Evaluation of Decay Kinetics of Black Elderberry Antioxidants from Fruits and Flowers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Samples for Testing
2.2. Characterization of Dry Elderberry Samples
2.2.1. Elemental Analysis
2.2.2. Determination of Antioxidant Properties
2.2.3. Radicals Used in Research
2.3. Calculation
3. Results and Discussion
3.1. Elemental Analysis of CHN
3.2. Determination of the Antioxidant Properties of Elderberry Flower and Fruit Infusions
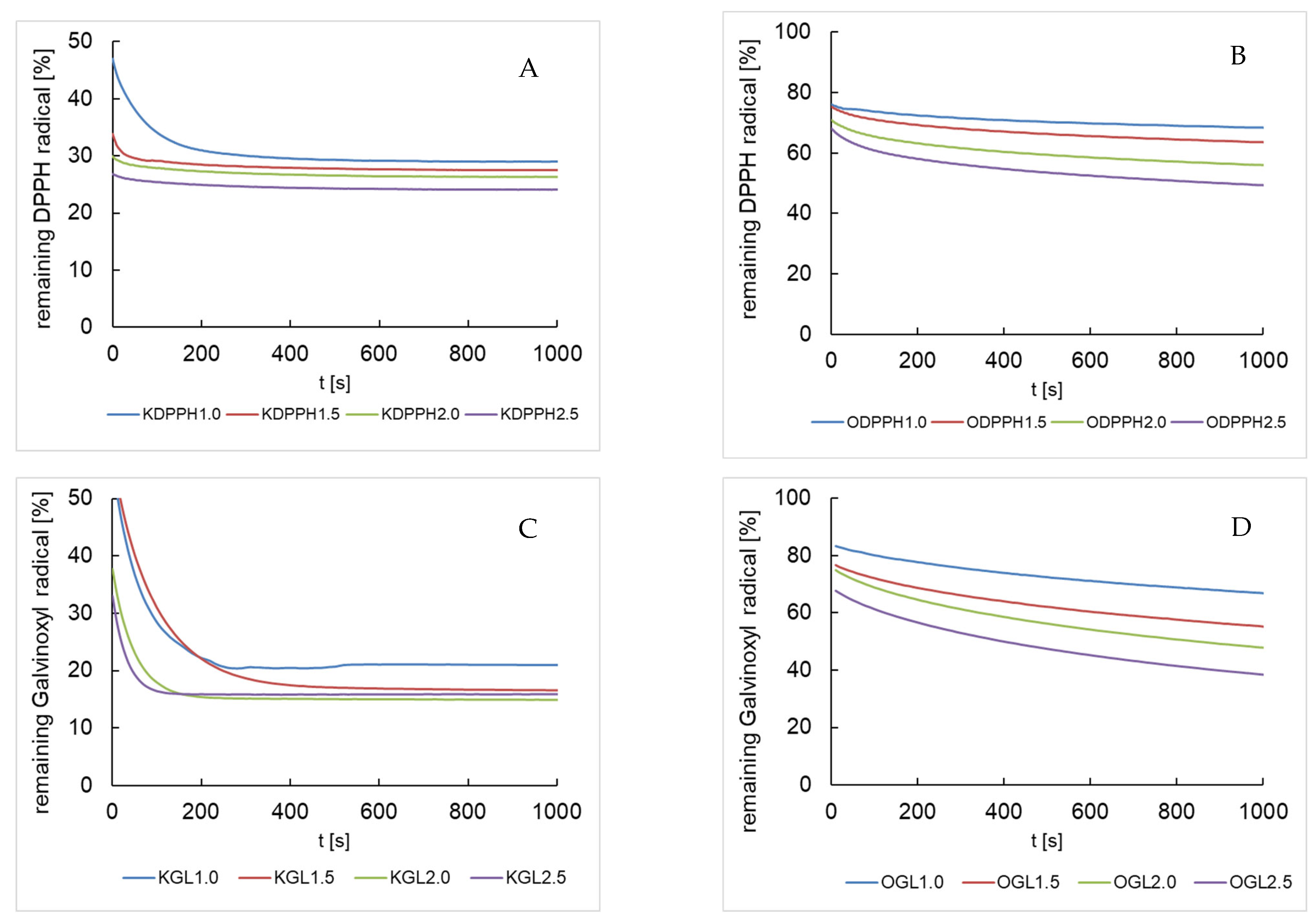
3.2.1. Scavenging Effect on DPPH· and Glv· Radicals
3.2.2. Evaluation of the Radical Decay Kinetics as Parallel Processes
3.2.3. Evaluation of the Radical Decay Kinetics as Second-Order Processes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Osman, A.G.; Avula, B.; Katragunta, K.; Ali, Z.; Chittiboyina, A.G.; Khan, I.A. Elderberry Extracts: Characterization of the Polyphenolic Chemical Composition, Quality Consistency, Safety, Adulteration, and Attenuation of Oxidative Stress- and Inflammation-Induced Health Disorders. Molecules 2023, 28, 3148. [Google Scholar] [CrossRef] [PubMed]
- Ulbricht, C.; Basch, E.; Cheung, L.; Goldberg, H.; Hammerness, P.; Isaac, R.; Khalsa, K.P.; Romm, A.; Rychlik, I.; Varghese, M.; et al. An evidence-based systematic review of elderberry and elderflower (Sambucus nigra) by the Natural Standard Research Collaboration. J. Diet. Suppl. 2014, 11, 80–120. [Google Scholar] [CrossRef] [PubMed]
- Tarahovsky, Y.S.; Kim, Y.A.; Yagolnik, E.A.; Muzafarov, E.N. Flavonoid-membrane interactions: Involvement of flavonoid-metal complexes in raft signaling. Biochim. Biophys. Acta (BBA)—Biomembr. 2014, 1838, 1235–1246. [Google Scholar] [CrossRef] [PubMed]
- Hussain, G.; Zhang, L.; Rasul, A.; Anwar, H.; Sohail, M.U.; Razzaq, A.; Aziz, N.; Shabbir, A.; Ali, M.; Sun, T. Role of plant-derived flavonoids and their mechanism in attenuation of Alzheimer’s and Parkinson’s diseases: An update of recent data. Molecules 2018, 23, 814. [Google Scholar] [CrossRef] [PubMed]
- Parcheta, M.; Świsłocka, R.; Orzechowska, S.; Akimowicz, M.; Choińska, R.; Lewandowski, W. Recent Developments in Effective Antioxidants: The Structure and Antioxidant Properties. Materials 2021, 14, 1984. [Google Scholar] [CrossRef] [PubMed]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Structure antioxidant activity relationships of flavonoids and phenolic acids. Free Radic. Biol. Med. 1996, 20, 933–956. [Google Scholar] [CrossRef] [PubMed]
- Paganga, G.; Miller, N.; Rice-Evans, C.A. The polyphenolic content of fruit and vegetables and their antioxidant activities. What does a serving constitute. Free Radic. Res. 1999, 30, 153–162. [Google Scholar] [CrossRef]
- Viapiana, A.; Wesolowski, M. The Phenolic Contents and Antioxidant Activities of Infusions of Sambucus nigra L. Plant Foods Hum. Nutr. 2017, 72, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Nevell, T.P.; Zeronian, S.H. Cellulose chemistry fundamentals. In Cellulose Chemistry and Its Applications; Nevell, T.P., Zeronian, S.H., Eds.; Ellis Horwood Limited/John Wiley & Sons: New York, NY, USA, 1985; pp. 15–29. [Google Scholar]
- Aravamudhan, A.; Ramos, D.M.; Nada, A.; Kumbar, S. Natural Polymers: Polysaccharides and Their Derivatives for Biomedical Applications. In Natural and Synthetic Biomedical Polymers; Elsevier: Amsterdam, The Netherlands, 2014; pp. 67–89. [Google Scholar]
- Pascariu, O.-E.; Israel-Roming, F. Bioactive Compounds from Elderberry: Extraction, Health Benefits and Food Applications. Processes 2022, 10, 2288. [Google Scholar] [CrossRef]
- Veberic, R.; Jakopic, J.; Stampar, F.; Schmitzer, V. European Elderberry (Sambucus nigra L.) Rich in Sugars, Organic Acids, Anthocyanins and Selected Polyphenols. Food Chem. 2009, 114, 511–515. [Google Scholar] [CrossRef]
- Ferreira, S.S.; Silva, P.; Silva, A.M.; Nunes, F.M. Effect of Harvesting Year and Elderberry Cultivar on the Chemical Composition and Potential Bioactivity: A Three-year Study. Food Chem. 2020, 302, 125366. [Google Scholar] [CrossRef] [PubMed]
- Mikulic-Petkovsek, M.; Ivancic, A.; Schmitzer, V.; Veberic, R.; Stampar, F. Comparison of Major Taste Compounds and Antioxidative Properties of Fruits and Flowers of Different Sambucus Species and Interspecific Hybrids. Food Chem. 2016, 200, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Mlynarczyk, K.; Walkowiak-Tomczak, D.; Lysiak, G.P. Bioactive properties of Sambucus nigra L. As a functional ingredient for food and pharmaceutical industry. J. Funct. Foods 2018, 40, 377–390. [Google Scholar] [CrossRef]
- Dumanović, J.; Nepovimova, E.; Natić, M.; Kuča, K.; Jaćević, V. The Significance of Reactive Oxygen Species and Antioxidant Defense System in Plants: A Concise Overview. Front. Plant Sci. 2021, 11, 552969. [Google Scholar] [CrossRef] [PubMed]
- Rai, K.K.; Kaushik, P. Free Radicals Mediated Redox Signaling in Plant Stress Tolerance. Life 2023, 13, 204. [Google Scholar] [CrossRef] [PubMed]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn. Rev. 2010, 4, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Niki, E. Assessment of antioxidant capacity in vitro and in vivo. Free Radic. Biol. Med. 2010, 15, 49, 503–515. [Google Scholar] [CrossRef] [PubMed]
- Honzel, D.; Carter, S.G.; Redman, K.A.; Schauss, A.G.; Endres, J.R.; Jensen, G.S. Comparison of chemical and cell-based antioxidant methods for evaluation of foods and natural products: Generating multifaceted data by parallel testing using erythrocytes and polymorphonuclear cells. J. Agric. Food Chem. 2008, 56, 8319–8325. [Google Scholar] [CrossRef] [PubMed]
- Wołosiak, R.; Rudny, M.; Skrobek, E.; Worobiej, E.; Drużyńska, B. Żywn. Nauk. Techn. Jakość. 2007, 3, 109–118. [Google Scholar]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. Lebensm. Wiss. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Paul, D. Bartlett and Toshio. Funahashi, Galvinoxyl (2,6-Di-tert-butyl-α-(3,5-di-tert-butyl-4-oxo-2,5-cyclohexadiene-1-ylidene)-p-tolyloxy) as a Scavenger of Shorter-lived Free Radicals. J. Am. Chem. Soc. 1962, 84, 2596–2601. [Google Scholar] [CrossRef]
- Shi, H.; Niki, E. Stoichiometric and kinetic studies on Ginkgo biloba extract and related antioxidants. Lipids 1998, 33, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Palanisamy, H.M.; Cheng, T.; Masilamani, T.; Subramaniam, T.; Ling, L.T.; Radhakrishnan, A. Rind of the rambutan, Nephelium lappaceum, a potential source of natural antioxidants. Food Chem. 2008, 109, 54. [Google Scholar] [CrossRef]
- Blois, M.S. Antioxidant determinations by the use of a stable free radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Flieger, J.; Flieger, M. The [DPPH●/DPPH-H]-HPLC-DAD Method on Tracking the Antioxidant Activity of Pure Antioxidants and Goutweed (Aegopodium podagraria L.) Hydroalcoholic Extracts. Molecules 2020, 25, 6005. [Google Scholar] [CrossRef] [PubMed]
- Gulcin, İ.; Alwasel, S.H. DPPH Radical Scavenging Assay. Processes 2023, 11, 2248. [Google Scholar] [CrossRef]
- Shi, H.L.; Noguchi, N.; Niki, E. Galvinoxyl method for standardizing electron and proton donation activity. Methods Enzymol. 2001, 335, 157–166. [Google Scholar]
- Foti, M.C. Use and abuse of the DPPH radical. J. Agric. Food Chem. 2015, 63, 8765–8776. [Google Scholar] [CrossRef]
- TIBCO Software Inc. Statistica (Data Analysis Software System), Version 13. 2017. Available online: http://statistica.io (accessed on 4 January 2024).
- Goel, P.; Bhuria, M.; Kaushal, M.; Singh, A.K. Carbon: Nitrogen Interaction Regulates Expression of Genes Involved in N-Uptake and Assimilation in Brassica juncea. L. PLoS ONE 2016, 11, 16. [Google Scholar] [CrossRef]
- Baslam, M.; Mitsui, T.; Sueyoshi, K.; Ohyama, T. Recent Advances in Carbon and Nitrogen Metabolism in C3 Plants. Int. J. Mol. Sci. 2021, 22, 318. [Google Scholar] [CrossRef]
- Wu, W.M.; Lu, L.; Long, Y.; Wang, T.; Liu, L.; Chen, Q.; Wang, R. Free radical scavenging and antioxidative activities of caffeic acid phenethyl ester (CAPE) and its related compounds in solution and membranes: A structure–activity insight. Food Chem. 2007, 105, 107–115. [Google Scholar] [CrossRef]
- van Acker, S.A.; de Groot, M.J.; van den Berg, D.J.; Tromp, M.N.; den Kelder, G.D.O.; van der Vijgh, W.J.; Bast, A. A Quantum Chemical Explanation of the Antioxidant Activity of Flavonoids. Chem. Res. Toxicol. 1996, 9, 1305–1312. [Google Scholar] [CrossRef] [PubMed]
- Todorov, L.; Saso, L.; Kostova, I. Antioxidant Activity of Coumarins and Their Metal Complexes. Pharmaceuticals 2023, 16, 651. [Google Scholar] [CrossRef] [PubMed]
- Dawidowicz, A.L.; Wianowska, D.; Baraniak, B. The antioxidant properties of alcoholic extracts from Sambucus nigra L. (antioxidant properties of extracts). LWT Food Sci. Technol. 2006, 39, 308–315. [Google Scholar] [CrossRef]
- Shahidi, F.; Wanasundara, P.K.J. Phenolic Antioxidants. Crit. Rev. Food Sci. Nutr. 1992, 32, 67–103. [Google Scholar] [CrossRef]
- Litwinienko, G.; Ingold, K.U.J. Abnormal Solvent Effects on Hydrogen Atom Abstraction. 3. Novel Kinetics in Sequential Proton Loss Electron Transfer Chemistry. Org. Chem. 2005, 70, 8982–8990. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Liu, Z.Q. Solvent-Free and Catalyst-Free Biginelli Reaction to Synthesize Ferrocenoyl Dihydropyrimidine and Kinetic Method to Express Radical-Scavenging Ability. J. Org. Chem. 2012, 77, 3952–3958. [Google Scholar] [CrossRef]
- Samodova, D.; Borodušķe, A.; Ramata-Stunda, A.; Mazarova, N.; Nikolajeva, V.; Boroduskis, M.; Nakurte, I. Anti-Bacterial Activity and Online HPLC-DPPH Based Antiradical Kinetics of Medicinal Plant Extracts of High Relevance for Cosmetics Production. Key Eng. Mat. 2018, 762, 8–13. [Google Scholar]
| Original Infusions Composition | Evaluated Dilutions Composition | Assessed Samples Composition | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Acronym | Composition | Acronym | Original Infusion of Flos Sambuci [mL] | Original Infusion of Fructus Sambuci [mL] | Water [mL] | Acronym | Dilution [mL] | DPPH· Solution [mL] | Acronym of Assessed Sample | Dilution [mL] | GLv· Solution [mL] |
| K | Flos Sambuci: 1 g Water: 100 mL | K1.0 | 1.0 | - | 9.0 | KDPPH1.0 | 0.5 | 3.0 | KGL1.0 | 0.5 | 3.0 |
| K1.5 | 1.5 | - | 8.5 | KDPPH1.5 | 0.5 | 3.0 | KGL1.5 | 0.5 | 3.0 | ||
| K2.0 | 2.0 | - | 8.0 | KDPPH2.0 | 0.5 | 3.0 | KGL2.0 | 0.5 | 3.0 | ||
| K2.5 | 2.5 | - | 7.5 | KDPPH2.5 | 0.5 | 3.0 | KGL2.5 | 0.5 | 3.0 | ||
| O | Fructus Sambuci: 1 g Water: 100 mL | O1.0 | - | 1.0 | 9.0 | ODPPH1.0 | 0.5 | 3.0 | OGL1.0 | 0.5 | 3.0 |
| O1.5 | - | 1.5 | 8.5 | ODPPH1.5 | 0.5 | 3.0 | OGL1.5 | 0.5 | 3.0 | ||
| O2.0 | - | 2.0 | 8.0 | ODPPH2.0 | 0.5 | 3.0 | OGL2.0 | 0.5 | 3.0 | ||
| O2.5 | - | 2.5 | 7.5 | ODPPH2.5 | 0.5 | 3.0 | OGL2.5 | 0.5 | 3.0 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Golonka, I.; Dryś, A.; Podgórska, K.; Polewska, J.; Musiał, W. Evaluation of Decay Kinetics of Black Elderberry Antioxidants from Fruits and Flowers. Antioxidants 2024, 13, 804. https://doi.org/10.3390/antiox13070804
Golonka I, Dryś A, Podgórska K, Polewska J, Musiał W. Evaluation of Decay Kinetics of Black Elderberry Antioxidants from Fruits and Flowers. Antioxidants. 2024; 13(7):804. https://doi.org/10.3390/antiox13070804
Chicago/Turabian StyleGolonka, Iwona, Andrzej Dryś, Katarzyna Podgórska, Joanna Polewska, and Witold Musiał. 2024. "Evaluation of Decay Kinetics of Black Elderberry Antioxidants from Fruits and Flowers" Antioxidants 13, no. 7: 804. https://doi.org/10.3390/antiox13070804







