Role of Myeloperoxidase, Oxidative Stress, and Inflammation in Bronchopulmonary Dysplasia
Abstract
1. Introduction
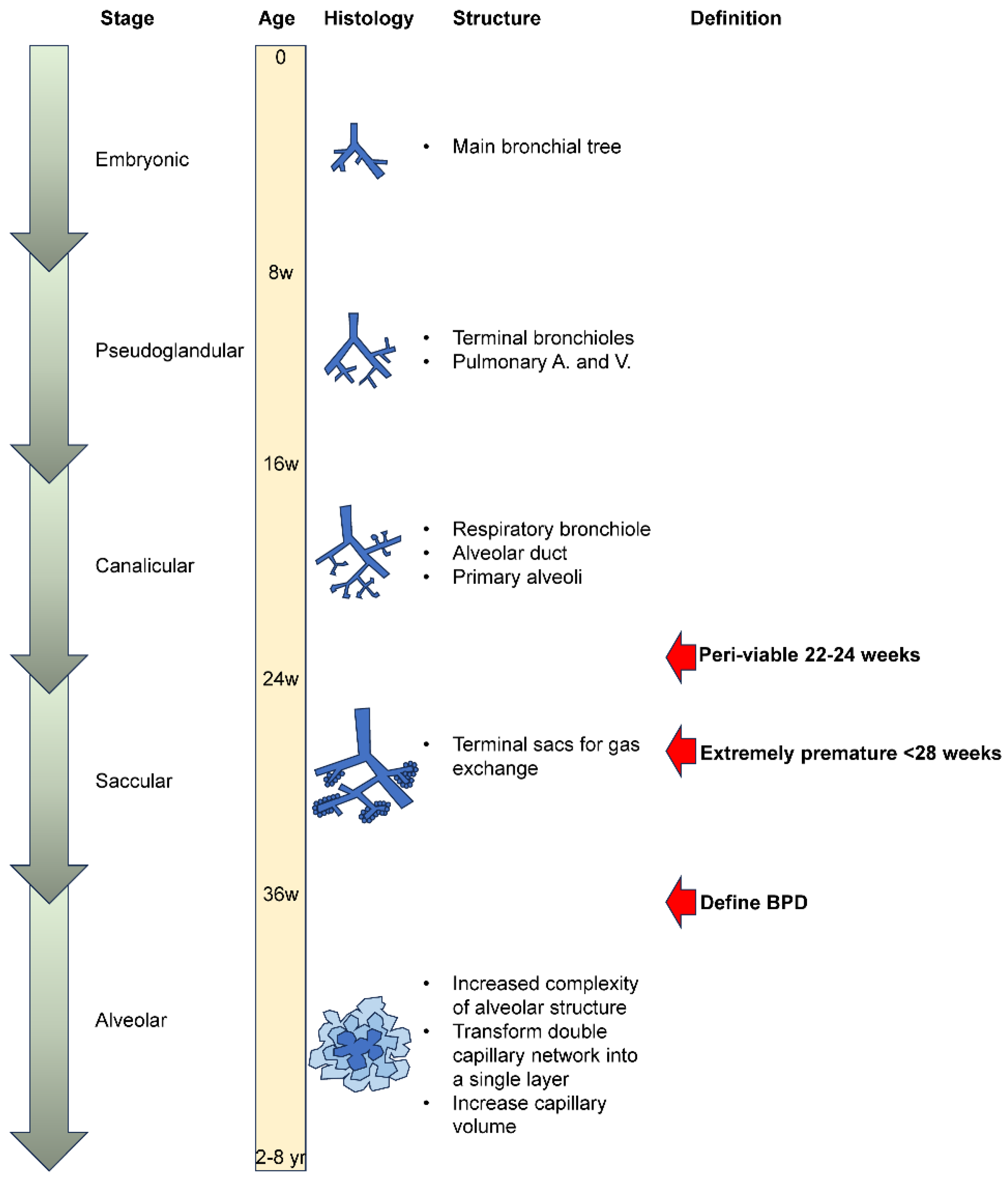
2. Changing Perspectives on BPD
| Author | Year | Criteria |
|---|---|---|
| Northway [28] | 1967 | Clinical course, X-ray, and histology |
| Tooley [29] | 1979 | X-ray and O2 use at 30 days, PaO2 < 60 mm-Hg in room air or PaCO2 < 45 mm-Hg |
| 28 days of O2 exposure with characteristic radiologic findings | ||
| Shennan [8] | 1988 | O2 requirement at 36 weeks PMA |
| Jobe [30] | 1999 | New BPD versus old BPD |
| Ehrenkranz [31] | 2001 | FiO2 > 0.21 for ≥28 days |
| GA < 32 weeks requires O2 at PMA 36 weeks, grade at DOL 56 | ||
| Mild: room air | ||
| Moderate: FiO2 <30% | ||
| Severe: FiO2 > 30% or CPAP or IMV | ||
| Walsh [32] | 2004 | Physiological BPD: O2 at precisely 36 weeks’ postmenstrual age |
| On PPV or >30% O2 with SpO2 90–96% | ||
| Fail to maintain SpO2 ≥ 90% for 30 min following the O2 reduction test | ||
| Higgins [33] | 2018 | GA < 32 weeks, (+) x-ay findings, and respiratory support at PMA 36 weeks to keep SpO2 90–96% |
| Gr 1: CPAP, NIPPV, NC ≥ 3 LPM 21% O2; NC 1–2 LPM 22–29% O2; NC 1 LPM 22–70% O2 | ||
| Gr 2: IMV 21% O2; CPAP, NIPPV, NC ≥ 3 LPM 22–29% O2; NC 1–2 LPM ≥30% O2; NC 1 LPM > 70% O2 | ||
| Gr 3: IMV > 21% O2; CPAP, NIPPV, NC > 3 LPM > 30% O2 | ||
| Gr 3a: Death > 14 days of life < 36 weeks PMA from respiratory failure | ||
| Jensen [34] | 2019 | GA < 32 weeks and grade at 36 weeks PMA or discharge |
| Gr 1: NC < 2 LPM | ||
| Gr 2: NC > 2 LPM, CPAP, or NIPPV | ||
| Gr 3: Invasive mechanical ventilation |
3. Pathology of Human BPD
4. Risk Factors for BPD Onset
4.1. Prenatal Risk Factors
- a.
- Intrauterine growth restriction (IUGR)
- b.
- Lack of antenatal corticosteroids
- c.
- Maternal smoking
- d.
- Chorioamnionitis
- e.
- Genetics
4.2. Risk Factors at Birth
- a.
- Gestational age and birth weight
- b.
- Gender
- c.
- Level of management
4.3. Postnatal Risk Factors
- a.
- Respiratory support
- b.
- Infections
- c.
- Patent ductus arteriosus (PDA)
- d.
- Gastroesophageal reflux (GER)
5. Biomarkers for BPD
6. Oxidative Stress and General Mechanistic Considerations of BPD Onset and Progression
6.1. OS in BPD
6.2. Animal Model OS Studies
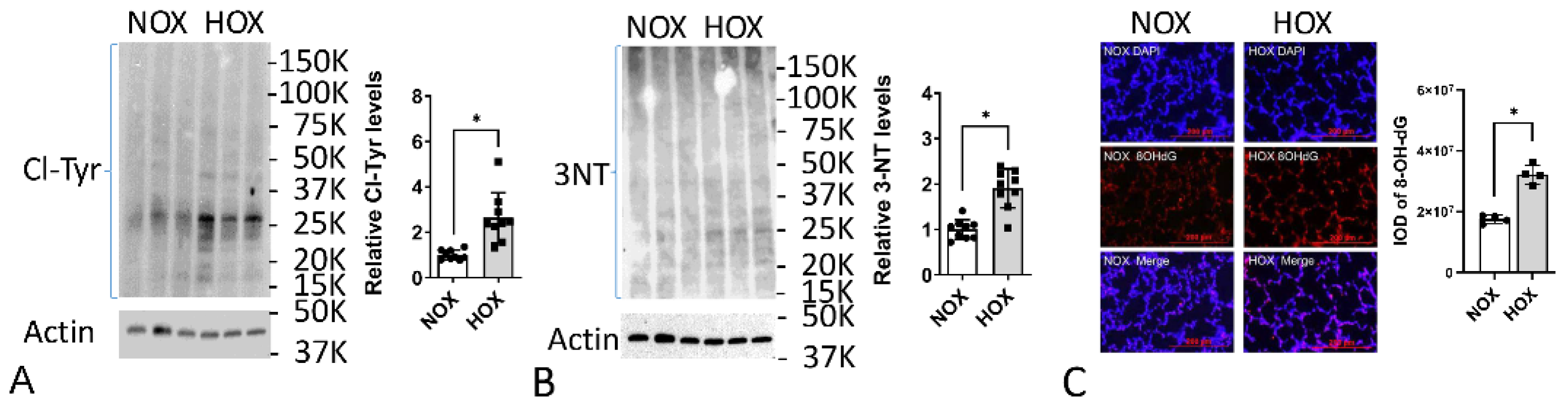
6.3. Evidence of OS in BPD Lungs
6.4. Increased ER Stress in BPD Lungs
6.5. Increased Cellular Senescence in BPD Lungs
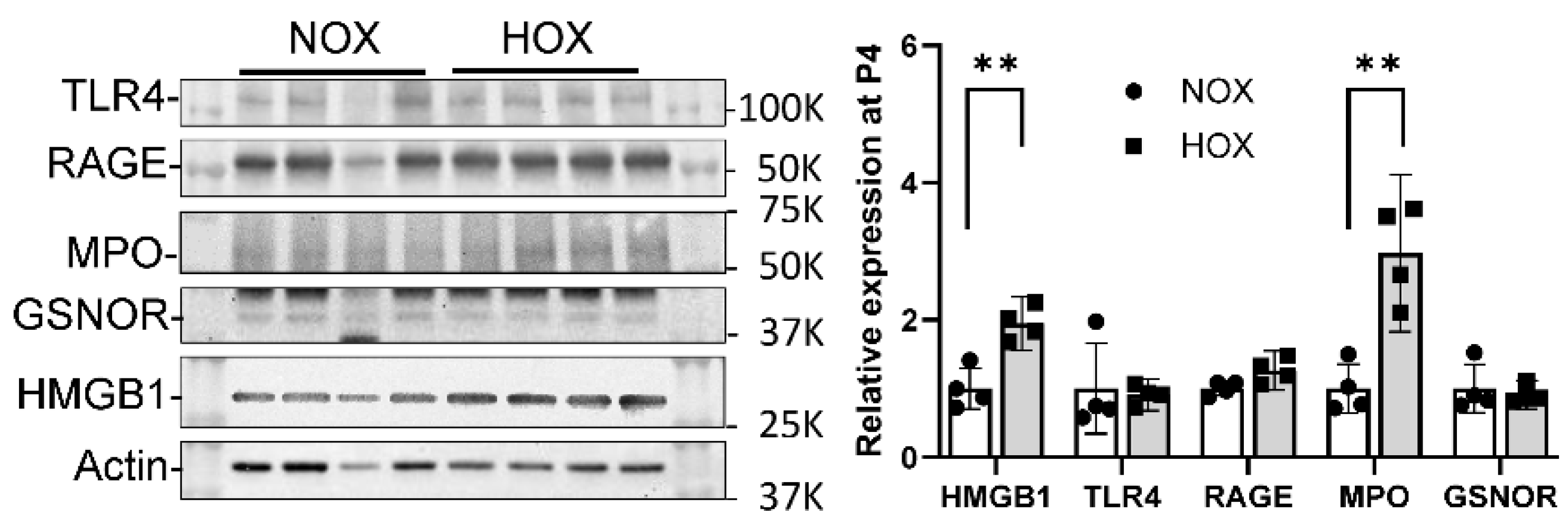
7. Specific Role of Myeloperoxidase and Sterile Inflammation in BPD
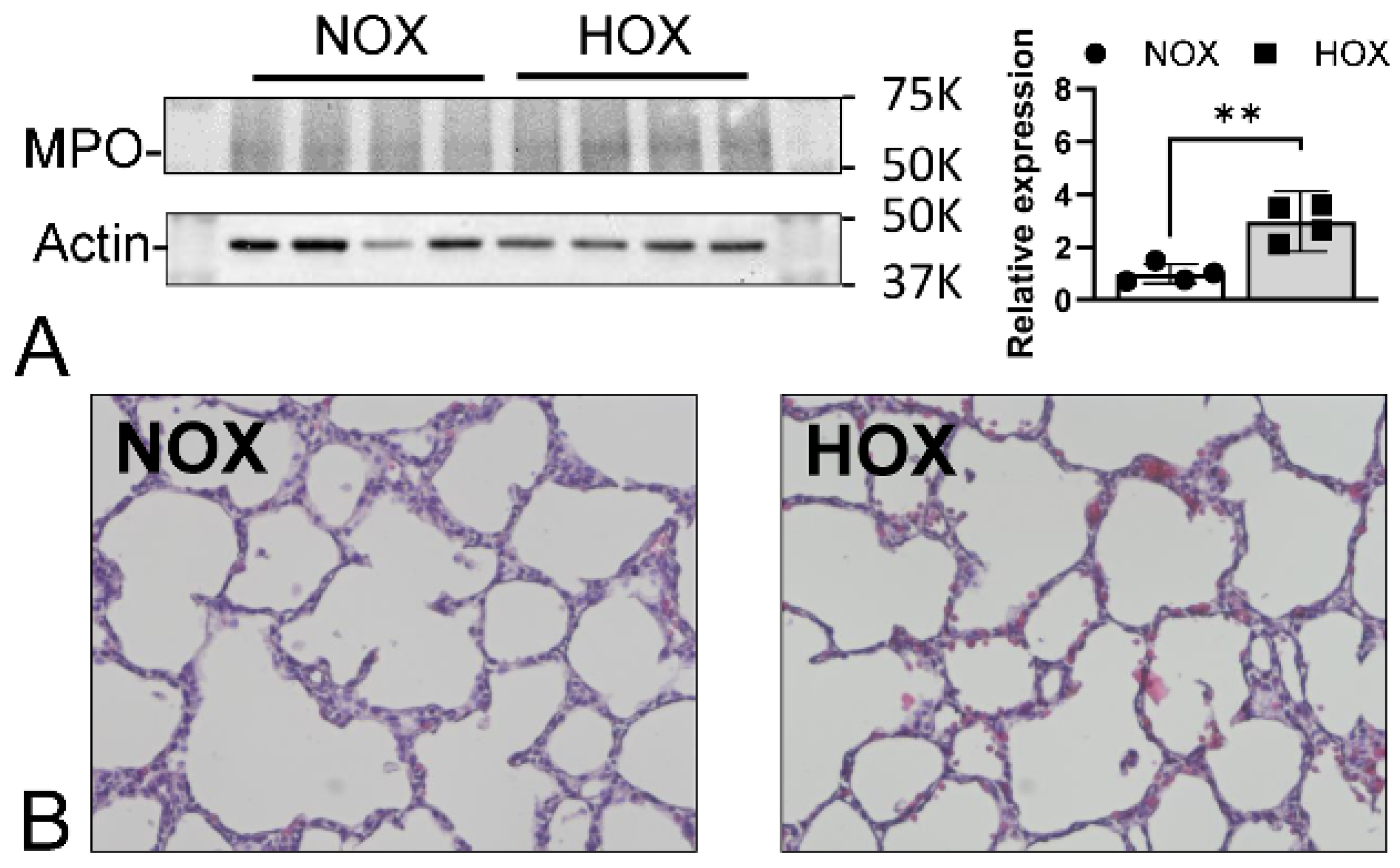
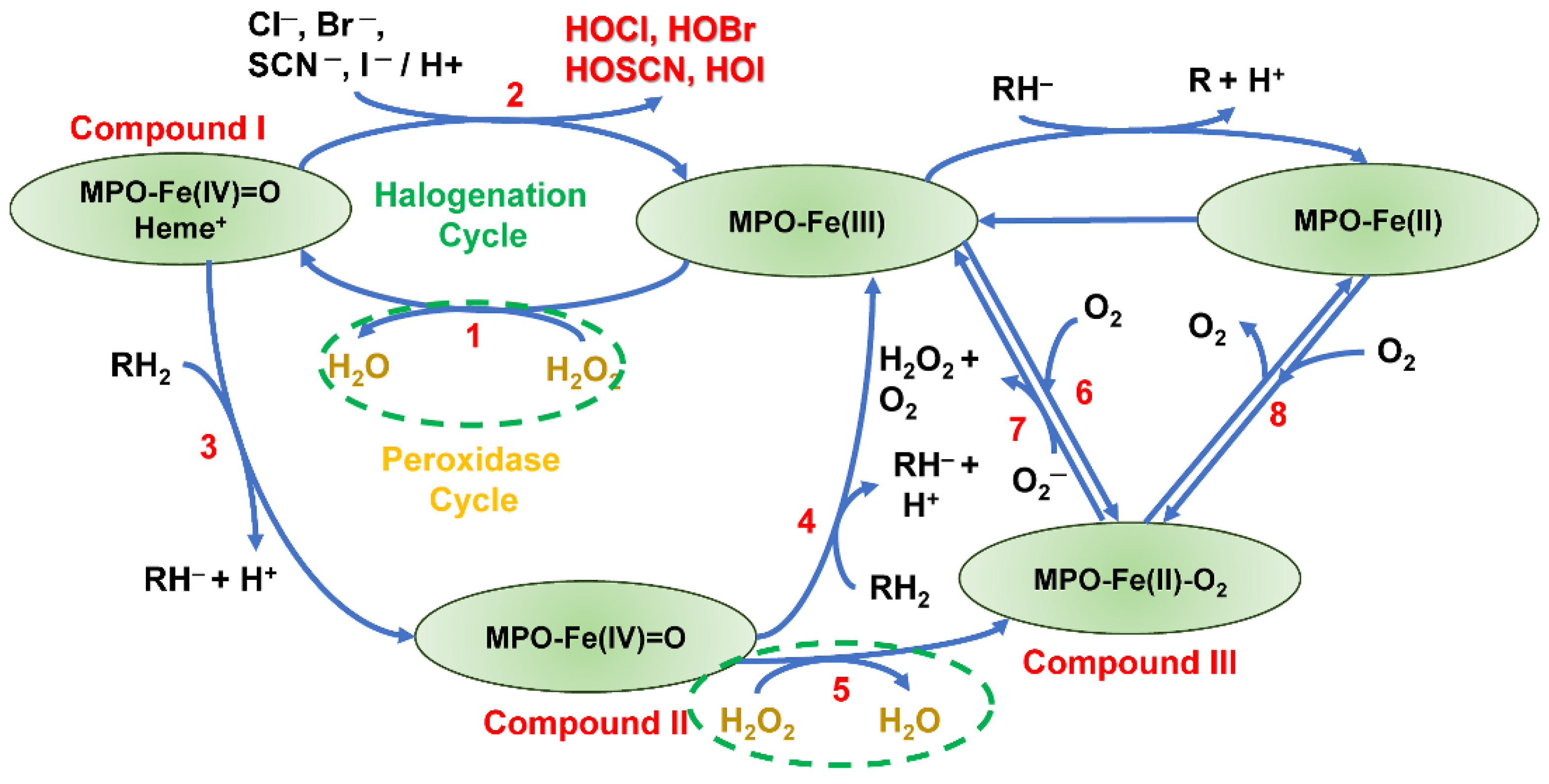
7.1. MPO
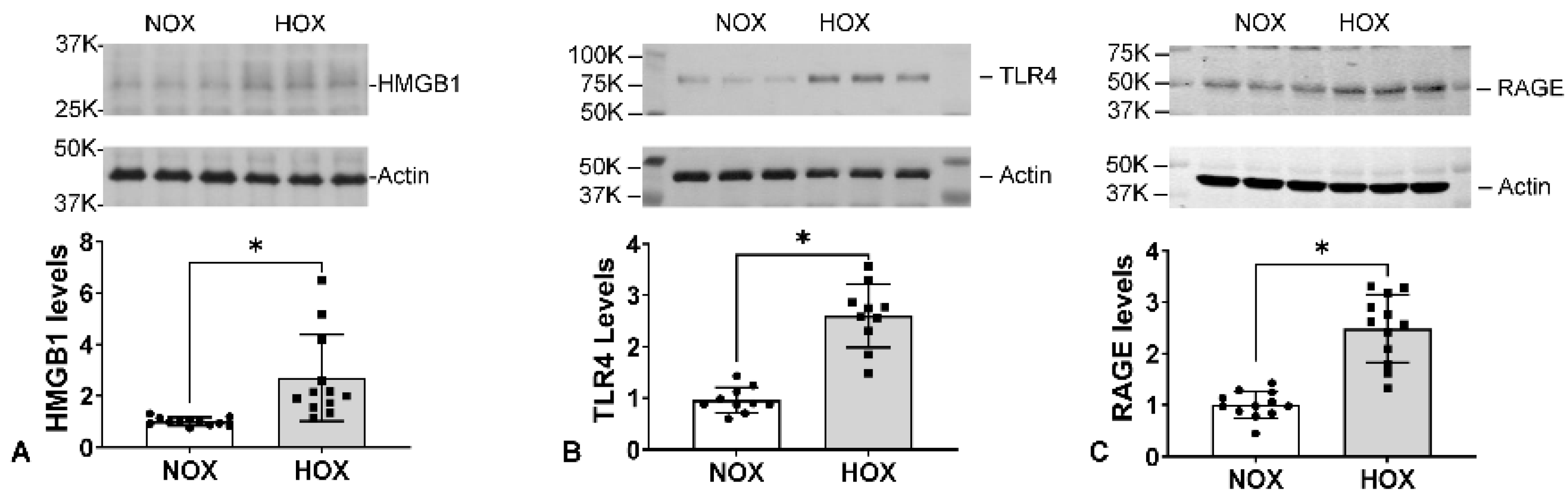
7.2. Other Inflammation-Related Proteins in BPD Lungs
- a.
- HMGB1
- b.
- TLR4
- c.
- RAGE
- d.
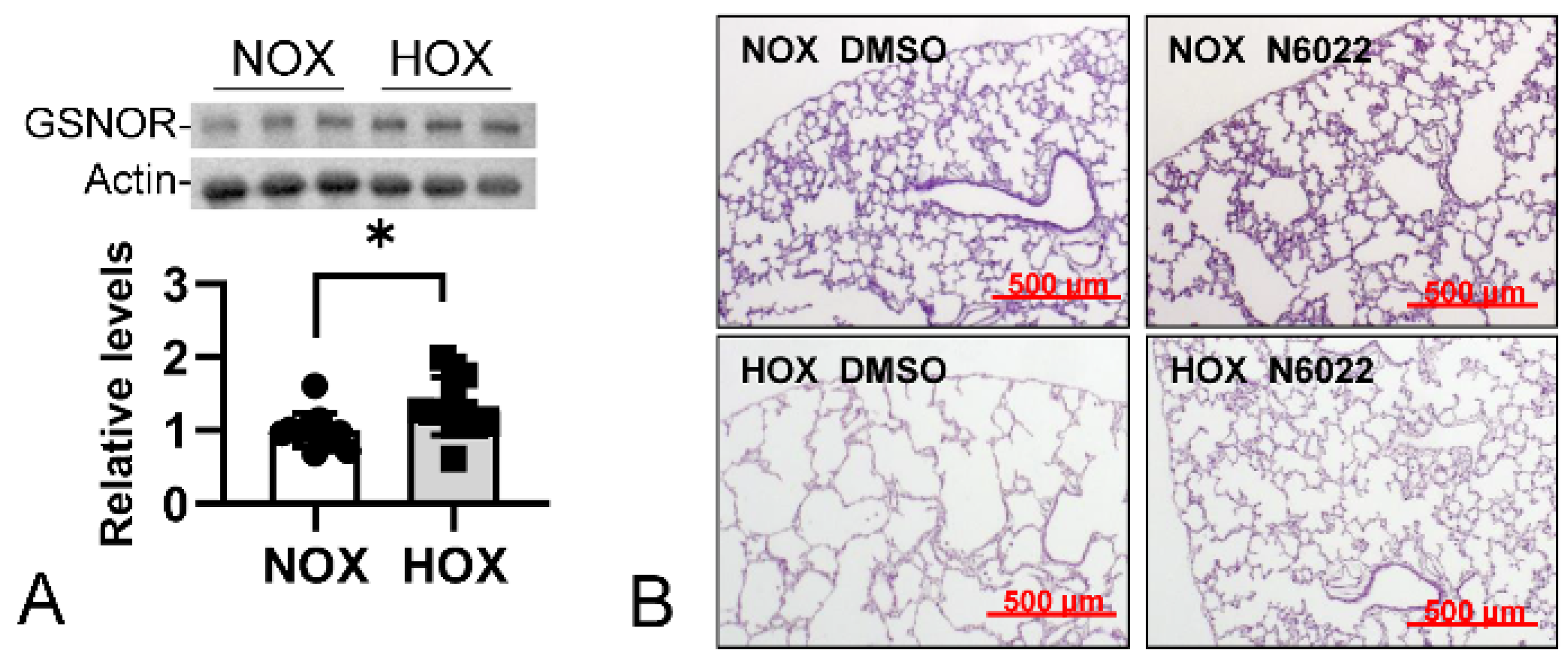
- GSNOR
7.3. The BPD Sterile Inflammatory Pathway
8. Targeted Therapeutic Intervention
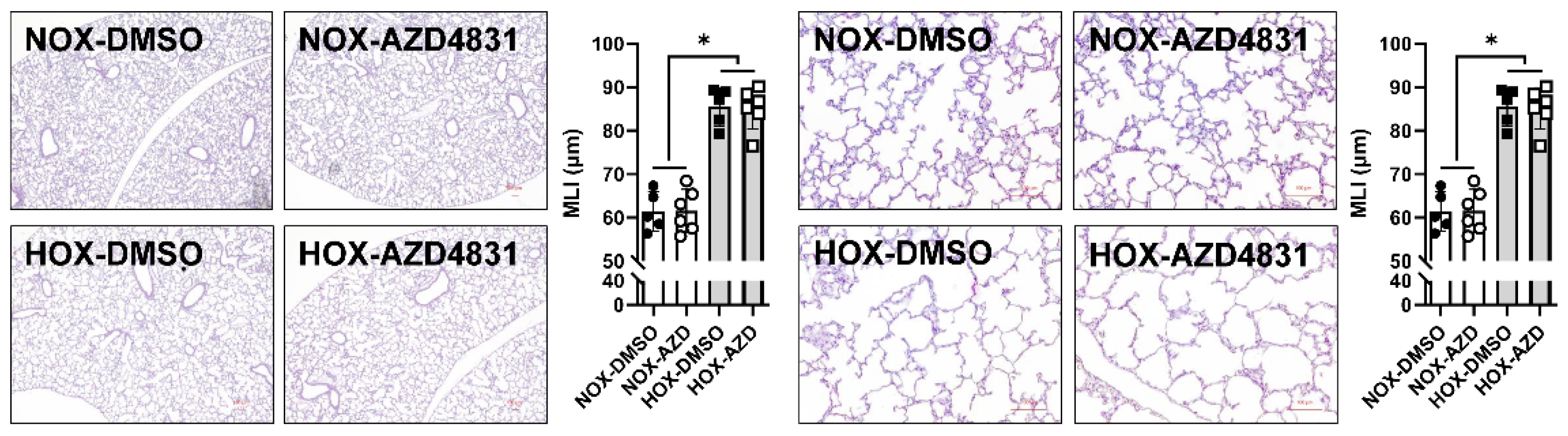
MPO Inhibitors
- a.
- Irreversible MPO inhibitor
- b.
- Reversible MPO inhibitor
- c.
- MPO inhibitors in BPD
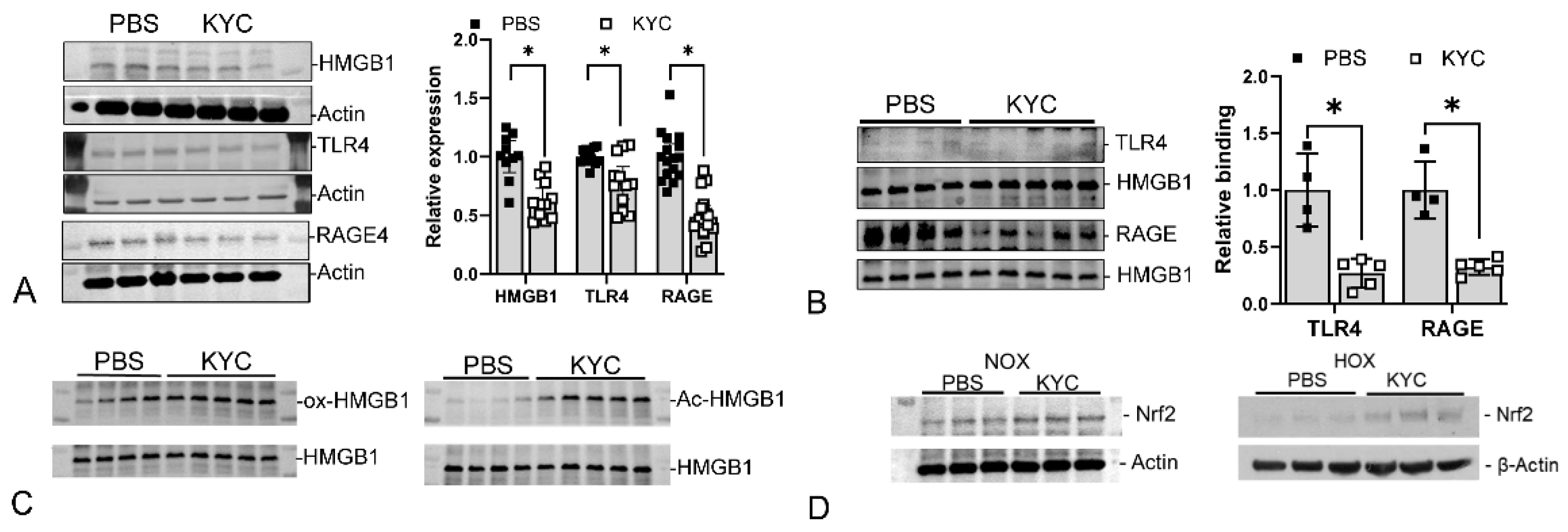
9. Discussion
- i.
- The SCPD (KYC) interacts with a first target that comprises a druggable biomolecular site, in this case, MPO.
- ii.
- The SCPD (KYC) modifies the properties and functions of the first target (MPO—inhibits the production of toxic oxidants and converts to a quasi-catalase) and is itself chemically modified (forms an initial tyrosyl radical, which through intramolecular transfer to cysteine forms a thiyl radical) and further activated by the first target.
- iii.
- The chemically modified and activated SCPD (KYC) interacts with one or more secondary targets (HMGB1, KEAP-1 Nrf2), thus modifying each of the second targets’ activity and functions.
10. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Thébaud, B.; Goss, K.N.; Laughon, M.; Whitsett, J.A.; Abman, S.H.; Steinhorn, R.H.; Aschner, J.L.; Davis, P.G.; McGrath-Morrow, S.A.; Soll, R.F.; et al. Bronchopulmonary dysplasia. Nat. Rev. Dis. Primers 2019, 5, 78. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nuthakki, S.; Ahmad, K.; Johnson, G.; Cuevas Guaman, M. Bronchopulmonary Dysplasia: Ongoing Challenges from Definitions to Clinical Care. J. Clin. Med. 2023, 12, 3864. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Stoll, B.J.; Hansen, N.I.; Bell, E.F.; Shankaran, S.; Laptook, A.R.; Walsh, M.C.; Hale, E.C.; Newman, N.S.; Schibler, K.; Carlo, W.A.; et al. Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics 2010, 126, 443–456. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Davidson, L.M.; Berkelhamer, S.K. Bronchopulmonary Dysplasia: Chronic Lung Disease of Infancy and Long-Term Pulmonary Outcomes. J. Clin. Med. 2017, 6, 4. [Google Scholar] [CrossRef] [PubMed Central]
- Humayun, J.; Löfqvist, C.; Ley, D.; Hellström, A.; Gyllensten, H. Systematic review of the healthcare cost of bronchopulmonary dysplasia. BMJ Open 2021, 11, e045729. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Perveen, S.; Chen, C.M.; Sobajima, H.; Zhou, X.; Chen, J.Y. Editorial: Bronchopulmonary dysplasia: Latest advances. Front. Pediatr. 2023, 11, 1303761. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bancalari, E.; Jain, D. Bronchopulmonary Dysplasia: 50 Years after the Original Description. Neonatology 2019, 115, 384–391. [Google Scholar] [CrossRef] [PubMed]
- Shennan, A.T.; Dunn, M.S.; Ohlsson, A.; Lennox, K.; Hoskins, E.M. Abnormal pulmonary outcomes in premature infants: Prediction from oxygen requirement in the neonatal period. Pediatrics 1988, 82, 527–532. [Google Scholar] [CrossRef]
- Bonadies, L.; Zaramella, P.; Porzionato, A.; Perilongo, G.; Muraca, M.; Baraldi, E. Present and Future of Bronchopulmonary Dysplasia. J. Clin. Med. 2020, 9, 1539. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rubarth, L.B.; Quinn, J. Respiratory Development and Respiratory Distress Syndrome. Neonatal Netw. 2015, 34, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Cheah, F.C.; Jobe, A.H.; Moss, T.J.; Newnham, J.P.; Kallapur, S.G. Oxidative stress in fetal lambs exposed to intra-amniotic endotoxin in a chorioamnionitis model. Pediatr. Res. 2008, 63, 274–279. [Google Scholar] [CrossRef] [PubMed]
- Zergeroglu, M.A.; McKenzie, M.J.; Shanely, R.A.; Van Gammeren, D.; DeRuisseau, K.C.; Powers, S.K. Mechanical ventilation-induced oxidative stress in the diaphragm. J. Appl. Physiol. (1985) 2003, 95, 1116–1124. [Google Scholar] [CrossRef] [PubMed]
- Murch, S.H.; Costeloe, K.; Klein, N.J.; MacDonald, T.T. Early production of macrophage inflammatory protein-1 alpha occurs in respiratory distress syndrome and is associated with poor outcome. Pediatr. Res. 1996, 40, 490–497. [Google Scholar] [CrossRef] [PubMed]
- Balany, J.; Bhandari, V. Understanding the Impact of Infection, Inflammation, and Their Persistence in the Pathogenesis of Bronchopulmonary Dysplasia. Front. Med. 2015, 2, 90. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hansmann, G.; Sallmon, H.; Roehr, C.C.; Kourembanas, S.; Austin, E.D.; Koestenberger, M.; European Pediatric Pulmonary Vascular Disease Network (EPPVDN). Pulmonary hypertension in bronchopulmonary dysplasia. Pediatr. Res. 2021, 89, 446–455. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Berkelhamer, S.K.; Mestan, K.K.; Steinhorn, R.H. Pulmonary hypertension in bronchopulmonary dysplasia. Semin. Perinatol. 2013, 37, 124–131. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Arjaans, S.; Haarman, M.G.; Roofthooft, M.T.R.; Fries, M.W.F.; Kooi, E.M.W.; Bos, A.F.; Berger, R.M.F. Fate of pulmonary hypertension associated with bronchopulmonary dysplasia beyond 36 weeks postmenstrual age. Arch. Dis. Child. Fetal Neonatal Ed. 2021, 106, 45–50. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Homan, T.D.; Nayak, R.P. Short- and Long-Term Complications of Bronchopulmonary Dysplasia. Respir. Care 2021, 66, 1618–1629. [Google Scholar] [CrossRef] [PubMed]
- Islam, J.Y.; Keller, R.L.; Aschner, J.L.; Hartert, T.V.; Moore, P.E. Understanding the Short- and Long-Term Respiratory Outcomes of Prematurity and Bronchopulmonary Dysplasia. Am. J. Respir. Crit. Care Med. 2015, 192, 134–156. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yeh, T.F.; Lin, Y.J.; Lin, H.C.; Huang, C.C.; Hsieh, W.S.; Lin, C.H.; Tsai, C.H. Outcomes at school age after postnatal dexamethasone therapy for lung disease of prematurity. N. Engl. J. Med. 2004, 350, 1304–1313. [Google Scholar] [CrossRef]
- Cummings, J.J.; Pramanik, A.K.; Committee on Fetus and Newborn. Postnatal Corticosteroids to Prevent or Treat Chronic Lung Disease Following Preterm Birth. Pediatrics 2022, 149, e2022057530. [Google Scholar] [CrossRef] [PubMed]
- Tyson, J.E.; Wright, L.L.; Oh, W.; Kennedy, K.A.; Mele, L.; Ehrenkranz, R.A.; Stoll, B.J.; Lemons, J.A.; Stevenson, D.K.; Bauer, C.R.; et al. Vitamin A supplementation for extremely-low-birth-weight infants. National Institute of Child Health and Human Development Neonatal Research Network. N. Engl. J. Med. 1999, 340, 1962–1968. [Google Scholar] [CrossRef] [PubMed]
- Meyer, S.; Bay, J.; Franz, A.R.; Erhardt, H.; Klein, L.; Petzinger, J.; Binder, C.; Kirschenhofer, S.; Stein, A.; Hüning, B.; et al. Early postnatal high-dose fat-soluble enteral vitamin A supplementation for moderate or severe bronchopulmonary dysplasia or death in extremely low birthweight infants (NeoVitaA): A multicentre, randomised, parallel-group, double-blind, placebo-controlled, investigator-initiated phase 3 trial. Lancet Respir. Med. 2024, 12, 544–555. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, B.; Roberts, R.S.; Davis, P.; Doyle, L.W.; Barrington, K.J.; Ohlsson, A.; Solimano, A.; Tin, W.; Caffeine for Apnea of Prematurity Trial Group. Caffeine therapy for apnea of prematurity. N. Engl. J. Med. 2006, 354, 2112–2121. [Google Scholar] [CrossRef] [PubMed]
- Simones, A.A.; Beisang, D.J.; Panoskaltsis-Mortari, A.; Roberts, K.D. Mesenchymal stem cells in the pathogenesis and treatment of bronchopulmonary dysplasia: A clinical review. Pediatr. Res. 2018, 83, 308–317. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- O’Reilly, M.; Thébaud, B. Animal models of bronchopulmonary dysplasia. The term rat models. Am. J. Physiol. Lung Cell Mol. Physiol. 2014, 307, L948–L958. [Google Scholar] [CrossRef] [PubMed]
- Berger, J.; Bhandari, V. Animal models of bronchopulmonary dysplasia. The term mouse models. Am. J. Physiol. Lung Cell Mol. Physiol. 2014, 307, L936–L947. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Northway, W.H., Jr.; Rosan, R.C.; Porter, D.Y. Pulmonary disease following respirator therapy of hyaline-membrane disease. Bronchopulmonary dysplasia. N. Engl. J. Med. 1967, 276, 357–368. [Google Scholar] [CrossRef] [PubMed]
- Tooley, W.H. Epidemiology of bronchopulmonary dysplasia. J. Pediatr. 1979, 95 Pt 2, 851–858. [Google Scholar] [CrossRef] [PubMed]
- Jobe, A.J. The new BPD: An arrest of lung development. Pediatr. Res. 1999, 46, 641–643. [Google Scholar] [CrossRef] [PubMed]
- Ehrenkranz, R.A.; Walsh, M.C.; Vohr, B.R.; Jobe, A.H.; Wright, L.L.; Fanaroff, A.A.; Wrage, L.A.; Poole, K.; National Institutes of Child Health and Human Development Neonatal Research Network. Validation of the National Institutes of Health consensus definition of bronchopulmonary dysplasia. Pediatrics 2005, 116, 1353–1360. [Google Scholar] [CrossRef] [PubMed]
- Walsh, M.C.; Yao, Q.; Gettner, P.; Hale, E.; Collins, M.; Hensman, A.; Everette, R.; Peters, N.; Miller, N.; Muran, G.; et al. Impact of a physiologic definition on bronchopulmonary dysplasia rates. Pediatrics 2004, 114, 1305–1311. [Google Scholar] [CrossRef] [PubMed]
- Higgins, R.D.; Jobe, A.H.; Koso-Thomas, M.; Bancalari, E.; Viscardi, R.M.; Hartert, T.V.; Ryan, R.M.; Kallapur, S.G.; Steinhorn, R.H.; Konduri, G.G.; et al. Bronchopulmonary Dysplasia: Executive Summary of a Workshop. J. Pediatr. 2018, 197, 300–308. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jensen, E.A.; Dysart, K.; Gantz, M.G.; McDonald, S.; Bamat, N.A.; Keszler, M.; Kirpalani, H.; Laughon, M.M.; Poindexter, B.B.; Duncan, A.F.; et al. The Diagnosis of Bronchopulmonary Dysplasia in Very Preterm Infants. An Evidence-based Approach. Am. J. Respir. Crit. Care Med. 2019, 200, 751–759. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ibrahim, J.; Bhandari, V. The definition of bronchopulmonary dysplasia: An evolving dilemma. Pediatr. Res. 2018, 84, 586–588. [Google Scholar] [CrossRef] [PubMed]
- Cerny, L.; Torday, J.S.; Rehan, V.K. Prevention and treatment of bronchopulmonary dysplasia: Contemporary status and future outlook. Lung 2008, 186, 75–89. [Google Scholar] [CrossRef] [PubMed]
- Younge, N.; Goldstein, R.F.; Bann, C.M.; Hintz, S.R.; Patel, R.M.; Smith, P.B.; Bell, E.F.; Rysavy, M.A.; Duncan, A.F.; Vohr, B.R.; et al. Survival and Neurodevelopmental Outcomes among Periviable Infants. N. Engl. J. Med. 2017, 376, 617–628. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jensen, E.A.; Schmidt, B. Epidemiology of bronchopulmonary dysplasia. Birth Defects Res. A Clin. Mol. Teratol. 2014, 100, 145–157. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lee, S.M.; Sie, L.; Liu, J.; Profit, J.; Lee, H.C. Evaluation of Trends in Bronchopulmonary Dysplasia and Respiratory Support Practice for Very Low Birth Weight Infants: A Population-Based Cohort Study. J. Pediatr. 2022, 243, 47–52.e2. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bäckström, E.; Hogmalm, A.; Lappalainen, U.; Bry, K. Developmental stage is a major determinant of lung injury in a murine model of bronchopulmonary dysplasia. Pediatr. Res. 2011, 69, 312–318. [Google Scholar] [CrossRef] [PubMed]
- Iliodromiti, Z.; Zygouris, D.; Sifakis, S.; Pappa, K.I.; Tsikouras, P.; Salakos, N.; Daniilidis, A.; Siristatidis, C.; Vrachnis, N. Acute lung injury in preterm fetuses and neonates: Mechanisms and molecular pathways. J. Matern. Fetal Neonatal Med. 2013, 26, 1696–1704. [Google Scholar] [CrossRef] [PubMed]
- Bonikos, D.S.; Bensch, K.G.; Northway, W.H., Jr.; Edwards, D.K. Bronchopulmonary dysplasia: The pulmonary pathologic sequel of necrotizing bronchiolitis and pulmonary fibrosis. Hum. Pathol. 1976, 7, 643–666. [Google Scholar] [CrossRef] [PubMed]
- Husain, A.N.; Siddiqui, N.H.; Stocker, J.T. Pathology of arrested acinar development in postsurfactant bronchopulmonary dysplasia. Hum. Pathol. 1998, 29, 710–717. [Google Scholar] [CrossRef] [PubMed]
- Lovering, A.T.; Riemer, R.K.; Thébaud, B. Intrapulmonary arteriovenous anastomoses. Physiological, pathophysiological, or both? Ann. Am. Thorac. Soc. 2013, 10, 504–508. [Google Scholar] [CrossRef] [PubMed]
- Sehgal, A.; Dassios, T.; Nold, M.F.; Nold-Petry, C.A.; Greenough, A. Fetal growth restriction and neonatal-pediatric lung diseases: Vascular mechanistic links and therapeutic directions. Paediatr. Respir. Rev. 2022, 44, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Groene, S.G.; Spekman, J.A.; Te Pas, A.B.; Heijmans, B.T.; Haak, M.C.; van Klink, J.M.M.; Roest, A.A.W.; Lopriore, E. Respiratory distress syndrome and bronchopulmonary dysplasia after fetal growth restriction: Lessons from a natural experiment in identical twins. eClinicalMedicine 2021, 32, 100725. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Valenzuela-Stutman, D.; Marshall, G.; Tapia, J.L.; Mariani, G.; Bancalari, A.; Gonzalez, Á.; Neocosur Neonatal Network. Bronchopulmonary dysplasia: Risk prediction models for very-low- birth-weight infants. J. Perinatol. 2019, 39, 1275–1281. [Google Scholar] [CrossRef] [PubMed]
- Hayes, D., Jr.; Feola, D.J.; Murphy, B.S.; Shook, L.A.; Ballard, H.O. Pathogenesis of bronchopulmonary dysplasia. Respiration 2010, 79, 425–436. [Google Scholar] [CrossRef] [PubMed]
- Morley, C.J.; Davis, P.G.; Doyle, L.W.; Brion, L.P.; Hascoet, J.M.; Carlin, J.B.; COIN Trial Investigators. Nasal CPAP or intubation at birth for very preterm infants. N. Engl. J. Med. 2008, 358, 700–708, Erratum in N. Engl. J. Med. 2008, 358, 1529. [Google Scholar] [CrossRef] [PubMed]
- Subramaniam, P.; Ho, J.J.; Davis, P.G. Prophylactic or very early initiation of continuous positive airway pressure (CPAP) for preterm infants. Cochrane Database Syst. Rev. 2021, 10, CD001243. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Laughon, M.; Allred, E.N.; Bose, C.; O’Shea, T.M.; Van Marter, L.J.; Ehrenkranz, R.A.; Leviton, A.; ELGAN Study Investigators. Patterns of respiratory disease during the first 2 postnatal weeks in extremely premature infants. Pediatrics 2009, 123, 1124–1131. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ryan, S.W.; Nycyk, J.; Shaw, B.N. Prediction of chronic neonatal lung disease on day 4 of life. Eur. J. Pediatr. 1996, 155, 668–671. [Google Scholar] [CrossRef] [PubMed]
- González-Luis, G.E.; van Westering-Kroon, E.; Villamor-Martinez, E.; Huizing, M.J.; Kilani, M.A.; Kramer, B.W.; Villamor, E. Tobacco Smoking During Pregnancy Is Associated with Increased Risk of Moderate/Severe Bronchopulmonary Dysplasia: A Systematic Review and Meta-Analysis. Front. Pediatr. 2020, 8, 160. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Coarfa, C.; Grimm, S.L.; Katz, T.; Zhang, Y.; Jangid, R.K.; Walker, C.L.; Moorthy, B.; Lingappan, K. Epigenetic response to hyperoxia in the neonatal lung is sexually dimorphic. Redox Biol. 2020, 37, 101718. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hammond, J.D., 2nd; Kielt, M.J.; Conroy, S.; Lingappan, K.; Austin, E.D.; Eldredge, L.C.; Truog, W.E.; Abman, S.H.; Nelin, L.D.; Guaman, M.C. Exploring the Association of Male Sex With Adverse Outcomes in Severe Bronchopulmonary Dysplasia: A Retrospective, Multicenter Cohort Study. Chest 2024, 165, 610–620. [Google Scholar] [CrossRef] [PubMed]
- Sohn, A.H.; Garrett, D.O.; Sinkowitz-Cochran, R.L.; Grohskopf, L.A.; Levine, G.L.; Stover, B.H.; Siegel, J.D.; Jarvis, W.R.; Pediatric Prevention Network. Prevalence of nosocomial infections in neonatal intensive care unit patients: Results from the first national point-prevalence survey. J. Pediatr. 2001, 139, 821–827. [Google Scholar] [CrossRef] [PubMed]
- Lapcharoensap, W.; Kan, P.; Powers, R.J.; Shaw, G.M.; Stevenson, D.K.; Gould, J.B.; Wirtschafter, D.D.; Lee, H.C. The Relationship of Nosocomial Infection Reduction to Changes in Neonatal Intensive Care Unit Rates of Bronchopulmonary Dysplasia. J. Pediatr. 2017, 180, 105–109.e1. [Google Scholar] [CrossRef] [PubMed]
- Travers, C.P.; Clark, R.H.; Spitzer, A.R.; Das, A.; Garite, T.J.; Carlo, W.A. Exposure to any antenatal corticosteroids and outcomes in preterm infants by gestational age: Prospective cohort study. BMJ 2017, 356, j1039. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gagliardi, L.; Bellù, R.; Rusconi, F.; Merazzi, D.; Mosca, F. Antenatal steroids and risk of bronchopulmonary dysplasia: A lack of effect or a case of over-adjustment? Paediatr. Perinat. Epidemiol. 2007, 21, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Lapcharoensap, W.; Gage, S.C.; Kan, P.; Profit, J.; Shaw, G.M.; Gould, J.B.; Stevenson, D.K.; O’Brodovich, H.; Lee, H.C. Hospital variation and risk factors for bronchopulmonary dysplasia in a population-based cohort. JAMA Pediatr. 2015, 169, e143676. [Google Scholar] [CrossRef] [PubMed]
- El-Khuffash, A.; Mullaly, R.; McNamara, P.J. Patent ductus arteriosus, bronchopulmonary dysplasia and pulmonary hypertension—A complex conundrum with many phenotypes? Pediatr. Res. 2023, 94, 416–417. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nawaytou, H.; Hills, N.K.; Clyman, R.I. Patent ductus arteriosus and the risk of bronchopulmonary dysplasia-associated pulmonary hypertension. Pediatr. Res. 2023, 94, 547–554. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gentle, S.J.; Travers, C.P.; Clark, M.; Carlo, W.A.; Ambalavanan, N. Patent Ductus Arteriosus and Development of Bronchopulmonary Dysplasia-associated Pulmonary Hypertension. Am. J. Respir. Crit. Care Med. 2023, 207, 921–928. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Villamor, E.; van Westering-Kroon, E.; Gonzalez-Luis, G.E.; Bartoš, F.; Abman, S.H.; Huizing, M.J. Patent Ductus Arteriosus and Bronchopulmonary Dysplasia-Associated Pulmonary Hypertension: A Bayesian Meta-Analysis. JAMA Netw. Open 2023, 6, e2345299. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jensen, E.A.; Foglia, E.E.; Schmidt, B. Association between prophylactic indomethacin and death or bronchopulmonary dysplasia: A systematic review and meta-analysis of observational studies. Semin. Perinatol. 2018, 42, 228–234. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hartling, L.; Liang, Y.; Lacaze-Masmonteil, T. Chorioamnionitis as a risk factor for bronchopulmonary dysplasia: A systematic review and meta-analysis. Arch. Dis. Child. Fetal Neonatal Ed. 2012, 97, F8–F17. [Google Scholar] [CrossRef] [PubMed]
- Villamor-Martinez, E.; Álvarez-Fuente, M.; Ghazi, A.M.T.; Degraeuwe, P.; Zimmermann, L.J.I.; Kramer, B.W.; Villamor, E. Association of Chorioamnionitis With Bronchopulmonary Dysplasia Among Preterm Infants: A Systematic Review, Meta-analysis, and Metaregression. JAMA Netw. Open 2019, 2, e1914611. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ballard, A.R.; Mallett, L.H.; Pruszynski, J.E.; Cantey, J.B. Chorioamnionitis and subsequent bronchopulmonary dysplasia in very-low-birth weight infants: A 25-year cohort. J. Perinatol. 2016, 36, 1045–1048. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.J.; Hu, Y.; Wang, W.; Zhang, C.Y.; Bai, Y.Z.; Zhang, S.C. Gastroesophageal Reflux Poses a Potential Risk for Late Complications of Bronchopulmonary Dysplasia: A Prospective Cohort Study. Chest 2020, 158, 1596–1605. [Google Scholar] [CrossRef] [PubMed]
- Gulati, I.K.; Jadcherla, S.R. Gastroesophageal Reflux Disease in the Neonatal Intensive Care Unit Infant: Who Needs to Be Treated and What Approach Is Beneficial? Pediatr. Clin. N. Am. 2019, 66, 461–473. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bhandari, V.; Bizzarro, M.J.; Shetty, A.; Zhong, X.; Page, G.P.; Zhang, H.; Ment, L.R.; Gruen, J.R.; Neonatal Genetics Study Group. Familial and genetic susceptibility to major neonatal morbidities in preterm twins. Pediatrics 2006, 117, 1901–1906. [Google Scholar] [CrossRef] [PubMed]
- Lavoie, P.M.; Pham, C.; Jang, K.L. Heritability of bronchopulmonary dysplasia, defined according to the consensus statement of the national institutes of health. Pediatrics 2008, 122, 479–485. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yu, K.H.; Li, J.; Snyder, M.; Shaw, G.M.; O’Brodovich, H.M. The genetic predisposition to bronchopulmonary dysplasia. Curr. Opin. Pediatr. 2016, 28, 318–323. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Parad, R.B.; Winston, A.B.; Kalish, L.A.; Gupta, M.; Thompson, I.; Sheldon, Y.; Morey, J.; Van Marter, L.J. Role of Genetic Susceptibility in the Development of Bronchopulmonary Dysplasia. J. Pediatr. 2018, 203, 234–241.e2. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bonadies, L.; Moschino, L.; Valerio, E.; Giordano, G.; Manzoni, P.; Baraldi, E. Early Biomarkers of Bronchopulmonary Dysplasia: A Quick Look to the State of the Art. Am. J. Perinatol. 2022, 39, S26–S30. [Google Scholar] [CrossRef] [PubMed]
- Lal, C.V.; Ambalavanan, N. Biomarkers, Early Diagnosis, and Clinical Predictors of Bronchopulmonary Dysplasia. Clin. Perinatol. 2015, 42, 739–754. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bhandari, A.; Bhandari, V. Biomarkers in bronchopulmonary dysplasia. Paediatr. Respir. Rev. 2013, 14, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Saugstad, O.D.; Oei, J.L.; Lakshminrusimha, S.; Vento, M. Oxygen therapy of the newborn from molecular understanding to clinical practice. Pediatr. Res. 2019, 85, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Falk, D.J.; Kavazis, A.N.; Whidden, M.A.; Smuder, A.J.; McClung, J.M.; Hudson, M.B.; Powers, S.K. Mechanical ventilation-induced oxidative stress in the diaphragm: Role of heme oxygenase-1. Chest 2011, 139, 816–824. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chacon-Cabrera, A.; Rojas, Y.; Martínez-Caro, L.; Vila-Ubach, M.; Nin, N.; Ferruelo, A.; Esteban, A.; Lorente, J.A.; Barreiro, E. Influence of mechanical ventilation and sepsis on redox balance in diaphragm, myocardium, limb muscles, and lungs. Transl. Res. 2014, 164, 477–495. [Google Scholar] [CrossRef] [PubMed]
- Andrés, C.M.C.; Pérez de la Lastra, J.M.; Juan, C.A.; Plou, F.J.; Pérez-Lebeña, E. Hypochlorous Acid Chemistry in Mammalian Cells-Influence on Infection and Role in Various Pathologies. Int. J. Mol. Sci. 2022, 23, 10735. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lu, G.; Zhang, R.; Geng, S.; Peng, L.; Jayaraman, P.; Chen, C.; Xu, F.; Yang, J.; Li, Q.; Zheng, H.; et al. Myeloid cell-derived inducible nitric oxide synthase suppresses M1 macrophage polarization. Nat. Commun. 2015, 6, 6676. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Reiter, C.D.; Teng, R.J.; Beckman, J.S. Superoxide reacts with nitric oxide to nitrate tyrosine at physiological pH via peroxynitrite. J. Biol. Chem. 2000, 275, 32460–32466. [Google Scholar] [CrossRef] [PubMed]
- Eiserich, J.P.; Hristova, M.; Cross, C.E.; Jones, A.D.; Freeman, B.A.; Halliwell, B.; van der Vliet, A. Formation of nitric oxide-derived inflammatory oxidants by myeloperoxidase in neutrophils. Nature 1998, 391, 393–397. [Google Scholar] [CrossRef] [PubMed]
- Buss, I.H.; Senthilmohan, R.; Darlow, B.A.; Mogridge, N.; Kettle, A.J.; Winterbourn, C.C. 3-Chlorotyrosine as a marker of protein damage by myeloperoxidase in tracheal aspirates from preterm infants: Association with adverse respiratory outcome. Pediatr. Res. 2003, 53, 455–462, Erratum in Pediatr. Res. 2003, 53, 868. [Google Scholar] [CrossRef] [PubMed]
- Laube, M.; Amann, E.; Uhlig, U.; Yang, Y.; Fuchs, H.W.; Zemlin, M.; Mercier, J.C.; Maier, R.F.; Hummler, H.D.; Uhlig, S.; et al. Inflammatory Mediators in Tracheal Aspirates of Preterm Infants Participating in a Randomized Trial of Inhaled Nitric Oxide. PLoS ONE 2017, 12, e0169352. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Banks, B.A.; Ischiropoulos, H.; McClelland, M.; Ballard, P.L.; Ballard, R.A. Plasma 3-nitrotyrosine is elevated in premature infants who develop bronchopulmonary dysplasia. Pediatrics 1998, 101, 870–874. [Google Scholar] [CrossRef] [PubMed]
- Lorch, S.A.; Banks, B.A.; Christie, J.; Merrill, J.D.; Althaus, J.; Schmidt, K.; Ballard, P.L.; Ischiropoulos, H.; Ballard, R.A. Plasma 3-nitrotyrosine and outcome in neonates with severe bronchopulmonary dysplasia after inhaled nitric oxide. Free Radic. Biol. Med. 2003, 34, 1146–1152. [Google Scholar] [CrossRef] [PubMed]
- Kimble, A.; Robbins, M.E.; Perez, M. Pathogenesis of Bronchopulmonary Dysplasia: Role of Oxidative Stress from ‘Omics’ Studies. Antioxidants 2022, 11, 2380. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ten, V.S.; Ratner, V. Mitochondrial bioenergetics and pulmonary dysfunction: Current progress and future directions. Paediatr. Respir. Rev. 2020, 34, 37–45. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Konduri, G.G.; Afolayan, A.J.; Eis, A.; Pritchard, K.A., Jr.; Teng, R.J. Interaction of endothelial nitric oxide synthase with mitochondria regulates oxidative stress and function in fetal pulmonary artery endothelial cells. Am. J. Physiol. Lung Cell Mol. Physiol. 2015, 309, L1009–L1017. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Course, C.W.; Lewis, P.A.; Kotecha, S.J.; Cousins, M.; Hart, K.; Heesom, K.J.; Watkins, W.J.; Kotecha, S. Evidence of abnormality in glutathione metabolism in the airways of preterm born children with a history of bronchopulmonary dysplasia. Sci. Rep. 2023, 13, 19465. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Teng, R.J.; Jing, X.; Michalkiewicz, T.; Afolayan, A.J.; Wu, T.J.; Konduri, G.G. Attenuation of endoplasmic reticulum stress by caffeine ameliorates hyperoxia-induced lung injury. Am. J. Physiol. Lung Cell Mol. Physiol. 2017, 312, L586–L598. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pritchard, K.A., Jr.; Jing, X.; Teng, M.; Wells, C.; Jia, S.; Afolayan, A.J.; Jarzembowski, J.; Day, B.W.; Naylor, S.; Hessner, M.J.; et al. Role of endoplasmic reticulum stress in impaired neonatal lung growth and bronchopulmonary dysplasia. PLoS ONE 2022, 17, e0269564. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Teng, R.J.; Jing, X.; Martin, D.P.; Hogg, N.; Haefke, A.; Konduri, G.G.; Day, B.W.; Naylor, S.; Pritchard, K.A., Jr. N-acetyl-lysyltyrosylcysteine amide, a novel systems pharmacology agent, reduces bronchopulmonary dysplasia in hyperoxic neonatal rat pups. Free Radic. Biol. Med. 2021, 166, 73–89. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Choo-Wing, R.; Syed, M.A.; Harijith, A.; Bowen, B.; Pryhuber, G.; Janér, C.; Andersson, S.; Homer, R.J.; Bhandari, V. Hyperoxia and interferon-γ-induced injury in developing lungs occur via cyclooxygenase-2 and the endoplasmic reticulum stress-dependent pathway. Am. J. Respir. Cell Mol. Biol. 2013, 48, 749–757. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhao, H.; Dennery, P.A.; Yao, H. Metabolic reprogramming in the pathogenesis of chronic lung diseases, including BPD, COPD, and pulmonary fibrosis. Am. J. Physiol. Lung Cell Mol. Physiol. 2018, 314, L544–L554. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Breitling, J.; Aebi, M. N-linked protein glycosylation in the endoplasmic reticulum. Cold Spring Harb. Perspect. Biol. 2013, 5, a013359. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lin, J.H.; Walter, P.; Yen, T.S. Endoplasmic reticulum stress in disease pathogenesis. Annu. Rev. Pathol. 2008, 3, 399–425. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ferreira, I.G.; Pucci, M.; Venturi, G.; Malagolini, N.; Chiricolo, M.; Dall’Olio, F. Glycosylation as a Main Regulator of Growth and Death Factor Receptors Signaling. Int. J. Mol. Sci. 2018, 19, 580. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chandler, K.B.; Leon, D.R.; Meyer, R.D.; Rahimi, N.; Costello, C.E. Site-Specific N-Glycosylation of Endothelial Cell Receptor Tyrosine Kinase VEGFR-2. J. Proteome Res. 2017, 16, 677–688. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yetkin-Arik, B.; Vogels, I.M.C.; Nowak-Sliwinska, P.; Weiss, A.; Houtkooper, R.H.; Van Noorden, C.J.F.; Klaassen, I.; Schlingemann, R.O. The role of glycolysis and mitochondrial respiration in the formation and functioning of endothelial tip cells during angiogenesis. Sci. Rep. 2019, 9, 12608. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ciccia, A.; Elledge, S.J. The DNA damage response: Making it safe to play with knives. Mol. Cell 2010, 40, 179–204. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Childs, B.G.; Baker, D.J.; Kirkland, J.L.; Campisi, J.; van Deursen, J.M. Senescence and apoptosis: Dueling or complementary cell fates? EMBO Rep. 2014, 15, 1139–1153. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bourgeois, B.; Madl, T. Regulation of cellular senescence via the FOXO4-p53 axis. FEBS Lett. 2018, 592, 2083–2097. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sano, R.; Reed, J.C. ER stress-induced cell death mechanisms. Biochim. Biophys. Acta 2013, 1833, 3460–3470. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Morishima, N.; Nakanishi, K.; Takenouchi, H.; Shibata, T.; Yasuhiko, Y. An endoplasmic reticulum stress-specific caspase cascade in apoptosis. Cytochrome c-independent activation of caspase-9 by caspase-12. J. Biol. Chem. 2002, 277, 34287–34294. [Google Scholar] [CrossRef] [PubMed]
- Kirkland, J.L.; Tchkonia, T. Cellular Senescence: A Translational Perspective. eBioMedicine 2017, 21, 21–28. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lopes-Paciencia, S.; Saint-Germain, E.; Rowell, M.C.; Ruiz, A.F.; Kalegari, P.; Ferbeyre, G. The senescence-associated secretory phenotype and its regulation. Cytokine 2019, 117, 15–22. [Google Scholar] [CrossRef] [PubMed]
- McHugh, D.; Gil, J. Senescence and aging: Causes, consequences, and therapeutic avenues. J. Cell Biol. 2018, 217, 65–77. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yao, H.; Wallace, J.; Peterson, A.L.; Scaffa, A.; Rizal, S.; Hegarty, K.; Maeda, H.; Chang, J.L.; Oulhen, N.; Kreiling, J.A.; et al. Timing and cell specificity of senescence drives postnatal lung development and injury. Nat. Commun. 2023, 14, 273. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jing, X.; Jia, S.; Teng, M.; Day, B.W.; Afolayan, A.J.; Jarzembowski, J.A.; Lin, C.W.; Hessner, M.J.; Pritchard, K.A., Jr.; Naylor, S.; et al. Cellular Senescence Contributes to the Progression of Hyperoxic Bronchopulmonary Dysplasia. Am. J. Respir. Cell Mol. Biol. 2024, 70, 94–109. [Google Scholar] [CrossRef] [PubMed]
- Nussbaum, C.; Klinke, A.; Adam, M.; Baldus, S.; Sperandio, M. Myeloperoxidase: A leukocyte-derived protagonist of inflammation and cardiovascular disease. Antioxid. Redox Signal 2013, 18, 692–713. [Google Scholar] [CrossRef] [PubMed]
- Hoy, A.; Leininger-Muller, B.; Kutter, D.; Siest, G.; Visvikis, S. Growing significance of myeloperoxidase in non-infectious diseases. Clin. Chem. Lab. Med. 2002, 40, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Siraki, A.G. The many roles of myeloperoxidase: From inflammation and immunity to biomarkers, drug metabolism and drug discovery. Redox Biol. 2021, 46, 102109. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Arnhold, J. The Dual Role of Myeloperoxidase in Immune Response. Int. J. Mol. Sci. 2020, 21, 8057. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kettle, A.J.; Winterbourn, C.C. A kinetic analysis of the catalase activity of myeloperoxidase. Biochemistry 2001, 40, 10204–10212. [Google Scholar] [CrossRef] [PubMed]
- Lanza, F. Clinical manifestation of myeloperoxidase deficiency. J. Mol. Med. 1998, 76, 676–681. [Google Scholar] [CrossRef] [PubMed]
- Ostanin, D.V.; Barlow, S.; Shukla, D.; Grisham, M.B. NADPH oxidase but not myeloperoxidase protects lymphopenic mice from spontaneous infections. Biochem. Biophys. Res. Commun. 2007, 355, 801–806. [Google Scholar] [CrossRef] [PubMed]
- Torres-Cuevas, I.; Kuligowski, J.; Cárcel, M.; Cháfer-Pericás, C.; Asensi, M.; Solberg, R.; Cubells, E.; Nuñez, A.; Saugstad, O.D.; Vento, M.; et al. Protein-bound tyrosine oxidation, nitration and chlorination by-products assessed by ultraperformance liquid chromatography coupled to tandem mass spectrometry. Anal. Chim. Acta 2016, 913, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Kang, R.; Tang, D. The mechanism of HMGB1 secretion and release. Exp. Mol. Med. 2022, 54, 91–102. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yang, H.; Lundbäck, P.; Ottosson, L.; Erlandsson-Harris, H.; Venereau, E.; Bianchi, M.E.; Al-Abed, Y.; Andersson, U.; Tracey, K.J. Redox modifications of cysteine residues regulate the cytokine activity of HMGB1. Mol. Med. 2021, 27, 58. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhong, H.; Li, X.; Zhou, S.; Jiang, P.; Liu, X.; Ouyang, M.; Nie, Y.; Chen, X.; Zhang, L.; Liu, Y.; et al. Interplay between RAGE and TLR4 Regulates HMGB1-Induced Inflammation by Promoting Cell Surface Expression of RAGE and TLR4. J. Immunol. 2020, 205, 767–775. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, L.A.; Bowie, A.G. The family of five: TIR-domain-containing adaptors in Toll-like receptor signalling. Nat. Rev. Immunol. 2007, 7, 353–364. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Bianchi, M.E.; Coleman, T.R.; Tracey, K.J.; Al-Abed, Y. Exploring the biological functional mechanism of the HMGB1/TLR4/MD-2 complex by surface plasmon resonance. Mol. Med. 2018, 24, 21, Erratum in Mol. Med. 2018, 24, 31. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lee, E.J.; Park, J.H. Receptor for Advanced Glycation Endproducts (RAGE), Its Ligands, and Soluble RAGE: Potential Biomarkers for Diagnosis and Therapeutic Targets for Human Renal Diseases. Genom. Inform. 2013, 11, 224–229. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Watanabe, H.; Son, M. The Immune Tolerance Role of the HMGB1-RAGE Axis. Cells 2021, 10, 564. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Schmidt, A.M. Soluble RAGEs—Prospects for treating & tracking metabolic and inflammatory disease. Vasc. Pharmacol. 2015, 72, 1–8. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Barnett, S.D.; Buxton, I.L.O. The role of S-nitrosoglutathione reductase (GSNOR) in human disease and therapy. Crit. Rev. Biochem. Mol. Biol. 2017, 52, 340–354. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Anand, P.; Stamler, J.S. Enzymatic mechanisms regulating protein S-nitrosylation: Implications in health and disease. J. Mol. Med. 2012, 90, 233–244. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhao, Y.; Vanhoutte, P.M.; Leung, S.W. Vascular nitric oxide: Beyond eNOS. J. Pharmacol. Sci. 2015, 129, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Nisoli, E.; Carruba, M.O. Nitric oxide and mitochondrial biogenesis. J. Cell Sci. 2006, 119 Pt 14, 2855–2862. [Google Scholar] [CrossRef] [PubMed]
- Raffay, T.M.; Bonilla-Fernandez, K.; Jafri, A.; Sopi, R.B.; Smith, L.A.; Cui, F.; O’Reilly, M.; Zhang, R.; Hodges, C.A.; MacFarlane, P.M.; et al. Bronchopulmonary Dysplasia and Pulmonary Hypertension. The Role of Smooth Muscle adh5. Am. J. Respir. Cell Mol. Biol. 2021, 65, 70–80. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Green, L.S.; Chun, L.E.; Patton, A.K.; Sun, X.; Rosenthal, G.J.; Richards, J.P. Mechanism of inhibition for N6022, a first-in-class drug targeting S-nitrosoglutathione reductase. Biochemistry 2012, 51, 2157–2168. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Zhang, Y.; Wang, P.; Zhang, L.; Wang, T.; Chen, C. Activation of GSNOR transcription by NF-κB negatively regulates NGF-induced PC12 differentiation. Free Radic. Res. 2014, 48, 1011–1017. [Google Scholar] [CrossRef] [PubMed]
- Rarick, K.R.; Li, K.; Teng, R.J.; Jing, X.; Martin, D.P.; Xu, H.; Jones, D.W.; Hogg, N.; Hillery, C.A.; Garcia, G.; et al. Sterile inflammation induces vasculopathy and chronic lung injury in murine sickle cell disease. Free Radic. Biol. Med. 2024, 215, 112–126. [Google Scholar] [CrossRef] [PubMed]
- Bhattarai, K.R.; Riaz, T.A.; Kim, H.R.; Chae, H.J. The aftermath of the interplay between the endoplasmic reticulum stress response and redox signaling. Exp. Mol. Med. 2021, 53, 151–167. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Garg, A.D.; Kaczmarek, A.; Krysko, O.; Vandenabeele, P.; Krysko, D.V.; Agostinis, P. ER stress-induced inflammation: Does it aid or impede disease progression? Trends Mol. Med. 2012, 18, 589–598. [Google Scholar] [CrossRef] [PubMed]
- Ferrante, G.; Montante, C.; Notarbartolo, V.; Giuffrè, M. Antioxidants: Role the in prevention and treatment of bronchopulmonary dysplasia. Paediatr. Respir. Rev. 2022, 42, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Galijasevic, S. The development of myeloperoxidase inhibitors. Bioorg. Med. Chem. Lett. 2019, 29, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Ward, J.; Spath, S.N.; Pabst, B.; Carpino, P.A.; Ruggeri, R.B.; Xing, G.; Speers, A.E.; Cravatt, B.F.; Ahn, K. Mechanistic characterization of a 2-thioxanthine myeloperoxidase inhibitor and selectivity assessment utilizing click chemistry--activity-based protein profiling. Biochemistry 2013, 52, 9187–9201. [Google Scholar] [CrossRef] [PubMed]
- Soubhye, J.; Prévost, M.; Van Antwerpen, P.; Zouaoui Boudjeltia, K.; Rousseau, A.; Furtmüller, P.G.; Obinger, C.; Vanhaeverbeek, M.; Ducobu, J.; Néve, J.; et al. Structure-based design, synthesis, and pharmacological evaluation of 3-(aminoalkyl)-5-fluoroindoles as myeloperoxidase inhibitors. J. Med. Chem. 2010, 53, 8747–8759. [Google Scholar] [CrossRef] [PubMed]
- Loke, W.M.; Proudfoot, J.M.; McKinley, A.J.; Needs, P.W.; Kroon, P.A.; Hodgson, J.M.; Croft, K.D. Quercetin and its in vivo metabolites inhibit neutrophil-mediated low-density lipoprotein oxidation. J. Agric. Food Chem. 2008, 56, 3609–3615. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Xu, Y.; Joseph, J.; Kalyanaraman, B. Intramolecular electron transfer between tyrosyl radical and cysteine residue inhibits tyrosine nitration and induces thiyl radical formation in model peptides treated with myeloperoxidase, H2O2, and NO2−: EPR SPIN trapping studies. J. Biol. Chem. 2005, 280, 40684–40698. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Jing, X.; Shi, Y.; Xu, H.; Du, J.; Guan, T.; Weihrauch, D.; Jones, D.W.; Wang, W.; Gourlay, D.; et al. N-acetyl lysyltyrosylcysteine amide inhibits myeloperoxidase, a novel tripeptide inhibitor. J. Lipid Res. 2013, 54, 3016–3029. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Weihrauch, D.; Martin, D.P.; Jones, D.; Krolikowski, J.; Struve, J.; Naylor, S.; Pritchard, K.A., Jr. Inhibition of myeloperoxidase increases revascularization and improves blood flow in a diabetic mouse model of hindlimb ischaemia. Diab. Vasc. Dis. Res. 2020, 17, 1479164120907971. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, H.; Xu, H.; Weihrauch, D.; Jones, D.W.; Jing, X.; Shi, Y.; Gourlay, D.; Oldham, K.T.; Hillery, C.A.; Pritchard, K.A., Jr. Inhibition of myeloperoxidase decreases vascular oxidative stress and increases vasodilatation in sickle cell disease mice. J. Lipid Res. 2013, 54, 3009–3015. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, H.; Ray, A.; Miller, N.M.; Hartwig, D.; Pritchard, K.A.; Dittel, B.N. Inhibition of myeloperoxidase at the peak of experimental autoimmune encephalomyelitis restores blood-brain barrier integrity and ameliorates disease severity. J. Neurochem. 2016, 136, 826–836. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Neu, S.D.; Strzepa, A.; Martin, D.; Sorci-Thomas, M.G.; Pritchard, K.A., Jr.; Dittel, B.N. Myeloperoxidase Inhibition Ameliorates Plaque Psoriasis in Mice. Antioxidants 2021, 10, 1338. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yu, G.; Liang, Y.; Huang, Z.; Jones, D.W.; Pritchard, K.A., Jr.; Zhang, H. Erratum to: Inhibition of myeloperoxidase oxidant production by N-acetyl lysyltyrosylcysteine amide reduces brain damage in a murine model of stroke. J. Neuroinflammation 2016, 13, 166, Erratum in J. Neuroinflammation 2016, 13, 119. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Inghardt, T.; Antonsson, T.; Ericsson, C.; Hovdal, D.; Johannesson, P.; Johansson, C.; Jurva, U.; Kajanus, J.; Kull, B.; Michaëlsson, E.; et al. Discovery of AZD4831, a Mechanism-Based Irreversible Inhibitor of Myeloperoxidase, As a Potential Treatment for Heart Failure with Preserved Ejection Fraction. J. Med. Chem. 2022, 65, 11485–11496, Erratum in J. Med. Chem. 2022, 65, 13482. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pritchard, K.A., Jr.; Martin, D.P.; Naylor, S. Systems Pharmacology: When Multi-Targeting Is Advantageous. Drug Discover World. 28 December 2018. Available online: https://www.ddw-online.com/systems-pharmacology-when-multi-targeting-is-advantageous-503-201812/ (accessed on 18 July 2024).






| Prenatal | At Birth | Postnatal |
|---|---|---|
| Intrauterine growth restriction [45,46] | Gestational age and birth weight [1,47] | Respiratory support [48,49,50,51,52] |
| Maternal smoking [53] | Gender [54,55] | Infection [56,57] |
| Lack of antenatal corticosteroid [58,59] | Level of management [60] | Patent ductus arteriosus [61,62,63,64,65] |
| Chorioamnionitis [66,67,68] | Gastroesophageal reflux [69,70] | |
| Genetics [71,72,73,74] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, T.-J.; Jing, X.; Teng, M.; Pritchard, K.A., Jr.; Day, B.W.; Naylor, S.; Teng, R.-J. Role of Myeloperoxidase, Oxidative Stress, and Inflammation in Bronchopulmonary Dysplasia. Antioxidants 2024, 13, 889. https://doi.org/10.3390/antiox13080889
Wu T-J, Jing X, Teng M, Pritchard KA Jr., Day BW, Naylor S, Teng R-J. Role of Myeloperoxidase, Oxidative Stress, and Inflammation in Bronchopulmonary Dysplasia. Antioxidants. 2024; 13(8):889. https://doi.org/10.3390/antiox13080889
Chicago/Turabian StyleWu, Tzong-Jin, Xigang Jing, Michelle Teng, Kirkwood A. Pritchard, Jr., Billy W. Day, Stephen Naylor, and Ru-Jeng Teng. 2024. "Role of Myeloperoxidase, Oxidative Stress, and Inflammation in Bronchopulmonary Dysplasia" Antioxidants 13, no. 8: 889. https://doi.org/10.3390/antiox13080889
APA StyleWu, T.-J., Jing, X., Teng, M., Pritchard, K. A., Jr., Day, B. W., Naylor, S., & Teng, R.-J. (2024). Role of Myeloperoxidase, Oxidative Stress, and Inflammation in Bronchopulmonary Dysplasia. Antioxidants, 13(8), 889. https://doi.org/10.3390/antiox13080889







