Investigation of Antioxidant Mechanisms of Novel Peptides Derived from Asian Swamp Eel Hydrolysate in Chemical Systems and AAPH-Induced Human Erythrocytes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Molecular Docking of Antioxidant Peptides with Free Radicals (ABTS, DPPH)
2.3. Cell Culture and Modeling
2.4. Scanning Electron Microscopy (SEM) Observation of Erythrocyte Morphology
2.5. Determination of Hemolysis Rate
2.6. Measurement of Hemoglobin Oxidation Rate
2.7. Quantification of Protein Concentration, MDA Levels, LDH Activity, GSH/GSSG Ratio, and Activities of SOD, CAT, and GSH-Px in Human Erythrocytes
2.8. Statistical Analysis
3. Results and Discussion
3.1. Molecular Docking of Antioxidant Peptides with the ABTS Radical
3.2. Molecular Docking of Antioxidant Peptides with the DPPH Radical
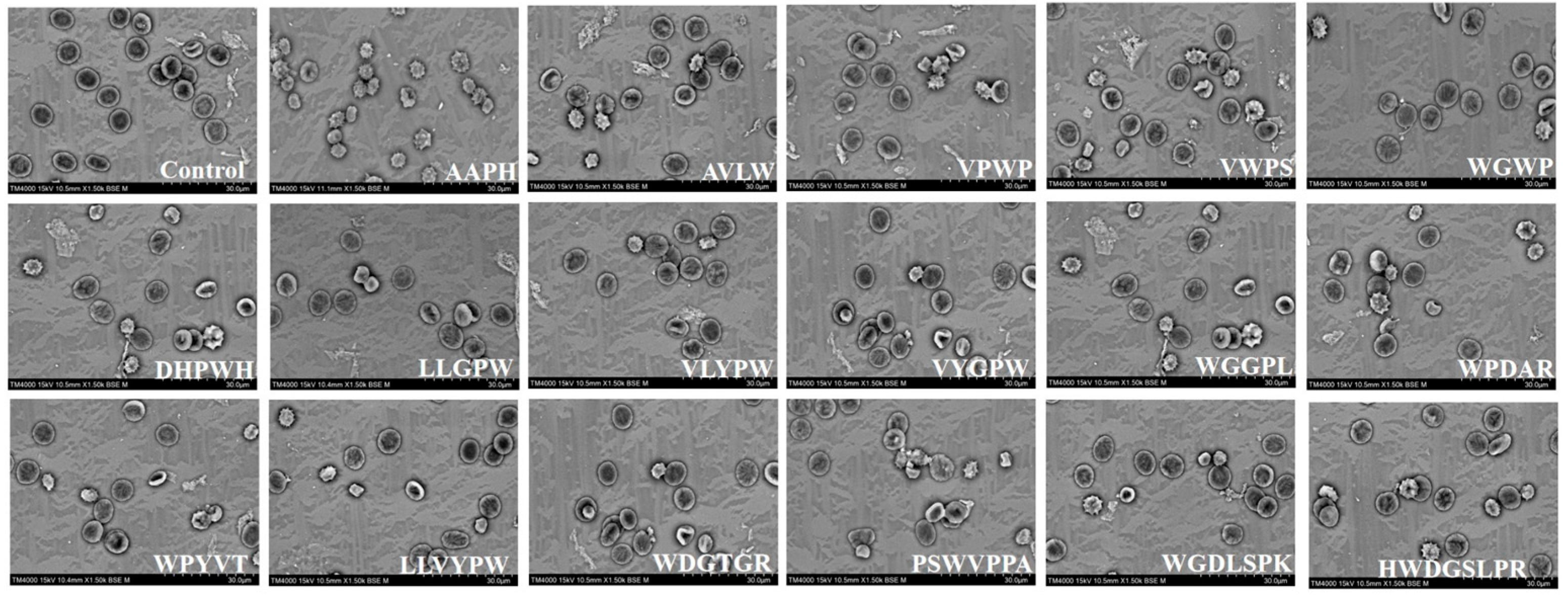
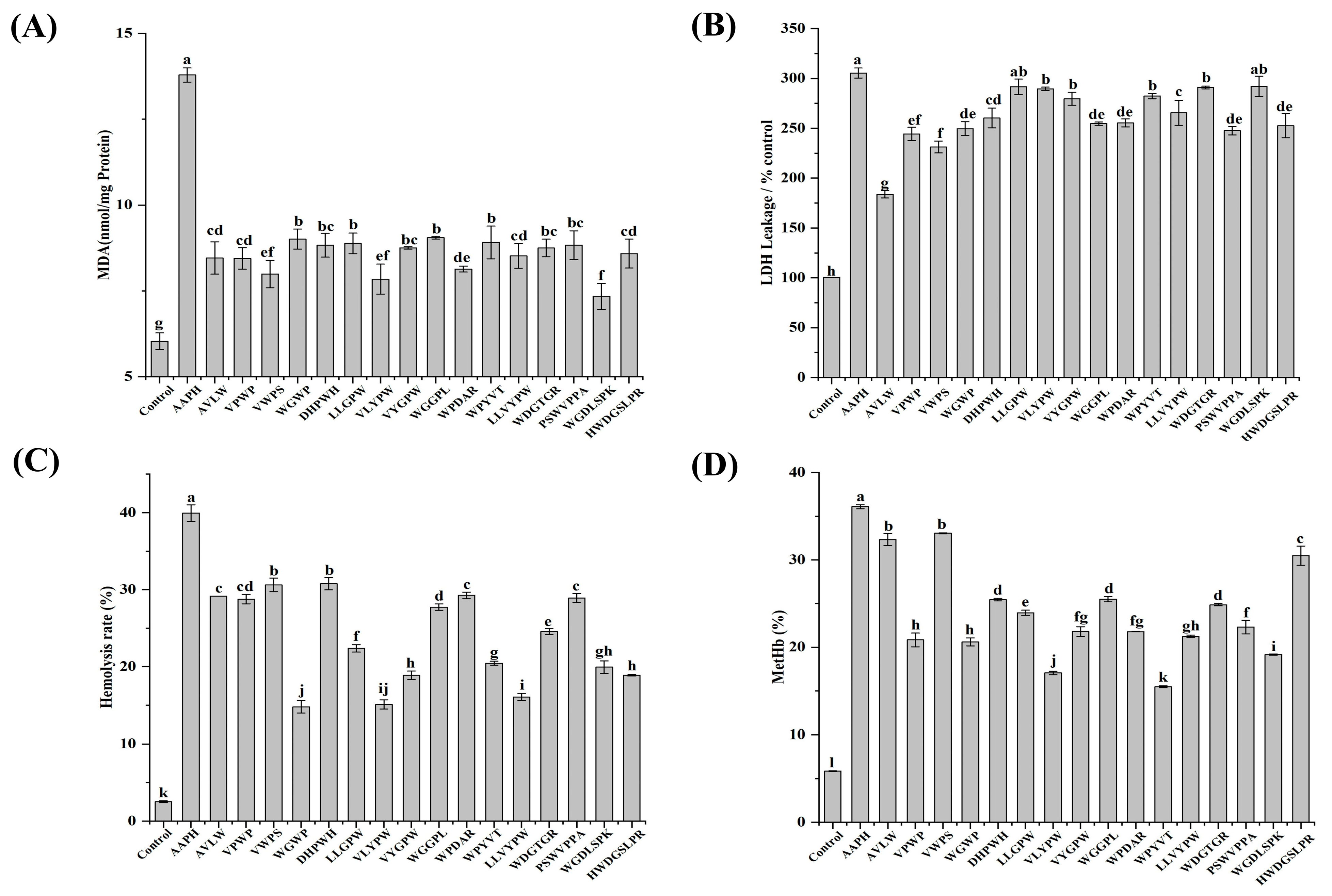
3.3. Protective Effects of Sixteen ASE Antioxidant Peptides on the Morphological Characteristics of Human Erythrocytes
3.4. Effect of Sixteen ASE Antioxidant Peptides on Erythrocyte Redox Systems (GSH/GSSG, SOD, CAT, GSH-Px) under AAPH-Induced Oxidative Stress
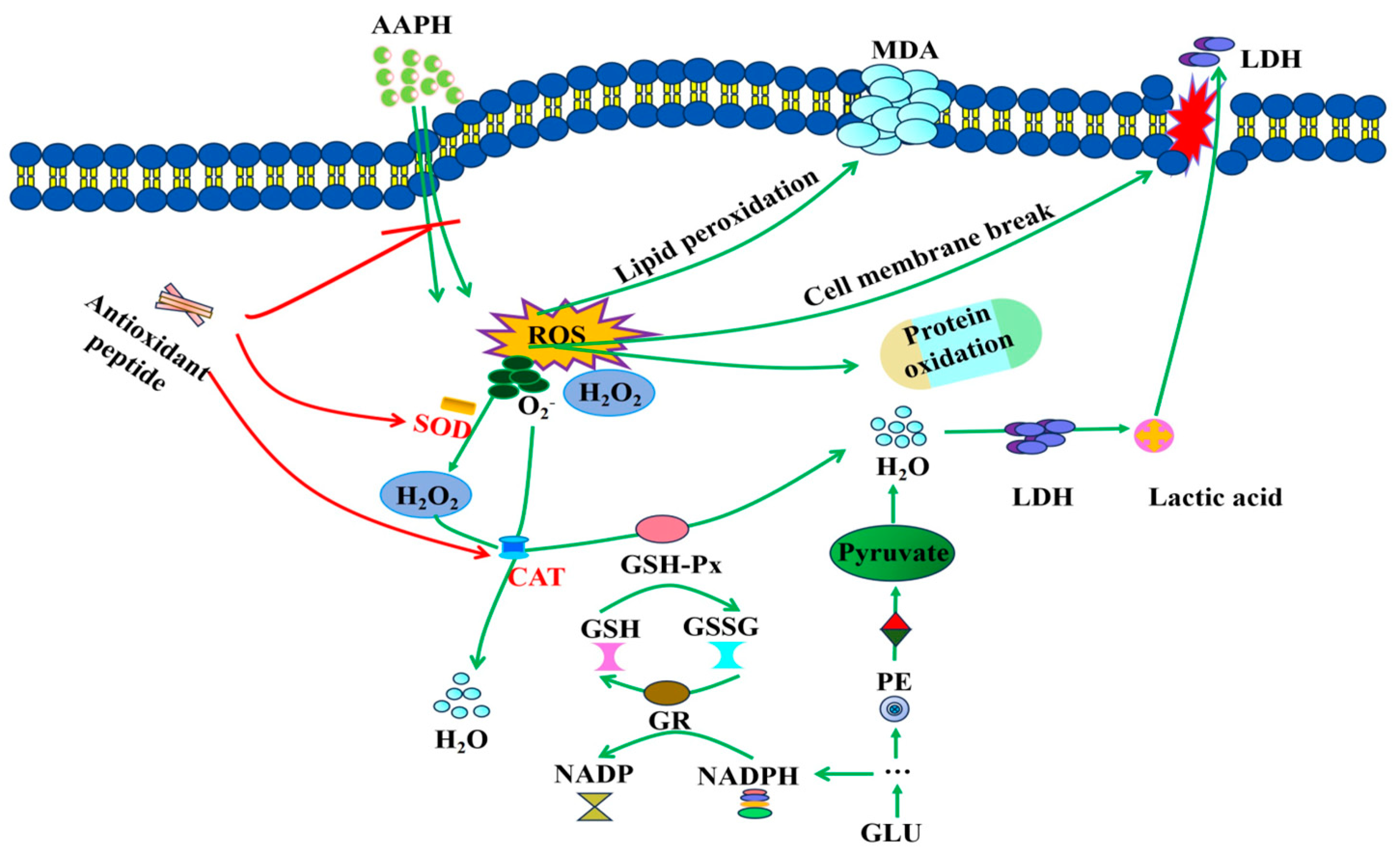
3.5. Mechanistic Study on the Inhibition of AAPH-Induced Oxidative Damage in Erythrocytes by ASE Antioxidant Peptides
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Waris, G.; Ahsan, H. Reactive oxygen species: Role in the development of cancer and various chronic conditions. J. Carcinog. 2006, 5, 14. [Google Scholar] [CrossRef]
- Martemucci, G.; Portincasa, P.; Centonze, V.; Mariano, M.; Khalil, M.; D’Alessandro, A.G. Prevention of oxidative stress and diseases by antioxidant supplementation. Med. Chem. 2023, 19, 509–537. [Google Scholar] [CrossRef] [PubMed]
- Wen, C.; Zhang, J.; Zhang, H.; Duan, Y.; Ma, H. Study on the structure-activity relationship of watermelon seed antioxidant peptides by using molecular simulations. Food Chem. 2021, 364, 130432. [Google Scholar] [CrossRef] [PubMed]
- Wen, C.; Zhang, J.; Zhang, H.; Duan, Y.; Ma, H. Plant protein-derived antioxidant peptides: Isolation, identification, mechanism of action and application in food systems: A review. Trends Food Sci. Technol. 2020, 105, 308–322. [Google Scholar] [CrossRef]
- Wang, X.; Fu, J.; Bhullar, K.S.; Chen, B.; Liu, H.; Zhang, Y.; Wang, C.; Liu, C.; Su, D.; Ma, X.; et al. Identification, in silico selection, and mechanistic investigation of antioxidant peptides from corn gluten meal hydrolysate. Food Chem. 2024, 446, 138777. [Google Scholar] [CrossRef] [PubMed]
- Lemes, A.C.; Sala, L.; Ores Jda, C.; Braga, A.R.; Egea, M.B.; Fernandes, K.F. A review of the latest advances in encrypted bioactive peptides from protein-rich waste. Int. J. Mol. Sci. 2016, 17, 950. [Google Scholar] [CrossRef]
- Wang, X.; Bhullar, K.S.; Fan, H.; Liao, W.; Qiao, Y.; Su, D.; Wu, J. Regulatory effects of a pea-derived peptide Leu-Arg-Trp (LRW) on dysfunction of rat aortic vascular smooth muscle cells against angiotensin II stimulation. J. Agric. Food Chem. 2020, 68, 3947–3953. [Google Scholar] [CrossRef] [PubMed]
- Herawati, V.E.; Nugroho, R.A.; Pinandoyo; Hutabarat, J.; Prayitno, B.; Karnaradjasa, O. The growth performance and nutrient quality of Asian swamp Eel Monopterus albus in central java Indonesia in a freshwater aquaculture system with different feeds. J. Aquat. Food Prod. Technol. 2018, 27, 658–666. [Google Scholar] [CrossRef]
- Yu, X.; Chen, Y.; Qi, Z.; Chen, Q.; Cao, Y.; Kong, Q. Preparation and identification of a novel peptide with high antioxidant activity from corn gluten meal. Food Chem. 2023, 424, 136389. [Google Scholar] [CrossRef]
- Jin, D.X.; Liu, X.L.; Zheng, X.Q.; Wang, X.J.; He, J.F. Preparation of antioxidative corn protein hydrolysates, purification and evaluation of three novel corn antioxidant peptides. Food Chem. 2016, 204, 427–436. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, J.; Zhu, Z.; Li, X.; Sun, S.; Wang, W.; Sadiq, F.A. Identification and characterization of two novel antioxidant peptides from silkworm pupae protein hydrolysates. Eur. Food Res. Technol. 2020, 247, 343–352. [Google Scholar] [CrossRef]
- He, P.; Zhang, Y.; Zhang, Y.; Zhang, L.; Lin, Z.; Sun, C.; Wu, H.; Zhang, M. Isolation, identification of antioxidant peptides from earthworm proteins and analysis of the structure-activity relationship of the peptides based on quantum chemical calculations. Food Chem. 2023, 431, 137137. [Google Scholar] [CrossRef]
- Rahmawati, I.; Amini, H.W.; Darmayanti, R.F. Molecular modelling of antioxidant agent by QSAR study of caffeic acidderivatives. Mater. Sci. Eng. 2020, 823, 012001. [Google Scholar]
- Ningrum, A.; Wardani, D.W.; Vanidia, N.; Sarifudin, A.; Kumalasari, R.; Ekafitri, R.; Kristanti, D.; Setiaboma, W.; Munawaroh, H.S.H. In silico approach of glycinin and conglycinin chains of soybean by-product (Okara) using papain and bromelain. Molecules 2022, 27, 6855. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Y.; Ren, T.; Xiao, P.; Duan, J.A. Novel and efficient techniques in the discovery of antioxidant peptides. Crit. Rev. Food Sci. Nutr. 2023, 10, 1–15. [Google Scholar] [CrossRef]
- Fan, X.; Han, Y.; Sun, Y.; Zhang, T.; Tu, M.; Du, L.; Pan, D. Preparation and characterization of duck liver-derived antioxidant peptides based on LC-MS/MS, molecular docking, and machine learning. LWT 2023, 175, 114479. [Google Scholar] [CrossRef]
- Agrawal, H.; Joshi, R.; Gupta, M. Purification, identification and characterization of two novel antioxidant peptides from finger millet (Eleusine coracana) protein hydrolysate. Food Res. Int. 2019, 120, 697–707. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, Z.; Wu, C.; Liu, S.; Wang, D.; Hu, C.; Chen, T.; Ran, Z.; Gan, W.; Li, G. Computational analysis on antioxidant activity of four characteristic structural units from persimmon tannin. Materials 2022, 16, 320. [Google Scholar] [CrossRef]
- Ren, L.; Fan, J.; Yang, Y.; Xu, Y.; Chen, F.; Bian, X.; Xing, T.; Liu, L.; Yu, D.; Zhang, N. Enzymatic hydrolysis of broken rice protein: Antioxidant activities by chemical and cellular antioxidant methods. Front. Nutr. 2021, 8, 788078. [Google Scholar] [CrossRef] [PubMed]
- Wong, F.C.; Xiao, J.; Wang, S.; Ee, K.Y.; Chai, T.T. Advances on the antioxidant peptides from edible plant sources. Trends Food Sci. Technol. 2020, 99, 44–57. [Google Scholar] [CrossRef]
- Aguilar-Toalá, J.E.; Liceaga, A.M. Cellular antioxidant effect of bioactive peptides and molecular mechanisms underlying: Beyond chemical properties. Int. J. Food Sci. Technol. 2020, 56, 2193–2204. [Google Scholar] [CrossRef]
- Balushi, H.A.; Hannemann, A.; Rees, D.; Brewin, J.; Gibson, J.S. The effect of antioxidants on the properties of red blood cells from patients with sickle cell anemias. Front. Physiol. 2019, 10, 976. [Google Scholar] [CrossRef] [PubMed]
- She, Y.; Liu, Q.; Xiong, X.; Li, N.; Zhang, J. Erythrocyte storage lesion improvements mediated by naringin screened from vegetable/fruit juice using cell extract and HPLC-MS. J. Anal. Methods Chem. 2022, 2022, 7556219. [Google Scholar] [CrossRef] [PubMed]
- Tzounakas, V.L.; Anastasiadi, A.T.; Arvaniti, V.; Lelli, V.; Fanelli, G.; Paronis, E.C. Supplementation with uric and ascorbic acid protects stored red blood cells through enhancement of non-enzymatic antioxidant activity and metabolic rewiring. Redox Biol. 2022, 57, 102477. [Google Scholar] [CrossRef] [PubMed]
- Möller, M.N.; Orrico, F.; Villar, S.F.; López, A.C.; Silva, N.; Donzé, M.; Thomson, L.; Denicola, A. Oxidants and antioxidants in the redox biochemistry of human red blood cells. ACS Omega 2023, 8, 147–168. [Google Scholar] [CrossRef] [PubMed]
- Lins, P.G.; Marina Piccoli Pugine, S.; Scatolini, A.M.; de Melo, M.P. In vitro antioxidant activity of olive leaf extract (Olea europaea L.) and its protective effect on oxidative damage in human erythrocytes. Heliyon 2018, 4, e00805. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Miao, J.; Ye, H.; Xia, Z.; Huang, W.; Guo, J.; Liang, X.; Yin, Y.; Zheng, Y.; Cao, Y. Purification, identification, and mechanistic investigation of novel selenium-enriched antioxidant peptides from moringa oleifera seeds. J. Agric. Food Chem. 2023, 71, 4625–4637. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, X.; Li, W.; Wu, D.; Zhang, Z.; Chen, W.; Yang, Y. A screening strategy for identifying umami peptides with multiple bioactivities from Stropharia rugosoannulata using in silico approaches and SPR sensing. Food Chem. 2024, 431, 137057. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Ouyang, X.; Cheng, C.; Chen, D.; Su, J.; Hu, Y.; Li, X. Bioactive peptides derived from Radix Angelicae sinensis inhibit ferroptosis in HT22 cells through direct Keap1-Nrf2 PPI inhibition. RSC Adv. 2023, 13, 22148–22157. [Google Scholar] [CrossRef]
- Du, R.; Liu, K.; Zhao, S.; Chen, F. Changes in antioxidant activity of peptides identified from brown rice hydrolysates under different conditions and their protective effects against AAPH-induced oxidative stress in human erythrocytes. ACS Omega 2020, 5, 12751–12759. [Google Scholar] [CrossRef]
- Wang, G.; Lei, Z.; Zhong, Q.; Wu, W.; Zhang, H.; Min, T.; Wu, H.; Lai, F. Enrichment of caffeic acid in peanut sprouts and evaluation of its in vitro effectiveness against oxidative stress-induced erythrocyte hemolysis. Food Chem. 2017, 217, 332–341. [Google Scholar] [CrossRef]
- Quds, R.; Iqbal, Z.; Arif, A.; Mahmood, R. Mancozeb-induced cytotoxicity in human erythrocytes: Enhanced generation of reactive species, hemoglobin oxidation, diminished antioxidant power, membrane damage and morphological changes. Pestic Biochem. Physiol. 2023, 193, 105453. [Google Scholar] [CrossRef]
- Ma, J.; Su, K.; Chen, M.; Wang, S. Study on the antioxidant activity of peptides from soybean meal by fermentation based on the chemical method and AAPH-induced oxidative stress. Food Sci. Nutr. 2023, 11, 6634–6647. [Google Scholar] [CrossRef]
- Liu, J.; Wu, G.; Yang, J.; He, C.; Xiong, H.; Ma, Y. Abalone visceral peptides containing Cys and Tyr exhibit strong in vitro antioxidant activity and cytoprotective effects against oxidative damage. Food Chem. X 2023, 17, 100582. [Google Scholar] [CrossRef]
- Chen, L.; Zhao, X.; He, Y.; Yang, H. Cloning, purification and characterisation of cytosolic fructose-1,6-bisphosphatase from mung bean (Vigna radiata). Food Chem. 2021, 347, 128973. [Google Scholar] [CrossRef]
- Fan, Y.; Yu, Z.; Zhao, W.; Ding, L.; Zheng, F.; Li, J.; Liu, J. Identification and molecular mechanism of angiotensin-converting enzyme inhibitory peptides from Larimichthys crocea titin. Food Sci. Hum. Wellness 2020, 9, 257–263. [Google Scholar] [CrossRef]
- Li, W.; Chen, W.; Ma, H.; Wu, D.; Zhang, Z.; Yang, Y. Structural characterization and angiotensin-converting enzyme (ACE) inhibitory mechanism of Stropharia rugosoannulata mushroom peptides prepared by ultrasound. Ultrason. Sonochem. 2022, 88, 106074. [Google Scholar] [CrossRef]
- Sun, C.; Wu, W.; Yin, Z.; Fan, L.; Ma, Y.; Lai, F.; Wu, H. Effects of simulated gastrointestinal digestion on the physicochemical properties, erythrocyte haemolysis inhibitory ability and chemical antioxidant activity of mulberry leaf protein and its hydrolysates. Int. J. Food Sci. Technol. 2017, 53, 282–295. [Google Scholar] [CrossRef]
- Biswas, S.; Das, R.; Ray Banerjee, E. Role of free radicals in human inflammatory diseases. AIMS Biophys. 2017, 4, 596–614. [Google Scholar] [CrossRef]
- Liu, H.; Ma, J.; Wu, H. Detoxifying effects of ultrafiltration fractions of Dendrobium aphyllum peptides on chemical and AAPH-induced oxidative stress. RSC Adv. 2017, 7, 48913–48924. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, X.J.; Chen, J.B.; Cao, J.P.; Li, X.; Sun, C.D. Citrus flavonoids and their antioxidant evaluation. Crit. Rev. Food Sci. Nutr. 2022, 62, 3833–3854. [Google Scholar] [CrossRef]
- Alara, O.R.; Ukaegbu, C.I.; Abdurahman, N.H.; Alara, J.A.; Ali, H.A. Plant-sourced antioxidants in human health: A state-of-art review. Curr. Nutr. Food Sci. 2023, 19, 817–830. [Google Scholar] [CrossRef]
- Chaudhary, P.; Janmeda, P.; Docea, A.O.; Yeskaliyeva, B.; Abdull Razis, A.F.; Modu, B.; Calina, D.; Sharifi-Rad, J. Oxidative stress, free radicals and antioxidants: Potential crosstalk in the pathophysiology of human diseases. Front. Chem. 2023, 11, 1158198. [Google Scholar] [CrossRef]
- Zheng, L.; Dong, H.; Su, G.; Zhao, Q.; Zhao, M. Radical scavenging activities of Tyr-, Trp-, Cys- and Met-Gly and their protective effects against AAPH-induced oxidative damage in human erythrocytes. Food Chem. 2016, 197, 807–813. [Google Scholar] [CrossRef]



| Peptide | CDOCKER Energy (kcal/mol) | Hydrogen Bond | Electrostatic Interaction | Hydrophobic Interaction | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Conventional Hydrogen Bond | Pi–Anion | Pi–Sulfur | Pi–Cation | Pi–Pi Stacked | Pi–Pi T-Shaped | Amide–Pi Stacked | Pi–Alkyl | Pi–Sigma | Alkyl | |||
| ABTS | AVLW | −3.2 | W4 | W4-2 | W4-2 | L3 | ||||||
| VPWP | −3.6 | V1-2 *, P4 | P4 | W3 | W3-2 | P2-2, P4 | ||||||
| VWPS | −3.7 | W2 | W2-4 | P3-2 | ||||||||
| WGWP | −3.8 | W1 | P4 | W1-4 | W1, P4-2 | P4 | ||||||
| DHPWH | −4.1 | H5 | W4, H5-2 | D1-2 | W4-4 | H2, W4 | ||||||
| LLGPW | −4.0 | W5 | L1-2 | W5 | W5-4 | L2 | ||||||
| VLYPW | −4.3 | W5-1 | V1-2 | W5-4 | V1-2 | |||||||
| VYGPW | −4.0 | V1, G3 | W5-3 | Y2-2 | Y2, W5 | P4 | ||||||
| WGGPL | −3.8 | W1 | W1-3 | W1-2, P4 | P4 | P4-2 | ||||||
| WPDAR | −3.9 | R5-2 | W1-2 | D3-2 | A4 | |||||||
| WPYVT | −4.1 | W1 | W1-4 | W1 | ||||||||
| LLVYPW | −4.2 | L1-2, Y4 | W6 | Y4 | L2-4 | |||||||
| WDGTGR | −4.7 | W1, G5 | R6-2 | W1-3 | W1 | |||||||
| PSWVPPA | −4.1 | W3 | W3-4 | W3-2 | V4 | P1 | ||||||
| WGDLSPK | −3.9 | W1-2, K7 | D3-2 | W1-2 | W1, L4-2 | |||||||
| HWDGSLPR | −4.4 | D3-2, S5-2, L6 | W2 | H1, W2, L6 | ||||||||
| DPPH | AVLW | −3.4 | W4-2 | W4-1 | L3 | |||||||
| VPWP | −3.3 | W3 | W3-2 | W3 | P2 | |||||||
| VWPS | −3.7 | S4 | W2-2 | |||||||||
| WGWP | −3.8 | W1-2 | W1-2 | |||||||||
| DHPWH | −4.4 | D1 | W4-2 | |||||||||
| LLGPW | −4.0 | L1 | W5-2 | L2 | ||||||||
| VLYPW | −3.8 | W3 | W3 | P4 | ||||||||
| VYGPW | −3.7 | Y2, W5 | Y2 | |||||||||
| WGGPL | −3.5 | W1 | W1-2 | P4-2 | ||||||||
| WPDAR | −3.7 | W1, D3 | W1-2 | |||||||||
| WPYVT | −3.7 | W1-2 | ||||||||||
| LLVYPW | −4.3 | W6 | Y4, W6-2 | |||||||||
| WDGTGR | −4.0 | T4, G5, R6 | W1 | W1 | ||||||||
| PSWVPPA | −4.2 | W3-2 | P1, V4 | |||||||||
| WGDLSPK | −4.3 | W1-2, K7 | L4 | |||||||||
| HWDGSLPR | −4.4 | W2-2 | W2 | L6 | ||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Chen, B.; Bhullar, K.S.; Yang, H.; Luo, X.; Fu, J.; Liu, H.; Su, D.; Sun, D.; Qiao, Y.; et al. Investigation of Antioxidant Mechanisms of Novel Peptides Derived from Asian Swamp Eel Hydrolysate in Chemical Systems and AAPH-Induced Human Erythrocytes. Antioxidants 2024, 13, 888. https://doi.org/10.3390/antiox13080888
Wang X, Chen B, Bhullar KS, Yang H, Luo X, Fu J, Liu H, Su D, Sun D, Qiao Y, et al. Investigation of Antioxidant Mechanisms of Novel Peptides Derived from Asian Swamp Eel Hydrolysate in Chemical Systems and AAPH-Induced Human Erythrocytes. Antioxidants. 2024; 13(8):888. https://doi.org/10.3390/antiox13080888
Chicago/Turabian StyleWang, Xiao, Bingjie Chen, Khushwant S. Bhullar, Hang Yang, Xiaohu Luo, Juan Fu, Hongru Liu, Di Su, Dapeng Sun, Yongjin Qiao, and et al. 2024. "Investigation of Antioxidant Mechanisms of Novel Peptides Derived from Asian Swamp Eel Hydrolysate in Chemical Systems and AAPH-Induced Human Erythrocytes" Antioxidants 13, no. 8: 888. https://doi.org/10.3390/antiox13080888






