Study on a Mechanism of Improving MaAPX1 Protein Activity by Mutating Methionine to Lysine
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Treatments
2.2. Measurement of Physiological Parameters
2.3. Plasmid Construction and Gene Expression
2.4. Site-Directed Mutagenesis of Cys32 and Met36 Residues
2.5. Purification of Recombinant Proteins
2.6. Enzymatic Activity of MaAPXs Measurement
2.7. Mass-Spectrometric Analysis of S-Nitrosylation Level of Proteins
2.8. Spectroscopic Analysis and Stopped-Flow Analysis
2.9. Molecular Docking Experiment
2.10. Statistical Analysis
3. Results
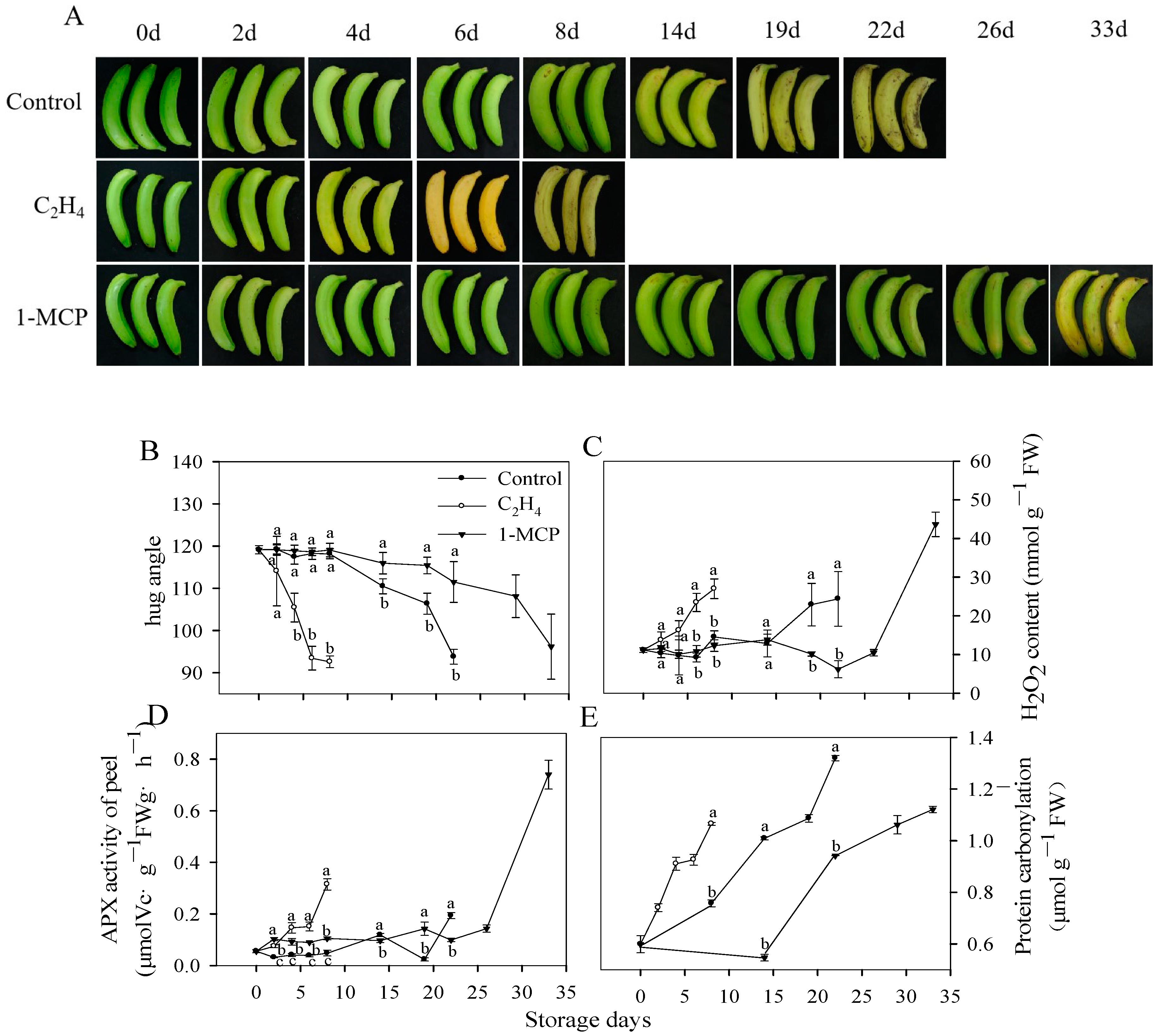
3.1. Ripening Characteristics and Redox Status of Harvested Banana Fruit during Ripening and Senescence
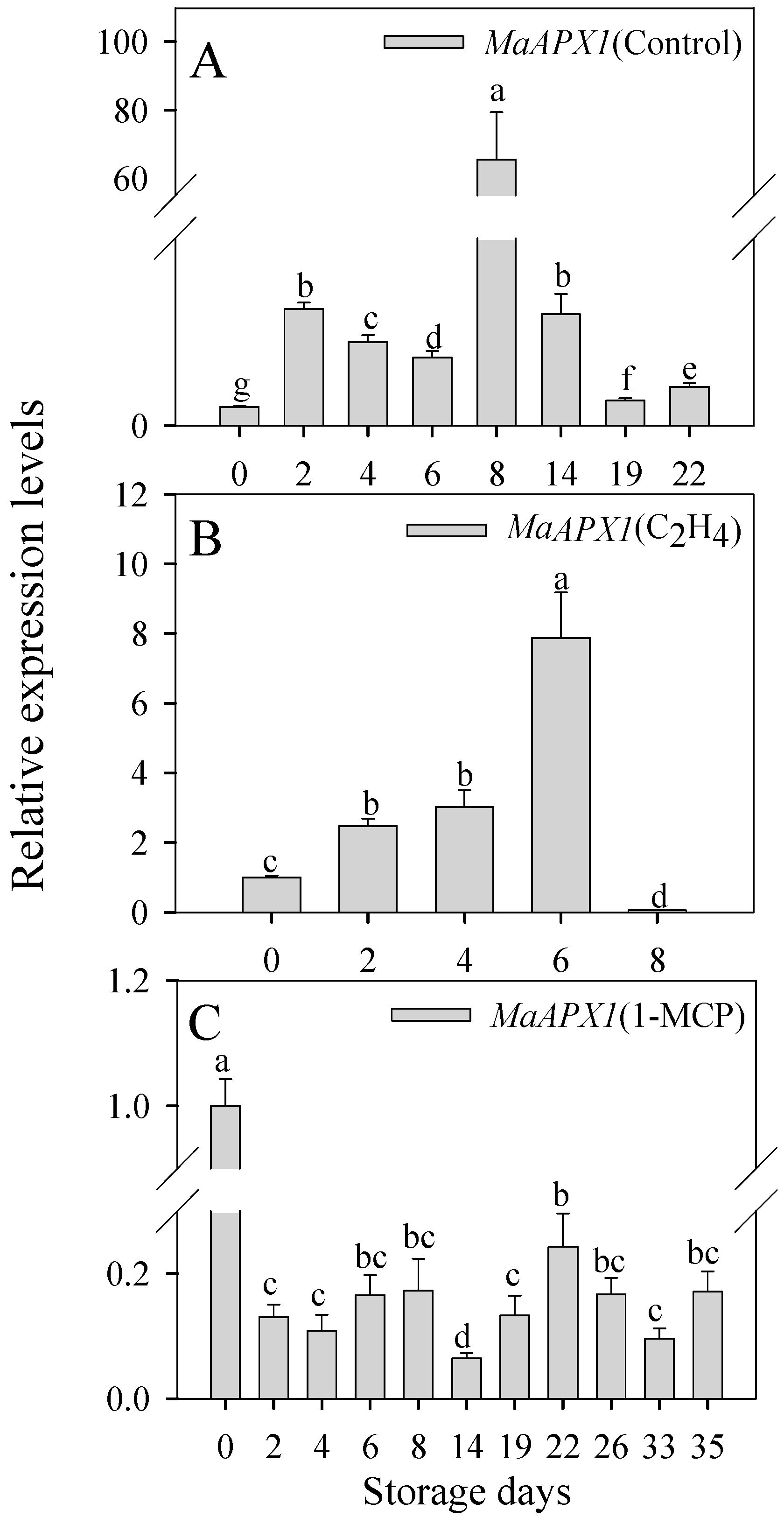
3.2. Expression Profiles of MaAPX1 Gene during Banana Ripening and Senescence
3.3. The MaAPX1 Activity Is Promoted by S-Nitrosylation of Cys32
3.4. Mutant MaAPX1M36K Activity Might Be Enhanced by Improving the S-Nitrosylation Level of Cys32
3.5. Visible Spectrum and Stopped-Flow Analysis of MaAPX1 and Mutant MaAPX1M36K Reaction with H2O2
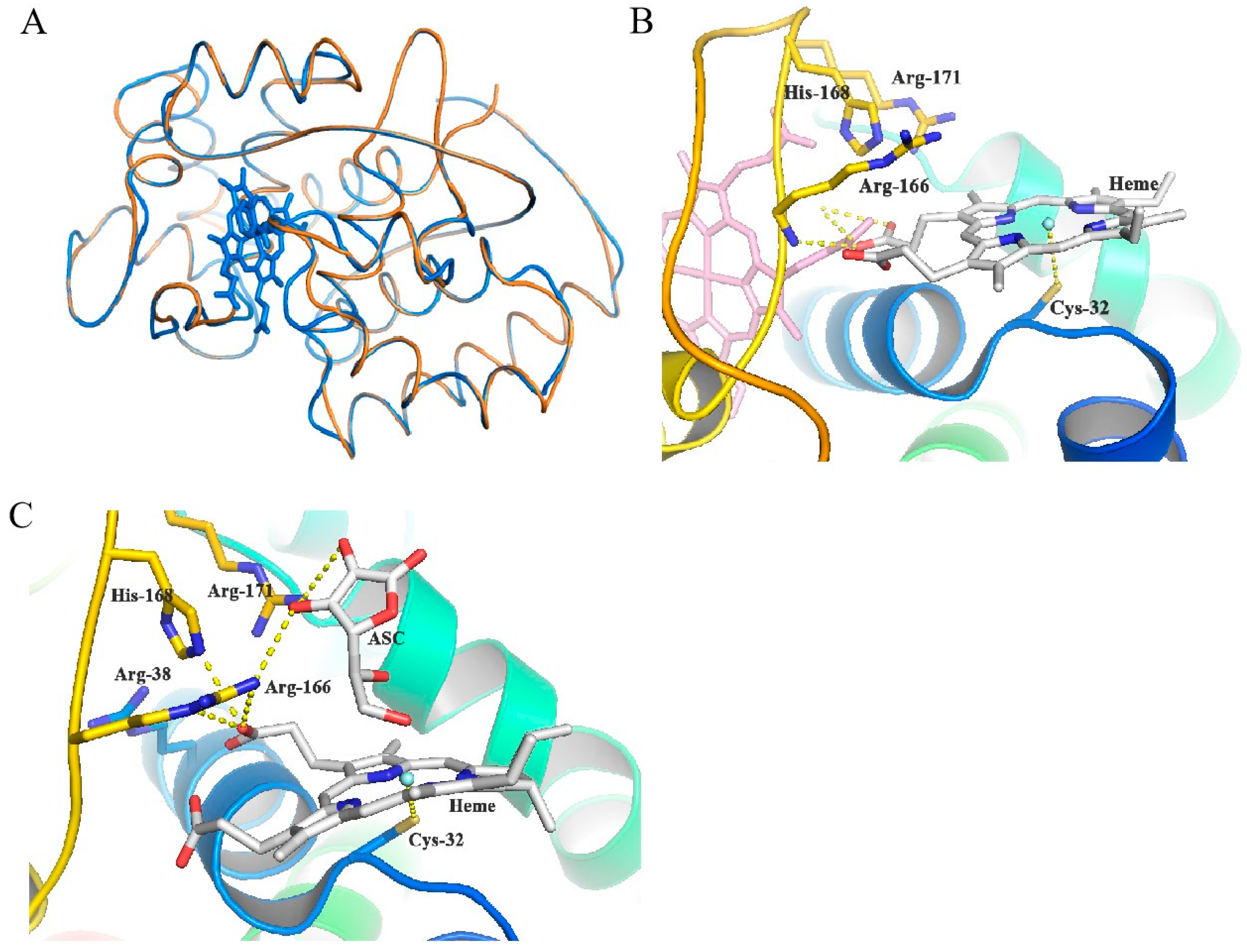
3.6. Molecular Docking Study of MaAPX1
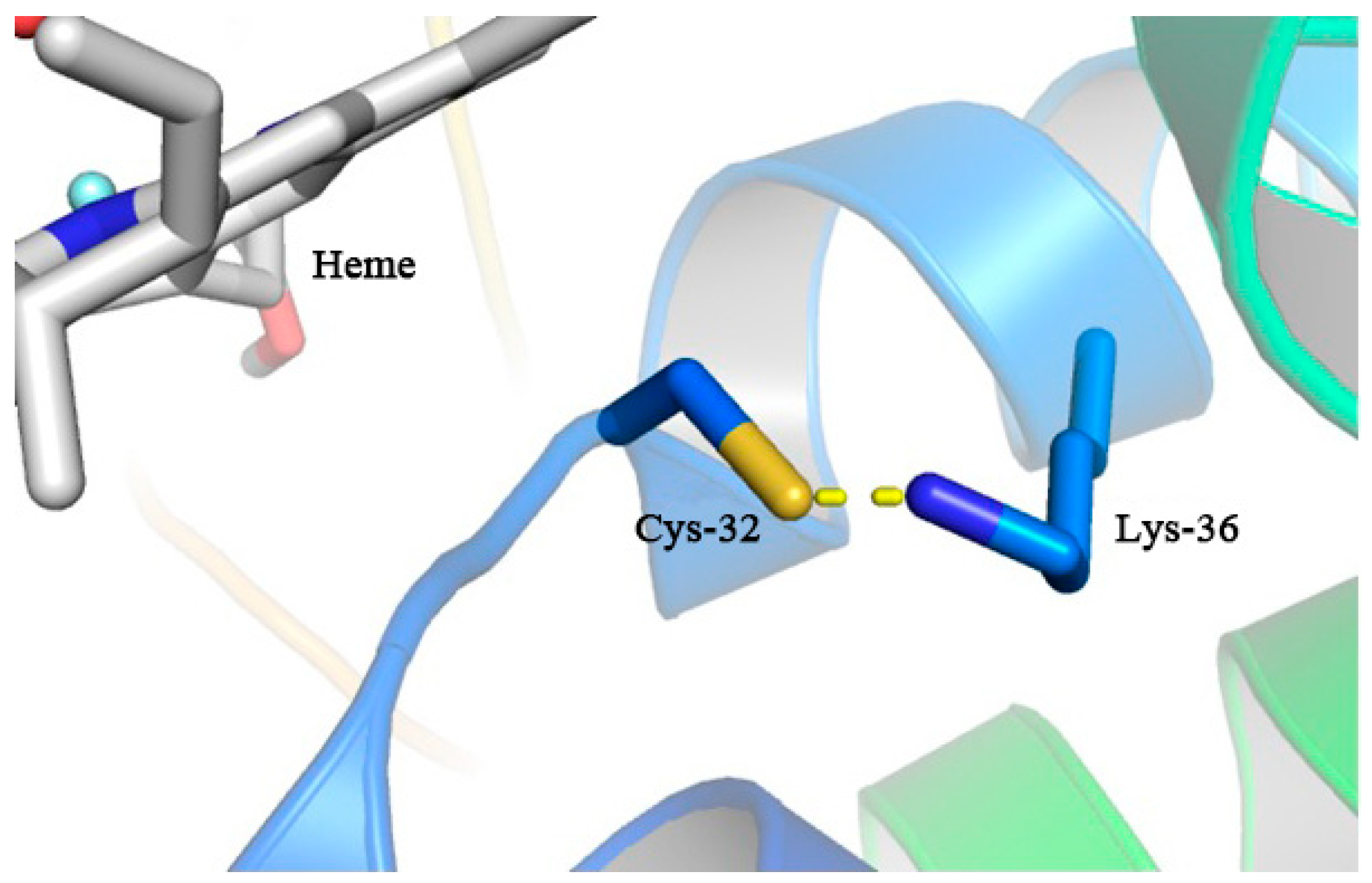
3.7. The Lys36 Residue of MaAPX1M36K Might Induce S-Nitrosylation of Cys32 via S-N Bonds to Enhance Enzymatic Activity
4. Conclusions
5. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yan, H.L.; Chen, H.X.; Zhao, J.L.; Yao, T.; Ding, X.C. Postharvest H2O2 treatment affects flavor quality, texture quality and ROS metabolism of ‘Hongshi’ kiwifruit fruit kept at ambient conditions. Food Chem. 2023, 405, 134908. [Google Scholar] [CrossRef]
- Juan, C.A.; Pérez de la Lastra, J.M.; Plou, F.J.; Pérez-Lebeña, E. The chemistry of Reactive Oxygen Species (ROS) revisited: Outlining their role in biological macromolecules (DNA, Lipids and Proteins) and induced pathologies. Int. J. Mol. Sci. 2021, 22, 4642. [Google Scholar] [CrossRef] [PubMed]
- Rohman, M.M.; Islam, M.R.; Monsur, M.B.; Amiruzzaman, M.; Fujita, M.; Hasanuzzaman, M. Trehalose protects Maize plants from salt stress and phosphorus deficiency. Plants 2019, 8, 568. [Google Scholar] [CrossRef] [PubMed]
- Hamurcu, M.; Khan, M.K.; Pandey, A.; Ozdemir, C.; Avsaroglu, Z.Z.; Elbasan, F.; Omay, A.H.; Gezgin, S. Nitric oxide regulates watermelon (Citrullus lanatus) responses to drought stress. 3 Biotech. 2020, 10, 494. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.; Rahman, K.; Sathi, K.S.; Alam, M.M.; Nahar, K.; Fujita, M.; Hasanuzzaman, M. Supplemental selenium and boron mitigate salt-induced oxidative damages in Glycine max L. Plants 2021, 10, 2224. [Google Scholar] [CrossRef]
- Liao, G.L.; Liu, Q.; Li, Y.Q.; Zhong, M.; Huang, C.H.; Jia, D.F.; Xu, X.B. Identification and expression profiling analysis of ascorbate peroxidase gene family in Actinidia chinensis (Hongyang). J. Plant Res. 2020, 133, 715–726. [Google Scholar] [CrossRef] [PubMed]
- Kuo, E.Y.; Cai, M.S.; Lee, T.M. Ascorbate peroxidase 4 plays a role in the tolerance of Chlamydomonas reinhardtii to photo-oxidative stress. Sci. Rep. 2020, 10, 13287. [Google Scholar] [CrossRef] [PubMed]
- Kizilkaya, I.T.; Sedef, A.; Unal, D. Determination of photosynthesis-related and ascorbate peroxidase gene expression in the green algae (Chlorella vulgaris) under high-temperature Conditions. Int. J. Second. Metab. 2021, 8, 59–69. [Google Scholar] [CrossRef]
- Huang, L.P.; Jia, J.; Zhao, X.X.; Zhang, M.Y.; Huang, X.X.; Ji, E.; Ni, L.; Jiang, M.Y. The ascorbate peroxidase APX1 is a direct target of a zinc finger transcription factor ZFP36 and a late embryogenesis abundant protein OsLEA5 interacts with ZFP36 to co-regulate OsAPX1 in seed germination in rice. Biochem. Biophys. Res. Commun. 2018, 495, 339–345. [Google Scholar] [CrossRef]
- Ribeiro, C.W.; Korbes, A.P.; Garighan, J.A.; Jardim-Messeder, D.; Carvalho, F.E.L.; Sousa, R.H.V.; Caverzan, A.; Teixeira, F.K.; Silveira, J.A.G.; Margis-Pinheiro, M. Rice peroxisomal ascorbate peroxidase knockdown affects ROS signaling and triggers early leaf senescence. Plant Sci. 2017, 263, 55–65. [Google Scholar] [CrossRef]
- Keyster, M.; Klein, A.; Ludidi, N. Endogenous NO levels regulate nodule functioning: Potential role of cGMP in nodule functioning? Plant Signal. Behav. 2010, 5, 1679–1681. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Correa-Aragunde, N.; Foresi, N.; Lamattina, L. Structure diversity of nitric oxide synthases (NOS): The emergence of new forms in photosynthetic organisms. Front. Plant Sci. 2013, 4, 232. [Google Scholar] [CrossRef] [PubMed]
- de Pinto, M.C.; Locato, V.; Sgobba, A.; Romero-Puertas, M.d.C.; Gadaleta, C.; Delledonne, M.; De Gara, L. S-Nitrosylation of ascorbate peroxidase is Part of programmed cell death signaling in tobacco bright yellow-2 cells. Plant Physiol. 2013, 163, 1766–1775. [Google Scholar] [CrossRef] [PubMed]
- Ben-Lulu, S.; Ziv, T.; Weisman-Shomer, P.; Benhar, M. Nitrosothiol-trapping-based proteomic analysis of S-nitrosylation in human lung carcinoma cells. PLoS ONE 2017, 12, e0169862. [Google Scholar] [CrossRef] [PubMed]
- Miller, G.; Suzuki, N.; Rizhsky, L.; Hegie, A.; Koussevitzky, S.; Mittler, R. Double mutants deficient in cytosolic and thylakoid ascorbate peroxidase reveal a complex mode of interaction between reactive oxygen species, plant development. and response to abiotic stresses. Plant Physiol. 2007, 144, 1777–1785. [Google Scholar] [CrossRef] [PubMed]
- Correa-Aragunde, N.; Foresi, N.; Lamattina, L. Nitric oxide is a ubiquitous signal for maintaining redox balance in plant cells: Regulation of ascorbate peroxidase as a case study. J. Exp. Bot. 2015, 66, 2913–2921. [Google Scholar] [CrossRef] [PubMed]
- Gelhaye, E.; Navrot, N.; Macdonald, I.K.; Rouhier, N.; Raven, E.L.; Jacquot, J.P. Ascorbate peroxidase-thioredoxin interaction. Photosynth. Res. 2006, 89, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Correa-Aragunde, N.; Foresi, N.; Delledonne, M.; Lamattina, L. Auxin induces redox regulation of ascorbate peroxidase 1 activity by S-nitrosylation/denitrosylation balance resulting in changes of root growth pattern in Arabidopsis. J. Exp. Bot. 2013, 64, 3339–3349. [Google Scholar] [CrossRef]
- Yang, H.J.; Mu, J.Y.; Chen, L.C.; Feng, J.; Hu, J.L.; Li, L.; Zhou, J.M.; Zuo, J.R. S-nitrosylation positively regulates ascorbate peroxidase activity during plant stress responses. Plant Physiol. 2015, 167, 1604–1615. [Google Scholar] [CrossRef]
- Bai, X.G.; Yang, L.M.; Tian, M.H.; Chen, J.H.; Shi, J.S.; Yang, Y.P.; Hu, X.Y. Nitric oxide enhances desiccation tolerance of recalcitrant Antiaris toxicaria seeds via protein S-nitrosylation and carbonylation. PLoS ONE 2011, 6, e20714. [Google Scholar] [CrossRef]
- Yamazaki, D.; Motohashi, K.; Kasama, T.; Hara, Y.; Hisabori, T. Target proteins of the cytosolic thioredoxins in Arabidopsis thaliana. Plant Cell Physiol. 2004, 45, 18–27. [Google Scholar] [CrossRef]
- Clark, D.; Durner, J.; Navarre, D.A.; Klessig, D.F. Nitric oxide inhibition of tobacco catalase and ascorbate peroxidase. Mol. Plant-Microbe Interact. MPMI 2000, 13, 1380–1384. [Google Scholar] [CrossRef]
- Ishikawa, T.; Madhusudhan, R.; Shigeoka, S. Effect of iron on the expression of ascorbate peroxidase in Euglena gracilis. Plant Sci. 2003, 165, 1363–1367. [Google Scholar] [CrossRef]
- Xiao, L.; Jiang, G.X.; Yan, H.L.; Lai, H.M.; Su, X.G.; Jiang, Y.M.; Duan, X.W. Methionine sulfoxide reductase B regulates the activity of ascorbate peroxidase of banana fruit. Antioxidants 2021, 10, 310. [Google Scholar] [CrossRef]
- Xiao, L.; Li, T.T.; Jiang, G.X.; John, A.; Zhang, D.D.; Jin, W.Y.; Duan, X.W.; Jiang, Y.M. Effects of dry fog humidification on pericarp browning and quality of litchi fruit stored at low temperature. Int. J. Agric. Biol. Eng. 2019, 12, 192–196. [Google Scholar] [CrossRef]
- Wan, C.Y.; Wilkins, T.A. A modified hot borate method significantly enhances the yield of high-quality RNA from cotton (Gossypium hirsutum L.). Anal. Biochem. 1994, 223, 7–12. [Google Scholar] [CrossRef]
- Xiao, L.; Jiang, X.Y.; Deng, Y.C.; Xu, K.H.; Duan, X.W.; Wan, K.; Tang, X.M. Study on characteristics and lignification mechanism of postharvest banana fruit during chilling injury. Foods 2023, 12, 1097. [Google Scholar] [CrossRef]
- Chen, L.; Zhong, H.Y.; Kuang, J.F.; Li, J.G.; Lu, W.J.; Chen, J.Y. Validation of reference genes for RT-qPCR studies of gene expression in banana fruit under different experimental conditions. Planta 2011, 234, 377–390. [Google Scholar] [CrossRef]
- Hugo, M.; Martínez, A.; Trujillo, M.; Estrad, D.; Mastrogiovanni, M.; Linares, E.; Augusto, O.; Issoglio, F.; Zeida, A.; Estrín, D.A. Kinetics, subcellular localization, and contribution to parasite virulence of a Trypanosoma cruzi hybrid type A heme peroxidase (TcAPx-CcP). Proc. Natl. Acad. Sci. USA 2017, 114, E1326–E1335. [Google Scholar] [CrossRef] [PubMed]
- Sharp, K.H.; Moody, P.C.; Brown, K.A.; Raven, E.L. Crystal structure of the ascorbate peroxidase-salicylhydroxamic acid complex. Biochemistry 2004, 43, 8644–8651. [Google Scholar] [CrossRef] [PubMed]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef]
- Orrenius, S.; Gogvadze, V.; Zhivotovsky, B. Mitochondrial oxidative stress: Implications for cell death. Annu. Rev. Pharmacol. Toxicol. 2007, 47, 143–183. [Google Scholar] [CrossRef]
- Ugarte, N.; Petropoulos, I.; Friguet, B. Oxidized mitochondrial protein degradation and repair in aging and oxidative stress. Antioxid. Redox Sign. 2010, 13, 539–549. [Google Scholar] [CrossRef]
- Fares, A.; Rossignol, M.; Peltier, J.B. Proteomics investigation of endogenous S-nitrosylation in Arabidopsis. Biochem. Biophys. Res. Commun. 2011, 416, 331–336. [Google Scholar] [CrossRef]
- Hu, J.L.; Huang, X.H.; Chen, L.C.; Sun, X.W.; Lu, C.M.; Zhang, L.X.; Wang, Y.C.; Zuo, J.R. Site-specific nitrosoproteomic identification of endogenously S-nitrosylated proteins in Arabidopsis. Plant Physiol. 2015, 167, 1731–1746. [Google Scholar] [CrossRef]
- Xue, Y.; Liu, Z.X.; Gao, X.J.; Jin, C.J.; Wen, L.P.; Yao, X.B.; Ren, J. GPS-SNO: Computational prediction of protein S-nitrosylation sites with a modified GPS algorithm. PLoS ONE 2010, 5, e11290. [Google Scholar]
- Yadav, R.K.; Dolai, S.; Pal, S.; Adak, S. Role of tryptophan-208 residue in cytochrome c oxidation by ascorbate peroxidase from Leishmania major-kinetic studies on Trp208Phe mutant and wild type enzyme. Biochim. Biophys. Acta-Proteins Proteom. 2008, 1784, 863–871. [Google Scholar] [CrossRef]
- Patterson, W.R.; Poulos, T.L. Crystal structure of recombinant pea cytosolic ascorbate peroxidase. Biochemistry 1995, 34, 4331–4341. [Google Scholar] [CrossRef] [PubMed]
- Erman, J.E.; Vitello, L.B.; Mauro, J.M.; Kraut, J. Detection of an oxyferryl porphyrin .pi.-cation-radical intermediate in the reaction between hydrogen peroxide and a mutant yeast cytochrome c peroxidase. Evidence for tryptophan-191 involvement in the radical site of compound I. Biochemistry 2002, 28, 7992–7995. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, Y.; Ishimori, K.; Uchida, T. Dual role of the active-center cysteine in human peroxiredoxin 1: Peroxidase activity and heme binding. Biochem. Biophys. Res. Commun. 2017, 483, 930–935. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Santamarina, S.; Boronat, S.; Hidalgo, E. Reversible cysteine oxidation in hydrogen peroxide sensing and signal transduction. Biochemistry 2014, 53, 2560–2580. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, L.; Jiang, G.; Lai, H.; Duan, X.; Yan, H.; Chen, S.; Chen, Z.; Duan, X. Study on a Mechanism of Improving MaAPX1 Protein Activity by Mutating Methionine to Lysine. Antioxidants 2024, 13, 843. https://doi.org/10.3390/antiox13070843
Xiao L, Jiang G, Lai H, Duan X, Yan H, Chen S, Chen Z, Duan X. Study on a Mechanism of Improving MaAPX1 Protein Activity by Mutating Methionine to Lysine. Antioxidants. 2024; 13(7):843. https://doi.org/10.3390/antiox13070843
Chicago/Turabian StyleXiao, Lu, Guoxiang Jiang, Hongmei Lai, Xiaoyan Duan, Huiling Yan, Shaoge Chen, Zexin Chen, and Xuewu Duan. 2024. "Study on a Mechanism of Improving MaAPX1 Protein Activity by Mutating Methionine to Lysine" Antioxidants 13, no. 7: 843. https://doi.org/10.3390/antiox13070843
APA StyleXiao, L., Jiang, G., Lai, H., Duan, X., Yan, H., Chen, S., Chen, Z., & Duan, X. (2024). Study on a Mechanism of Improving MaAPX1 Protein Activity by Mutating Methionine to Lysine. Antioxidants, 13(7), 843. https://doi.org/10.3390/antiox13070843






