The Combination of Molecular Hydrogen and Heme Oxygenase 1 Effectively Inhibits Neuropathy Caused by Paclitaxel in Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. PTX-Derived Neuropathy
2.3. Behavioral Tests
2.4. Western Blot Analysis
2.5. Analysis of mRNA Levels by Quantitative Real-Time PCR (qRT-PCR)
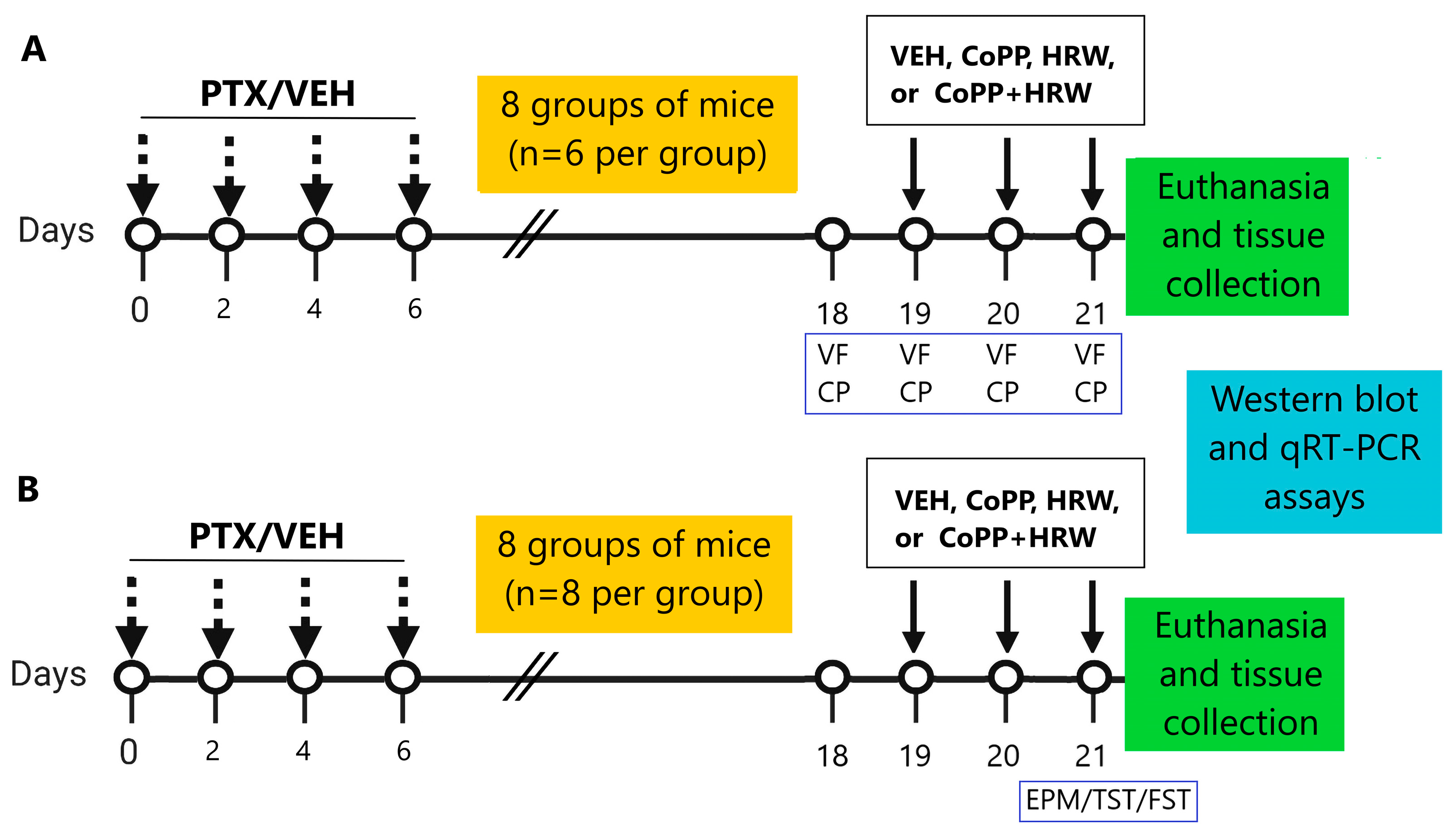
2.6. Experimental Procedures
2.7. Drugs
2.8. Statistical Analyses
3. Results
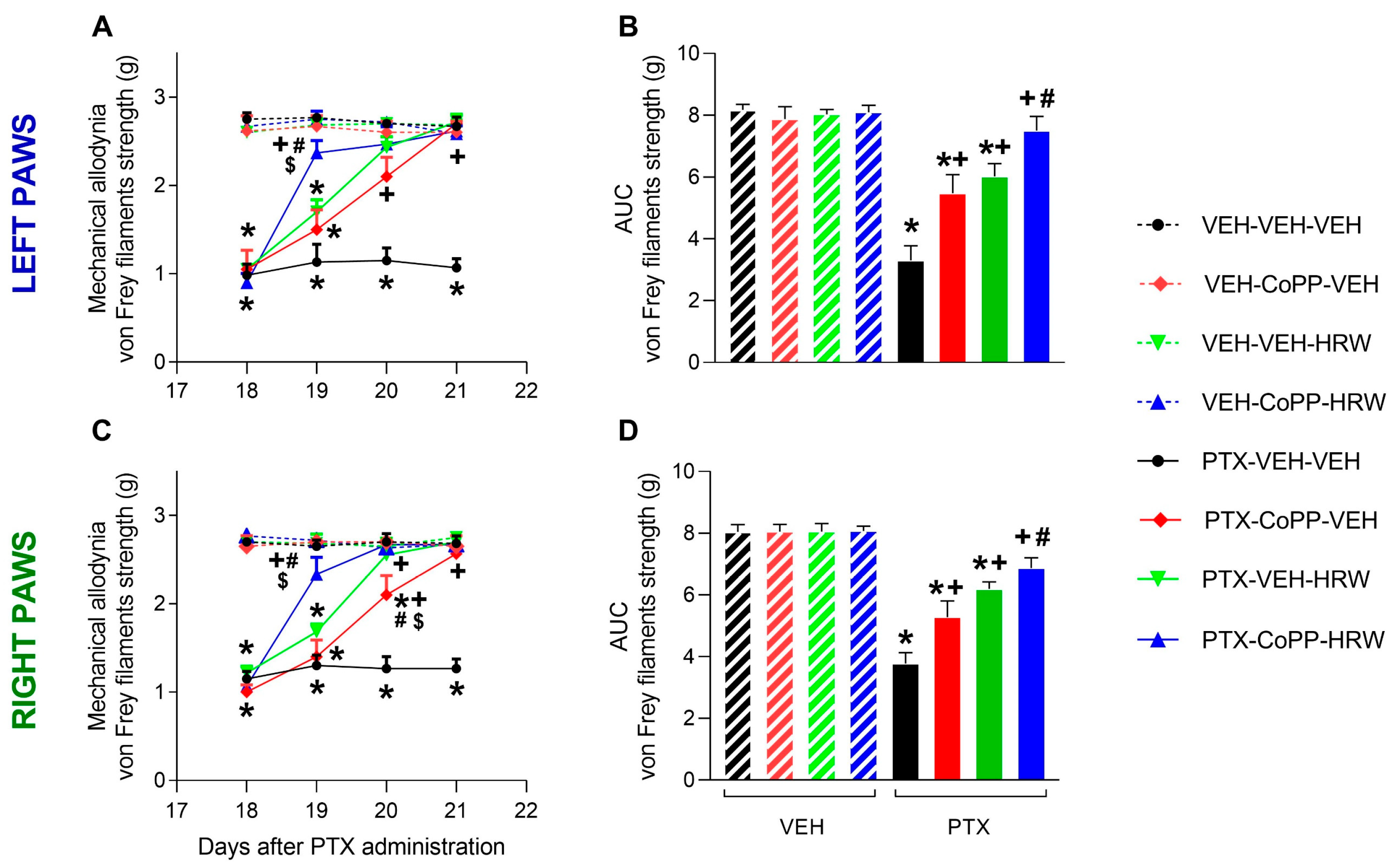
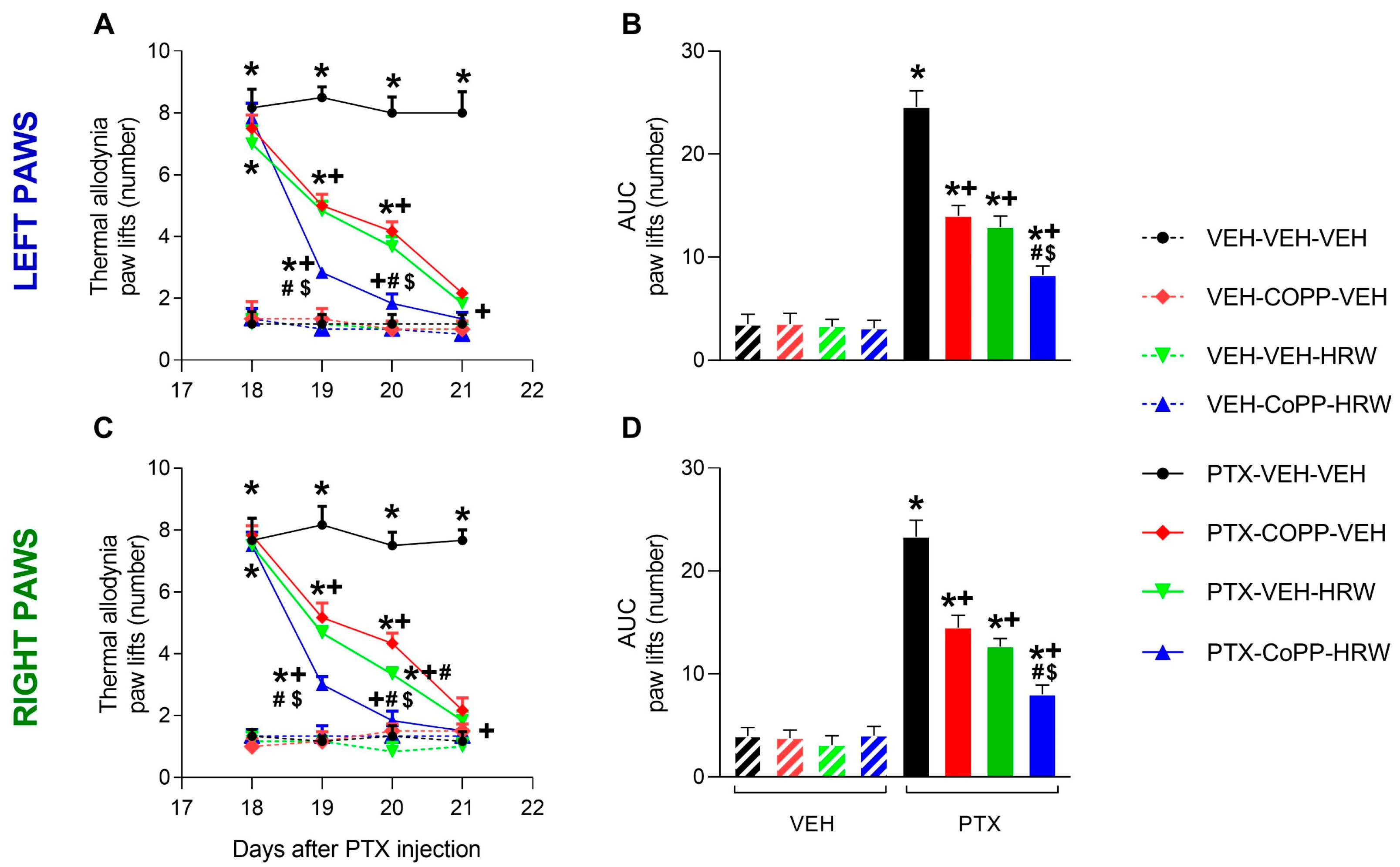
3.1. The Co-Treatment of CoPP with HRW Enhanced the Antiallodynic Effects Produced by Each Treatment Separately
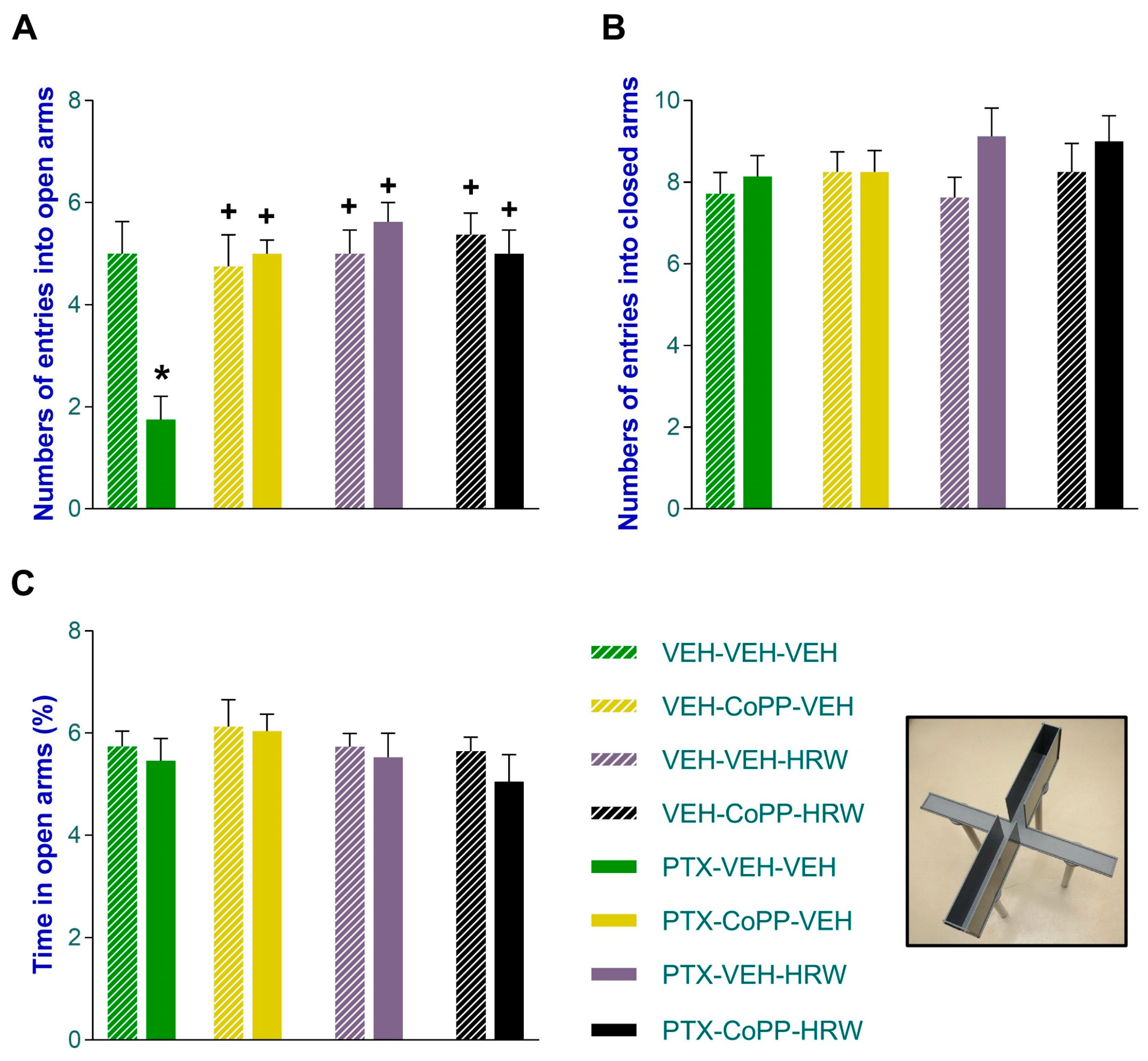
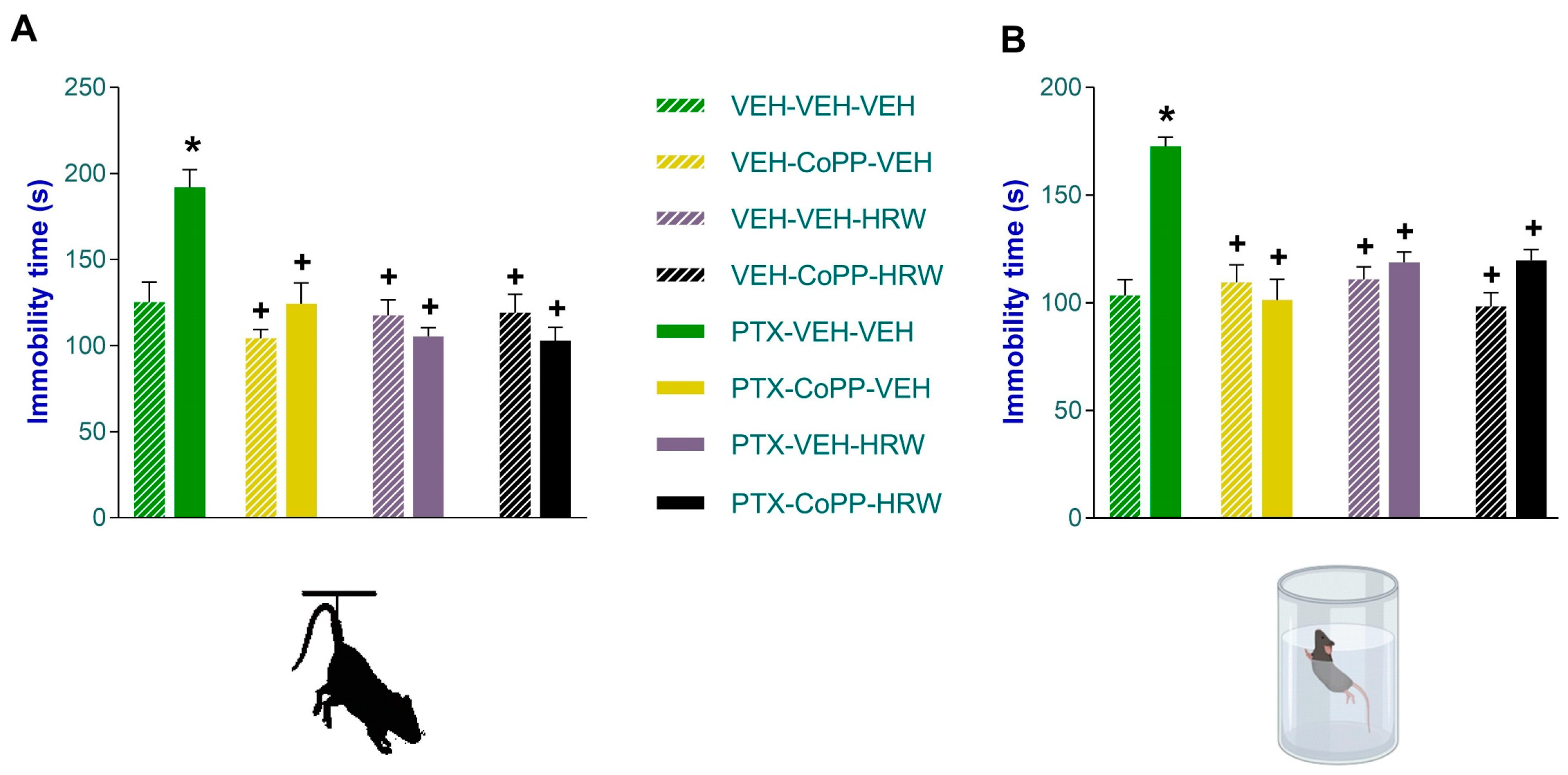
3.2. The Concurrent Administration of CoPP and HRW Normalized the Anxious- and Depressant-like Comportments Linked to PIPN
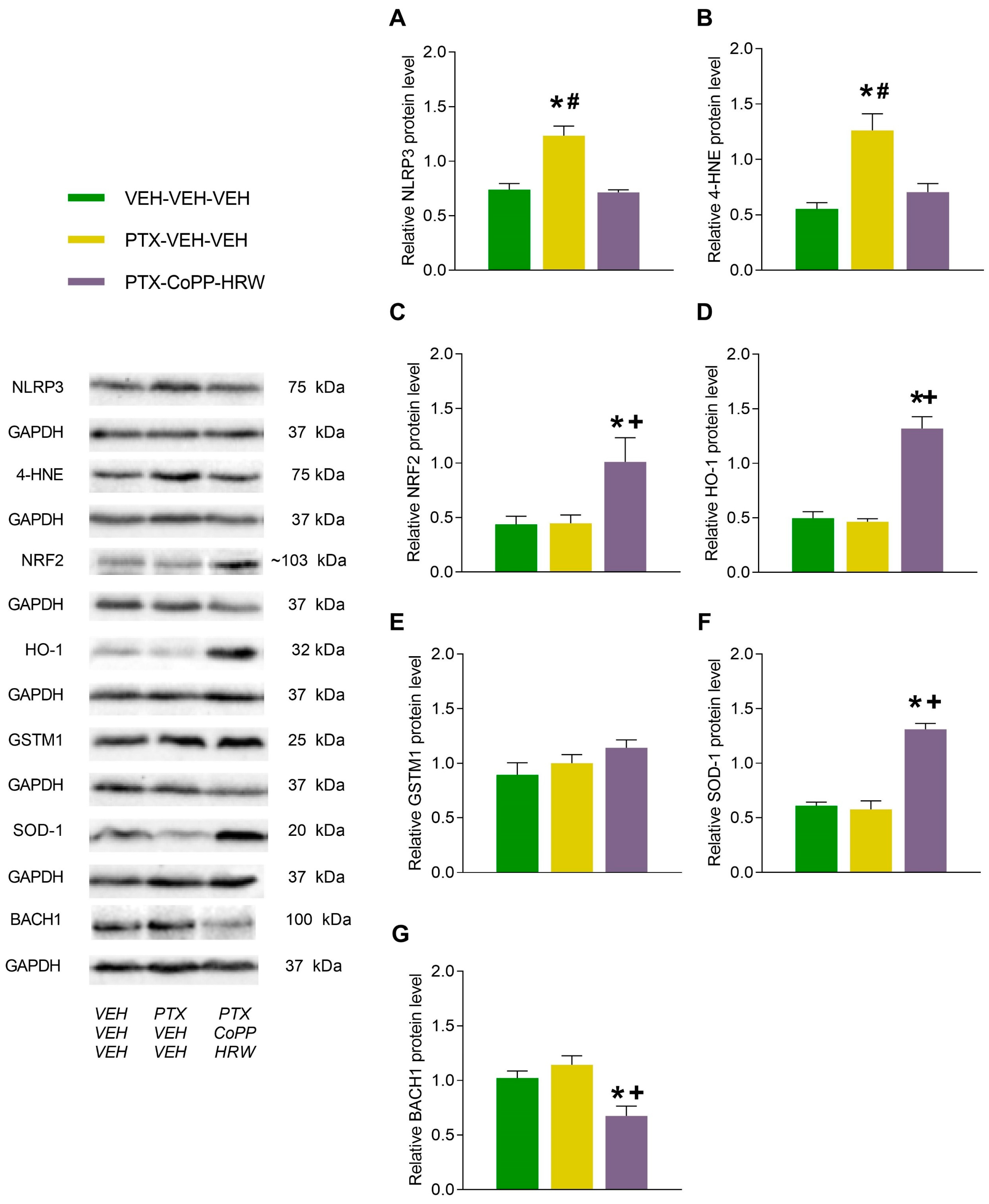
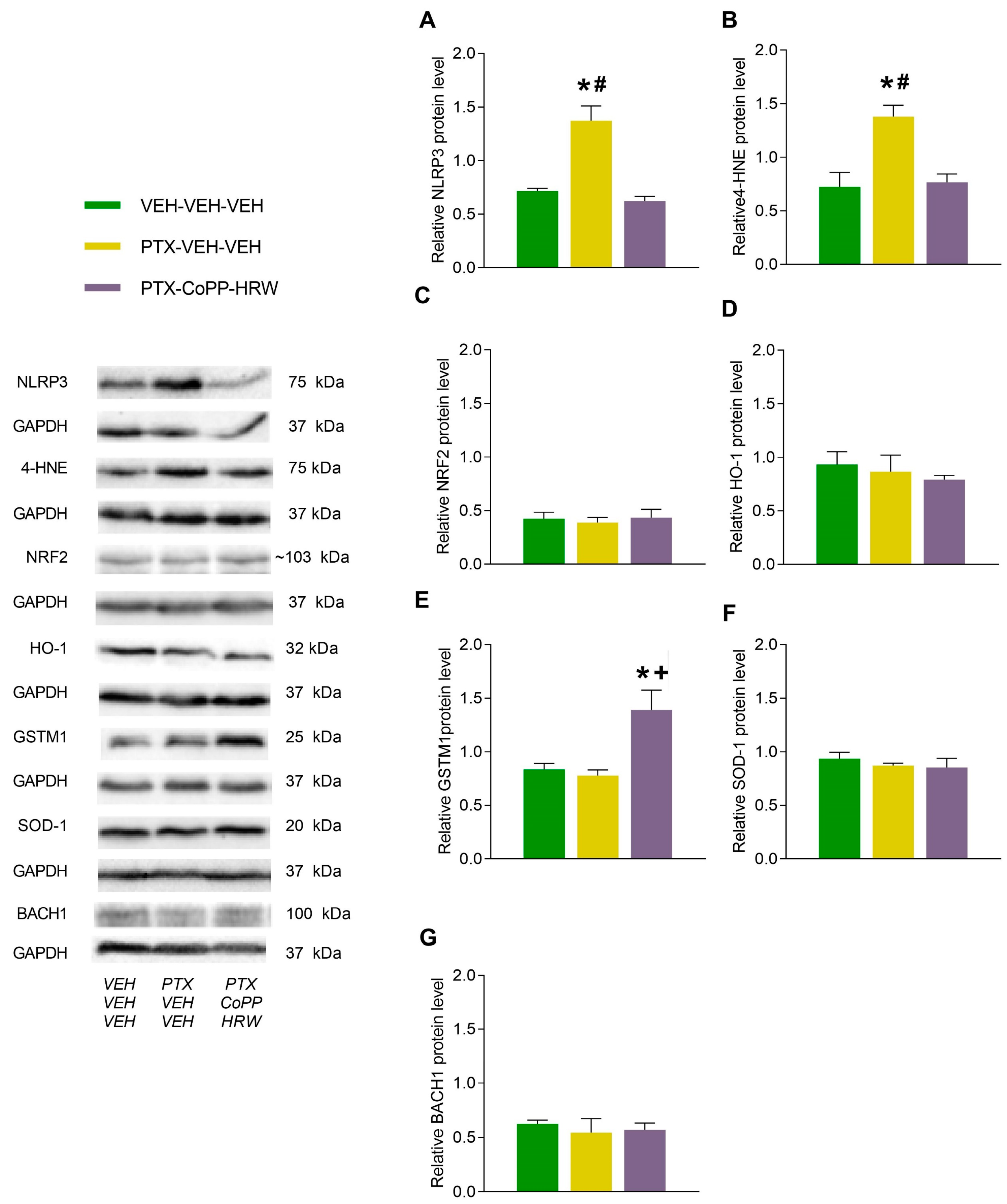
3.3. The Effects of Combining CoPP with HRW on the Levels of NLRP3, 4-HNE, NRF2, HO-1, GSTM1, SOD-1, and BACH1 in the DRG and AMG of PTX-Injected Mice
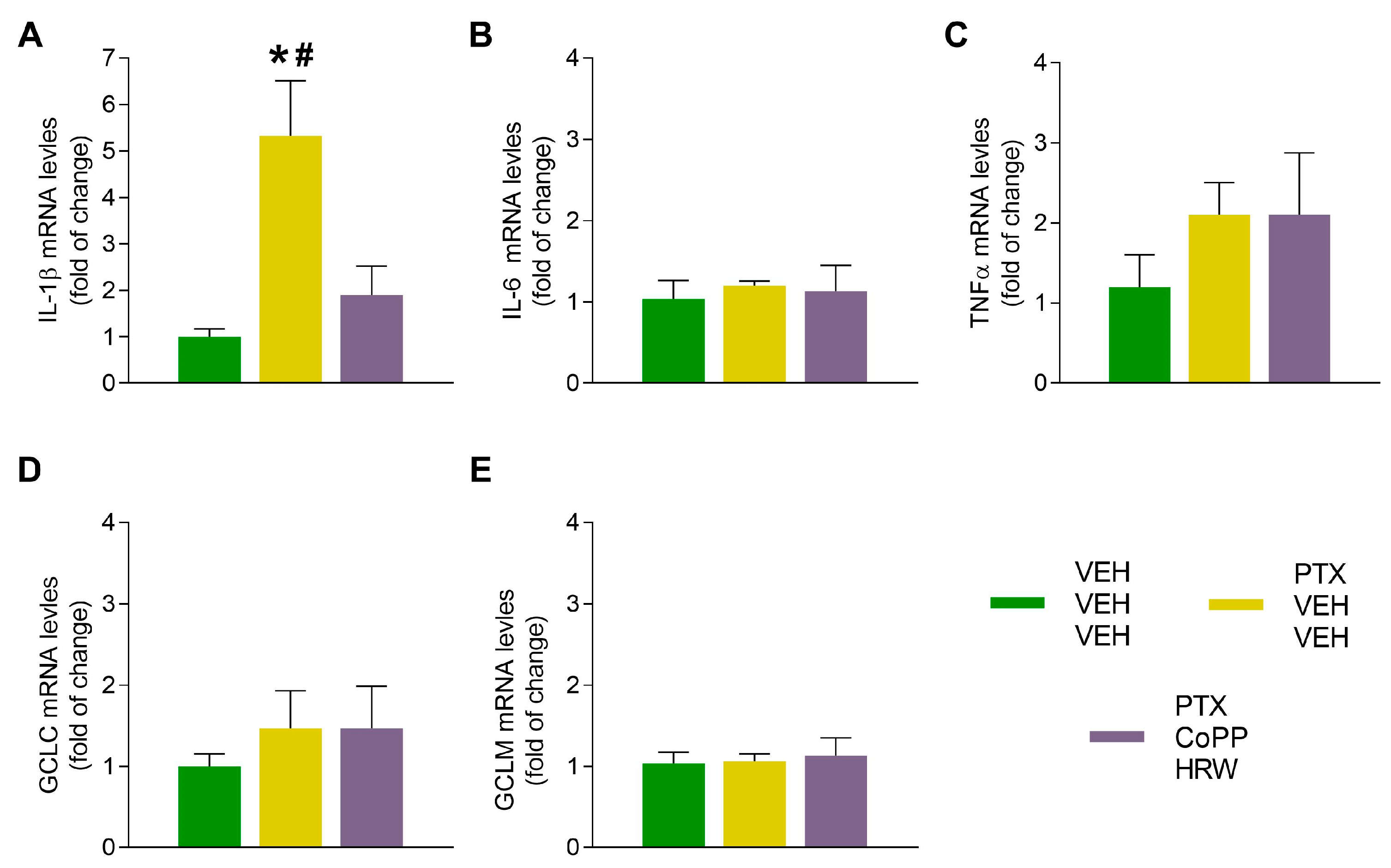
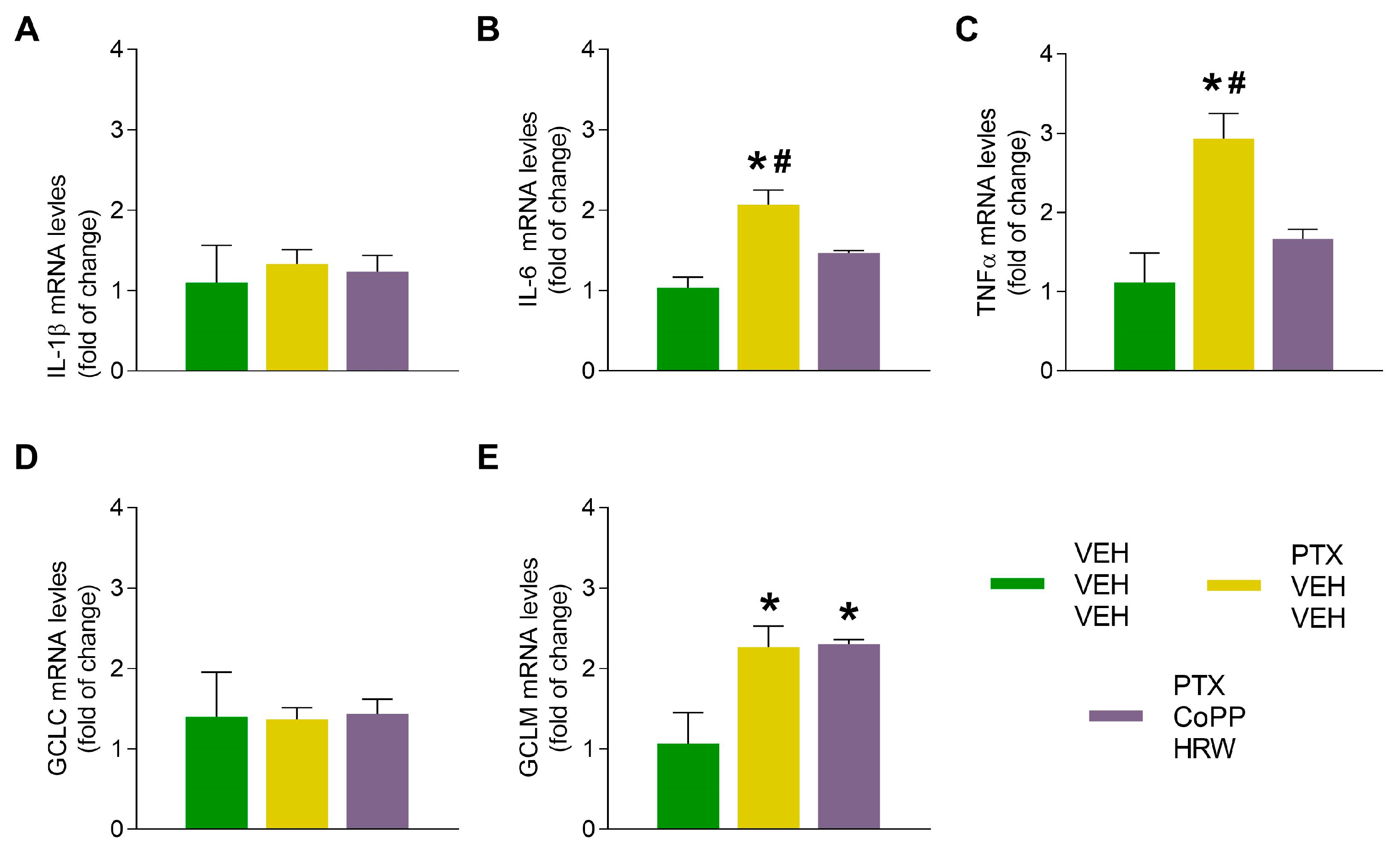
3.4. The Effects of CoPP Combined with HRW on the mRNA Levels of IL-1β, IL-6, TNFα, CGLC, and GCLM in the DRG and AMG of PTX-Injected Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Flatters, S.J.L.; Dougherty, P.M.; Colvin, L.A. Clinical and preclinical perspectives on Chemotherapy-Induced Peripheral Neuropathy (CIPN): A narrative review. Br. J. Anaesth. 2017, 119, 737–749. [Google Scholar] [CrossRef] [PubMed]
- Stage, T.B.; Bergmann, T.K.; Kroetz, D.L. Clinical Pharmacokinetics of Paclitaxel Monotherapy: An Updated Literature Review. Clin. Pharmacokinet. 2018, 57, 7–19. [Google Scholar] [CrossRef] [PubMed]
- Engvall, K.; Gréen, H.; Fredrikson, M.; Lagerlund, M.; Lewin, F.; Åvall-Lundqvist, E. Impact of persistent peripheral neuropathy on health-related quality of life among early-stage breast cancer survivors: A population-based cross-sectional study. Breast Cancer Res. Treat. 2022, 195, 379–391. [Google Scholar] [CrossRef] [PubMed]
- Seretny, M.; Currie, G.L.; Sena, E.S.; Ramnarine, S.; Grant, R.; MacLeod, M.R.; Colvin, L.A.; Fallon, M. Incidence, prevalence, and predictors of chemotherapy-induced peripheral neuropathy: A systematic review and meta-analysis. Pain 2014, 155, 2461–2470. [Google Scholar] [CrossRef] [PubMed]
- Banach, M.; Juranek, J.K.; Zygulska, A.L. Chemotherapy-induced neuropathies—A growing problem for patients and health care providers. Brain Behav. 2016, 7, e00558. [Google Scholar] [CrossRef] [PubMed]
- Staff, N.P.; Grisold, A.; Grisold, W.; Windebank, A.J. Chemotherapy-induced peripheral neuropathy: A current review. Ann. Neurol. 2017, 81, 772–781. [Google Scholar] [CrossRef] [PubMed]
- Gewandter, J.S.; Kleckner, A.S.; Marshall, J.H.; Brown, J.S.; Curtis, L.H.; Bautista, J.; Dworkin, R.H.; Kleckner, I.R.; Kolb, N.; Mohile, S.G.; et al. Chemotherapy-induced peripheral neuropathy (CIPN) and its treatment: An NIH Collaboratory study of claims data. Support. Care Cancer 2020, 28, 2553–2562. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Yu, Y.; Li, B.; Gu, X.; Xie, K.; Wang, G.; Yu, Y. Protective effects of hydrogen-rich saline against experimental diabetic peripheral neuropathy via activation of the mitochondrial ATP-sensitive potassium channel channels in rats. Mol. Med. Rep. 2020, 21, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Choi, J.I. Hydrogen-Rich Water Improves Cognitive Ability and Induces Antioxidative, Antiapoptotic, and Anti-Inflammatory Effects in an Acute Ischemia-Reperfusion Injury Mouse Model. BioMed Res. Int. 2021, 2021, 9956938. [Google Scholar] [CrossRef] [PubMed]
- Baskin, V.; Eroglu, E.; Harmanci, N.; Erol, K. Antinociceptive, anxiolytic, and depression-like effects of hydrogen sulfide, nitric oxide, and carbon monoxide in rats and the role of opioidergic and serotonergic systems in antinociceptive activity. Fundam. Clin. Pharmacol. 2022, 36, 674–686. [Google Scholar] [CrossRef]
- Lin, Y.T.; Shi, Q.Q.; Zhang, L.; Yue, C.P.; He, Z.J.; Li, X.X.; He, Q.J.; Liu, Q.; Du, X.B. Hydrogen-rich water ameliorates neuropathological impairments in a mouse model of Alzheimer’s disease through reducing neuroinflammation and modulating intestinal microbiota. Neural ReGrn Res. 2022, 17, 409–417. [Google Scholar]
- Abraham, N.G.; Kappas, A. Pharmacological and clinical aspects of heme oxygenase. Pharmacol. Rev. 2008, 60, 79–127. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Zhang, Z.J.; Zhu, M.D.; Jiang, B.C.; Yang, T.; Gao, Y.J. Exogenous induction of HO-1 alleviates vincristine-induced neuropathic pain by reducing spinal glial activation in mice. Neurobiol. Dis. 2015, 79, 100–110. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.Q.; Liu, D.Q.; Chen, S.P.; Chenm, N.; Sun, J.; Wang, X.M.; Cao, F.; Tian, Y.K.; Ye, D.W. Nrf2 activation ameliorates mechanical allodynia in paclitaxel-induced neuropathic pain. Acta Pharmacol. Sin. 2020, 41, 1041–1048. [Google Scholar] [CrossRef] [PubMed]
- Cazuza, R.A.; Pol, O.; Leite-Panissi, C.R.A. Enhanced expression of heme oxygenase-1 in the locus coeruleus can be associated with anxiolytic-like effects. Behav. Brain Res. 2018, 336, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Ullah, R.; Wang, J.; Du, Y.; Huang, S.; Meng, L.; Gao, Y.; Gong, M.; Galaj, E.; Yin, X.; et al. Exogenous carbon monoxide produces rapid antidepressant- and anxiolytic-like effects. Front. Pharmacol. 2021, 12, 757417. [Google Scholar] [CrossRef]
- Ge, Y.; Wu, F.; Sun, X.; Xiang, Z.; Yang, L.; Huang, S.; Lu, Z.; Sun, Y.; Yu, W.F. Intrathecal infusion of hydrogen-rich normal saline attenuates neuropathic pain via inhibition of activation of spinal astrocytes and microglia in rats. PLoS ONE 2014, 9, e97436. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, M.; Satoh, Y.; Otsubo, Y.; Kazama, T. Molecular hydrogen attenuates neuropathic pain in mice. PLoS ONE 2014, 9, e100352. [Google Scholar] [CrossRef]
- Wang, H.; Huo, X.; Chen, H.; Li, B.; Liu, J.; Ma, W.; Wang, X.; Xie, K.; Yu, Y.; Shi, K. Hydrogen-Rich Saline Activated Autophagy via HIF-1 α Pathways in Neuropathic Pain Model. BioMed Res. Int. 2018, 2018, 4670834. [Google Scholar] [CrossRef]
- Zhang, Y.; Su, W.J.; Chen, Y.; Wu, T.Y.; Gong, H.; Shen, X.L.; Wang, Y.X.; Sun, X.J.; Jiang, C.L. Effects of hydrogen-rich water on depressive-like behavior in mice. Sci. Rep. 2016, 6, 23742. [Google Scholar] [CrossRef]
- Masuda, K.; Tanaka, Y.; Kanehisa, M.; Ninomiya, T.; Inoue, A.; Higuma, H.; Kawashima, C.; Nakanishi, M.; Okamoto, K.; Akiyoshi, J. Natural reduced water suppressed anxiety and protected the heightened oxidative stress in rats. Neuropsychiatr. Dis. Treat. 2017, 13, 2357–2362. [Google Scholar] [CrossRef] [PubMed]
- Roch, G.; Batallé, G.; Bai, X.; Pouso-Vázquez, E.; Rodríguez, L.; Pol, O. The Beneficial Effects of Heme Oxygenase 1 and Hydrogen Sulfide Activation in the Management of Neuropathic Pain, Anxiety- and Depressive-like Effects of Paclitaxel in Mice. Antioxidants 2022, 11, 122. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Martel, I.; Bai, X.; Batallé, G.; Pol, O. New Treatment for the Cognitive and Emotional Deficits Linked with Paclitaxel-Induced Peripheral Neuropathy in Mice. Antioxidants 2022, 11, 2387. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Chen, P.; Zhou, S.; Yan, X.; Zhang, J.; Sun, X.; Yuan, H.; Yu, W. Hydrogen-rich saline attenuated neuropathic pain by reducing oxidative stress. Can. J. Neurol. Sci. 2013, 40, 857–863. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, H.; Xie, K.; Liu, L.; Li, Y.; Yu, Y.; Wang, G. H2 Treatment Attenuated Pain Behavior and Cytokine Release Through the HO-1/CO Pathway in a Rat Model of Neuropathic Pain. Inflammation 2015, 38, 1835–1846. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Serrat, M.; Martínez-Martel, I.; Coral-Pérez, S.; Bai, X.; Batallé, G.; Pol, O. Hydrogen-Rich Water as a Novel Therapeutic Strategy for the Affective Disorders Linked with Chronic Neuropathic Pain in Mice. Antioxidants 2022, 11, 1826. [Google Scholar] [CrossRef] [PubMed]
- Jia, M.; Wu, C.; Gao, F.; Xiang, H.; Sun, N.; Peng, P.; Li, J.; Yuan, X.; Li, H.; Meng, X.; et al. Activation of NLRP3 inflammasome in peripheral nerve contributes to paclitaxel-induced neuropathic pain. Mol. Pain 2017, 13, 1744806917719804. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Y.; Meng, X.; Ye, T.; Xie, W.; Sun, G.; Sun, X. Inhibiting the NLRP3 Inflammasome Activation with MCC950 Ameliorates Diabetic Encephalopathy in db/db Mice. Molecules 2018, 23, 522. [Google Scholar] [CrossRef]
- Arioz, B.I.; Tastan, B.; Tarakcioglu, E.; Tufekci, K.U.; Olcum, M.; Ersoy, N.; Bagriyanik, A.; Genc, K.; Genc, S. Melatonin Attenuates LPS-Induced Acute Depressive-like Behaviors and Microglial NLRP3 Inflammasome Activation Through the SIRT1/Nrf2 Pathway. Front. Immunol. 2019, 10, 1511. [Google Scholar] [CrossRef]
- Starobova, H.; Vetter, I. Pathophysiology of chemotherapy-induced peripheral neuropathy. Front. Mol. Neurosci. 2017, 10, 174. [Google Scholar] [CrossRef]
- Neugebauer, V.; Mazzitelli, M.; Cragg, B.; Ji, G.; Navratilova, E.; Porreca, F. Amygdala, neuropeptides, and chronic pain-related affective behaviors. Neuropharmacology 2020, 170, 108052. [Google Scholar] [CrossRef]
- Sałat, K.; Cios, A.; Wyska, E.; Sałat, R.; Mogilski, S.; Filipek, B.; Więckowski, K.; Malawska, B. Antiallodynic and antihyperalgesic activity of 3-[4-(3-trifluoromethyl-phenyl)-piperazin-1-yl]-dihydrofuran-2-one compared to pregabalin in chemotherapy-induced neuropathic pain in mice. Pharmacol. Biochem. Behav. 2014, 122, 173–181. [Google Scholar] [CrossRef]
- Gao, W.; Zan, Y.; Wang, Z.J.; Hu, X.Y.; Huang, F. Quercetin ameliorates paclitaxel-induced neuropathic pain by stabilizing mast cells, and subsequently blocking PKCε-dependent activation of TRPV1. Acta Pharmacol. Sin. 2016, 37, 1166–1177. [Google Scholar] [CrossRef]
- Singh, J.; Saha, L.; Singh, N.; Kumari, P.; Bhatia, A.; Chakrabarti, A. Study of nuclear factor-2 erythroid related factor-2 activator, berberine, in paclitaxel induced peripheral neuropathy pain model in rats. J. Pharm. Pharmacol. 2019, 71, 797–805. [Google Scholar] [CrossRef]
- Carvalho, L.F.; Silva, A.M.F.; Carvalho, A.A. The use of antioxidant agents for chemotherapy-induced peripheral neuropathy treatment in animal models. Clin. Exp. Pharmacol. Physiol. 2017, 44, 971–979. [Google Scholar] [CrossRef]
- Zhou, Y.Q.; Liu, D.Q.; Chen, S.P.; Chen, N.; Sun, J.; Wang, X.M.; Li, D.Y.; Tian, Y.K.; Ye, D.W. PPARγ activation mitigates mechanical allodynia in paclitaxel-induced neuropathic pain via induction of Nrf2/HO-1 signaling pathway. Biomed. Pharmacother. 2020, 129, 110356. [Google Scholar] [CrossRef]
- Su, C.J.; Zhang, J.T.; Zhao, F.L.; Xu, D.L.; Pan, J.; Liu, T. Resolvin D1/N-formyl peptide receptor 2 ameliorates paclitaxel-induced neuropathic pain through the activation of IL-10/Nrf2/HO-1 pathway in mice. Front. Immunol. 2023, 14, 1091753. [Google Scholar] [CrossRef]
- Suárez-Rojas, I.; Pérez-Fernández, M.; Bai, X.; Martínez-Martel, I.; Intagliata, S.; Pittalà, V.; Salerno, L.; Pol, O. The Inhibition of Neuropathic Pain Incited by Nerve Injury and Accompanying Mood Disorders by New Heme Oxygenase-1 Inducers: Mechanisms Implicated. Antioxidants 2023, 12, 1859. [Google Scholar] [CrossRef]
- Toma, W.; Kyte, S.L.; Bagdas, D.; Alkhlaif, Y.; Alsharari, S.D.; Lichtman, A.H.; Chen, Z.J.; Del Fabbro, E.; Bigbee, J.W.; Gewirtz, D.A.; et al. Effects of paclitaxel on the development of neuropathy and affective behaviors in the mouse. Neuropharmacology 2017, 117, 305–315. [Google Scholar] [CrossRef]
- Chaplan, S.R.; Bach, F.W.; Pogrel, J.W.; Chung, J.M.; Yaksh, T.L. Quantitative assessment of tactile allodynia in the rat paw. J. Neurosci. Methods 1994, 53, 55–63. [Google Scholar] [CrossRef]
- Walf, A.A.; Frye, C.A. The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nat. Protoc. 2007, 2, 322–328. [Google Scholar] [CrossRef]
- Steru, L.; Chermat, R.; Thierry, B.; Simon, P. The tail suspension test: A new method for screening antidepressants in mice. Psychopharmacology 1985, 85, 367–370. [Google Scholar] [CrossRef]
- Porsolt, R.D.; Le Pichon, M.; Jalfre, M. Depression: A new animal model sensitive to antidepressant treatments. Nature 1977, 266, 730–732. [Google Scholar] [CrossRef]
- Manavi, M.A.; Fathian Nasab, M.H.; Mohammad Jafari, R.; Dehpour, A.R. Mechanisms underlying dose-limiting toxicities of conventional chemotherapeutic agents. J. Chemother. 2023. [Google Scholar] [CrossRef]
- Flatters, S.J.; Bennett, G.J. Ethosuximide reverses paclitaxel- and vincristine-induced painful peripheral neuropathy. Pain 2004, 109, 150–161. [Google Scholar] [CrossRef]
- Ward, S.J.; McAllister, S.D.; Kawamura, R.; Murase, R.; Neelakantan, H.; Walker, E.A. Cannabidiol inhibits paclitaxel-induced neuropathic pain through 5-HT(1A) receptors without diminishing nervous system function or chemotherapy efficacy. Br. J. Pharmacol. 2014, 171, 636–645. [Google Scholar] [CrossRef]
- Brandolini, L.; Benedetti, E.; Ruffini, P.A.; Russo, R.; Cristiano, L.; Antonosante, A.; d’Angelo, M.; Castelli, V.; Giordano, A.; Allegretti, M.; et al. CXCR1/2 pathways in paclitaxel-induced neuropathic pain. Oncotarget 2017, 8, 23188–23201. [Google Scholar]
- Galley, H.F.; McCormick, B.; Wilson, K.L.; Lowes, D.A.; Colvin, L.; Torsney, C. Melatonin limits paclitaxel-induced mitochondrial dysfunction in vitro and protects against paclitaxel-induced neuropathic pain in the rat. J. Pineal Res. 2017, 63, e12444. [Google Scholar] [CrossRef]
- Miao, H.; Li, R.; Chen, D.; Hu, J.; Chen, Y.; Xu, C.; Wen, Z. Pro-tective Effects of Vitamin E on Chemotherapy-Induced Peripheral Neuropathy: A Me-ta-Analysis of Randomized Controlled Trials. Ann. Nutr. Metab. 2021, 77, 127–137. [Google Scholar] [CrossRef]
- Uher, T.; Bob, P. Neuropathic pain, depressive symptoms, and C-reactive protein in sciatica patients. Int. J. Neurosci. 2013, 123, 204–208. [Google Scholar] [CrossRef]
- Fidanboylu, M.; Griffiths, L.A.; Flatters, S.J. Global inhibition of reactive oxygen species (ROS) inhibits paclitaxel-induced painful peripheral neuropathy. PLoS ONE 2011, 6, e25212. [Google Scholar] [CrossRef]
- Coral-Pérez, S.; Martínez-Martel, I.; Martínez-Serrat, M.; Batallé, G.; Bai, X.; Leite-Panissi, C.R.A.; Pol, O. Treatment with Hydrogen-Rich Water Improves the Nociceptive and Anxio-Depressive-like Behaviors Associated with Chronic Inflammatory Pain in Mice. Antioxidants 2022, 11, 2153. [Google Scholar] [CrossRef]
- Ramos-Hryb, A.B.; Pazini, F.L.; Costa, A.P.; Cunha, M.P.; Kaster, M.P.; Rodrigues, A.L.S. Role of heme oxygenase-1 in the antidepressant-like effect of ursolic acid in the tail suspension test. J. Pharm. Pharmacol. 2022, 74, 13–21. [Google Scholar] [CrossRef]
- Bae, E.H.; Greenwald, M.K.; Schwartz, A.G. Chemotherapy-Induced Peripheral Neuropathy: Mechanisms and Therapeutic Avenues. Neurotherapeutics 2021, 18, 2384–2396. [Google Scholar] [CrossRef]
- Duggett, N.A.; Griffiths, L.A.; McKenna, O.E.; de Santis, V.; Yongsanguanchai, N.; Mokori, E.B.; Flatters, S.J. Oxidative stress in the development, maintenance and resolution of paclitaxel-induced painful neuropathy. Neuroscience 2016, 333, 13–26. [Google Scholar] [CrossRef]
- Shim, H.S.; Bae, C.; Wang, J.; Lee, K.H.; Hankerd, K.M.; Kim, H.K.; Chung, J.M.; La, J.H. Peripheral and central oxidative stress in chemotherapy-induced neuropathic pain. Mol. Pain 2019, 15, 1744806919840098. [Google Scholar] [CrossRef]
- Catanzaro, E.; Calcabrini, C.; Turrini, E.; Sestili, P.; Fimognari, C. Nrf2: A potential therapeutic target for naturally occurring anticancer drugs? Expert Opin. Ther. Targets 2017, 21, 781–793. [Google Scholar] [CrossRef]
- Raghunath, A.; Sundarraj, K.; Nagarajan, R.; Arfuso, F.; Bian, J.; Kumar, A.P.; Sethi, G.; Perumal, E. Antioxidant response elements: Discovery, classes, regulation and potential applications. Redox Biol. 2018, 17, 297–314. [Google Scholar] [CrossRef]
- Schipper, H.M.; Song, W.; Tavitian, A.; Cressatti, M. The sinister face of heme oxygenase-1 in brain aging and disease. Prog. Neurobiol. 2019, 172, 40–70. [Google Scholar] [CrossRef]
- Liu, R.; Yang, J.; Li, Y.; Xie, J.; Wang, J. Heme oxygenase 1: The roles of both good and evil in neurodegenerative diseases. J. Neurochem. 2023, 167, 347–361. [Google Scholar] [CrossRef]
- Shan, Y.; Lambrecht, R.W.; Donohue, S.E.; Bonkovsky, H.L. Role of Bach1 and Nrf2 in up-regulation of the heme oxygenase-1 gene by cobalt protoporphyrin. FASEB J. 2006, 20, 2651–2653. [Google Scholar] [CrossRef]
- Rosa, P.; Zerbinati, C.; Crestini, A.; Canudas, A.M.; Ragona, G.; Confaloni, A.; Iuliano, L.; Calogero, A. Heme Oxygenase-1 and Brain Oxysterols Metabolism Are Linked to Egr-1 Expression in Aged Mice Cortex, but Not in Hippocampus. Front. Aging Neurosci. 2018, 10, 363. [Google Scholar] [CrossRef]
- Pérez-Fernández, M.; Suárez-Rojas, I.; Bai, X.; Martínez-Martel, I.; Ciaffaglione, V.; Pittalà, V.; Salerno, L.; Pol, O. Novel Heme Oxygenase-1 Inducers Palliate Inflammatory Pain and Emotional Disorders by Regulating NLRP3 Inflammasome and Activating the Antioxidant Pathway. Antioxidants 2023, 12, 1794. [Google Scholar] [CrossRef]
- Neis, V.B.; Rosa, P.B.; Moretti, M.; Rodrigues, A.L.S. Involvement of Heme Oxygenase-1 in Neuropsychiatric and Neurodegenerative Diseases. Curr. Pharm. Des. 2018, 24, 2283–2302. [Google Scholar] [CrossRef]
- Majkutewicz, I. Dimethyl fumarate: A review of preclinical efficacy in models of neurodegenerative diseases. Eur. J. Pharmacol. 2022, 926, 175025. [Google Scholar] [CrossRef]
- Shah, S.; Pushpa Tryphena, K.; Singh, G.; Kulkarni, A.; Pinjala, P.; Kumar Khatri, D. Neuro-protective role of Carvacrol via Nrf2/HO-1/NLRP3 axis in Rotenone-induced PD mice model. Brain Res. 2024, 1836, 148954. [Google Scholar] [CrossRef]
- Scibetta, S.; Miceli, M.; Iuliano, M.; Stefanuto, L.; Carbone, E.; Piscopo, P.; Petrozza, V.; Romeo, G.; Mangino, G.; Calogero, A.; et al. In Vitro Evaluation of the Antioxidant Capacity of 3,3-Disubstituted-3H-benzofuran-2-one Derivatives in a Cellular Model of Neurodegeneration. Life 2024, 14, 422. [Google Scholar] [CrossRef]
- Zafar, S.; Luo, Y.; Zhang, L.; Li, C.H.; Khan, A.; Khan, M.I.; Shah, K.; Seo, E.K.; Wang, F.; Khan, S. Daidzein attenuated paclitaxel-induced neuropathic pain via the down-regulation of TRPV1/P2Y and up-regulation of Nrf2/HO-1 signaling. Inflammopharmacology 2023, 31, 1977–1992. [Google Scholar] [CrossRef]
- Starobova, H.; Monteleone, M.; Adolphe, C.; Batoon, L.; Sandrock, C.J.; Tay, B.; Deuis, J.R.; Smith, A.V.; Mueller, A.; Nadar, E.I.; et al. Vincristine-induced peripheral neuropathy is driven by canonical NLRP3 activation and IL-1β release. J. Exp. Med. 2021, 218, e20201452. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Wang, X.; Sun, Q.; Zhang, Y.; Liu, J.; Hu, T.; Wu, W.; Wei, C.; Liu, M.; Ding, Y.; et al. The upregulation of NLRP3 inflammasome in dorsal root ganglion by ten-eleven translocation methylcytosine dioxygenase 2 (TET2) contributed to diabetic neuropathic pain in mice. J. Neuroinflamm. 2022, 19, 302. [Google Scholar] [CrossRef] [PubMed]
- Silva Santos Ribeiro, P.; Willemen, H.L.D.M.; Versteeg, S.; Martin Gil, C.; Eijkelkamp, N. NLRP3 inflammasome activation in sensory neurons promotes chronic inflammatory and osteoarthritis pain. Immunother. Adv. 2023, 3, ltad022. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Smith, M.T. The NLRP3 inflammasome: Role in the pathobiology of chronic pain. Inflammopharmacology 2023, 31, 1589–1603. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Yin, C.; Fang, J.; Liu, B. The NLRP3 inflammasome: An emerging therapeutic target for chronic pain. J. Neuroinflamm. 2021, 18, 84. [Google Scholar] [CrossRef] [PubMed]
- Tapia-Abellán, A.; Angosto-Bazarra, D.; Martínez-Banaclocha, H.; de Torre-Minguela, C.; Cerón-Carrasco, J.P.; Pérez-Sánchez, H.; Arostegui, J.I.; Pelegrin, P. MCC950 closes the active conformation of NLRP3 to an inactive state. Nat. Chem. Biol. 2019, 15, 560–564. [Google Scholar] [CrossRef] [PubMed]
- Mokhtari, T.; Uludag, K. Role of NLRP3 Inflammasome in Post-Spinal-Cord-Injury Anxiety and Depression: Molecular Mechanisms and Therapeutic Implications. ACS Chem. Neurosci. 2024, 15, 56–70. [Google Scholar] [CrossRef]
| Gene Product | Forward Primer (5′-3′) | Reverse Primer (5′-3′) |
|---|---|---|
| Il1b | CTGGTGTGTGACGTTCCCATTA | CCGACAGCACGAGGCTTT |
| Il6 | CCTACCCCAATTTCCAATGCT | TATTTTCTGACCACAGTGAGGAATG |
| Tnfa | CATCTTCTCAAAATTCGAGTGACAA | TGGGAGTAGACAAGGTACAACCC |
| Gclc | TTACCGAGGCTACGTGTCAGAC | TATCGATGGTCAGGTCGATGTC |
| Gclm | AATCAGCCCCGATTTAGTCAGG | CCAGCGTGCAACTCCAAGGAC |
| Gapdh | CGACTTCAACAGCAACTCCCACTCTTCC | TGGGTGGTCCAGGGTTTCTTACTCCTT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Martel, I.; Bai, X.; Kordikowski, R.; Leite-Panissi, C.R.A.; Pol, O. The Combination of Molecular Hydrogen and Heme Oxygenase 1 Effectively Inhibits Neuropathy Caused by Paclitaxel in Mice. Antioxidants 2024, 13, 856. https://doi.org/10.3390/antiox13070856
Martínez-Martel I, Bai X, Kordikowski R, Leite-Panissi CRA, Pol O. The Combination of Molecular Hydrogen and Heme Oxygenase 1 Effectively Inhibits Neuropathy Caused by Paclitaxel in Mice. Antioxidants. 2024; 13(7):856. https://doi.org/10.3390/antiox13070856
Chicago/Turabian StyleMartínez-Martel, Ignacio, Xue Bai, Rebecca Kordikowski, Christie R. A. Leite-Panissi, and Olga Pol. 2024. "The Combination of Molecular Hydrogen and Heme Oxygenase 1 Effectively Inhibits Neuropathy Caused by Paclitaxel in Mice" Antioxidants 13, no. 7: 856. https://doi.org/10.3390/antiox13070856






