Qualitative, Quantitative, In Vitro Antioxidant Activity and Chemical Profiling of Leptadenia pyrotechnica (Forssk.) Decne Using Advanced Analytical Techniques
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Drugs Utilized
2.2. Collection of Experimental Plant Material
2.3. Extract Preparation of the Plant Sample
2.4. Proximate Analyses
2.5. Qualitative Screening
2.6. Quantitative Analysis of Phytocompounds
2.6.1. Total Phenolic Estimation
2.6.2. Total Saponin Estimation
2.6.3. Total Tannin Estimation
2.6.4. Total Flavonoid Estimation
2.6.5. Total Alkaloid Estimation
2.7. Estimation of the In Vitro Antioxidant Activity
2.7.1. 2,2-Diphenyl-1-picrylhydrazyl (DPPH) Free Radical Scavenging Assay
2.7.2. Nitric Oxide (NO) Scavenging Assay
2.7.3. Hydrogen Peroxide (H2O2) Radical Scavenging Activity Analysis
2.7.4. Metal Chelation (MC) Activity
2.7.5. Ferric Reducing Antioxidant Power (FRAP) Assay
2.8. Elemental Analysis
2.9. Comparison between Conventional and Non-Conventional Methods
Experimental Design
2.10. Analytical Methods
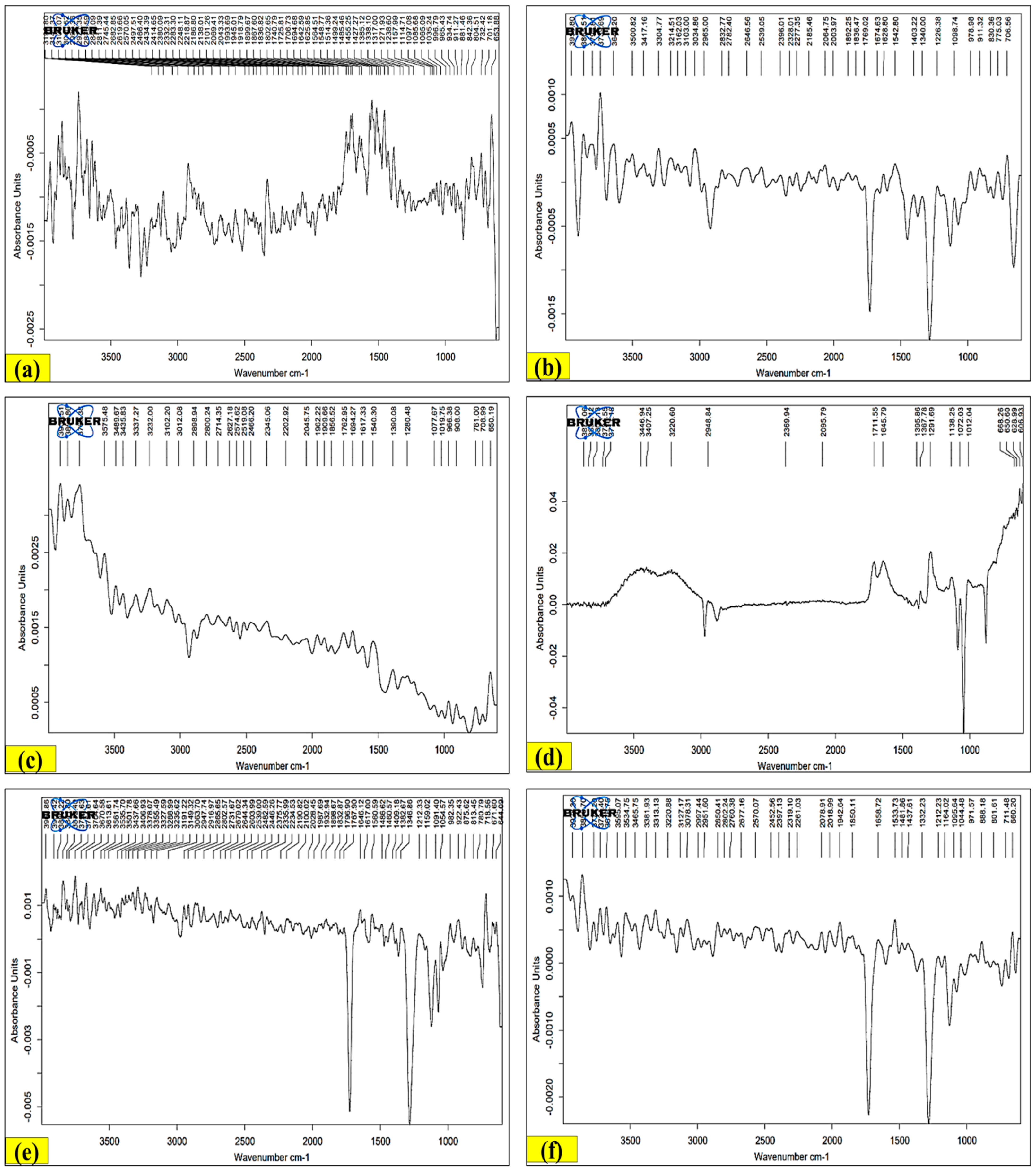
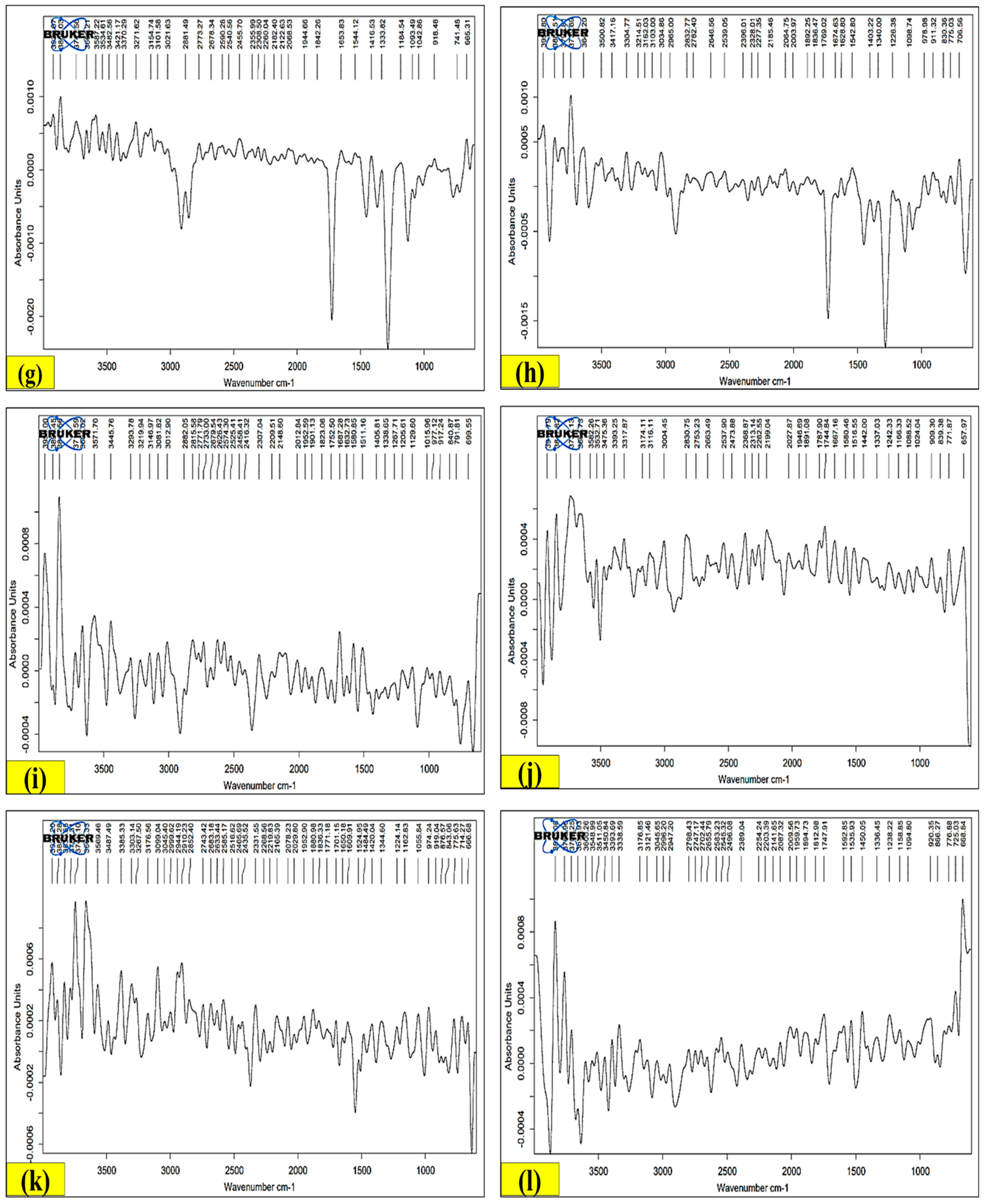
2.10.1. Fourier-Transform Infrared Spectroscopy (FT-IR)
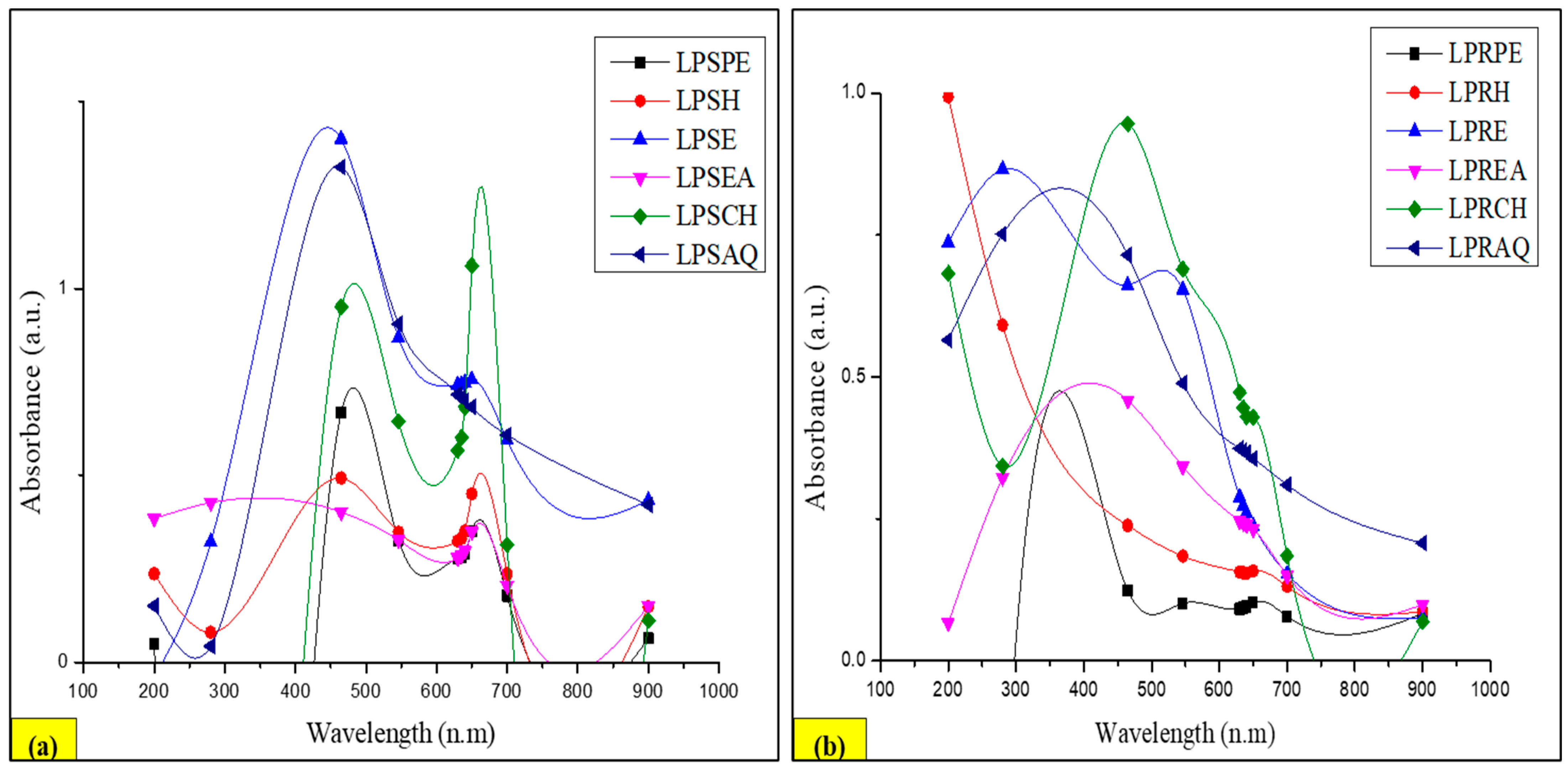
2.10.2. UV–Visible Spectroscopy
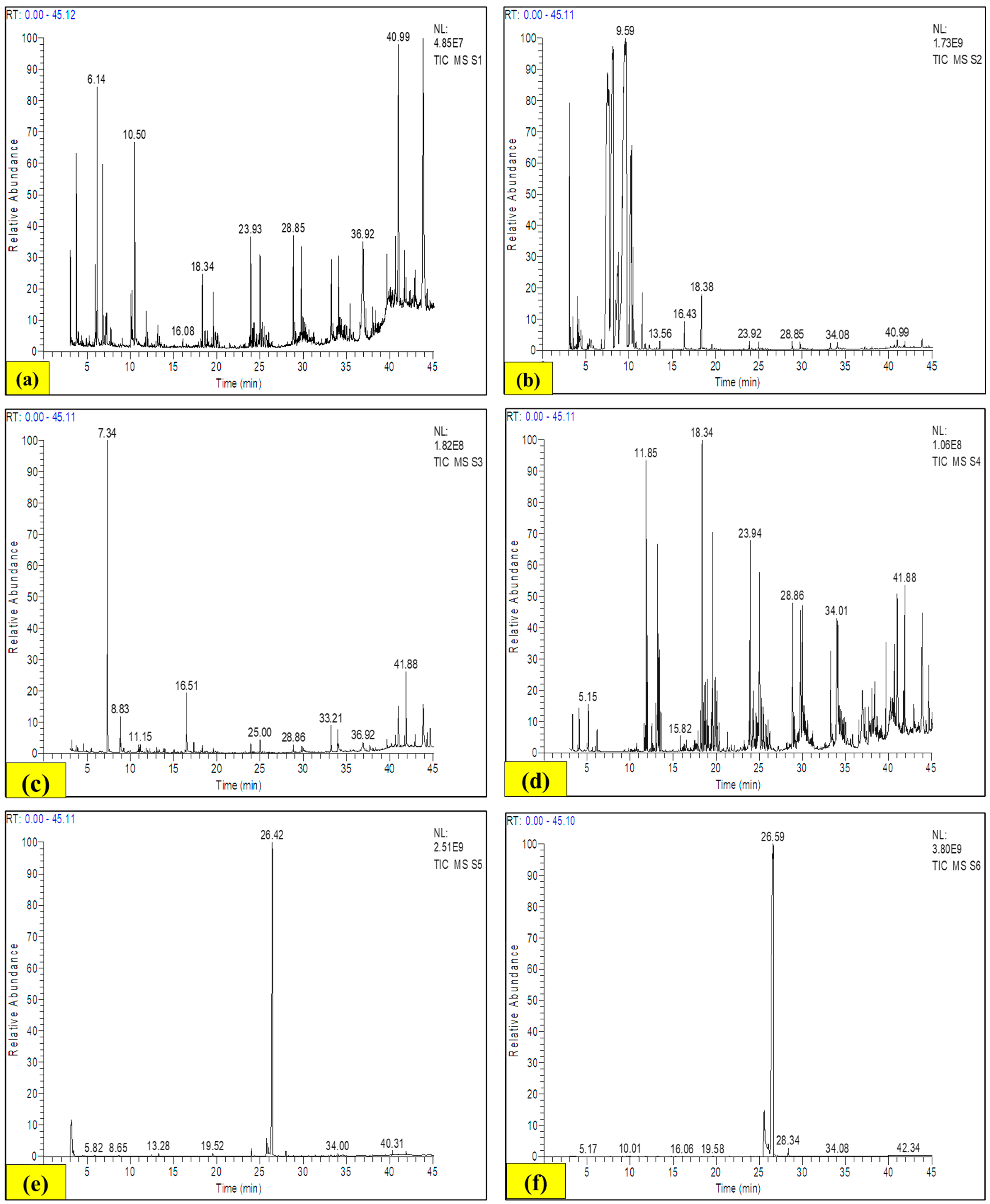
2.10.3. GC-MS
2.11. Statistical Calculations
3. Results and Discussion
3.1. Extraction Yield Values of LP Extracts
3.2. Physicochemical Analysis of LP
3.3. Qualitative Screening of Phytochemical Compounds in Various LP Extracts
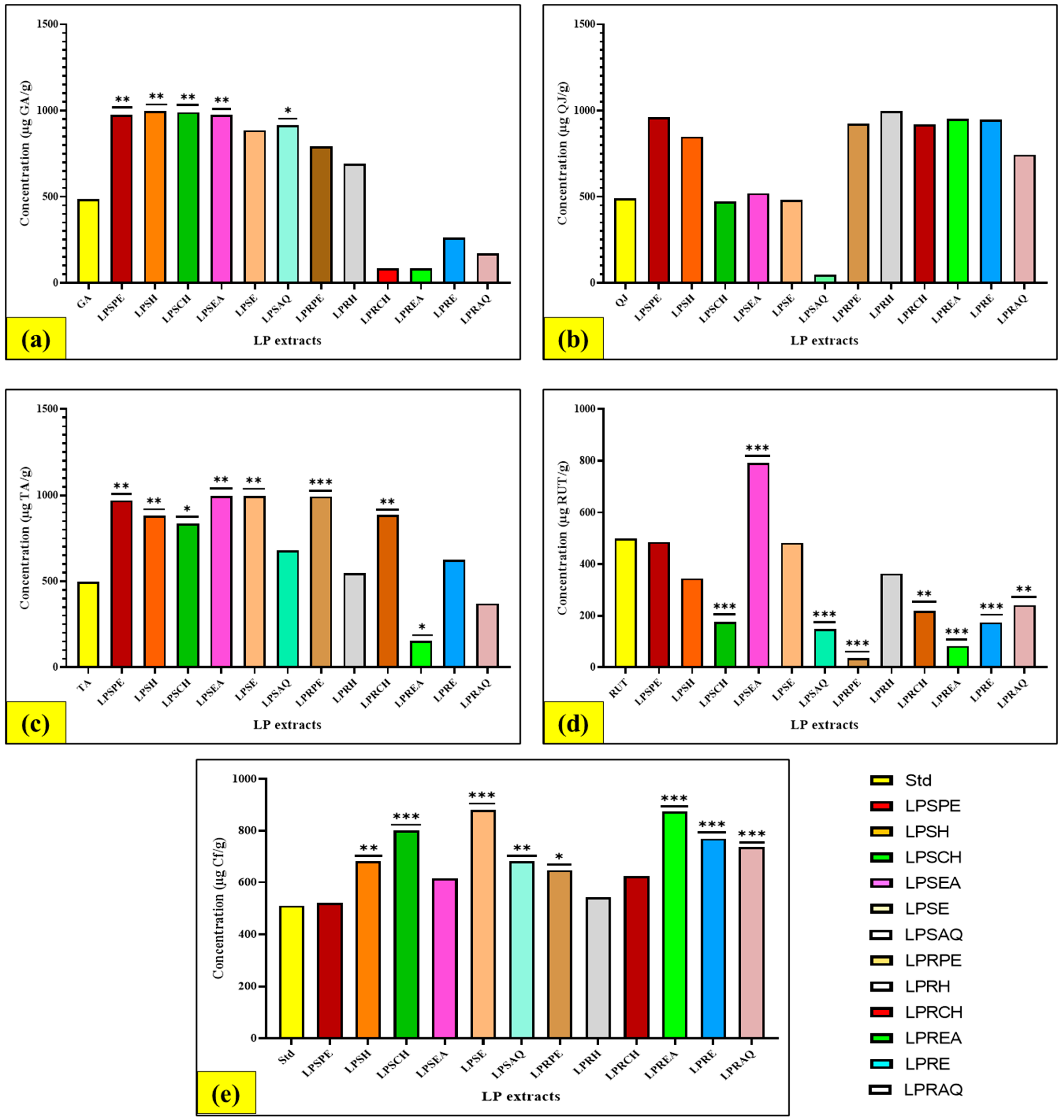
3.4. Quantitative Evaluation of Phytochemical Compounds in Various LP Extracts
3.4.1. Evaluation of Total Phenolic (TPC) Content
3.4.2. Evaluation of Total Saponin (TSC) Content
3.4.3. Evaluation of Total Tannin (TTC) Content
3.4.4. Evaluation of Total Flavonoid (TFC) Content
3.4.5. Evaluation of Total Alkaloid (TAC) Content
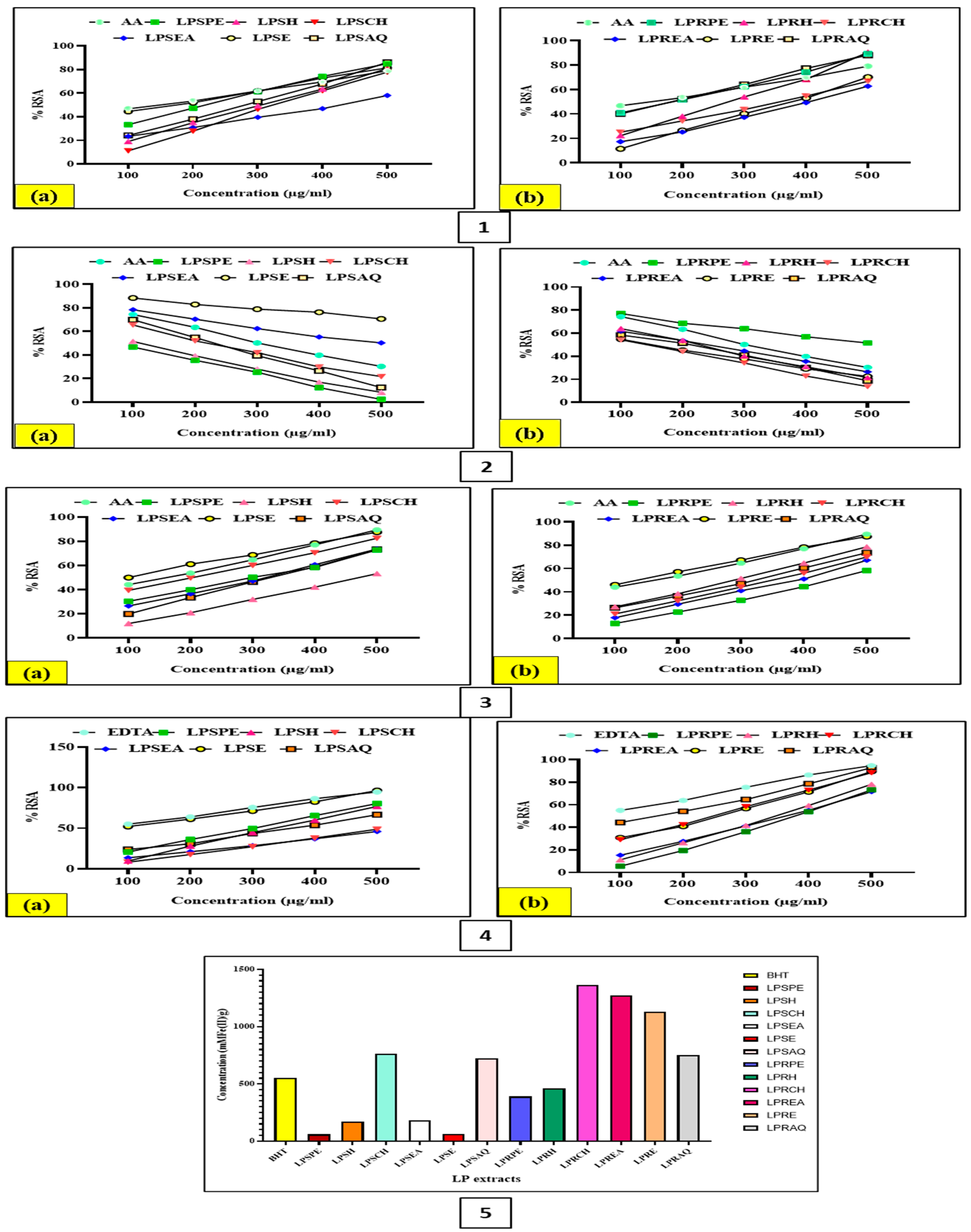
3.5. Assessment of In Vitro Antioxidant Activities of Different LP Extracts
3.5.1. DPPH Free Radical Scavenging Assay
3.5.2. Hydrogen Peroxide (H2O2) Radical Scavenging Activity Analysis
3.5.3. Metal Chelation Activity
3.5.4. Nitric Oxide (NO) Scavenging Assay
3.5.5. Ferric Reducing Antioxidant Power (FRAP) Assay
3.6. Correlation of Phytochemical Contents with Their In Vitro Antioxidant Activities
3.7. Elemental Analysis
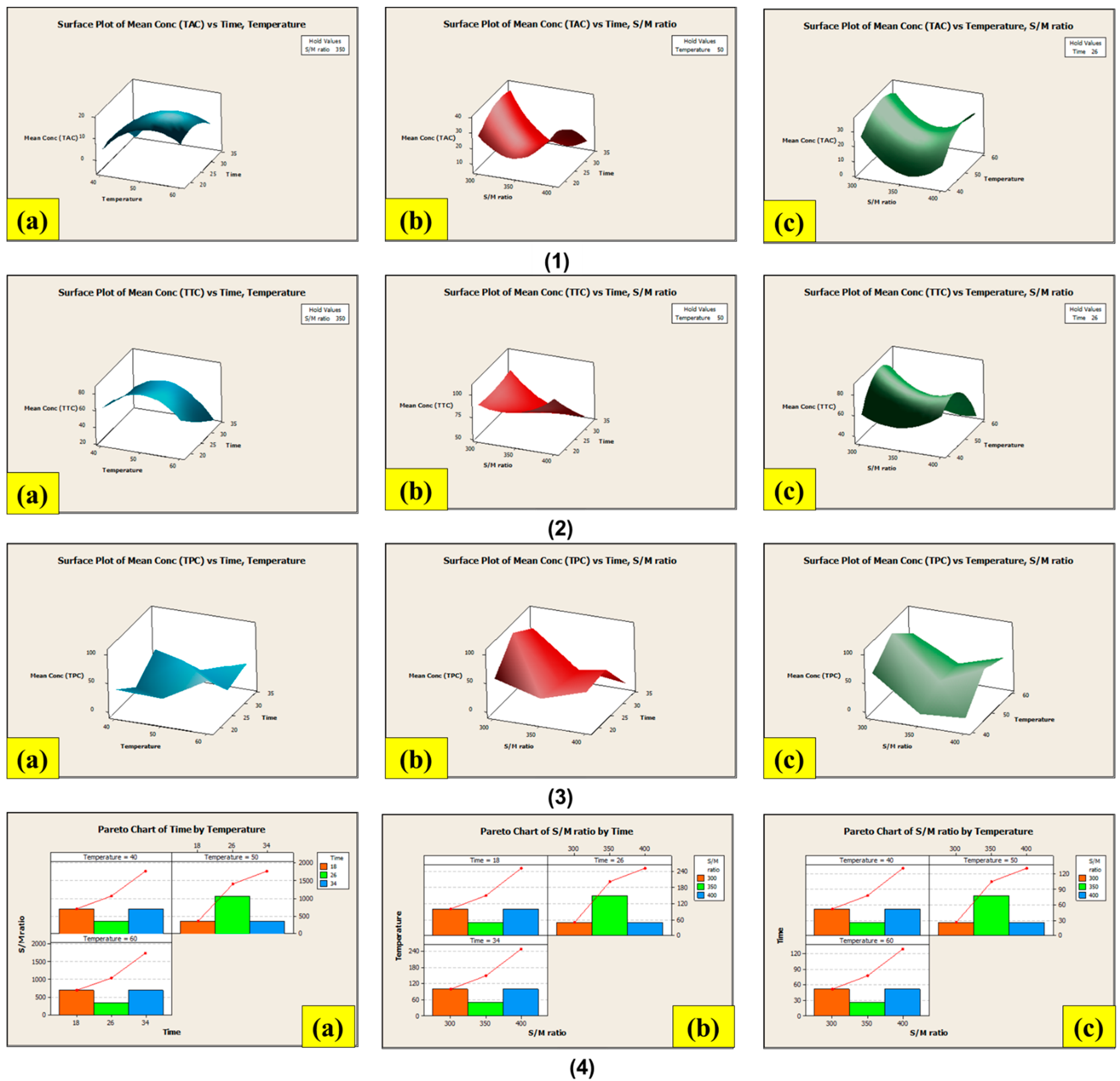
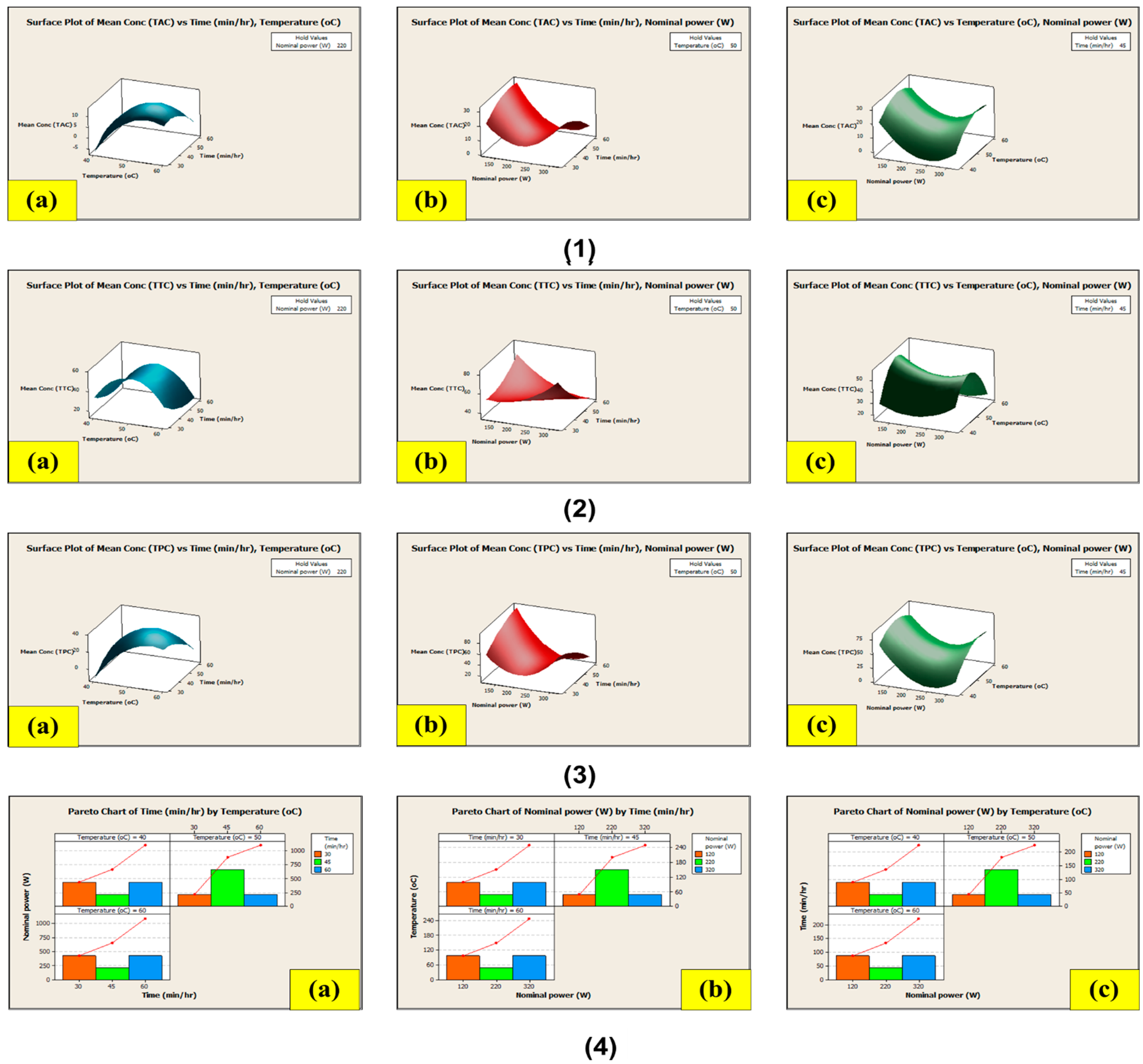
3.8. Extraction Using Conventional Soxhlet Extraction (CSE) and Non-Conventional Ultrasound-Assisted Extraction (NCUSAE) Methods
3.8.1. Extraction Using the Conventional Soxhlet Extraction (CSE) Method
3.8.2. Extraction Using the Non-Conventional Ultrasound-Assisted Extraction (NCUSAE) Method
3.9. Analytical Methods
3.9.1. FT-IR
3.9.2. UV–Visible Profile
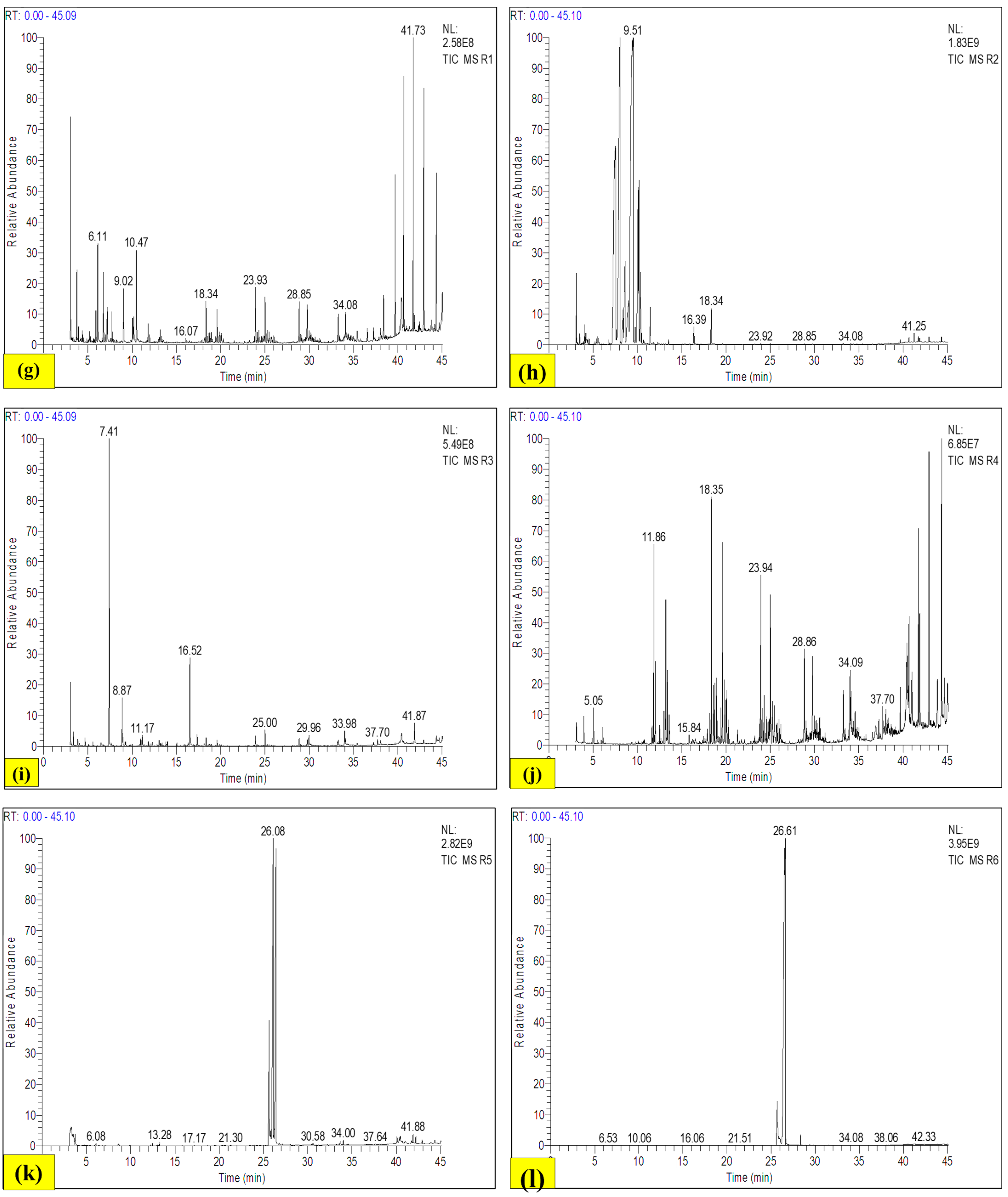
3.9.3. Gas Chromatography–Mass Spectrometry (GC-MS)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Kumar, R.; Sharma, N.; Rolta, R.; Lal, U.R.; Sourirajan, A.; Dev, K.; Kumar, V. Thalictrum foliolosum DC: An unexplored medicinal herb from the North Western Himalayas with potential against fungal pathogens and scavenger of reactive oxygen species. Biocatal. Agric. Biotechnol. 2020, 26, 101621. [Google Scholar] [CrossRef]
- Eddouks, M.; Chattopadhyay, D.; De Feo, V.; Cho, W.C. Medicinal plants in the prevention and treatment of chronic diseases 2013. Evid. Based Complement. Altern. Med. 2014, 2014, 180981. [Google Scholar] [CrossRef]
- Zehra, A.; Meena, M.; Jadhav, D.M.; Swapnil, P. Harish Regulatory mechanisms for the conservation of endangered plant species, Chlorophytum tuberosum—Potential medicinal plant species. Sustainability 2023, 15, 6406. [Google Scholar] [CrossRef]
- Yadav, G.; Sonigra, P.; Meena, M. A review on pharmaceutical and medicinal importance of Anethum graveolens L. Acta Sci. Nutr. Health 2022, 6, 23–28. [Google Scholar]
- Yadav, G.; Meena, M. Bioprospecting of endophytes in medicinal plants of Thar Desert: An attractive resource for biopharmaceuticals. Biotechnol. Rep. 2021, 30, e00629. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Singh, J.; Sharma, D. Traditional wisdom to treat the most common ailments in Chopal region of Shimla district, Himachal Pradesh, India. Plant Arch. 2018, 18, 2759–2769. [Google Scholar]
- Shantabi, L.; Jagetia, G.C.; Vabeiryureilai, M.; Lalrinzuali, K. Phytochemical screening of certain medicinal plants of Mizoram, India and their folklore use. J. Biodivers. Biopros. Dev. 2014, 2, 1. [Google Scholar] [CrossRef]
- Meena, M.; Singh, S.K.; Swapnil, P. The role of PGPRs in medicinal plants under abiotic stress. In Medicinal Plants: Their Response to Abiotic Stress; Husen, A., Iqbal, M., Eds.; Springer: Singapore, 2023; pp. 267–285. [Google Scholar] [CrossRef]
- El-Fitiany, R.A.; Khasawneh, M.A. Leptadenia pyrotechnica (Forsk.) Decne: From edibility to drug discovery (a comprehensive review). Food Rev. Int. 2023, 39, 6834–6886. [Google Scholar] [CrossRef]
- Idrees, S.; Qureshi, R.; Bibi, Y.; Ishfaq, A.; Khalid, N.; Iftikhar, A.; Shabir, A.; Riaz, I.; Ahmad, S.N. Ethnobotanical and biological activities of Leptadenia pyrotechnica (Forssk.) Decne.: A review. Afr. J. Tradit. Complement. Altern. Med. 2016, 13, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Abd-ElGawad, A.M.; Assaeed, A.M.; Bonanomi, G.; El-Amier, Y.A. Ecological insight, anatomical features, and fiber characterization of Leptadenia pyrotechnica (Forrsk.) Decne. as a promising resource. Sustainability 2022, 14, 16895. [Google Scholar] [CrossRef]
- IPNI (International Plant Names Index). The Royal Botanic Gardens, Kew, Harvard University Herbaria & Libraries and Australian National Herbarium. Available online: http://www.ipni.org (accessed on 1 June 2024).
- Sharma, V.; Janmeda, P. Extraction, isolation and identification of flavonoids from Euphorbia neriifolia leaves. Arab. J. Chem. 2014, 10, 509–514. [Google Scholar] [CrossRef]
- Pavithra, K.; Vadivukkarasi, S. Evaluation of free radical scavenging activity of various extracts of leaves from Kedrostis foetidissima (Jacq.), Cogn. Food Sci. Hum. Wellness 2015, 4, 42–46. [Google Scholar] [CrossRef]
- Sharma, V.; Janmeda, P. Microscopic studies and preliminary pharmacognostical evaluation of Euphorbia neriifolia L. leaves. Indian J. Nat. Prod. Resour. 2013, 4, 348–357. [Google Scholar]
- Çetin, N.; Saglam, C. Rapid detection of total phenolics, antioxidant activity and ascorbic acid of dried apples by chemometric algorithms. Food Biosci. 2022, 2022, 101670. [Google Scholar] [CrossRef]
- Le, A.V.; Parks, S.E.; Nguyen, M.H.; Roach, P.D. Optimisation of the microwave-assisted ethanol extraction of saponin from Gac (Momordica cochinchinensis Spreng.) seeds. Medicines 2018, 5, 70. [Google Scholar] [CrossRef] [PubMed]
- Haile, M.; Kang, W.H. Antioxidant activity, total polyphenol, flavonoid and tannin contents of fermented green coffee beans with selected yeast. Fermentation 2019, 5, 29. [Google Scholar] [CrossRef]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 55–59. [Google Scholar] [CrossRef]
- Gan, J.; Feng, Y.; He, Z.; Li, X.; Zhang, H. Correlations between antioxidant activity and alkaloids and phenols of Maca (Lepidium meyenii). J. Food Qual. 2017, 2017, 3185945. [Google Scholar] [CrossRef]
- Habibian, M.; Sadeghi, G.; Karimi, A. Phytochemicals and antioxidant properties of solvent extracts from Purslane (Portulaca oleracea L.): A preliminary study. Food Sci. Eng. 2019, 1, 12. [Google Scholar] [CrossRef]
- Partap, S.; Tewari, U.; Sharma, K.; Jha, K.K. In vitro antioxidant activity of whole plant of Leptadenia pyrotechnica. J. Drug Deliv. Ther. 2014, 4, 40–44. [Google Scholar] [CrossRef]
- Devi, K.R.; Patnaik, K.S.K.R.; Ashok, D.; Bakshi, V.; Bathula, R.; Rani, S.S. Microwave irradiation synthesis and antioxidant activity of isatin-oxadiazole derivatives. Int. J. Adv. Res. 2017, 5, 2329–2336. [Google Scholar] [CrossRef] [PubMed]
- Wetchakul, P.; Chonsut, P.; Punsawad, C.; Sanpinit, S. LC-QTOF-MS characterization, antioxidant activity, and in vitro toxicity of medicinal plants from the Tri-Than-Thip Remedy. Evid. Based Complement. Alternat. Med. 2022, 2022, 4477003. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, R.P.; Trindade, M.A.; Tonin, F.G.; Lima, C.G.; Pugine, S.M.; Munekata, P.E.; Lorenzo, J.M.; de Melo, M.P. Evaluation of antioxidant capacity of 13 plant extracts by three different methods: Cluster analyses applied for selection of the natural extracts with higher antioxidant capacity to replace synthetic antioxidant in lamb burgers. J. Food Sci. Technol. 2015, 53, 451–460. [Google Scholar] [CrossRef] [PubMed]
- Zafar, M.; Khan, M.A.; Ahmad, M.; Jan, G.; Sultana, S.; Ullah, K.; Marwat, S.K.; Ahmad, F.; Jabeen, A.; Nazir, A.; et al. Elemental analysis of some medicinal plants used in traditional medicine by atomic absorption spectrophotometer (AAS). J. Med. Plant Res. 2010, 4, 1987–1990. [Google Scholar] [CrossRef]
- de Castro, M.D.L.; García-Ayuso, L.E. Soxhlet extraction of solid materials: An outdated technique with a promising innovative future. Anal. Chim. Acta 1998, 369, 1–10. [Google Scholar] [CrossRef]
- Chemat, F.; Tomao, V.; Virot, M. Ultrasound-assisted extraction in food analysis. In Handbook of Food Analysis Instruments; Otles, S., Ed.; CRC Press: Boca Raton, FL, USA, 2008; pp. 85–94. [Google Scholar] [CrossRef]
- Kalil, S.J.; Maugeri, F.; Rodrigues, M.I. Response surface analysis and simulation as a tool for bioprocess design and optimization. Proc. Biochem. 2000, 35, 539–550. [Google Scholar] [CrossRef]
- Rao, K.J.; Kim, C.H.; Rhee, S.K. Statistical optimization of medium for the production of recombinant hirudin from Saccharomyces cerevisiae using response surface methodology. Proc. Biochem. 2000, 35, 639–647. [Google Scholar] [CrossRef]
- Puri, S.; Beg, Q.K.; Gupta, R. Optimization of alkaline protease production from Bacillus sp. by response surface methodology. Curr. Microbiol. 2002, 44, 286–290. [Google Scholar] [CrossRef]
- Vohra, A.; Satyanarayana, T. Statistical optimization of the medium components by response surface methodology to enhance phytase production by Pichia anomala. Proc. Biochem. 2002, 37, 999–1004. [Google Scholar] [CrossRef]
- Burkert, J.F.; Maugeri, F.; Rodrigues, M.I. Optimization of extracellular lipase production by Geotrichum sp. using factorial design. Bioresour. Technol. 2004, 91, 77–84. [Google Scholar] [CrossRef]
- Jain, D.; Meena, M.; Singh, D.; Janmeda, P. Structural characterization of bioactive compounds of Gymnosporia senegalensis (Lam.) Loes. using advanced analytical technique like FT-IR, GC-MS and 1H-NMR spectroscopy. Nat. Prod. Res. 2023, 37, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Serna-Jiménez, J.A.; Torres-Valenzuela, L.S.; Sanín Villarreal, A.; Roldan, C.; Martín, M.A.; Siles, J.A.; Chica, A.F. Advanced extraction of caffeine and polyphenols from coffee pulp: Comparison of conventional and ultrasound-assisted methods. LWT 2023, 177, 114571. [Google Scholar] [CrossRef]
- Mabasa, X.E.; Mathomu, L.M.; Madala, N.E.; Musie, E.M.; Sigidi, M.T. Molecular spectroscopic (FTIR and UV-Vis) and hyphenated chromatographic (UHPLC-qTOF-MS) analysis and in vitro bioactivities of the Momordica balsamina leaf extract. Biochem. Res. Int. 2021, 2021, 2854217. [Google Scholar] [CrossRef] [PubMed]
- Jirvankar, P.S.; Khobragade, D.; Chandewar, S.; Pimpale, A.; Gawali, R.; Lokade, A.; Maske, A.; Agrawal, R. Phytochemical, UV-visible and FTIR assessment along with in vitro antioxidant activity of methanolic extract of Tephrosia purpurea Linn Root. J. Pharm. Res. Int. 2022, 34, 26–33. [Google Scholar] [CrossRef]
- Monika, S.; Thirumal, M.; Kumar, P.R. Identification of bioactive phytochemicals by GC-MS profiling, phytochemical screening, and TLC using methanolic extract of Carissa carandas Linn. leaf. J. Pharm. Negat. Results 2022, 13, 4439–4444. [Google Scholar] [CrossRef]
- Migahid, A.M.; Batanoun, K.; Abdelwah, A. Eco-physiological studies on desert plants. VII. Water relations of Leptadenia pyrotechnica (Forsk.) Decne. growing in Egyptian desert. Oecologia 1972, 10, 79–91. [Google Scholar] [CrossRef] [PubMed]
- Al-Easa, H.S. Fatty acids composition of fifty-one plant species growing in Qatar. Int. J. Chem. 2004, 14, 195–204. [Google Scholar]
- Schmelzer, G.H.; Gurib-Fakim, A. Medicinal Plants 2; Prota: Wageningen, The Netherlands, 2008; Volume 11. [Google Scholar]
- Javid, S.; Khalil, S.; Munir, I.; Amjad, M.S.; Akbar, K.; Yasmeen, F.; Akhtar, M.S. Plant metabolites and pharmacological activities of Leptadenia pyrotechnica (Forssk.) Decne. In Natural Bio-Active Compounds; Sayeed, M., Swamy, A.M.K., Sinniah, U.R., Eds.; Springer: Singapore, 2019; pp. 551–560. [Google Scholar]
- Verma, N.; Jha, K.K.; Chaudhary, S.; Singh, O.; Kumar, A. Phytochemistry, pharmacology and traditional uses of Leptadenia pyrotechnica-An important medicinal plant. Indian J. Pharm. Biol. Res. 2014, 2, 128. [Google Scholar] [CrossRef]
- Ullah, F.; Uzair, M.; Chaudhry, B.A.; Zafar, Z.U. Phytochemical and pharmacological studiof Leptadenia pyrotechnica. Pak. J. Pharm. Res. 2015, 1, 78–85. [Google Scholar] [CrossRef]
- Purohit, S. Phytochemical screening, free radical scavenging, antimicrobial activity of ethanolic extract of Leptadenia pyrotechnica. Int. J. Green Pharm. 2017, 11, 180–187. [Google Scholar]
- Rekha, R.; Singh, S.; Kumar, M.; Kumar, K. In vivo and in vitro comparative study of primary metabolites of Leptadenia pyrotechnia. J. Microbiol. Biotechnol. Res. 2013, 3, 51–54. [Google Scholar]
- Tanira, M.; Bashir, A.K.; Dib, R.; Goodwin, C.S.; Wasfi, I.A.; Banna, N.R. Antimicrobial and phytochemical screening of medicinal plants of the United Arab Emirates. J. Ethnopharmacol. 1994, 41, 201–205. [Google Scholar] [CrossRef] [PubMed]
- Soliman, G.A.; Donia, A.E.R.M.; Awaad, A.S.; Alqasoumi, S.I.; Yusufoglu, H. Effect of Emex spinosa, Leptadenia pyrotechnica, Haloxylon salicornicum and Ochradenus baccatus extracts on the reproductive organs of adult male rats. Pharm. Biol. 2012, 50, 105–112. [Google Scholar] [CrossRef]
- Kumar, D.; Dhayal, K.; Sharma, R.A. Biochemical estimation of primary metabolites and antioxidant activity of Corchorus olitorius L. and Leptadenia pyrotechnica (Forsk) Decne. Int. J. Res. Pharm. Chem. 2018, 8, 183–187. [Google Scholar]
- Zubair, M.; Nazir, M.; Saleem, M.; Raiz, N.; Touseef, S.; Khan, S.; Zengin, G.; Mazhar, M.E.; Tousif, M.I. Chemodiversity, biological activities and molecular docking studies of Leptadenia pyrotechnica (Forssk.) Decne: A comprehensive approach to validate its medicinal use. Chem. Biodivers. 2022, 19, e202100884. [Google Scholar] [CrossRef]
- Khasawneh, M.A.; Elwy, H.M.; Hamza, A.A.; Fawzi, N.M.; Hassan, A.H. Antioxidant, anti-lipoxygenase and cytotoxic activity of Leptadenia pyrotechnica (Forssk.) Decne polyphenolic constituents. Molecules 2011, 16, 7510–7521. [Google Scholar] [CrossRef] [PubMed]
- Khasawneh, M.A.; Koch, A.; Elwy, H.M.; Hamza, A.A.; Schneider-Stock, R. Leptadenia pyrotechnica induces P53-dependent apoptosis in colon cancer cells. Nat. Prod. Chem. Res. 2015, 3, 3. [Google Scholar] [CrossRef]
- Qasim, M.; Abideen, Z.; Adnan, M.Y.; Gulzar, S.; Gul, B.; Rasheed, M.; Khan, M.A. Antioxidant properties, phenolic composition, bioactive compounds and nutritive value of medicinal halophytes commonly used as herbal teas. S. Afr. J. Bot. 2017, 110, 240–250. [Google Scholar] [CrossRef]
- Hossain, S.; El-Sayed, M.; Aoshima, H. Antioxidative and anti-α-amylase activities of four wild plants consumed by pastoral nomads in Egypt. Orient. Pharm. Exp. Med. 2009, 9, 217–224. [Google Scholar] [CrossRef]
- Munazir, M.; Qureshi, R.; Munir, M. Preliminary phytochemical screening of roots and aerial parts of Leptadenia pyrotechnica. Pak. J. Bot. 2015, 47, 659–664. [Google Scholar]
- Alqasoumi, S.I.; Soliman, G.A.E.H.; Awaad, A.S.; Donia, A.E.R.M. Anti-inflammatory activity, safety and protective effects of Leptadenia pyrotechnica, Haloxylon salicornicum and Ochradenus baccatus in ulcerative colitis. Phytopharmacology 2012, 2, 58–71. [Google Scholar]
- Munazir, M.; Qureshi, R.; Munir, M. In vitro antioxidant activity of methanolic extracts of various parts of Leptadenia pyrotechnica (Forssk.) Decne. Pak. J. Pharm. Sci. 2015, 28, 535–539. [Google Scholar] [PubMed]
- Yadav, S.S.; Rao, A.S.; Sheoran, A.; Singh, N.; Nandal, A.; Bhandoria, M.S.; Ganaie, S.A.; Bansal, P. An ethnomedicinal survey of traditionally used medicinal plants from Charkhi Dadri District, Haryana: An attempt towards documentation and preservation of ethnic knowledge. Indian J. Tradit. Knowl. IJTK 2021, 20, 436–450. [Google Scholar]
- Halliwell, B.; Gutteridge, J.M.C. Formation of a thiobarbituric-acid-reactive substance from deoxyribose in the presence of iron salts. The role of superoxide and hydroxyl radicals. FEBS Lett. 1981, 128, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Yoshiki, K.; Nonaka, G.; Nishioka, I.; Chang, J.J.; Lee, K.H. Antitumor agents, 129. Tannins and related compounds as selective cytotoxic agents. J. Nat. Prod. 1992, 55, 1033–1043. [Google Scholar] [CrossRef]
- Berard, N.C.; Wang, Y.; Wittenberg, K.M.; Krause, D.O.; Coulman, B.E.; Mcallister, T.A.; Ominski, K.H. Condensed tannin concentrations found in vegetative and mature forage legumes grown in western Canada. Can. J Plant Sci. 2015, 91, 669–675. [Google Scholar] [CrossRef]
- Tong, Z.; He, W.; Fan, X.; Guo, A. Biological function of plant tannin and its application in animal health. Front. Vet. Sci. 2021, 8, 803657. [Google Scholar] [CrossRef] [PubMed]
- Masood, N.; Jamil, Q.; Aslam, M.I.; Masood, M.I.; Shirazi, J.H.; Jamil, Q.A.; Jan, M.S.; Alsuwayt, B.; Ahmad, A.; Abdullah Alnasser, S.M.; et al. Antioxidant, carbonic anhydrase inhibition and diuretic activity of Leptadenia pyrotechnica Forssk. Decne. Heliyon 2023, 9, e22485. [Google Scholar] [CrossRef] [PubMed]
- Nandal, U.; Bhardwaj, R.L. The role of underutilized fruits in nutritional and economic security of tribals: A review. Crit. Rev. Food Sci. Nutr. 2014, 54, 880–890. [Google Scholar] [CrossRef]
- NSRDS-NBS: National Standard Reference Data Series, National Bureau of Standards (PDF); U.S. Government Printing Office: Washington, DC, USA, 1972.
- Jacox, M.E. Vibrational and electronic energy levels of polyatomic transient molecules. Supplement B. J. Phys. Chem. Ref. Data 2003, 32, 1–441. [Google Scholar] [CrossRef]
- Joshi, B.; Agrawal, V.; Kishore, D. Chemical investigation of Leptadenia spartium Part II. Herba Pol. 1988, 34, 85–86. [Google Scholar]
- Manavalan, R.; Mithal, B. Constituents of the aerial parts of Leptadenia pyrotechnica. Planta Med. 1980, 39, 95. [Google Scholar] [CrossRef]
- Moustafa, A.M.Y.; Khodair, A.; Saleh, M. Phytochemical investigation and toxicological studies of lipid constituents isolated from Leptadenia pyrotechnica. J. Pharmacol. Toxicol. 2007, 2, 681–697. [Google Scholar] [CrossRef]
- Kumar, D.D.; Dhayal, K.; Shrama, R.A. Phytosterols from Leptadenia pyrotechnica (Forsk) Decne. Elixir Int. J. 2017, 48714–48716. [Google Scholar]
- Moustafa, A.M.Y.; Khodair, A.I.; Saleh, M.A. GC-MS investigation and toxicological evaluation of alkaloids from Leptadenia pyrotechnica. Pharm. Biol. 2009, 47, 994–1003. [Google Scholar] [CrossRef]
- Iyer, A.; Bhasin, V.; Joshi, B.C. Chemical investigation of Leptadenia Spartium. I. Herba Pol. 1974, 20, 321–324. [Google Scholar]
- Preet, R.; Gupta, R.C.; Pradhan, S.K. Chromatographic determination of β-sitosterol, lupeol, and oleanolic acid in Leptadenia pyrotechnica (Forsk.) Decne.—A botanical source of the ayurvedic drug Jivanti. J. Planar Chromatogr. Mod. TLC 2018, 31, 150–154. [Google Scholar] [CrossRef]
- Preet, R.; Gupta, R.C. Simultaneous determination of phenolic compounds in Leptadenia pyrotechnica (Forssk.) Decne. by using high-performance liquid chromatography (HPLC-DAD-UV). Adv. Pharmacol. Sci. 2018, 2018, 9604972. [Google Scholar] [CrossRef]
- Dhawan, A.K.; Singh, H. Free pools of amino acids and sugars in Leptadaenia pyrotechnica F. Curr. Sci. 1976, 45, 198. [Google Scholar]


| CSE | NCUSAE | |||||||
|---|---|---|---|---|---|---|---|---|
| Sources of Variation | TPC | TAC | TTC | % Yield | TPC | TAC | TTC | % Yield |
| R | 99.90% | 91.33% | 96.24% | 99.25% | 98.72% | 95.15% | 99.79% | 99.04% |
| Predicted R | 99.29% | 0.00% | 67.22% | 92.07% | 70.89% | 0.00% | 97.04% | 86.88% |
| Adjusted R | 99.73% | 75.73% | 89.47% | 97.91% | 96.40% | 86.42% | 99.41% | 97.32% |
| PRESS | 103.409 | 3814.42 | 2330.39 | 25.1612 | 3582.92 | 1782.86 | 129.972 | 41.0171 |
| S | 1.68121 | 5.92274 | 7.31087 | 0.688518 | 5.62258 | 3.98130 | 1.35556 | 0.773193 |
| S. No. | Phytochemical Compounds | RT (min) | Molecular Formulae | Molecular Weight | Peak Area (%) | Category | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| LPSPE | LPSH | LPSCH | LPSEA | LPSE | LPSAQ | ||||||
| 1. | 1-Propanol, 2-(2-hydroxypropoxy)- | 10.19 | C6H14O3 | 134 | 1.72 | - | - | - | - | - | Glycol ether |
| 2. | Hexadecane, 2,6,10,14-tetramethyl | 25.00 | C20H42 | 282 | 3.07 | - | - | - | - | - | Phytane (diterpenoid hydrocarbon) |
| 3. | Heptacosane | 23.93 | C27H56 | 380 | 3.33 | 0.14 | 0.97 | 4.35 | 0.14 | - | Alkane |
| 4. | Heneicosane | 28.85 | C21H44 | 296 | 3.47 | - | 1.13 | 4.35 | - | - | Alkane |
| 5. | 7,9-Di-tert-butyl-1-oxaspiro(4,5)deca-6,9-diene-2,8-dione | 33.21 | C17H24O3 | 276 | 1.13 | - | - | - | - | - | Flavonoid |
| 6. | Ursolic aldehyde | 33.21 | C30H48O2 | 440 | 1.13 | - | - | - | - | - | Triterpenoid |
| 7. | Propanoic acid, 2-methyl-, (dodecahydro-6a-hydroxy-9a-methyl-3-methylene-2,9-dioxoazuleno[4,5-b]fu ran-6-yl)methyl ester | 33.21 | C19H26O6 | 350 | 1.13 | - | - | - | - | - | Sesquiterpenoid |
| 8. | Dihydromorphine, 2TMS derivative | 39.78 | C23H37NO3Si2 | 431 | 1.00 | - | - | - | - | - | Morphinane alkaloid |
| 9. | à-D-Glucopyranosiduronic acid, 3-(5-ethylhexahydro-2,4,6-trioxo-5-py rimidinyl)-1,1-dimethylpropyl 2,3,4-tris-O-(trimethylsilyl)-, methyl ester | 39.78 | C27H52N2O10Si3 | 648 | 1.00 | - | - | - | - | - | Alpha-D-glucuronic acid (Steroid) |
| 10. | 1,2-Propanediol, 3-(hexadecyloxy)-, diacetate | 39.78 | C23H44O5 | 400 | 1.00 | - | - | - | - | - | Phenol |
| 11. | 25-Norisopropyl-9,19-cyclolanostan-2 2-en-24-one, 3-acetoxy-24-phenyl-4,4,14-trimethyl- | 39.89 | C35H48O3 | 516 | 1.33 | - | - | - | - | - | Steroid |
| 12. | 2,2,4-Trimethyl-3-pentanol | 3.53 | C8H18O | 130 | - | 0.38 | - | - | - | - | Terpenoid |
| 13. | Cyclohexane, 1,4-dimethyl- | 4.03 | C8H16 | 112 | - | 0.62 | - | - | - | - | Cycloalkane |
| 14. | 2-Octanol | 4.42 | C8H18O | 130 | - | 0.45 | - | - | - | - | Fatty alcohol |
| 15. | 3-Hexene, 2-methyl-, (Z)- | 5.62 | C7H14 | 98 | - | 0.33 | - | - | - | - | Terpenoid |
| 16. | 3-Methyl-3-hexene | 5.62 | C7H14 | 98 | - | 0.33 | - | - | - | - | Alkene |
| 17. | 2,4-Hexanedione, 5,5-dimethyl- | 7.64 | C8H14O2 | 142 | - | 4.44 | - | - | - | - | Keto-enol tautomer of pivaloylacetone |
| 18. | 1-Butene-3-ethoxy | 8.16 | C6H12O | 100 | - | 21.47 | - | - | - | - | Dialkyl ether |
| 19. | Hexanoic acid, 2-oxo-, methyl ester | 8.75 | C7H12O3 | 144 | - | 3.48 | - | - | - | - | Oxo fatty acid |
| 20. | Pentanoic acid, 2-propenyl ester | 8.90 | C8H14O2 | 142 | - | 0.20 | - | - | - | - | Fatty acid |
| 21. | Oxirane, [(hexyloxy)methyl] | 9.20 | C9H18O2 | 158 | - | 0.42 | - | - | - | - | Hexyl glycidyl ether |
| 22. | 6,6-Dimethyl-1,3-heptadien-5-ol | 9.20 | C9H16O | 140 | - | 0.42 | - | - | - | - | Terpenoid |
| 23. | 3-Ethyl-3-methyl-2-pentanol | 9.20 | C8H18O | 130 | - | 0.42 | - | - | - | - | Terpenoid |
| 24. | Hexane, 1,1′-oxybis | 9.20 | C12H26O | 186 | - | 0.42 | - | - | - | - | Ether |
| 25. | 1,5-Heptadien-4-one, 3,3,6-trimethyl- | 9.69 | C10H16O | 152 | - | 28.20 | - | - | - | - | Terpenoid |
| 26. | Butane, 2,2,3,3-tetramethyl- | 9.89 | C8H18 | 144 | - | 0.22 | - | - | - | - | Terpenoid |
| 27. | Cyclopropane, 1,1,2,2-tetramethyl- | 10.23 | C7H14 | 98 | - | 5.52 | - | - | - | - | Terpenoid |
| 28. | 1-Butene, 2,3,3-trimethyl- | 10.23 | C7H14 | 98 | - | 5.52 | - | - | - | - | Terpene |
| 29. | 2-Pentene, 4,4-dimethyl-, (E)- | 10.23 | C7H14 | 98 | - | 5.52 | - | - | - | - | Acyclic olefin |
| 30. | Oxalic acid, cyclohexyl pentyl ester | 10.23 | C13H22O4 | 242 | - | 5.52 | - | - | - | - | Ester of oxalic acid |
| 31. | 1-Hexanol, 2,2-dimethyl- | 10.59 | C8H18O | 130 | - | 0.16 | - | - | - | - | Tertiary alcohol |
| 32. | 3-Heptanone, 5-methyl- | 10.59 | C8H16O | 128 | - | 0.16 | - | - | - | - | Ketone |
| 33. | N-Formylglycine | 18.37 | C3H5NO3 | 103 | - | 1.28 | - | - | - | - | Carbohydrate derivative |
| 34. | Carbamic acid, methyl ester | 18.37 | C2H5NO2 | 75 | - | 1.28 | - | - | - | - | Ester of Carbamic acid |
| 35. | Hexadecane, 2,6,11,15-tetramethyl | 28.86 | C20H42 | 282 | - | - | 0.97 | 5.51 | - | - | Isoprenoid hydrocarbon |
| 36. | Tetradecanoic acid | 29.94 | C14H28O2 | 228 | - | - | 1.17 | 4.90 | - | - | Saturated fatty acid |
| 37. | n-Hexadecanoic acid | 33.98 | C16H32O2 | 256 | - | - | 2.96 | 4.28 | - | - | Palmitic acid |
| 38. | Octadecanoic acid | 37.71 | C18H36O2 | 284 | - | - | 0.93 | - | - | - | Saturated fatty acid |
| 39. | 4,4,5,7,8-Pentamethyl-6-chromanol | 33.21 | C14H20O2 | 220 | - | - | 3.59 | - | - | - | Vitamin E |
| 40. | Benzo[e]isobenzofuran-1,4-dione,1,3,4,5,5a,6,7,8,9,9a-decahydro-6,6,9a-trimethyl | 33.21 | C15H20O3 | 248 | - | - | 3.59 | - | - | - | Terpenoid |
| 41. | l-(+)-Ascorbic acid 2,6-dihexadecanoate | 33.98 | C38H68O8 | 652 | - | - | 2.96 | 4.28 | - | - | Vitamin C |
| 42. | 10-Acetoxy-2-hydroxy-1,2,6a,6b,9,9,1 2a-heptamethyl-1,3,4,5,6,6a,6b,7,8,8a, 9,10,11,12,12a,12b,13,14b-octadecahy dro-2H-picene-4a-carboxylic acid, | 40.19 | C33H52O5 | 528 | - | - | 0.62 | - | - | - | Terpenoid |
| 43. | 2,4-Di-tert-butylphenol | 24.31 | C14H22O | 206 | - | - | - | 1.69 | 0.15 | - | Phenol |
| 44. | Phenol, 3,5-bis(1,1-dimethylethyl)- | 24.31 | C14H22O | 206 | - | - | - | 1.69 | 0.15 | - | Phenol |
| 45. | 2,6,10-Trimethyltridecane | 25.46 | C16H34 | 226 | - | - | - | 1.55 | - | - | Terpenoid |
| 46. | Tridecane, 2-methyl- | 25.46 | C14H30 | 198 | - | - | - | 1.55 | - | - | Terpenoid |
| 47. | Hexadecanoic acid, 2-hydroxy-1-(hydroxymethyl)ethyl ester | 41.88 | C19H38O4 | 330 | - | - | - | 3.56 | - | - | Fatty acid ester |
| 48. | Glycerol 1-palmitate | 41.88 | C19H38O4 | 330 | - | - | - | 3.56 | - | - | Monoacylglycerols |
| 49. | Octadecanoic acid, 2,3-dihydroxypropyl ester | 41.88 | C21H42O4 | 358 | - | - | - | 3.56 | - | - | Stearic acid |
| 50. | Benzoic acid, 2-benzoyl | 27.12 | C14H10O3 | 226 | - | - | - | - | 0.10 | - | Aromatic carboxylic acid |
| 51. | Myristic acid | 29.99 | C14H28O2 | 228 | - | - | - | - | 4.90 | - | Fatty acid |
| 52. | Phosphonoacetic Acid, 3TMS derivative | 19.52 | C11H29O5Psi3 | 356 | - | - | - | - | 0.83 | - | Phosphonic acid |
| 53. | 1,1-Diphenyl-2-phenylthiobut-3-en-1-ol | 27.12 | C22H20OS | 332 | - | - | - | - | 0.10 | - | Thioether |
| 54. | Benzophenone | 27.12 | C13H10O | 182 | - | - | - | - | 0.10 | - | Flavonoid |
| 55. | Propanoic acid, 2-methyl-, (dodecahydro-6a-hydroxy-9a-methyl-3-methylene-2,9-dioxoazuleno[4,5-b] furan-6-yl) methyl ester, [3aS-(3aà,6á,6aà,9aá,9bà)]- | 33.20 | C19H26O6 | 350 | - | - | - | - | 0.22 | - | Terpenoid |
| 56. | 1,2-Benzenedicarboxylic acid, butyl octyl ester | 34.00 | C20H30O4 | 334 | - | - | - | - | 0.33 | - | Phthalic acid ester |
| 57. | Erucic acid | 34.58 | C22H42O2 | 338 | - | - | - | - | 0.22 | - | Fatty acid |
| 58. | 18,19-Secoyohimban-19-oic acid, 16,17,20,21-tetradehydro-16-(hydroxy methyl)-, methyl ester, (15á,16E)- | 34.58 | C21H24N2O3 | 352 | - | - | - | - | 0.22 | - | Alkaloid (Yohimbine, an indole alkaloid) |
| 59. | 13-Docosenamide, (Z) | 34.58 | C22H43NO | 337 | - | - | - | - | 0.22 | - | Fatty acid amide |
| 60. | Oxalic acid, mono-(5-[(2-bromophenyl)(2,2-dimeth ylpropionyloxy)methyl]-7,8-dihydro-5 H-[1,3]dioxolo[4,5-g]isoquinolin-6-yl) ester | 42.20 | C24H26BrNO8 | 535 | - | - | - | - | 0.14 | - | Dicarboxylic acid |
| 61. | 5-Benzofuranacetic acid, 6-ethenyl-2,4,5,6,7,7a-hexahydro-3,6-d imethyl-à-methylene-2-oxo-, methyl ester | 42.20 | C16H20O4 | 276 | - | - | - | - | 0.14 | - | Flavonoid |
| 62. | 2,6-Dihydroxybenzoic acid, 3TMS derivative | 14.80 | C16H30O4Si3 | 370 | - | - | - | - | - | 0.01 | Hydroxybenzoic acid |
| 63. | 2,6-Dihydroxyacetophenone, 2TMS derivative | 24.01 | C14H24O3Si2 | 296 | - | - | - | - | - | 0.01 | Phenol |
| 64. | 3,3a-Epoxydicyclopenta[a,d]cyclooctan-4á-ol, 9,10a-dimethyl-6-methylene-3á-isopropyl- | 33.22 | C20H32O2 | 304 | - | - | - | - | - | 0.01 | Sesquiterpene |
| 65. | 25-Norisopropyl-9,19-cyclolanostan-22-en-24-one, 3-acetoxy-24-phenyl-4,4,14-trimethyl- | 40.15 | C35H48O3 | 516 | - | - | - | - | - | 0.06 | Triterpenoid |
| 66. | 24,25-Dihydroxycholecalciferol | 40.15 | C27H44O3 | 416 | - | - | - | - | - | 0.06 | Vitamin D |
| S. No. | Phytochemical Compound | RT (min) | Molecular Formulae | Molecular Weight | Peak Area (%) | Category | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| LPRPE | LPRH | LPRCH | LPREA | LPRE | LPRAQ | ||||||
| 1. | 1,5-Heptadien-4-one, 3,3,6-trimethyl- | 9.02 | C10H16O | 152 | 2.18 | - | - | - | - | - | Ketone |
| 2. | Hexadecane, 2,6,10,14-tetramethyl- | 33.26 | C20H42 | 282 | 1.74 | - | 1.12 | 3.02 | - | - | Phytane |
| 3. | Tetratetracontane | 34.08 | C44H90 | 618 | 1.44 | - | 0.88 | - | - | - | Alkane |
| 4. | Eicosane | 39.68 | C20H42 | 282 | 5.18 | - | - | 2.90 | - | - | Alkane |
| 5. | Heptacosane | 28.85 | C21H44 | 296 | 2.16 | - | 0.87 | - | 0.31 | - | Alkane |
| 6. | 2-Hexanone | 4.18 | C6H12O | 100 | - | 0.26 | - | - | - | - | Ketone |
| 7. | (R)-(+)-3-Methylcyclopentanone 8 | 5.52 | C6H10O | 98 | - | 0.24 | - | - | - | - | Cyclic ketone |
| 8. | Oxalic acid, cyclohexyl pentyl ester | 9.51 | C13H22O4 | 242 | - | 40.90 | - | - | - | - | Ester of oxalic acid and cyclohexyl pentanol |
| 9. | 2,2,4-Trimethyl-3-pentanol | 3.48 | C8H18O | 130 | - | 0.20 | - | - | - | - | Phenol |
| 10. | 2-Hexanol, 2,5-dimethyl-, (S)- | 13.51 | C8H18O | 130 | - | 0.10 | - | - | - | - | Terpenoid |
| 11. | 2-Heptanol, 2-methyl- | 13.51 | C8H18O | 130 | - | 0.10 | - | - | - | - | Alcohol |
| 12. | 3-Allyloxy-1,2 propanediol | 16.38 | C6H12O3 | 132 | - | 0.47 | - | - | - | - | Glycerol 1-allyl ether |
| 13. | 1,2,3-Butanetriol | 16.38 | C4H10O3 | 106 | - | 0.47 | - | - | - | - | Polyol |
| 14. | Heptacosane | 40.66 | C27H56 | 380 | - | 0.08 | 0.70 | 2.68 | 0.76 | 0.02 | Alkane |
| 15. | Tetratetracontane | 40.66 | C44H90 | 618 | - | 0.08 | 1.33 | 4.00 | 0.76 | - | Hydrocarbon compound |
| 16. | N-Formylglycine | 18.34 | C3H5NO3 | 103 | - | 0.93 | - | - | - | - | Carbohydrate derivative |
| 17. | (2,3-Diphenylcyclopropyl) methyl phenyl sulfoxide, trans | 41.24 | C22H20OS | 332 | - | 0.23 | - | - | - | - | Sulfoxide |
| 18. | 3-Benzyl-2-phenyl-2,3,4,5-tetrahydro-1H-benzo[d]azepin | 41.24 | C23H23N | 313 | - | 0.23 | - | - | - | - | Benzodiazepine derivative |
| 19. | Carbamic acid, methyl ester | 18.34 | C2H5NO2 | 75 | - | 0.93 | - | - | - | - | Ester of Carbamic acid |
| 20. | 1,1′,1″-[5-methyl-1-pentene-1,3,5-triyl] tris- | 41.24 | C24H24 | 312 | - | 0.23 | - | - | - | - | Terpenoids |
| 21. | 2-Propanone, 1,1-dichloro- | 3.38 | C3H4Cl2O | 126 | - | - | 1.16 | - | - | - | Dichloroacetone |
| 22. | Propane, 1,1,3,3-tetrachloro-2-methyl-5 | 4.69 | C4H6Cl4 | 194 | - | - | 0.90 | - | - | - | Chloroalkane |
| 23. | Acetyl chloride, dichloro- | 11.16 | C2HCl3O | 146 | - | - | 1.59 | - | - | - | Acetic acid |
| 24. | Phenol, 4-chloro-2,6-bis(1,1-dimethylethyl)- | 25.00 | C14H21ClO | 240 | - | - | 2.45 | - | - | - | Phenol |
| 25. | Tetradecanoic acid | 29.96 | C14H28O2 | 228 | - | - | 2.13 | - | - | - | Fatty acid |
| 26. | n-Hexadecanoic acid | 33.99 | C16H32O2 | 256 | - | - | 2.81 | 2.14 | - | - | Palmitic acid |
| 27. | l-(+)-Ascorbic acid 2,6-dihexadecanoate | 33.99 | C38H68O8 | 652 | - | - | 2.81 | 2.14 | - | - | Vitamin C |
| 28. | Octadecanoic acid | 37.70 | C18H36O2 | 284 | - | - | 1.04 | - | - | - | Stearic acid |
| 29. | Hexadecanoic acid, 2-hydroxy-1-(hydroxymethyl)ethyl ester | 41.87 | C19H38O4 | 330 | - | - | 2.93 | 3.69 | - | - | Fatty acid ether |
| 30. | Glycerol 1-palmitate | 41.87 | C19H38O4 | 330 | - | - | 2.93 | 3.69 | - | - | Monoacylglycerol |
| 31. | Octadecanoic acid, 2,3-dihydroxypropyl ester | 41.87 | C21H24O4 | 358 | - | - | 2.93 | - | - | - | Glycerol ester of stearic acid |
| 32. | Octadecanoic acid, 2-hydroxy-1,3-propanediyl ester | 41.87 | C39H76O5 | 624 | - | - | 2.93 | - | - | - | Stearic acid |
| 33. | Tetrapentacontane, 1,54-dibromo- | 42.92 | C54H108Br2 | 914 | - | - | 0.70 | - | - | - | Alkane |
| 34. | Octadecanoic acid, 2-hydroxy-1-(hydroxymethyl)ethyl ester | 44.67 | C21H24O4 | 358 | - | - | 0.96 | 1.63 | - | - | Fatty acid ester |
| 35. | Phenol, 3,5-bis(1,1-dimethylethyl) | 24.31 | C14H22O | 206 | - | - | - | 1.58 | - | - | Butylated hydroxytoluene (BHT) |
| 36. | 2,4-Di-tert-butylphenol | 24.31 | C14H22O | 206 | - | - | - | 1.58 | - | - | Phenol |
| 37. | Hexadecane, 2,6,11,15-tetramethyl | 25.01 | C20H42 | 282 | - | - | - | 4.63 | - | - | Crocetane |
| 38. | Hexadecanoic acid, 1-(hydroxymethyl)-1,2-ethanediyl ester | 41.88 | C35H68O5 | 568 | - | - | - | 3.69 | - | - | Palmitic acid |
| 39. | Octadecanoic acid, 2-hydroxy-1-(hydroxymethyl)ethyl ester | 44.68 | C21H42O4 | 358 | - | - | - | 1.63 | - | - | Fatty acid ester |
| 40. | 3-Buten-1-ol, TMS derivative | 13.04 | C7H16OSi | 144 | - | - | - | - | 0.11 | - | Homoallyl alcohol |
| 41. | 1,1-Diphenyl-2-phenylthiobut-3-en-1-ol | 26.76 | C22H20OS | 332 | - | - | - | - | 0.15 | - | Thioether (phenylpropanoid) |
| 42. | Benzophenone | 26.76 | C13H10O | 182 | - | - | - | - | 0.15 | - | Flavonoid |
| 43. | Benzoic acid, 2-benzoyl | 26.76 | C14H10O3 | 226 | - | - | - | - | 0.15 | - | Aromatic carboxylic acid |
| 44. | Heptacos-1-ene | 30.48 | C27H54 | 378 | - | - | - | - | 0.18 | - | Alkene |
| 45. | 1-Hexadecanol, 2-methyl | 30.48 | C17H36O | 256 | - | - | - | - | 0.18 | - | Fatty alcohol |
| 46. | 1,2-Benzenedicarboxylic acid, butyl 8-methylnonyl ester | 33.65 | C22H34O4 | 362 | - | - | - | - | 0.28 | 0.01 | Phthalate |
| 47. | Phthalic acid, butyl nonyl ester | 33.65 | C21H32O4 | 348 | - | - | - | - | 0.28 | - | Phthalic acid ester |
| 48. | Tetrapentacontane, 1,54-dibromo- | 40.65 | C54H108Br2 | 914 | - | - | - | - | 0.16 | - | Fatty acid |
| 49. | Ethanol, 2-(octadecyloxy) | 40.65 | C20H42O2 | 314 | - | - | - | - | 0.16 | - | Glycol ether |
| 50. | 1,2-Propanediol, 3-(octadecyloxy)-, diacetate | 41.00 | C25H48O5 | 428 | - | - | - | - | 0.42 | 0.02 | Fatty acid ester |
| 51. | Oxalic acid, mono-(5-[(2 bromophenyl) (2,2-dimeth yl propionyloxy) methyl]-7,8-dihydro-5 H-[1,3] dioxolo[4,5-g] isoquinolin-6-yl) ester | 41.88 | C24H26BrNO8 | 535 | - | - | - | - | 0.61 | - | Dicarboxylic acid |
| 52. | Oleic acid, 3-(octadecyloxy)propyl ester | 43.90 | C39H76O3 | 592 | - | - | - | - | 0.22 | 0.02 | Fatty acid ester |
| 53. | Ethanol, 2-(octadecyloxy)-6 | 28.85 | C20H42O2 | 314 | - | - | - | - | - | 0.02 | Fatty alcohol |
| 54. | 1,2-Benzenedicarboxylic acid, butyl 8-methylnonyl ester | 32.16 | C22H34O4 | 362 | - | - | - | - | - | 0.01 | Phthalate |
| 55. | 7,9-Di-tert-butyl-1-oxaspiro (4,5) deca-6,9-diene-2,8-dione | 33.20 | C17H24O3 | 276 | - | - | - | - | - | 0.02 | Flavonoid |
| 56. | Propanoic acid, 2-methyl-, (dodecahydro-6a-hydroxy-9a-methyl-3-methylene-2,9-dioxoazuleno [4,5-b] furan-6-yl)methyl ester, [3aS-(3aà,6á,6aà,9aá,9bà)]- | 33.20 | C19H26O6 | 350 | - | - | - | - | - | 0.02 | Sesquiterpenoid |
| 57. | Betulinaldehyde | 33.20 | C30H48O2 | 440 | - | - | - | - | - | 0.02 | Triterpenoid |
| 58. | 9,10-Secocholesta-5,7,10(19)-triene-3, 24,25-triol, (3á,5Z,7E)- | 38.06 | C27H44O3 | 416 | - | - | - | - | - | 0.02 | Steroid |
| 59. | 24,25-Dihydroxycholecalciferol | 38.06 | C27H44O3 | 416 | - | - | - | - | - | 0.02 | Vitamin D3 derivative |
| 60. | Dodecyl cis-9,10-epoxyoctadecanoate | 39.02 | C30H58O3 | 466 | - | - | - | - | - | 0.01 | Epoxy fatty acid |
| 61. | Spirost-8-en-11-one, 3-hydroxy-, (3á,5à,14á,20á,22á,25R)- | 40.61 | C27H40O4 | 428 | - | - | - | - | - | 0.02 | Steroid |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumari, D.; Singh, D.; Meena, M.; Janmeda, P.; Siddiqui, M.H. Qualitative, Quantitative, In Vitro Antioxidant Activity and Chemical Profiling of Leptadenia pyrotechnica (Forssk.) Decne Using Advanced Analytical Techniques. Antioxidants 2024, 13, 794. https://doi.org/10.3390/antiox13070794
Kumari D, Singh D, Meena M, Janmeda P, Siddiqui MH. Qualitative, Quantitative, In Vitro Antioxidant Activity and Chemical Profiling of Leptadenia pyrotechnica (Forssk.) Decne Using Advanced Analytical Techniques. Antioxidants. 2024; 13(7):794. https://doi.org/10.3390/antiox13070794
Chicago/Turabian StyleKumari, Divya, Devendra Singh, Mukesh Meena, Pracheta Janmeda, and Manzer H. Siddiqui. 2024. "Qualitative, Quantitative, In Vitro Antioxidant Activity and Chemical Profiling of Leptadenia pyrotechnica (Forssk.) Decne Using Advanced Analytical Techniques" Antioxidants 13, no. 7: 794. https://doi.org/10.3390/antiox13070794









