limosilactobacillus fermentum, Current Evidence on the Antioxidant Properties and Opportunities to be Exploited as a Probiotic Microorganism. Probiotics Antimicrob. Proteins 2022, 14, 960–979. [Google Scholar] [CrossRef]
- Naghmouchi, K.; Belguesmia, Y.; Bendali, F.; Spano, G.; Seal, B.S.; Drider, D. Lactobacillus fermentum: A bacterial species with potential for food preservation and biomedical applications. Crit. Rev. Food Sci. Nutr. 2020, 60, 3387–3399. [Google Scholar] [CrossRef]
- Wei, T.; Chen, H.; Wu, D.; Gao, D.; Cai, Y.; Cao, X.; Xu, H.; Yang, J.; Guo, P. Response surface methodology for the mixed fungal fermentation of Codonopsis pilosula straw using Trichoderma reesei and Coprinus comatus. PeerJ 2023, 11, e15757. [Google Scholar] [CrossRef]
- Liu, Y.; Wei, S.; Liao, M. Optimization of ultrasonic extraction of phenolic compounds from euryale ferox seed shells using response surface methodology. Ind. Crops Prod. 2013, 49, 837–843. [Google Scholar] [CrossRef]
- Dulf, F.V.; Vodnar, D.C.; Socaciu, C. Effects of solid-state fermentation with two filamentous fungi on the total phenolic contents, flavonoids, antioxidant activities and lipid fractions of plum fruit (Prunus domestica L.) by-products. Food Chem. 2016, 209, 27–36. [Google Scholar] [CrossRef]
- Rasera, G.B.; Bridi, R.; Danielski, R.; Shahidi, F.; de Camargo, A.C. Phenolic antioxidants in the framework of Sustainable Development Goals: How far are we from zero waste? Curr. Opin. Food Sci. 2024, 57, 101163. [Google Scholar] [CrossRef]
- Zhou, X.; Du, H.H.; Jiang, M.; Zhou, C.; Deng, Y.; Long, X.; Zhao, X. Antioxidant Effect of Lactobacillus fermentum CQPC04-Fermented Soy Milk on D-Galactose-Induced Oxidative Aging Mice. Front. Nutr. 2021, 8, 727467. [Google Scholar] [CrossRef]
- Filannino, P.; Cavoski, I.; Thlien, N.; Vincentini, O.; De Angelis, M.; Silano, M.; Gobbetti, M.; Di Cagno, R. Lactic Acid Fermentation of Cactus Cladodes (Opuntia ficus-indica L.) Generates Flavonoid Derivatives with Antioxidant and Anti-Inflammatory Properties. PLoS ONE 2016, 11, e0152575. [Google Scholar] [CrossRef]
- Du, Z.; Yamasaki, S.; Oya, T.; Cai, Y. Cellulase-lactic acid bacteria synergy action regulates silage fermentation of woody plant. Biotechnol. Biofuels Bioprod. 2023, 16, 125. [Google Scholar] [CrossRef]
- Feng, T.; Wang, J. Oxidative stress tolerance and antioxidant capacity of lactic acid bacteria as probiotic: A systematic review. Gut Microbes 2020, 2, 1801944. [Google Scholar] [CrossRef] [PubMed]
- Krautkramer, K.A.; Fan, J.; Bäckhed, F. Gut microbial metabolites as multi-kingdom intermediates. Nat. Rev. Microbiol. 2021, 19, 77–94. [Google Scholar] [CrossRef]
- Zhou, G.; Xu, X.; Qiu, X.; Zhang, J. Biochar influences the succession of microbial communities and the metabolic functions during rice straw composting with pig manure. Bioresour. Technol. 2019, 272, 10–18. [Google Scholar] [CrossRef]
- Wang, Y.; Ying, G.; Zhang, Z.; Tang, Y.; Zhang, Y.; Chen, L. Bacillus velezensis promotes the proliferation of lactic acid bacteria and influences the fermentation quality of whole-plant corn silage. Front. Plant Sci. 2024, 15, 1285582. [Google Scholar] [CrossRef]
- Iorjiim, W.M.; Omale, S.; Etuh, M.A.; Ubani, A.; Alemika, E.T.; Gyang, S.S. Senescence and oxidative stress toxicities induced by lamivudine and tenofovir in Drosophila melanogaster. Ann. Pharm. Fr. 2022, 80, 864–875. [Google Scholar] [CrossRef]
- Wen, D.; Xie, J.; Yuan, Y.; Shen, L.; Yang, Y.; Chen, W. The endogenous antioxidant ability of royal jelly in Drosophila is independent of Keap1/Nrf2 by activating oxidoreductase activity. Insect Sci. 2024, 31, 503–523. [Google Scholar] [CrossRef]
- Aksu, U.; Yanar, K.; Terzioglu, D.; Erkol, T.; Ece, E.; Aydin, S.; Uslu, E.; Çakatay, U. Effect of tempol on redox homeostasis and stress tolerance in mimetically aged Drosophila. Arch. Insect Biochem. Physiol. 2014, 87, 13–25. [Google Scholar] [CrossRef]
- Dai, X.; Zhang, Q.; Zhang, G.; Ma, C.; Zhang, R. Protective effect of agar oligosaccharide on male Drosophila melanogaster suffering from oxidative stress via intestinal microflora activating the Keap1-Nrf2 signaling pathway. Carbohydr. Polym. 2023, 313, 120878. [Google Scholar] [CrossRef]
- Nielsen, F.; Mikkelsen, B.B.; Nielsen, J.B.; Andersen, H.R.; Grandjean, P. Plasma malondialdehyde as biomarker for oxidative stress: Reference interval and effects of life-style factors. Clin. Chem. 1997, 43, 1209–1214. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Tan, F.; Liu, X.; Yi, R.; Zhao, X. Grape skin fermentation by Lactobacillus fermentum CQPC04 has anti-oxidative effects on human embryonic kidney cells and apoptosis-promoting effects on human hepatoma cells. RSC Adv. 2020, 10, 4607–4620. [Google Scholar] [CrossRef] [PubMed]
- Letizia, F.; Fratianni, A.; Cofelice, M.; Testa, B.; Albanese, G.; Di Martino, C.; Panfili, G.; Lopez, F.; Iorizzo, M. Antioxidative Properties of Fermented Soymilk Using Lactiplantibacillus plantarum LP95. Antioxidants 2023, 12, 1442. [Google Scholar] [CrossRef] [PubMed]
- He, M.; He, T.; Farrar, S.; Ji, L.; Liu, T.; Ma, X. Antioxidants Maintain Cellular Redox Homeostasis by Elimination of Reactive Oxygen Species. Cell Physiol. Biochem. 2017, 44, 532–553. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Xia, Y.; Wang, G.; Xiong, Z.; Zhang, H.; Lai, F.; Ai, L. Lactobacillus plantarum AR501 Alleviates the Oxidative Stress of D-Galactose-Induced Aging Mice Liver by Upregulation of Nrf2-Mediated Antioxidant Enzyme Expression. J. Food Sci. 2018, 83, 1990–1998. [Google Scholar] [CrossRef] [PubMed]
- Nordberg, J.; Arnér, E.S.J. Reactive oxygen species, antioxidants, and the mammalian thioredoxin system. Free Radic. Biol. Med. 2001, 31, 1287–1312. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, C.; Li, D.; Zhao, Y.; Zhang, X.; Zeng, X.; Yang, Z. Antioxidant activity of an exopolysaccharide isolated from Lactobacillus plantarum C88. Int. J. Biol. Macromol. 2013, 54, 270–275. [Google Scholar] [CrossRef] [PubMed]
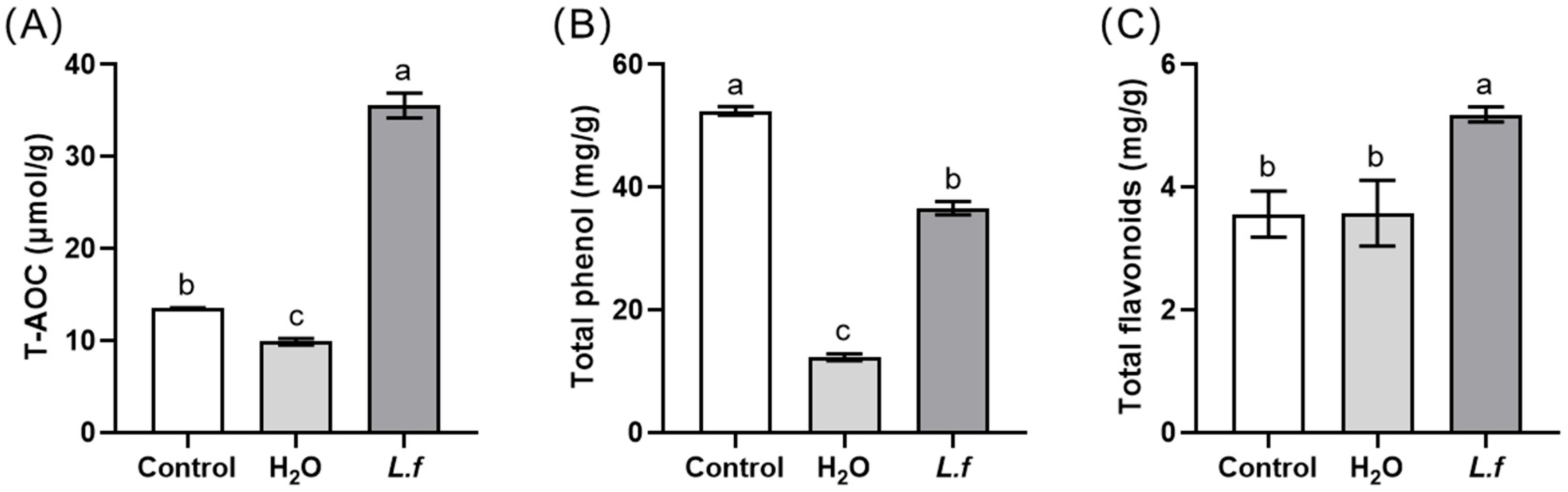
| Components | Content | References |
|---|---|---|
| tannins | 17.53% ± 3.97 mg/g | [4] |
| total phenols | 18.36 ± 0.32 mg/g | [5] |
| total flavonoids | 7.33 ± 2.63 mg/g | [6] |
| 4-hydroxybenzyl alcohol | 1.3 ± 0.12 mg/g | [7] |
| 3,4-dehydroxybenzoic acid | 0.19 ± 0.001 mg/g | |
| p-hydroxybenzoic acid | 0.046 ± 0.003 mg/g | |
| p-hydroxybenzaldehyde | 0.15 ± 0.009 mg/g |
| Factor | Coded Symbol | Level | ||
|---|---|---|---|---|
| −1 | 0 | 1 | ||
| Cellulase dosage (%) | A | 0.6 | 0.9 | 1.2 |
| Inoculation size (mL/100 g) | B | 3 | 4 | 5 |
| Fermentation time (day) | C | 5 | 7 | 9 |
| Flies | Groups | Treatment |
|---|---|---|
| H2O2-induced oxidative stress flies | Low group (L group) | fed with 6.25% FMIPE containing 5% sucrose |
| Medium group (M group) | fed with 12.5% FMIPE containing 5% sucrose | |
| High group (H group) | fed with 25% FMIPE containing 5% sucrose | |
| H2O2 group | negative control, feed with 5% sucrose solution (normal feed) | |
| Vc group | positive control, fed with 0.5% Vc solution containing 5% sucrose | |
| Healthy flies | Control group | blank control, feed with 5% sucrose solution (normal feed) |
| Experimental No. | A | B | C | T-AOC (μmol/g) |
|---|---|---|---|---|
| 1 | −1 | 0 | 1 | 23.18 |
| 2 | 0 | 0 | 0 | 35.27 |
| 3 | 0 | 0 | 0 | 35.38 |
| 4 | 1 | 0 | 1 | 26.51 |
| 5 | 0 | 1 | −1 | 23.26 |
| 6 | 0 | −1 | 1 | 23.71 |
| 7 | 0 | 1 | 1 | 28.76 |
| 8 | 0 | −1 | −1 | 20.20 |
| 9 | −1 | −1 | 0 | 18.96 |
| 10 | 0 | 0 | 0 | 34.21 |
| 11 | 0 | 0 | 0 | 37.80 |
| 12 | −1 | 0 | −1 | 20.79 |
| 13 | 0 | 0 | 0 | 34.86 |
| 14 | −1 | 1 | 0 | 30.15 |
| 15 | 1 | 0 | −1 | 26.58 |
| 16 | 1 | 1 | 0 | 29.37 |
| 17 | 1 | −1 | 0 | 26.36 |
| Source | Sum of Squares | df | Mean Squares | F-Value | p-Value | Significance |
|---|---|---|---|---|---|---|
| Model | 543.21 | 9 | 60.36 | 22.95 | 0.0002 | ** |
| A | 30.98 | 1 | 30.98 | 11.78 | 0.0110 | * |
| B | 62.29 | 1 | 62.29 | 23.68 | 0.0018 | ** |
| C | 16.05 | 1 | 16.05 | 6.10 | 0.0428 | * |
| AB | 16.75 | 1 | 16.75 | 6.37 | 0.0396 | * |
| AC | 1.51 | 1 | 1.51 | 0.5753 | 0.4729 | |
| BC | 0.9943 | 1 | 0.9943 | 0.3780 | 0.5581 | |
| A2 | 85.38 | 1 | 85.38 | 32.46 | 0.0007 | ** |
| B2 | 96.52 | 1 | 96.52 | 36.70 | 0.0005 | ** |
| C2 | 190.94 | 1 | 190.94 | 72.60 | <0.0001 | ** |
| Residual | 18.41 | 7 | 2.63 | |||
| Lack of Fit | 10.99 | 3 | 3.66 | 1.98 | 0.2597 | |
| Pure Error | 7.42 | 4 | 1.85 | |||
| Cor total | 561.62 | 16 | ||||
| R2 | 0.9672 | |||||
| R2Adj | 0.9251 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, C.; Huang, H.; Liu, B.; Tang, X.; Tan, B.; Jiang, Q.; Yin, Y. Optimizing Cellulase—Limosilactobacillus fermentum ZC529 Synergy Fermentation for Preserving Macadamia integrifolia Pericarp’s Potential Use as Antioxidants. Antioxidants 2024, 13, 783. https://doi.org/10.3390/antiox13070783
Zhang C, Huang H, Liu B, Tang X, Tan B, Jiang Q, Yin Y. Optimizing Cellulase—Limosilactobacillus fermentum ZC529 Synergy Fermentation for Preserving Macadamia integrifolia Pericarp’s Potential Use as Antioxidants. Antioxidants. 2024; 13(7):783. https://doi.org/10.3390/antiox13070783
Chicago/Turabian StyleZhang, Chen, Haibo Huang, Bifan Liu, Xiongzhuo Tang, Bi’e Tan, Qian Jiang, and Yulong Yin. 2024. "Optimizing Cellulase—Limosilactobacillus fermentum ZC529 Synergy Fermentation for Preserving Macadamia integrifolia Pericarp’s Potential Use as Antioxidants" Antioxidants 13, no. 7: 783. https://doi.org/10.3390/antiox13070783
APA StyleZhang, C., Huang, H., Liu, B., Tang, X., Tan, B., Jiang, Q., & Yin, Y. (2024). Optimizing Cellulase—Limosilactobacillus fermentum ZC529 Synergy Fermentation for Preserving Macadamia integrifolia Pericarp’s Potential Use as Antioxidants. Antioxidants, 13(7), 783. https://doi.org/10.3390/antiox13070783











