The Potential Health Benefits of Gallic Acid: Therapeutic and Food Applications
Abstract
:1. Introduction
2. Chemical Structure and Various Sources
3. Identification and Quantification Techniques
3.1. Chromatography Techniques
3.1.1. High-Performance Liquid Chromatography (HPLC)
3.1.2. Gas Chromatography (GC)
3.1.3. High-Performance Thin-Layer Chromatography (HPTLC)
3.2. Capillary Electrophoresis (CE)
3.3. Spectroscopic Techniques
Nuclear Magnetic Resonance (NMR)
3.4. Other Techniques
4. Biological and Therapeutic Properties
4.1. Antibacterial Activity
4.1.1. Modification of Cytoplasmic Membrane Function
4.1.2. Disruption of Intracellular Functions
| Sample/Matrix Type | Bacterial Strains | Antibacterial Assay | Results | Mechanism of Action | References |
|---|---|---|---|---|---|
| GA-grafted chitosan | Staphylococcus aureus Bacillus subtilis Bacillus cereus Enterococcus faecalis Listeria monocytogenes Escherichia coli Klebsiella pneumoniae Pseudomonas aeruginosa Salmonella typhimurium Shigella flexneri | Broth dilution method Time–kill experiment against E. coli and S. aureus OM and IM permeabilization assay | MIC values from unmodified chitosan are 64–128 μg/mL against Gram-positive bacteria and 512–1024 μg/mL against Gram-negative bacteria while MIC values from GA-grafted chitosan are 16 to 64 μg/mL against Gram-positive bacteria and 128 to 512 μg/mL against Gram-negative bacteria. GA-grafted chitosan (I) at MIC suppressed both E. coli bacterial growth for 24 h and S. aureus bacteria. Moreover, over the MIC values, no viable cells were observed. OM and IM permeabilization experiments indicated that GA-crafted chitosan influenced the integrity of the membrane | Disruption of the cell membranes by GA-crafted chitosan. S. aureus and E. coli cells undergo cell membrane damage resulting in the release of their cellular components into the surrounding environment, becoming finally empty | [110] |
| 3D chitosan–GA complexes | E. coli S. aureus | Broth dilution method | 3D chitosan–GA complexes demonstrated a higher antimicrobial capacity than 3D chitosan alone, with an inhibition percentage of around 83% more than 3D chitosan alone, regardless of the bacterial strain and concentration used, indicating that the adsorption of GA effectively increases the antimicrobial activity of 3D chitosan | ND | [103] |
| Native pectin (Na-Pe) acylated with GA (Ac-Pe) | E. coli S. aureus | OD method | The inhibition rate of the pectin against E. coli and S. aureus improved from 2.93% and 8.92% (Na-Pe) to 26.95% and 42.18% (Ac1-Pe) and 31.56% and 47.87% (Ac2-Pe), respectively | ND | [104] |
| GA-loaded ovalbumin (OVA)–chitosan (CS) nanoparticles | Morganella morganii E. coli | Plate count method | The number of M. morganii was 1.7 × 109 CFU/mL for pectin film, 8.7 × 108 CFU/mL for GA–pectin film, 5.5 × 108 CFU/mL for pectin film with OVA/CS nanoparticles, and 3.2 × 108 for GA-loaded OVA/CS nanoparticles. On the other hand, compared with pure pectin film (2.7 × 1010 CFU/mL), the pectin film with GA-loaded OVA/CS nanoparticles (5.8 × 109 CFU/mL) could retard the growth of E. coli Moreover, the quantity of histamine (toxic compound produced during food spoilage) was also measured, showing that the growth rate of this amine in salmon fillets treated with the pectin coating with GA-loaded OVA/CS nanoparticles was the lowest (51.6%) compared with the control group (140.0%) | ND | [105] |
| Chitosan (Ch) and zinc oxide nanoparticle-loaded gallic acid films, (Ch-ZnO@gal | B. subtilis E. coli | Agar well diffusion assay | The results of the antibacterial activity of Ch-ZnO@gal revealed that the antimicrobial activity is linearly related to the amount of GA in the nanoparticles, Ch-ZnO@gal3 (70 mg of ZnO@gal) being the most efficient film against both B. subtilis and E. coli bacteria | The released ROS from the ZnO@gal, together with Zn2+ ions, attack the negatively charged cell wall, which leads to leakage and ultimately death of bacteria | [121] |
| PVDF-grafted GA (PVDF-g-PGAL) | S. aureus E. coli | Plate growth inhibition assay | M0 (unmodified PVDF) showed minimal or no inhibition zones, indicating no antibacterial activity. In contrast, M3 (PVDF-grafted GA) showed clear zones of inhibition around the membrane, indicating antibacterial activity against E. coli and S. aureus. Furthermore, M3-SO3Na (PVDF-grafted GA + sodium sulphonate) showed higher inhibition zones compared to M3, due to the increased hydrophilicity of the membrane | The hydrophilic membrane reduces the chances of bacterial colonies establishing themselves and proliferating on its surface. In addition, hydrophilic surfaces attract water molecules, creating a thin aqueous layer on the membrane surface. This layer can inhibit bacterial adhesion and biofilm formation, as bacteria prefer to adhere to dry surfaces | [122] |
| Commercially obtained GA | K. pneumoniae | Broth dilution method | K. pneumoniae growth was reduced at 5 and 10 mM GA concentrations, but not at 2.5 mM | GA may affect the iron availability in K. pneumoniae, thus possibly repressing the cps transcription (the inhibition in the production of capsules reduces bacterial virulence) | [123] |
| Commercially obtained GA | E. coli P. aeruginosa S. aureus L. monocytogenes | Broth dilution method, physicochemical characterization of the bacterial surfaces | GA had antimicrobial activity against the bacteria tested, with a MIC of 1500 mg/mL for E. coli, 500 mg/mL for P. aeruginosa, 1750 mg/mL for S. aureus, and 2000 mg/mL for L. monocytogenes. In addition, GA also had bactericidal activity due to the MBC values for GA: 5000 mg/mL for E. coli, 500 mg/mL for P. aeruginosa, 5250 mg/mL for S. aureus, and 5500 mg/mL for L. monocytogenes | GA led to irreversible changes in membrane properties (charge, intra- and extracellular permeability, and physicochemical properties) through hydrophobicity changes, a decrease in negative surface charge, and the occurrence of local ruptures or pore formation in the cell membranes, with a consequent leakage of essential intracellular constituents | [124] |
| Commercially obtained GA | S. flexneri | - Broth dilution method - Time-dependent killing assay Antibacterial assays to elucidate the mechanism of action: viability assay, integrity of cell membrane, FESEM analysis | - GA showed a MIC value of 2 mg/mL and MBC value of 8 mg/mL against Sh. Flexneri. It showed that GA led to inhibitory effects, which was evidenced by reduced cell viability, destroyed cell membranes, and changes in bacterial morphology | GA effectively inhibited Sh. flexneri activity and its biofilm formation by regulating the expression of the mdoH gene and the OpgH protein (mutations in mdoH that affect OpgH function may reduce bacterial virulence) | [125] |
| Commercially obtained GA | Proteus spp. E. coli Pseudomonas spp. Salmonella spp. Streptococcus spp. S. aureus | Bacterial growth inhibition assay with OD measurement Petri dish biofilm assay Measurement of cell biomass concentration and EPS quantification | Different concentrations (1–200 mg/L) of GA showed antimicrobial effects by reducing the growth of single and multispecies bacteria (12–86%). Higher concentrations (100–200 mg/L) of GA had prominent inhibitory effects on biofilm formation. Further, GA (20–200 mg/L) exhibited a 93.43% biomass reduction and 88.6% EPS (polysaccharide) reduction | GA can reduce biofilm formation and EPS, where it is suspected to be the major reason of biofilm development | [126] |
| Commercially obtained GA | Streptococcus mutans | Broth dilution method Antibiofilm assays (pH drop test and proton permeability test) | GA showed a MIC value at 250 μg/mL, although GA did not inhibit the adherence of S. mutans in sub-MIC values. Regarding the antibiofilm assay, GA showed antimicrobial activity, reducing the number of viable cells ( vs. ). Moreover, GA sensitized the cells to acidification, thereby reducing the glycolysis and acid production in the biofilm | The biofilms treated with GA showed a different architecture–structure: less compact and less dense due to a reduction in the synthesis of glucans (produced by a downregulated expression of gtfB, gtfC, and gtfD genes in the biofilms). These changes in the biofilm structure occurred because of the bactericidal activity, reduction of soluble alkaline glucans, and acidogenicity of S. mutans by GA | [127] |
| Commercially obtained GA | Paenibacillus larvae | Microdilution method Agar well diffusion assay Spore germination assay | The MIC and MBC values of GA against P. larvae were 125 and 250 μg/mL GA (200 mg/mL) produced an average inhibition halo of 17.7 ± 0.39 mm against P. larvae in the agar well diffusion assay. In the presence of 125 and 250 μg/mL GA, spore germination rates were reduced to 83.9% and 18.7%, respectively | GA resulted in the leakage of proteins and nucleic acids (vital intracellular components of the bacteria), leading to bacterial death. Moreover, GA-mediated membrane and intracellular damage, together with its capacity for restricting biofilm formation, increase bacterial susceptibility to other antibacterial agents and eventually cause lethal effects | [128] |
4.1.3. Programmed Cell Death (PCD)
4.2. Antioxidant Activity
4.3. Anticancer Mechanisms of GA
4.4. Antiviral Activity
4.5. Anti-Alzheimer Activity
4.6. Anti-Inflammatory
4.7. Anti-Diabetes
| Model | Condition | Main Findings | Reference |
|---|---|---|---|
| Anti-inflammatory | |||
| Rats | In vivo | - Dose-dependent decreases in IL-6 and TNF-α levels. | [250] |
| Suppressing NF-κB signaling pathway in IPEC-J2 cells | In vitro | - In IPEC-J2 cells, GA pretreatment significantly decreased the elevated expression of tumor necrosis factor-α and interleukin-8 genes induced by LPS. | [26] |
| Control inflammation in NAFLD and NASH | In vitro | - By activating AMP-activated protein kinase (AMPK) in HepG2 cells, GA reduced the fat accumulation induced by palmitic acid. | [251] |
| Nrf2 antioxidant response element signaling pathway | In vitro and in vivo | - The PM10 groups exhibited a substantial increase in epithelial permeability and inflammatory markers. - Additionally, there was a notable reduction in the expression of Nrf2 and its upstream regulator genes. | [147] |
| Mice, suppressed interleukin-33 and group 2 innate lymphoid cells | - | - GA was found to lower IL-13 and IL-5 levels in bronchoalveolar lavage fluid (BALF) and to reduce IL-33 expression in lung tissue. This effect is accomplished by inhibiting MyD88 expression and downregulating NF-κB signaling pathways, leading to a reduction in IL-33 production. | [252] |
| Al2O3-induced myocardial injury | - | - ↓ CPK, LDH, CK-MB, MDA, LDL, TNF-α, and TG - ↑ SOD, HDL, CAT, and GSH | [253] |
| STZ-induced oxidative stress in testis of rats | - | ↓ TNF-α, NOS2, VEGF, and MDA | [254] |
| Antidiabetic | |||
| Mice, enhances insulin sensitivity and glucose transporters via Akt and PPAR-γ signaling pathways. | 2–20 µM | - GA treatment enhanced insulin sensitivity by activating the Akt signaling pathway rather than the AMPK signaling pathway. | [255] |
| Mice, enhances lipid profile, glycemic and liver function | 8.436 mg | - Promotes the repair of tissue damage in the pancreas and liver. - GA regulates autophagy in a diabetic cell model using Rin-5F cells. | [256] |
| Mice | 50 mg/kg | - GA regulated lipid peroxidation (measured by TBARS) and antioxidant enzymes (GPX, superoxide dismutase, and catalase) in the liver and kidney, which are affected by diabetes-related complications caused by hyperglycemia. | [257] |
| - Mice 4- and 9-month-old groups - APPswe/PS1dE9 transgenic | 30 mg/kg through gavage | - LTP - Aβ1–42 aggregation - Cognitive deficits - Expression of synaptic marker proteins | [258] |
| STZ-induced diabetic rat | 20 mg/kg | - Reduces TNF-α levels, while increasing the upregulation of adiponectin and PPARγ mRNA. | [259] |
| Rat, Aβ hippocampal injection | 50, 100, 200 mg/kg | - Hippocampal LTP | [260] |
| Pheochromocytoma12 cells | GA: Aβ 2.0: 1.0 M | - Toxicity - K-CN fibril formation | [261] |
| Mice, scopolamine-induced amnesia | 10 mg/kg | - AChE activity - Transfer latency in the elevated plus maze (EPM) test - Duration spent in the target quadrant during the Morris water maze (MWM) test | [262] |
| Rat, i.p. injection of TMT 8 mg/kg | 50, 100 mg/kg | - Hippocampal level of TNF-a - Hippocampal level of BDNF | [263] |
| Mice, via mTOR/PPARγ/AMPK signaling | 3 mg | - Decreased expression levels of p-AMPK and increased levels of peroxisome proliferator-activated receptor gamma (PPARγ), LOX-1, NF-κB, COX-2, and p-mTOR. | [236] |
4.8. Anti-Obesity
4.9. Anti-Hypertensive
5. Food Applications
5.1. Active Packaging Systems
5.1.1. Fish and Seafood Products
| Food Products | Film/Coating Matrix | GA Concentration | Other Active Compounds | Storage Conditions | Highlights | References |
|---|---|---|---|---|---|---|
| Fish and seafood products | ||||||
| Japanese sea bass (Lateolabrax japonicus) fillets | Pectin | 5% (w/v) | - | 20 days, 4 °C | □ Lower TVB-N, lipid oxidation, and total sulfhydryl groups □ The coated samples showed the highest sensory quality rating | [282] |
| CS | 15 mM | PA | 10 days, 4 °C | □ CS-grafted GA showed a higher GR (110.82 mg GA/g) than CS-grafted PA (62.63 mg PA/g) □ Higher thermal, rheological, and antioxidant properties than pure CS □ Delayed the deterioration of texture, color, and sensory quality | [283] | |
| Tilapia (Orechromis niloticus) fillets | PE + CS | 1% (w/w) | - | 14 days, 4 °C | □ Higher antioxidant and antimicrobial activities □ Inhibited TVC and TVB-N □ Lower TBARS value on day 14 | [284] |
| Collagen + zein | 1–10% (w/w) | - | 10 days, 4 °C | □ The electrospun fibers exhibited a smooth nanostructure with no beads □ GA formed hydrogen bonds with the protein matrix □ Prolonged the shelf life of the fillets for at least two days, especially at a concentration of 8% | [285] | |
| Pacific mackerel (Pneumatophorus japonicus) fillets | CS | 5% (w/v) | - | 12 days, 4 °C | □ Inhibited protein decomposition, nucleotide breakdown, microbial growth, and lipid oxidation up to 6 days □ Delayed the deterioration of sensory quality | [286] |
| Horse mackerel (Trachurus trachurus) fillets | 10 wt% | - | 14 days, 4 °C | □ Decreased microbial growth in more than two log cycles □ Lower TVB-N and TBARS values | [287] | |
| Yellowfin tuna (Thunnus albacares) fillets | Zein + gelatin | 1 g | PL | 15 days, 4 °C | □ Higher average diameter with well-distributed morphology □ Improved thermal, antioxidant, and antimicrobial properties □ The combined films effectively inhibited TVC, TVB-N, lipid oxidation, and texture deterioration up to 3 days | [288] |
| Atlantic salmon (Salmo salar) fillets | Gelatin + CS | 0.2% (w/v) | CO | 15 days, 4 °C | □ Higher antioxidant and antimicrobial activities □ The combined coatings prolonged the shelf life of the fillets up to 5 days | [289] |
| Grass carp (Ctenopharyngodon idellus) fillets | Agarose | 0.0350–0.1373 g | - | 15 days, 4 °C | □ GA was grafted onto the C6-OH of D-galactose in agarose, with a highest GR of 13.73% □ Higher antioxidant and antimicrobial activities □ Lower viscosity, gel strength, and gelling temperature □ Inhibited microbial growth and lipid oxidation | [31] |
| Meat products | ||||||
| Pork | CS | 5 mL | - | 18 days, 4 °C | □ Higher antioxidant and antimicrobial activities □ Lower TVB-N and TBARS values □ Prolonged the shelf life of the pork meat from 6 to 18 days | [290] |
| 0.2 and 0.4% (w/w) | - | 20 days, 4 °C | □ Higher antioxidant activity □ Lower lipid oxidation and myoglobin oxidation □ Improved the safety and quality of samples in MAP environment | [291] | ||
| Collagen + CS | NS | PL | 15 days, 4 °C | □ Improved structural and UV barrier properties □ Higher antioxidant and antimicrobial activities □ Prolonged the shelf life of the pork meat by approximately 5 days | [292] | |
| CYS + CS | 40 g | - | One day, 25 °C | □ Better light transmittance and thinner thickness □ Improved the tensile strength □ Improved the quality of pork during storage compared to PE film packaging | [293] | |
| Beef | CS | 0.1 and 0.3% (w/v) | - | 21 days, 4 °C | □ Reduced spoilage bacteria count, TVB-N, and TBARS □ Delayed lipid oxidation and color deterioration | [294] |
| Gelatin + CS | 0.5% | CGA and RES | 12 days, 4 °C | □ Lower TVC, TVB-N, and TBARS values □ The combined coatings prolonged the shelf life of beef at least 3–6 days | [295] | |
| CA | NS | ZIF-8 | 12 days, 4 °C | □ Improved mechanical strength and UV barrier properties □ Reduced WVP, MC, and SR □ Higher antioxidant and antimicrobial activities | [296] | |
| Fruits and vegetables | ||||||
| Strawberry | CS | 15 mM | - | 14 days, 4 °C, under UV-A light | □ Higher reduction of E. coli compared to the control after 180 min exposure to UV-A □ The photo-irradiated coatings did not significantly affect the mold decay incidence in strawberries □ The firmness value did not show significant differences during storage | [297] |
| Banana | mPLA | 0.0301 g | - | 14 days, 25 °C | □ Improved mechanical and antioxidant properties □ Retained the firmness and green peel color of bananas after storing for 14 days | [298] |
| Mango | CS or CG | 0.075 or 0.15% (w/v) | - | 14 days, 20 °C, 60–70% RH | □ Lower pH, TSS, and TSS/acid ratio □ Higher antioxidant activity (lower IC50 values) after one week of storage □ Delayed ripening during two weeks of storage | [299] |
| Cherry tomato | CS | GA/CS ratio of 1:3 (w/w) | - | 10 days, 15 °C | □ Improved antioxidant activities in scavenging hydroxyl, DPPH, and superoxide anion radicals □ Protected the ascorbate–glutathione cycle of cherry tomatoes □ Inhibited enzymatic browning | [300] |
| PLA-PBAT | 1, 5, and 10 wt% | TA | 20 days, 25 °C | □ Improved tensile strength and UV barrier properties □ High antimicrobial activity against E. coli and L. monocytogenes, especially for those containing 10 wt% GA □ Enhanced the shelf life of cherry tomatoes for up to 20 days of storage at room temperature | [301] | |
| Green chili | CS + pullulan | 5, 10, and 15 wt% | - | 18 days, 25 °C | □ Improved tensile strength, WVP, and oxygen and UV barrier properties, especially for those containing 15 wt% GA □ Lower overall migration than the acceptable limit of 10 mg dm−2 □ Higher antioxidant and antimicrobial activities | [302] |
5.1.2. Meat Products
5.1.3. Fruits and Vegetables
5.2. Functional Foods
6. Disadvantages of GA in Human Health and Food Products
6.1. Toxicity
6.2. Interaction with Drugs and Nutrients
6.3. Sensory Impact in Food Products
7. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Rahman, M.M.; Rahaman, M.S.; Islam, M.R.; Rahman, F.; Mithi, F.M.; Alqahtani, T.; Almikhlafi, M.A.; Alghamdi, S.Q.; Alruwaili, A.S.; Hossain, M.S.; et al. Role of Phenolic Compounds in Human Disease: Current Knowledge and Future Prospects. Molecules 2021, 27, 233. [Google Scholar] [CrossRef] [PubMed]
- McClements, D.J. Future Foods: Is It Possible to Design a Healthier and More Sustainable Food Supply? Nutr. Bull. 2020, 45, 341–354. [Google Scholar] [CrossRef]
- Haghani, S.; Hadidi, M.; Pouramin, S.; Adinepour, F.; Hasiri, Z.; Moreno, A.; Munekata, P.E.S.; Lorenzo, J.M. Application of Cornelian Cherry (Cornus mas L.) Peel in Probiotic Ice Cream: Functionality and Viability during Storage. Antioxidants 2021, 10, 1777. [Google Scholar] [CrossRef]
- Gueffai, A.; Gonzalez-Serrano, D.J.; Christodoulou, M.C.; Orellana-Palacios, J.C.; Ortega, M.L.S.; Ouldmoumna, A.; Kiari, F.Z.; Ioannou, G.D.; Kapnissi-Christodoulou, C.P.; Moreno, A.; et al. Phenolics from Defatted Black Cumin Seeds (Nigella sativa L.): Ultrasound-Assisted Extraction Optimization, Comparison, and Antioxidant Activity. Biomolecules 2022, 12, 1311. [Google Scholar] [CrossRef] [PubMed]
- Martins, N.; Barros, L.; Ferreira, I.C.F.R. In Vivo Antioxidant Activity of Phenolic Compounds: Facts and Gaps. Trends Food Sci. Technol. 2016, 48, 1–12. [Google Scholar] [CrossRef]
- Silva, B.N.; Cadavez, V.; Caleja, C.; Pereira, E.; Calhelha, R.C.; Molina, A.K.; Finimundy, T.; Kostić, M.; Soković, M.; Teixeira, J.A.; et al. Chemical Profiles and Bioactivities of Polyphenolic Extracts of Lavandula stoechas L., Artemisia dracunculus L. and Ocimum basilicum L. Food Chem. 2024, 451, 139308. [Google Scholar] [CrossRef]
- Rodríguez-Díaz, M.; Pérez, F.E.; Manosalva, P.M.; Cerda, J.I.; Martínez-Contreras, C.F.; Mora, A.Y.; Villagra, N.A.; Bucarey, S.A.; Barriga, A.; Escobar, J.; et al. Antimicrobial Activity and Phytochemical Characterization of Baccharis Concava Pers., a Native Plant of the Central Chilean Coast. Molecules 2024, 29, 1654. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Goel, N. Phenolic Acids: Natural Versatile Molecules with Promising Therapeutic Applications. Biotechnol. Rep. 2019, 24, e00370. [Google Scholar] [CrossRef]
- Pratyusha, S.; Pratyusha, S. Phenolic Compounds in the Plant Development and Defense: An Overview; Intechopen: London, UK, 2022. [Google Scholar] [CrossRef]
- Xiang, Z.; Zhu, B.; Yang, X.; Deng, J.; Zhu, Y.; Gan, L.; Yu, M.; Chen, J.; Xia, C.; Chen, S. Comprehensive Analysis of Phenolic Constituents, Biological Activities, and Derived Aroma Differences of Penthorum Chinense Pursh Leaves after Processing into Green and Black Tea. Foods 2024, 13, 399. [Google Scholar] [CrossRef]
- Valencia-Avilés, E.; García-Pérez, M.E.; Garnica-Romo, M.G.; Figueroa-Cárdenas, J.D.D.; Meléndez-Herrera, E.; Salgado-Garciglia, R.; Martínez-Flores, H.E. Antioxidant Properties of Polyphenolic Extracts from Quercus Laurina, Quercus Crassifolia, and Quercus Scytophylla Bark. Antioxidants 2018, 7, 81. [Google Scholar] [CrossRef]
- Lamien, C.E.; Meda, A.; Mans, J.; Romito, M.; Nacoulma, O.G.; Viljoen, G.J. Inhibition of Fowlpox Virus by an Aqueous Acetone Extract from Galls of Guiera Senegalensis J. F. Gmel (Combretaceae). J. Ethnopharmacol. 2005, 96, 249–253. [Google Scholar] [CrossRef]
- Rodríguez, H.; de las Rivas, B.; Gómez-Cordovés, C.; Muñoz, R. Degradation of Tannic Acid by Cell-Free Extracts of Lactobacillus Plantarum. Food Chem. 2008, 107, 664–670. [Google Scholar] [CrossRef]
- Suleria, H.A.R.; Barrow, C.J.; Dunshea, F.R. Screening and Characterization of Phenolic Compounds and Their Antioxidant Capacity in Different Fruit Peels. Foods 2020, 9, 1206. [Google Scholar] [CrossRef] [PubMed]
- Saifullah, M.; McCullum, R.; McCluskey, A.; Vuong, Q. Effects of Different Drying Methods on Extractable Phenolic Compounds and Antioxidant Properties from Lemon Myrtle Dried Leaves. Heliyon 2019, 5, e03044. [Google Scholar] [CrossRef]
- Dadwal, V.; Joshi, R.; Gupta, M. A Comparative Metabolomic Investigation in Fruit Sections of Citrus medica L. and Citrus maxima L. Detecting Potential Bioactive Metabolites Using UHPLC-QTOF-IMS. Food Res. Int. 2022, 157, 111486. [Google Scholar] [CrossRef]
- Junior, T.K.; de Moura, C.; Do Carmo, M.A.V.; Azevedo, L.; Esmerino, L.A.; Tardivo, R.C.; Kilpeläinen, P.; Granato, D. Chemical Composition, Antioxidant, Antimicrobial and Cytotoxic/Cytoprotective Activity of Non-Polar Extracts of Grape (Vitis labrusca Cv. Bordeaux) and Blackberry (Rubus fruticosus) Seeds. Molecules 2021, 26, 4057. [Google Scholar] [CrossRef] [PubMed]
- Kosuru, R.Y.; Roy, A.; Das, S.K.; Bera, S. Gallic Acid and Gallates in Human Health and Disease: Do Mitochondria Hold the Key to Success? Mol. Nutr. Food Res. 2018, 62, 1700699. [Google Scholar] [CrossRef]
- Saliu, J.A.; Oyeleye, S.I.; Olasehinde, T.A.; Oboh, G. Modulatory Effects of Stonebreaker (Phyllanthus amarus) and Bitter Gourd (Momordica charantia) on Enzymes Linked with Cardiac Function in Heart Tissue of Doxorubicin-Stressed Rats. Drug Chem. Toxicol. 2022, 45, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, T.A.; El-Hefnawy, H.M.; El-Hela, A.A. Antioxidant Potential and Phenolic Acid Content of Certain Cucurbitaceous Plants Cultivated in Egypt. Nat. Prod. Res. 2010, 24, 1537–1545. [Google Scholar] [CrossRef] [PubMed]
- Mohamed Ahmed, I.A.; Al Juhaimi, F.; Uslu, N.; Özcan, M.M.; Karrar, E. The Role of Environmental Air and Microwave Drying on Colour Values, Bioactive Properties and Phenolic Compounds of Jalapeno Pepper. Int. J. Food Sci. Technol. 2024, 59, 3613–3623. [Google Scholar] [CrossRef]
- Hu, D.B.; Xue, R.; Zhuang, X.C.; Zhang, X.S.; Shi, S.L. Ultrasound-Assisted Extraction Optimization of Polyphenols from Boletus Bicolor and Evaluation of Its Antioxidant Activity. Front. Nutr. 2023, 10, 1135712. [Google Scholar] [CrossRef]
- Dimitrijević, A.; Marić, S.; Jocić, A.; Tekić, D.; Mušović, J.; Amaral, J.S. Green Extraction Strategy Using Bio-Based Aqueous Biphasic Systems for Polyphenol Valorization from Grape By-Product. Foods 2024, 13, 954. [Google Scholar] [CrossRef] [PubMed]
- Bak, E.J.; Kim, J.; Jang, S.; Woo, G.H.; Yoon, H.G.; Yoo, Y.J.; Cha, J.H. Gallic Acid Improves Glucose Tolerance and Triglyceride Concentration in Diet-Induced Obesity Mice. Scand. J. Clin. Lab. Investig. 2013, 73, 607–614. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.L.; Yen, G.C. Effect of Gallic Acid on High Fat Diet-Induced Dyslipidaemia, Hepatosteatosis and Oxidative Stress in Rats. Br. J. Nutr. 2007, 98, 727–735. [Google Scholar] [CrossRef]
- Cai, L.; Wei, Z.; Zhao, X.; Li, Y.; Li, X.; Jiang, X. Gallic Acid Mitigates LPS-Induced Inflammatory Response via Suppressing NF-ΚB Signalling Pathway in IPEC-J2 Cells. J. Anim. Physiol. Anim. Nutr. 2022, 106, 1000–1008. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Li, Y.P.; Wei, Z.X.; Li, X.L.; Jiang, X.R. Effects of Dietary Gallic Acid on Growth Performance, Diarrhea Incidence, Intestinal Morphology, Plasma Antioxidant Indices, and Immune Response in Weaned Piglets. Anim. Feed. Sci. Technol. 2020, 261, 114391. [Google Scholar] [CrossRef]
- Luo, W.; Zhao, M.; Yang, B.; Shen, G.; Rao, G. Identification of Bioactive Compounds in Phyllenthus Emblica L. Fruit and Their Free Radical Scavenging Activities. Food Chem. 2009, 114, 499–504. [Google Scholar] [CrossRef]
- Yang, D.J.; Moh, S.H.; Son, D.H.; You, S.; Kinyua, A.W.; Ko, C.M.; Song, M.; Yeo, J.; Choi, Y.H.; Kim, K.W. Gallic Acid Promotes Wound Healing in Normal and Hyperglucidic Conditions. Molecules 2016, 21, 899. [Google Scholar] [CrossRef]
- Lemos, M.R.B.; Zambiazi, R. Gallic Acid: Occurrences, Antioxidant Activity and Health Implications. In Handbook on Gallic Acid: Natural Occurrences, Antioxidant Properties and Health Implications; Thompson, M.A., Collins, R.P., Eds.; Nova: Hauppauge, NY, USA, 2013; pp. 193–213. [Google Scholar]
- Fu, L.; Xiao, Q.; Ru, Y.; Hong, Q.; Weng, H.; Zhang, Y.; Chen, J.; Xiao, A. Bio-Based Active Packaging: Gallic Acid Modified Agarose Coatings in Grass Carp (Ctenopharyngodon Idellus) Preservation. Int. J. Biol. Macromol. 2024, 255, 128196. [Google Scholar] [CrossRef] [PubMed]
- de Jesus, G.A.M.; Berton, S.B.R.; Simões, B.M.; Zola, R.S.; Monteiro, J.P.; Martins, A.F.; Bonafé, E.G. κ-Carrageenan/Poly(Vinyl Alcohol) Functionalized Films with Gallic Acid and Stabilized with Metallic Ions. Int. J. Biol. Macromol. 2023, 253, 127087. [Google Scholar] [CrossRef] [PubMed]
- Zheng, T.; Tang, P.; Li, G. Development of Composite Film Based on Collagen and Phenolic Acid-Grafted Chitosan for Food Packaging. Int. J. Biol. Macromol. 2023, 241, 124494. [Google Scholar] [CrossRef] [PubMed]
- Panich, U.; Onkoksoong, T.; Limsaengurai, S.; Akarasereenont, P.; Wongkajornsilp, A. UVA-Induced Melanogenesis and Modulation of Glutathione Redox System in Different Melanoma Cell Lines: The Protective Effect of Gallic Acid. J. Photochem. Photobiol. B 2012, 108, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Panich, U.; Pluemsamran, T.; Tangsupa-a-nan, V.; Wattanarangsan, J.; Phadungrakwittaya, R.; Akarasereenont, P.; Laohapand, T. Protective Effect of AVS073, a Polyherbal Formula, against UVA-Induced Melanogenesis through a Redox Mechanism Involving Glutathione-Related Antioxidant Defense. BMC Complement. Altern. Med. 2013, 13, 159. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Liu, M.; Staker, B.L.; Buchko, G.W.; Quinn, R.J. Drug-Repurposing Screening Identifies a Gallic Acid Binding Site on SARS-CoV-2 Non-Structural Protein 7. ACS Pharmacol. Transl. Sci. 2023, 6, 578–586. [Google Scholar] [CrossRef] [PubMed]
- Ye, S.; Su, F.; Li, J.; Yu, B.; Xu, L.; Xiong, T.; Shao, K.; Yuan, X. Enhanced in Vivo Antiviral Activity against Pseudorabies Virus through Transforming Gallic Acid into Graphene Quantum Dots with Stimulation of Interferon-Related Immune Responses. J. Mater. Chem. B 2023, 12, 122–130. [Google Scholar] [CrossRef]
- Vo, T.S.; Ngo, D.H.; Kim, S.K. Gallic Acid-Grafted Chitooligosaccharides Suppress Antigen-Induced Allergic Reactions in RBL-2H3 Mast Cells. Eur. J. Pharm. Sci. 2012, 47, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Yun, S.S.; Young, K.M.; Park, J.C.; Nam, S.H. Comparison of Anti-Allergenic Activities of Various Polyphenols in Cell Assays. J. Appl. Biol. Chem. 2010, 53, 139–146. [Google Scholar] [CrossRef]
- Dong, X.; Luo, S.; Hu, D.; Cao, R.; Wang, Q.; Meng, Z.; Feng, Z.; Zhou, W.; Song, W. Gallic Acid Inhibits Neuroinflammation and Reduces Neonatal Hypoxic-Ischemic Brain Damages. Front. Pediatr. 2022, 10, 973256. [Google Scholar] [CrossRef] [PubMed]
- Thong-Asa, W.; Wassana, C.; Sukkasem, K.; Innoi, P.; Dechakul, M.; Timda, P. Neuroprotective Effect of Gallic Acid in Mice with Rotenone-Induced Neurodegeneration. Exp. Anim. 2024, 73, 23–0165. [Google Scholar] [CrossRef]
- Badhani, B.; Sharma, N.; Kakkar, R. Gallic Acid: A Versatile Antioxidant with Promising Therapeutic and Industrial Applications. RSC Adv. 2015, 5, 27540–27557. [Google Scholar] [CrossRef]
- Skeva, E.; Girousi, S. Gallic Acid: Applications, Analysis and Electrochemical Characterisation. In Handbook on Gallic Acid: Natural Occurrences, Antioxidant Properties and Health Implications; Thompson, M.A., Collins, R.P., Eds.; Nova: Hauppauge, NY, USA, 2013; pp. 111–134. [Google Scholar]
- Masoud, M.S.; Hagagg, S.S.; Ali, A.E.; Nasr, N.M. Synthesis and Spectroscopic Characterization of Gallic Acid and Some of Its Azo Complexes. J. Mol. Struct. 2012, 1014, 17–25. [Google Scholar] [CrossRef]
- Lamuela-Raventós, R.M. Folin–Ciocalteu Method for the Measurement of Total Phenolic Content and Antioxidant Capacity. Meas. Antioxid. Act. Capacit. Recent Trends Appl. 2017, 6, 107–115. [Google Scholar] [CrossRef]
- Seymour-Jones, F.L.; Road, K.; Jersey, N. The beginnings of leather chemistry. J. Chem. Educ. 1927, 4, 831. [Google Scholar] [CrossRef]
- Cordeiro, A.; Damasceno, S.; Costa, J.G.M.; Rosenhaim, R.; Soledade, L.; Souza, A.; Santos, N. Gallic Acid: Thermal and Antioxidant Properties. In Handbook on Gallic Acid: Natural Occurrences, Antioxidant Properties and Health Implications; Thompson, M.A., Collins, R.P., Eds.; Nova: Hauppauge, NY, USA, 2013; pp. 57–85. [Google Scholar]
- Grundhöfer, P.; Niemetz, R.; Schilling, G.; Gross, G.G. Biosynthesis and Subcellular Distribution of Hydrolyzable Tannins. Phytochemistry 2001, 57, 915–927. [Google Scholar] [CrossRef] [PubMed]
- Baiano, A. Gallic Acid: Occurrence in Plant Foods and Effects of Agricultural Practices, Vegetative Stage and Processing. In Handbook on Gallic Acid: Natural Occurrences, Antioxidant Properties and Health Implications; Thompson, M.A., Collins, R.P., Eds.; Nova: Hauppauge, NY, USA, 2013; pp. 177–192. [Google Scholar]
- Govea-Salas, M.; González-Castillo, M.; Aguilar, C.N.; Rodríguez-Herrera, R.; Zugasti-Cruz, A.; Silva-Belmares, S.Y.; Morlett-Chávez, J.A. Gallic Acid Extraction and Its Application to Prevention and Treatment of Cancer. In Handbook on Gallic Acid: Natural Occurrences, Antioxidant Properties and Health Implications; Thompson, M.A., Collins, R.P., Eds.; Nova: Hauppauge, NY, USA, 2013; pp. 157–176. [Google Scholar]
- Amirah; Reddy Prasad, D.M.; Khan, M.R. Comparison of Extraction Techniques on Extraction of Gallic Acid from Stem Bark of Jatropha Curcas. J. Appl. Sci. 2012, 12, 1106–1111. [Google Scholar] [CrossRef]
- Baite, T.N.; Mandal, B.; Purkait, M.K. Ultrasound Assisted Extraction of Gallic Acid from Ficus Auriculata Leaves Using Green Solvent. Food Bioprod. Process. 2021, 128, 1–11. [Google Scholar] [CrossRef]
- Markom, M.; Hasan, M.; Wan Daud, W.R.; Anuar, N.; Hassan, O.; Singh, H. Chemical Profiling and Quantification of Tannins in Phyllanthus Niruri Linn. Fractionated by SFE Method. Sep. Sci. Technol. 2010, 46, 71–78. [Google Scholar] [CrossRef]
- Cláudio, A.F.M.; Ferreira, A.M.; Freire, C.S.R.; Silvestre, A.J.D.; Freire, M.G.; Coutinho, J.A.P. Optimization of the Gallic Acid Extraction Using Ionic-Liquid-Based Aqueous Two-Phase Systems. Sep. Purif. Technol. 2012, 97, 142–149. [Google Scholar] [CrossRef]
- López-Giral, N.; González-Arenzana, L.; González-Ferrero, C.; López, R.; Santamaría, P.; López-Alfaro, I.; Garde-Cerdán, T. Pulsed Electric Field Treatment to Improve the Phenolic Compound Extraction from Graciano, Tempranillo and Grenache Grape Varieties during Two Vintages. Innov. Food Sci. Emerg. Technol. 2015, 28, 31–39. [Google Scholar] [CrossRef]
- De Camargo, A.C.; Regitano-D’Arce, M.A.B.; Biasoto, A.C.T.; Shahidi, F. Enzyme-Assisted Extraction of Phenolics from Winemaking by-Products: Antioxidant Potential and Inhibition of Alpha-Glucosidase and Lipase Activities. Food Chem. 2016, 212, 395–402. [Google Scholar] [CrossRef]
- Khodaie, F.; Ghoreishi, S.M. Experimental Extraction of Gallic Acid from Brown Sumac Seed (Rhus Coriaria) Using Supercritical Carbon Dioxide and Ethanol as Co-Solvent: Modeling and Optimization. J. Supercrit. Fluids 2021, 175, 105266. [Google Scholar] [CrossRef]
- Aguilar-Zarate, P.; Chávez, M.L.; Herrera, R.; Aguilar, C. Biotechnological Productionof Gallic Acid. In Handbook on Gallic Acid: Natural Occurrences, Antioxidant Properties and Health Implications; Thompson, M.A., Collins, R.P., Eds.; Nova: Hauppauge, NY, USA, 2013; ISBN 978-1-62618-921-8. [Google Scholar]
- Saeed, S.; Aslam, S.; Mehmood, T.; Naseer, R.; Nawaz, S.; Mujahid, H.; Firyal, S.; Anjum, A.A.; Sultan, A. Production of Gallic Acid Under Solid-State Fermentation by Utilizing Waste from Food Processing Industries. Waste Biomass Valoriz. 2021, 12, 155–163. [Google Scholar] [CrossRef]
- Banerjee, D.; Pati, B.R. Optimization of Tannase Production by Aureobasidium Pullulans DBS66. J. Microbiol. Biotechnol. 2007, 17, 1049–1053. [Google Scholar]
- Aguilar-Zárate, P.; Cruz-Hernández, M.A.; Montañez, J.C.; Belmares-Cerda, R.E.; Aguilar, C.N. Bacterial Tannases: Production, Properties and Applications. Rev. Mex. Ing. Quim. 2014, 13, 63–74. [Google Scholar]
- Aguilar-Zárate, P.; Cruz, M.A.; Montañez, J.; Rodríguez-Herrera, R.; Wong-Paz, J.E.; Belmares, R.E.; Aguilar, C.N. Gallic Acid Production under Anaerobic Submerged Fermentation by Two Bacilli Strains. Microb. Cell Fact. 2015, 14, 209. [Google Scholar] [CrossRef]
- Zhao, D.; Zeng, H.; Xiao, S.; Yu, Y.; Wang, J.; Zhang, P.; Deng, Z. Characterization of a Recombinant Tannase from Pseudoduganella Albidiflava with High Substance Affinity for Propyl Gallate. Process Biochem. 2024, 138, 150–158. [Google Scholar] [CrossRef]
- Ristinmaa, A.S.; Coleman, T.; Cesar, L.; Weinmann, A.L.; Mazurkewich, S.; Branden, G.; Hasani, M.; Larsbrink, J. Structural Diversity and Substrate Preferences of Three Tannase Enzymes Encoded by the Anaerobic Bacterium Clostridium Butyricum. J. Biol. Chem. 2022, 298, 101758. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Durán, L.V.; Valdivia-Urdiales, B.; Contreras-Esquivel, J.C.; Rodríguez-Herrera, R.; Aguilar, C.N. Novel Strategies for Upstream and Downstream Processing of Tannin Acyl Hydrolase. Enzym. Res. 2011, 2011, 823619. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, O.D.; Kulkarni, Y.A. Mini-Review of Analytical Methods Used in Quantification of Ellagic Acid. Rev. Anal. Chem. 2020, 39, 31–44. [Google Scholar] [CrossRef]
- Soong, Y.Y.; Barlow, P.J. Quantification of Gallic Acid and Ellagic Acid from Longan (Dimocarpus longan Lour.) Seed and Mango (Mangifera indica L.) Kernel and Their Effects on Antioxidant Activity. Food Chem. 2006, 97, 524–530. [Google Scholar] [CrossRef]
- Bhishnurkar, P.; Deo, S.S.; Inam, F.S.; Mahmood, S.H.; Taher, D.; Lambat, T.L. Simultaneous Determination of β-Sitosterol and Gallic Acid in Nigella Sativa Seeds Using Reverse Phase High Performance Liquid Chromatography. SN Appl. Sci. 2020, 2, 1–7. [Google Scholar] [CrossRef]
- Zaripour, M.; Zare-Shahabadi, V.; Jahromi, H.J. Application of Ultrasonic-Assisted Inclusion Complex Formation with α–Cyclodextrin for Simultaneous Spectrophotometric Determination of Gallic Acid and Vanillic Acids in Fruit Samples. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2019, 222, 117197. [Google Scholar] [CrossRef]
- Padilha, C.V.D.S.; Miskinis, G.A.; de Souza, M.E.A.O.; Pereira, G.E.; de Oliveira, D.; Bordignon-Luiz, M.T.; dos Santos Lima, M. Rapid Determination of Flavonoids and Phenolic Acids in Grape Juices and Wines by RP-HPLC/DAD: Method Validation and Characterization of Commercial Products of the New Brazilian Varieties of Grape. Food Chem. 2017, 228, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Farhadi, K.; Esmaeilzadeh, F.; Hatami, M.; Forough, M.; Molaie, R. Determination of Phenolic Compounds Content and Antioxidant Activity in Skin, Pulp, Seed, Cane and Leaf of Five Native Grape Cultivars in West Azerbaijan Province, Iran. Food Chem. 2016, 199, 847–855. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zeng, Q.; del Mar Contreras, M.; Wang, L. Profiling and Quantification of Phenolic Compounds in Camellia Seed Oils: Natural Tea Polyphenols in Vegetable Oil. Food Res. Int. 2017, 102, 184–194. [Google Scholar] [CrossRef] [PubMed]
- Díaz-de-Cerio, E.; Gómez-Caravaca, A.M.; Verardo, V.; Fernández-Gutiérrez, A.; Segura-Carretero, A. Determination of Guava (Psidium guajava L.) Leaf Phenolic Compounds Using HPLC-DAD-QTOF-MS. J. Funct. Foods 2016, 22, 376–388. [Google Scholar] [CrossRef]
- Gómez-Caravaca, A.M.; López-Cobo, A.; Verardo, V.; Segura-Carretero, A.; Fernández-Gutiérrez, A. HPLC-DAD-q-TOF-MS as a Powerful Platform for the Determination of Phenolic and Other Polar Compounds in the Edible Part of Mango and Its by-Products (Peel, Seed, and Seed Husk). Electrophoresis 2016, 37, 1072–1084. [Google Scholar] [CrossRef]
- Mallmann, L.P.; Tischer, B.; Vizzotto, M.; Rodrigues, E.; Manfroi, V. Comprehensive Identification and Quantification of Unexploited Phenolic Compounds from Red and Yellow Araçá (Psidium cattleianum Sabine) by LC-DAD-ESI-MS/MS. Food Res. Int. 2020, 131, 108978. [Google Scholar] [CrossRef] [PubMed]
- de Souza Dias, F.; Silva, M.F.; David, J.M. Determination of Quercetin, Gallic Acid, Resveratrol, Catechin and Malvidin in Brazilian Wines Elaborated in the Vale Do São Francisco Using Liquid-Liquid Extraction Assisted by Ultrasound and GC-MS. Food Anal. Methods 2013, 6, 963–968. [Google Scholar] [CrossRef]
- Balkrishna, A.; Sharma, P.; Joshi, M.; Srivastava, J.; Varshney, A. Development and Validation of a Rapid High-Performance Thin-Layer Chromatographic Method for Quantification of Gallic Acid, Cinnamic Acid, Piperine, Eugenol, and Glycyrrhizin in Divya-Swasari-Vati, an Ayurvedic Medicine for Respiratory Ailments. J. Sep. Sci. 2021, 44, 3146–3157. [Google Scholar] [CrossRef]
- Lawag, I.L.; Islam, M.K.; Sostaric, T.; Lim, L.Y.; Hammer, K.; Locher, C. Antioxidant Activity and Phenolic Compound Identification and Quantification in Western Australian Honeys. Antioxidants 2023, 12, 189. [Google Scholar] [CrossRef] [PubMed]
- Tessema, F.B.; Gonfa, Y.H.; Asfaw, T.B.; Tadesse, M.G.; Tadesse, T.G.; Bachheti, A.; Alshaharni, M.O.; Kumar, P.; Kumar, V.; Širić, I.; et al. Targeted HPTLC Profile, Quantification of Flavonoids and Phenolic Acids, and Antimicrobial Activity of Dodonaea Angustifolia (L.f.) Leaves and Flowers. Molecules 2023, 28, 2870. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhang, J.; Chen, G. Simultaneous Determination of Flavones and Phenolic Acids in the Leaves of Ricinus Communis Linn. by Capillary Electrophoresis with Amperometric Detection. J. Chromatogr. B 2008, 863, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Priego-Capote, F.; Ruiz-Jiménez, J.; Luque De Castro, M.D. Fast Separation and Determination of Phenolic Compounds by Capillary Electrophoresis–Diode Array Detection: Application to the Characterisation of Alperujo after Ultrasound-Assisted Extraction. J. Chromatogr. A 2004, 1045, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Nerantzaki, A.A.; Tsiafoulis, C.G.; Charisiadis, P.; Kontogianni, V.G.; Gerothanassis, I.P. Novel Determination of the Total Phenolic Content in Crude Plant Extracts by the Use of 1H NMR of the –OH Spectral Region. Anal. Chim. Acta 2011, 688, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Song, Y.; Jing, W.; Wang, Y.; Yang, X.; Liu, D. Simultaneous Determination of Caffeine, Gallic Acid, Theanine, (−)-Epigallocatechin and (−)-Epigallocatechin-3-Gallate in Green Tea Using Quantitative 1H-NMR Spectroscopy. Anal. Methods 2014, 6, 907–914. [Google Scholar] [CrossRef]
- de Fátima, C.; Santos, M.; Rech, K.S.; Dutra, L.M.; Menezes, L.R.A.; Alan, A.D.; Nagata, N.; Stefanello, M.É.A.; Barison, A. 1H HR-MAS NMR Chemical Profile and Chemometric Analysis as a Tool for Quality Control of Different Cultivars of Green Tea (Camellia sinensis). Food Chem. 2023, 408, 135016. [Google Scholar] [CrossRef] [PubMed]
- Pooralhossini, J.; Ghaedi, M.; Zanjanchi, M.A.; Asfaram, A. The Choice of Ultrasound Assisted Extraction Coupled with Spectrophotometric for Rapid Determination of Gallic Acid in Water Samples: Central Composite Design for Optimization of Process Variables. Ultrason. Sonochem. 2017, 34, 692–699. [Google Scholar] [CrossRef] [PubMed]
- Paranavitana, L.; Oh, W.Y.; Yeo, J.D.; Shahidi, F. Determination of Soluble and Insoluble-Bound Phenolic Compounds in Dehulled, Whole, and Hulls of Green and Black Lentils Using Electrospray Ionization (ESI)-MS/MS and Their Inhibition in DNA Strand Scission. Food Chem. 2021, 361, 130083. [Google Scholar] [CrossRef]
- Dmitrienko, S.G.; Medvedeva, O.M.; Ivanov, A.A.; Shpigun, O.A.; Zolotov, Y.A. Determination of Gallic Acid with 4-Nitrobenzenediazonium Tetrafluoroborate by Diffuse Reflectance Spectrometry on Polyurethane Foam. Anal. Chim. Acta 2002, 469, 295–301. [Google Scholar] [CrossRef]
- Fink, D.W.; Stong, J.D. The Electronic Spectral Properties of Gallic Acid. Spectrochim. Acta A 1982, 38, 1295–1298. [Google Scholar] [CrossRef]
- Mir-Cerdà, A.; Nuñez, O.; Granados, M.; Sentellas, S.; Saurina, J. An Overview of the Extraction and Characterization of Bioactive Phenolic Compounds from Agri-Food Waste within the Framework of Circular Bioeconomy. TrAC Trends Anal. Chem. 2023, 161, 116994. [Google Scholar] [CrossRef]
- Dong, M.W.; Boyes, B.E. Modern Trends and Best Practices in Mobile-Phase Selection in Reversed-Phase Chromatography: This Installment Provides an Overview of the Modern Trends and Best Practices in Mobile-Phase Selection for Reversed-Phase Liquid Chromatography. In Particular, We Focus on Selection Criteria and Rationale for Enhancing Analytical Performance and Ease of Preparation. LC-GC N. Am. 2018, 36, 752. [Google Scholar]
- Rohloff, J. Analysis of Phenolic and Cyclic Compounds in Plants Using Derivatization Techniques in Combination with GC-MS-Based Metabolite Profiling. Molecules 2015, 20, 3431–3462. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, F.H.A.; Salgado, H.R.N. Gallic Acid: Review of the Methods of Determination and Quantification. Crit. Rev. Anal. Chem. 2016, 46, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Capriotti, A.L.; Cavaliere, C.; Foglia, P.; Piovesana, S.; Ventura, S. Chromatographic Methods Coupled to Mass Spectrometry Detection for the Determination of Phenolic Acids in Plants and Fruits. J. Liq. Chromatogr. Relat. Technol. 2015, 38, 353–370. [Google Scholar] [CrossRef]
- Jug, U.; Glavnik, V.; Kranjc, E.; Vovk, I. High-Performance Thin-Layer Chromatography and High-Performance Thin-Layer Chromatography–Mass Spectrometry Methods for the Analysis of Phenolic Acids. J. Planar Chromatogr. Mod. TLC 2018, 31, 13–22. [Google Scholar] [CrossRef]
- Jain, A.; Parashar, A.K.; Nema, R.K.; Ankita Jain, C.; Narsinghani, T. High Performance Thin Layer Chromatography (HPTLC): A Modern Analytical Tool for Chemical Analysis. Curr. Res. Pharm. Sci. 2014, 4, 8–14. [Google Scholar]
- Chaphalkar, R.; Apte, K.G.; Talekar, Y.; Ojha, S.K.; Nandave, M. Antioxidants of Phyllanthus Emblica L. Bark Extract Provide Hepatoprotection against Ethanol-Induced Hepatic Damage: A Comparison with Silymarin. Oxid. Med. Cell Longev. 2017, 2017, 3876040. [Google Scholar] [CrossRef]
- Parimala, M.; Shoba, F.G. In Vitro Antimicrobial Activity and HPTLC Analysis of Hydroalcoholic Seed Extract of Nymphaea Nouchali Burm. f. BMC Complement. Altern. Med. 2014, 14, 361. [Google Scholar] [CrossRef]
- Ignat, I.; Volf, I.; Popa, V.I. A Critical Review of Methods for Characterisation of Polyphenolic Compounds in Fruits and Vegetables. Food Chem. 2011, 126, 1821–1835. [Google Scholar] [CrossRef] [PubMed]
- Hemwech, P.; Obma, A.; Detsangiamsak, S.; Wirasate, S.; Chaiyen, P.; Wilairat, P.; Chantiwas, R. Capillary Electrophoresis-UV Analysis Using Silica-Layer Coated Capillary for Separation of Seven Phenolic Acids and Caffeine and Its Application to Tea Analysis. SN Appl. Sci. 2021, 3, 872. [Google Scholar] [CrossRef]
- Amargianitaki, M.; Spyros, A. NMR-Based Metabolomics in Wine Quality Control and Authentication. Chem. Biol. Technol. Agric. 2017, 4, 9. [Google Scholar] [CrossRef]
- Lommen, A.; Godejohann, M.; Venema, D.P.; Hollman, P.C.H.; Spraul, M. Application of Directly Coupled HPLC-NMR-MS to the Identification and Confirmation of Quercetin Glycosides and Phloretin Glycosides in Apple Peel. Anal. Chem. 2000, 72, 1793–1797. [Google Scholar] [CrossRef] [PubMed]
- Keyvani-Ghamsari, S.; Rahimi, M.; Khorsandi, K. An Update on the Potential Mechanism of Gallic Acid as an Antibacterial and Anticancer Agent. Food Sci. Nutr. 2023, 11, 5856–5872. [Google Scholar] [CrossRef]
- Marzano, M.; Borbone, N.; Amato, F.; Oliviero, G.; Fucile, P.; Russo, T.; Sannino, F. 3D Chitosan-Gallic Acid Complexes: Assessment of the Chemical and Biological Properties. Gels 2022, 8, 124. [Google Scholar] [CrossRef]
- Zhang, G.; Zheng, C.; Huang, B.; Fei, P. Preparation of Acylated Pectin with Gallic Acid through Enzymatic Method and Their Emulsifying Properties, Antioxidation Activities and Antibacterial Activities. Int. J. Biol. Macromol. 2020, 165, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, J.; Li, H.; Wang, Y. Nanocomplexes Film Composed of Gallic Acid Loaded Ovalbumin/Chitosan Nanoparticles and Pectin with Excellent Antibacterial Activity: Preparation, Characterization and Application in Coating Preservation of Salmon Fillets. Int. J. Biol. Macromol. 2024, 259, 128934. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Lan, W.; Xie, J. Natural Phenolic Compounds: Antimicrobial Properties, Antimicrobial Mechanisms, and Potential Utilization in the Preservation of Aquatic Products. Food Chem. 2024, 440, 138198. [Google Scholar] [CrossRef]
- Žagar, T.; Frlan, R.; Kočevar Glavač, N. Using Subcritical Water to Obtain Polyphenol-Rich Extracts with Antimicrobial Properties. Antibiotics 2024, 13, 334. [Google Scholar] [CrossRef]
- Erental, A.; Sharon, I.; Engelberg-Kulka, H. Two Programmed Cell Death Systems in Escherichia Coli: An Apoptotic-Like Death Is Inhibited by the MazEF-Mediated Death Pathway. PLoS Biol. 2012, 10, e1001281. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Lee, D.G. Programmed Cell Death in Bacterial Community: Mechanisms of Action, Causes and Consequences. J. Microbiol. Biotechnol. 2019, 29, 1014–1021. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.S.; Je, J.Y. Gallic Acid-Grafted-Chitosan Inhibits Foodborne Pathogens by a Membrane Damage Mechanism. J. Agric. Food Chem. 2013, 61, 6574–6579. [Google Scholar] [CrossRef] [PubMed]
- Rohde, M. The Gram-Positive Bacterial Cell Wall. Microbiol. Spectr. 2019, 7, 10–128. [Google Scholar] [CrossRef] [PubMed]
- Cao-Hoang, L.; Marechal, P.A.; Lê-Thanh, M.; Gervais, P.; Waché, Y. Fluorescent Probes to Evaluate the Physiological State and Activity of Microbial Biocatalysts: A Guide for Prokaryotic and Eukaryotic Investigation. Biotechnol. J. 2008, 3, 890–903. [Google Scholar] [CrossRef] [PubMed]
- Tokuda, H. Biogenesis of Outer Membranes in Gram-Negative Bacteria. Biosci. Biotechnol. Biochem. 2009, 73, 465–473. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Fu, J.; Wu, M.; Liao, S.; Jia, X.; Wang, J.; Yang, S.; Liu, Z.; Liu, Z.; Xue, Z.; et al. Evaluation of Gallic Acid on Membrane Damage of Yersinia Enterocolitica and Its Application as a Food Preservative in Pork. Int. J. Food Microbiol. 2022, 374, 109720. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Lin, K.; Zhang, X.; Ma, H.; Yang, L.; Wei, L.; Yang, L.; Jiang, M. Antibacterial Effect of Gallic Acid in UV-C Light Treatment Against Escherichia Coli O157:H7 and the Underlying Mechanism. Food Bioproc Tech. 2023, 1, 1–16. [Google Scholar] [CrossRef]
- Freeman, Z.N.; Dorus, S.; Waterfield, N.R. The KdpD/KdpE Two-Component System: Integrating K+ Homeostasis and Virulence. PLoS Pathog. 2013, 9, e1003201. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Y.; Chen, X.; Jin, J.; Liu, H.; Hao, Y.; Zhang, H.; Xie, Y. BasS/BasR Two-Component System Affects the Sensitivity of Escherichia Coli to Plantaricin BM-1 by Regulating the Tricarboxylic Acid Cycle. Front. Microbiol. 2022, 13, 874789. [Google Scholar] [CrossRef]
- Zhu, Y.; Dou, Q.; Du, L.; Wang, Y. QseB/QseC: A Two-Component System Globally Regulating Bacterial Behaviors. Trends Microbiol. 2023, 31, 749–762. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, B.; Grenier, D.; Yi, L. Regulatory Mechanisms of the LuxS/AI-2 System and Bacterial Resistance. Antimicrob. Agents Chemother. 2019, 63, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; De Oliveira, E.F.; Alborzi, S.; Bastarrachea, L.J.; Tikekar, R.V. On Mechanism behind UV-A Light Enhanced Antibacterial Activity of Gallic Acid and Propyl Gallate against Escherichia Coli O157:H7. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.; Mehrotra, G.K.; Dutta, P.K. Chitosan Based ZnO Nanoparticles Loaded Gallic-Acid Films for Active Food Packaging. Food Chem. 2021, 334, 127605. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Chen, X.; Luo, J.; Wang, F.; Liu, G.; Zhu, H.; Guo, Y. PVDF Grafted Gallic Acid to Enhance the Hydrophilicity and Antibacterial Properties of PVDF Composite Membrane. Sep. Purif. Technol. 2021, 259, 118127. [Google Scholar] [CrossRef]
- Lin, T.H.; Wu, C.C.; Tseng, C.Y.; Fang, J.H.; Lin, C.T. Effects of Gallic Acid on Capsular Polysaccharide Biosynthesis in Klebsiella Pneumoniae. J. Microbiol. Immunol. Infect. 2022, 55, 1255–1262. [Google Scholar] [CrossRef] [PubMed]
- Borges, A.; Ferreira, C.; Saavedra, M.J.; Simões, M. Antibacterial Activity and Mode of Action of Ferulic and Gallic Acids against Pathogenic Bacteria. Microb. Drug Resist. 2013, 19, 256–265. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Liu, L.; Liu, M.; Wu, X.; Li, J. Antibacterial Activity of Gallic Acid against Shigella Flexneri and Its Effect on Biofilm Formation by Repressing MdoH Gene Expression. Food Control 2018, 94, 147–154. [Google Scholar] [CrossRef]
- Albutti, A.; Gul, M.S.; Siddiqui, M.F.; Maqbool, F.; Adnan, F.; Ullah, I.; Rahman, Z.; Qayyum, S.; Shah, M.A.; Salman, M. Combating Biofilm by Targeting Its Formation and Dispersal Using Gallic Acid against Single and Multispecies Bacteria Causing Dental Plaque. Pathogens 2021, 10, 1486. [Google Scholar] [CrossRef] [PubMed]
- Passos, M.R.; Almeida, R.S.; Lima, B.O.; de Souza Rodrigues, J.Z.; de Macêdo Neres, N.S.; Pita, L.S.; Marinho, P.D.O.F.; Santos, I.A.; da Silva, J.P.; Oliveira, M.C.; et al. Anticariogenic Activities of Libidibia Ferrea, Gallic Acid and Ethyl Gallate against Streptococcus Mutans in Biofilm Model. J. Ethnopharmacol. 2021, 274, 114059. [Google Scholar] [CrossRef]
- Ye, M.; Li, X.; Ren, Z.; Tarequl, I.M.; Ji, C.; Ji, J.; Ji, F.; Zhou, B.; Yang, S. Identification of Gallic Acid in Trapa Bispinosa as an Effective Inhibitor of the Vegetative Growth and Spore Germination of Paenibacillus Larvae. Apidologie 2022, 53, 1–13. [Google Scholar] [CrossRef]
- Amitai, S.; Yassin, Y.; Engelberg-Kulka, H. MazF-Mediated Cell Death in Escherichia Coli: A Point of No Return. J. Bacteriol. 2004, 186, 8295–8300. [Google Scholar] [CrossRef] [PubMed]
- Erental, A.; Kalderon, Z.; Saada, A.; Smith, Y.; Engelberg-Kulka, H. Apoptosis-Like Death, An Extreme SOS Response in Escherichia Coli. Mbio 2014, 5, 1426–1440. [Google Scholar] [CrossRef] [PubMed]
- Yun, D.G.; Lee, D.G. Antibacterial Activity of Curcumin via Apoptosis-like Response in Escherichia Coli. Appl. Microbiol. Biotechnol. 2016, 100, 5505–5514. [Google Scholar] [CrossRef] [PubMed]
- Gong, S.; Jiao, C.; Liu, B.; Qu, W.; Guo, L.; Jiang, Y. Beetroot (Beta vulgaris) Extract Exerts an Antibacterial Effect by Inducing Apoptosis-like Death in Bacillus Cereus. J. Funct. Foods 2023, 105, 105571. [Google Scholar] [CrossRef]
- Shih, Y.H.; Tsai, P.J.; Chen, Y.L.; Pranata, R.; Chen, R.J. Assessment of the Antibacterial Mechanism of Pterostilbene against Bacillus Cereus through Apoptosis-like Cell Death and Evaluation of Its Beneficial Effects on the Gut Microbiota. J. Agric. Food Chem. 2021, 69, 12219–12229. [Google Scholar] [CrossRef] [PubMed]
- Jin, C.; Kang, S.-M.; Kim, D.-H.; Lee, Y.; Lee, B.-J. Discovery of Antimicrobial Agents Based on Structural and Functional Study of the Klebsiella Pneumoniae MazEF Toxin–Antitoxin System. Antibiotics 2024, 13, 398. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.G.; Lin, S.; Chen, W.X.; Jiang, L.; Gu, Q.; Li, D.H.; Chen, Y.W. Dual-Stage Blue-Light-Guided Membrane and DNA-Targeted Photodynamic Inactivation Using Octyl Gallate for Ultraefficient Eradication of Planktonic Bacteria and Sessile Biofilms. J. Agric. Food Chem. 2022, 70, 7547–7565. [Google Scholar] [CrossRef] [PubMed]
- Noor Mohammadi, T.; Maung, A.T.; Sato, J.; Sonoda, T.; Masuda, Y.; Honjoh, K.; Miyamoto, T. Mechanism for Antibacterial Action of Epigallocatechin Gallate and Theaflavin-3,3′-digallate on Clostridium Perfringens. J. Appl. Microbiol. 2019, 126, 633–640. [Google Scholar] [CrossRef]
- Liñán-Atero, R.; Aghababaei, F.; García, S.R.; Hasiri, Z.; Ziogkas, D.; Moreno, A.; Hadidi, M. Clove Essential Oil: Chemical Profile, Biological Activities, Encapsulation Strategies, and Food Applications. Antioxidants 2024, 13, 488. [Google Scholar] [CrossRef]
- Hadidi, M.; Rostamabadi, H.; Moreno, A.; Jafari, S.M. Nanoencapsulation of Essential Oils from Industrial Hemp (Cannabis sativa L.) by-Products into Alfalfa Protein Nanoparticles. Food Chem. 2022, 386, 132765. [Google Scholar] [CrossRef] [PubMed]
- Christodoulou, M.C.; Orellana Palacios, J.C.; Hesami, G.; Jafarzadeh, S.; Lorenzo, J.M.; Domínguez, R.; Moreno, A.; Hadidi, M. Spectrophotometric Methods for Measurement of Antioxidant Activity in Food and Pharmaceuticals. Antioxidants 2022, 11, 2213. [Google Scholar] [CrossRef] [PubMed]
- Gregoriou, G.; Neophytou, C.M.; Vasincu, A.; Gregoriou, Y.; Hadjipakkou, H.; Pinakoulaki, E.; Christodoulou, M.C.; Ioannou, G.D.; Stavrou, I.J.; Christou, A.; et al. Anti-Cancer Activity and Phenolic Content of Extracts Derived from Cypriot Carob (Ceratonia Siliqua L.) Pods Using Different Solvents. Molecules 2021, 26, 5017. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Z.; Guan, H.; Zhao, X.; Xie, Q.; Xie, Z.; Cai, F.; Dang, R.; Li, M.; Wang, C. Dietary Gallic Acid as an Antioxidant: A Review of Its Food Industry Applications, Health Benefits, Bioavailability, Nano-Delivery Systems, and Drug Interactions. Food Res. Int. 2024, 180, 114068. [Google Scholar] [CrossRef] [PubMed]
- Yen, G.C.; Duh, P.D.; Tsai, H.L. Antioxidant and Pro-Oxidant Properties of Ascorbic Acid and Gallic Acid. Food Chem. 2002, 79, 307–313. [Google Scholar] [CrossRef]
- Abrantes, T.; Moura-Nunes, N.; Perrone, D. Gallic Acid Mitigates 5-Hydroxymethylfurfural Formation While Enhancing or Preserving Browning and Antioxidant Activity Development in Glucose/Arginine and Sucrose/Arginine Maillard Model Systems. Molecules 2022, 27, 848. [Google Scholar] [CrossRef] [PubMed]
- Mojadami, S.; Ahangarpour, A.; Mard, S.A.; Khorsandi, L. Diabetic Nephropathy Induced by Methylglyoxal: Gallic Acid Regulates Kidney MicroRNAs and Glyoxalase1–Nrf2 in Male Mice. Arch. Physiol. Biochem. 2023, 129, 655–662. [Google Scholar] [CrossRef] [PubMed]
- Sohrabi, F.; Dianat, M.; Badavi, M.; Radan, M.; Mard, S.A. Gallic Acid Suppresses Inflammation and Oxidative Stress through Modulating Nrf2-HO-1-NF-ΚB Signaling Pathways in Elastase-Induced Emphysema in Rats. Environ. Sci. Pollut. Res. 2021, 28, 56822–56834. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Kishimoto, Y.; Sasaki, M.; Sato, A.; Kamiya, T.; Kondo, K.; Iida, K. Terminalia bellirica (Gaertn.) Roxb. Extract and Gallic Acid Attenuate LPS-Induced Inflammation and Oxidative Stress via MAPK/NF-κ B and Akt/AMPK/Nrf2 Pathways. Oxid. Med. Cell Longev. 2018, 2018, 9364364. [Google Scholar] [CrossRef]
- Radan, M.; Dianat, M.; Badavi, M.; Mard, S.A.; Bayati, V.; Goudarzi, G. In Vivo and in Vitro Evidence for the Involvement of Nrf2-Antioxidant Response Element Signaling Pathway in the Inflammation and Oxidative Stress Induced by Particulate Matter (PM10): The Effective Role of Gallic Acid. Free Radic. Res. 2019, 53, 210–225. [Google Scholar] [CrossRef]
- Hashemzaei, M.; Tabrizian, K.; Alizadeh, Z.; Pasandideh, S.; Rezaee, R.; Mamoulakis, C.; Tsatsakis, A.; Skaperda, Z.; Kouretas, D.; Shahraki, J. Resveratrol, Curcumin and Gallic Acid Attenuate Glyoxal-Induced Damage to Rat Renal Cells. Toxicol. Rep. 2020, 7, 1571–1577. [Google Scholar] [CrossRef] [PubMed]
- Ola-Davies, O.E.; Olukole, S.G. Gallic Acid Protects against Bisphenol A-Induced Alterations in the Cardio-Renal System of Wistar Rats through the Antioxidant Defense Mechanism. Biomed. Pharmacother. 2018, 107, 1786–1794. [Google Scholar] [CrossRef] [PubMed]
- Nouri, A.; Salehi-Vanani, N.; Heidarian, E. Antioxidant, Anti-Inflammatory and Protective Potential of Gallic Acid against Paraquat-Induced Liver Toxicity in Male Rats. Avicenna J. Phytomed 2021, 11, 633–644. [Google Scholar] [CrossRef] [PubMed]
- Doğan, D.; Meydan, İ.; Kömüroğlu, A.U. Protective Effect of Silymarin and Gallic Acid against Cisplatin-Induced Nephrotoxicity and Hepatotoxicity. Int. J. Clin. Pract. 2022, 2022, 6541026. [Google Scholar] [CrossRef] [PubMed]
- Kaur, S.; Muthuraman, A. Ameliorative Effect of Gallic Acid in Paclitaxel-Induced Neuropathic Pain in Mice. Toxicol. Rep. 2019, 6, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Maya, S.; Prakash, T.; Goli, D. Effect of Wedelolactone and Gallic Acid on Quinolinic Acid-Induced Neurotoxicity and Impaired Motor Function: Significance to Sporadic Amyotrophic Lateral Sclerosis. Neurotoxicology 2018, 68, 1–12. [Google Scholar] [CrossRef]
- Ojeaburu, S.I.; Oriakhi, K. Hepatoprotective, Antioxidant and, Anti-Inflammatory Potentials of Gallic Acid in Carbon Tetrachloride-Induced Hepatic Damage in Wistar Rats. Toxicol. Rep. 2021, 8, 177–185. [Google Scholar] [CrossRef]
- Fanaei, H.; Mard, S.A.; Sarkaki, A.; Goudarzi, G.; Khorsandi, L. Gallic Acid Treats Dust-Induced NAFLD in Rats by Improving the Liver’s Anti-Oxidant Capacity and Inhibiting ROS/NFκβ/TNFα Inflammatory Pathway. Iran. J. Basic. Med. Sci. 2021, 24, 240–247. [Google Scholar] [CrossRef]
- Pereira, M.M.; de Morais, H.; dos Santos Silva, E.; Corso, C.R.; Adami, E.R.; Carlos, R.M.; Acco, A.; Zanoveli, J.M. The Antioxidant Gallic Acid Induces Anxiolytic-, but Not Antidepressant-like Effect, in Streptozotocin-Induced Diabetes. Metab. Brain Dis. 2018, 33, 1573–1584. [Google Scholar] [CrossRef]
- Behdarvand-Margha, Z.; Ahangarpour, A.; Shahraki, M.; Komeili, G.; Khorsandi, L. The Effects of Gallic Acid and Metformin on Male Reproductive Dysfunction in Diabetic Mice Induced by Methylglyoxal: An Experimental Study. Int. J. Reprod. Biomed. 2021, 19, 715–724. [Google Scholar] [CrossRef] [PubMed]
- Panghal, A.; Sathua, K.B.; Flora, S.J.S. Gallic Acid and MiADMSA Reversed Arsenic Induced Oxidative/Nitrosative Damage in Rat Red Blood Cells. Heliyon 2020, 6, e03431. [Google Scholar] [CrossRef] [PubMed]
- Hosseinzadeh, A.; Houshmand, G.; Goudarzi, M.; Sezavar, S.H.; Mehrzadi, S.; Mansouri, E.; Kalantar, M. Ameliorative Effect of Gallic Acid on Sodium Arsenite-Induced Spleno-, Cardio- and Hemato-Toxicity in Rats. Life Sci. 2019, 217, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Shackebaei, D.; Hesari, M.; Ramezani-Aliakbari, S.; Hoseinkhani, Z.; Ramezani-Aliakbari, F. Gallic Acid Protects against Isoproterenol-Induced Cardiotoxicity in Rats. Hum. Exp. Toxicol. 2022, 41, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Diaz, A.; Muñoz-Arenas, G.; Caporal-Hernandez, K.; Vázquez-Roque, R.; Lopez-Lopez, G.; Kozina, A.; Espinosa, B.; Flores, G.; Treviño, S.; Guevara, J. Gallic Acid Improves Recognition Memory and Decreases Oxidative-Inflammatory Damage in the Rat Hippocampus with Metabolic Syndrome. Synapse 2021, 75, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Singla, E.; Dharwal, V.; Naura, A.S. Gallic Acid Protects against the COPD-Linked Lung Inflammation and Emphysema in Mice. Inflamm. Res. 2020, 69, 423–434. [Google Scholar] [CrossRef] [PubMed]
- Jalili, C.; Korani, M.; Pazhouhi, M.; Ghanbari, A.; Zhaleh, M.; Davoudi, S.; Rashidi, I. Protective Effect of Gallic Acid on Nicotine-Induced Testicular Toxicity in Mice. Res. Pharm. Sci. 2021, 16, 414–424. [Google Scholar] [CrossRef] [PubMed]
- Garud, M.S.; Kulkarni, Y.A. Gallic Acid Attenuates Type I Diabetic Nephropathy in Rats. Chem. Biol. Interact. 2018, 282, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Saleh, S.M.M.; Mahmoud, A.B.; Al-Salahy, M.B.; Mohamed Moustafa, F.A. Morphological, Immunohistochemical, and Biochemical Study on the Ameliorative Effect of Gallic Acid against Bisphenol A-Induced Nephrotoxicity in Male Albino Rats. Sci. Rep. 2023, 13, 1–20. [Google Scholar] [CrossRef]
- Nouri, A.; Heibati, F.; Heidarian, E. Gallic Acid Exerts Anti-Inflammatory, Anti-Oxidative Stress, and Nephroprotective Effects against Paraquat-Induced Renal Injury in Male Rats. Naunyn Schmiedeberg’s Arch. Pharmacol. 2021, 394, 1–9. [Google Scholar] [CrossRef]
- Moradi, A.; Abolfathi, M.; Javadian, M.; Heidarian, E.; Roshanmehr, H.; Khaledi, M.; Nouri, A. Gallic Acid Exerts Nephroprotective, Anti-Oxidative Stress, and Anti-Inflammatory Effects Against Diclofenac-Induced Renal Injury in Malerats. Arch. Med. Res. 2021, 52, 380–388. [Google Scholar] [CrossRef]
- Baharmi, S.; Kalantari, H.; Kalantar, M.; Goudarzi, M.; Mansouri, E.; Kalantar, H. Pretreatment with Gallic Acid Mitigates Cyclophosphamide Induced Inflammation and Oxidative Stress in Mice. Curr. Mol. Pharmacol. 2021, 15, 204–212. [Google Scholar] [CrossRef]
- Amini, N.; Badavi, M.; Mard, S.A.; Dianat, M.; Moghadam, M.T. The Renoprotective Effects of Gallic Acid on Cisplatin-Induced Nephrotoxicity through Anti-Apoptosis, Anti-Inflammatory Effects, and Downregulation of LncRNA TUG1. Naunyn Schmiedeberg’s Arch. Pharmacol. 2022, 395, 691–701. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Wu, Y.; Yan, H.; Shen, T.; Li, S.; Gong, J.; Li, G.; Mai, H.; Wang, D.; Tan, X. Gallic Acid Ameliorates Calcium Oxalate Crystal-Induced Renal Injury via Upregulation of Nrf2/HO-1 in the Mouse Model of Stone Formation. Phytomedicine 2022, 106, 154429. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Wu, Y.; Qu, C.; Lin, Y.; Yi, X.; Gao, C.; Cai, J.; Su, Z.; Zeng, H. Hypouricemic Effect of Gallic Acid, a Bioactive Compound from Sonneratia Apetala Leaves and Branches, on Hyperuricemic Mice. Food Funct. 2022, 13, 10275–10290. [Google Scholar] [CrossRef]
- Blas-Valdivia, V.; Franco-Colín, M.; Rojas-Franco, P.; Chao-Vazquez, A.; Cano-Europa, E. Gallic Acid Prevents the Oxidative and Endoplasmic Reticulum Stresses in the Hippocampus of Adult-Onset Hypothyroid Rats. Front. Pharmacol. 2021, 12, 1–9. [Google Scholar] [CrossRef]
- Schimites, P.I.; Segat, H.J.; Teixeira, L.G.; Martins, L.R.; Mangini, L.T.; Baccin, P.S.; Rosa, H.Z.; Milanesi, L.H.; Burger, M.E.; Soares, A.V. Gallic Acid Prevents Ketamine-Induced Oxidative Damages in Brain Regions and Liver of Rats. Neurosci. Lett. 2020, 714, 134560. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.T.; Yang, M.Y.; Lee, Y.J.; Yang, T.W.; Wang, C.C.; Wang, C.J. Gallic Acid Improves Diabetic Steatosis by Downregulating Microrna-34a-5p through Targeting Nfe2l2 Expression in High-Fat Diet-Fed Db/Db Mice. Antioxidants 2022, 11, 92. [Google Scholar] [CrossRef]
- Shruthi, S.; Shenoy, K.B. Gallic Acid: A Promising Genoprotective and Hepatoprotective Bioactive Compound against Cyclophosphamide Induced Toxicity in Mice. Environ. Toxicol. 2021, 36, 123–131. [Google Scholar] [CrossRef]
- Hussein, R.M.; Anwar, M.M.; Farghaly, H.S.; Kandeil, M.A. Gallic Acid and Ferulic Acid Protect the Liver from Thioacetamide-Induced Fibrosis in Rats via Differential Expression of MiR-21, MiR-30 and MiR-200 and Impact on TGF-Β1/Smad3 Signaling. Chem. Biol. Interact. 2020, 324, 109098. [Google Scholar] [CrossRef]
- Owumi, S.E.; Bello, S.A.; Najophe, S.E.; O Nwozo, S.; O Esan, I. Coadministration of Gallic Acid Abates Zearalenone-Mediated Defects in Male Rat’s Reproductive Function. J. Biochem. Mol. Toxicol. 2022, 36, e22940. [Google Scholar] [CrossRef] [PubMed]
- Ayazoglu Demir, E.; Mentese, A.; Livaoglu, A.; Turkmen Alemdar, N.; Demir, S. Ameliorative Effect of Gallic Acid on Cisplatin-Induced Ovarian Toxicity in Rats. Drug Chem. Toxicol. 2023, 46, 97–103. [Google Scholar] [CrossRef]
- Yan, X.; Zhang, Y.L.; Zhang, L.; Zou, L.X.; Chen, C.; Liu, Y.; Xia, Y.L.; Li, H.H. Gallic Acid Suppresses Cardiac Hypertrophic Remodeling and Heart Failure. Mol. Nutr. Food Res. 2019, 63, 1800807. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Pan, W.; Liu, S.; Shen, Z.; Xu, Y.; Hu, L. ERK/MAPK Signalling Pathway and Tumorigenesis (Review). Exp. Ther. Med. 2020, 19, 1997–2007. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Wang, Y.; Zhou, C.; Mei, W.; Zeng, C. PI3K/Akt/MTOR Pathway and Its Role in Cancer Therapeutics: Are We Making Headway? Front. Oncol. 2022, 12, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Shaik, M.; Vanapatla, S.R. Enhanced Oral Bioavailability of Linagliptin by the Influence of Gallic Acid and Ellagic Acid in Male Wistar Albino Rats: Involvement of p-Glycoprotein Inhibition. Drug Metab. Pers. Ther. 2019, 34, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Basu, S.; Jana, S.; Patel, V.B.; Patel, H. Effects of Piperine, Cinnamic Acid and Gallic Acid on Rosuvastatin Pharmacokinetics in Rats. Phytother. Res. 2013, 27, 1548–1556. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wang, K.; Zheng, J.; Cheung, F.S.G.; Chan, T.; Zhu, L.; Zhou, F. Interactions of the Active Components of Punica Granatum (Pomegranate) with the Essential Renal and Hepatic Human Solute Carrier Transporters. Pharm. Biol. 2014, 52, 1510–1517. [Google Scholar] [CrossRef]
- Subramanian, A.P.; John, A.A.; Vellayappan, M.V.; Balaji, A.; Jaganathan, S.K.; Supriyanto, E.; Yusof, M. Gallic Acid: Prospects and Molecular Mechanisms of Its Anticancer Activity. RSC Adv. 2015, 5, 35608–35621. [Google Scholar] [CrossRef]
- Subramanian, A.P.; Jaganathan, S.K.; Mandal, M.; Supriyanto, E.; Muhamad, I.I. Gallic Acid Induced Apoptotic Events in HCT-15 Colon Cancer Cells. World J. Gastroenterol. 2016, 22, 3952–3961. [Google Scholar] [CrossRef]
- Pedra, N.S.; Bona, N.P.; de Aguiar, M.S.S.; Spohr, L.; Alves, F.L.; dos Santos, F.d.S.; Saraiva, J.T.; Stefanello, F.M.; Braganhol, E.; Spanevello, R.M. Impact of Gallic Acid on Tumor Suppression: Modulation of Redox Homeostasis and Purinergic Response in in Vitro and a Preclinical Glioblastoma Model. J. Nutr. Biochem. 2022, 110, 109156. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.M.; Wu, Y.C.; Chia, Y.C.; Chang, F.R.; Hsu, H.K.; Hsieh, Y.C.; Chen, C.C.; Yuan, S.S. Gallic Acid, a Major Component of Toona Sinensis Leaf Extracts, Contains a ROS-Mediated Anti-Cancer Activity in Human Prostate Cancer Cells. Cancer Lett. 2009, 286, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Ho, P.C.L.; Wong, F.C.; Sethi, G.; Wang, L.Z.; Goh, B.C. Garcinol: Current Status of Its Anti-Oxidative, Anti-Inflammatory and Anti-Cancer Effects. Cancer Lett. 2015, 362, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Lo, C.; Lai, T.Y.; Yang, J.S.; Yang, J.H.; Ma, Y.S.; Weng, S.W.; Lin, H.Y.; Chen, H.Y.; Lin, J.G.; Chung, J.G. Gallic Acid Inhibits the Migration and Invasion of A375.S2 Human Melanoma Cells through the Inhibition of Matrix Metalloproteinase-2 and Ras. Melanoma Res. 2011, 21, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, K.; Kataoka, T.; Hayashi, T.; Hasegawa, M.; Ishi, Y.; Hibasami, H. Induction of Apoptosis by Gallic Acid in Human Stomach Cancer KATO III and Colon Adenocarcinoma COLO 205 Cell Lines. Oncol. Rep. 2000, 7, 1221–1223. [Google Scholar] [CrossRef]
- Htay, H.H.; Tsubouchi, R.; Haneda, M.; Murakami, K.; Yoshino, M. Induction of Apotosis of HL60 Cells by Gallic Acid Derivatives. Biomed. Res. 2002, 23, 127–134. [Google Scholar] [CrossRef]
- Madlener, S.; Illmer, C.; Horvath, Z.; Saiko, P.; Losert, A.; Herbacek, I.; Grusch, M.; Elford, H.L.; Krupitza, G.; Bernhaus, A.; et al. Gallic Acid Inhibits Ribonucleotide Reductase and Cyclooxygenases in Human HL-60 Promyelocytic Leukemia Cells. Cancer Lett. 2007, 245, 156–162. [Google Scholar] [CrossRef]
- Chandramohan Reddy, T.; Bharat Reddy, D.; Aparna, A.; Arunasree, K.M.; Gupta, G.; Achari, C.; Reddy, G.V.; Lakshmipathi, V.; Subramanyam, A.; Reddanna, P. Anti-Leukemic Effects of Gallic Acid on Human Leukemia K562 Cells: Downregulation of COX-2, Inhibition of BCR/ABL Kinase and NF-ΚB Inactivation. Toxicology in Vitro 2012, 26, 396–405. [Google Scholar] [CrossRef] [PubMed]
- You, B.R.; Moon, H.J.; Han, Y.H.; Park, W.H. Gallic Acid Inhibits the Growth of HeLa Cervical Cancer Cells via Apoptosis and/or Necrosis. Food Chem. Toxicol. 2010, 48, 1334–1340. [Google Scholar] [CrossRef]
- Ashrafizadeh, M.; Zarrabi, A.; Mirzaei, S.; Hashemi, F.; Samarghandian, S.; Zabolian, A.; Hushmandi, K.; Ang, H.L.; Sethi, G.; Kumar, A.P.; et al. Gallic Acid for Cancer Therapy: Molecular Mechanisms and Boosting Efficacy by Nanoscopical Delivery. Food Chem. Toxicol. 2021, 157, 112576. [Google Scholar] [CrossRef] [PubMed]
- Khorsandi, K.; Kianmehr, Z.; Hosseinmardi, Z.; Hosseinzadeh, R. Anti-Cancer Effect of Gallic Acid in Presence of Low Level Laser Irradiation: ROS Production and Induction of Apoptosis and Ferroptosis. Cancer Cell Int. 2020, 20, 18. [Google Scholar] [CrossRef]
- Aborehab, N.M.; Elnagar, M.R.; Waly, N.E. Gallic Acid Potentiates the Apoptotic Effect of Paclitaxel and Carboplatin via Overexpression of Bax and P53 on the MCF-7 Human Breast Cancer Cell Line. J. Biochem. Mol. Toxicol. 2021, 35, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Goulart, K.; Catyana, G.; Daniele, A.; Velasque, A.; Souza, B.; Cristina, F.; Mesquita, D.; Pedrazza, L.; Simon, E.; Andrade, B.; et al. ScienceDirect Gallic Acid Reduces Cell Growth by Induction of Apoptosis and Reduction of IL-8 in HepG2 Cells. Biomed. Et Pharmacother. 2016, 84, 1282–1290. [Google Scholar] [CrossRef]
- Mann, M.; Cortez, V.; Vadlamudi, R. PELP1 Oncogenic Functions Involve CARM1 Regulation. Carcinogenesis 2013, 34, 1468–1475. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Jiang, F.; Jiang, H.; Wu, K.; Zheng, X.; Cai, Y.; Katakowski, M.; Chopp, M.; To, S.T. Gallic Acid Suppresses Cell Viability, Proliferation, Invasion and Angiogenesis in Human Glioma Cells. Eur. J. Pharmacol. 2010, 641, 102–107. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Chen, A.Y.; Rojanasakul, Y.; Rankin, G.O.; Chen, Y.C. Gallic Acid, a Phenolic Compound, Exerts Anti-Angiogenic Effects via the PTEN/AKT/HIF-1 α/VEGF Signaling Pathway in Ovarian Cancer Cells. Oncol. Rep. 2016, 35, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Al-ani, L.A.; Yehye, W.A.; Kadir, F.A.; Hashim, N.M.; Alsaadi, M.A.; Julkapli, N.M.; Hsiao, V.K.S. Hybrid Nanocomposite Curcumin-Capped Gold Nanoparticle-Reduced Graphene Oxide: Anti-Oxidant Potency and Selective Cancer Cytotoxicity. PLoS ONE 2019, 50, e0216725. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Lin, K.; Jhang, L.; Huang, C.; Lee, Y.; Chang, L. Chemico-Biological Interactions Gallic Acid Abolishes the EGFR/Src/Akt/Erk-Mediated Expression of Matrix Metalloproteinase-9 in MCF-7 Breast Cancer Cells. Chem. Biol. Interact. 2016, 252, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Rattanata, N.; Daduang, S.; Wongwattanakul, M.; Limpaiboon, T.; Lekphrom, R.; Sandee, A.; Boonsiri, P.; Chio-srichan, S.; Daduang, J. Gold Nanoparticles Enhance the Anticancer Activity of Gallic Acid against Cholangiocarcinoma Cell Lines. Asian Pac. J. Cancer Prev. 2015, 16, 7143–7147. [Google Scholar] [CrossRef] [PubMed]
- Coelho, C.; Reis, D.P.; Pereira, M.C.; Coelho, M.A.N. Doxorubicin and Varlitinib Delivery by Functionalized Gold Nanoparticles Against Human Pancreatic Adenocarcinoma. Pharmaceutics 2019, 11, 551. [Google Scholar] [CrossRef]
- Yang, K.; Zhang, L.; Liao, P.; Xiao, Z.; Zhang, F.; Sindaye, D.; Xin, Z.; Tan, C.; Deng, J.; Yin, Y.; et al. Impact of Gallic Acid on Gut Health: Focus on the Gut Microbiome, Immune Response, and Mechanisms of Action. Front. Immunol. 2020, 11, 1–13. [Google Scholar] [CrossRef]
- Dorniani, D.; Saifullah, B.; Barahuie, F.; Arulselvan, P.; Hussein, M.Z.; Fakurazi, S.; Twyman, L.J. Graphene Oxide-Gallic Acid Nanodelivery System for Cancer Therapy. Nanoscale Res. Lett. 2016, 11, 491. [Google Scholar] [CrossRef] [PubMed]
- Design, D.; Wang, D.; Bao, B. Gallic Acid Impedes Non-Small Cell Lung Cancer Progression via Suppression of EGFR-Dependent CARM1-PELP1 Complex. Drug Des. Dev. Ther. 2020, 14, 1583–1592. [Google Scholar]
- Giftson, J.S.; Jayanthi, S.; Nalini, N. Chemopreventive Efficacy of Gallic Acid, an Antioxidant and Anticarcinogenic Polyphenol, against 1,2-Dimethyl Hydrazine Induced Rat Colon Carcinogenesis. Investig. New Drugs 2010, 28, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Zeng, M.; Su, Y.; Li, K.; Jin, D.; Li, Q.; Li, Y.; Zhou, B. Gallic Acid Inhibits Bladder Cancer T24 Cell Progression Through Mitochondrial Dysfunction and PI3K/Akt/NF-ΚB Signaling Suppression. Front. Pharmacol. 2020, 11, 1222. [Google Scholar] [CrossRef] [PubMed]
- Maruszewska, A.; Tarasiuk, J. Antitumour Effects of Selected Plant Polyphenols, Gallic Acid and Ellagic Acid, on Sensitive and Multidrug-Resistant Leukaemia HL60 Cells. Phytother. Res. 2019, 33, 1208–1221. [Google Scholar] [CrossRef] [PubMed]
- Umar, H.I.; Siraj, B.; Ajayi, A.; Jimoh, T.O.; Chukwuemeka, P.O. Molecular Docking Studies of Some Selected Gallic Acid Derivatives against Five Non-Structural Proteins of Novel Coronavirus. J. Genet. Eng. Biotechnol. 2021, 19, 16. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Kola, P.; Kaur, D.; Singla, G.; Mishra, V.; Panesar, P.S.; Mallikarjunan, K.; Krishania, M. Therapeutic Potential of Nutraceuticals and Dietary Supplements in the Prevention of Viral Diseases: A Review. Front. Nutr. 2021, 8, 679312. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.S.; Quispe, C.; Hossain, R.; Islam, M.T.; Al-Harrasi, A.; Al-Rawahi, A.; Martorell, M.; Mamurova, A.; Seilkhan, A.; Altybaeva, N.; et al. Neuropharmacological Effects of Quercetin: A Literature-Based Review. Front. Pharmacol. 2021, 12, 665031. [Google Scholar] [CrossRef] [PubMed]
- Jia, J.; Xu, J.; Liu, J.; Wang, Y.; Wang, Y.; Cao, Y.; Guo, Q.; Qu, Q.; Wei, C.; Wei, W.; et al. Comprehensive Management of Daily Living Activities, Behavioral and Psychological Symptoms, and Cognitive Function in Patients with Alzheimer’s Disease: A Chinese Consensus on the Comprehensive Management of Alzheimer’s Disease. Neurosci. Bull. 2021, 37, 1025–1038. [Google Scholar] [CrossRef]
- Van Marum, R.J. Current and Future Therapy in Alzheimer’s Disease. Fundam. Clin. Pharmacol. 2008, 22, 265–274. [Google Scholar] [CrossRef]
- Tõugu, V.; Tiiman, A.; Palumaa, P. Interactions of Zn(Ii) and Cu(Ii) Ions with Alzheimer’s Amyloid-Beta Peptide. Metal Ion Binding, Contribution to Fibrillization and Toxicity. Metallomics 2011, 3, 250–261. [Google Scholar] [CrossRef] [PubMed]
- Selkoe, D.J. The Molecular Pathology of Alzheimer’s Disease. Neuron 1991, 6, 487–498. [Google Scholar] [CrossRef] [PubMed]
- Savelieff, M.G.; Detoma, A.S.; Derrick, J.S.; Lim, M.H. The Ongoing Search for Small Molecules to Study Metal-Associated Amyloid-β Species in Alzheimers Disease. Acc. Chem. Res. 2014, 47, 2475–2482. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.N.; Hassan, M.N.; Khan, R.H. Gallic Acid: A Naturally Occurring Bifunctional Inhibitor of Amyloid and Metal Induced Aggregation with Possible Implication in Metal-Based Therapy. J. Mol. Liq. 2019, 285, 27–37. [Google Scholar] [CrossRef]
- Atri, A. Current and Future Treatments in Alzheimer’s Disease. Semin. Neurol. 2019, 39, 227–240. [Google Scholar] [CrossRef] [PubMed]
- Colucci-D’amato, L.; Speranza, L.; Volpicelli, F. Neurotrophic Factor BDNF, Physiological Functions and Therapeutic Potential in Depression, Neurodegeneration and Brain Cancer. Int. J. Mol. Sci. 2020, 21, 7777. [Google Scholar] [CrossRef] [PubMed]
- Amidfar, M.; de Oliveira, J.; Kucharska, E.; Budni, J.; Kim, Y.K. The Role of CREB and BDNF in Neurobiology and Treatment of Alzheimer’s Disease. Life Sci. 2020, 257, 118020. [Google Scholar] [CrossRef]
- Banerjee, M.; Shenoy, R.R. Emphasizing Roles of BDNF Promoters and Inducers in Alzheimer’s Disease for Improving Impaired Cognition and Memory. J. Basic. Clin. Physiol. Pharmacol. 2023, 34, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Serafini, S.; Ferretti, G.; Monterosso, P.; Angiolillo, A.; Di Costanzo, A.; Matrone, C. TNF-α Levels Are Increased in Patients with Subjective Cognitive Impairment and Are Negatively Correlated with β Amyloid-42. Antioxidants 2024, 13, 216. [Google Scholar] [CrossRef]
- Bhuia, M.S.; Rahaman, M.M.; Islam, T.; Bappi, M.H.; Sikder, M.I.; Hossain, K.N.; Akter, F.; Al Shamsh Prottay, A.; Rokonuzzman, M.; Gürer, E.S.; et al. Neurobiological Effects of Gallic Acid: Current Perspectives. Chin. Med. 2023, 18, 27. [Google Scholar] [CrossRef] [PubMed]
- Mansouri, M.T.; Naghizadeh, B.; Ghorbanzadeh, B.; Farbood, Y.; Sarkaki, A.; Bavarsad, K. Gallic Acid Prevents Memory Deficits and Oxidative Stress Induced by Intracerebroventricular Injection of Streptozotocin in Rats. Pharmacol. Biochem. Behav. 2013, 111, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Megha, K.B.; Joseph, X.; Akhil, V.; Mohanan, P.V. Cascade of Immune Mechanism and Consequences of Inflammatory Disorders. Phytomedicine 2021, 91, 153712. [Google Scholar] [CrossRef] [PubMed]
- Shabani, S.; Rabiei, Z.; Amini-Khoei, H. Exploring the Multifaceted Neuroprotective Actions of Gallic Acid: A Review. Int. J. Food Prop. 2020, 23, 736–752. [Google Scholar] [CrossRef]
- Kroes, B.H.; Van Den Berg, A.J.J.; Quarles Van Ufford, H.C.; Van Dijk, H.; Labadie, R.P. Anti-Inflammatory Activity of Gallic Acid. Planta Med. 1992, 58, 499–504. [Google Scholar] [CrossRef] [PubMed]
- BenSaad, L.A.; Kim, K.H.; Quah, C.C.; Kim, W.R.; Shahimi, M. Anti-Inflammatory Potential of Ellagic Acid, Gallic Acid and Punicalagin A&B Isolated from Punica Granatum. BMC Complement. Altern. Med. 2017, 17, 47. [Google Scholar] [CrossRef]
- Seo, C.S.; Jeong, S.J.; Yoo, S.R.; Lee, N.R.; Shin, H.K. Quantitative Analysis and In Vitro Anti-Inflammatory Effects of Gallic Acid, Ellagic Acid, and Quercetin from Radix Sanguisorbae. Pharmacogn. Mag. 2016, 12, 104. [Google Scholar] [CrossRef] [PubMed]
- Sripanidkulchai, B.; Junlatat, J. Bioactivities of Alcohol Based Extracts of Phyllanthus Emblica Branches: Antioxidation, Antimelanogenesis and Anti-Inflammation. J. Nat. Med. 2014, 68, 615–622. [Google Scholar] [CrossRef]
- Singla, E.; Puri, G.; Dharwal, V.; Naura, A.S. Gallic Acid Ameliorates COPD-Associated Exacerbation in Mice. Mol. Cell Biochem. 2021, 476, 293–302. [Google Scholar] [CrossRef]
- Gwon, M.H.; Yun, J.M. Phenethyl Isothiocyanate Improves Lipid Metabolism and Inflammation via MTOR/PPARγ/AMPK Signaling in the Adipose Tissue of Obese Mice. J. Med. Food 2021, 24, 666–669. [Google Scholar] [CrossRef]
- Tanaka, M.; Sugama, A.; Sumi, K.; Shimizu, K.; Kishimoto, Y.; Kondo, K.; Iida, K. Gallic Acid Regulates Adipocyte Hypertrophy and Suppresses Inflammatory Gene Expression Induced by the Paracrine Interaction between Adipocytes and Macrophages in Vitro and in Vivo. Nutr. Res. 2020, 73, 58–66. [Google Scholar] [CrossRef]
- Dehghani, M.A.; Maram, N.S.; Moghimipour, E.; Khorsandi, L.; Atefi khah, M.; Mahdavinia, M. Protective Effect of Gallic Acid and Gallic Acid-Loaded Eudragit-RS 100 Nanoparticles on Cisplatin-Induced Mitochondrial Dysfunction and Inflammation in Rat Kidney. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2020, 1866, 165911. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Gu, P.Q.; Shen, H. Gallic Acid Improved Inflammation via NF-ΚB Pathway in TNBS-Induced Ulcerative Colitis. Int. Immunopharmacol. 2019, 67, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Pandurangan, A.K.; Mohebali, N.; Esa, N.M.; Looi, C.Y.; Ismail, S.; Saadatdoust, Z. Gallic Acid Suppresses Inflammation in Dextran Sodium Sulfate-Induced Colitis in Mice: Possible Mechanisms. Int. Immunopharmacol. 2015, 28, 1034–1043. [Google Scholar] [CrossRef] [PubMed]
- Rahimifard, M.; Baeeri, M.; Bahadar, H.; Moini-Nodeh, S.; Khalid, M.; Haghi-Aminjan, H.; Mohammadian, H.; Abdollahi, M. Therapeutic Effects of Gallic Acid in Regulating Senescence and Diabetes; an In Vitro Study. Molecules 2020, 25, 5875. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.L.; Hsu, C.C.; Huang, H.J.; Chang, C.J.; Sun, S.H.; Lin, A.M.Y. Gallic Acid Attenuated LPS-Induced Neuroinflammation: Protein Aggregation and Necroptosis. Mol. Neurobiol. 2020, 57, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Reyes, A.W.B.; Arayan, L.T.; Hop, H.T.; Ngoc Huy, T.X.; Vu, S.H.; Min, W.G.; Lee, H.J.; Kim, S. Effects of Gallic Acid on Signaling Kinases in Murine Macrophages and Immune Modulation against Brucella Abortus 544 Infection in Mice. Microb. Pathog. 2018, 119, 255–259. [Google Scholar] [CrossRef] [PubMed]
- Olufunmilayo, E.O.; Gerke-Duncan, M.B.; Holsinger, R.M.D. Oxidative Stress and Antioxidants in Neurodegenerative Disorders. Antioxidants 2023, 12, 517. [Google Scholar] [CrossRef]
- Siddiqui, S.; Kamal, A.; Khan, F.; Jamali, K.S.; Saify, Z.S. Gallic and Vanillic Acid Suppress Inflammation and Promote Myelination in an in Vitro Mouse Model of Neurodegeneration. Mol. Biol. Rep. 2019, 46, 997–1011. [Google Scholar] [CrossRef] [PubMed]
- Uddin, S.J.; Afroz, M.; Zihad, S.M.N.K.; Rahman, M.S.; Akter, S.; Khan, I.N.; Al-Rabbi, S.M.S.; Rouf, R.; Islam, M.T.; Shilpi, J.A.; et al. A Systematic Review on Anti-Diabetic and Cardioprotective Potential of Gallic Acid: A Widespread Dietary Phytoconstituent. Food Rev. Int. 2022, 38, 420–439. [Google Scholar] [CrossRef]
- Paul, S.; Ali, A.; Katare, R. Molecular Complexities Underlying the Vascular Complications of Diabetes Mellitus—A Comprehensive Review. J. Diabetes Complicat. 2020, 34, 107613. [Google Scholar] [CrossRef]
- Farkhondeh, T.; Samarghandian, S.; Borji, A. An Overview on Cardioprotective and Anti-Diabetic Effects of Thymoquinone. Asian Pac. J. Trop. Med. 2017, 10, 849–854. [Google Scholar] [CrossRef] [PubMed]
- Volpe, C.M.O.; Villar-Delfino, P.H.; Dos Anjos, P.M.F.; Nogueira-Machado, J.A. Cellular Death, Reactive Oxygen Species (ROS) and Diabetic Complications. Cell Death Dis. 2018, 9, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Li, K.; Zeng, M.; Qiao, B.; Zhou, B. Serum Metabolomics Analysis of the Anti-Inflammatory Effects of Gallic Acid on Rats With Acute Inflammation. Front. Pharmacol. 2022, 13, 830439. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Sato, A.; Kishimoto, Y.; Mabashi-Asazuma, H.; Kondo, K.; Iida, K. Gallic Acid Inhibits Lipid Accumulation via AMPK Pathway and Suppresses Apoptosis and Macrophage-Mediated Inflammation in Hepatocytes. Nutrients 2020, 12, 1479. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhao, H.; Ma, C.; Lv, L.; Feng, J.; Han, S. Gallic Acid Attenuates Allergic Airway Inflammation via Suppressed Interleukin-33 and Group 2 Innate Lymphoid Cells in Ovalbumin-Induced Asthma in Mice. Int. Forum Allergy Rhinol. 2018, 8, 1284–1290. [Google Scholar] [CrossRef] [PubMed]
- El-Hussainy, E.H.M.A.; Hussein, A.M.; Abdel-Aziz, A.; El-Mehasseb, I. Effects of Aluminum Oxide (Al2O3) Nanoparticles on ECG, Myocardial Inflammatory Cytokines, Redox State, and Connexin 43 and Lipid Profile in Rats: Possible Cardioprotective Effect of Gallic Acid. Can. J. Physiol. Pharmacol. 2016, 94, 868–878. [Google Scholar] [CrossRef] [PubMed]
- Yigitturk, G.; Acara, A.C.; Erbas, O.; Oltulu, F.; Yavasoglu, N.U.K.; Uysal, A.; Yavasoglu, A. The Antioxidant Role of Agomelatine and Gallic Acid on Oxidative Stress in STZ Induced Type I Diabetic Rat Testes. Biomed. Pharmacother. 2017, 87, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Variya, B.C.; Bakrania, A.K.; Patel, S.S. Antidiabetic Potential of Gallic Acid from Emblica Officinalis: Improved Glucose Transporters and Insulin Sensitivity through PPAR-γ and Akt Signaling. Phytomedicine 2020, 73, 152906. [Google Scholar] [CrossRef]
- Bahramsoltani, R.; Farzaei, M.H.; Sajadimajd, S.; Iranpanah, A.; Khazaei, M.; Pourjabar, Z.; Hajimahmoodi, M.; Rahimi, R. In Vitro and in Vivo Antidiabetic Activity of Tamarix Stricta Boiss.: Role of Autophagy. J. Ethnopharmacol. 2021, 269, 113692. [Google Scholar] [CrossRef]
- Bouzghaya, S.; Amri, M.; Homble, F. Improvement of Diabetes Symptoms and Complications by an Aqueous Extract of Linum usitatissimum (L.) Seeds in Alloxan-Induced Diabetic Mice. J. Med. Food 2020, 23, 1077–1082. [Google Scholar] [CrossRef]
- Yu, M.; Chen, X.; Liu, J.; Ma, Q.; Zhuo, Z.; Chen, H.; Zhou, L.; Yang, S.; Zheng, L.; Ning, C.; et al. Gallic Acid Disruption of Aβ1–42 Aggregation Rescues Cognitive Decline of APP/PS1 Double Transgenic Mouse. Neurobiol. Dis. 2019, 124, 67–80. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Moneim, A.; El-Twab, S.M.A.; Yousef, A.I.; Reheim, E.S.A.; Ashour, M.B. Modulation of Hyperglycemia and Dyslipidemia in Experimental Type 2 Diabetes by Gallic Acid and P-Coumaric Acid: The Role of Adipocytokines and PPARγ. Biomed. Pharmacother. 2018, 105, 1091–1097. [Google Scholar] [CrossRef]
- Hajipour, S.; Sarkaki, A.; Farbood, Y.; Eidi, A.; Mortazavi, P.; Valizadeh, Z. Effect of Gallic Acid on Dementia Type of Alzheimer Disease in Rats: Electrophysiological and Histological Studies. Basic. Clin. Neurosci. 2016, 7, 97. [Google Scholar] [CrossRef]
- Liu, Y.; Pukala, T.L.; Musgrave, I.F.; Williams, D.M.; Dehle, F.C.; Carver, J.A. Gallic Acid Is the Major Component of Grape Seed Extract That Inhibits Amyloid Fibril Formation. Bioorg Med. Chem. Lett. 2013, 23, 6336–6340. [Google Scholar] [CrossRef]
- Nagpal, K.; Singh, S.K.; Mishra, D.N. Optimization of Brain Targeted Gallic Acid Nanoparticles for Improved Antianxiety-like Activity. Int. J. Biol. Macromol. 2013, 57, 83–91. [Google Scholar] [CrossRef]
- Baziyar, Y.; Amin Edalatmanesh, M.; Hosseini, S.A.; Zar, A. The Effects of Endurance Training and Gallic Acid. on BDNF and TNF-a in Male Rats. with Alzheimer. Int. J. Appl. Exerc. Physiol. 2016, 5, 2322–3537. [Google Scholar]
- Bayes, W. Gallic Acid in Hæmoptysis. Prov. Med. Surg. J. 1852, 16, 498. [Google Scholar] [CrossRef]
- Ong, K.C.; Khoo, H.E.; Das, N.P. Tannic Acid Inhibits Insulin-Stimulated Lipogenesis in Rat Adipose Tissue and Insulin Receptor Function in Vitro. Experientia 1995, 51, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Dludla, P.V.; Nkambule, B.B.; Jack, B.; Mkandla, Z.; Mutize, T.; Silvestri, S.; Orlando, P.; Tiano, L.; Louw, J.; Mazibuko-Mbeje, S.E. Inflammation and Oxidative Stress in an Obese State and the Protective Effects of Gallic Acid. Nutrients 2018, 11, 23. [Google Scholar] [CrossRef]
- Oi, Y.; Hou, I.C.; Fujita, H.; Yazawa, K. Antiobesity Effects of Chinese Black Tea (Pu-Erh Tea) Extract and Gallic Acid. Phytother. Res. 2012, 26, 475–481. [Google Scholar] [CrossRef]
- Chao, J.; Huo, T.I.; Cheng, H.Y.; Tsai, J.C.; Liao, J.W.; Lee, M.S.; Qin, X.M.; Hsieh, M.T.; Pao, L.H.; Peng, W.H. Gallic Acid Ameliorated Impaired Glucose and Lipid Homeostasis in High Fat Diet-Induced NAFLD Mice. PLoS ONE 2014, 9, e96969. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A. Anti-Obesity Potential of Gallic Acid from Labisia Pumila, through Augmentation of Adipokines in High Fat Diet Induced Obesity in C57BL/6 Mice. Adv. Res. 2014, 2, 556–570. [Google Scholar] [CrossRef]
- Huang, D.W.; Chang, W.C.; Yang, H.J.; Wu, J.S.B.; Shen, S.C. Gallic Acid Alleviates Hypertriglyceridemia and Fat Accumulation via Modulating Glycolysis and Lipolysis Pathways in Perirenal Adipose Tissues of Rats Fed a High-Fructose Diet. Int. J. Mol. Sci. 2018, 19, 254. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, G.R.; Jothi, G.; Antony, P.J.; Balakrishna, K.; Paulraj, M.G.; Ignacimuthu, S.; Stalin, A.; Al-Dhabi, N.A. Gallic Acid Attenuates High-Fat Diet Fed-Streptozotocin-Induced Insulin Resistance via Partial Agonism of PPARγ in Experimental Type 2 Diabetic Rats and Enhances Glucose Uptake through Translocation and Activation of GLUT4 in PI3K/p-Akt Signaling Pathway. Eur. J. Pharmacol. 2014, 745, 201–216. [Google Scholar] [CrossRef]
- Doan, K.V.; Ko, C.M.; Kinyua, A.W.; Yang, D.J.; Choi, Y.H.; Oh, I.Y.; Nguyen, N.M.; Ko, A.; Choi, J.W.; Jeong, Y.; et al. Gallic Acid Regulates Body Weight and Glucose Homeostasis Through AMPK Activation. Endocrinology 2015, 156, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Liang, X.; Yang, Q.; Fu, X.; Rogers, C.J.; Zhu, M.; Rodgers, B.D.; Jiang, Q.; Dodson, M.V.; Du, M. Resveratrol Induces Brown-like Adipocyte Formation in White Fat through Activation of AMP-Activated Protein Kinase (AMPK) A1. Int. J. Obes. 2015, 39, 967–976. [Google Scholar] [CrossRef] [PubMed]
- Punithavathi, V.R.; Stanely Mainzen Prince, P.; Kumar, M.R.; Selvakumari, C.J. Protective Effects of Gallic Acid on Hepatic Lipid Peroxide Metabolism, Glycoprotein Components and Lipids in Streptozotocin-Induced Type II Diabetic Wistar Rats. J. Biochem. Mol. Toxicol. 2011, 25, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Kang, N.; Lee, J.H.; Lee, W.W.; Ko, J.Y.; Kim, E.A.; Kim, J.S.; Heu, M.S.; Kim, G.H.; Jeon, Y.J. Gallic Acid Isolated from Spirogyra Sp. Improves Cardiovascular Disease through a Vasorelaxant and Antihypertensive Effect. Environ. Toxicol. Pharmacol. 2015, 39, 764–772. [Google Scholar] [CrossRef]
- Yan, X.; Zhang, Q.Y.; Zhang, Y.L.; Han, X.; Guo, S.B.; Li, H.H. Gallic Acid Attenuates Angiotensin II-Induced Hypertension and Vascular Dysfunction by Inhibiting the Degradation of Endothelial Nitric Oxide Synthase. Front. Pharmacol. 2020, 11, 558032. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Hadidi, M.; Karaca, A.C.; Hedayati, S.; Tarahi, M.; Assadpour, E.; Jafari, S.M. Chitosan-Grafted Phenolic Acids as an Efficient Biopolymer for Food Packaging Films/Coatings. Carbohydr. Polym. 2023, 314, 120901. [Google Scholar] [CrossRef]
- Shah, Y.A.; Bhatia, S.; Al-Harrasi, A.; Tarahi, M.; Almasi, H.; Chawla, R.; Ali, A.M.M. Insights into Recent Innovations in Barrier Resistance of Edible Films for Food Packaging Applications. Int. J. Biol. Macromol. 2024, 271, 132354. [Google Scholar] [CrossRef]
- Hadidi, M.; Jafarzadeh, S.; Forough, M.; Garavand, F.; Alizadeh, S.; Salehabadi, A.; Khaneghah, A.M.; Jafari, S.M. Plant Protein-Based Food Packaging Films; Recent Advances in Fabrication, Characterization, and Applications. Trends Food Sci. Technol. 2022, 120, 154–173. [Google Scholar] [CrossRef]
- Zhang, W.; Hedayati, S.; Tarahi, M.; Can Karaca, A.; Hadidi, M.; Assadpour, E.; Jafari, S.M. Advances in Transglutaminase Cross-Linked Protein-Based Food Packaging Films; a Review. Int. J. Biol. Macromol. 2023, 253, 127399. [Google Scholar] [CrossRef] [PubMed]
- Umaraw, P.; Munekata, P.E.S.; Verma, A.K.; Barba, F.J.; Singh, V.P.; Kumar, P.; Lorenzo, J.M. Edible Films/Coating with Tailored Properties for Active Packaging of Meat, Fish and Derived Products. Trends Food Sci. Technol. 2020, 98, 10–24. [Google Scholar] [CrossRef]
- Nie, X.; Gao, Z.; Ren, X.; Jiang, Q.; Li, S.; Jiang, C.; Liu, B.; Liu, X.; He, F. Effect of Pectin Coating Infused with Gallic Acid on the Quality and Shelf Life of Japanese Sea Bass (Lateolabrax japonicas) Fillets. Food Bioproc Tech. 2020, 13, 300–307. [Google Scholar] [CrossRef]
- Liu, J.; Lan, W.; Wu, Y.; Sun, X.; Mei, J.; Chen, Y.; Xie, J. The Preservation Effects of Chitosan Copolymers (gallic acid and protocatechuic acid) on Sea Bass (Lateolabrax japonicus) Fillets. Aquac. Fish. 2023, 8, 305–315. [Google Scholar] [CrossRef]
- Wong, L.W.; Loke, X.J.; Chang, C.K.; Ko, W.C.; Hou, C.Y.; Hsieh, C.W. Use of the Plasma-Treated and Chitosan/Gallic Acid-Coated Polyethylene Film for the Preservation of Tilapia (Orechromis niloticus) Fillets. Food Chem. 2020, 329, 126989. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Liu, H.; Huang, A.; Zhou, C.; Hong, P.; Deng, C. Collagen/Zein Electrospun Films Incorporated with Gallic Acid for Tilapia (Orechromis niloticus) Muscle Preservation. J. Food Eng. 2022, 317, 110860. [Google Scholar] [CrossRef]
- Wu, C.; Li, Y.; Wang, L.; Hu, Y.; Chen, J.; Liu, D.; Ye, X. Efficacy of Chitosan-Gallic Acid Coating on Shelf Life Extension of Refrigerated Pacific Mackerel Fillets. Food Bioproc. Tech. 2016, 9, 675–685. [Google Scholar] [CrossRef]
- Zarandona, I.; López-Caballero, M.E.; Montero, M.P.; Guerrero, P.; de la Caba, K.; Gómez-Guillén, M.C. Horse Mackerel (Trachurus Trachurus) Fillets Biopreservation by Using Gallic Acid and Chitosan Coatings. Food Control 2021, 120, 107511. [Google Scholar] [CrossRef]
- Li, Q.; Zhou, W.; Yu, X.; Cui, F.; Tan, X.; Sun, T.; Li, J. Preparation and Characterization of Zein/Gelatin Electrospun Film Loaded with ε-Polylysine and Gallic Acid as Tuna Packaging System. J. Sci. Food Agric. 2024, 104, 1942–1952. [Google Scholar] [CrossRef]
- Xiong, Y.; Kamboj, M.; Ajlouni, S.; Fang, Z. Incorporation of Salmon Bone Gelatine with Chitosan, Gallic Acid and Clove Oil as Edible Coating for the Cold Storage of Fresh Salmon Fillet. Food Control 2021, 125, 107994. [Google Scholar] [CrossRef]
- Zheng, M.; Zhang, C.; Zhou, Y.; Lu, Z.; Zhao, H.; Bie, X.; Lu, F. Preparation of Gallic Acid-Grafted Chitosan Using Recombinant Bacterial Laccase and Its Application in Chilled Meat Preservation. Front. Microbiol. 2018, 9, 01729. [Google Scholar] [CrossRef]
- Fang, Z.; Lin, D.; Warner, R.D.; Ha, M. Effect of Gallic Acid/Chitosan Coating on Fresh Pork Quality in Modified Atmosphere Packaging. Food Chem. 2018, 260, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Zheng, T.; Tang, P.; Yang, C.; Ran, R.; Li, G. Development of Active Packaging Films Based on Collagen/Gallic Acid-Grafted Chitosan Incorporating with ε-Polylysine for Pork Preservation. Food Hydrocoll. 2023, 140, 108590. [Google Scholar] [CrossRef]
- Rong, L.; Ji, X.; Shen, M.; Chen, X.; Qi, X.; Li, Y.; Xie, J. Characterization of Gallic Acid-Chinese Yam Starch Biodegradable Film Incorporated with Chitosan for Potential Use in Pork Preservation. Food Research Int. 2023, 164, 112331. [Google Scholar] [CrossRef] [PubMed]
- Hoa, V.B.; Song, D.H.; Seol, K.H.; Kim, Y.S.; Kim, H.W.; Bae, I.S.; Cho, S.H. Effect of Coating with Combined Chitosan and Gallic Acid on Shelf-Life Stability of Jeju Black Cattle Beef. Anim. Biosci. 2024, 37, 123–130. [Google Scholar] [CrossRef]
- Zou, J.; Liu, X.; Wang, X.; Yang, H.; Cheng, J.; Lin, Y.; Tang, D. Influence of Gelatin-Chitosan-Glycerol Edible Coating Incorporated with Chlorogenic Acid, Gallic Acid, and Resveratrol on the Preservation of Fresh Beef. Foods 2022, 11, 3813. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Yi, J.; Yuan, X.; Zhang, Z.; Shan, Z.; Wang, H. Fabrication and Characterization of Carrageenan-Based Multifunctional Films Integrated with Gallic Acid@ZIF-8 for Beef Preservation. Int. J. Biol. Macromol. 2024, 274, 133319. [Google Scholar] [CrossRef]
- Zhang, H.; Montemayor, A.M.; Wimsatt, S.T.; Tikekar, R.V. Effect of Combination of UV-A Light and Chitosan-Gallic Acid Coating on Microbial Safety and Quality of Fresh Strawberries. Food Control 2022, 140, 109106. [Google Scholar] [CrossRef]
- Taechutrakul, S.; Piroonpan, T.; Pasanphan, W. Active Film Strips to Extend the Shelf Life of Fruits: Multibranched PLA-Gallic Acid as an Antioxidant/Oxygen Scavenger in a Case Study of Bananas (Musa AAA Group). J. Food Eng. 2024, 364, 111794. [Google Scholar] [CrossRef]
- Awad, M.A.; Al-Qurashi, A.D.; Mohamed, S.A.; El-Shishtawy, R.M. Quality and Biochemical Changes of ‘Hindi-Besennara’ Mangoes during Shelf Life as Affected by Chitosan, Gallic Acid and Chitosan Gallate. J. Food Sci. Technol. 2017, 54, 4139–4148. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wu, H.; Zhang, L.; Sun, Q. Horseradish Peroxidase-Mediated Synthesis of an Antioxidant Gallic Acid-G-Chitosan Derivative and Its Preservation Application in Cherry Tomatoes. RSC Adv. 2018, 8, 20363–20371. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Perera, K.Y.; Pradhan, D.; Duffy, B.; Jaiswal, A.K.; Jaiswal, S. Active Packaging Film Based on Poly Lactide-Poly (Butylene Adipate-Co-Terephthalate) Blends Incorporated with Tannic Acid and Gallic Acid for the Prolonged Shelf Life of Cherry Tomato. Coatings 2022, 12, 1902. [Google Scholar] [CrossRef]
- Gasti, T.; Dixit, S.; Chougale, R.B.; Masti, S.P. Fabrication of Novel Gallic Acid Functionalized Chitosan/Pullulan Active Bio-Films for the Preservation and Shelf-Life Extension of Green Chillies. Sustain. Food Technol. 2023, 1, 390–403. [Google Scholar] [CrossRef]
- Hadidi, M.; Orellana-Palacios, J.C.; Aghababaei, F.; Gonzalez-Serrano, D.J.; Moreno, A.; Lorenzo, J.M. Plant By-Product Antioxidants: Control of Protein-Lipid Oxidation in Meat and Meat Products. Lebensm. Wiss. Technol. 2022, 169, 114003. [Google Scholar] [CrossRef]
- Li, X.; Zhang, R.; Hassan, M.M.; Cheng, Z.; Mills, J.; Hou, C.; Realini, C.E.; Chen, L.; Day, L.; Zheng, X. Active Packaging for the Extended Shelf-Life of Meat: Perspectives from Consumption Habits, Market Requirements and Packaging Practices in China and New Zealand. Foods 2022, 11, 2903. [Google Scholar] [CrossRef] [PubMed]
- Aghababaei, F.; McClements, D.J.; Martinez, M.M.; Hadidi, M. Electrospun Plant Protein-Based Nanofibers in Food Packaging. Food Chem. 2024, 432, 137236. [Google Scholar] [CrossRef] [PubMed]
- Nian, L.; Wang, M.; Sun, X.; Zeng, Y.; Xie, Y.; Cheng, S.; Cao, C. Biodegradable Active Packaging: Components, Preparation, and Applications in the Preservation of Postharvest Perishable Fruits and Vegetables. Crit. Rev. Food Sci. Nutr. 2024, 64, 2304–2339. [Google Scholar] [CrossRef] [PubMed]
- Tarahi, M.; Mohamadzade Fakhr-davood, M.; Ghaedrahmati, S.; Roshanak, S.; Shahidi, F. Physicochemical and Sensory Properties of Vegan Gummy Candies Enriched with High-Fiber Jaban Watermelon Exocarp Powder. Foods 2023, 12, 1478. [Google Scholar] [CrossRef] [PubMed]
- Melini, V.; Vescovo, D.; Melini, F.; Raffo, A. Bakery Product Enrichment with Phenolic Compounds as an Unexplored Strategy for the Control of the Maillard Reaction. Appl. Sci. 2024, 14, 2647. [Google Scholar] [CrossRef]
- Mildner-Szkudlarz, S.; Różańska, M.; Piechowska, P.; Waśkiewicz, A.; Zawirska-Wojtasiak, R. Effects of Polyphenols on Volatile Profile and Acrylamide Formation in a Model Wheat Bread System. Food Chem. 2019, 297, 125008. [Google Scholar] [CrossRef] [PubMed]
- Villanova, F.A.; Lin, A.H.M. Modification of Pea Starch Digestibility through the Complexation with Gallic Acid via High-Pressure Homogenization. Polymers 2022, 14, 2623. [Google Scholar] [CrossRef] [PubMed]
- Chi, C.; Li, X.; Zhang, Y.; Chen, L.; Xie, F.; Li, L.; Bai, G. Modulating the in Vitro Digestibility and Predicted Glycemic Index of Rice Starch Gels by Complexation with Gallic Acid. Food Hydrocoll. 2019, 89, 821–828. [Google Scholar] [CrossRef]
- Deng, N.; Bian, X.; Luo, S.; Liu, C.; Hu, X. The Starch-Polyphenol Inclusion Complex: Preparation, Characterization and Digestion. Food Biosci. 2023, 53, 102655. [Google Scholar] [CrossRef]
- Wu, D.; Ge, F.; Ma, H.; Xia, R.; Cheng, W.; Tang, X. Gallic Acid-Fortified Buckwheat Wantuo: Characteristics of in Vitro Starch Digestibility, Antioxidant and Eating Quality. J. Food Sci. Technol. 2023, 60, 292–302. [Google Scholar] [CrossRef] [PubMed]
- Luo, D.; Xie, Q.; Chen, C.; Mu, K.; Wang, Z.; Gu, S.; Xue, W. Increasing the Pressure during High Pressure Homogenization Regulates the Starch Digestion of the Resulting Pea Starch-Gallic Acid Complexes. Int. J. Biol. Macromol. 2023, 235, 123820. [Google Scholar] [CrossRef] [PubMed]
- Saphier, O.; Barak, T.; Hamou, B.; Silberstein, T. Caffeic and Gallic Acids Protect Commercial Infant Milk Formula from Oxidative Damage. Adv. Dairy Res. 2019, 7, 1–4. [Google Scholar] [CrossRef]
- Delfanian, M.; Sahari, M.A.; Barba, F.J. Effect of Lipophilized Gallic Acid on the Oxidative Stability of Omega-3 Fatty Acids Rich Soy and Cow Milk. Lebensm. Wiss. Technol. 2023, 190, 115475. [Google Scholar] [CrossRef]
- Harbourne, N.; Jacquier, J.C.; O’Riordan, D. Effects of Addition of Phenolic Compounds on the Acid Gelation of Milk. Int. Dairy. J. 2011, 21, 185–191. [Google Scholar] [CrossRef]
- Mittal, A.; Benjakul, S.; Palamae, S.; Saetang, J.; Singh, A. Milk Fortified with Chitooligosaccharide–Gallic Acid Conjugate and Its Liposome: Characterisation, Storage Stability, and in Vitro Digestibility. Int. J. Food Sci. Technol. 2024, 59, 1466–1480. [Google Scholar] [CrossRef]
- Al-Younis, Z.K.; Al-Hatim, R.R.; Fadhal Abbas, S.; Altemimi, A.B.; Hesarinejad, M.A.; Younis, M.I.; Abedelmaksoud, T.G. The Use of Transglutaminase and Gallic Acid as Stabilizers and Thickeners in the Production of Ice Cream. Res. Innov. Food Sci. Technol. 2024. [Google Scholar] [CrossRef]
- Du, M.; Ahn, D.U. Effect of Antioxidants on the Quality of Irradiated Sausages Prepared with Turkey Thigh Meat 1. Poult. Sci. 2002, 81, 1251–1256. [Google Scholar] [CrossRef] [PubMed]
- López-Romero, J.C.; Valenzuela-Melendres, M.; Juneja, V.K.; García-Dávila, J.; Camou, J.P.; Peña-Ramos, A.; González-Ríos, H. Effects and Interactions of Gallic Acid, Eugenol and Temperature on Thermal Inactivation of Salmonella Spp. in Ground Chicken. Food Res. Int. 2018, 103, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Anvar, N.; Nateghi, L.; Shariatifar, N.; Mousavi, S.A. The Effect of Essential Oil of Anethum graveolens L. Seed and Gallic Acid (Free and Nano Forms) on Microbial, Chemical and Sensory Characteristics in Minced Meat during Storage at 4 °C. Food Chem. X 2023, 19, 100842. [Google Scholar] [CrossRef] [PubMed]
- Sik, B.; Székelyhidi, R.; Lakatos, E.; Kapcsándi, V.; Ajtony, Z. Analytical Procedures for Determination of Phenolics Active Herbal Ingredients in Fortified Functional Foods: An Overview. Eur. Food Res. Technol. 2022, 248, 329–344. [Google Scholar] [CrossRef]
- Silva, I.C.; Polaquini, C.R.; Regasini, L.O.; Ferreira, H.; Pavan, F.R. Evaluation of Cytotoxic, Apoptotic, Mutagenic, and Chemopreventive Activities of Semi-Synthetic Esters of Gallic Acid. Food Chem. Toxicol. 2017, 105, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Kahkeshani, N.; Farzaei, F.; Fotouhi, M.; Alavi, S.S.; Bahramsoltani, R.; Naseri, R.; Momtaz, S.; Abbasabadi, Z.; Rahimi, R.; Farzaei, M.H.; et al. Pharmacological Effects of Gallic Acid in Health and Diseases: A Mechanistic Review. Iran. J. Basic Med. Sci. 2019, 22, 225. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Zhang, Y.; Tang, C.; Hou, Y.; Ai, X.; Chen, X.; Zhang, Y.; Wang, X.; Meng, X. Gallic Acid: Pharmacological Activities and Molecular Mechanisms Involved in Inflammation-Related Diseases. Biomed. Pharmacother. 2021, 133, 110985. [Google Scholar] [CrossRef]
- Lesschaeve, I.; Noble, A.C. Polyphenols: Factors Influencing Their Sensory Properties and Their Effects on Food and Beverage Preferences. Am. J. Clin. Nutr. 2005, 81, 330S–335S. [Google Scholar] [CrossRef]
- Ferrer-Gallego, R.; Miguel Hernández-Hierro, J.; Rivas-Gonzalo, J.C.; Teresa Escribano-Bailón, M. Sensory Evaluation of Bitterness and Astringency Sub-Qualities of Wine Phenolic Compounds: Synergistic Effect and Modulation by Aromas. Food Res. Int. 2014, 62, 1100–1107. [Google Scholar] [CrossRef]
- Chuang, W.-P.; Hsieh, B.-C. Development of a Gallic Acid Based Time Temperature Indicator with Adjustable Activation Energy. Food Control 2023, 144, 109396. [Google Scholar] [CrossRef]

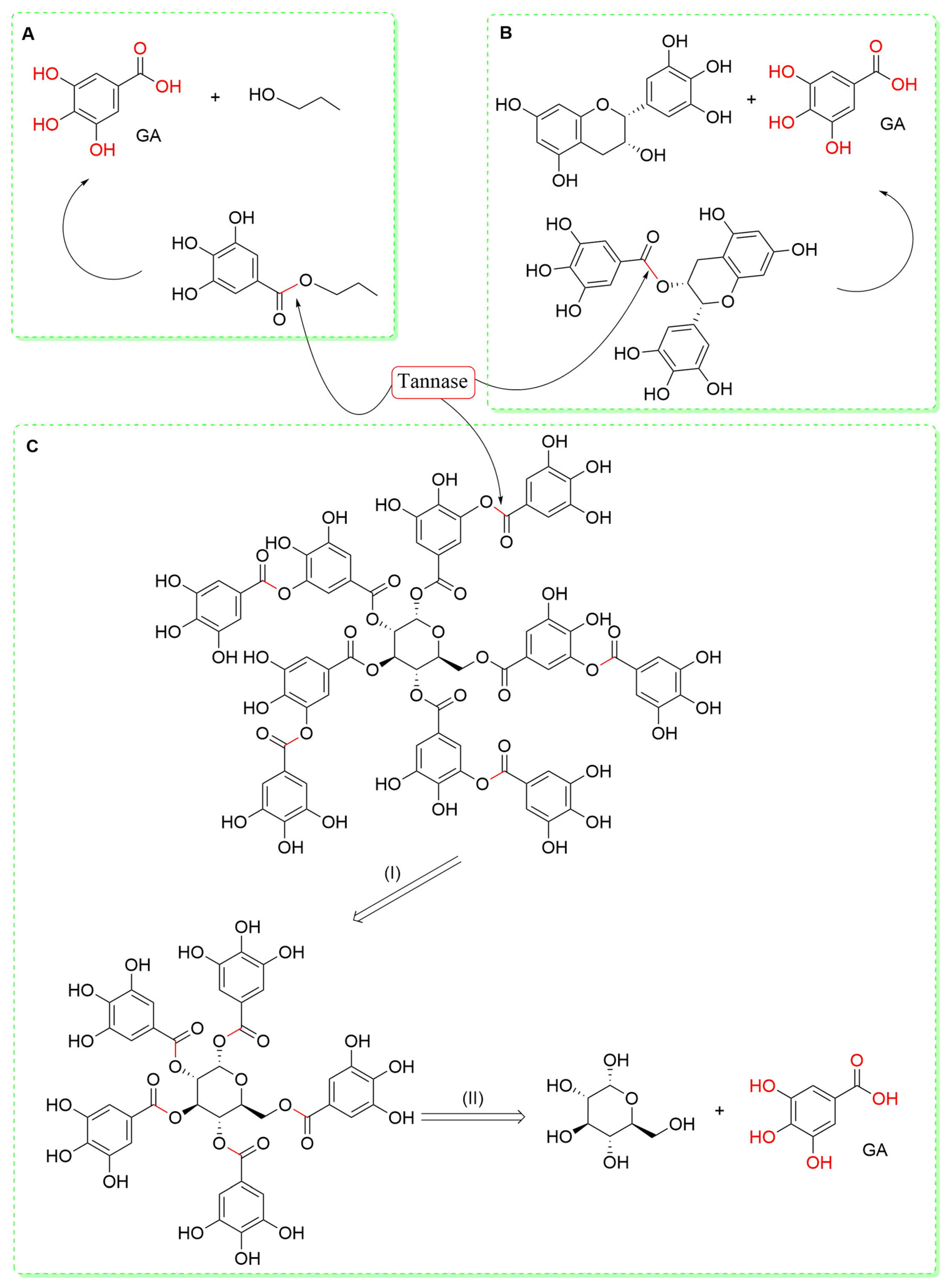
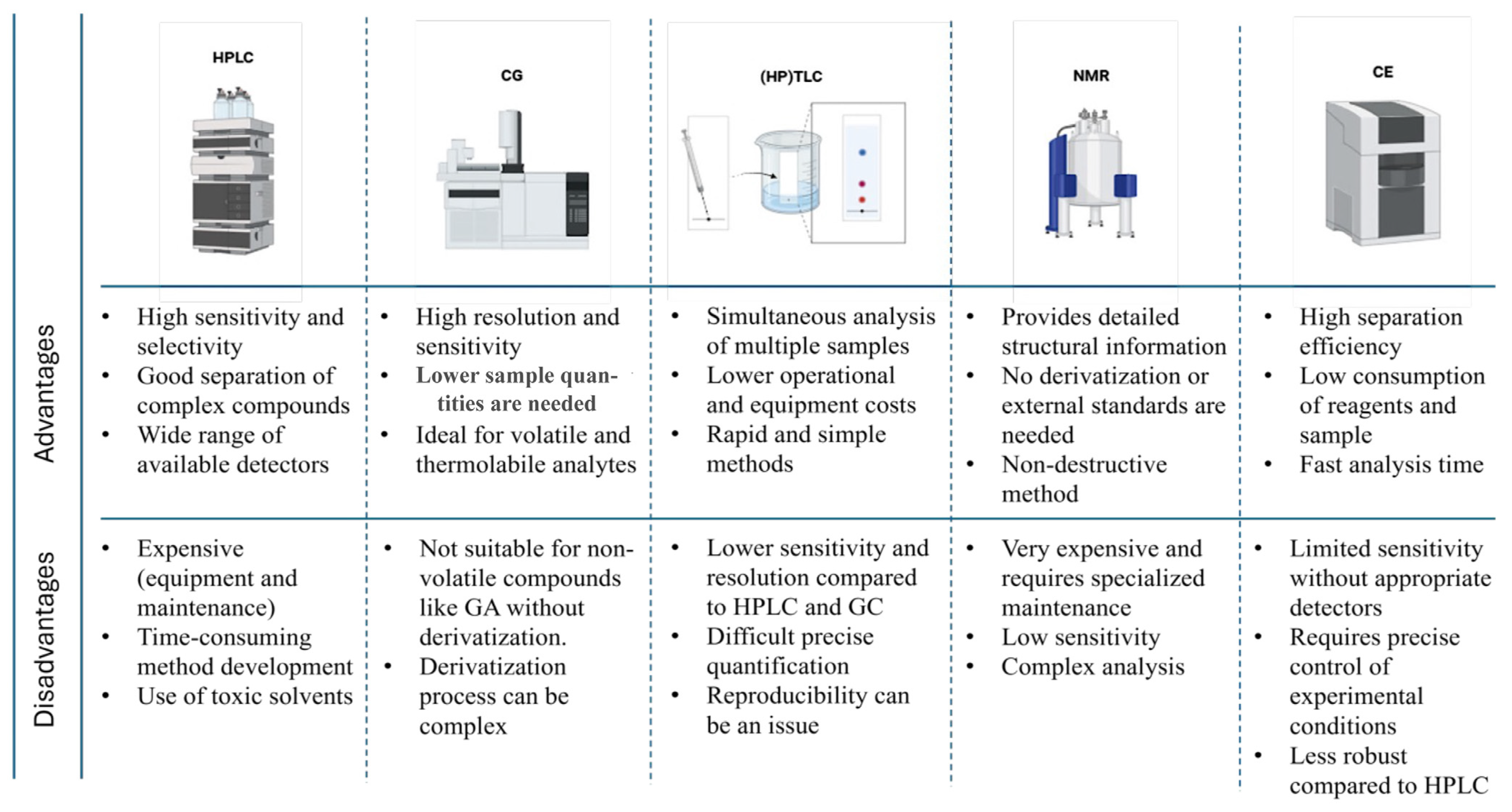

| Sample/Matrix Type | Sample Pretreatment | Sample Preparation | Analytical Method | Analytical Conditions | GA Concentration | References |
|---|---|---|---|---|---|---|
| Longan seed (Dimocarpus longan Lour.) and mango kernel (Mangifera indica L.) (Singapore) | Longan seed and mango kernel: freeze-dried (FD) (−50 °C, 24 h) Mango kernel: dried at 160 °C, then ground and stored at −20 °C | Longan seed: (1) FD/EtOH extraction al 70 °C, 1 h Mango kernel (2) FD/EtOH extraction at 70 °C, 1 h (3) FD/MeOH extraction at 70 °C, 1 h (4) FD/MeOH and hydrolysis at 35 °C, 16 h (5) FD/MeOH and hydrolysis at 85 °C, 2 h (6) 160 °C-dried/MeOH extraction at 70 °C, 1 h (7) 160 °C-heated/MeOH extraction and hydrolysis at 35 °C, 16 h (8) 160 °C-heated/MeOH extraction and hydrolysis at 85 °C, 2 h | RP-HPLC/DAD | Column: Shim-Pack VP-ODS column (250 × 4.6 mm) with a guard column GCP-ODS (10 × 4.6 mm); MP: H2O–AA (97:3, v/v) (A) and MeOH (B) in gradient mode: 10% B for 10 min to 70% B in 40 min; Tª: 40 °C; FR: 0.9 mL/min; λ = 280 and 360 nm | [GA] (mg/100 g seeds) (1) 23.3 (2) 185 (3) 20.0 (4) 84.1 (5) 163 (6) 414 (7) 535 (8) 838 | [67] |
| Nigella sativa seeds (Nagpur, India) | Seeds were dried in air, ground, sieved, weighed and stored in an airtight container at room temperature | N. sativa powder was dissolved in 10 mL of MeOH, filtered, and distilled under reduced pressure. | RP-HPLC/DAD | Column: C18 (MakeGrace) (250 × 4.6 mm, 5 µm); MP: ACN:H2O (60:40, v/v) adjusted to pH 3.00 with 0.05% PA was used in isocratic mode; Tª: RT; FR: 0.5 mL/min; λ = 210 nm | [GA]: 0.4736 µg/mL | [68] |
| Five commercial pomegranate juices (PJ) with different brands (Mahshahr, Iran) | Samples were stored at 4 °C | 5 mL of each sample was centrifuged at 3000 rpm for 20 min. The supernatant was filtered and diluted to 50 mL with distilled water; 10 mL of the above solution was transferred into a volumetric flask, and after the addition of α–cyclodextrin, adjusting the pH to 3.0 and filling it to the mark with 10% EtOH:H2O, it was sonicated for 20 min. | RP-HPLC/DAD | Column: C18 (250 mm × 4.6 mm, 5 µm); MP: ACN/diluted with PA 25.0 × 10−3 mol/L (15:85, v/v) in isocratic mode; FR: 1.0 mL/min; λ = 240 nm | [GA] (mg/L) PJ1: 3.98 PJ2: 2.41 PJ3: 1.30 PJ4: 3.00 PJ5: 4.98 | [69] |
| Grape juice (GJ) and wine (W) (Sub-Middle São Francisco Valley, Brazil) | Sorting and separation of GJ varieties (GJA, GJB, GJC, GJD, GJE) and W varieties: WF, WG, WH | GJ/W previously diluted 500 μL + 1000 μL in phase A. | RP-HPLC/DAD | Column: Zorbax Eclipse Plus RP-C18 (100 × 4.6 mm, 3.5 µm) with a guard column C18 (12.6 × 4.6 mm, 5 µm); MP: 0.1 M PA in H2O (pH 2.0) (A) and 0.5% PA in MeOH (B) in a gradient mode: 0–5 min: 5% B; 5–14 min: 23% B; 14–22 min: 26% B; 22–25 min: 80% B; Tª: 35 °C; FR: 0.8 mL/min; λ = 280 nm | [GA] (mg/mL) GJA: 4.5 ± 0.4 GJB: 3.6 ± 2.9 GJC: 16.7 ± 3.5 GJD: 7.6 ± 1.2 GJE: 6.5 ± 1.0 WF: 26.4 ± 1.1 WG: 24.7 ± 1.0 WH: 16.9 ± 2.6 | [70] |
| Skins, pulps, seeds, canes, and leaves from grapes (V. vinifera L) (Urmia, Iran) | Sorting, separation, and crushing of different parts of grape varieties: Muscat Alexanderia (MA), Hosseini (Hos), Ghara Shira (GShi), Agh Shani (AG), Ghara Shani (GSha), and Ghara Ghandome (GG) | UAE: Powdered grape parts were weighed and mixed with 5 mL. MeOH:HCl (99:1, v/v) for ultrasonic extraction at 25 °C and a frequency of 35 kHz for 20 min. Then, samples were taken out and left at RT for 30 min. The extract was filtered and the remaining solids were extracted again under the same conditions. | RP-HPLC/DAD | Column: C18, (250 × 4.6 mm, 5 µm) and pre-column; MP: H2O:THF:TFA (98:2:0.1, v/v/v) (A) and MeOH:THF:TFA (98:2:0.1, v/v/v) (B) in a gradient mode: 17% B for 2 min: 17% B for 2 min increasing to 25% B after 5 min to 35% B after a further 8 min and to 50% B after a further 5 min; Tª: 25 °C; FR: 1 mL/min; λ = 278 nm | [GA] (μg/g) MA: skin: 122 ± 7, pulp: 109 ± 5; seed: 87 ± 3; cane: 118 ± 4; leaf: 1.4 ± 0.1; Hos: skin: 143 ± 6, pulp: 128 ± 7, seed: 87 ± 5, cane: 102 ± 4, leaf: 0.9 ± 0.1; GShi: skin: 238 ± 13, Pulp:153 ± 8, seed: 77 ± 4, cane: 132 ± 8, leaf: 1.3 ± 0.2; AG: skin: 220 ± 13, pulp: 178 ± 8, seed: 77 ± 4, cane: 141 ± 7, leaf: 1.9 ± 0.2; GSha: skin: 319 ± 17, pulp: 192 ± 10, seed: 91 ± 5, cane: 153 ± 8, leaf: 2.6 ± 0.2; GG: skin: 127 ± 7, pulp: 87 ± 4, seed: 67 ± 4, cane: 101 ± 6, leaf: 1.1 ± 0.1 | [71] |
| Three different varieties of Camellia seed oils (China) | C. sinensis (CS): (1) H. Xinyang; (2) J. Lushan; (3) S. Rizhao; (4) H. Enshi; (5) Z. Hangzhou; (6) F. Quanzhou C. oleifera (CO): (7) A. Huangshan; (8) J. Ganzhou; (9) G. Liuzhou; (10) H. Chenzhou; (11) H. Huaihua; (12) F. Shanming C. chekiangoleosa (CC): (13) J. Dexing; (14) Z. Kaihua; (15) H. Loudi All kinds of Camellia seeds were dried, unshelled, and crushed to obtain oil. The oil samples were placed in glass bottles, and stored in the dark at RT until further extraction | Liquid–liquid extraction assisted by centrifugation. | HPLC-ESI-Q-TOF-MS | HPLC conditions: Column: SPURSIL-C18 (150 × 2.1 mm, 2.1 µm); MP: 0.1% AA in H2O (A) and 0.1% AA in ACN (B) in a gradient mode: 0–20 min, 0–50% B; 20–25 min, 50–100% B; Tª: 30 °C; FR: 0.4 mL/min. ESI conditions: negative ionization mode; capillary: 4000 V; nebulizer pressure: 30 psi; fragment voltage: 140 V; drying gas FR: 9 L/min; gas Tª: 190 °C; N2 sheath gas Tª: 350 °C; N2 sheath gas FR: 10 L/min; Scan range, m/z: 100–1000 in full scan mode; collision energy: 10–40 eV. Quantitative analysis was performed by HPLC-QqQ-MS with the following gradient elution: 0–30 min, 0–10% B; 30–50 min, 10–40% B; 50–55 min, 40–0% B. | [GA] (μg/g) CS1: 2.2114 ± 0.0069 CS2: 2.3097 ± 0.0239 CS3: 1.9689 ± 0.0113 CS4: 2.5764 ± 0.0099 CS5: 1.8805 ± 0.0019 CS6: 2.3964 ± 0.0201 CO7: 0.5150 ± 0.0011 CO8: 0.4640 ± 0.0051 CO9: 0.9720 ± 0.0102 CO10: 1.5580 ± 0.0014 CO11: 0.5169 ± 0.0051 CO12: 0.7012 ± 0.0009 CC13: 1.7910 ± 0.0062 CC14: 1.4881 ± 0.0098 CC15: 1.1243 ± 0.024 | [72] |
| Two varieties of Psidium guajava L. (Motril, España) | Pyrifera (Pyr) and pomifera (Pom) varieties of P. guajava L. were air-dried and crushed | UAE: samples were extracted (×3 times) with 15 mL of EtOH: H2O mixture (80:20, v/v) for 10 min at RT, centrifuged for 15 min at 6000 rpm. The pooled supernatants were evaporated, dissolved in 2 mL of MeOH/H2O (50:50, v/v), and stored at −20 °C in the dark until analysis. | HPLC-ESI-Q-TOF-MS | HPLC conditions: Column: Poroshell 120 EC-C18 (100 × 4.6 mm, 2.7 µm); MP: 1% AA in H2O (A) and ACN (B) in a gradient mode: 0 min, 0.8% B; 2.5 min, 0.8% B; 5.5 min, 6.8% B; 11 min, 14.4% B; 17 min, 24% B; 22 min, 40% B; 26 min, 100% B; Tª: 25 °C; FR: 0.8 mL/min. ESI conditions: negative ionization mode; capillary: 3500 V; nebulizer pressure: 50 psi; fragment voltage: 3500 V; drying gas FR: 12.0 L/min; gas Tª: 370 °C; N2 sheath gas Tª: 350 °C; N2 sheath gas FR: 10 L/min; Scan range, m/z: 50–1500 in full scan mode; collision energy: 30–45 eV. | [GA] (mg/g leaf d.w) (Pyr): 0.060 ± 0.008 (Pom): 0.223 ± 0.003 | [73] |
| Pulp, peel, seed, and husk of Keitt mango | Samples were separated, FD, milled, and kept at −18 °C until use | Solid–liquid extraction: Free polar fraction of mango (FPF): FD powder samples were dissolved in 10 mL of solution of MeOH/H2O (80:20, v/v) and placed for 15 min at RT in an ultrasonic bath. The mixture was centrifuged for 15 min at 1000× g, and the supernatant was stored. This process was repeated twice. | HPLC-DAD-ESI-Q-TOF-MS | HPLC conditions: Column: Poroshell 120 EC-C18 (100 × 4.6 mm, 2.7 µm); MP: 1% AA in H2O (A) and ACN (B) in a gradient mode: 0 min, 0.8% B; 5.5 min, 6.8% B; 16 min, 20% B; 20 min, 25% B; 25 min, 35% B; 29 min, 100%; Tª: 25 °C; FR: 0.8 mL/min. λ: 240, 280, and 330 nm. ESI conditions: negative ionization mode; capillary: 3500 V; nebulizer pressure: 50 psi; fragment voltage: 3500 V; drying gas FR: 12.0 L/min; gas Tª: 370 °C; N2 sheath gas Tª: 370 °C; N2 sheath gas FR: 10 L/min; Scan range, m/z: 50–1500 in full scan mode; collision energy: 30–45 eV. | [GA] (mg/100 g leaf d.w) FPF Pulp: 2.08 ± 0.02 Peel: 12.18 ± 0.39 Seed: 17.55 ± 0.74 Husk: 2.48 ± 0.05 BPF Pulp: 0.019 ± 0.001 Peel: 0.718 ± 0.062 Seed: 0.310 ± 0.015 Husk: 0.487 ± 0.017 | [74] |
| Red and yellow araçá (Psidium cattleianum Sabine) (Pelotas, Brazil) | Red araçá (RA) and yellow araçá (YA) were separated and lyophilized for 72 h. FD samples were milled and stored at −18 °C until analysis | Extraction of extractable phenolic compounds (EPC): 5 mL of MeOH: H2O (8:2, v/v) acidified with 0.35% FA was added to FD samples and vortexed for 3 min. The extract was centrifuged at 3000× g for 5 min (4 °C), and the supernatant was placed in a rotary evaporator to remove the MeOH. Extraction of non-extractable phenolic compounds (NEPC): The NEPC fraction was obtained from acid hydrolysis of the solid product generated in the EPC extraction. The pellet was added to 20 mL of MeOH acidified with HCl (15%, v/v) for 15 min at 90 °C. The extract was centrifuged and placed in a rotary evaporator as before. | LC-DAD-ESI-Q-TOF-MS/MS | HPLC conditions: Column: C18 Synergy Hydro-RP column (250 × 4.6 mm, 4 µm); MP: 0.5% FA in H2O (A) and 0.5% FA in ACN (B) in a gradient mode: 99:1 (v/v) A/B to 50:50 (v/v) A/B over 50 min and then from 50:50 (v/v) A/B to 1:99 (v/v) A/B over 5 min. λ: 280 and 320 nm. ESI conditions: negative ionization mode; capillary: 3000 V; nebulizer pressure: 50 psi; fragment voltage: 3500 V; drying gas FR: 8.0 L/min; gas Tª: 370 °C; N2 sheath gas Tª: 310 °C; N2 sheath gas FR: 10 L/min; Scan range, m/z: 50–1500 in full scan mode; collision energy: 30–45 eV. | [GA] (μg/g) RA-EPC: 9.9 ± 0.5 RA-NEPC: 34.1 ± 2.1 YA-EPC: 7.4 ± 0.3 YA-NEPC: 11.1 ± 0.9 | [75] |
| Seven samples of different types of red wine (Bahia, Brazil) | Samples were stored at 4 °C in the dark: Shiraz (1), Cabernet Sauvignon (2), Cabernet Sauvignon/Shiraz (3), Cabernet Sauvignon/Shiraz (4), Shiraz (5), Shiraz (6), Cabernet Sauvignon/Shiraz (7) | Liquid extraction: NaCl, Na2S2O5, and acidified ethyl acetate were added to each wine, and samples were subjected to ultrasonication for 7 min. After that, the samples were dried and the solid residue was spiked with pyridine, BSTFA, and 1% TMS. | GS-MS | The Tª program was the following: initial temperature of 80 °C, for 1 min, from 80 to 250 °C with a rate of 20 °C/min, 6 °C/min to 300 °C, and finally increased at 20 °C/min to 320 °C, and held for 24 min. ND about GS-MS procedure | [GA] (mg/L) (1): 21.4 ± 1.7 (2): 27.1 ± 4.0 (3): 47.2 ± 5.7 (4): 49.4 ± 6.0 (5): 46.4 ± 6.3 (6): 56.3 ± 5.6 (7): 54.1 ± 3.7 | [76] |
| Divya-Swasari-Vati (DSV) (Haridwar, India) | ND | Powdered DSV were dissolved in 10 mL H2O:MeOH (20:80, v/v) while sonicated for 20 min. | HPTLC | HPTLC plates: 10 × 10 cm plates for fingerprinting, and 20 × 10 cm plates, for quantification on aluminum-backed plates coated with a 0.20 mm layer of silica gel 60 F254; solvent system: two solvent systems, ethyl acetate/toluene/formic acid (10:9:1, v/v/v), and ethyl acetate/formic acid/acetic acid/water (10:1:1:2.3, v/v/v/v). | [GA]: 3226.0 ± 610.4 µg/g | [77] |
| Honey (Western Australia) | Four different varieties of honey were collected: (A) Calothamnus spp. honey (B) Agonis flexuosa honey (C) Corymbia calophylla honey (D) Eucalyptus marginata honey | Liquid–liquid extraction: 2 mL of deionized water was added to the honey sample and vortexed. The resulting solution was then extracted three times with 5 mL DCM and ACN (1:1, v/v). The combined extracts were dried and stored at 4 °C. | HPTLC | HPTLC plates: silica gel 60 F254 plates 10 × 20 cm; solvent system: toluene/ethyl acetate/formic acid (2:8:1, v/v/v) and toluene/ethyl acetate/formic acid (6:5:1, v/v/v); λ: 254 nm. | [GA] (µg/g) (A): 1.64 ± 0.00 (B): n.d. (C): 5.84 ± 0.00 (D): n.d. | [78] |
| Dodonaea angustifolia leaves (DALs) and flower (DAF) (Ethiopia) | Samples were washed, cut into smaller pieces (<45 µm), dried at RT, and ground | UAE: samples were extracted (twice) in 25 mL of MeOH at 35 °C for 15 min. The extracts were centrifuged at 10× g for 20 min and the supernatant was filtered and stored. | HPTLC | HPTLC plates: precoated silica gel 60 F254 aluminum plates (20 × 10 cm, 100 µm); solvent system: toluene/ethyl acetate/FA/MeOH (20:12:4:8, volume ratio); λ: 254 to 450 nm; humidity: 44–46% | [GA] (mg/100 g) DAL: 32.26 ± 1.55 DAF: 53.64 ± 1.21 | [79] |
| Leaves of Ricinus communis Linn (Shanghai, China) | Three samples of the leaves of R. communis L (1, 2, 3) were all dried at 60 °C for 2 h and then pulverized. | Powdered samples were dispersed in MeOH in a water bath at 60 °C for 3 h. After cooling, it was sonicated for 30 min and filtered. The extract was diluted using 50 mM borate buffer (pH 9.0) before CE analysis. | CE-AD | A ±30 kV high-voltage dc power supply provided a separation voltage between the ends of the capillary. The inlet of the capillary was held at a positive potential and the outlet of the capillary was maintained at ground. The separations were carried out in a 40 cm length of 25 µm i.d. and 360 µm o.d. fused silica capillary. The CE system was assembled at Tª 25 °C. The detection electrode was a 300 µm diameter carbon disc electrode at a detection potential of +0.90 V and a saturated calomel electrode (SCE), as the reference electrode was used in combination with a BAS LC-4C amperometric detector. | [GA] (mg/g) (1): 11.48 (2): 9.627 (3): 6.778 | [80] |
| Alperujo samples from one olive oil (Córdoba, Spain) | Samples were taken directly from the production line and stored at −20 °C until analysis | Alperujo was placed in MeOH–H2O (1:3, v/v) for 13 min under ultrasonic irradiation (duty cycle 0.5 s, output amplitude 10%, applied power of 450 W, with the probe placed at 3 cm from the top surface of the extraction cell). During extraction, FR changed at 2 mL/min every 40 s, and after extraction was completed, DMF was added to the extract. Then, the sample was diluted using MeOH, and before introduction into the CE system, the extract was centrifuged for 3 min at 3000 rpm. | CE-DAD | The running buffer used was a solution of 45 mM H3BO3 (pH 9.6), adjusted with NaOH to pH 10 and with 5% MeOH. Extracts were electrokinetically injected by application of 25 kV for 4 s. The analysis voltage was 27 kV, being the average current ∼110 A, Tª 30 °C, and λ: 210 nm. To maintain the capillary under optimal working conditions, its surface was regenerated after each run by sequential washing with water (2 min), 0.1 M sodium hydroxide (2 min), 1 min waiting, followed by the running buffer (10 min). | [GA]: 12.48 ± 0.4 µg/g | [81] |
| Leaves from rosemary (Rosmarinus officinalis), sage (Salvia officinalis), oregano (Origanum vulgare), and Ligustrum lucidum (Ioannina, Greece) | The samples were washed and pulverized into a fine powder | Oregano leaves were extracted with acetone in a Soxhlet apparatus for 6 h, while sage, rosemary, and L. lucidum leaves were subsequently extracted with hexane and ethyl acetate in a Soxhlet apparatus for 6 h. Furthermore, L. lucidum leaves were subjected to two more treatments: macerated in MeOH for 7 days in the dark at RT and boiled with distilled water for 1 h. All the extracts were filtered, freeze-dried, and stored at −20 °C. | 1H-NMR | NMR experiments were performed at 295 K on a Bruker 500 spectrometer equipped with a TXI cryoprobe. Samples were dissolved in DMSO-d6. All chemical shifts were measured with reference to the internal standard TSP-d4 (δ = 0.000 ppm) of a given concentration (0.03 mM). All spectra were acquired with an acquisition time of 1.818 s, relaxation delay 5 s, 64 K data points, 90° pulse length, and optimum low-power radiofrequency irradiation for the water signal pre-saturation. | 1H NMR spectrum of the artificial mixture of phenolic acids found 2.94 mM GA | [82] |
| Green tea samples (C. sinensis) (Guangzhou, China) | Different varieties of green tea were pulverized and stored until use. | 1.5 mL Milli-Q water was added to 50 mg pulverized green tea and kept at 70 °C with continuous shaking for 25 min. The extract was centrifuged at 13,000 rpm for 20 min, and the supernatant was stored until analysis. | 1H-NMR | 600 µL of the sample was mixed with 100 µL of TSP-d4–D2O solution and analyzed in a Bruker 600 spectrometer at 600.13 MHz proton frequency; 128 scans of 38.460 data points were acquired, with a spectral width of 9600 Hz (16 ppm), pulse width of 12.34 ms, acquisition time of 4.0 s, relaxation D1 of 10 s, flip angle of 90°, and constant gain of 181. | [GA] (mg/g) (1) 0.34 (2) 1.58 (3) 1.88 (4) 1.31 (5) 1.73 (6) 1.63 | [83] |
| Leaves from green tea of the cultivars C. sinensis Yabukita and Yutakamidori | ND | 100 mg of green tea from each cultivar was sprayed with liquid N2 and subjected to microextraction in 1 mL of CD3OD (0.05% TMS). Then, sonication and centrifugation were performed at 12,000 rpm for 10 min Each, and the supernatant was stored until analysis. | 1H HR-MAS NMR | 1H HR-MAS qNMR spectra were acquired on a Bruker 400 (9.4 T) spectrometer at 400.13 MHz, equipped with 4 mm four channel (1H; 13C; 15N; 2H) HR-MAS probe and gradient field in the direction of the magic angle (θ = 54.74°). All acquisitions were performed with an interpulse delay time of 5 × T1, a recycle delay of 20 s (D1), 256 transients, AQ of 4.89 s, and 64 K points during the acquisition, using a spectral window of 8012.820 Hz. | GA was identified via a singlet at δH 7.10 | [84] |
| Different water samples matrices, including tap (1), mineral (2), and river (3) (Iran) | Before the determination of GA in samples, 500 mL of each sample were filtered through Whatman filter paper, and the samples were stored at 4 °C until analysis and processed within 1 week of collection | An ultrasonic processor operated at 40 kHz with a power of 130 W was used as the source of ultrasound for the enhanced recovery of GA. | UV–Vis | The volume of eluent (EtOH), sonication time, amount of sorbent, and pH were the parameters to optimize. | [GA] (ng/mL) (1): 995.43 (2): 984.35 (3): 990.71 | [85] |
| Green lentils (Saskatoon, SK, Canada) | Initial preparation of the samples involved the removal of lipids: samples were dried, dehulled. and defatted with hexane (1:5, w/v) for 5 min at RT. This procedure was repeated twice more, and samples were stored at −20 °C. Black hull soluble (BHS); black whole soluble (BWS); black dehull soluble (BDS); green hull soluble (GHS); green whole soluble (GWS); green dehull soluble (GDS); black hull insoluble-bound (BHI); black whole insoluble-bound (BWI); black dehull insoluble-bound (BDI); green hull insoluble-bound (GHI); green whole insoluble-bound (GWI); green dehull insoluble-bound (GDI) | Soluble phenolic compounds (SPCs): 10 mL of MeOH/acetone/H2O (1:1:1, v/v/v) was added to defatted samples and then sonicated for 20 min at 40 °C. The supernatant was then filtered and stored. Non-soluble phenolic compounds (NSPCs): Residues after the extraction of soluble phenolics were hydrolyzed using 2 M NaOH while stirring for 4 h at RT, then using 6 M HCl, and then centrifuged at 2000× g for 5 min. The supernatant was then extracted with hexane and then with diethyl ether/ethyl acetate 1:1 (v/v), four times. The solvent was then removed using a rotary evaporator, and then reconstituted in MeOH and stored at −20 °C until use. | (ESI)-MS-MS | 500 µL of both SPC and NSPC were injected into a mass spectrometer through direct diffusion at a rate of 10 µL/min. The individual phenolic compounds were identified and quantified in the negative mode along with 4045 (v) ion spray voltage, 16.1 (arb) sheath gas, 2.4 aux gas, 325 °F ion transfer tube Tª, and 30 °C vaporizer Tª. | [GA] (μg/g) SPC: (BHS): 96.0 ± 2.0 (BWS): 2.8 ± 0.5 (BDS): 0.9 ± 0.2 (GHS): 4.0 ± 0.2 (GHS): 1.4 ± 0.1 (GDS): 1.1 ± 0.0 NSPC: (BHI): 489.9 ± 38.9 (BWI): 1.6 ± 0.5 (BDI): 1.5 ± 0.2 (GHI): 104.1 ± 2.4 (GHI): 0.9 ± 0.0 (GDI): 2.6 ± 0.2 | [86] |
| Alcohol beverages (sherry and fruit wines and cognac 1 and 2) | No sample pretreatment is necessary and the procedure is rapid | A test solution containing 5–150 µg of GA, 1.5 mL of fresh 8 × 10−3 M 4-nitrobenzenediazonium tetrafluoroborate, 2.5 mL of 1 M HCl, and water up to a volume of 25 mL was sequentially added to vessels with ground stoppers. A single (polyurethane foam) PUF tablet was placed in each vessel, and after an unspecified period, tablets were removed and dried, and their diffuse reflectance was measured. | DRS | Diffuse reflectance values were measured on “Uniphot” portable reflectometer–colorimeter. The GA concentration was obtained using a Kubelka–Munk calibration curve, F (R) = f (C), where F (R) is the Kubelka–Munk function at 410 nm and C is the concentration of GA in µg/mL | [GA] (µg/mL) Sherry wine: 43 ± 3 Fruit wine: 77 ± 5 Cognac 1: 18 ± 2 Cognac 2: 38 ± 2 | [87] |
| Study Model and Administration Way | Dose mg/kg | GA Antioxidant Activity | Therapeutic Outcome | References |
|---|---|---|---|---|
| Mice with diabetic nephropathy induced with methylglyoxal, oral administration | 30 | Decreases MDA, miR-192, miR-204, albuminuria, and Nrf2; increases antioxidant enzymes such as SOD, CAT, glyoxalase1, and miR-29a | Mitigates kidney damage by reducing oxidative stress markers | [144] |
| Elastase emphysema in rats, oral administration | 30 | Lowers MDA and NF-κB levels; increases GS, SOD, CAT, Nrf2, and HO-1 levels | Reduces ischemia/reperfusion injury and histological damage | [145] |
| Paraquat liver injury in rats, oral administration | 50 or 100 | Lowers TG, AST, ALT, ALP, MDA, PC, IL-1β, LDL-C, and VLDL-C levels; increases HDL-C, FRAP, SOD, and CAT levels | Improves histological damage | [150] |
| Metabolic syndrome in rats, oral administration | 20 | Increases SOD, CAT, GPx, and GSH; decreases ROS, LPO, TNF-α, and IL-1β | Reduces oxidative stress and enhances recognition memory, hippocampal dendritic spines, and antioxidant enzymes | [161] |
| Paclitaxel neuropathy in mice, intravenous injection | 20 or 40 | Lowers LPO, TNF-α, and MPO levels; increases GSH level | Reduces thermal and mechanical hyperalgesia and allodynia symptoms | [152] |
| Quinolinic acid-induced neurotoxicity in rats, oral administration | 200 | Increases GPx and CAT levels; decreases caspase-3, IL-1β, IL-6, and TNF-α levels | Protects against oxidative damage | [153] |
| Carbon tetrachloride liver fibrosis in rats, oral administration | 100 | Lowers AST, ALT, ALP, bilirubin, albumin, and MDA; increases SOD, CAT, and GSH levels | Prevents oxidative damage in emphysema | [154] |
| Streptozotocin diabetes in rats, oral administration | 10, 50, 100 | Decreases LPO and increases GSH levels | Lowers oxidative stress and improves depressive-like behavior | [156] |
| COPD-linked lung inflammation/emphysema in mice, intraperitoneal injection | 100 | Lowers IL-6, TNF-α, IL-1β, ROS, LPO, PC, MMP-9, MMP-2; increases GSH and TIMP-1 levels | Decreases oxidative stress and histological damage | [162] |
| Nicotine-induced testicular injury in mice, intraperitoneal injection | 20 | Lowers NO and MDA levels; increases FRAP and SOD levels | Reduces histological damage and increases sperm quality and testosterone | [163] |
| Sodium arsenite-induced toxicity in rats, oral administration | 30 | Lowers creatine kinase-MB, MDA, and NO levels; increases white blood cells, platelets, MCV, hemoglobin, MCH, GPx, GSH and SOD levels | Reduces histological damage of the heart | [159] |
| Isoproterenol-induced ischemia/reperfusion in rats, oral administration | 50 | Increases SERCA2a and SOD levels; lowers LDH and creatine kinase-MB levels | Improves cardiac function and reduces cardiac hypertrophy | [160] |
| STZ diabetes in rats, oral administration | 20 or 40 | Reduces MDA markers; enhances antioxidant enzymes like GSH, SOD, and CAT | Reduces oxidative stress and prevents glomerular damage and tubulo-interstitial fibrosis | [164] |
| Bisphenol A toxicity in rats, oral administration | 50 or 200 | Scavenges ROS; boosts SOD, CAT, and GSH antioxidants; decreases levels of urea, LPO, creatinine, uric acid, IL-6, and IL-1β | Mitigates oxidative damage and reduces kidney morphological damage | [165] |
| Paraquat toxicity in rats, oral administration | 50 or 100 | Lowers uric acid, creatinine, PCI, IL-1β, and MDA levels; enhances SOD and CAT activity | Reduces oxidative stress and kidney morphological damage | [166] |
| Diclofenac toxicity in rats, oral administration | 50 or 100 | Reduces serum urea, uric acid, creatinine, MDA, IL-1β, and NO levels; enhances SOD, CAT, GPx, and GSH levels | Prevents oxidative damage and kidney morphological damage | [167] |
| Cyclophosphamide toxicity in mice, oral administration | 30 | Reduces BUN, KIM-1, NGAL, IL-1β, TNF-α, and creatinine levels; enhances antioxidants like SOD, CAT, GPx, and GSH | Mitigates kidney damage by reducing oxidative stress markers | [168] |
| Cisplatin toxicity in rats, oral administration | 40 | Lowers creatinine, LncRNA TUG1, Bax, caspase-3, Bcl-2, BUN, MDA, IL-1β, and TNF-α levels | Prevents oxidative damage and kidney morphological damage | [169] |
| Glyoxylic acid kidney stone formation in mice, intraperitoneal injection | 50 | Decreases MDA, Lcn2 mRNA, KIM1 mRNA, creatinine, BUN, OPN, TNF-α, IL-1β, renal tubular injury, and CaOx crystal deposition; increases 4-HNE, Nrf2, HO-1 levels | Reduces deposition of kidney stones and oxidative stress | [170] |
| Hyperuricemia in mice, oral administration | 100 | Decreases BUN, uric acid, Cys-C, MDA, IL-1β, COX-2, TGF-β1, and GLUT9 levels; increases CAT, OAT1, OAT3, and GPx levels | Reduces kidney morphological damage | [171] |
| Hypoxic-ischemic brain damage in rats, intraperitoneal injection | 50 | Decreases IL-1β, ROS, and LPO levels; increases SOD and CAT levels | Reduces neuronal loss, motor ability issues, and oxidative stress markers, and learning and memory improved | [40] |
| Hypothyroidism in rats, oral administration | 50 | Lowers ATF4, PERK1, p-eIF2α, GADD153, GADD34, caspase-12, Bax, ATF6α, IRE1a, and p53; increases eIF2α and Bcl-2 levels | Prevents oxidative damage and reduces morphological damage in CA3 hippocampal region | [172] |
| Ketamine toxicity in rats, oral administration | 10, 25, 50 | Lowers ROS, PC, and LPO levels; increases NPSH levels | Reduces oxidative stress | [173] |
| HFD obesity in db/db mice, oral administration | 100 | Lowers AST, ALT, cholesterol, TG, LPO, SREBP1, and SREBP2 levels; increases GST, GPx, SOD, and CAT levels | Reduces oxidative stress in pulmonary fibrosis and histological damage | [174] |
| Cyclophosphamide toxicity in mice, oral administration | 100, 200, or 400 | Increases SOD and GSH levels | Improves histological damage, reduces micronucleus and DNA strand breaks, and decreases oxidative stress markers by increasing antioxidant enzymes | [175] |
| Thioacetamide liver fibrosis in rats, oral administration | 20 | Lowers AST, total bilirubin, ALT, ALP, MDA, TGF-β1, p-Smad3, and miR-21 levels; increases miR-30, SOD, CAT, and miR-200 levels | Improves histological damage | [176] |
| Zearalenone reproductive dysfunction in rats, oral administration | 40 | Increases CAT, GPx, GST, GSH, SOD and TSH levels; decreases RONS, LPO, MPO, NO, and TNF-α levels | Increases testicular function enzymes, reproductive hormones, and sperm quality; reduces histological damage | [177] |
| Cisplatin ovarian damage in rats, oral administration | 2.5 or 5 | Lowers MDA, TNF-α, and caspase-3 levels; increases CAT level | Reduces histological damage | [178] |
| TAC cardiac hypertrophic remodeling in mice, oral administration | 20 | Lowers IL-1β, IL-6, gp130, CaNA, p-ERK1/2, MCP-1, EGFR, p-STAT3, and p-AKT levels | Reduces myocardial dysfunction, cardiac hypertrophy, and histological damage | [179] |
| Study Model | Dose | Anticancer Activity | Therapeutic Outcome | References |
|---|---|---|---|---|
| Glioblastoma cell in rats | 50 and 100 mg/kg | Activates mitochondrial apoptotic pathway | 90% reduction in tumor size and lower brain oxidative damage | [187] |
| DU145 prostate cancer cells in mice | 25, 50, and 100 µg/mL | Initiates mitochondria-mediated apoptosis, inhibits cell growth at G2/M phases by activating Chk1 and Chk2 and inhibiting Cdc25C and Cdc2 activities | Reduced cancer cell survival and induced cell cycle arrest | [188] |
| A375S2 human melanoma cells in mice | 100 µmol/L | Promotes apoptosis, downregulates Bcl-2, upregulates Bax | Marked inhibition of cell proliferation and induction of apoptosis | [190] |
| Breast cancer MCF-7 human cell line | GA, paclitaxel, and carboplatin in various concentrations | Cell cycle arrest at the G2/M phase | GA boosted the efficacy of paclitaxel and carboplatin, induced apoptotic cell death in MCF-7 cells, and increased the expression of P53, Bax, and CASP-3 | [198] |
| Non-small-cell lung cancer (NSCLC) in human cells and rats | 10, 20, and 40 µg/kg | Inhibits EGFR activation and reduces the CARM1-PELP1 complex | Inhibits proliferation and promotes apoptosis in NSCLC human cells, contributing to reduced tumor growth in vivo in rats | [209] |
| Colon cancer in rats | 20 and 50 mg/kg body weight | Reduces lipid peroxidation products like TBARS, LOOH, and CD, increases antioxidant levels like SOD, CAT, GSH, GR, and GPx, and lowers ascorbic acid and α-tocopherol levels in DMH-treated rats | Strong chemopreventive effect on DMH-induced colon carcinogenesis | [210] |
| Human bladder cancer T24 cell line | 6.25, 12.5, 25 µg/mL | Inhibits cell proliferation by disrupting PI3K/Akt/NF-κB signaling pathways | Inhibits T24 cell proliferation and metastasis, and leads to apoptosis; pro-apoptotic activity linked with mitochondrial dysfunction and PI3K/Akt/NF-κB signaling inhibition | [211] |
| Leukemia and its resistant sublines (HL60 cell HL60/VINC HL60/M2) in human cells | Eleven concentrations ranging from 10–500 µM | Alters cell cycle distribution and increases cell population in sub-G1 phase, modulates ROS production in a time- and dose-dependent manner | Cytotoxic activity against human promyelocytic leukemia-sensitive HL60 line and its resistant sublines, showing different MDR phenotypes: HL60/VINC (overexpressing P-gp) and HL60/M2 | [212] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hadidi, M.; Liñán-Atero, R.; Tarahi, M.; Christodoulou, M.C.; Aghababaei, F. The Potential Health Benefits of Gallic Acid: Therapeutic and Food Applications. Antioxidants 2024, 13, 1001. https://doi.org/10.3390/antiox13081001
Hadidi M, Liñán-Atero R, Tarahi M, Christodoulou MC, Aghababaei F. The Potential Health Benefits of Gallic Acid: Therapeutic and Food Applications. Antioxidants. 2024; 13(8):1001. https://doi.org/10.3390/antiox13081001
Chicago/Turabian StyleHadidi, Milad, Rafael Liñán-Atero, Mohammad Tarahi, Marios C. Christodoulou, and Fatemeh Aghababaei. 2024. "The Potential Health Benefits of Gallic Acid: Therapeutic and Food Applications" Antioxidants 13, no. 8: 1001. https://doi.org/10.3390/antiox13081001
APA StyleHadidi, M., Liñán-Atero, R., Tarahi, M., Christodoulou, M. C., & Aghababaei, F. (2024). The Potential Health Benefits of Gallic Acid: Therapeutic and Food Applications. Antioxidants, 13(8), 1001. https://doi.org/10.3390/antiox13081001










