Paraoxonase I Activity and Its Relationship with Nutrition in Amyotrophic Lateral Sclerosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Serum Extraction Procedure
2.3. Determination of the Lipid Profile and PON 1 Activity
2.4. Measure of Functional Capacity
2.5. Assessment of Respiratory Capacity: Spirometry Forced Expiratory Volume in 1 S (FEV1)
2.6. Heart Rate Variability (HRV)
2.7. Nutritional Analysis
2.7.1. Dietary-Nutritional Anamnesis and Collection of Data
2.7.2. Assessment of Diet and Eating Habits
2.8. Statistical Analysis
2.9. Ethical Concerns
3. Results
3.1. Descriptive Analysis
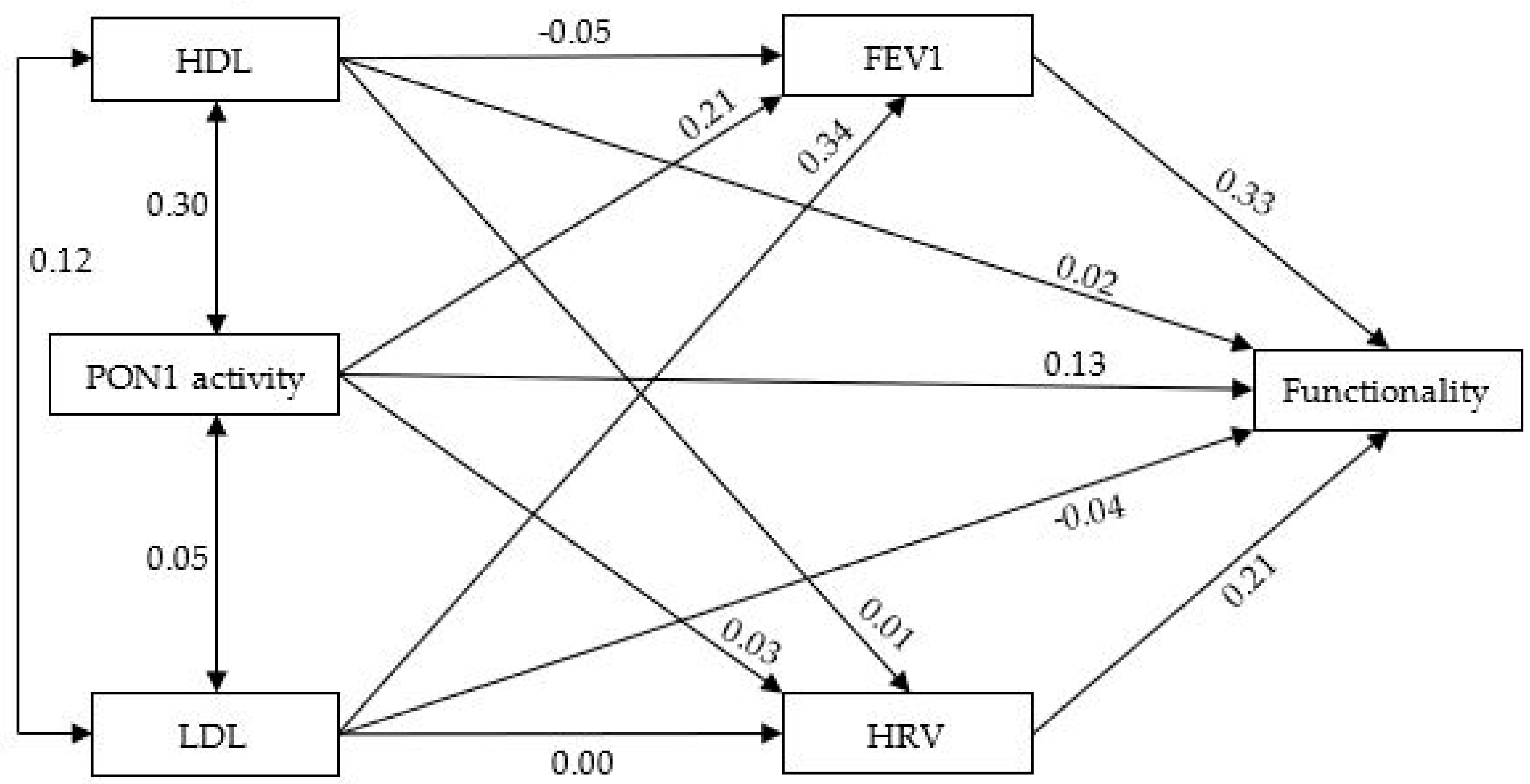
3.2. Correlations between PON1 Activity and the Other Variables in ALS
3.3. PON1 Activity in ALS: Predictive Model
3.4. Relation between Nutrition and PON1 Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rojas, P.; Ramírez, A.I.; Fernández-Albarral, J.A.; López-Cuenca, I.; Salobrar-García, E.; Cadena, M.; Elvira-Hurtado, L.; Salazar, J.J.; de Hoz, R.; Ramírez, J.M. Amyotrophic Lateral Sclerosis: A Neurodegenerative Motor Neuron Disease with Ocular Involvement. Front. Neurosci. 2020, 14, 566858. [Google Scholar] [CrossRef]
- Barber, S.C.; Mead, R.J.; Shaw, P.J. Oxidative stress in ALS: A mechanism of neurodegeneration and a therapeutic target. Biochim. Biophys. Acta 2006, 1762, 1051–1067. [Google Scholar] [CrossRef]
- Zhang, Q.J.; Huang, J.C.; Chen, J.; Hu, W.; Xu, L.Q.; Guo, Q.F. Peak expiratory flow is a reliably household pulmonary function parameter correlates with disease severity and survival of patients with amyotrophic lateral sclerosis. BMC Neurol. 2022, 22, 105. [Google Scholar] [CrossRef]
- Quereshi, M.; Schoenfeld, D.A.; Paliwal, Y.; Shui, A.; Cudkowicz, M.E. The natural history of ALS is changing: Improved survival. Amyotroph Lateral. Scler. 2009, 10, 324–331. [Google Scholar] [CrossRef]
- Motataianu, A.; Serban, G.; Barcutean, L.; Balasa, R. Oxidative Stress in Amyotrophic Lateral Sclerosis: Synergy of Genetic and Environmental Factors. Int. J. Mol. Sci. 2022, 23, 9339. [Google Scholar] [CrossRef]
- Liu, Z.; Zhou, T.; Ziegler, A.C.; Dimitrion, P.; Zuo, L. Oxidative Stress in Neurodegenerative Diseases: From Molecular Mechanisms to Clinical Applications. Oxid. Med. Cell Longev. 2017, 2017, 2525967. [Google Scholar] [CrossRef]
- Ticozzi, N.; LeClerc, A.L.; Keagle, P.J.; Glass, J.D.; Wills, A.M.; van Blitterswijk, M.; Bosco, D.A.; Rodriguez-Leyva, I.; Gellera, C.; Ratti, A.; et al. Paraoxonase gene mutations in amyotrophic lateral sclerosis. Ann. Neurol. 2010, 68, 102–107. [Google Scholar] [CrossRef]
- Arab, Z.N.; Khayatan, D.; Razavi, S.M.; Zare, K.; Kheradkhah, E.; Momtaz, S.; Ferretti, G.; Bacchetti, T.; Sathyapalan, T.; Emami, S.A.; et al. Phytochemicals as Modulators of Paraoxonase-1 in Health and Diseases. Antioxidants 2022, 11, 1273. [Google Scholar] [CrossRef]
- Khalaf, F.K.; Connolly, J.; Khatib-Shahidi, B.; Albehadili, A.; Tassavvor, I.; Ranabothu, M.; Eid, N.; Dube, P.; Khouri, S.J.; Malhotra, D.; et al. Paraoxonases at the Heart of Neurological Disorders. Int. J. Mol. Sci. 2023, 24, 6881. [Google Scholar] [CrossRef]
- Sentí, M.; Tomás, M.; Fitó, M.; Weinbrenner, T.; Covas, M.-I.; Sala, J.; Masiá, R.; Marrugat, J. Antioxidant paraoxonase 1 activity in the metabolic syndrome. J. Clin. Endocrinol. Metab. 2003, 88, 5422–5426. [Google Scholar] [CrossRef]
- Reichert, C.O.; Levy, D.; Bydlowski, S.P. Paraoxonase Role in Human Neurodegenerative Diseases. Antioxidants 2020, 10, 11. [Google Scholar] [CrossRef]
- Asare, G.A.; Andam, S.E.; Asare-Anane, H.; Ammanquah, S.; Anang-Quartey, Y.; Afriyie, D.K.; Musah, I. Lipid associated antioxidants: Arylesterase and paraoxonase-1 in benign prostatic hyperplasia treatment-naïve patients. Prostate Int. 2018, 6, 36–40. [Google Scholar] [CrossRef]
- Verde, F.; Tiloca, C.; Morelli, C.; Doretti, A.; Poletti, B.; Maderna, L.; Messina, S.; Gentilini, D.; Fogh, I.; Ratti, A. PON1 is a disease modifier gene in amyotrophic lateral sclerosis: Association of the Q192R polymorphism with bulbar onset and reduced survival. Neurol. Sci. 2019, 40, 1469–1473. [Google Scholar] [CrossRef]
- Rydell, A.; Nerpin, E.; Zhou, X.; Lind, L.; Lindberg, E.; Theorell Haglöw, J.; Fall, T.; Janson, C.; Lisspers, K.; Elmståhl, S.; et al. Cardiovascular disease-linked plasma proteins are mainly associated with lung volume. ERJ Open Res. 2023, 9, 00321–02022. [Google Scholar] [CrossRef]
- Lavie, L.; Vishnevsky, A.; Lavie, P. Evidence for lipid peroxidation in obstructive sleep apnea. Sleep 2004, 27, 123–128. [Google Scholar]
- Watanabe, J.; Kotani, K.; Gugliucci, A. Paraoxonase 1 and chronic obstructive pulmonary disease: A meta-analysis. Antioxidants 2021, 10, 1891. [Google Scholar] [CrossRef]
- Pimentel, R.M.M.; Macedo, H., Jr.; Valenti, V.E.; Rocha, F.O.; Abreu, L.C.; de M Monteiro, C.B.; Ferreira, C. Decreased Heart Rate Variability in Individuals with Amyotrophic Lateral Sclerosis. Respir. Care 2019, 64, 1088–1095. [Google Scholar] [CrossRef]
- Durrington, P.N.; Mackness, B.; Mackness, M.I. The hunt for nutritional and pharmacological modulators of paraoxonase. Arterioscler. Thromb. Vasc. Biol. 2002, 22, 1248–1250. [Google Scholar] [CrossRef]
- Costa, L.G.; Giordano, G.; Furlong, C.E. Pharmacological and dietary modulators of paraoxonase 1 (PON1) activity and expression: The hunt goes on. Biochem. Pharmacol. 2011, 81, 337–344. [Google Scholar] [CrossRef]
- Sznajder, J.; Lefarska-Wasilewska, M.S.; Kłek, S. The influence of the initial state of nutrition on the lifespan of patients with amyotrophic lateral sclerosis (ALS) during home enteral nutrition. Nutr. Hosp. 2016, 33, 3–7. (In English) [Google Scholar] [CrossRef] [PubMed]
- Ceron, J.J.; Tecles, F.; Tvarijonaviciute, A. Serum paraoxonase 1 (PON1) measurement: An update. BMC Vet. Res. 2014, 10, 74. [Google Scholar] [CrossRef]
- Kollewe, K.; Mauss, U.; Krampfl, K.; Petri, S.; Dengler, R.; Mohammadi, B. ALSFRS-R score and its ratio: A useful predictor for ALS-progression. J. Neurol. Sci. 2008, 275, 69–73. [Google Scholar] [CrossRef]
- Heiman-Patterson, T.D.; Khazaal, O.; Yu, D.; Sherman, M.E.; Kasarskis, E.J.; Jackson, C.E.; PEG NIV study Group. Pulmonary function decline in amyotrophic lateral sclerosis. Amyotroph. Lateral Scler. Front. Degener. 2021, 22 (Suppl. S1), 54–61. [Google Scholar] [CrossRef] [PubMed]
- Lechtzin, N.; Rothstein, J.; Clawson, L.; Diette, G.B.; Wiener, C.M. Amyotrophic lateral sclerosis: Evaluation and treatment of respiratory impairment. Amyotrophic lateral sclerosis and other motor neuron disorders: Official publication of the World Federation of Neurology. Res. Group Mot. Neuron Dis. 2002, 3, 5–13. [Google Scholar] [CrossRef]
- Sociedad de Neumología y Cirugía Torácica [Internet]. Guía de Procedimiento para la Espirometría en Atención Primaria. Available online: https://www. semfyc.es/actualidad (accessed on 2 February 2024).
- Miller, M.R.; Hankinson, J.; Brusasco, V.; Burgos, F.; Casaburi, R.; Coates, A.; Crapo, R.; Enright, P.; van der Grinten, C.P.; Gustafsson, P.; et al. Standardisation of spirometry. Eur. Respir. J. 2005, 26, 319–338. [Google Scholar] [CrossRef] [PubMed]
- Shaffer, F.; Ginsberg, J.P. An Overview of Heart Rate Variability Metrics and Norms. Front. Public Health 2017, 5, 258. [Google Scholar] [CrossRef] [PubMed]
- Freedson, P.S.; Melanson, E.; Sirard, J. Calibration of the computer science and applications, Inc. accelerometer. Med. Sci. Sports Exerc. 1998, 30, 777–781. [Google Scholar] [CrossRef]
- Ortega, R.M.; Pérez-Rodrigo, C.; López-Sobaler, A.M. Métodos de evaluación de la ingesta actual: Registro o diario diétetico. Nutr. Hosp. 2015, 21, 34–41. [Google Scholar]
- Trinidad Rodríguez, I.; Fernández Ballart, J.; Cucó Pastor, G.; BiarnésJordà, E.; Arija Val, V. Validación de un cuestionario de frecuencia de consumo alimentario corto: Reproducibilidad y validez. Nutr. Hosp. 2008, 23, 242–252. [Google Scholar]
- Dapcich, V.; Salvador, G.; Ribas, L.; Pérez, C.; Aranceta, J.; Serra, L.L. Guía de Alimentación Saludable; Sociedad Española de Nutrición Comunitaria: Barcelona, España, 2004. [Google Scholar]
- Cuervo, M.; Abete, I.; Baladia, E.; Corbalán, M.; Manera, M.; Basulto, J.; Martínez, A. Ingestas Dietéticas de Referencia Para la Población Española; Ediciones Universidad de Navarra, SA (EUNSA): Barañáin, Spain, 2010. [Google Scholar]
- Aranceta, J.; Ll, S.M. Grupo Colaborativo para la actualización de los Objetivos Nutricionales para la Población Española. Objetivos Nutricionales para la Población Española 2011. Consenso de la Sociedad Española de Nutrición Comunitaria (SENC). Rev. Esp. Nutr. Comunitaria 2011, 17, 178–199. [Google Scholar]
- Arbuckle, J.L. Amos 7.0 User’s Guide; SPSS: Chicago, IL, USA, 2006. [Google Scholar]
- Bentler, P.M.; Bonett, D.G. Significance tests and goodness of fit in the analysis of covariance structures. Acad. Psychol. Bull. 1980, 88, 588–606. [Google Scholar] [CrossRef]
- Jöreskog, K.G.; Sörbom, D. LISREL 8: User’s Guide; Scientific Software International: Skokie, IL, USA, 1993. [Google Scholar]
- Hu, L.; Bentler, P.M. Cutoff criteria for fi t indexes in covariance structure analysis: Conventional criteria versus new alternatives. Struct. Equ. Model. A Multidiscip. J. 1999, 6, 1–55. [Google Scholar] [CrossRef]
- Steiger, J.H. Structural model evaluation modification: An interval estimation approach. Multivar. Behav. Res. 1990, 25, 173–180. [Google Scholar] [CrossRef]
- Hair, J.F.; Anderson, R.E.; Tatham, R.L.; Black, W.C. Análisis Multivariante; Prentice Hall: Upper Saddle River, NJ, USA, 1999. [Google Scholar]
- Bentler, P.M. Comparative fit indexes in structural models. Psychol. Bull. 1990, 107, 238–246. [Google Scholar] [CrossRef] [PubMed]
- Byrne, B.M. Structural Equation Modeling with AMOS Basic Concepts, Applications, and Programming; Lawrence Erlbaum: Mahwah, NJ, USA, 2001. [Google Scholar]
- World Medical Association. World medical association declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef] [PubMed]
- West, S.G.; Finch, J.F.; Curran, P.J. Structural equation models with non-normal variables. In Structural Equation Modeling: Concepts, Issues and Applications; Hoyle, R.H., Ed.; Sage: Greenville, SC, USA, 1995; pp. 56–75. [Google Scholar]
- Cohen, J. A Power Primer. Psychol. Bull. 1992, 112, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Van Es, M.A.; Hardiman, O.; Chio, A.; Al-Chalabi, A.; Pasterkamp, R.J.; Veldink, J.H.; van den Berg, L.H. Amyotrophic lateral sclerosis. Lancet 2017, 390, 2084–2098. [Google Scholar] [CrossRef]
- Haley, R.W. Excess incidence of ALS in young Gulf War veterans. Neurology 2003, 61, 750–756. [Google Scholar] [CrossRef]
- Efrat, M.; Aviram, M. Paraoxonase 1 interactions with HDL, antioxidants and macrophages regulate atherogenesis—A protective role for HDL phospholipids. Adv. Exp. Med. Biol. 2010, 660, 153–166. [Google Scholar] [CrossRef]
- Janse van Mantgem, M.R.; van Rheenen, W.; Hackeng, A.V.; van Es, M.A.; Veldink, J.H.; van den Berg, L.H.; van Eijk, R.P.A. Association between Serum Lipids and Survival in Patients with Amyotrophic Lateral Sclerosis: A Meta-analysis and Population-Based Study. Neurology 2023, 100, e1062–e1071. [Google Scholar] [CrossRef]
- He, X.; Feng, J.; Cong, X.; Huang, H.; Zhao, Q.; Shen, Q.; Xu, F.; Xu, Y. A Prediction Model for Peak Expiratory Flow Derived From Venous Blood Biomarkers and Clinical Factors in Amyotrophic Lateral Sclerosis. Front. Public Health 2022, 10, 899027. [Google Scholar] [CrossRef] [PubMed]
- Cavallero, A.; Puccini, P.; Aprile, V.; Lucchi, M.; Gervasi, P.G.; Longo, V.; Gabriele, M. Presence, enzymatic activity, and subcellular localization of paraoxonases 1, 2, and 3 in human lung tissues. Life Sci. 2022, 311, 121147. [Google Scholar] [CrossRef] [PubMed]
- Dupuis, L.; Corcia, P.; Fergani, A.; Gonzalez De Aguilar, J.L.; Bonnefont-Rousselot, D.; Bittar, R.; Seilhean, D.; Hauw, J.J.; Lacomblez, L.; Loeffler, J.P.; et al. Dyslipidemia is a protective factor in amyotrophic lateral sclerosis. Neurology 2008, 70, 1004–1009. [Google Scholar] [CrossRef] [PubMed]
- Kelly, M.; Lavrov, A.; Garcia-Gancedo, L.; Parr, J.; Hart, R.; Chiwera, T.; Shaw, C.E.; Al-Chalabi, A.; Marsden, R.; Turner, M.R.; et al. The use of biotelemetry to explore disease progression markers in amyotrophic lateral sclerosis. Amyotroph Lateral Scler Front. Degener 2020, 21, 563–573. [Google Scholar] [CrossRef]
- Kim, D.S.; Maden, S.K.; Burt, A.A.; Ranchalis, J.E.; Furlong, C.E.; Jarvik, G.P. Dietary fatty acid intake is associated with paraoxonase 1 activity in a cohort-based analysis of 1548 subjects. Lipids Health Dis. 2013, 12, 183. [Google Scholar] [CrossRef]
- Ferretti, G.; Bachetti, T. Effect of dietary lipids on paraoxonase-1 activity and gene expression. Nutr. Metab. Cardiovasc. Dis. 2012, 22, 88–94. [Google Scholar] [CrossRef]
- Daniels, J.A.; Mulligan, C.; McCance, D.; Woodside, J.V.; Patterson, C.; Young, I.S.; McEneny, J. A randomised controlled trial of increasing fruit and vegetable intake and how this influences the carotenoid concentration and activities of PON-1 and LCAT in HDL from subjects with type 2 diabetes. Cardiovasc. Diabetol. 2014, 13, 16. [Google Scholar] [CrossRef]
- Govindarajan, S.; Vellingiri, K. Effect of red yeast rice and coconut, rice bran or sunflower oil combination in rats on hypercholesterolemic diet. J. Clin. Diagn. Res. 2016, 10, BF05–BF07. [Google Scholar] [CrossRef]
- Morassutti, I.; Giometto, M.; Baruffi, C.; Marcon, M.L.; Michieletto, S.; Giometto, B.; Spinella, N.; Paccagnella, A. Nutritional intervention for amyotrophic lateral sclerosis. Minerva Gastroenterol. Dietol. 2012, 58, 253–260. [Google Scholar]
- Drehmer, E.; Platero, J.L.; Carrera-Juliá, S.; Moreno, M.L.; Tvarijonaviciute, A.; Navarro, M.Á.; López-Rodríguez, M.M.; Ortí, J.E.R. The Relation between Eating Habits and Abdominal Fat, Anthropometry, PON1 and IL-6 Levels in Patients with Multiple Sclerosis. Nutrients 2020, 12, 744. [Google Scholar] [CrossRef]
- Nguyen, S.D.; Sok, D.E. beneficial effect of oleolylated lipids on paraoxonase 1: Protection against oxidative inactivation and stabilization. Biochem. J. 2003, 375, 275–285. [Google Scholar] [CrossRef]
- Schleithoff, S.S.; Zittermann, A.; Tenderich, G.; Berthold, H.K.; Stehle, P.; Koerfer, R. Vitamin D supplementation improves cytokine profiles in patients with congestive heart failure: A double-blind, randomized, placebo-controlled trial. Am. J. Clin. Nutr. 2006, 83, 754–759. [Google Scholar] [CrossRef]
- Hamden, K.; Carreau, S.; Jamoussi, K.; Miladi, S.; Lajmi, S.; Aloulou, D.; Ayadi, F.; Elfeki, A. 1Alpha,25 dihydroxyvitamin D3: Therapeutic and preventive effects against oxidative stress, hepatic, pancreatic and renal injury in alloxan-induced diabetes in rats. J. Nutr. Sci. Vitaminol. 2009, 55, 215–222. [Google Scholar] [CrossRef]
- Taniura, H.; Ito, M.; Sanada, N.; Kuramoto, N.; Ohno, Y.; Nakamichi, N.; Yoneda, Y. Chronic vitamin D3 treatment protects against neurotoxicity by glutamate in association with upregulation of vitamin D receptor mRNA expression in cultured rat cortical neurons. J. Neurosci. Res. 2006, 83, 1179–1189. [Google Scholar] [CrossRef]
- Mohammadshahi, M.; Haidari, F.; Saei, A.A.; Rashidi, B.; Mahboob, S.; Rashidi, M.R. Soy protein, genistein, and daidzein improve serum paraoxonase activity and lipid profiles in rheumatoid arthritis in rats. J. Med. Food 2013, 16, 147–154. [Google Scholar] [CrossRef]
- Beer, S.; Moren, X.; Ruiz, J.; James, R.W. Postprandial modulation of serum paraoxonase activity and concentration in diabetic and non-diabetic subjects. Nutr. Metab. Cardiovasc. Dis. 2006, 16, 457–465. [Google Scholar] [CrossRef]
- Ergun, M.A.; Yurtcu, E.; Demirci, H.; Ilhan, M.N.; Barkar, V.; Yetkin, I.; Menevse, A. PON1 55 and 192 gene polymorphisms in type 2 diabetes mellitus patients in a Turkish population. Biochem. Genet. 2011, 49, 1–8. [Google Scholar] [CrossRef]
- Kotur-Stevuljevic, J.; Vujovic, A.; Spasic, S.; Spasoje-vic-Kaliomanvska, V.; Jelic-Ivanovic, Z.; Mandic-Markovic, V.; Mikovic, Z.; Cerovic, N. The influence of maternal smoking habits before pregnancy and antioxidative supplementation during pregnancy on oxidative stress status in a non-complicated pregnancy. Adv. Clin. Exp. Med 2014, 23, 575–583. [Google Scholar] [CrossRef]
- Begcevic, I.; Simundic, A.M.; Nikolac, N.; Dobrijevic, S.; Rajkovic, M.G.; Tesija-Kuna, A. Can cranberry extract and vitamin C + Zn supplements affect the in vivo activity of paraoxonase 1, antioxidant potential, and lipid status? Clin. Lab. 2013, 59, 1053–1060. [Google Scholar] [CrossRef]
- Ferre, N.; Camps, J.; Fernandez-Ballart, J.; Arija, V.; Murphy, M.M.; Marsillach, J.; Joven, J. Longitudinal changes in serum paraoxonase-1 activity throughout normal pregnancy. Clin. Chem. Lab. Med. 2006, 44, 880–882. [Google Scholar] [CrossRef]
| Variables | N | Mean | SD | Asymmetry | Kurtosis |
|---|---|---|---|---|---|
| HDL | 70 | 51.80 | 12.42 | 0.96 | 0.96 |
| LDL | 70 | 140.78 | 37.60 | 1.04 | 1.85 |
| PON1 | 70 | 3.12 | 0.86 | 0.65 | 0.36 |
| FEV1 | 70 | 1.77 | 0.83 | 0.56 | 0.30 |
| HRV | 70 | 42.30 | 11.33 | 0.12 | 1.13 |
| Functionality (ALSFRS-R) | 70 | 28.57 | 8.92 | −0.19 | −0.40 |
| Energy (kcal) | 65 | 2294.72 | 853.30 | 1.39 | 3.85 |
| Total Carbohydrates (g) | 65 | 222.27 | 93.52 | 0.82 | 0.62 |
| Total Protein (g) | 65 | 115.96 | 41.30 | 0.57 | 0.42 |
| Total fat (g) | 65 | 136.01 | 136.23 | 2.89 | 8.22 |
| SAFA (g) | 65 | 31.22 | 17.27 | 1.20 | 2.34 |
| PUFA (g) | 65 | 39.06 | 160.03 | 7.95 | 63.76 |
| MUFA (g) | 65 | 29.23 | 27.66 | 0.73 | −0.15 |
| PUFA/SAFAs | 65 | 9.83 | 32.49 | 5.95 | 39.04 |
| (PUFA + MUFA)/SAFA | 65 | 62.91 | 378.77 | 8.00 | 64.26 |
| Cholesterol (g) | 65 | 459.72 | 231.62 | 0.36 | 0.34 |
| Sodium (g) | 65 | 3661.49 | 10,567.02 | 7.88 | 63.02 |
| Fiber (g) | 65 | 30.01 | 51.56 | 6.88 | 51.33 |
| Ethanol (g) | 65 | 86.02 | 602.09 | 7.98 | 64.03 |
| Iodine (µg) | 65 | 226.01 | 249.30 | 3.24 | 11.70 |
| Potassium (mg) | 65 | 3983.11 | 2287.92 | 1.83 | 4.62 |
| Calcium (mg) | 65 | 1022.08 | 520.22 | 0.87 | 0.19 |
| Magnesium (mg) | 65 | 396.29 | 218.68 | 1.36 | 2.71 |
| Phosphorus (mg) | 65 | 1514.69 | 725.64 | 1.13 | 3.09 |
| Iron (mg) | 65 | 33.29 | 82.87 | 5.40 | 28.69 |
| Selenium (µg) | 65 | 126.44 | 102.57 | 4.64 | 29.48 |
| Zinc (mg) | 65 | 18.49 | 48.19 | 7.79 | 61.96 |
| Vitamin B1 (mg) | 65 | 7.67 | 46.82 | 8.05 | 64.83 |
| Vitamin B2 (mg) | 65 | 8.70 | 47.03 | 7.93 | 63.46 |
| Vitamin B6 (mg) | 65 | 13.60 | 62.32 | 5.59 | 30.34 |
| Vitamin B12 (µg) | 65 | 15.58 | 53.13 | 7.80 | 62.09 |
| Folate (µg) | 65 | 325.63 | 163.30 | 1.28 | 2.39 |
| Vitamin B3 (mg) | 65 | 73.92 | 269.52 | 7.64 | 59.93 |
| Vitamin C (mg) | 65 | 175.09 | 113.99 | 1.34 | 2.51 |
| Vitamin A (µg) | 65 | 1127.22 | 989.45 | 2.41 | 6.62 |
| Vitamin D (µg) | 65 | 34.65 | 166.09 | 7.27 | 54.98 |
| Vitamin E (mg) | 65 | 20.56 | 48.53 | 7.59 | 59.76 |
| Calcium/Phosphorus | 65 | 7.3 | 12.79 | 4.19 | 23.77 |
| Vitamin E/PUFA | 65 | 9.16 | 21.64 | 3.93 | 17.77 |
| Vitamin B6/Protein | 65 | 5.37 | 39.8 | 8.06 | 64.97 |
| HDL | LDL | PON1 | FEV1 | HRV | Functionality | |
|---|---|---|---|---|---|---|
| HDL | 1.00 | |||||
| LDL | 0.12 | 1.00 | ||||
| PON1 | 0.30 | 0.05 | 1.00 | |||
| FEV1 | 0.05 | 0.34 | 0.21 | 1.00 | ||
| HRV | 0.01 | 0.01 | 0.03 | 0.02 | 1.00 | |
| Functionality (ALSFRS-R) | 0.08 | 0.08 | 0.21 | 0.34 | 0.22 | 1.00 |
| Variables | PON1 | Variables | PON1 |
|---|---|---|---|
| Energy (kcal) | −0.09 | Phosphorus (mg) | −0.16 |
| Total Carbohydrates (g) | −0.21 | Iron (mg) | −0.06 |
| Total Protein (g) | −0.22 | Selenium (µg) | −0.18 |
| Total fat (g) | 0.08 | Zinc (mg) | −0.21 |
| SAFA (g) | 0.04 | Vitamin B1 (mg) | −0.20 |
| PUFA (g) | 0.03 | Vitamin B2 (mg) | −0.21 |
| MUFA (g) | 0.21 | Vitamin B6 (mg) | −0.17 |
| PUFA/SAFA | 0.01 | Vitamin B12 (µg) | −0.20 |
| (PUFA + MUFA)/SAFA | −0.03 | Folate (µg) | −0.19 |
| Cholesterol (g) | 0.02 | Vitamin B3 (mg) | −0.07 |
| Sodium (g) | −0.16 | Vitamin C (mg) | −0.15 |
| Fiber (g) | −0.23 | Vitamin A (µg) | −0.11 |
| Ethanol (g) | −0.05 | Vitamin D (µg) | 0.22 |
| Iodine (µg) | −0.20 | Vitamin E (mg) | −0.20 |
| Potassium (mg) | −0.10 | Calcium/Phosphorus | 0.08 |
| Calcium (mg) | −0.03 | Vitamin E/PUFA | 0.00 |
| Magnesium (mg) | −0.31 | Vitamin B6/Protein | 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Proaño, B.; Benlloch, M.; Sancho-Castillo, S.; Privado, J.; Bargues-Navarro, G.; Sanchis-Sanchis, C.E.; Martínez Bolós, P.; Carriquí-Suárez, A.B.; Cubero-Plazas, L.; Platero Armero, J.L.; et al. Paraoxonase I Activity and Its Relationship with Nutrition in Amyotrophic Lateral Sclerosis. Antioxidants 2024, 13, 1021. https://doi.org/10.3390/antiox13081021
Proaño B, Benlloch M, Sancho-Castillo S, Privado J, Bargues-Navarro G, Sanchis-Sanchis CE, Martínez Bolós P, Carriquí-Suárez AB, Cubero-Plazas L, Platero Armero JL, et al. Paraoxonase I Activity and Its Relationship with Nutrition in Amyotrophic Lateral Sclerosis. Antioxidants. 2024; 13(8):1021. https://doi.org/10.3390/antiox13081021
Chicago/Turabian StyleProaño, Belén, María Benlloch, Sandra Sancho-Castillo, Jesús Privado, Guillermo Bargues-Navarro, Claudia Emmanuela Sanchis-Sanchis, Palmira Martínez Bolós, Ana Belén Carriquí-Suárez, Laura Cubero-Plazas, Jose Luis Platero Armero, and et al. 2024. "Paraoxonase I Activity and Its Relationship with Nutrition in Amyotrophic Lateral Sclerosis" Antioxidants 13, no. 8: 1021. https://doi.org/10.3390/antiox13081021
APA StyleProaño, B., Benlloch, M., Sancho-Castillo, S., Privado, J., Bargues-Navarro, G., Sanchis-Sanchis, C. E., Martínez Bolós, P., Carriquí-Suárez, A. B., Cubero-Plazas, L., Platero Armero, J. L., Escriva, D., Ceron, J. J., Tvarijonaviciute, A., & de la Rubia Ortí, J. E. (2024). Paraoxonase I Activity and Its Relationship with Nutrition in Amyotrophic Lateral Sclerosis. Antioxidants, 13(8), 1021. https://doi.org/10.3390/antiox13081021









