Isolation, Characterization, and Functional Properties of Antioxidant Peptides from Mulberry Leaf Enzymatic Hydrolysates
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Preparation of Mulberry Leaf Protein (MLP) and Mulberry Leaf Protein Hydrolysates (MLPHs)
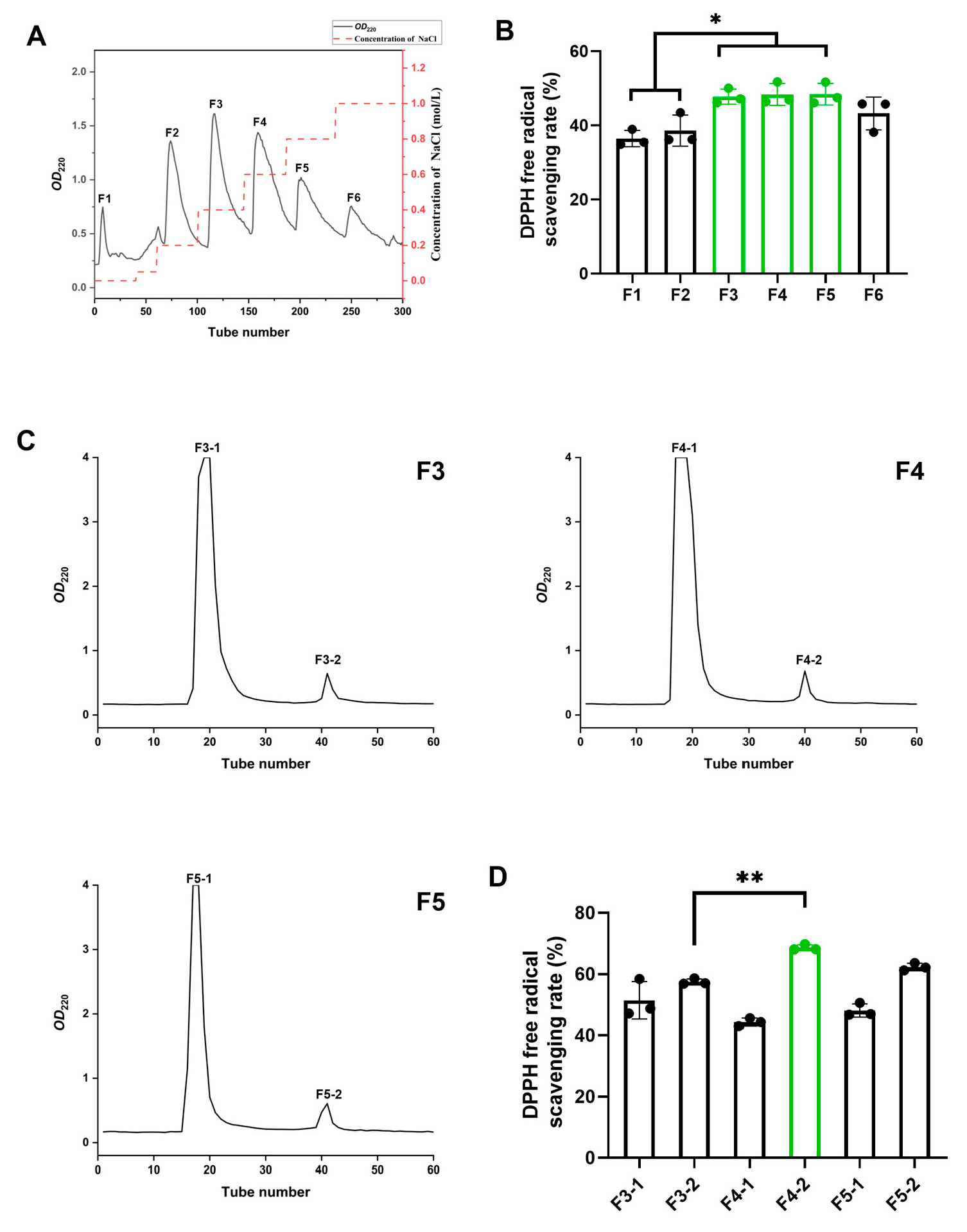
2.3. Separation of Antioxidant Components of MLPHs
2.4. Identification of Bioactive Mulberry Peptides (BMPs) by HPLC-MS
2.5. In Silico Analysis of Physicochemical Properties of BMPs
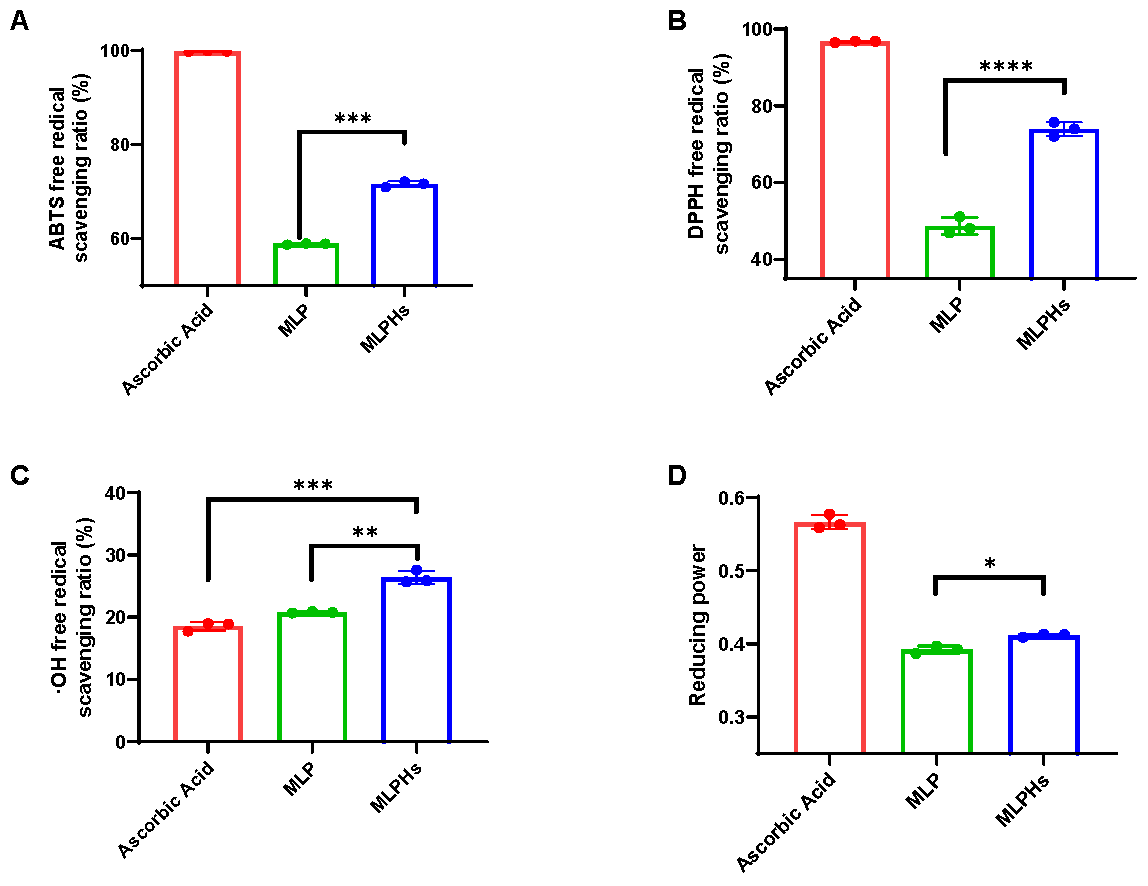
2.6. Determination of ABTS, DPPH, and OH Scavenging Capacities
2.7. Determination of Reducing Power
2.8. Cell Culture and Cell Viability Assay
2.9. The Protective Effect of BMPs against H2O2-Induced Oxidative Damage in HepG2 Cells
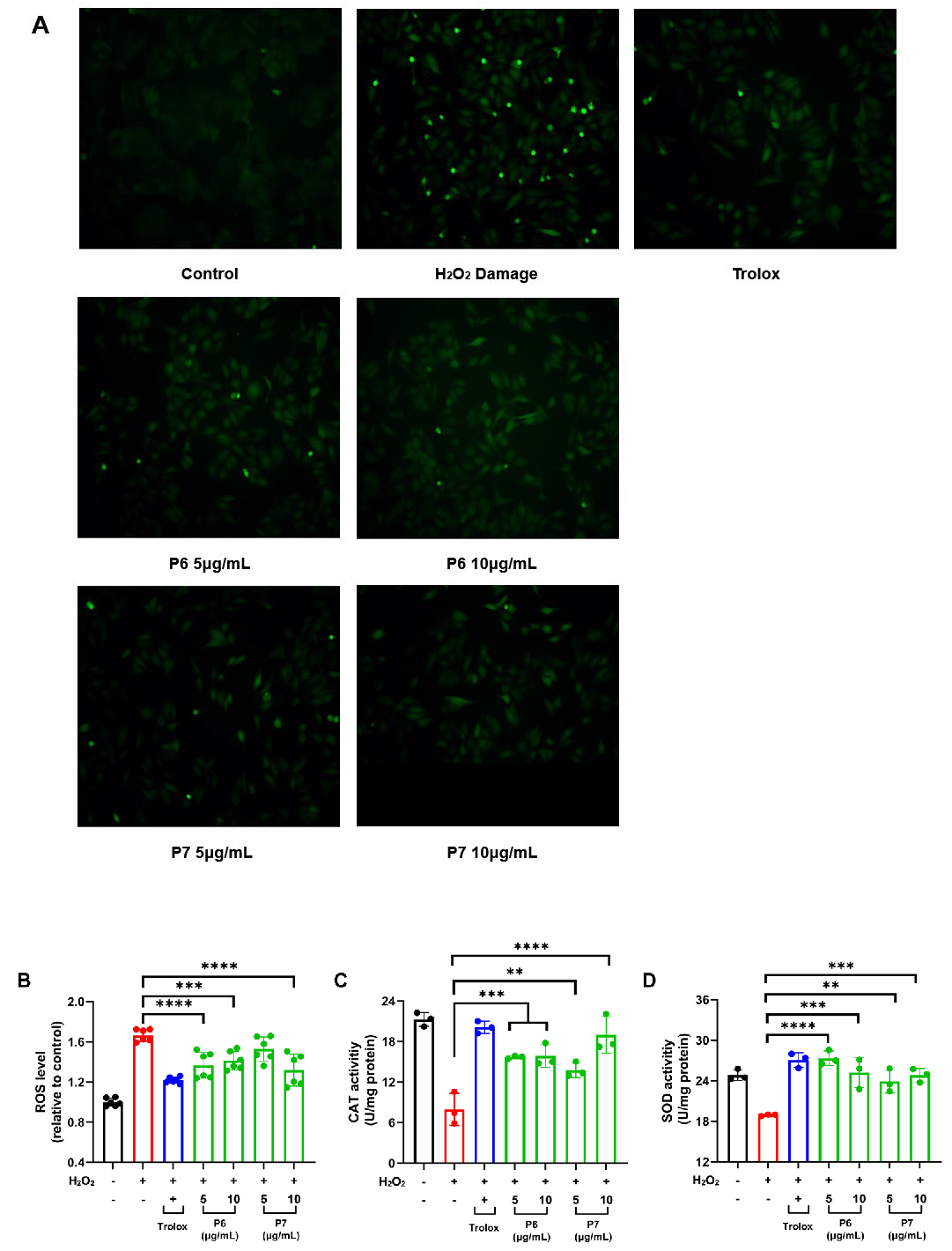
2.10. Determination of SOD and CAT Activities in HepG2 Cells
2.11. Determination of ROS Levels in HepG2 Cells
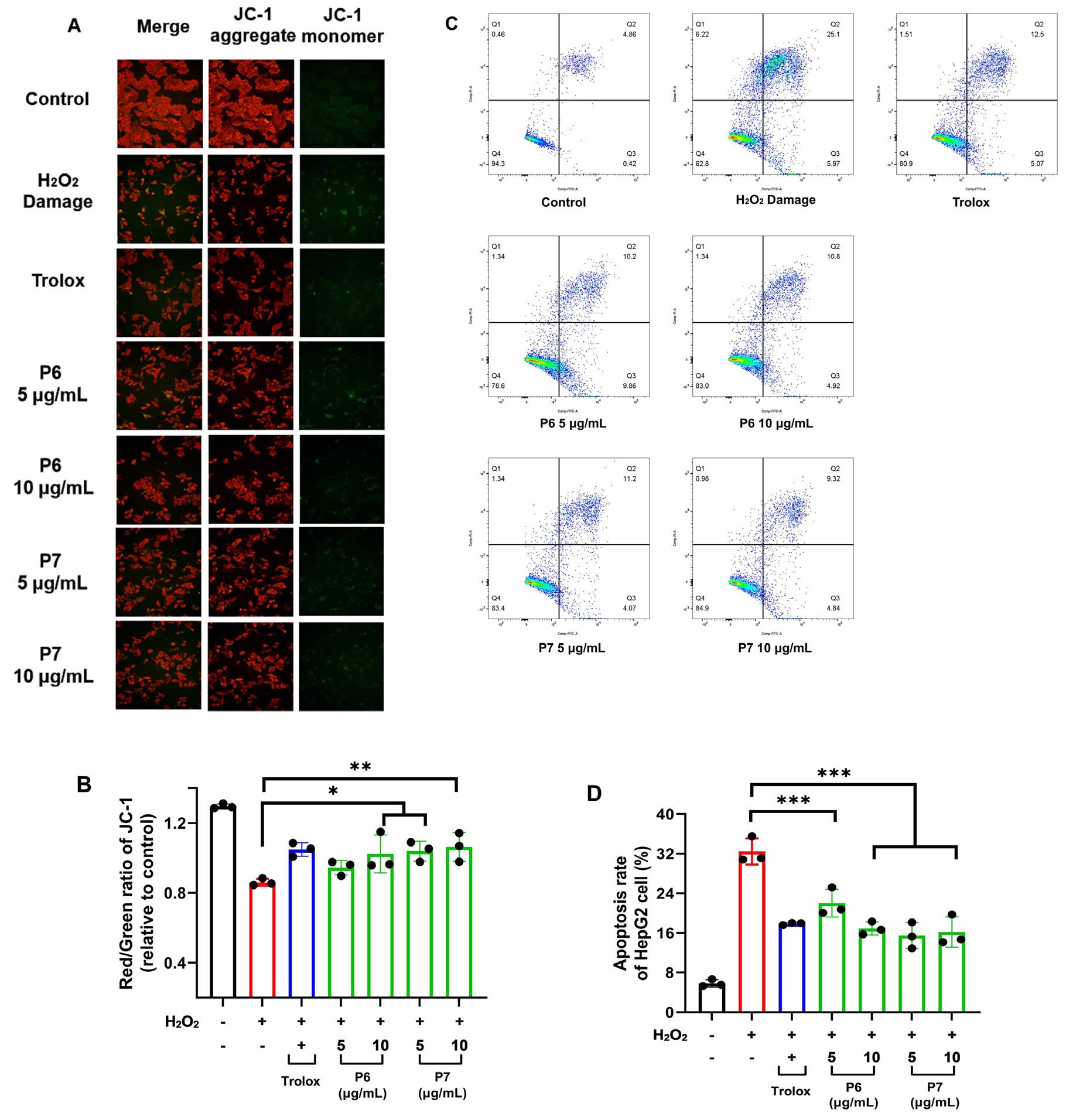
2.12. Hoechst 33258 and JC-1 Staining
2.13. Determination of Apoptosis Rate of HepG2 Cells
2.14. Statistical Analysis
3. Results
3.1. Antioxidant Capacity of MLP and MLPHs
3.2. Isolation and Characterization of BMPs from MLPHs
3.3. Virtual Screening of BMPs
3.4. Screening of BMPs with Antioxidant Activity by Cellular Assay
3.5. P6 and P7 Reduce H2O2-Induced ROS Accumulation and Oxidative Damage
3.6. Protective Effect of P6 and P7 against Chromatin Damage
3.7. Protective Effect of P6 and P7 against Cell Apoptosis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kim, G.N.; Jang, H.D. Protective mechanism of quercetin and rutin using glutathione metabolism on HO-induced oxidative stress in HepG2 cells. Ann. N. Y. Acad. Sci. 2009, 1171, 530–537. [Google Scholar] [CrossRef]
- Wang, L.; Ding, L.; Yu, Z.; Zhang, T.; Ma, S.; Liu, J. Intracellular ROS scavenging and antioxidant enzyme regulating capacities of corn gluten meal-derived antioxidant peptides in HepG2 cells. Food Res. Int. 2016, 90, 33–41. [Google Scholar] [CrossRef]
- Nwachukwu, I.D.; Aluko, R.E. Structural and functional properties of food protein-derived antioxidant peptides. J. Food Biochem. 2019, 43, e12761. [Google Scholar] [CrossRef]
- Ruan, J.; Chen, J.; Zeng, J.; Yang, Z.; Wang, C.; Hong, Z.; Zuo, Z. The protective effects of Nile tilapia (Oreochromis niloticus) scale collagen hydrolysate against oxidative stress induced by tributyltin in HepG2 cells. Environ. Sci. Pollut. Res. Int. 2019, 26, 3612–3620. [Google Scholar] [CrossRef]
- Hajam, Y.A.; Rani, R.; Ganie, S.Y.; Sheikh, T.A.; Javaid, D.; Qadri, S.S.; Pramodh, S.; Alsulimani, A.; Alkhanani, M.F.; Harakeh, S.; et al. Oxidative Stress in Human Pathology and Aging: Molecular Mechanisms and Perspectives. Cells 2022, 11, 552. [Google Scholar] [CrossRef] [PubMed]
- Jomova, K.; Raptova, R.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Reactive oxygen species, toxicity, oxidative stress, and antioxidants: Chronic diseases and aging. Arch. Toxicol. 2023, 97, 2499–2574. [Google Scholar]
- Alkadi, H. A Review on Free Radicals and Antioxidants. Infect. Disord. Drug Targets 2020, 20, 16–26. [Google Scholar]
- Liu, C.; Ren, D.; Li, J.; Fang, L.; Wang, J.; Liu, J.; Min, W. Cytoprotective effect and purification of novel antioxidant peptides from hazelnut (C. heterophylla Fisch) protein hydrolysates. J. Funct. Foods 2018, 42, 203–215. [Google Scholar] [CrossRef]
- Fan, W.; Duan, H.; Ren, X.; Guo, X.; Zhang, Y.; Li, J.; Zhang, F.; Chen, J.; Yang, X. Optimization of ultrasound-assisted cellulase degradation method on the extraction of mulberry leaf protein and its effect on the functional characteristics. Ultrason. Sonochem. 2023, 99, 106561. [Google Scholar] [CrossRef]
- Zhao, L.; Cheng, X.; Song, X.; Ouyang, D.; Wang, J.; Wu, Q.; Jia, J. Ultrasonic assisted extraction of mulberry leaf protein: Kinetic model, structural and functional properties, in vitro digestion. Process. Biochem. 2023, 128, 12–21. [Google Scholar] [CrossRef]
- Ma, G.; Chai, X.; Hou, G.; Zhao, F.; Meng, Q. Phytochemistry, bioactivities and future prospects of mulberry leaves: A review. Food Chem. 2022, 372, 131335. [Google Scholar] [CrossRef]
- Wen, P.; Hu, T.; Linhardt, R.J.; Liao, S.; Wu, H.; Zou, Y. Mulberry: A review of bioactive compounds and advanced processing technology. Trends Food Sci. Technol. 2019, 83, 138–158. [Google Scholar] [CrossRef]
- Kirac, F.T.; Sahingil, D.; Hayaloglu, A.A. Isolation and characterization of a new potential source of bioactive peptides: White mulberry (Morus alba) fruits and its leaves. Food Chem. Adv. 2024, 4, 100597. [Google Scholar] [CrossRef]
- Chen, Y.; Zheng, Z.; Ai, Z.; Zhang, Y.; Tan, C.P.; Liu, Y. Exploring the Antioxidant and Structural Properties of Black Bean Protein Hydrolysate and Its Peptide Fractions. Front. Nutr. 2022, 9, 884537. [Google Scholar] [CrossRef] [PubMed]
- Chalamaiah, M.; Yu, W.; Wu, J. Immunomodulatory and anticancer protein hydrolysates (peptides) from food proteins: A review. Food Chem. 2018, 245, 205–222. [Google Scholar] [CrossRef]
- Wu, Z.; Wang, H.; Fang, S.; Xu, C. Roles of endoplasmic reticulum stress and autophagy on H2O2-induced oxidative stress injury in HepG2 cells. Mol. Med. Rep. 2018, 18, 4163–4174. [Google Scholar] [CrossRef] [PubMed]
- Pachaiappan, R.; Tamboli, E.; Acharya, A.; Su, C.H.; Gopinath, S.; Chen, Y.; Velusamy, P. Separation and identification of bioactive peptides from stem of Tinospora cordifolia (Willd.) Miers. PLoS ONE 2018, 13, e0193717. [Google Scholar] [CrossRef] [PubMed]
- Xiao, F.; Xu, T.; Lu, B.; Liu, R. Guidelines for antioxidant assays for food components. Food Front. 2020, 1, 60–69. [Google Scholar] [CrossRef]
- Zhuang, Y.; Ma, Q.; Guo, Y.; Sun, L. Protective effects of rambutan (Nephelium lappaceum) peel phenolics on H2O2-induced oxidative damages in HepG2 cells and d-galactose-induced aging mice. Food Chem. Toxicol. 2017, 108, 554–562. [Google Scholar] [CrossRef]
- Sarkar, J.; Das, M.; Howlader, M.; Prateeksha, P.; Barthels, D.; Das, H. Epigallocatechin-3-gallate inhibits osteoclastic differentiation by modulating mitophagy and mitochondrial functions. Cell Death Dis. 2022, 13, 908. [Google Scholar] [CrossRef]
- Hong, S.; Lin, Y.; Dia, V.P. Anti-inflammatory and antioxidant properties of hempseed protein enzymatic hydrolysates. Food Hydrocoll. Health 2022, 2, 100082. [Google Scholar] [CrossRef]
- Yin, H.; Zhang, S.; Yue, H.; Wang, M.; Zeng, J.; Wu, W.; Wang, J.; Zheng, H.; Xue, C.; Zhao, Y. Isolation, identification and in silico analysis of two novel cytoprotective peptides from tilapia skin against oxidative stress-induced ovarian granulosa cell damage. J. Funct. Foods 2023, 107, 105629. [Google Scholar] [CrossRef]
- Zhang, Q.; Tong, X.; Sui, X.; Wang, Z.; Qi, B.; Li, Y.; Jiang, L. Antioxidant activity and protective effects of Alcalase-hydrolyzed soybean hydrolysate in human intestinal epithelial Caco-2 cells. Food Res. Int. 2018, 111, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.M.; Wang, Y.M.; Zhao, Y.Q.; Chi, C.F.; Wang, B. Antioxidant Peptides from the Protein Hydrolysate of Monkfish (Lophius litulon) Muscle: Purification, Identification, and Cytoprotective Function on HepG2 Cells Damage by H(2)O(2). Mar. Drugs 2020, 18, 153. [Google Scholar] [CrossRef] [PubMed]
- Wong, F.; Xiao, J.; Wang, S.; Ee, K.; Chai, T. Advances on the antioxidant peptides from edible plant sources. Trends Food Sci. Technol. 2020, 99, 44–57. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, T.; Liang, X.; Yuan, Q.; Zeng, X.; Wu, Z.; Pan, D.; Tao, M.; Guo, Y. Production and transepithelial transportation of casein-derived peptides and identification a novel antioxidant peptide LHSMK. Food Sci. Technol. 2021, 151, 112194. [Google Scholar] [CrossRef]
- Song, R.; Wei, R.; Ruan, G.; Luo, H. Isolation and identification of antioxidative peptides from peptic hydrolysates of half-fin anchovy (Setipinna taty). Food Sci. Technol. 2015, 60, 221–229. [Google Scholar] [CrossRef]
- Guo, H.; Hao, Y.; Richel, A.; Everaert, N.; Chen, Y.; Liu, M.; Yang, X.; Ren, G. Antihypertensive effect of quinoa protein under simulated gastrointestinal digestion and peptide characterization. J. Sci. Food Agric. 2020, 100, 5569–5576. [Google Scholar] [CrossRef]
- Montone, C.M.; Capriotti, A.L.; Cavaliere, C.; La Barbera, G.; Piovesana, S.; Zenezini, C.R.; Lagana, A. Peptidomic strategy for purification and identification of potential ACE-inhibitory and antioxidant peptides in Tetradesmus obliquus microalgae. Anal. Bioanal. Chem. 2018, 410, 3573–3586. [Google Scholar] [CrossRef]



| Peptide | Score | Bioactivity | Solubility | Toxicity | Length | m/z | Z | RT | Mass | Ppm |
|---|---|---|---|---|---|---|---|---|---|---|
| P1 | 97 | 0.861147 | good | Non-Toxin | 11 | 507.7345 | 2 | 25.69 | 1013.445 | 8.9 |
| P2 | 95 | 0.860728 | good | Non-Toxin | 9 | 390.2128 | 2 | 6.95 | 778.4085 | 3.3 |
| P3 | 99 | 0.833139 | good | Non-Toxin | 9 | 434.7379 | 2 | 10.68 | 867.4562 | 5.7 |
| P4 | 99 | 0.819956 | good | Non-Toxin | 9 | 418.7236 | 2 | 7.52 | 835.43 | 3.2 |
| P5 | 95 | 0.803338 | good | Non-Toxin | 7 | 321.1724 | 2 | 5.85 | 640.3292 | 1.5 |
| P6 | 99 | 0.795404 | good | Non-Toxin | 11 | 448.2379 | 2 | 7.09 | 894.4559 | 6 |
| P7 | 95 | 0.744693 | good | Non-Toxin | 19 | 845.8934 | 2 | 14.89 | 1689.771 | 1.1 |
| P8 | 97 | 0.725901 | good | Non-Toxin | 13 | 588.8252 | 2 | 14.9 | 1175.641 | −4.5 |
| Peptide | Sequence |
|---|---|
| P1 | GPAGPAGPR |
| P2 | GSPPGEGPAGF |
| P3 | GPSGPQGLR |
| P4 | SGPAGPR |
| P5 | GPAGPSGAPGK |
| P6 | EGDAGAQGPPGPAGPAGER |
| P7 | RPGPSPGVGAPGK |
| P8 | GPAGPQGPR |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Y.; Zhang, R.; Wang, J.; Tong, Y.; Zhang, J.; Li, Z.; Zhang, H.; Abbas, Z.; Si, D.; Wei, X. Isolation, Characterization, and Functional Properties of Antioxidant Peptides from Mulberry Leaf Enzymatic Hydrolysates. Antioxidants 2024, 13, 854. https://doi.org/10.3390/antiox13070854
Zhou Y, Zhang R, Wang J, Tong Y, Zhang J, Li Z, Zhang H, Abbas Z, Si D, Wei X. Isolation, Characterization, and Functional Properties of Antioxidant Peptides from Mulberry Leaf Enzymatic Hydrolysates. Antioxidants. 2024; 13(7):854. https://doi.org/10.3390/antiox13070854
Chicago/Turabian StyleZhou, Yichen, Rijun Zhang, Junyong Wang, Yucui Tong, Jing Zhang, Zhenzhen Li, Haosen Zhang, Zaheer Abbas, Dayong Si, and Xubiao Wei. 2024. "Isolation, Characterization, and Functional Properties of Antioxidant Peptides from Mulberry Leaf Enzymatic Hydrolysates" Antioxidants 13, no. 7: 854. https://doi.org/10.3390/antiox13070854
APA StyleZhou, Y., Zhang, R., Wang, J., Tong, Y., Zhang, J., Li, Z., Zhang, H., Abbas, Z., Si, D., & Wei, X. (2024). Isolation, Characterization, and Functional Properties of Antioxidant Peptides from Mulberry Leaf Enzymatic Hydrolysates. Antioxidants, 13(7), 854. https://doi.org/10.3390/antiox13070854







