Nrf2 Deficiency Accelerates IL-17-Dependent Neutrophilic Airway Inflammation in Asthmatic Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Model
2.2. Experimental Protocols
2.3. Measurement of Airway Hyperresponsiveness (AHR)
2.4. Bronchoalveolar Lavage
2.5. Histopathology and Immunohistochemistry
2.6. Purification of Helper T (Th) Cells
2.7. RNA Isolation and Real-Time PCR
2.8. Multiplex Immunology
2.9. Western Blot Analysis
2.10. Statistical Analysis
3. Results
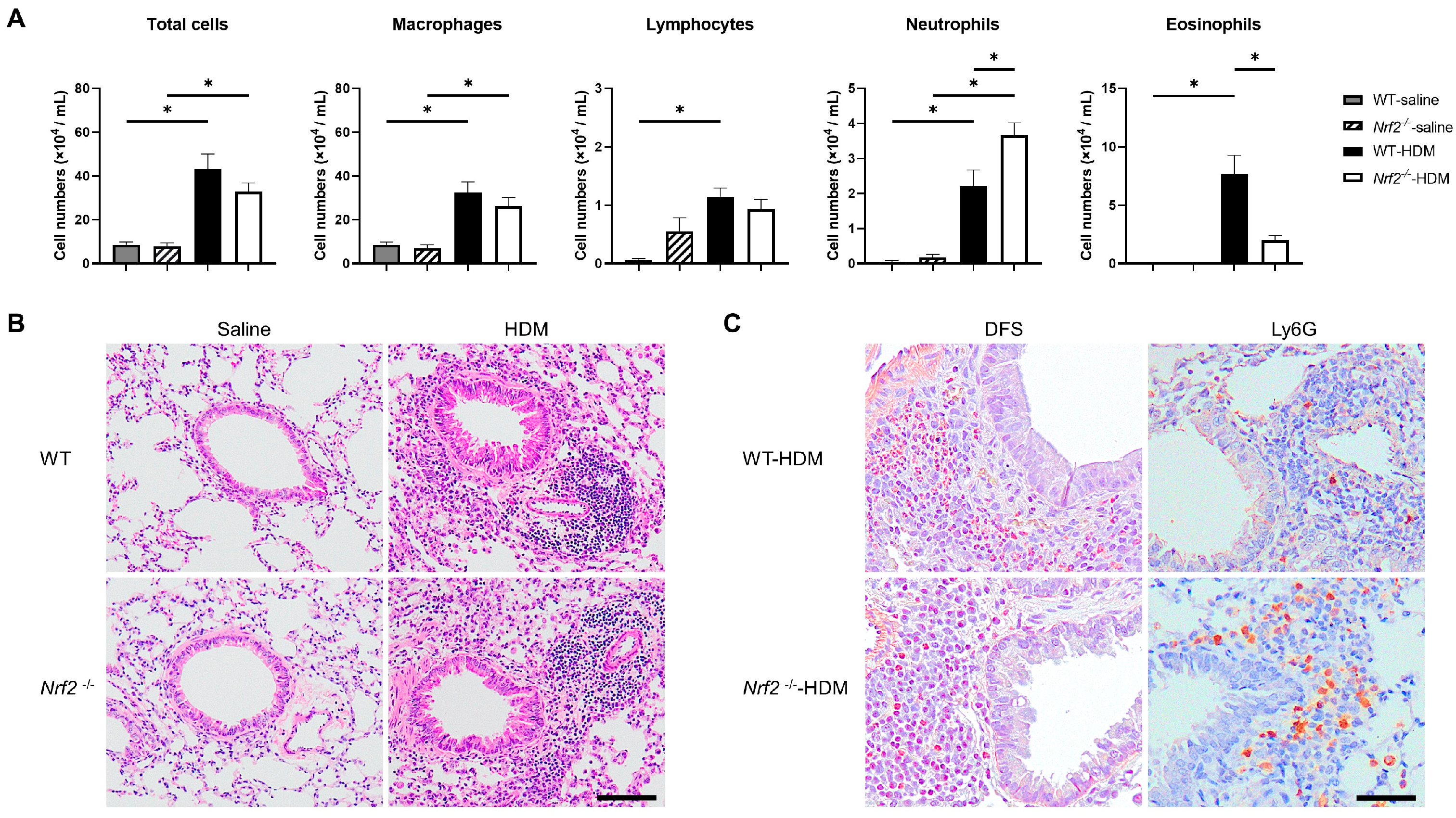
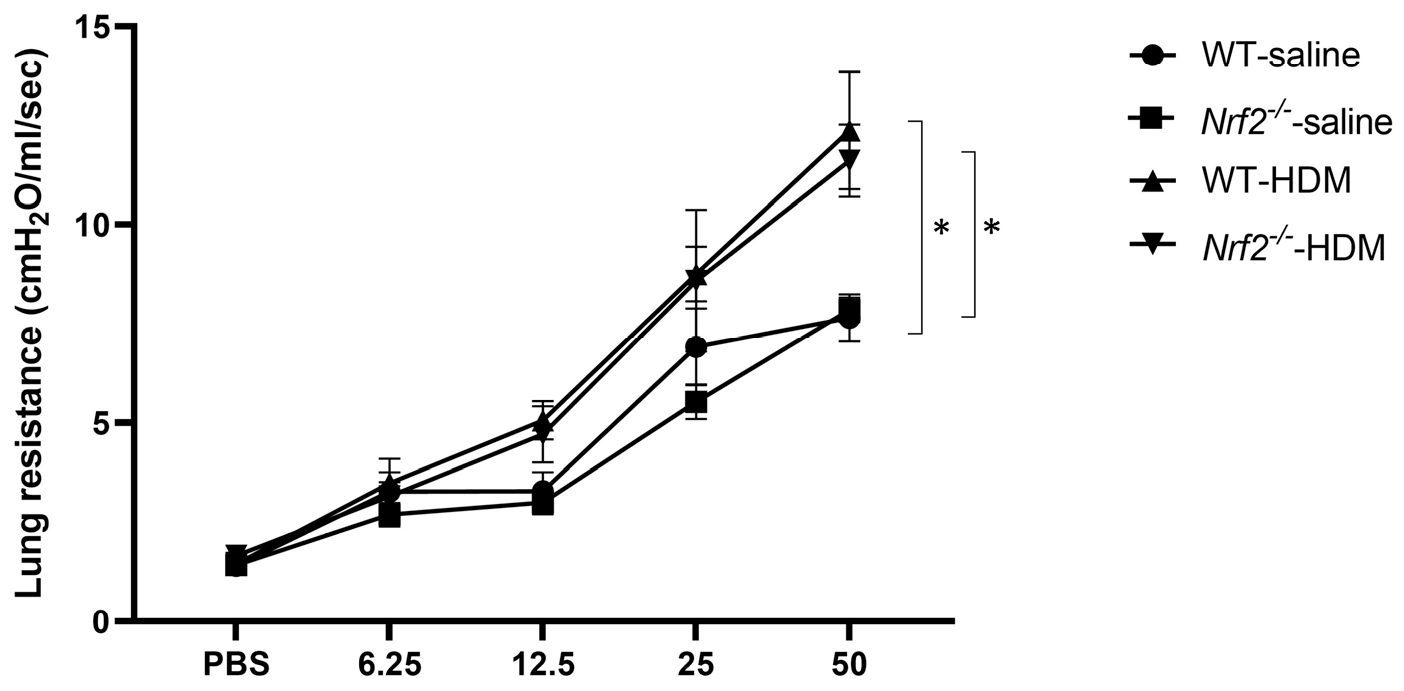
3.1. Nrf2 Deficiency Exacerbates Neutrophilic Airway Inflammation in a Mouse Model of Asthma
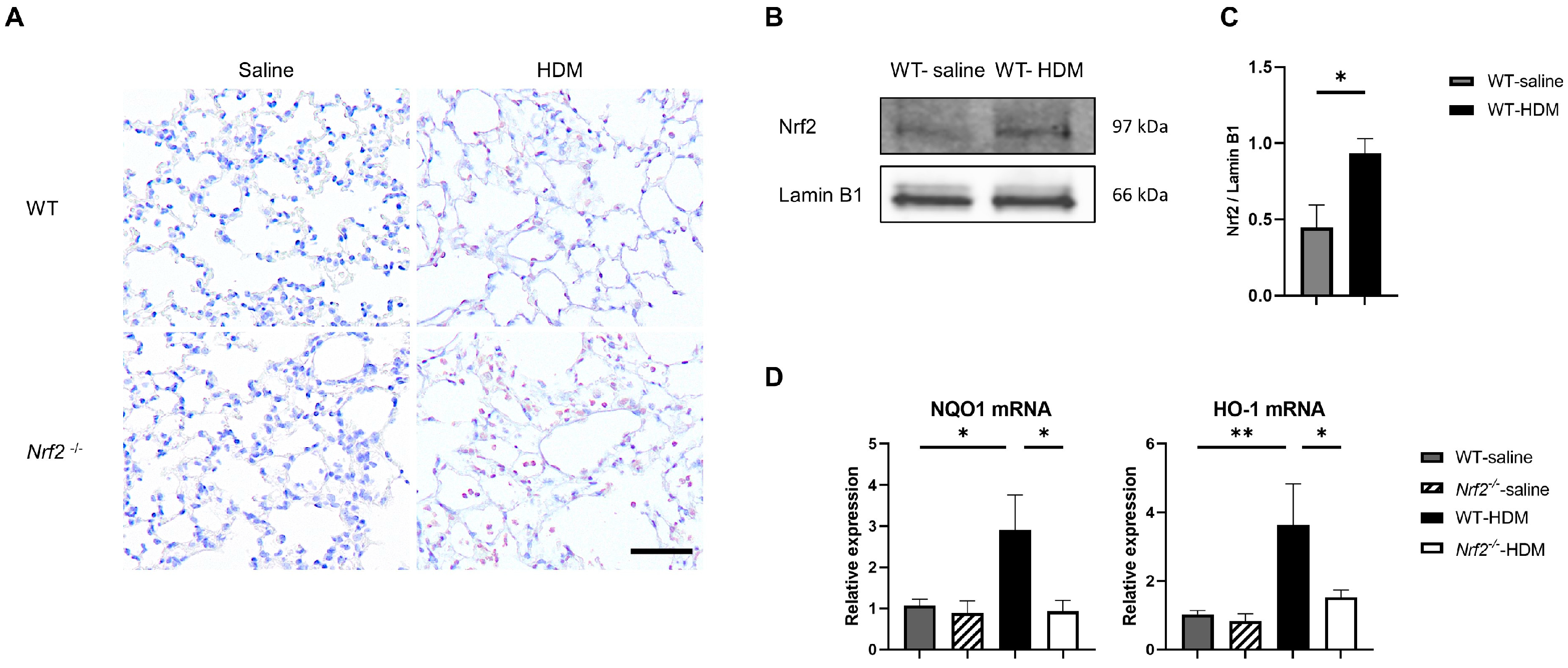
3.2. Oxidative Stress Is Exacerbated in an Nrf2-Deficient Mouse Model of Asthma
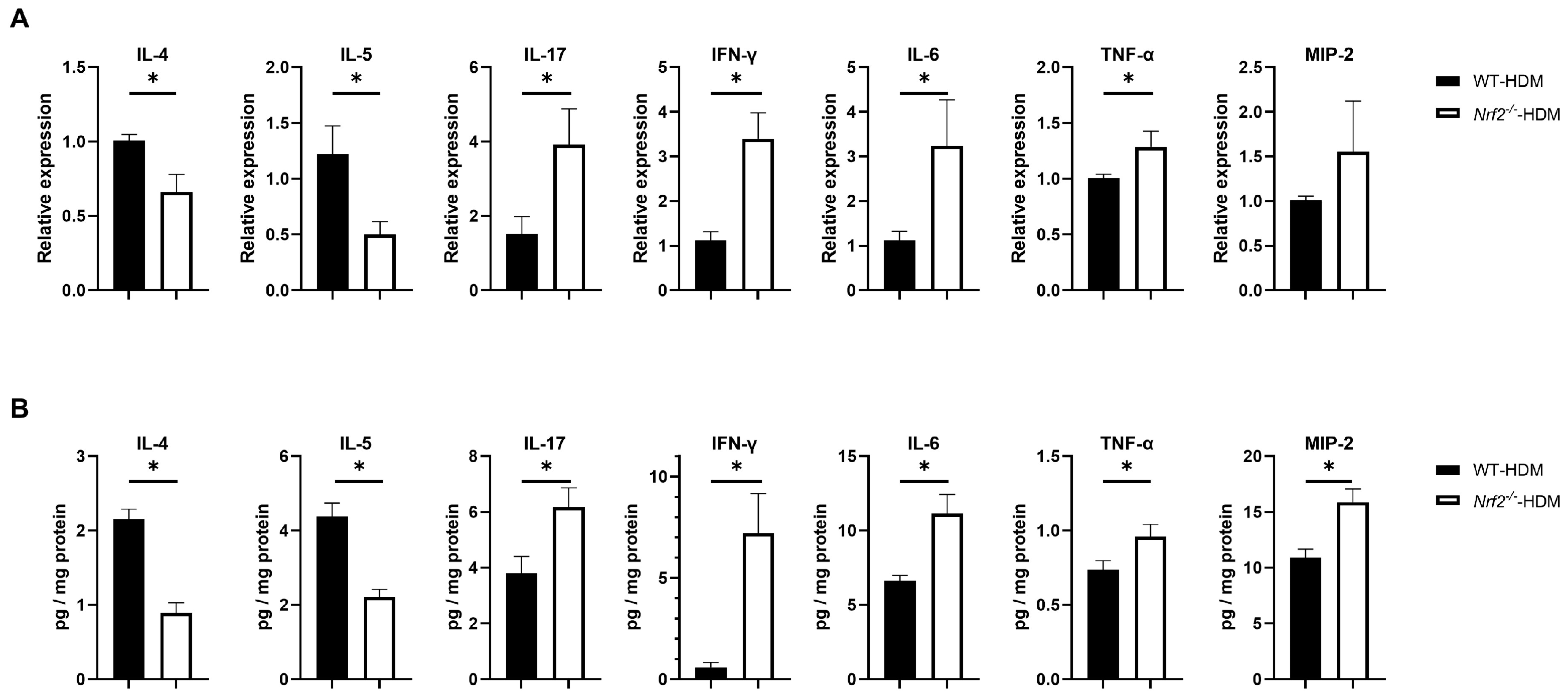
3.3. Nrf2 Deletion Alters the Expression of Inflammatory Cytokines and Chemokines
3.4. The RORγt Gene Is Overexpressed in Lung Th Cells in HDM-Stimulated Nrf2−/− Mice
3.5. Treatment with Anti-IL17 Antibody Improves Neutrophilic Airway Inflammation in HDM-Stimulated Nrf2−/− Mice
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hammad, H.; Lambrecht, B.N. The basic immunology of asthma. Cell 2021, 184, 1469–1485. [Google Scholar] [CrossRef]
- 2024 GINA Report, Global Strategy for Asthma Management and Prevention. Available online: https://ginasthma.org/reports/ (accessed on 16 May 2024).
- Heaney, L.G.; Perez de Llano, L.; Al-Ahmad, M.; Backer, V.; Busby, J.; Canonica, G.W.; Christoff, G.C.; Cosio, B.G.; FitzGerald, J.M.; Heffler, E.; et al. Eosinophilic and noneosinophilic asthma: An expert consensus framework to characterize phenotypes in a global real-life severe asthma cohort. Chest 2021, 160, 814–830. [Google Scholar] [CrossRef] [PubMed]
- Itoh, K.; Wakabayashi, N.; Katoh, Y.; Ishii, T.; Igarashi, K.; Engel, J.D.; Yamamoto, M. Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes Dev. 1999, 13, 76–86. [Google Scholar] [CrossRef] [PubMed]
- Itoh, K.; Chiba, T.; Takahashi, S.; Ishii, T.; Igarashi, K.; Katoh, Y.; Oyake, T.; Hayashi, N.; Satoh, K.; Hatayama, I. An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem. Biophys. Res. Commun. 1997, 236, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Ishii, T.; Itoh, K.; Takahashi, S.; Sato, H.; Yanagawa, T.; Katoh, Y.; Bannai, S.; Yamamoto, M. Transcription factor Nrf2 coordinately regulates a group of oxidative stress-inducible genes in macrophages. J. Biol. Chem. 2000, 275, 16023–16029. [Google Scholar] [CrossRef] [PubMed]
- Ishii, Y.; Itoh, K.; Morishima, Y.; Kimura, T.; Kiwamoto, T.; IIzuka, T.; Hegab, A.E.; Hosoya, T.; Nomura, A.; Sakamoto, T. Transcription factor Nrf2 plays a pivotal role in protection against elastase-induced pulmonary inflammation and emphysema. J. Immunol. 2005, 175, 6968–6975. [Google Scholar] [CrossRef] [PubMed]
- Iizuka, T.; Ishii, Y.; Itoh, K.; Kiwamoto, T.; Kimura, T.; Matsuno, Y.; Morishima, Y.; Hegab, A.E.; Homma, S.; Nomura, A. Nrf2-deficient mice are highly susceptible to cigarette smoke-induced emphysema. Genes Cells 2005, 10, 1113–1125. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.Y.; Reddy, S.P.; Yamamoto, M.; Kleeberger, S.R. The transcription factor NRF2 protects against pulmonary fibrosis. FASEB J. 2004, 18, 1258–1260. [Google Scholar] [CrossRef]
- Kikuchi, N.; Ishii, Y.; Morishima, Y.; Yageta, Y.; Haraguchi, N.; Itoh, K.; Yamamoto, M.; Hizawa, N. Nrf2 protects against pulmonary fibrosis by regulating the lung oxidant level and Th1/Th2 balance. Respir. Res. 2010, 11, 31. [Google Scholar] [CrossRef] [PubMed]
- Mochizuki, M.; Ishii, Y.; Itoh, K.; Iizuka, T.; Morishima, Y.; Kimura, T.; Kiwamoto, T.; Matsuno, Y.; Hegab, A.E.; Nomura, A.; et al. Role of 15-deoxy delta(12,14) prostaglandin J2 and Nrf2 pathways in protection against acute lung injury. Am. J. Respir. Crit. Care Med. 2005, 171, 1260–1266. [Google Scholar] [CrossRef]
- Yageta, Y.; Ishii, Y.; Morishima, Y.; Masuko, H.; Ano, S.; Yamadori, T.; Itoh, K.; Takeuchi, K.; Yamamoto, M.; Hizawa, N. Role of Nrf2 in host defense against influenza virus in cigarette smoke-exposed mice. J. Virol. 2011, 85, 4679–4690. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, M.; Matsuyama, M.; Kawaguchi, M.; Kiwamoto, T.; Matsuno, Y.; Morishima, Y.; Yoshida, K.; Sherpa, M.; Yazaki, K.; Osawa, H.; et al. Nrf2 regulates granuloma formation and macrophage activation during mycobacterium avium infection via mediating Nramp1 and HO-1 expressions. mBio 2021, 12, e01947-20. [Google Scholar] [CrossRef] [PubMed]
- Yoo, S.; Kim, H.B.; Lee, S.Y.; Kim, B.S.; Kim, J.H.; Yu, J.; Kim, B.J.; Lee, D.H.; Seong, M.W.; Hong, S.J. Effect of active smoking on asthma symptoms, pulmonary function, and BHR in adolescents. Pediatr. Pulmonol. 2009, 44, 954–961. [Google Scholar] [CrossRef] [PubMed]
- Boulet, L.P.; Lemière, C.; Archambault, F.; Carrier, G.; Descary, M.C.; Deschesnes, F. Smoking and asthma: Clinical and radiologic features, lung function, and airway inflammation. Chest 2006, 129, 661–668. [Google Scholar] [CrossRef]
- Chalmers, G.W.; MacLeod, K.J.; Thomson, L.; Little, S.A.; McSharry, C.; Thomson, N.C. Smoking and airway inflammation in patients with mild asthma. Chest 2001, 120, 1917–1922. [Google Scholar] [CrossRef] [PubMed]
- Shimoda, T.; Obase, Y.; Kishikawa, R.; Iwanaga, T. Influence of cigarette smoking on airway inflammation and inhaled corticosteroid treatment in patients with asthma. Allergy Asthma Proc. 2016, 37, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Min, M.G.; Song, D.J.; Miller, M.; Cho, J.Y.; McElwain, S.; Ferguson, P.; Broide, D.H. Coexposure to environmental tobacco smoke increases levels of allergen-induced airway remodeling in mice. J. Immunol. 2007, 178, 5321–5328. [Google Scholar] [CrossRef] [PubMed]
- Osborne, M.L.; Pedula, K.L.; O’Hollaren, M.; Ettinger, K.M.; Stibolt, T.; Buist, A.S.; Vollmer, W.M. Assessing future need for acute care in adult asthmatics: The Profile of Asthma Risk Study: A prospective health maintenance organization-based study. Chest 2007, 132, 1151–1161. [Google Scholar] [CrossRef] [PubMed]
- Kauppi, P.; Kupiainen, H.; Lindqvist, A.; Haahtela, T.; Laitinen, T. Long-term smoking increases the need for acute care among asthma patients: A case control study. BMC Pulm. Med. 2014, 14, 119. [Google Scholar] [CrossRef] [PubMed]
- Tomlinson, J.E.; McMahon, A.D.; Chaudhuri, R.; Thompson, J.M.; Wood, S.F.; Thomson, N.C. Efficacy of low and high dose inhaled corticosteroid in smokers versus non-smokers with mild asthma. Thorax 2005, 60, 282–287. [Google Scholar] [CrossRef]
- Dijkstra, A.; Vonk, J.M.; Jongepier, H.; Koppelman, G.H.; Schouten, J.P.; ten Hacken, N. H: Timens, W: Postma, D.S. Lung function decline in asthma: Association with inhaled corticosteroids, smoking and sex. Thorax 2006, 61, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, H.; Morishima, Y.; Ishii, Y.; Yoshida, K.; Nakajima, M.; Tsunoda, Y.; Hayashi, S.Y.; Kiwamoto, T.; Matsuno, Y.; Kawaguchi, M.; et al. Sulforaphane ameliorates steroid insensitivity through an Nrf2-dependent pathway in cigarette smoke-exposed asthmatic mice. Free Radic. Biol. Med. 2018, 129, 473–485. [Google Scholar] [CrossRef] [PubMed]
- Klein, D.K.; Silberbrandt, A.; Frøssing, L.; Hvidtfeldt, M.; von Bülow, A.; Nair, P.; Mukherjee, M.; Porsbjerg, C. Impact of former smoking exposure on airway eosinophilic activation and autoimmunity in patients with severe asthma. Eur. Respir. J. 2022, 60, 2102446. [Google Scholar] [CrossRef]
- Dong, C.; Yang, X.; Luo, W.; Fan, E.; Assam, N.P.; Kang, J.; Zhang, Y.; Huang, M.; Xu, J.; Huang, K.; et al. C–BIOPRED consortium. Influence of sex, cigarette smoking and airway inflammation on treatable traits in CBIOPRED severe asthma. Clin. Transl. Allergy 2022, 12, e12189. [Google Scholar] [CrossRef]
- Cowan, D.C.; Cowan, J.O.; Palmay, R.; Williamson, A.; Taylor, D.R. Effects of steroid therapy on inflammatory cell subtypes in asthma. Thorax 2010, 65, 384–390. [Google Scholar] [CrossRef] [PubMed]
- Cox, G. Glucocorticoid treatment inhibits apoptosis in human neutrophils. Separation of survival and activation outcomes. J. Immunol. 1995, 154, 4719–4725. [Google Scholar] [CrossRef]
- Hyde, E.J.; Wakelin, K.A.; Daniels, N.J.; Ghosh, S.; Ronchese, F. Similar immune mechanisms control experimental airway eosinophilia elicited by different allergens and treatment protocols. BMC Immunol. 2019, 20, 18. [Google Scholar] [CrossRef] [PubMed]
- Rangasamy, T.; Guo, J.; Mitzner, W.A.; Roman, J.; Singh, A.; Fryer, A.D.; Yamamoto, M.; Kensler, T.W.; Tuder, R.M.; Georas, S.N.; et al. Disruption of Nrf2 enhances susceptibility to severe airway inflammation and asthma in mice. J. Exp. Med. 2005, 202, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Abu Khweek, A.; Kim, E.; Joldrichsen, M.R.; Amer, A.O.; Boyaka, P.N. Insights into mucosal innate immune responses in house dust mite-mediated allergic asthma. Front Immunol. 2020, 11, 534501. [Google Scholar] [CrossRef]
- Zhao, S.; Jiang, Y.; Yang, X.; Guo, D.; Wang, Y.; Wang, J.; Wang, R.; Wang, C. Lipopolysaccharides promote a shift from Th2-derived airway eosinophilic inflammation to Th17-derived neutrophilic inflammation in an ovalbumin-sensitized murine asthma model. J. Asthma 2017, 54, 447–455. [Google Scholar] [CrossRef]
- Tan, H.T.; Hagner, S.; Ruchti, F.; Radzikowska, U.; Tan, G.; Altunbulakli, C.; Eljaszewicz, A.; Moniuszko, M.; Akdis, M.; Akdis, C.A.; et al. Tight junction, mucin, and inflammasome-related molecules are differentially expressed in eosinophilic, mixed, and neutrophilic experimental asthma in mice. Allergy 2019, 74, 294–307. [Google Scholar] [CrossRef] [PubMed]
- Nadeem, A.; Chhabra, S.K.; Masood, A.; Raj, H.G. Increased oxidative stress and altered levels of antioxidants in asthma. J. Allergy Clin. Immunol. 2003, 111, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Ammar, M.; Bahloul, N.; Amri, O.; Omri, R.; Ghozzi, H.; Kammoun, S.; Zeghal, K.; Ben Mahmoud, L. Oxidative stress in patients with asthma and its relation to uncontrolled asthma. J. Clin. Lab. Anal. 2022, 36, e24345. [Google Scholar] [CrossRef] [PubMed]
- Fernando, Y.; Wickramasinghe, P.; De Silva, U.; Alahakoon, M.; Anuradha, K.W.D.A.; Handunnetti, S. Differences in serum markers of oxidative stress in well controlled and poorly controlled asthma in Sri Lankan children: A pilot study. Allergy Asthma Clin. Immunol. 2020, 16, 66. [Google Scholar] [CrossRef] [PubMed]
- Jasemi, S.V.; Khazaei, H.; Fakhri, S.; Mohammadi-Noori, E.; Farzaei, M.H. Naringenin Improves Ovalbumin-Induced Allergic Asthma in Rats through Antioxidant and Anti-Inflammatory Effects. Evid. Based Complement. Alternat. Med. 2022, 2022, 9110798. [Google Scholar] [CrossRef] [PubMed]
- Sussan, T.E.; Gajghate, S.; Chatterjee, S.; Mandke, P.; McCormick, S.; Sudini, K.; Kumar, S.; Breysse, P.N.; Diette, G.B.; Sidhaye, V.K.; et al. Nrf2 reduces allergic asthma in mice through enhanced airway epithelial cytoprotective function. Am. J. Physiol. Lung Cell. Mol. Physiol. 2015, 309, L27–L36. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Li, W.; Feng, Y.; Xu, J.; Cao, H. Edaravone attenuates experimental asthma in mice through induction of HO-1 and the Keap1/Nrf2 pathway. Exp. Ther. Med. 2020, 19, 1407–1416. [Google Scholar] [CrossRef]
- Zhang, J.H.; Yang, X.; Chen, Y.P.; Zhang, J.F.; Li, C.Q. Nrf2 Activator RTA-408 protects against ozone-induced acute asthma exacerbation by suppressing ROS and gammadeltaT17 cells. Inflammation 2019, 42, 1843–1856. [Google Scholar] [CrossRef]
- Hellings, P.W.; Kasran, A.; Liu, Z.; Vandekerckhove, P.; Wuyts, A.; Overbergh, L.; Mathieu, C.; Ceuppens, J.L. Interleukin-17 orchestrates the granulocyte influx into airways after allergen inhalation in a mouse model of allergic asthma. Am. J. Respir. Cell Mol. Biol. 2003, 28, 42–50. [Google Scholar] [CrossRef]
- Xie, Y.; Abel, P.W.; Casale, T.B.; Tu, Y. TH17 cells and corticosteroid insensitivity in severe asthma. J. Allergy Clin. Immunol. 2022, 149, 467–479. [Google Scholar] [CrossRef]
- McKinley, L.; Alcorn, J.F.; Peterson, A.; Dupont, R.B.; Kapadia, S.; Logar, A.; Henry, A.; Irvin, C.G.; Piganelli, J.D.; Ray, A.; et al. TH17 cells mediate steroid-resistant airway inflammation and airway hyperresponsiveness in mice. J. Immunol. 2008, 181, 4089–4097. [Google Scholar] [CrossRef] [PubMed]
- Ano, S.; Morishima, Y.; Ishii, Y.; Yoh, K.; Yageta, Y.; Ohtsuka, S.; Matsuyama, M.; Kawaguchi, M.; Takahashi, S.; Hizawa, N. Transcription factors GATA-3 and RORγt are important for determining the phenotype of allergic airway inflammation in a murine model of asthma. J. Immunol. 2013, 190, 1056–1065. [Google Scholar] [CrossRef] [PubMed]
- Al-Harbi, N.O.; Nadeem, A.; Ahmad, S.F.; AlThagfan, S.S.; Alqinyah, M.; Alqahtani, F.; Ibrahim, K.E.; Al-Harbi, M.M. Sulforaphane treatment reverses corticosteroid resistance in a mixed granulocytic mouse model of asthma by upregulation of antioxidants and attenuation of Th17 immune responses in the airways. Eur. J. Pharmacol. 2019, 855, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Chesné, J.; Braza, F.; Mahay, G.; Brouard, S.; Aronica, M.; Magnan, A. IL-17 in severe asthma. Where do we stand? Am. J. Respir. Crit. Care Med. 2014, 190, 1094–1101. [Google Scholar] [CrossRef] [PubMed]
- Mills, K.H.G. IL-17 and IL-17-producing cells in protection versus pathology. Nat. Rev. Immunol. 2023, 23, 38–54. [Google Scholar] [CrossRef]
- Ivanov, I.I.; McKenzie, B.S.; Zhou, L.; Tadokoro, C.E.; Lepelley, A.; Lafaille, J.J.; Cua, D.J.; Littman, D.R. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell 2006, 126, 1121–1133. [Google Scholar] [CrossRef] [PubMed]
- Weng, C.M.; Lee, M.J.; He, J.R.; Chao, M.W.; Wang, C.H.; Kuo, H.P. Diesel exhaust particles up-regulate interleukin-17A expression via ROS/NF-κB in airway epithelium. Biochem. Pharmacol. 2018, 151, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Su, B.; Tao, P.; Yang, X.; Zheng, L.; Lin, Y.; Zou, X.; Yang, H.; Wu, W.; Zhang, T.; et al. Interplay of IL-33 and IL-35 modulates Th2/Th17 responses in cigarette smoke exposure HDM-induced asthma. Inflammation 2024, 47, 173–190. [Google Scholar] [CrossRef] [PubMed]
- Brandt, E.B.; Kovacic, M.B.; Lee, G.B.; Gibson, A.M.; Acciani, T.H.; Le Cras, T.D.; Ryan, P.H.; Budelsky, A.L.; Khurana Hershey, G.K. Diesel exhaust particle induction of IL-17A contributes to severe asthma. J. Allergy Clin. Immunol. 2013, 132, 1194–1204.e2. [Google Scholar] [CrossRef]
- Liang, Y.; Shen, Y.; Kuang, L.; Zhou, G.; Zhang, L.; Zhong, X.; Zhang, J.; Liu, J. Cigarette smoke exposure promotes differentiation of CD4+ T cells toward Th17 cells by CD40-CD40L costimulatory pathway in mice. Int. J. Chron. Obstruct. Pulmon. Dis. 2018, 13, 959–968. [Google Scholar] [CrossRef]
- McGovern, T.K.; Goldberger, M.; Allard, B.; Farahnak, S.; Hamamoto, Y.; O’Sullivan, M.; Hirota, N.; Martel, G.; Rousseau, S.; Martin, J.G. Neutrophils mediate airway hyperresponsiveness after chlorine-induced airway injury in the mouse. Am. J. Respir. Cell Mol. Biol. 2015, 52, 513–522. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Sun, L.; Jiang, T.; Zhang, D.; He, D.; Nie, H. TNFα promotes Th17 cell differentiation through IL-6 and IL-1β produced by monocytes in rheumatoid arthritis. J. Immunol. Res. 2014, 2014, 85352. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, S.; Ghilardi, N.; Xie, M.H.; de Sauvage, F.J.; Gurney, A.L. Interleukin-23 promotes a distinct CD4 T cell activation state characterized by the production of interleukin-17. J. Biol. Chem. 2003, 278, 1910–1914. [Google Scholar] [CrossRef] [PubMed]
- Williams, M.A.; Rangasamy, T.; Bauer, S.M.; Killedar, S.; Karp, M.; Kensler, T.W.; Yamamoto, M.; Breysse, P.; Biswal, S.; Georas, S.N. Disruption of the transcription factor Nrf2 promotes pro-oxidative dendritic cells that stimulate Th2-like immunoresponsiveness upon activation by ambient particulate matter. J. Immunol. 2008, 181, 4545–4559. [Google Scholar] [CrossRef] [PubMed]
- Rockwell, C.E.; Zhang, M.; Fields, P.E.; Klaassen, C.D. Th2 skewing by activation of Nrf2 in CD4+ T cells. J. Immunol. 2012, 188, 1630–1637. [Google Scholar] [CrossRef] [PubMed]
- Hwang, E.S.; Szabo, S.J.; Schwartzberg, P.L.; Glimcher, L.H. T helper cell fate specified by kinase-mediated interaction of T-bet with GATA-3. Science 2005, 307, 430–433. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, M.; Hirose, K.; Kagami, S.; Takatori, H.; Wakashin, H.; Tamachi, T.; Watanabe, N.; Saito, Y.; Iwamoto, I.; Nakajima, H. T-bet inhibits both TH2 cell-mediated eosinophil recruitment and TH17 cell-mediated neutrophil recruitment into the airways. J. Allergy Clin. Immunol. 2007, 119, 662–670. [Google Scholar] [CrossRef] [PubMed]
- Busse, W.W.; Holgate, S.; Kerwin, E.; Chon, Y.; Feng, J.; Lin, J.; Lin, S.L. Randomized, double-blind, placebo-controlled study of brodalumab, a human anti-IL-17 receptor monoclonal antibody, in moderate to severe asthma. Am. J. Respir. Crit. Care Med. 2013, 188, 1294–1302. [Google Scholar] [CrossRef]
- Thiruvengadam, M.; Venkidasamy, B.; Subramanian, U.; Samynathan, R.; Ali Shariati, M.; Rebezov, M.; Girish, S.; Thangavel, S.; Dhanapal, A.R.; Fedoseeva, N.; et al. Bioactive Compounds in Oxidative Stress-Mediated Diseases: Targeting the NRF2/ARE Signaling Pathway and Epigenetic Regulation. Antioxidants 2021, 10, 1859. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuramoto, K.; Morishima, Y.; Yoshida, K.; Ano, S.; Kawashima, K.; Yabuuchi, Y.; Sakai, C.; Matsumura, S.; Nishino, K.; Yazaki, K.; et al. Nrf2 Deficiency Accelerates IL-17-Dependent Neutrophilic Airway Inflammation in Asthmatic Mice. Antioxidants 2024, 13, 818. https://doi.org/10.3390/antiox13070818
Kuramoto K, Morishima Y, Yoshida K, Ano S, Kawashima K, Yabuuchi Y, Sakai C, Matsumura S, Nishino K, Yazaki K, et al. Nrf2 Deficiency Accelerates IL-17-Dependent Neutrophilic Airway Inflammation in Asthmatic Mice. Antioxidants. 2024; 13(7):818. https://doi.org/10.3390/antiox13070818
Chicago/Turabian StyleKuramoto, Kenya, Yuko Morishima, Kazufumi Yoshida, Satoshi Ano, Kai Kawashima, Yuki Yabuuchi, Chio Sakai, Sosuke Matsumura, Kengo Nishino, Kai Yazaki, and et al. 2024. "Nrf2 Deficiency Accelerates IL-17-Dependent Neutrophilic Airway Inflammation in Asthmatic Mice" Antioxidants 13, no. 7: 818. https://doi.org/10.3390/antiox13070818
APA StyleKuramoto, K., Morishima, Y., Yoshida, K., Ano, S., Kawashima, K., Yabuuchi, Y., Sakai, C., Matsumura, S., Nishino, K., Yazaki, K., Matsuyama, M., Kiwamoto, T., Ishii, Y., & Hizawa, N. (2024). Nrf2 Deficiency Accelerates IL-17-Dependent Neutrophilic Airway Inflammation in Asthmatic Mice. Antioxidants, 13(7), 818. https://doi.org/10.3390/antiox13070818







