Thermal-Responsive Antibacterial Hydrogel with Photothermal Therapy and Improving Wound Microenvironment for Promote Healing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Au Seeds
2.3. Preparation of Au BNPs
2.4. Preparation of Au@Pt BNPs
2.5. Preparation of Au@Pt@MgSiO3
2.6. Preparation of Thermal-Responsive Hydrogel
2.7. Photothermal Performance of APM NPs
2.8. Ability of APM NPs to Remove H2O2
2.9. Ability to Release Magnesium Ions
2.10. Degradation of GAG Hydrogels
2.11. The Photothermal Performance of GAG Hydrogels
2.12. The Release of APM NPs and GM
2.13. Bacterial Culture
2.14. In Vitro Antibacterial Activity of GAG Hydrogels
2.15. Determination of Live/Dead Ratio
2.16. The Cytotoxicity of APM NPs
2.17. Cell Migration
2.18. In Vivo Wound Healing
2.19. Statistical Analysis
3. Results and Discussion
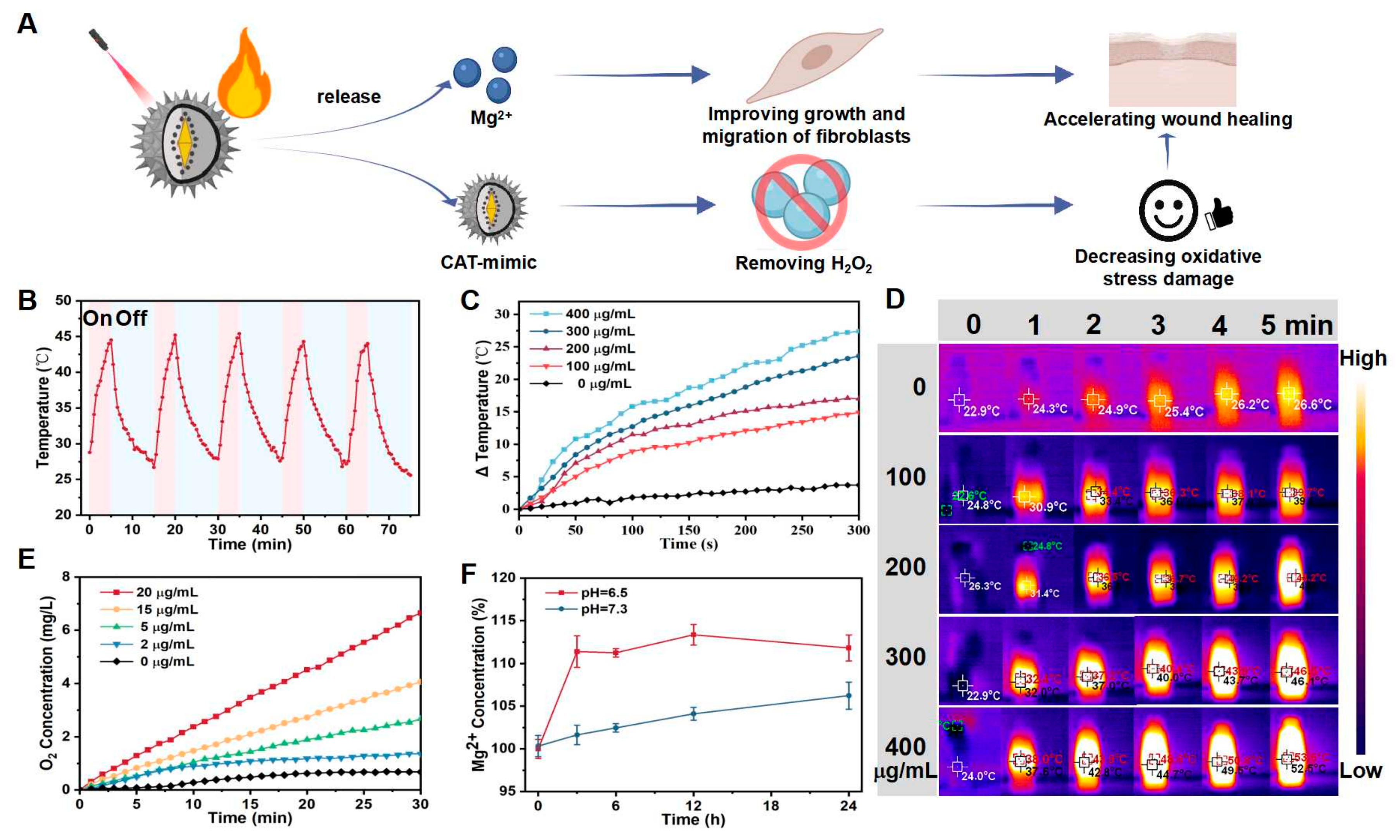
3.1. Synthesis and Characterization of APM NPs
3.2. Property Analysis of APM NPs
3.2.1. The Photothermal Performance of APM NPs
3.2.2. The CAT-like Performance of APM NPs
3.2.3. Mg2+ Release in Acidic Environment
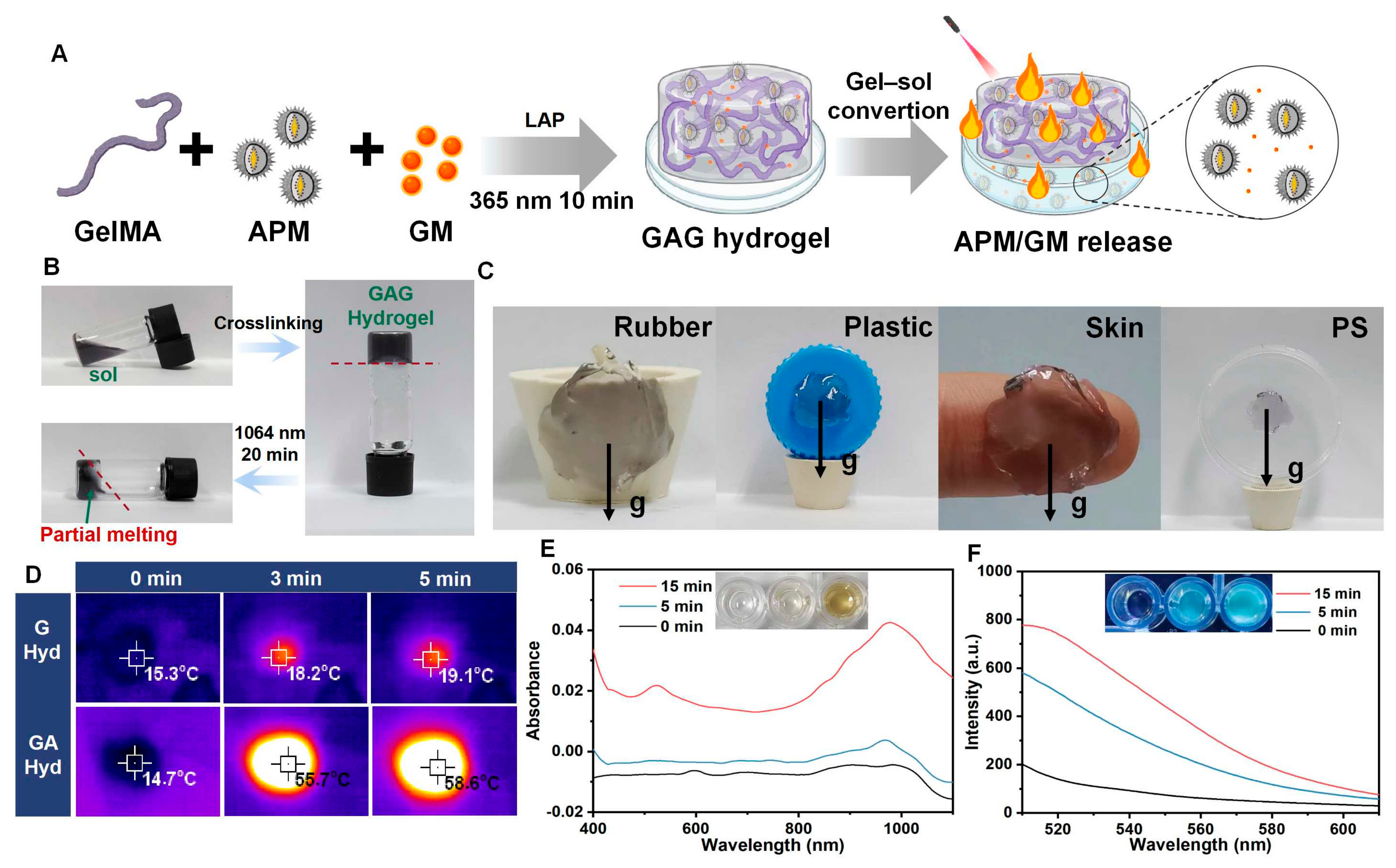
3.3. The Performance of GAG Hydrogels
3.3.1. The Synthesis and Characterization of GAG Hydrogels
3.3.2. Biodegradation of GAG Hydrogels
3.3.3. Photothermal Performance of GAG Hydrogels
3.3.4. APM NPs and GM Release of GAG Hydrogels
3.4. Antibacterial and Microenvironment-Improving Activity of GAG Hydrogels
3.4.1. Antibacterial Activity of GAG Hydrogels In Vitro
3.4.2. Cytotoxicity and Cell Migration Ability
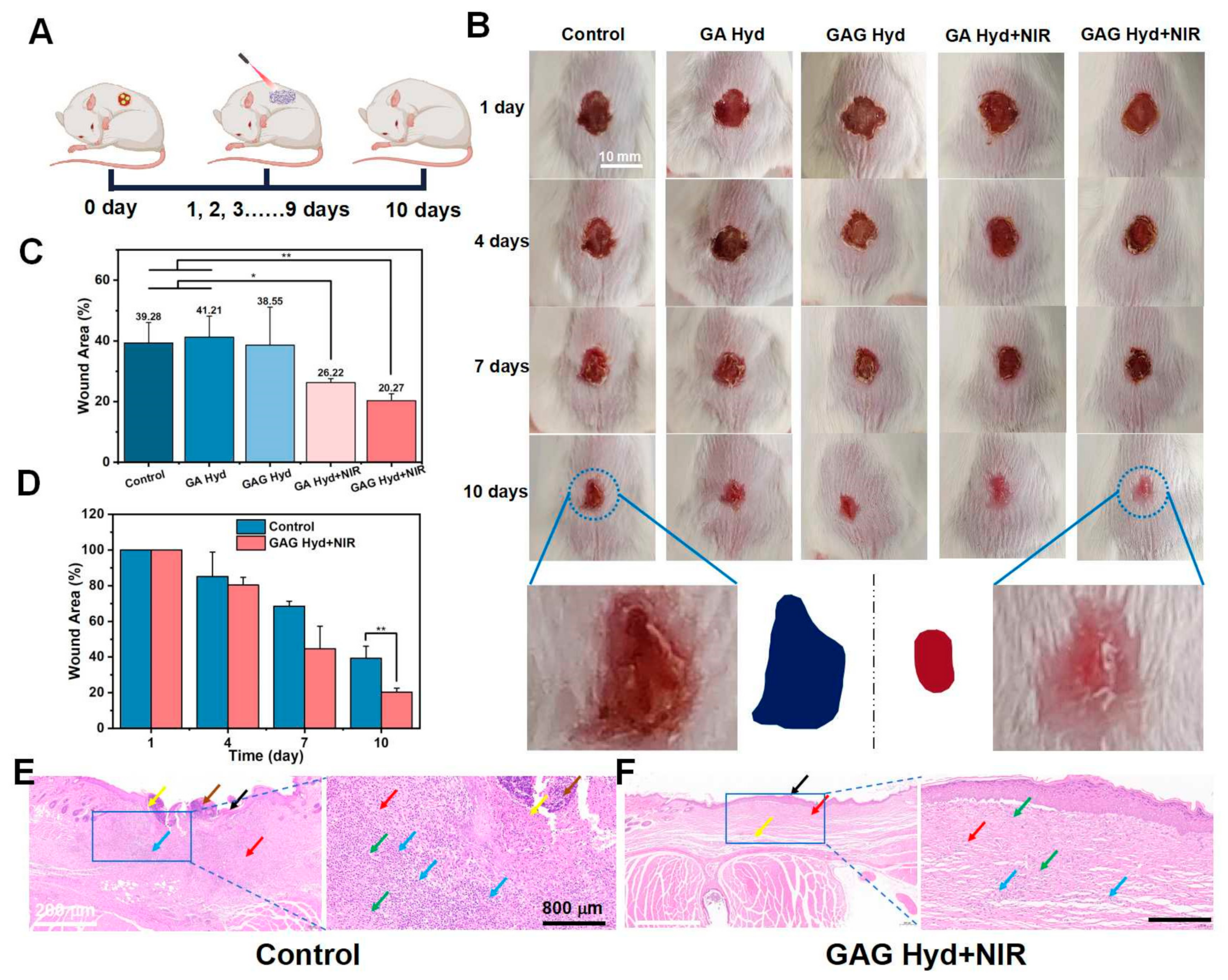
3.5. In Vivo Wound Healing Effects of GAG Hydrogels
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jin, L.; Guo, X.; Gao, D.; Liu, Y.; Ni, J.; Zhang, Z.; Huang, Y.; Xu, G.; Yang, Z.; Zhang, X.; et al. An NIR photothermal-responsive hybrid hydrogel for enhanced wound healing. Bioact. Mater. 2022, 16, 162–172. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Mu, L.; Zhao, X.; Han, Y.; Guo, B. Bacterial Growth-Induced Tobramycin Smart Release Self-Healing Hydrogel for Pseudomonas aeruginosa-Infected Burn Wound Healing. ACS Nano 2022, 16, 13022–13036. [Google Scholar] [CrossRef] [PubMed]
- Shan, J.; Zhang, X.; Cheng, Y.; Song, C.; Chen, G.; Gu, Z.; Zhao, Y. Glucose metabolism-inspired catalytic patches for NIR-II phototherapy of diabetic wound infection. Acta Biomater. 2023, 157, 200–209. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Zhong, D.; Li, W.; Lin, X.; He, J.; Sun, Y.; Wu, Y.; Shi, M.; Chen, X.; Xu, F.; et al. Microalgae-based bioactive hydrogel loaded with quorum sensing inhibitor promotes infected wound healing. Nano Today 2022, 42, 101368. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, J.; Huang, S.; Li, M.; Chen, J.; Pei, D.; Tang, Z.; Guo, B. Bacteria-responsive programmed self-activating antibacterial hydrogel to remodel regeneration microenvironment for infected wound healing. Natl. Sci. Rev. 2024, 11, nwae044. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Li, M.; Pan, G.; Chen, J.; Guo, B. Multiple Stimuli-Responsive Nanozyme-Based Cryogels with Controlled NO Release as Self-Adaptive Wound Dressing for Infected Wound Healing. Adv. Funct. Mater. 2023, 33, 2214089. [Google Scholar] [CrossRef]
- Liu, M.; Ding, R.; Li, Z.; Xu, N.; Gong, Y.; Huang, Y.; Jia, J.; Du, H.; Yu, Y.; Luo, G. Hyaluronidase-Responsive Bactericidal Cryogel for Promoting Healing of Infected Wounds: Inflammatory Attenuation, ROS Scavenging, and Immune Regulation. Adv. Sci. 2024, 11, 2306602. [Google Scholar] [CrossRef]
- Yu, L.; Sun, Y.; Niu, Y.; Zhang, P.; Hu, J.; Chen, Z.; Zhang, G.; Xu, Y. Microenvironment-Adaptive Nanozyme for Accelerating Drug-Resistant Bacteria-Infected Wound Healing. Adv. Healthc. Mater. 2023, 12, 2202596. [Google Scholar] [CrossRef] [PubMed]
- Geng, H.; Zheng, X.; Zhang, Y.; Cui, X.; Li, Z.; Zhang, X.; Cui, J.; Meng, F.; Sun, L.; Ni, S. Microenvironment-Responsive Hydrogels with Detachable Skin Adhesion and Mild-Temperature Photothermal Property for Chronic Wound Healing. Adv. Funct. Mater. 2023, 33, 2305154. [Google Scholar] [CrossRef]
- Hu, X.; He, J.; Qiao, L.; Wang, C.; Wang, Y.; Yu, R.; Xu, W.; Wang, F.; Yang, S.; Zhang, X.; et al. Multifunctional Dual Network Hydrogel Loaded with Novel Tea Polyphenol Magnesium Nanoparticles Accelerates Wound Repair of MRSA Infected Diabetes. Adv. Funct. Mater. 2024, 34, 2312140. [Google Scholar] [CrossRef]
- Shao, Z.; Yin, T.; Jiang, J.; He, Y.; Xiang, T.; Zhou, S. Wound microenvironment self-adaptive hydrogel with efficient angiogenesis for promoting diabetic wound healing. Bioact. Mater. 2023, 20, 561–573. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Lai, T.-P.; Gao, P.; Zhang, H.; Ho, P.-L.; Woo, P.C.-Y.; Ma, G.; Kao, R.Y.-T.; Li, H.; Sun, H. Bismuth antimicrobial drugs serve as broad-spectrum metallo-β-lactamase inhibitors. Nat. Commun. 2018, 9, 439. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Zheng, H.; Yin, H.; Zhou, L.; Zhang, Q.; Zhang, B. Niobium carbide doped ROS/temperature dual-responsive multifunctional hydrogel for facilitating MRSA-infected wound healing. Chem. Eng. J. 2023, 471, 144634. [Google Scholar] [CrossRef]
- Li, Y.; Xu, Z.H.; Tang, L.Q.; Wang, F.J.; Wang, L. Nanofibers fortified with synergistic defense route: A potent wound dressing against drug-resistant bacterial infections. Chem. Eng. J. 2023, 475, 146492. [Google Scholar] [CrossRef]
- Kim, H.S.; Sun, X.; Lee, J.H.; Kim, H.W.; Fu, X.; Leong, K.W. Advanced drug delivery systems and artificial skin grafts for skin wound healing. Adv. Drug Deliv. Rev. 2019, 146, 209–239. [Google Scholar] [CrossRef] [PubMed]
- Zare, I.; Chevrier, D.M.; Cifuentes-Rius, A.; Moradi, N.; Xianyu, Y.; Ghosh, S.; Trapiella-Alfonso, L.; Tian, Y.; Shourangiz-Haghighi, A.; Mukherjee, S.; et al. Protein-protected metal nanoclusters as diagnostic and therapeutic platforms for biomedical applications. Mater. Today 2023, 66, 159–193. [Google Scholar] [CrossRef]
- Liu, Z.; Zhao, X.; Yu, B.; Zhao, N.; Zhang, C.; Xu, F.-J. Rough Carbon–Iron Oxide Nanohybrids for Near-Infrared-II Light-Responsive Synergistic Antibacterial Therapy. ACS Nano 2021, 15, 7482–7490. [Google Scholar] [CrossRef] [PubMed]
- Zha, K.; Zhang, W.; Hu, W.; Tan, M.; Zhang, S.; Yu, Y.; Gou, S.; Bu, P.; Zhou, B.; Zou, Y.; et al. Three-Step Regenerative Strategy: Multifunctional Bilayer Hydrogel for Combined Photothermal/Photodynamic Therapy to Promote Drug-Resistant Bacteria-Infected Wound Healing. Adv. Funct. Mater. 2023, 34, 2308145. [Google Scholar] [CrossRef]
- Maleki, A.; He, J.; Bochani, S.; Nosrati, V.; Shahbazi, M.A.; Guo, B. Multifunctional Photoactive Hydrogels for Wound Healing Acceleration. ACS Nano 2021, 15, 18895–18930. [Google Scholar] [CrossRef]
- Yu, X.; Zhao, J.; Fan, D. A dissolving microneedle patch for Antibiotic/Enzymolysis/Photothermal triple therapy against bacteria and their biofilms. Chem. Eng. J. 2022, 437, 135475. [Google Scholar] [CrossRef]
- Tan, L.; Zhou, Z.; Liu, X.; Li, J.; Zheng, Y.; Cui, Z.; Yang, X.; Liang, Y.; Li, Z.; Feng, X.; et al. Overcoming Multidrug-Resistant MRSA Using Conventional Aminoglycoside Antibiotics. Adv. Sci. 2020, 7, 1902070. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Chen, Z.; Tu, H.; Liu, H.; Liu, Y.; Chen, S.; Cai, D.; Liu, C.; Zhang, X.; Zou, G.; et al. Multifunctional carbon quantum dots decorated self-healing hydrogel for highly effective treatment of superbug infected wounds. Chem. Eng. J. 2024, 480, 148218. [Google Scholar] [CrossRef]
- Yan, Y.; Wang, M.; Zhao, M.; Zhang, J.; Liu, Y.; Gao, X. pH Switchable Nanozyme Platform for Healing Skin Tumor Wound Infected with Drug-Resistant Bacteria. Adv. Healthc. Mater. 2023, 12, e2301375. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, M.; Cao, L.; Zhuang, J.; Wang, D.; Wu, M.; Liu, B. Living Artificial Skin: Photosensitizer and Cell Sandwiched Bacterial Cellulose for Chronic Wound Healing. Adv. Mater. 2024, 36, 2403355. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Fu, R.; Duan, Z.; Zhu, C.; Fan, D. Construction of multifunctional hydrogel based on the tannic acid-metal coating decorated MoS2 dual nanozyme for bacteria-infected wound healing. Bioact. Mater. 2022, 9, 461–474. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Chen, Y.; He, X.; Yu, Y.; Han, R.; Li, Y.; Yang, C.; Hu, D.; Qian, Z. Polymeric Nanoparticles with ROS-Responsive Prodrug and Platinum Nanozyme for Enhanced Chemophotodynamic Therapy of Colon Cancer. Adv. Sci. 2020, 7, 2001853. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Zhang, L.; Luo, Y.; Cai, Z.; Zeng, H.; Wang, T.; Liu, Z.; Chen, Y.; Sheng, X.; Mandlate, A.E.d.G.; et al. Charge-Driven Self-Assembled Microspheres Hydrogel Scaffolds for Combined Drug Delivery and Photothermal Therapy of Diabetic Wounds. Adv. Funct. Mater. 2023, 33, 2214036. [Google Scholar] [CrossRef]
- Norahan, M.H.; Pedroza-Gonzalez, S.C.; Sanchez-Salazar, M.G.; Alvarez, M.M.; Trujillo de Santiago, G. Structural and biological engineering of 3D hydrogels for wound healing. Bioact. Mater. 2023, 24, 197–235. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.; Li, M.; Chen, H.; Zhang, Y.; Liu, Y.; Chen, M.; Luo, Z.; Cai, K.; Hu, Y. Dynamic hydrogel with environment-adaptive autonomous wound-compressing ability enables rapid hemostasis and inflammation amelioration for hemorrhagic wound healing. Nano Today 2023, 52, 101962. [Google Scholar] [CrossRef]
- Yang, F.; Xue, Y.; Wang, F.; Guo, D.; He, Y.; Zhao, X.; Yan, F.; Xu, Y.; Xia, D.; Liu, Y. Sustained release of magnesium and zinc ions synergistically accelerates wound healing. Bioact. Mater. 2023, 26, 88–101. [Google Scholar] [CrossRef]
- Chen, S.; Wang, Y.; Lai, J.; Tan, S.; Wang, M. Structure and Properties of Gelatin Methacryloyl (GelMA) Synthesized in Different Reaction Systems. Biomacromolecules 2023, 24, 2928–2941. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Wang, P.; Cao, R.; Wu, J.; Ji, H.; Wang, M.; Hu, C.; Huang, P.; Wang, X. Exudate Absorbing and Antimicrobial Hydrogel Integrated with Multifunctional Curcumin-Loaded Magnesium Polyphenol Network for Facilitating Burn Wound Healing. ACS Nano 2023, 17, 22355–22370. [Google Scholar] [CrossRef] [PubMed]
- Yin, M.; Wu, J.; Deng, M.; Wang, P.; Ji, G.; Wang, M.; Zhou, C.; Blum, N.T.; Zhang, W.; Shi, H.; et al. Multifunctional Magnesium Organic Framework-Based Microneedle Patch for Accelerating Diabetic Wound Healing. ACS Nano 2021, 15, 17842–17853. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Iglesias, A.; Winckelmans, N.; Altantzis, T.; Bals, S.; Grzelczak, M.; Liz-Marzán, L.M. High-Yield Seeded Growth of Monodisperse Pentatwinned Gold Nanoparticles through Thermally Induced Seed Twinning. J. Am. Chem. Soc. 2016, 139, 107–110. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.U.; Zhang, R.; Li, L.; You, H.; Fang, J. Solution-Based SERS Detection of Weak Surficial Affinity Molecules Using Cysteamine-Modified Au Bipyramids. Anal. Chem. 2021, 93, 7657–7664. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhang, X.; Zhou, H.; Liu, G.; Kong, M.; Wang, G. Microwave-assisted synthesis of magnetic Fe3O4-mesoporous magnesium silicate core-shell composites for the removal of heavy metal ions. Microporous Mesoporous Mater. 2017, 242, 50–58. [Google Scholar] [CrossRef]
- Zou, B.; Chen, K.; Wang, Y.; Niu, C.; Zhou, S. Amino-functionalized magnetic magnesium silicate double-shelled hollow microspheres for enhanced removal of lead ions. RSC Adv. 2015, 5, 22973–22979. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, L.; Deng, J.; Su, Y.; Hu, X.; Zhang, Y.; Hong, S.; Lin, X. Thermal-Responsive Antibacterial Hydrogel with Photothermal Therapy and Improving Wound Microenvironment for Promote Healing. Antioxidants 2024, 13, 857. https://doi.org/10.3390/antiox13070857
Huang L, Deng J, Su Y, Hu X, Zhang Y, Hong S, Lin X. Thermal-Responsive Antibacterial Hydrogel with Photothermal Therapy and Improving Wound Microenvironment for Promote Healing. Antioxidants. 2024; 13(7):857. https://doi.org/10.3390/antiox13070857
Chicago/Turabian StyleHuang, Linjie, Jingwen Deng, Yina Su, Xueqi Hu, Yichao Zhang, Shanni Hong, and Xiahui Lin. 2024. "Thermal-Responsive Antibacterial Hydrogel with Photothermal Therapy and Improving Wound Microenvironment for Promote Healing" Antioxidants 13, no. 7: 857. https://doi.org/10.3390/antiox13070857




