Protective Effects of Beta-3 Adrenoceptor Agonism on Mucosal Integrity in Hyperoxia-Induced Ileal Alterations
Abstract
:1. Introduction
2. Materials and Methods
2.1. Exposure to Hyperoxia and Normoxia and Drug Administration
2.2. Sample Collection and 5-Epi-5-F2t-IsoP Measurement
2.3. Flow Cytometric Analysis
2.4. Histological Evaluations
2.5. Immunofluorescence Analysis
2.6. Transmission Electron Microscopy Analysis
2.7. Statistical Analysis
3. Results
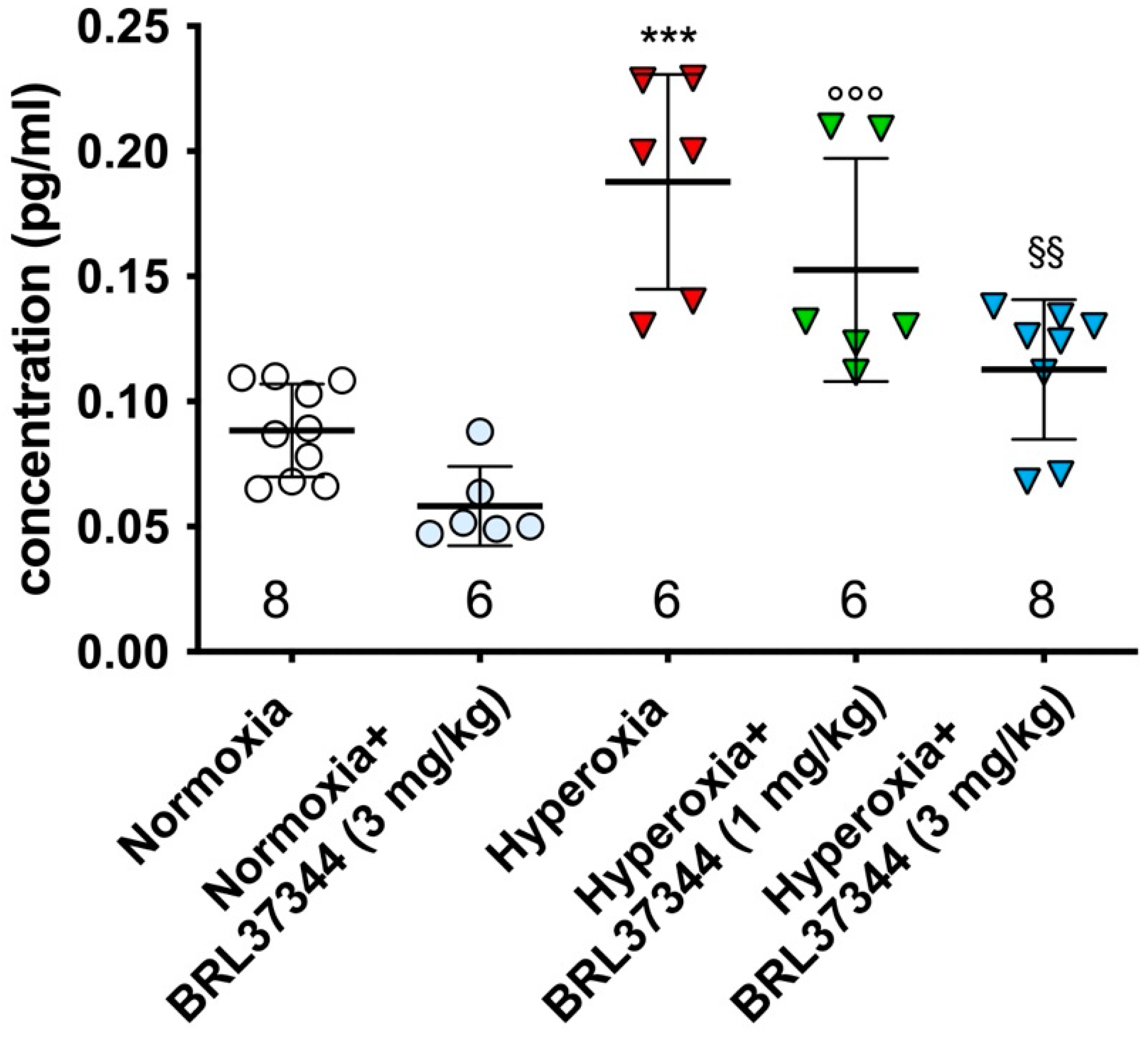
3.1. Plasma Levels of 5-Epi-5-F2t-IsoP
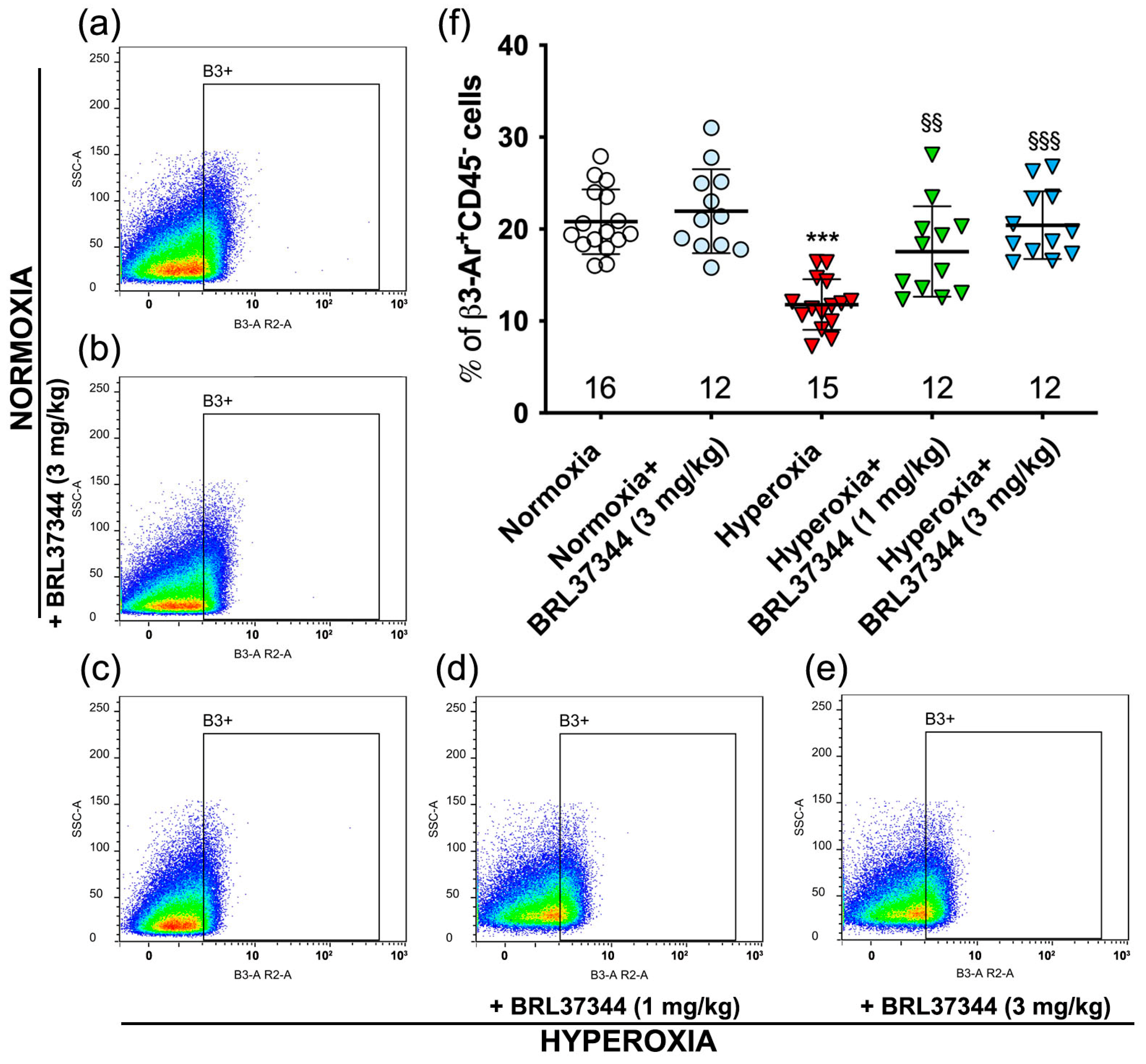
3.2. β3-AR Expression on Ileal Resident Cells
3.3. Body Weight
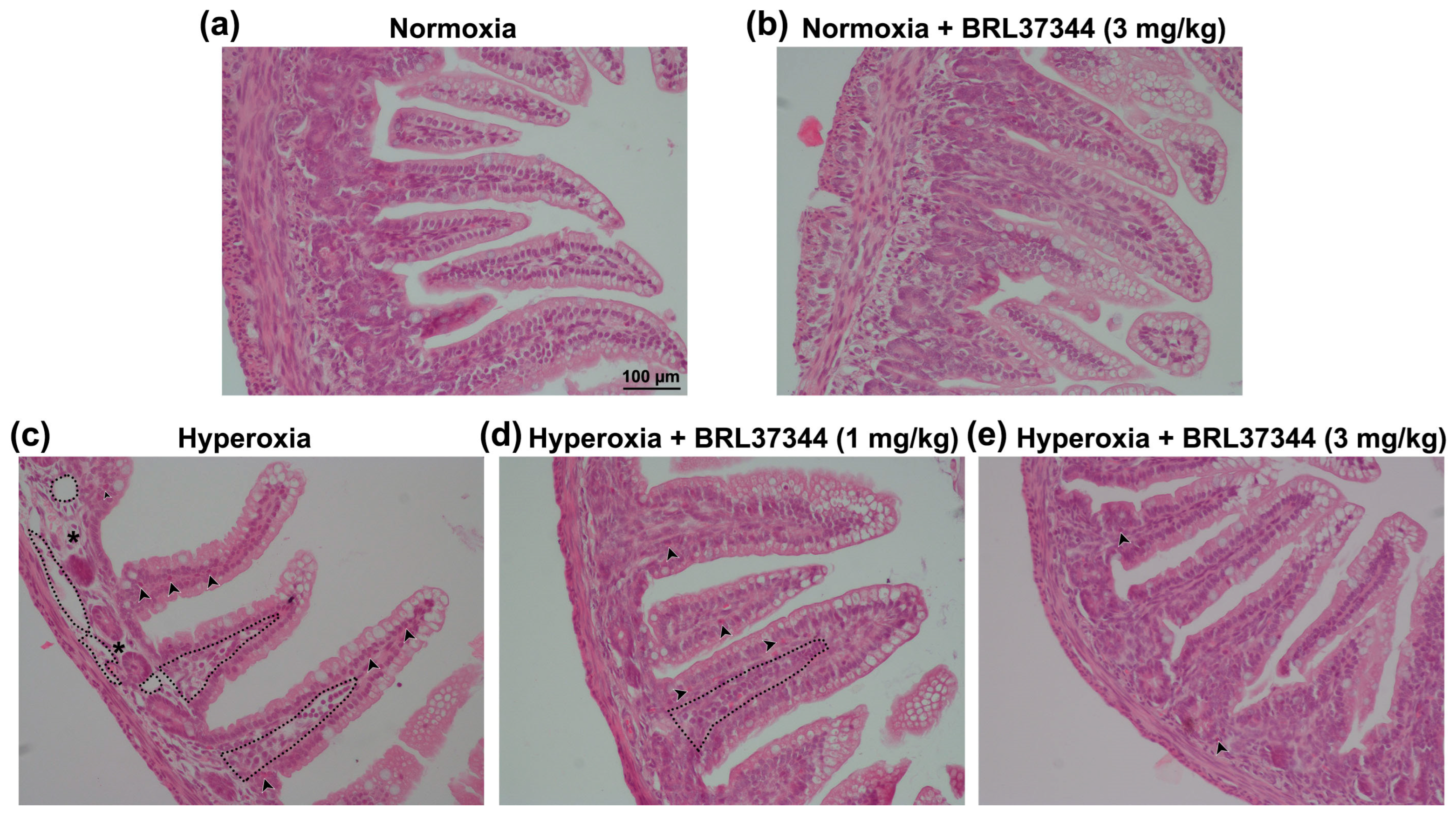
3.4. Histological Assessment of Mucosal Morphology
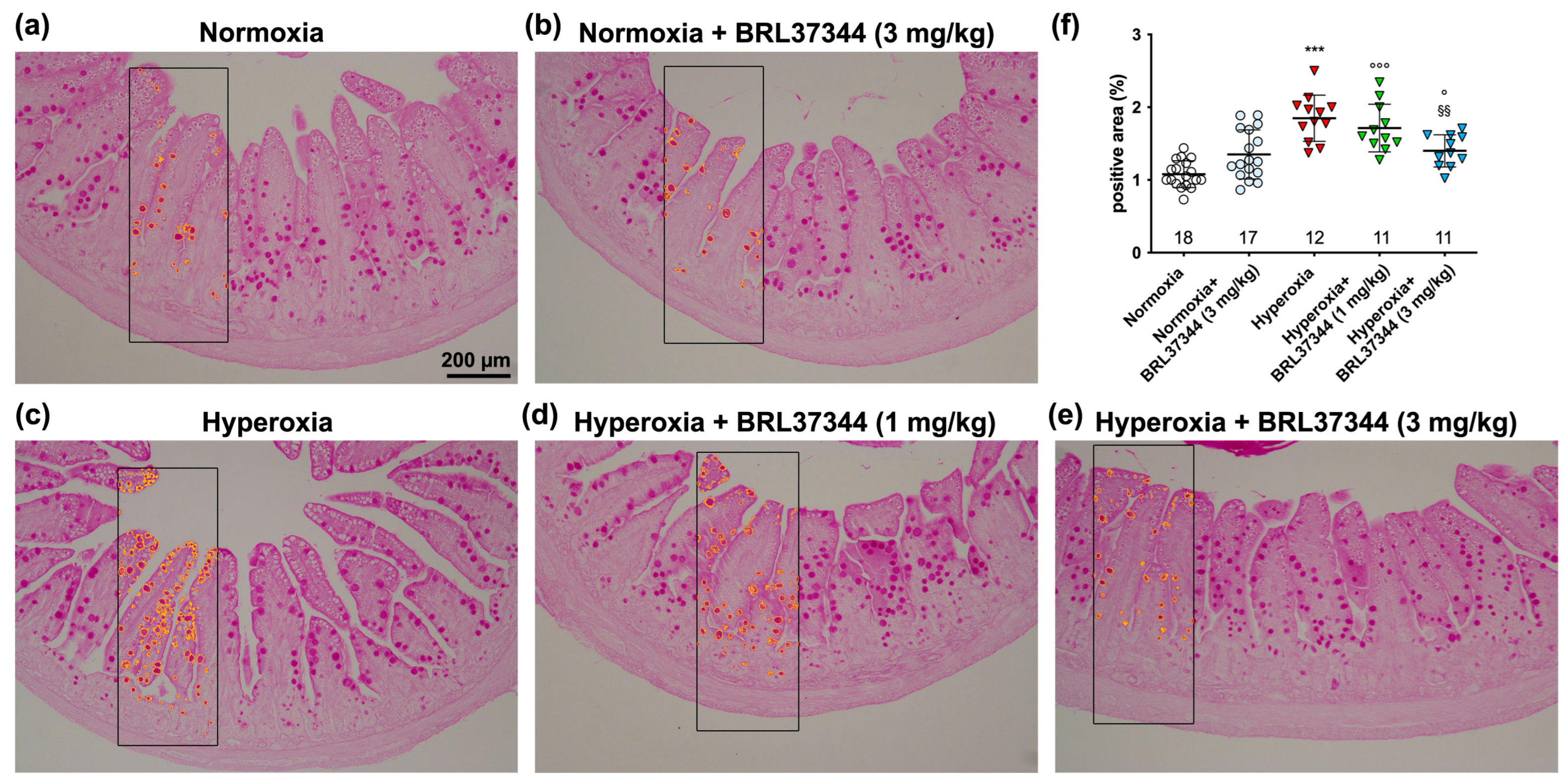
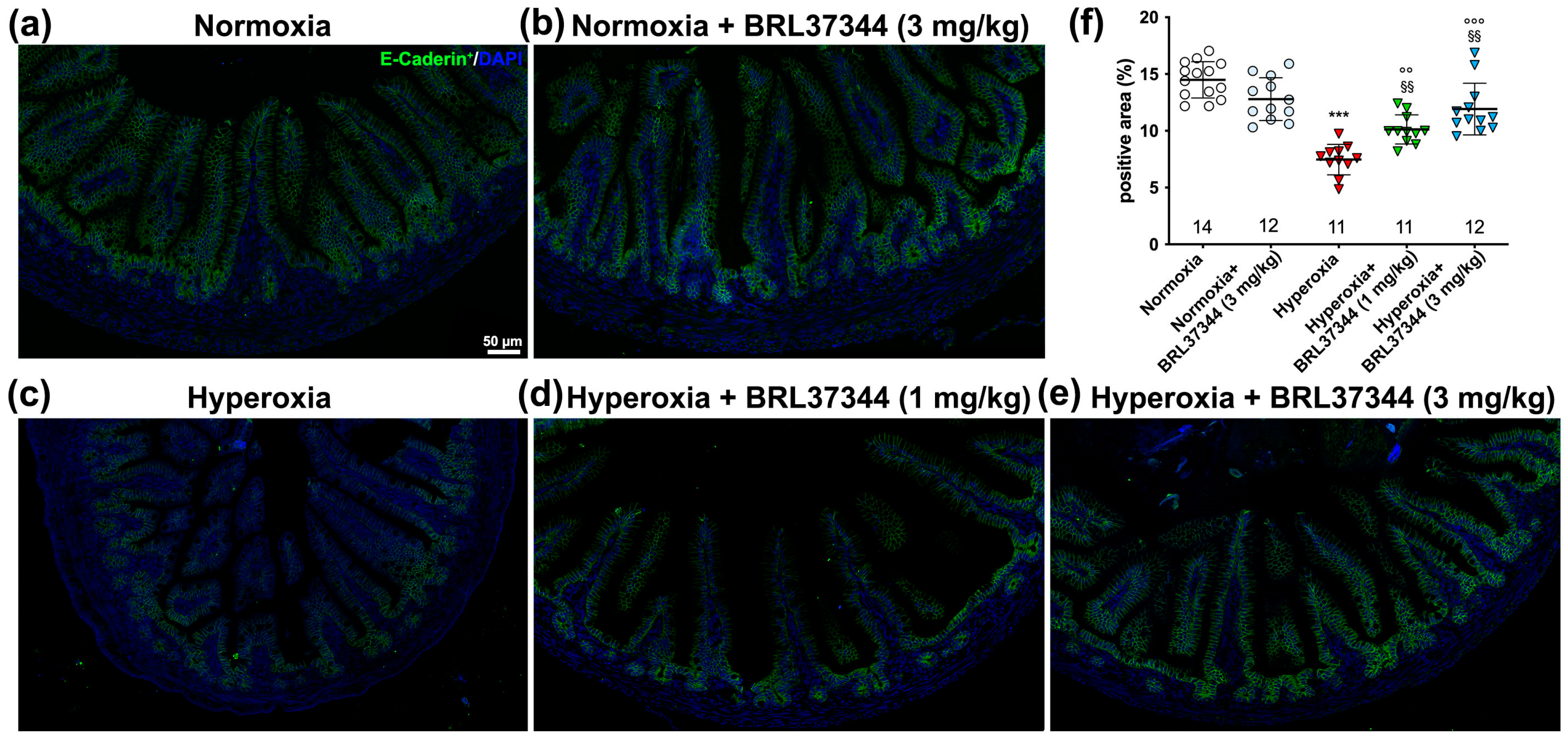
3.5. Immunofluorescent Analysis and Ultrastructural Evaluation of the Ileal Lining Epithelium
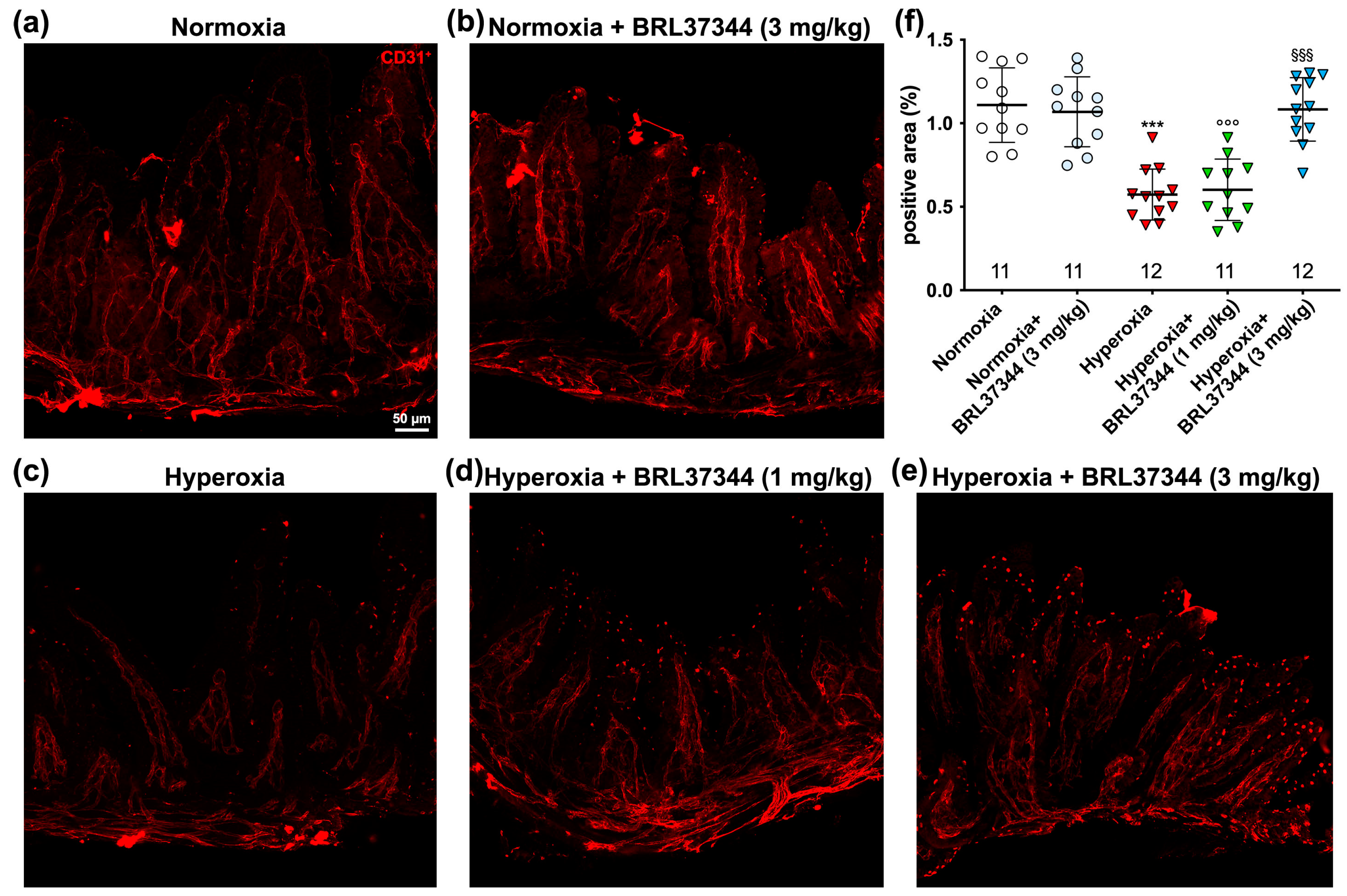
3.6. Immunofluorescent Analysis of Vascularization
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Imanirad, P.; Dzierzak, E. Hypoxia and HIFs in Regulating the Development of the Hematopoietic System. Blood Cells Mol. Dis. 2013, 51, 256–263. [Google Scholar] [CrossRef]
- Coelho-Santos, V.; Shih, A.Y. Postnatal Development of Cerebrovascular Structure and the Neurogliovascular Unit. Wiley Interdiscip. Rev. Dev. Biol. 2020, 9, e363. [Google Scholar] [CrossRef] [PubMed]
- O’Reilly, M.; Thébaud, B. Animal Models of Bronchopulmonary Dysplasia. The Term Rat Models. Am. J. Physiol. Lung Cell Mol. Physiol. 2014, 307, L948–L958. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-M.; Chou, H.-C. Hyperoxia Disrupts the Intestinal Barrier in Newborn Rats. Exp. Mol. Pathol. 2016, 101, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Filippi, L.; Pini, A.; Cammalleri, M.; Bagnoli, P.; Dal Monte, M. Β3-Adrenoceptor, a Novel Player in the Round-Trip from Neonatal Diseases to Cancer: Suggestive Clues from Embryo. Med. Res. Rev. 2022, 42, 1179–1201. [Google Scholar] [CrossRef]
- Hellström, A.; Smith, L.E.H.; Dammann, O. Retinopathy of Prematurity. Lancet 2013, 382, 1445–1457. [Google Scholar] [CrossRef]
- Giannone, P.J.; Bauer, J.A.; Schanbacher, B.L.; Reber, K.M. Effects of Hyperoxia on Postnatal Intestinal Development. Biotech. Histochem. 2007, 82, 17–22. [Google Scholar] [CrossRef]
- Schmidt, A.R.; Ramamoorthy, C. Bronchopulmonary Dysplasia. Paediatr. Anaesth. 2022, 32, 174–180. [Google Scholar] [CrossRef]
- Filippi, L.; Nardini, P.; Zizi, V.; Molino, M.; Fazi, C.; Calvani, M.; Carrozzo, F.; Cavallaro, G.; Giuseppetti, G.; Calosi, L.; et al. Β3 Adrenoceptor Agonism Prevents Hyperoxia-Induced Colonic Alterations. Biomolecules 2023, 13, 1755. [Google Scholar] [CrossRef]
- Li, N.; Ma, L.; Liu, X.; Shaw, L.; Li Calzi, S.; Grant, M.B.; Neu, J. Arginyl-Glutamine Dipeptide or Docosahexaenoic Acid Attenuates Hyperoxia-Induced Small Intestinal Injury in Neonatal Mice. J. Pediatr. Gastroenterol. Nutr. 2012, 54, 499–504. [Google Scholar] [CrossRef]
- Liu, D.Y.; Lou, W.J.; Zhang, D.Y.; Sun, S.Y. ROS Plays a Role in the Neonatal Rat Intestinal Barrier Damages Induced by Hyperoxia. Biomed. Res. Int. 2020, 2020, 8819195. [Google Scholar] [CrossRef]
- Torbati, D.; Tan, G.H.; Smith, S.; Frazier, K.S.; Gelvez, J.; Fakioglu, H.; Totapally, B.R. Multiple-Organ Effect of Normobaric Hyperoxia in Neonatal Rats. J. Crit. Care 2006, 21, 85–93; discussion 93–94. [Google Scholar] [CrossRef] [PubMed]
- Bundgaard, H.; Axelsson, A.; Hartvig Thomsen, J.; Sørgaard, M.; Kofoed, K.F.; Hasselbalch, R.; Fry, N.A.S.; Valeur, N.; Boesgaard, S.; Gustafsson, F.; et al. The First-in-Man Randomized Trial of a Beta3 Adrenoceptor Agonist in Chronic Heart Failure: The BEAT-HF Trial. Eur. J. Heart Fail. 2017, 19, 566–575. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, Y.; Kadoi, H.; Yamamuro, A.; Ishimaru, Y.; Maeda, S. Noradrenaline Increases Intracellular Glutathione in Human Astrocytoma U-251 MG Cells by Inducing Glutamate-Cysteine Ligase Protein via Β3-Adrenoceptor Stimulation. Eur. J. Pharmacol. 2016, 772, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Civantos Calzada, B.; Aleixandre de Artiñano, A. Alpha-Adrenoceptor Subtypes. Pharmacol. Res. 2001, 44, 195–208. [Google Scholar] [CrossRef] [PubMed]
- Summers, R.J.; Kompa, A.; Roberts, S.J. Beta-Adrenoceptor Subtypes and Their Desensitization Mechanisms. J. Auton. Pharmacol. 1997, 17, 331–343. [Google Scholar] [CrossRef] [PubMed]
- Emorine, L.J.; Marullo, S.; Briend-Sutren, M.M.; Patey, G.; Tate, K.; Delavier-Klutchko, C.; Strosberg, A.D. Molecular Characterization of the Human Beta 3-Adrenergic Receptor. Science 1989, 245, 1118–1121. [Google Scholar] [CrossRef] [PubMed]
- Fujinaga, M.; Scott, J.C. Gene Expression of Catecholamine Synthesizing Enzymes and Beta Adrenoceptor Subtypes during Rat Embryogenesis. Neurosci. Lett. 1997, 231, 108–112. [Google Scholar] [CrossRef] [PubMed]
- Resch, B.E.; Ducza, E.; Gáspár, R.; Falkay, G. Role of Adrenergic Receptor Subtypes in the Control of Human Placental Blood Vessels. Mol. Reprod. Dev. 2003, 66, 166–171. [Google Scholar] [CrossRef]
- Hynes, P.G.; Friel, A.M.; Smith, T.J.; Morrison, J.J. Beta-Adrenoceptor Subtype Expression in Human Placenta and Umbilical Arteries in Normal and Preeclamptic Pregnancies. Hypertens. Pregnancy 2008, 27, 169–181. [Google Scholar] [CrossRef]
- Pini, A.; Fazi, C.; Nardini, P.; Calvani, M.; Fabbri, S.; Guerrini, A.; Forni, G.; La Marca, G.; Rosa, A.C.; Filippi, L. Effect of Beta 3 Adrenoreceptor Modulation on Patency of the Ductus Arteriosus. Cells 2020, 9, 2625. [Google Scholar] [CrossRef] [PubMed]
- Cammalleri, M.; Amato, R.; Dal Monte, M.; Filippi, L.; Bagnoli, P. The Β3 Adrenoceptor in Proliferative Retinopathies: “Cinderella” Steps out of Its Family Shadow. Pharmacol. Res. 2023, 190, 106713. [Google Scholar] [CrossRef] [PubMed]
- Cero, C.; Lea, H.J.; Zhu, K.Y.; Shamsi, F.; Tseng, Y.-H.; Cypess, A.M. Β3-Adrenergic Receptors Regulate Human Brown/Beige Adipocyte Lipolysis and Thermogenesis. JCI Insight 2021, 6, e139160. [Google Scholar] [CrossRef] [PubMed]
- Michel, M.C.; Harding, S.E.; Bond, R.A. Are There Functional Β3-Adrenoceptors in the Human Heart? Br. J. Pharmacol. 2011, 162, 817–822. [Google Scholar] [CrossRef] [PubMed]
- Rouget, C.; Bardou, M.; Breuiller-Fouché, M.; Loustalot, C.; Qi, H.; Naline, E.; Croci, T.; Cabrol, D.; Advenier, C.; Leroy, M.J. Beta3-Adrenoceptor Is the Predominant Beta-Adrenoceptor Subtype in Human Myometrium and Its Expression Is up-Regulated in Pregnancy. J. Clin. Endocrinol. Metab. 2005, 90, 1644–1650. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.-C.; Hsieh, W.-C.; Lo, T.-S.; Huang, T.-X.; Chou, Y.-C.; Huang, J.-Y.; Huang, Y.-H. Urinary Beta 3-Adrenoceptor as a Diagnostic Biomarker for Overactive Bladder in Women. Sci. Rep. 2023, 13, 19368. [Google Scholar] [CrossRef] [PubMed]
- De Paermentier, F.; Cheetham, S.C.; Crompton, M.R.; Horton, R.W. Beta-Adrenoceptors in Human Brain Labelled with [3H]Dihydroalprenolol and [3H]CGP 12177. Eur. J. Pharmacol. 1989, 167, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.-K.; Tao, Y.-X. Physiology and Pathophysiology of the Beta3-Adrenergic Receptor. Prog. Mol. Biol. Transl. Sci. 2019, 161, 91–112. [Google Scholar] [CrossRef] [PubMed]
- Collins, J.M.; Hyland, N.P.; Clarke, G.; Fitzgerald, P.; Julio-Pieper, M.; Bulmer, D.C.; Dinan, T.G.; Cryan, J.F.; O’Mahony, S.M. Beta 3-Adrenoceptor Agonism Ameliorates Early-Life Stress-Induced Visceral Hypersensitivity in Male Rats. J. Neurochem. 2023, 1–14. [Google Scholar] [CrossRef]
- Zhang, M.; Xu, Y.; Chen, J.; Qin, C.; Liu, J.; Guo, D.; Wang, R.; Hu, J.; Zou, Q.; Yang, J.; et al. Beta3-Adrenergic Receptor Activation Alleviates Cardiac Dysfunction in Cardiac Hypertrophy by Regulating Oxidative Stress. Oxid. Med. Cell Longev. 2021, 2021, 3417242. [Google Scholar] [CrossRef]
- Yoshioka, Y.; Negoro, R.; Kadoi, H.; Motegi, T.; Shibagaki, F.; Yamamuro, A.; Ishimaru, Y.; Maeda, S. Noradrenaline Protects Neurons against H2O2-Induced Death by Increasing the Supply of Glutathione from Astrocytes via β3-Adrenoceptor Stimulation. J. Neurosci. Res. 2021, 99, 621–637. [Google Scholar] [CrossRef] [PubMed]
- Dyall, S.C.; Balas, L.; Bazan, N.G.; Brenna, J.T.; Chiang, N.; da Costa Souza, F.; Dalli, J.; Durand, T.; Galano, J.-M.; Lein, P.J.; et al. Polyunsaturated Fatty Acids and Fatty Acid-Derived Lipid Mediators: Recent Advances in the Understanding of Their Biosynthesis, Structures, and Functions. Prog. Lipid Res. 2022, 86, 101165. [Google Scholar] [CrossRef] [PubMed]
- Biagini, D.; Franzini, M.; Oliveri, P.; Lomonaco, T.; Ghimenti, S.; Bonini, A.; Vivaldi, F.; Macera, L.; Balas, L.; Durand, T.; et al. MS-Based Targeted Profiling of Oxylipins in COVID-19: A New Insight into Inflammation Regulation. Free Radic. Biol. Med. 2022, 180, 236–243. [Google Scholar] [CrossRef]
- Paavonsalo, S.; Subashi, Y.; Lackman, M.H.; Karaman, S. Whole-Mount Immunofluorescence Staining of Blood and Lymphatic Vessels in Murine Small Intestine. STAR Protoc. 2023, 4, 102310. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yuan, P.-Q.; Taché, Y. Vasculature in the Mouse Colon and Spatial Relationships with the Enteric Nervous System, Glia, and Immune Cells. Front. Neuroanat. 2023, 17, 1130169. [Google Scholar] [CrossRef] [PubMed]
- Durand, T.; Cracowski, J.L.; Guy, A.; Rossi, J.C. Syntheses and Preliminary Pharmacological Evaluation of the Two Epimers of the 5-F2t-Isoprostane. Bioorg Med. Chem. Lett. 2001, 11, 2495–2498. [Google Scholar] [CrossRef] [PubMed]
- Misharin, A.V.; Morales-Nebreda, L.; Mutlu, G.M.; Budinger, G.R.S.; Perlman, H. Flow Cytometric Analysis of Macrophages and Dendritic Cell Subsets in the Mouse Lung. Am. J. Respir. Cell Mol. Biol. 2013, 49, 503–510. [Google Scholar] [CrossRef]
- Calvani, M.; Bruno, G.; Dal Monte, M. Beta3-Adrenoceptor as a Potential Immuno-Suppressor Agent in Melanoma. Br. J. Pharmacol. 2019, 176, 2509–2524. [Google Scholar] [CrossRef] [PubMed]
- Bruno, G.; Nastasi, N.; Subbiani, A.; Boaretto, A.; Ciullini Mannurita, S.; Mattei, G.; Nardini, P.; Della Bella, C.; Magi, A.; Pini, A.; et al. Β3-Adrenergic Receptor on Tumor-Infiltrating Lymphocytes Sustains IFN-γ-Dependent PD-L1 Expression and Impairs Anti-Tumor Immunity in Neuroblastoma. Cancer Gene Ther. 2023, 30, 890–904. [Google Scholar] [CrossRef]
- Rosa, A.C.; Nardini, P.; Sgambellone, S.; Gurrieri, M.; Spampinato, S.F.; Dell’Accio, A.; Chazot, P.L.; Obara, I.; Liu, W.L.; Pini, A. CNS-Sparing Histamine H3 Receptor Antagonist as a Candidate to Prevent the Diabetes-Associated Gastrointestinal Symptoms. Biomolecules 2022, 12, 184. [Google Scholar] [CrossRef]
- Chou, H.-C.; Chen, C.-M. Neonatal Hyperoxia Disrupts the Intestinal Barrier and Impairs Intestinal Function in Rats. Exp. Mol. Pathol. 2017, 102, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Mödl, B.; Awad, M.; Zwolanek, D.; Scharf, I.; Schwertner, K.; Milovanovic, D.; Moser, D.; Schmidt, K.; Pjevac, P.; Hausmann, B.; et al. Defects in Microvillus Crosslinking Sensitize to Colitis and Inflammatory Bowel Disease. EMBO Rep. 2023, 24, e57084. [Google Scholar] [CrossRef] [PubMed]
- Mammoto, T.; Chen, J.; Jiang, E.; Jiang, A.; Smith, L.E.; Ingber, D.E.; Mammoto, A. LRP5 Regulates Development of Lung Microvessels and Alveoli through the Angiopoietin-Tie2 Pathway. PLoS ONE 2012, 7, e41596. [Google Scholar] [CrossRef] [PubMed]
- Filippi, L.; Cammalleri, M.; Amato, R.; Ciantelli, M.; Pini, A.; Bagnoli, P.; Dal Monte, M. Decoupling Oxygen Tension From Retinal Vascularization as a New Perspective for Management of Retinopathy of Prematurity. New Opportunities From β-Adrenoceptors. Front. Pharmacol. 2022, 13, 835771. [Google Scholar] [CrossRef] [PubMed]
- Hannah, R.S.; Hannah, K.J. Hyperoxia: Effects on the Vascularization of the Developing Central Nervous System. Acta Neuropathol. 1980, 51, 141–144. [Google Scholar] [CrossRef] [PubMed]
- Attaye, I.; Smulders, Y.M.; de Waard, M.C.; Oudemans-van Straaten, H.M.; Smit, B.; Van Wijhe, M.H.; Musters, R.J.; Koolwijk, P.; Spoelstra-de Man, A.M.E. The Effects of Hyperoxia on Microvascular Endothelial Cell Proliferation and Production of Vaso-Active Substances. Intensive Care Med. Exp. 2017, 5, 22. [Google Scholar] [CrossRef] [PubMed]
- Nechuta, S.; Cai, Q.; Zheng, Y.; Milne, G.L.; Cai, H.; Dai, Q.; Yang, G.; Zheng, W.; Lu, W.; Shu, X.O. Urinary Biomarkers of Oxidative Stress and Breast Cancer Survival. Cancer Causes Control 2014, 25, 701–707. [Google Scholar] [CrossRef] [PubMed]
- Barden, A.; Mas, E.; Henry, P.; Durand, T.; Galano, J.-M.; Roberts, L.J.; Croft, K.D.; Mori, T.A. The Effects of Oxidation Products of Arachidonic Acid and N3 Fatty Acids on Vascular and Platelet Function. Free Radic. Res. 2011, 45, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Pasha, A.; Vignoli, M.; Subbiani, A.; Nocentini, A.; Selleri, S.; Gratteri, P.; Dabraio, A.; Casini, T.; Filippi, L.; Fotzi, I.; et al. Β3-Adrenoreceptor Activity Limits Apigenin Efficacy in Ewing Sarcoma Cells: A Dual Approach to Prevent Cell Survival. Int. J. Mol. Sci. 2019, 20, 2149. [Google Scholar] [CrossRef]
- Amato, R.; Pisani, F.; Laudadio, E.; Cammalleri, M.; Lucchesi, M.; Marracci, S.; Filippi, L.; Galeazzi, R.; Svelto, M.; Dal Monte, M.; et al. HIF-1-Dependent Induction of Β3 Adrenoceptor: Evidence from the Mouse Retina. Cells 2022, 11, 1271. [Google Scholar] [CrossRef]
- Henning, S.J. Postnatal Development: Coordination of Feeding, Digestion, and Metabolism. Am. J. Physiol. 1981, 241, G199–G214. [Google Scholar] [CrossRef] [PubMed]
- Gephart, S.M.; McGrath, J.M.; Effken, J.A.; Halpern, M.D. Necrotizing Enterocolitis Risk: State of the Science. Adv. Neonatal Care 2012, 12, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Kanzler, S.A.; Januario, A.C.; Paschoalini, M.A. Involvement of Β3-Adrenergic Receptors in the Control of Food Intake in Rats. Braz. J. Med. Biol. Res. 2011, 44, 1141–1147. [Google Scholar] [CrossRef] [PubMed]
- Richard, J.E.; López-Ferreras, L.; Chanclón, B.; Eerola, K.; Micallef, P.; Skibicka, K.P.; Wernstedt Asterholm, I. CNS β3-Adrenergic Receptor Activation Regulates Feeding Behavior, White Fat Browning, and Body Weight. Am. J. Physiol. Endocrinol. Metab. 2017, 313, E344–E358. [Google Scholar] [CrossRef] [PubMed]
- Elgin, T.G.; Fricke, E.M.; Gong, H.; Reese, J.; Mills, D.A.; Kalantera, K.M.; Underwood, M.A.; McElroy, S.J. Fetal Exposure to Maternal Inflammation Interrupts Murine Intestinal Development and Increases Susceptibility to Neonatal Intestinal Injury. Dis. Model. Mech. 2019, 12, dmm040808. [Google Scholar] [CrossRef] [PubMed]
- Clark, J.A.; Doelle, S.M.; Halpern, M.D.; Saunders, T.A.; Holubec, H.; Dvorak, K.; Boitano, S.A.; Dvorak, B. Intestinal Barrier Failure during Experimental Necrotizing Enterocolitis: Protective Effect of EGF Treatment. Am. J. Physiol. Gastrointest. Liver Physiol. 2006, 291, G938–G949. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Hock, A.; Wu, R.Y.; Minich, A.; Botts, S.R.; Lee, C.; Antounians, L.; Miyake, H.; Koike, Y.; Chen, Y.; et al. Bovine Milk-Derived Exosomes Enhance Goblet Cell Activity and Prevent the Development of Experimental Necrotizing Enterocolitis. PLoS ONE 2019, 14, e0211431. [Google Scholar] [CrossRef]
- Hackam, D.J.; Sodhi, C.P. Bench to Bedside—New Insights into the Pathogenesis of Necrotizing Enterocolitis. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 468–479. [Google Scholar] [CrossRef]
- Wang, H.-C.; Chou, H.-C.; Chen, C.-M. Molecular Mechanisms of Hyperoxia-Induced Neonatal Intestinal Injury. Int. J. Mol. Sci. 2023, 24, 4366. [Google Scholar] [CrossRef]
- Li, L.; Peng, P.; Ding, N.; Jia, W.; Huang, C.; Tang, Y. Oxidative Stress, Inflammation, Gut Dysbiosis: What Can Polyphenols Do in Inflammatory Bowel Disease? Antioxidants 2023, 12, 967. [Google Scholar] [CrossRef]
- Sim, K.; Shaw, A.G.; Randell, P.; Cox, M.J.; McClure, Z.E.; Li, M.-S.; Haddad, M.; Langford, P.R.; Cookson, W.O.C.M.; Moffatt, M.F.; et al. Dysbiosis Anticipating Necrotizing Enterocolitis in Very Premature Infants. Clin. Infect. Dis. 2015, 60, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Khandagale, A.; Reinhardt, C. Gut Microbiota—Architects of Small Intestinal Capillaries. Front Biosci. (Landmark Ed.) 2018, 23, 752–766. [Google Scholar] [CrossRef]
- Goldie, L.C.; Nix, M.K.; Hirschi, K.K. Embryonic Vasculogenesis and Hematopoietic Specification. Organogenesis 2008, 4, 257–263. [Google Scholar] [CrossRef]
- Kim, K.E.; Sung, H.-K.; Koh, G.Y. Lymphatic Development in Mouse Small Intestine. Dev. Dyn. 2007, 236, 2020–2025. [Google Scholar] [CrossRef]
- Brueckl, C.; Kaestle, S.; Kerem, A.; Habazettl, H.; Krombach, F.; Kuppe, H.; Kuebler, W.M. Hyperoxia-Induced Reactive Oxygen Species Formation in Pulmonary Capillary Endothelial Cells in Situ. Am. J. Respir. Cell Mol. Biol. 2006, 34, 453–463. [Google Scholar] [CrossRef] [PubMed]
- Yung, L.M.; Leung, F.P.; Yao, X.; Chen, Z.-Y.; Huang, Y. Reactive Oxygen Species in Vascular Wall. Cardiovasc. Hematol. Disord. Drug Targets 2006, 6, 1–19. [Google Scholar] [CrossRef]
- D’Amore, P.A.; Sweet, E. Effects of Hyperoxia on Microvascular Cells in Vitro. Vitro Cell Dev. Biol. 1987, 23, 123–128. [Google Scholar] [CrossRef]
- Dal Monte, M.; Cammalleri, M.; Mattei, E.; Filippi, L.; Bagnoli, P. Protective Effects of Beta1/2 Adrenergic Receptor Deletion in a Model of Oxygen-Induced Retinopathy. Investig. Ophthalmol. Vis. Sci. 2014, 56, 59–73. [Google Scholar] [CrossRef]
- Buczynski, B.W.; Maduekwe, E.T.; O’Reilly, M.A. The Role of Hyperoxia in the Pathogenesis of Experimental BPD. Semin. Perinatol. 2013, 37, 69–78. [Google Scholar] [CrossRef]
- Bhatt, A.J.; Pryhuber, G.S.; Huyck, H.; Watkins, R.H.; Metlay, L.A.; Maniscalco, W.M. Disrupted Pulmonary Vasculature and Decreased Vascular Endothelial Growth Factor, Flt-1, and TIE-2 in Human Infants Dying with Bronchopulmonary Dysplasia. Am. J. Respir. Crit. Care Med. 2001, 164, 1971–1980. [Google Scholar] [CrossRef]
- Gerstner, B.; DeSilva, T.M.; Genz, K.; Armstrong, A.; Brehmer, F.; Neve, R.L.; Felderhoff-Mueser, U.; Volpe, J.J.; Rosenberg, P.A. Hyperoxia Causes Maturation-Dependent Cell Death in the Developing White Matter. J. Neurosci. 2008, 28, 1236–1245. [Google Scholar] [CrossRef]
- Li, Y.; Tao, Y.; Xu, J.; He, Y.; Zhang, W.; Jiang, Z.; He, Y.; Liu, H.; Chen, M.; Zhang, W.; et al. Hyperoxia Provokes Time- and Dose-Dependent Gut Injury and Endotoxemia and Alters Gut Microbiome and Transcriptome in Mice. Front Med. (Lausanne) 2021, 8, 732039. [Google Scholar] [CrossRef]
- Lo, Y.-C.; Chen, K.-Y.; Chou, H.-C.; Lin, I.-H.; Chen, C.-M. Neonatal Hyperoxia Induces Gut Dysbiosis and Behavioral Changes in Adolescent Mice. J. Chin. Med. Assoc. 2021, 84, 290–298. [Google Scholar] [CrossRef]
- Arboleya, S.; Rios-Covian, D.; Maillard, F.; Langella, P.; Gueimonde, M.; Martín, R. Preterm Delivery: Microbial Dysbiosis, Gut Inflammation and Hyperpermeability. Front. Microbiol. 2021, 12, 806338. [Google Scholar] [CrossRef]
- Hoffmann, C.; Leitz, M.R.; Oberdorf-Maass, S.; Lohse, M.J.; Klotz, K.-N. Comparative Pharmacology of Human Beta-Adrenergic Receptor Subtypes--Characterization of Stably Transfected Receptors in CHO Cells. Naunyn Schmiedebergs Arch. Pharmacol. 2004, 369, 151–159. [Google Scholar] [CrossRef]
- Schena, G.; Caplan, M.J. Everything You Always Wanted to Know about β3-AR * (* But Were Afraid to Ask). Cells 2019, 8, 357. [Google Scholar] [CrossRef] [PubMed]
- Barbier, M.; Attoub, S.; Joubert, M.; Bado, A.; Laboisse, C.; Cherbut, C.; Galmiche, J.P. Proinflammatory Role of Leptin in Experimental Colitis in Rats Benefit of Cholecystokinin-B Antagonist and Beta3-Agonist. Life Sci. 2001, 69, 567–580. [Google Scholar] [CrossRef]
- Ngala, R.A.; O’Dowd, J.; Wang, S.J.; Stocker, C.; Cawthorne, M.A.; Arch, J.R.S. Beta2-Adrenoceptors and Non-Beta-Adrenoceptors Mediate Effects of BRL37344 and Clenbuterol on Glucose Uptake in Soleus Muscle: Studies Using Knockout Mice. Br. J. Pharmacol. 2009, 158, 1676–1682. [Google Scholar] [CrossRef]
- Li, T.-M.; Liu, D.-Y. Mechanism of Neonatal Intestinal Injury Induced by Hyperoxia Therapy. J. Immunol. Res. 2022, 2022, 2316368. [Google Scholar] [CrossRef]
- Wang, P.; Li, T.; Niu, C.; Sun, S.; Liu, D. ROS-Activated MAPK/ERK Pathway Regulates Crosstalk between Nrf2 and Hif-1α to Promote IL-17D Expression Protecting the Intestinal Epithelial Barrier under Hyperoxia. Int. Immunopharmacol. 2023, 116, 109763. [Google Scholar] [CrossRef]



| Experimental Group | Age (d) | Body Weight (g) | Age (d) | Body Weight (g) |
|---|---|---|---|---|
| Normoxia | 0 | 6.4 ± 0.1 | 14 | 33.1 ± 0.6 |
| Normoxia + BRL37344 (3 mg/kg) | 0 | 6.5 ± 0.2 | 14 | 31.9 ± 1.1 |
| Hyperoxia | 0 | 6.3 ± 0.2 | 14 | 21.0 ± 0.8 *** |
| Hyperoxia + BRL37344 (1 mg/kg) | 0 | 6.3 ± 0.3 | 14 | 21.7 ± 0.7 °°° |
| Hyperoxia + BRL37344 (3 mg/kg) | 0 | 6.4 ± 0.2 | 14 | 23.6 ± 1.6 °°° |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nardini, P.; Zizi, V.; Molino, M.; Fazi, C.; Calvani, M.; Carrozzo, F.; Giuseppetti, G.; Calosi, L.; Guasti, D.; Biagini, D.; et al. Protective Effects of Beta-3 Adrenoceptor Agonism on Mucosal Integrity in Hyperoxia-Induced Ileal Alterations. Antioxidants 2024, 13, 863. https://doi.org/10.3390/antiox13070863
Nardini P, Zizi V, Molino M, Fazi C, Calvani M, Carrozzo F, Giuseppetti G, Calosi L, Guasti D, Biagini D, et al. Protective Effects of Beta-3 Adrenoceptor Agonism on Mucosal Integrity in Hyperoxia-Induced Ileal Alterations. Antioxidants. 2024; 13(7):863. https://doi.org/10.3390/antiox13070863
Chicago/Turabian StyleNardini, Patrizia, Virginia Zizi, Marta Molino, Camilla Fazi, Maura Calvani, Francesco Carrozzo, Giorgia Giuseppetti, Laura Calosi, Daniele Guasti, Denise Biagini, and et al. 2024. "Protective Effects of Beta-3 Adrenoceptor Agonism on Mucosal Integrity in Hyperoxia-Induced Ileal Alterations" Antioxidants 13, no. 7: 863. https://doi.org/10.3390/antiox13070863







