Antioxidant and Neuroprotective Effects of Seed Oils from Trichosanthes kirilowii and T. laceribractea in Caenorhabditis elegans: A Comparative Analysis and Mechanism Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Seed Oil Preparation
2.2. C. elegans Strains and Maintenance Conditions
2.3. Measurement of Reactive Oxygen Species (ROS)
2.4. Paralysis Assay
2.5. Thrashing Assay
2.6. Chemotaxis Assay
2.7. 5-HT Sensitivity Assay
2.8. Chemotaxis Assay
2.9. RNA Sequencing
2.10. Determination of GSH Level and CAT Activity
2.11. Statistical Analysis
3. Results
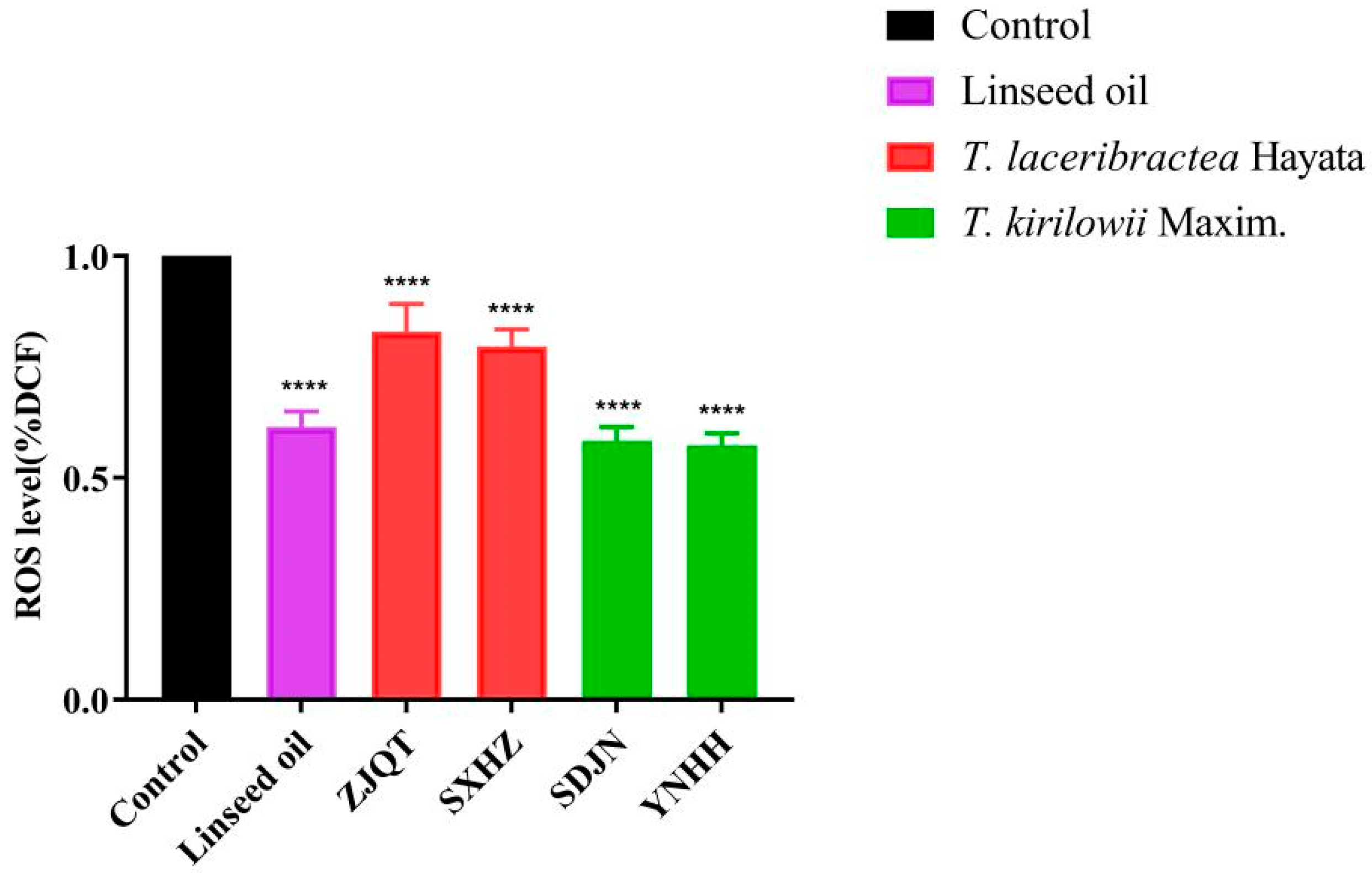
3.1. Seed Oils Reduced ROS Levels
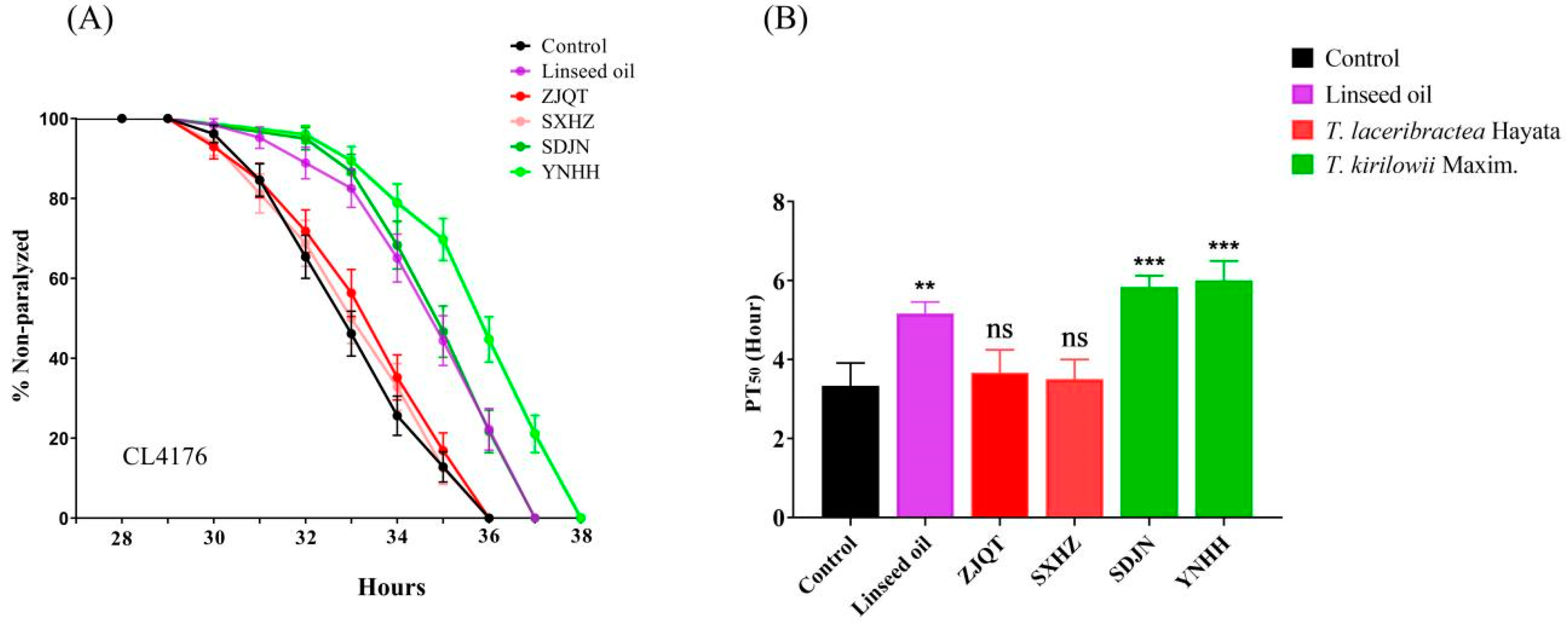
3.2. Seed Oils Delayed Aβ-Induced Paralysis
3.3. Seed Oils Alleviated Tau-Induced Toxicity in Locomotion
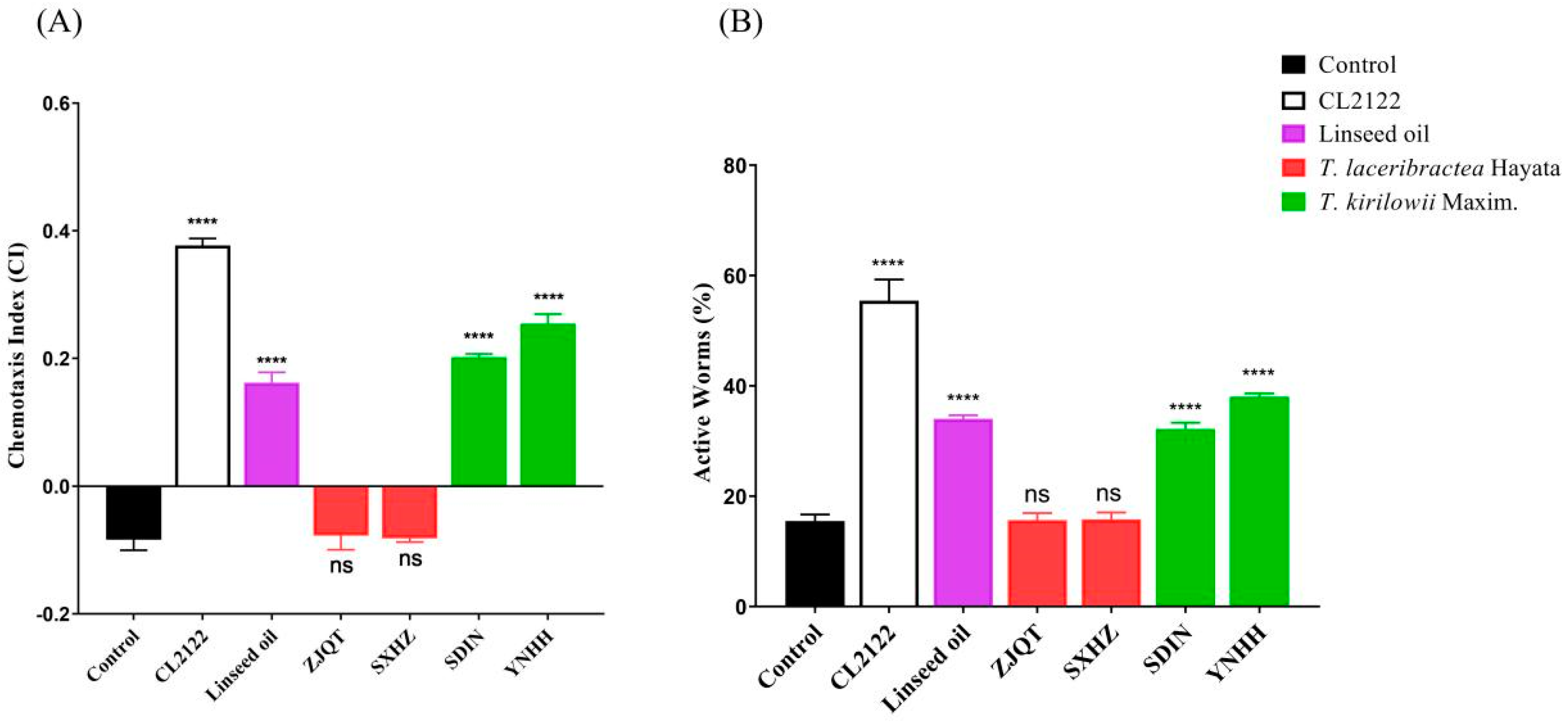
3.4. Seed Oils Suppressed Neuronal Aβ-Expression-Induced Defects in Chemotaxis Behavior and 5-HT Sensitivity
3.5. Seed Oils Attenuated Aβ-Induced Damage in GABA Neurons
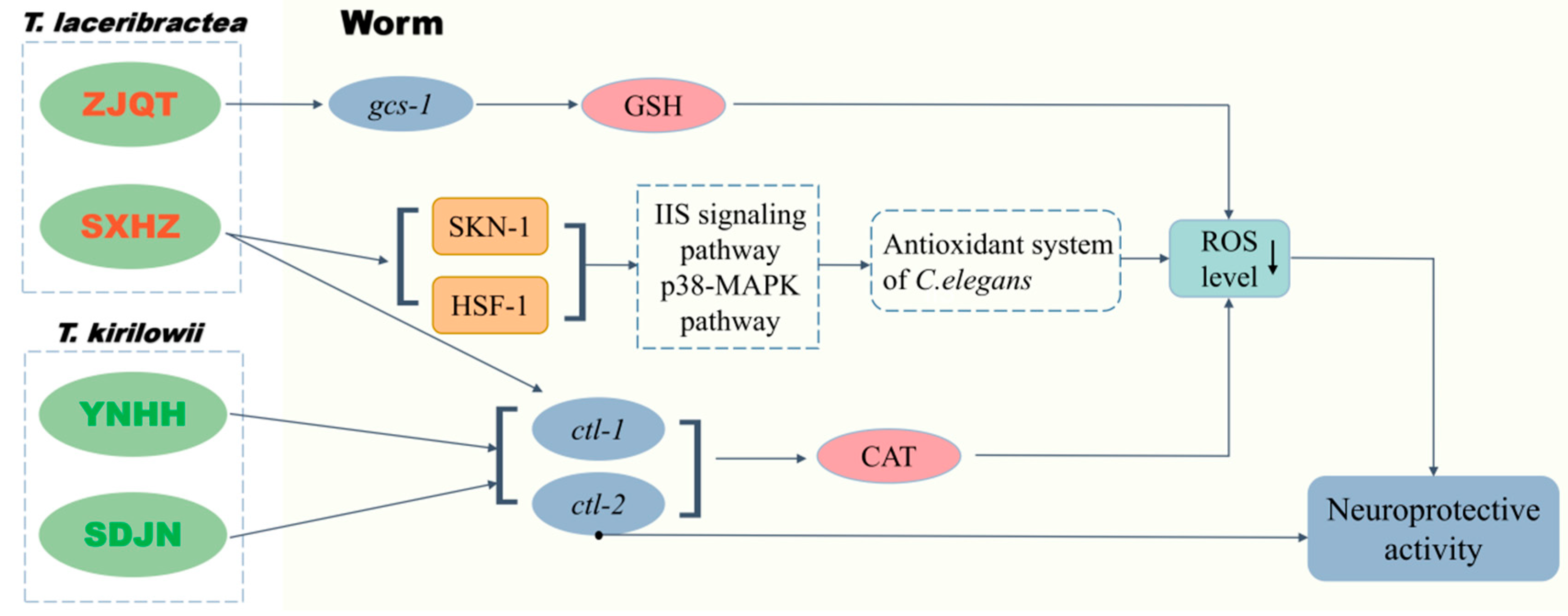
3.6. Seed Oils from Different Trichosanthes Germplasms Exerted Neuroprotective Effect Based on Different Mechanisms
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Huang, L.; Yue, C.; Cheng, J.; Lou, Z. Taxonomy of Medicinal Plants in Genus Trichosanthes L.; Shanghai Science and Technology Press: Shanghai, China, 2009. [Google Scholar]
- Committee for the Pharmacopoeia of China. Pharmacopoeia of the People’s Republic of China; Part 1; China Medical Science and Technology Press: Beijing, China, 2020. [Google Scholar]
- Jin, X.; Sang, G.; Du, X. Fatty acid composition and heavy mental accumulation characteristics of Fructus Trichosanthis seed. Fresen. Environ. Bull. 2019, 28, 1978–1980. [Google Scholar]
- Zhang, H.-Q.; Liu, P.; Duan, J.-A.; Dong, L.; Shang, E.-X.; Qian, D.-W.; Zhu, Z.-H.; Li, H.-W.; Li, W.-W. Comparative analysis of carbohydrates nucleosides and amino acids in different parts of Trichosanthes kirilowii Maxim. by (ultra) high-performance liquid chromatography coupled with tandem mass spectrometry and evaporative light scattering detector methods. Molecules 2019, 24, 1440. [Google Scholar] [CrossRef]
- Wang, W.; Li, S.; Zhu, Y.; Zhu, R.; Du, X.; Cui, X.; Wang, H.; Cheng, Z. Effect of different edible Trichosanthes germplasm on its seed oil to enhance antioxidant and anti-aging activity in Caenorhabditis elegans. Foods 2024, 13, 503. [Google Scholar] [CrossRef]
- Majumder, D.; Nath, P.; Debnath, R.; Maiti, D. Understanding the complicated relationship between antioxidants and carcinogenesis. J. Biochem. Mol. Toxicol. 2021, 35, e22643. [Google Scholar] [CrossRef]
- Lee, Y.; He, W.; Liou, Y. The redox language in neurodegenerative diseases: Oxidative post-translational modifications by hydrogen peroxide. Cell Death. Dis. 2021, 12, 58. [Google Scholar] [CrossRef]
- Ashrafian, H.; Zadeh, E.; Khan, R. Review on Alzheimer’s disease: Inhibition of amyloid beta and tau tangle formation. Int. J. Biol. Macromol. 2021, 167, 382–394. [Google Scholar] [CrossRef]
- Yu, Z.; Luo, F. The role of reactive oxygen species in Alzheimer’s disease: From mechanism to biomaterials therapy. Adv. Healthc. Mater. 2024, e2304373. [Google Scholar] [CrossRef]
- Jeong, S.; Park, J.; Oh, G. Peroxiredoxins as potential targets for cardiovascular disease. Antioxidants 2021, 10, 101244. [Google Scholar] [CrossRef]
- Honisch, C.; Rodella, U.; Gatto, C.; Ruzza, P.; Tóthová, J. Oxidative stress and antioxidant-based interventional medicine in ophthalmology. Pharmaceuticals 2023, 16, 1146. [Google Scholar] [CrossRef]
- Lin, L.; Li, C.; Li, T.; Zheng, J.; Shu, Y.; Zhang, J.; Ren, D. Plant-derived peptides for the improvement of Alzheimer’s disease: Production, functions, and mechanisms. Food Front. 2023, 4, 677–699. [Google Scholar] [CrossRef]
- Hulme, S.; Whitesides, G. Chemistry and the worm: Caenorhabditis elegans as a platform for integrating chemical and biological research. Angew. Chem. Int. Ed. 2011, 50, 4774–4807. [Google Scholar] [CrossRef]
- Tataridas-Pallas, N.; Thompson, M.; Howard, A.; Brown, I.; Ezcurra, M.; Wu, Z. Neuronal SKN-1B modulates nutritional signalling pathways and mitochondrial networks to control satiety. PLoS Genet. 2021, 17, e1009358. [Google Scholar] [CrossRef]
- Sorrentino, V.; Romani, M.; Mouchiroud, L.; Beck, J.; Zhang, H.; D’Amico, D. Enhancing mitochondrial proteostasis reduces amyloid-beta proteotoxicity. Nature 2017, 552, 187–193. [Google Scholar] [CrossRef]
- Desai, S.; Jadhav, A.; Holkar, C.; Pawar, B.; Pinjari, D. Extraction and microencapsulation of Buchanania lanzan Spreng seed oil. Chem. Pap. 2022, 76, 3521–3530. [Google Scholar] [CrossRef]
- Stiernagle, T. Maintenance of C. elegans. In WormBook: The Online Review of C. elegans Biology; The C. elegans Research Community, Ed.; WormBook: Pasadena, CA, USA, 2006. [Google Scholar]
- Cheslow, L.; Byrne, M.; Kopenhaver, J. GUCY2C signaling limits dopaminergic neuron vulnerability to toxic insults. NPJ Park. Dis. 2024, 10, 83. [Google Scholar] [CrossRef]
- Ye, Z.; Liu, Y.; Jin, X.; Wu, Y.; Zhao, H.; Gao, T.; Tong, Z. Aβ-binding with alcohol dehydrogenase drives Alzheimer’s disease pathogenesis: A review. Int. J. Biol. Macromol. 2024, 264, 130580. [Google Scholar] [CrossRef]
- Gutierrez-Zepeda, A.; Santell, R.; Wu, Z. Soy isoflavone glycitein protects against beta amyloid-induced toxicity and oxidative stress in transgenic Caenorhabditis elegans. BMC Neurosci. 2005, 6, 54. [Google Scholar] [CrossRef]
- Brandt, R.; Gergou, A.; Wacker, I.; Fath, T.; Hutter, H. A Caenorhabditis elegans model of tau hyperphosphorylation: Induction of developmental defects by transgenic overexpression of Alzheimer’s disease-like modified tau. Neurobiol. Aging 2009, 30, 22–33. [Google Scholar] [CrossRef]
- Liu, P.; Chen, B.; Wang, Z. GABAergic motor neurons bias locomotor decision-making in C. elegans. Nat. Commun. 2020, 11, 5076. [Google Scholar] [CrossRef]
- Wu, Y.; Wu, Z.; Butko, P.; Christen, Y.; Lambert, M.; Klein, W.L.; Link, C.D.; Luo, Y. Amyloid-β-induced pathological behaviors are suppressed by Ginkgo biloba extract EGb 761 and ginkgolides in transgenic Caenorhabditis elegans. J. Neurosci. 2006, 26, 13102–13113. [Google Scholar] [CrossRef]
- Cruz-Corchado, J.; Ooi, F.; Das, S.; Prahlad, V. Global transcriptome changes that accompany alterations in serotonin levels in Caenorhabditis elegans. G3 Genes Genomes Genet. 2020, 10, 1225–1246. [Google Scholar] [CrossRef]
- Weishaupt, A.; Kubens, L.; Ruecker, L.; Schwerdtle, T.; Aschner, M.; Bornhorst, J. A reliable method based on liquid chromatography-tandem mass spectrometry for the simultaneous quantification of neurotransmitters in Caenorhabditis elegans. Molecules 2023, 28, 5373. [Google Scholar] [CrossRef]
- Korovesis, D.; Rubio-Tomás, T.; Tavernarakis, N. Oxidative stress in age-related neurodegenerative diseases: An overview of recent tools and findings. Antioxidants 2023, 12, 131. [Google Scholar] [CrossRef]
- Islam, F.; Nafady, M.; Islam, M. Resveratrol and neuroprotection: An insight into prospective therapeutic approaches against Alzheimer’s disease from bench to bedside. Mol. Neurobiol. 2022, 59, 4384–4404. [Google Scholar] [CrossRef]
- Akbari, B.; Baghaei-Yazdi, N.; Bahmaie, M.; Mahdavi, F. The role of plant-derived natural antioxidants in reduction of oxidative stress. BioFactors 2022, 48, 611–633. [Google Scholar] [CrossRef]
- Dhavamani, S.; Rao, Y.; Lokesh, B. Total antioxidant activity of selected vegetable oils and their influence on total antioxidant values in vivo: A photochemiluminescence based analysis. Food Chem. 2014, 164, 551–555. [Google Scholar] [CrossRef]
- Mannino, F.; Imbesi, C.; Bitto, A.; Minutoli, L.; Squadrito, F.; D’Angelo, T.; Booz, C.; Pallio, G.; Irrera, N. Anti-oxidant and anti-inflammatory effects of ellagic and punicic acid in an in vitro model of cardiac fibrosis. Biomed. Pharm. 2023, 162, 114666. [Google Scholar] [CrossRef]
- Tantipaiboonwong, P.; Chaiwangyen, W.; Suttajit, M.; Kangwan, N.; Kaowinn, S.; Khanaree, C.; Punfa, W.; Pintha, K. Molecular mechanism of antioxidant and anti-inflammatory effects of omega-3 fatty acids in perilla seed oil and rosmarinic acid rich fraction extracted from perilla seed meal on TNF-α induced A549 lung adenocarcinoma cells. Molecules 2021, 26, 6757. [Google Scholar] [CrossRef]
- Liu, S.; Xiao, C.; Wang, F. Comparison of two varieties fig (peggy red and green) peel extracts by liquid chromatography–tandem mass spectrometry analysis and for neuroprotective efficacy in Caenorhabditis elegans. J. Med. Food 2023, 26, 14–26. [Google Scholar] [CrossRef]
- Li, Y.; Zou, Z.; An, J.; Liu, X.; Wu, Q.; Sun, J.; Liu, X.; Du, J.; Xiong, Y.; Wu, C.; et al. Folic acid-functionalized chitosan nanoparticles with bioenzyme activity for the treatment of spinal cord injury. Eur. J. Pharm. Sci. 2024, 192, 106667. [Google Scholar] [CrossRef]
- Xu, J.; Guo, Y.; Sui, T.; Wang, Q.; Zhang, Y.; Zhang, R.; Wang, M.; Guan, S.; Wang, L. Molecular mechanisms of antioxidant and anti-aging effects induced by convallatoxin in Caenorhabditis elegans. Free Radic. Res. 2017, 51, 529–544. [Google Scholar] [CrossRef]
- Zhang, J.; Xue, X.; Qiao, Y.; Li, D.; Wei, Q.; Zhang, F.; Qin, X. Astragaloside IV extends lifespan of Caenorhabditis elegans by improving age-related functional declines and triggering antioxidant responses. Rejuv. Res. 2021, 24, 120–130. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Yu, X.; Li, H.; Wu, G.; Luo, H. D-chiro-inositol increases antioxidant capacity and longevity of Caenorhabditis elegans via activating Nrf-2/SKN-1 and FOXO/DAF-16. Exp. Gerontol. 2023, 175, 112145. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zhou, J.; Xu, Z.; Kong, Q.; Zhang, J.; Wang, H.; Xiang, Z.; Chen, T.; Zhou, L.; Feng, S.; et al. The positive effects of Camellia oleifera oil on lifespan in Caenorhabditis elegans. J. Funct. Foods 2023, 110, 105869. [Google Scholar] [CrossRef]
- Ferguson, G.; Bridge, W. The glutathione system and the related thiol network in Caenorhabditis elegans. Redox Biol. 2019, 24, 101171. [Google Scholar] [CrossRef] [PubMed]
- Ayyadevara, S.; Bharill, P.; Dandapat, A.; Hu, C.; Khaidakov, M.; Mitra, S.; Reis, R.J.S.; Mehta, J.L. Aspirin inhibits oxidant stress, reduces age-associated functional declines, and extends lifespan of Caenorhabditis elegans. Antioxid. Redox Sign. 2013, 18, 481–490. [Google Scholar] [CrossRef] [PubMed]
- Arjeh, E.; Akhavan, H.; Barzegar, M.; Carbonell-Barrachina, Á. Bio-active compounds and functional properties of pistachio hull: A review. Trends Food Sci. Tech. 2020, 97, 55–64. [Google Scholar] [CrossRef]


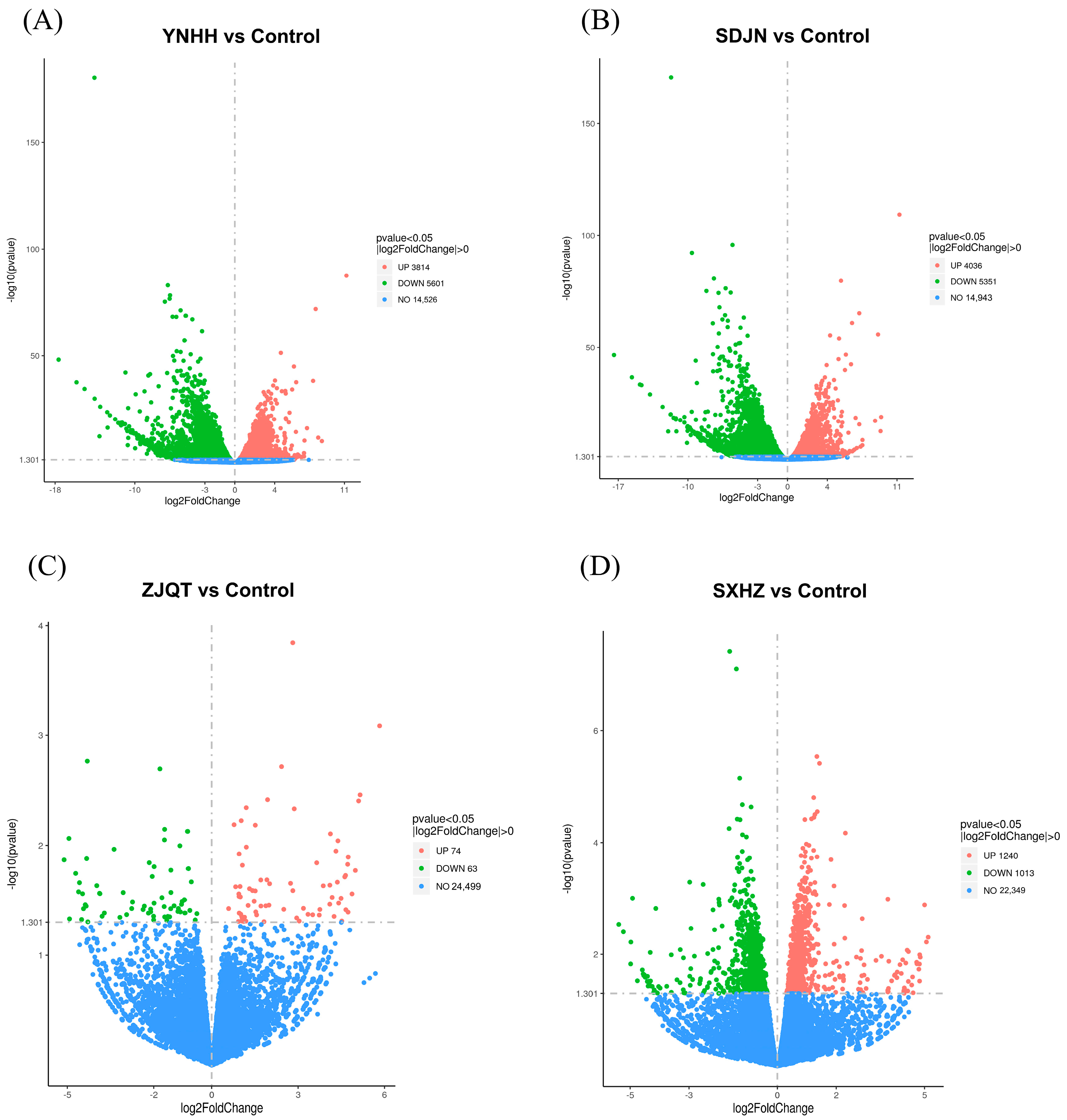
| Groups | Total | Up-Regulating | Down-Regulating |
|---|---|---|---|
| SDJN vs. Control | 7146 | 4511 | 2635 |
| YNHH vs. Control | 7065 | 4680 | 2385 |
| ZJQT vs. Control | 119 | 55 | 64 |
| SXHZ vs. Control | 415 | 247 | 168 |
| Inter-Species | Total | Up-Regulating | Down-Regulating |
| SDJN vs. YNHH | 574 | 297 | 277 |
| ZJQT vs. SXHZ | 327 | 193 | 134 |
| Groups | log2Foldchange (p Value) | ||||
|---|---|---|---|---|---|
| gcs-1 | ctl-1 | ctl-2 | hsf-1 | skn-1 | |
| SDJN | 0.3989 (0.12) | 1.2632 (0.01) | 1.0020 (0.01) | 0.4389 (0.08) | 0.1237 (0.66) |
| YNHH | 0.2042 (0.39) | 0.5416 (0.02) | 0.6183 (0.02) | 0.3950 (0.10) | 0.2246 (0.40) |
| SXHZ | 0.3614 (0.09) | 0.4787 (0.01) | 0.0657 (0.72) | 0.0165 (0.02) | 0.7193 (0.01) |
| ZJQT | 0.2411 (0.04) | 0.1000 (0.63) | 0.0059 (0.97) | 0.1332 (0.61) | 0.0005 (1.00) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Li, S.; Zhu, Y.; Cui, X.; Sheng, Z.; Wang, H.; Cheng, Z. Antioxidant and Neuroprotective Effects of Seed Oils from Trichosanthes kirilowii and T. laceribractea in Caenorhabditis elegans: A Comparative Analysis and Mechanism Study. Antioxidants 2024, 13, 861. https://doi.org/10.3390/antiox13070861
Wang W, Li S, Zhu Y, Cui X, Sheng Z, Wang H, Cheng Z. Antioxidant and Neuroprotective Effects of Seed Oils from Trichosanthes kirilowii and T. laceribractea in Caenorhabditis elegans: A Comparative Analysis and Mechanism Study. Antioxidants. 2024; 13(7):861. https://doi.org/10.3390/antiox13070861
Chicago/Turabian StyleWang, Wenqian, Shan Li, Yunguo Zhu, Xianghuan Cui, Zhejin Sheng, Hongbing Wang, and Zhou Cheng. 2024. "Antioxidant and Neuroprotective Effects of Seed Oils from Trichosanthes kirilowii and T. laceribractea in Caenorhabditis elegans: A Comparative Analysis and Mechanism Study" Antioxidants 13, no. 7: 861. https://doi.org/10.3390/antiox13070861





