Synergistic Antioxidant Effects of Cysteine Derivative and Sm-Cluster for Food Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Instruments
2.3. Preparation of Sm-Cluster
2.4. Scanning Electron Microscope (SEM) of Synthesized Sm-Cluster
2.5. Ultraviolet–Visible (UV–Vis) Diffuse Reflectance Spectroscopy of Sm-Cluster
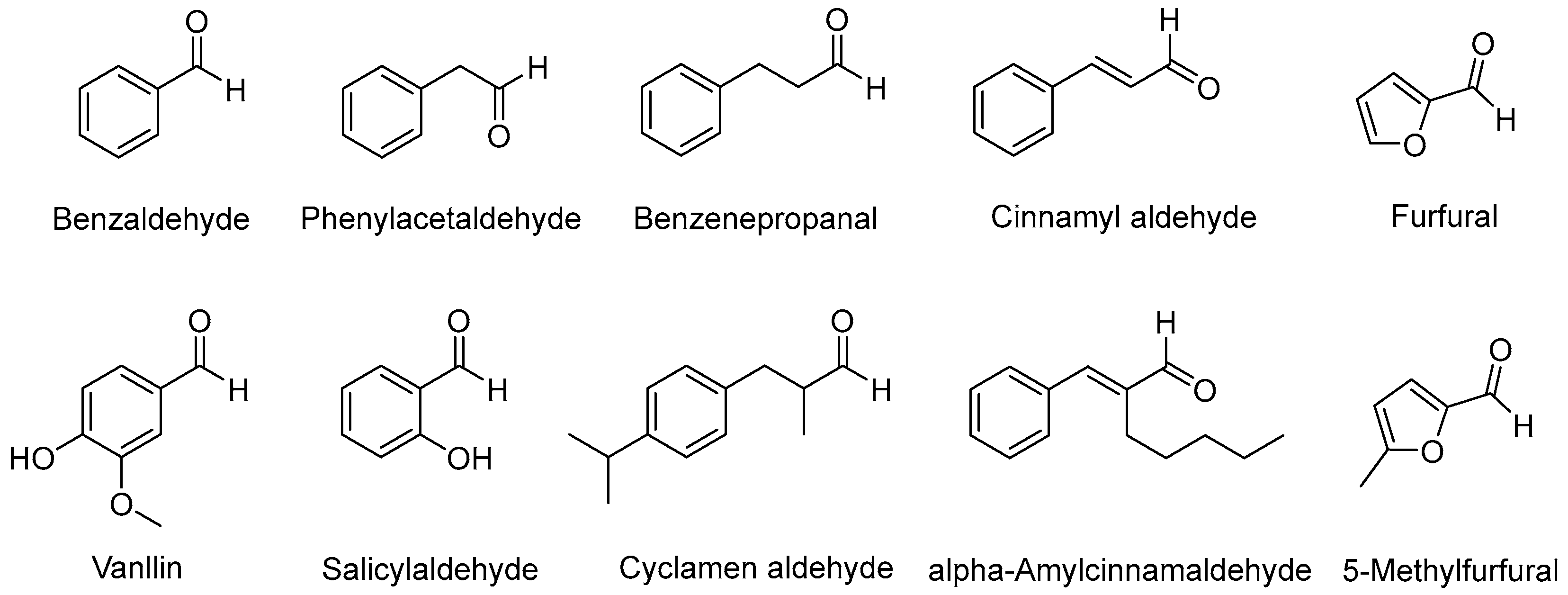
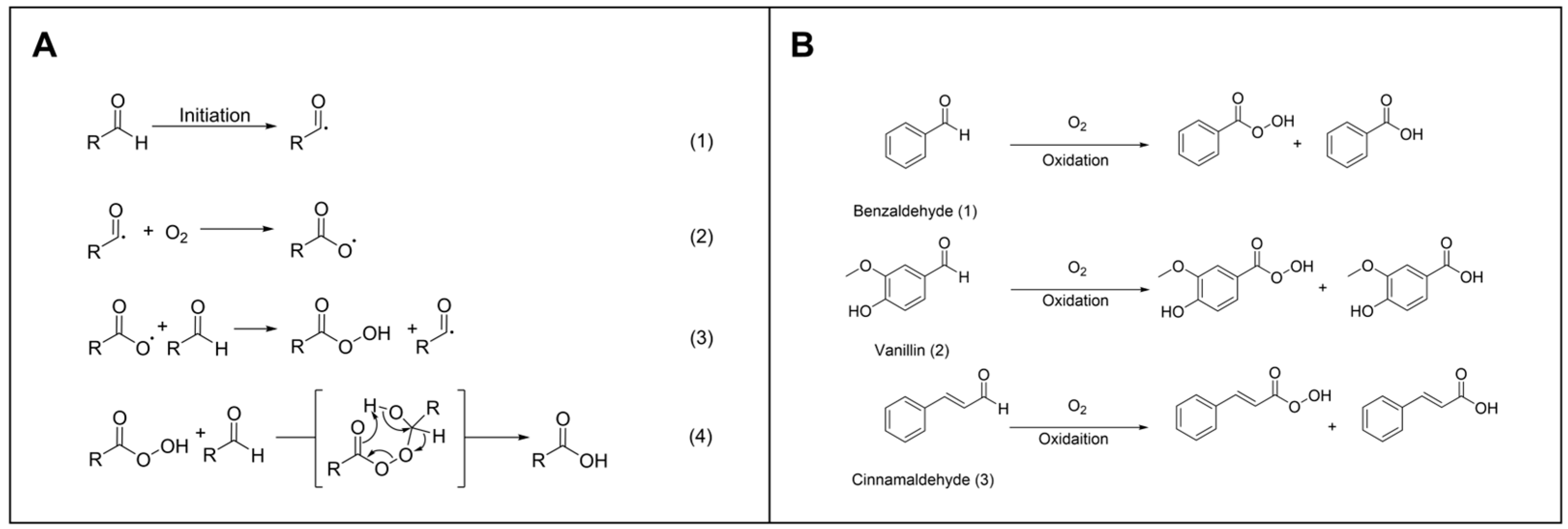
2.6. Evaluation of the Antioxidation Effect on Aromatic Aldehydes
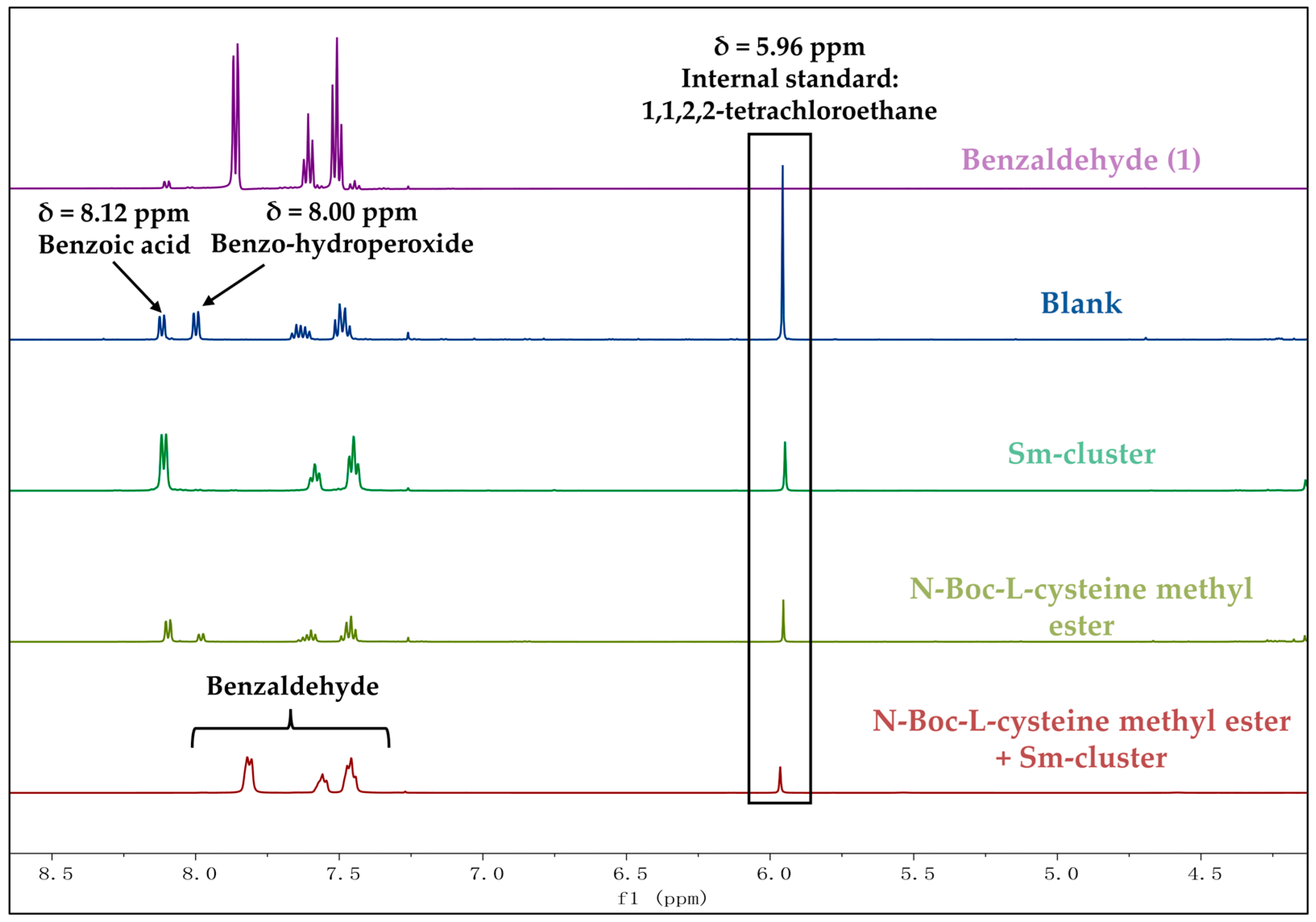
2.6.1. 1H NMR Acquisition Method and Parameters
2.6.2. Calculation of the Recovery Rate by Internal Standard Using 1H NMR
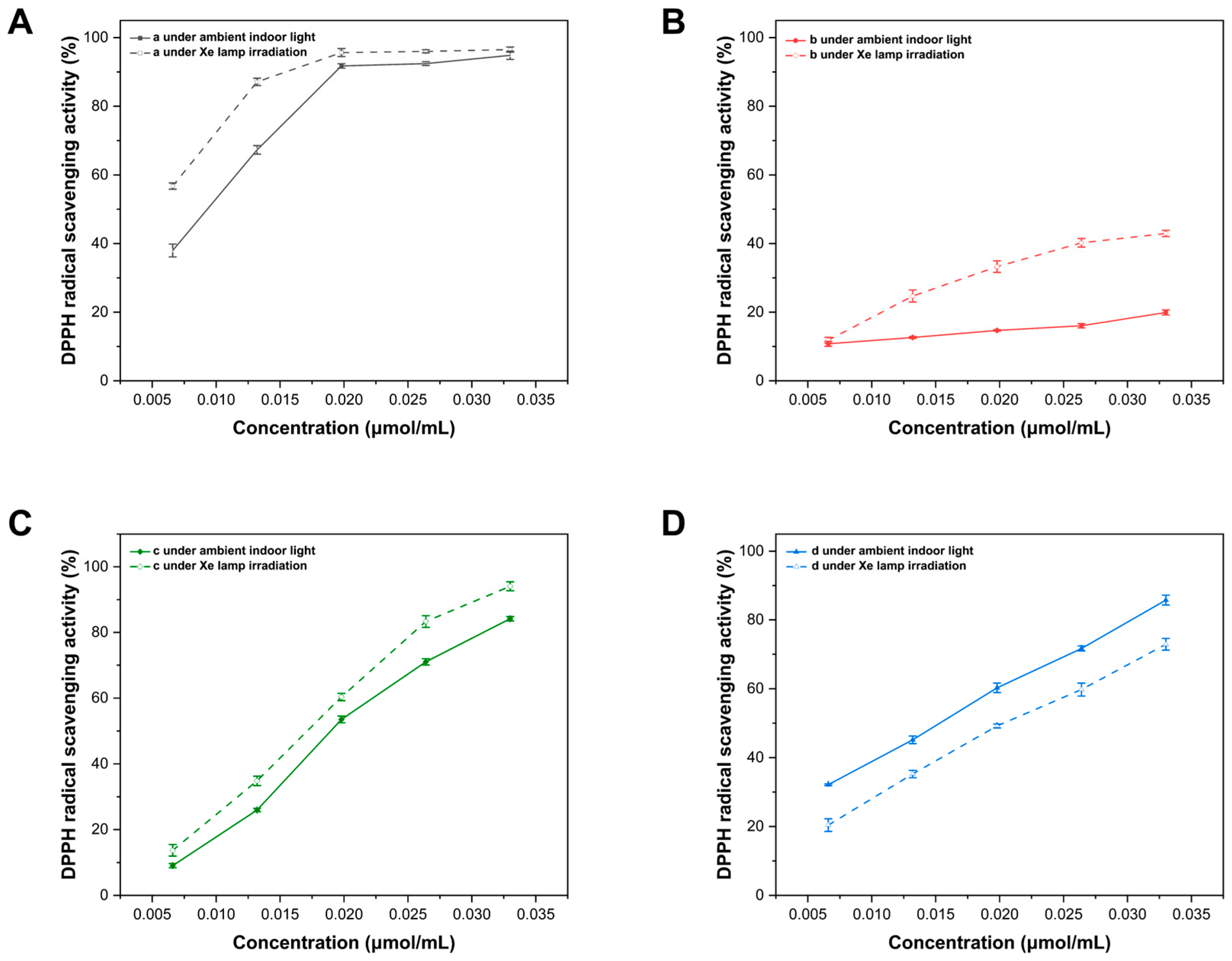
2.7. Evaluation of DPPH Free Radical Scavenging Activity
2.7.1. General Method
2.7.2. Statistical Analysis
3. Results
3.1. Synthesis and Structural Characterization of Sm-Cluster
3.2. Evaluation of the Antioxidation Efficiency of Sm-Cluster/Cysteine Derivative
3.3. Determination of Radical Scavenging Ability of Sm-Cluster/Cysteine Derivative
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zeb, A. Concept, Mechanism, and Applications of Phenolic Antioxidants in Foods. J. Food Biochem. 2020, 44, e13394. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Wang, J.; Dong, M.; Zhang, H.; Li, L.; Wang, L. Food Spoilage, Bioactive Food Fresh-Keeping Films and Functional Edible Coatings: Research Status, Existing Problems and Development Trend. Trends Food Sci. Technol. 2022, 119, 122–132. [Google Scholar] [CrossRef]
- Bayram, I.; Decker, E.A. Underlying Mechanisms of Synergistic Antioxidant Interactions during Lipid Oxidation. Trends Food Sci. Technol. 2023, 133, 219–230. [Google Scholar] [CrossRef]
- Demirkol, O.; Adams, C.; Ercal, N. Biologically Important Thiols in Various Vegetables and Fruits. J. Agric. Food Chem. 2004, 52, 8151–8154. [Google Scholar] [CrossRef] [PubMed]
- Neal, R.; Matthews, R.H.; Lutz, P.; Ercal, N. Antioxidant Role of N-Acetyl Cysteine Isomers Following High Dose Irradiation. Free Radic. Biol. Med. 2003, 34, 689–695. [Google Scholar] [CrossRef] [PubMed]
- Meister, A.; Anderson, M.E. Glutathione. Annu. Rev. Biochem. 1983, 52, 711–760. [Google Scholar] [CrossRef] [PubMed]
- Yehye, W.A.; Rahman, N.A.; Ariffin, A.; Abd Hamid, S.B.; Alhadi, A.A.; Kadir, F.A.; Yaeghoobi, M. Understanding the Chemistry behind the Antioxidant Activities of Butylated Hydroxytoluene (BHT): A Review. Eur. J. Med. Chem. 2015, 101, 295–312. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Liu, A.; Hu, S.; Ares, I.; Martínez-Larrañaga, M.-R.; Wang, X.; Martínez, M.; Anadón, A.; Martínez, M.-A. Synthetic Phenolic Antioxidants: Metabolism, Hazards and Mechanism of Action. Food Chem. 2021, 353, 129488. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, S.N.; Pirsa, S.; Farzi, J. Biodegradable Nano Composite Film Based on Modified Starch-Albumin/MgO; Antibacterial, Antioxidant and Structural Properties. Polym. Test 2021, 97, 107182. [Google Scholar] [CrossRef]
- Nunes, M.R.; de Souza Maguerroski Castilho, M.; de Lima Veeck, A.P.; da Rosa, C.G.; Noronha, C.M.; Maciel, M.V.O.B.; Barreto, P.M. Antioxidant and Antimicrobial Methylcellulose Films Containing Lippia Alba Extract and Silver Nanoparticles. Carbohydr. Polym. 2018, 192, 37–43. [Google Scholar] [CrossRef]
- Clark, A.; Zhu, A.; Sun, K.; Petty, H.R. Cerium Oxide and Platinum Nanoparticles Protect Cells from Oxidant-Mediated Apoptosis. J. Nanopart. Res. 2011, 13, 5547–5555. [Google Scholar] [CrossRef]
- Singh, K.; Chopra, D.S.; Singh, D.; Singh, N. Optimization and Ecofriendly Synthesis of Iron Oxide Nanoparticles as Potential Antioxidant. Arab. J. Chem. 2020, 13, 9034–9046. [Google Scholar] [CrossRef]
- Tee, J.K.; Ong, C.N.; Bay, B.H.; Ho, H.K.; Leong, D.T. Oxidative Stress by Inorganic Nanoparticles. WIREs Nanomed Nanobi 2016, 8, 414–438. [Google Scholar] [CrossRef]
- Gil, D.; Rodriguez, J.; Ward, B.; Vertegel, A.; Ivanov, V.; Reukov, V. Antioxidant Activity of SOD and Catalase Conjugated with Nanocrystalline Ceria. Bioengineering 2017, 4, 18. [Google Scholar] [CrossRef]
- Vieira, I.R.S.; de Carvalho, A.P.A.d.; Conte-Junior, C.A. Recent Advances in Biobased and Biodegradable Polymer Nanocomposites, Nanoparticles, and Natural Antioxidants for Antibacterial and Antioxidant Food Packaging Applications. Compr. Rev. Food. Sci. Food Saf. 2022, 21, 3673–3716. [Google Scholar] [CrossRef]
- Bi, F.; Zhang, X.; Liu, J.; Yong, H.; Gao, L.; Liu, J. Development of Antioxidant and Antimicrobial Packaging Films Based on Chitosan, D-α-Tocopheryl Polyethylene Glycol 1000 Succinate and Silicon Dioxide Nanoparticles. Food Packag. Shelf Life 2020, 24, 100503. [Google Scholar] [CrossRef]
- Bumbudsanpharoke, N.; Ko, S. The Green Fabrication, Characterization and Evaluation of Catalytic Antioxidation of Gold Nanoparticle-Lignocellulose Composite Papers for Active Packaging. Int. J. Biol. Macromol. 2018, 107, 1782–1791. [Google Scholar] [CrossRef]
- Pachaiappan, R.; Rajendran, S.; Show, P.L.; Manavalan, K.; Naushad, M. Metal/Metal Oxide Nanocomposites for Bactericidal Effect: A Review. Chemosphere 2021, 272, 128607. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, N.; Hsu, H.-S.; Liang, S.-T.; Roldan, M.J.M.; Lee, J.-S.; Ger, T.-R.; Hsiao, C.-D. An Updated Review of Toxicity Effect of the Rare Earth Elements (REEs) on Aquatic Organisms. Animals 2020, 10, 1663. [Google Scholar] [CrossRef]
- Chen, J.; Guo, Y.; Zhang, X.; Liu, J.; Gong, P.; Su, Z.; Fan, L.; Li, G. Emerging Nanoparticles in Food: Sources, Application, and Safety. J. Agric. Food Chem. 2023, 71, 3564–3582. [Google Scholar] [CrossRef]
- Abbasi, R.; Shineh, G.; Mobaraki, M.; Doughty, S.; Tayebi, L. Structural Parameters of Nanoparticles Affecting Their Toxicity for Biomedical Applications: A Review. J. Nanopart. Res. 2023, 25, 43. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, S.; Wang, B.; Dutta, P. Nanoparticle Processing: Understanding and Controlling Aggregation. Adv. Colloid Interface Sci. 2020, 279, 102162. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Chen, L.; Qin, Z.; Zhao, B.; Ni, K.; Li, H.; Li, J.; Duan, H.; Ren, F.; An, J. Samarium-Oxo/Hydroxy Cluster: A Solar Photocatalyst for Chemoselective Aerobic Oxidation of Thiols for Disulfide Synthesis. J. Org. Chem. 2024, 89, 8357–8362. [Google Scholar] [CrossRef] [PubMed]
- Arias-Pérez, I.; Sáenz-Navajas, M.P.; de-la-Fuente-Blanco, A.; Ferreira, V.; Escudero, A. Insights on the Role of Acetaldehyde and Other Aldehydes in the Odour and Tactile Nasal Perception of Red Wine. Food Chem. 2021, 361, 130081. [Google Scholar] [CrossRef] [PubMed]
- Alcolado, C.I.; Garcia-Rio, L.; Mejuto, J.C.; Moreno, I.; Poblete, F.J.; Tejeda, J. Oxidation of Aldehydes Used as Food Additives by Peroxynitrite. Antioxidants 2023, 12, 743. [Google Scholar] [CrossRef] [PubMed]
- Baliyan, S.; Mukherjee, R.; Priyadarshini, A.; Vibhuti, A.; Gupta, A.; Pandey, R.P.; Chang, C.-M. Determination of Antioxidants by DPPH Radical Scavenging Activity and Quantitative Phytochemical Analysis of Ficus Religiosa. Molecules 2022, 27, 1326. [Google Scholar] [CrossRef]
- Sun, G.; Lv, X.; Zhang, Y.; Lei, M.; Hu, L. Palladium-Catalyzed Formylation of Aryl Iodides with HCOOH as CO Source. Org. Lett. 2017, 19, 4235–4238. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Pu, W.; Zhang, G.; Wang, C. Synthesis of Salicylaldehydes from Phenols via Copper-Mediated Duff Reaction. Res. Chem. Intermed. 2015, 41, 8147–8158. [Google Scholar] [CrossRef]
- Sultane, P.R.; Bielawski, C.W. Burgess Reagent Facilitated Alcohol Oxidations in DMSO. J. Org. Chem. 2017, 82, 1046–1052. [Google Scholar] [CrossRef]
- Yin, H.; Xu, L.; Porter, N.A. Free Radical Lipid Peroxidation: Mechanisms and Analysis. Chem. Rev. 2011, 111, 5944–5972. [Google Scholar] [CrossRef]


| Groups | Minimal Light Exposure | Xenon Lamp Irradiation |
|---|---|---|
| a | 0.897 ** | 0.820 ** |
| b | 0.973 ** | 0.968 ** |
| c | 0.993 ** | 0.992 ** |
| d | 0.998 ** | 0.995 ** |
| Light Condition | Sample | IC50 (1 × 10−3 μmol/mL) |
|---|---|---|
| ambient indoor light | a | 8.49 ± 0.16 b |
| b | 1049 ± 43 a | |
| c | 18.48 ± 0.26 b | |
| d | 14.4 ± 2.4 b | |
| Xe lamp irradiation | a | 5.70 ± 0.17 d |
| b | 40.0 ± 1.2 a | |
| c | 15.35 ± 0.46 c | |
| d | 18.96 ± 0.37 b | |
| sample | ** | |
| light condition | ** | |
| sample × light condition | ** | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, L.; Wang, L.; Ma, L.; Wang, C.; Qin, X.; Wang, M.; Zhang, X.; Yang, R.; Fang, B.; An, J. Synergistic Antioxidant Effects of Cysteine Derivative and Sm-Cluster for Food Applications. Antioxidants 2024, 13, 910. https://doi.org/10.3390/antiox13080910
Chen L, Wang L, Ma L, Wang C, Qin X, Wang M, Zhang X, Yang R, Fang B, An J. Synergistic Antioxidant Effects of Cysteine Derivative and Sm-Cluster for Food Applications. Antioxidants. 2024; 13(8):910. https://doi.org/10.3390/antiox13080910
Chicago/Turabian StyleChen, Lingxia, Lijun Wang, Lifu Ma, Chao Wang, Xinshu Qin, Minlong Wang, Xiaohe Zhang, Ruoyan Yang, Bing Fang, and Jie An. 2024. "Synergistic Antioxidant Effects of Cysteine Derivative and Sm-Cluster for Food Applications" Antioxidants 13, no. 8: 910. https://doi.org/10.3390/antiox13080910
APA StyleChen, L., Wang, L., Ma, L., Wang, C., Qin, X., Wang, M., Zhang, X., Yang, R., Fang, B., & An, J. (2024). Synergistic Antioxidant Effects of Cysteine Derivative and Sm-Cluster for Food Applications. Antioxidants, 13(8), 910. https://doi.org/10.3390/antiox13080910






