Exploring Agro-Industrial By-Products: Phenolic Content, Antioxidant Capacity, and Phytochemical Profiling via FI-ESI-FTICR-MS Untargeted Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Agro-Industrial By-Product Materials, Reagents, and Solvents
2.2. Extract Preparation
2.3. Total Phenolic Content (TPC) and Total Flavonoid Content (TFC) Determinations
2.4. Antioxidant Assays
2.5. Untargeted Phytochemical Profiling by FI-ESI-FTICR-MS
2.6. Statistical Analyses
3. Results and Discussion
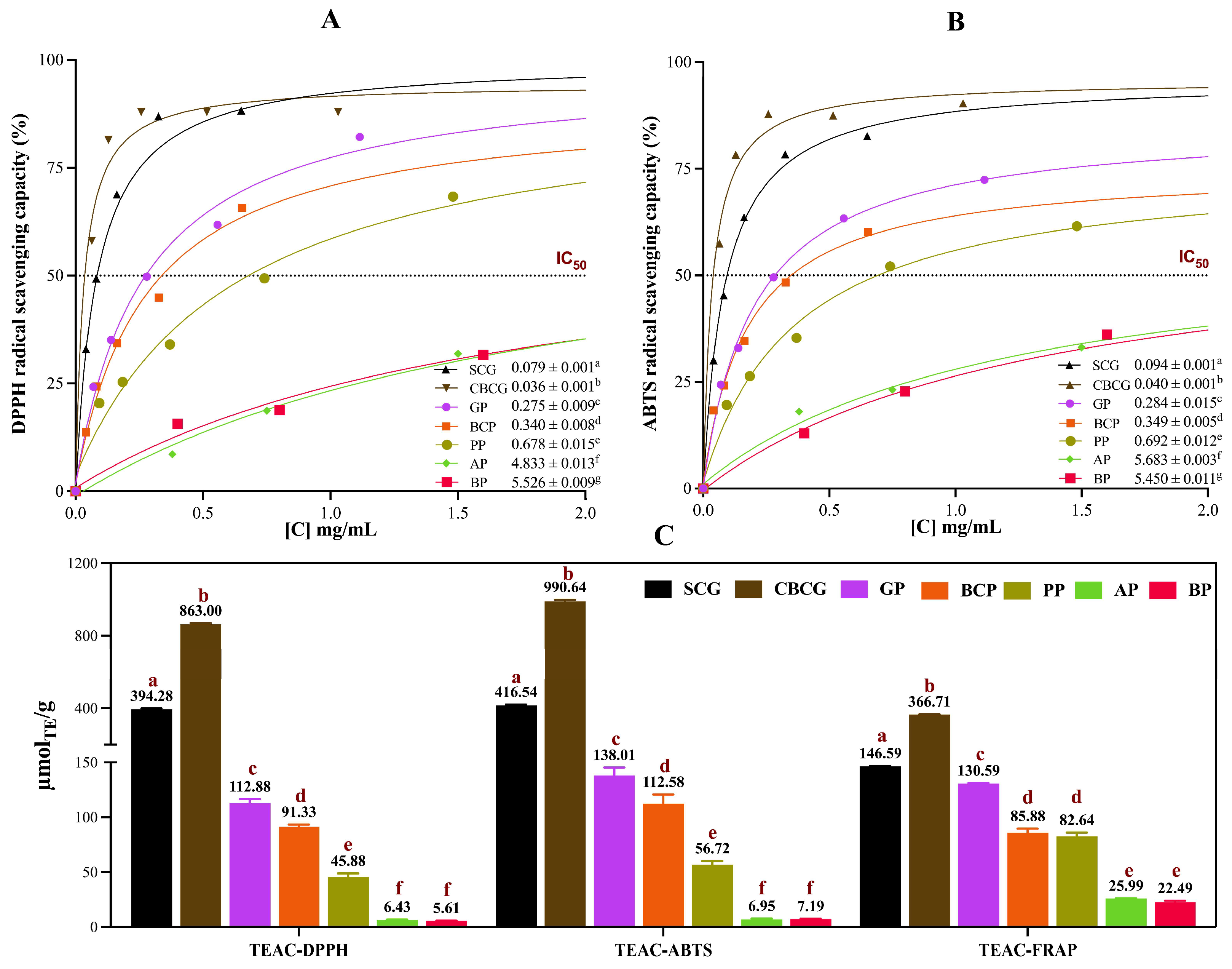
3.1. Total Phenolic Content, Total Flavonoid Content, and Antioxidant Capacity
3.2. Untargeted Phytochemical Profiling by FI-ESI-FTICR-MS
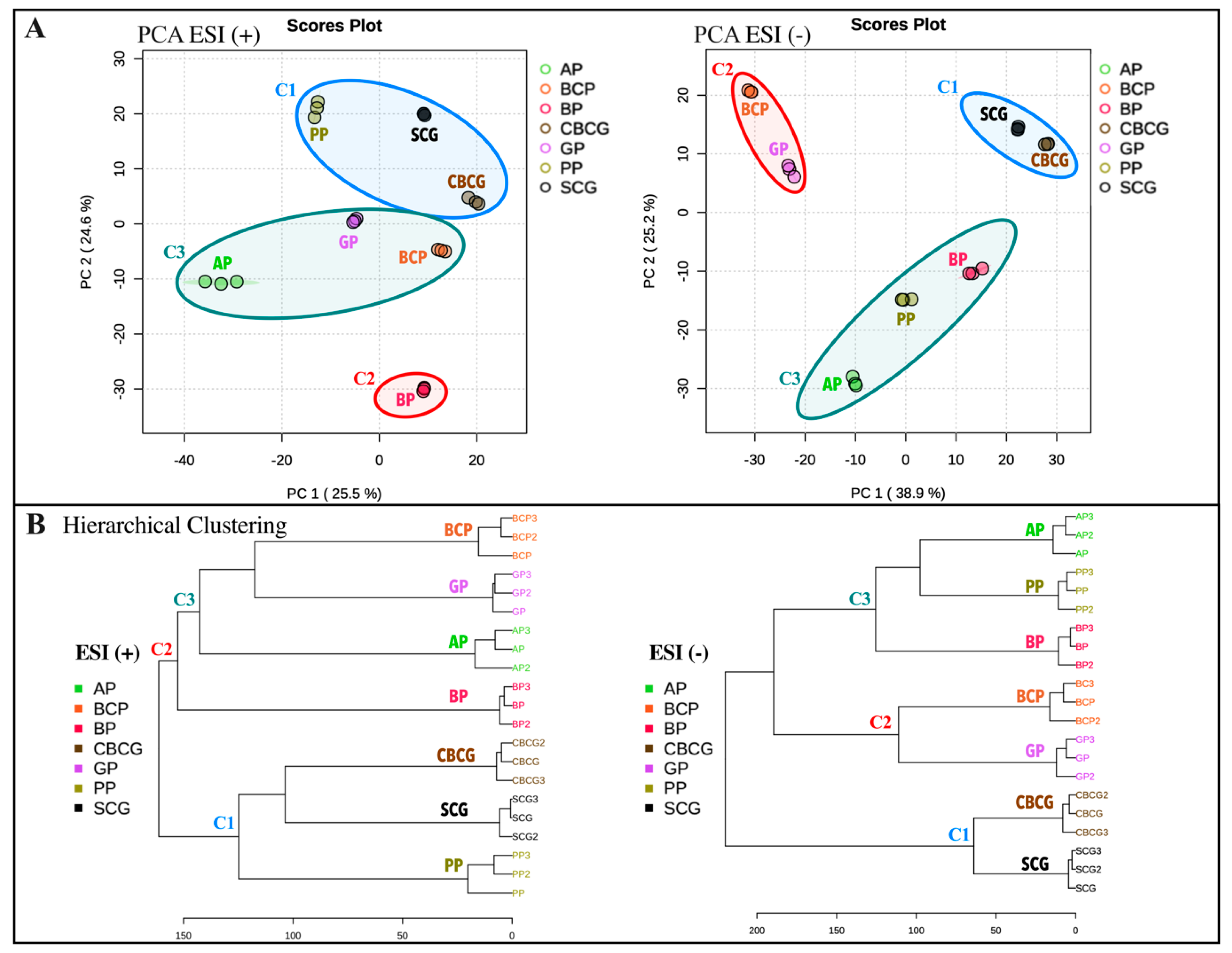
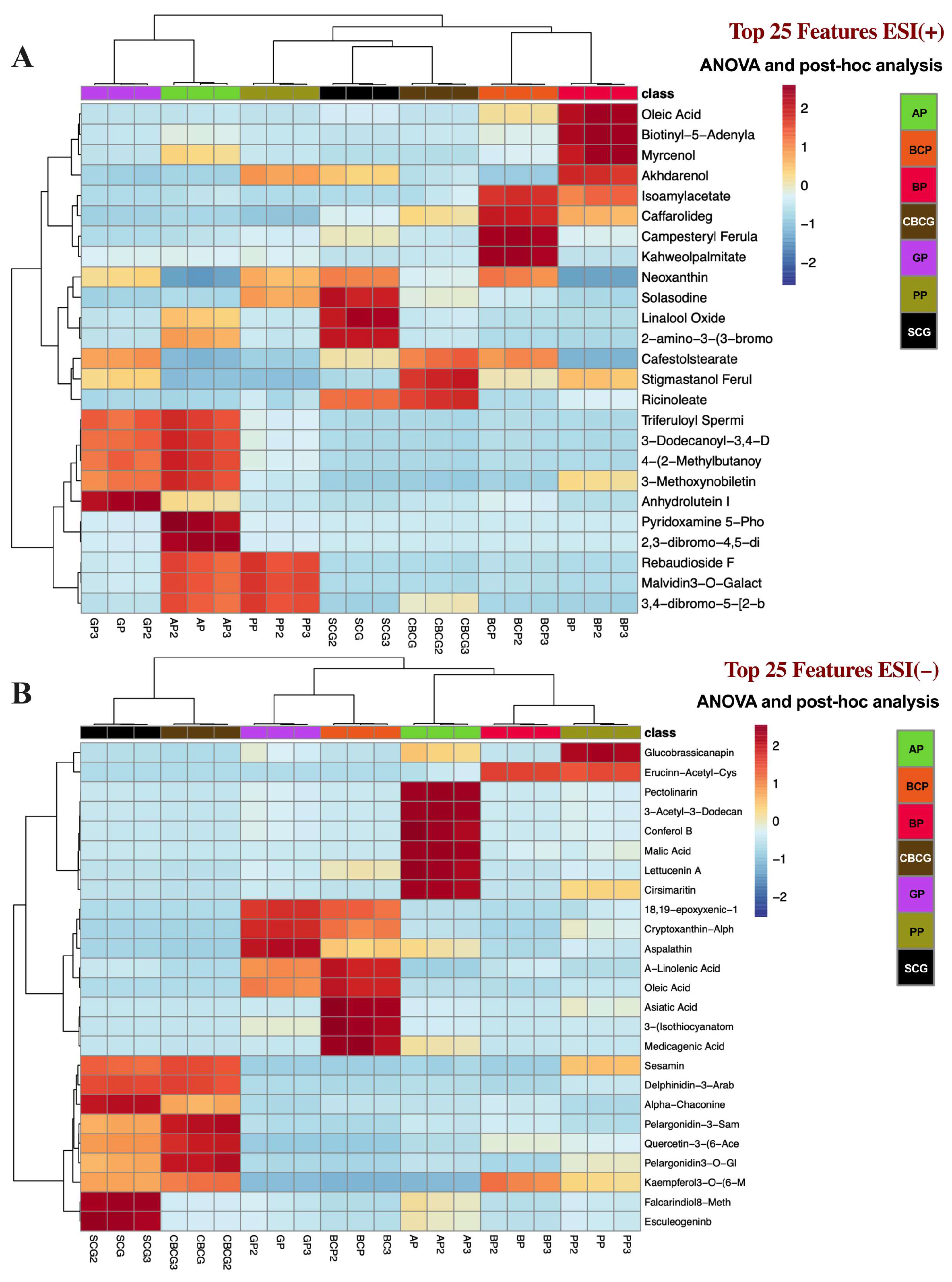
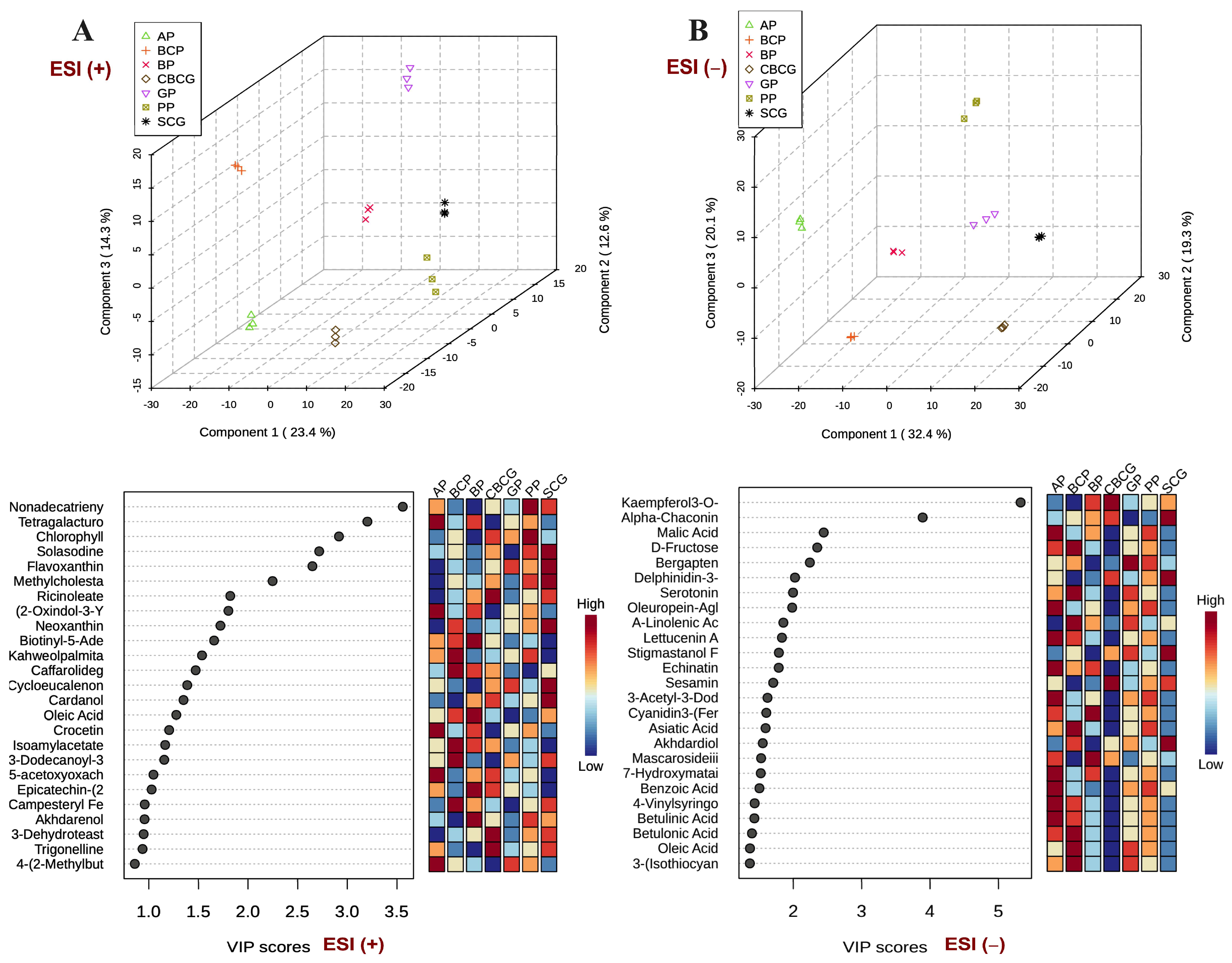
3.3. Univariate and Multivariate Analyses of Phytochemical Profiles in Agro-Industrial Sub-Products
3.4. Bioactive Properties and Phytochemical Content Correlation Matrix
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Altemimi, A.; Lakhssassi, N.; Baharlouei, A.; Watson, D.G.; Lightfoot, D.A. Phytochemicals: Extraction, Isolation, and Identification of Bioactive Compounds from Plant Extracts. Plants 2017, 6, 42. [Google Scholar] [CrossRef] [PubMed]
- Alara, O.R.; Abdurahman, N.H.; Ukaegbu, C.I. Extraction of Phenolic Compounds: A Review. Curr. Res. Food Sci. 2021, 4, 200–214. [Google Scholar] [CrossRef]
- Maia, M.; Ferreira, A.E.N.; Laureano, G.; Marques, A.P.; Torres, V.M.; Silva, A.B.; Matos, A.R.; Cordeiro, C.; Figueiredo, A.; Silva, M.S. Vitis Vinifera ‘Pinot Noir’ Leaves as a Source of Bioactive Nutraceutical Compounds. Food Funct. 2019, 10, 3822–3827. [Google Scholar] [CrossRef] [PubMed]
- Yust, B.G.; Rao, N.Z.; Schwarzmann, E.T.; Peoples, M.H. Quantification of Spent Coffee Ground Extracts by Roast and Brew Method, and Their Utility in a Green Synthesis of Gold and Silver Nanoparticles. Molecules 2022, 27, 5124. [Google Scholar] [CrossRef] [PubMed]
- Meena, L.; Sengar, A.S.; Neog, R.; Sunil, C.K. Pineapple Processing Waste (PPW): Bioactive Compounds, Their Extraction, and Utilisation: A Review. J. Food Sci. Technol. 2022, 59, 4152–4164. [Google Scholar] [CrossRef] [PubMed]
- Pollini, L.; Cossignani, L.; Juan, C.; Mañes, J. Extraction of Phenolic Compounds from Fresh Apple Pomace by Different Non-Conventional Techniques. Molecules 2021, 26, 4272. [Google Scholar] [CrossRef] [PubMed]
- Majerska, J.; Michalska, A.; Figiel, A. A Review of New Directions in Managing Fruit and Vegetable Processing By-Products. Trends Food Sci. Technol. 2019, 88, 207–219. [Google Scholar] [CrossRef]
- Blando, F.; Marchello, S.; Maiorano, G.; Durante, M.; Signore, A.; Laus, M.N.; Soccio, M.; Mita, G. Bioactive Compounds and Antioxidant Capacity in Anthocyanin-Rich Carrots: A Comparison between the Black Carrot and the Apulian Landrace “Polignano” Carrot. Plants 2021, 10, 564. [Google Scholar] [CrossRef] [PubMed]
- Suzme, S.; Boyacioglu, D.; Toydemir, G.; Capanoglu, E. Effect of Industrial Juice Concentrate Processing on Phenolic Profile and Antioxidant Capacity of Black Carrots. Int. J. Food Sci. Technol. 2014, 49, 819–829. [Google Scholar] [CrossRef]
- Carreón-Delgado, D.F.; Hernández-Montesinos, I.Y.; Rivera-Hernández, K.N.; del Sugeyrol Villa-Ramírez, M.; Ochoa-Velasco, C.E.; Ramírez-López, C. Evaluation of Pretreatments and Extraction Conditions on the Antifungal and Antioxidant Effects of Garlic (Allium Sativum) Peel Extracts. Plants 2023, 12, 217. [Google Scholar] [CrossRef]
- Hernández-Montesinos, I.Y.; Carreón-Delgado, D.F.; Ocaranza-Sánchez, E.; Ochoa-Velasco, C.E.; Suárez-Jacobo, Á.; Ramírez-López, C. Garlic (Allium sativum) Peel Extracts and Their Potential as Antioxidant and Antimicrobial Agents for Food Applications: Influence of Pretreatment and Extraction Solvent. Int. J. Food Sci. Technol. 2023, 58, 6794–6805. [Google Scholar] [CrossRef]
- Vijaya Kumar Reddy, C.; Sreeramulu, D.; Raghunath, M. Antioxidant Activity of Fresh and Dry Fruits Commonly Consumed in India. Food Res. Int. 2010, 43, 285–288. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Patiny, L.; Borel, A. ChemCalc: A Building Block for Tomorrow’s Chemical Infrastructure. J. Chem. Inf. Model. 2013, 53, 1223–1228. [Google Scholar] [CrossRef] [PubMed]
- Andrade, C.; Perestrelo, R.; Câmara, J.S. Bioactive Compounds and Antioxidant Activity from Spent Coffee Grounds as a Powerful Approach for Its Valorization. Molecules 2022, 27, 7504. [Google Scholar] [CrossRef]
- Alves, R.C.; Costa, A.S.G.; Jerez, M.; Casal, S.; Sineiro, J.; Núñez, M.J.; Oliveira, B. Antiradical Activity, Phenolics Profile, and Hydroxymethylfurfural in Espresso Coffee: Influence of Technological Factors. J. Agric. Food Chem. 2010, 58, 12221–12229. [Google Scholar] [CrossRef]
- Pan, L.; Xiao, Y.; Jiang, F.; Jiang, T.; Zhu, J.; Tang, W.; Liu, X.; Zhou, Y.; Yu, L. Comparison of Characterization of Cold Brew and Hot Brew Coffee Prepared at Various Roasting Degrees. J. Food Process. Preserv. 2023, 2023, 1–15. [Google Scholar] [CrossRef]
- Angeloni, G.; Guerrini, L.; Masella, P.; Bellumori, M.; Daluiso, S.; Parenti, A.; Innocenti, M. What Kind of Coffee Do You Drink? An Investigation on Effects of Eight Different Extraction Methods. Food Res. Int. 2019, 116, 1327–1335. [Google Scholar] [CrossRef]
- Rao, N.Z.; Fuller, M.; Grim, M.D. Physiochemical Characteristics of Hot and Cold Brew Coffee Chemistry: The Effects of Roast Level and Brewing Temperature on Compound Extraction. Foods 2020, 9, 902. [Google Scholar] [CrossRef]
- Gölge, E.; Ova, G.; Kemahlıoğlu, Ö.K.; Demirağ, M.K. Effect of Black Carrot (Daucus carota L.) Pomace in Cake and Cookie Formulations as a Functional Ingredient on Sensory Analysis. Food Health 2022, 8, 103–110. [Google Scholar] [CrossRef]
- Kumar, M.; Dahuja, A.; Sachdev, A.; Kaur, C.; Varghese, E.; Saha, S.; Sairam, K.V.S.S. Valorisation of Black Carrot Pomace: Microwave Assisted Extraction of Bioactive Phytoceuticals and Antioxidant Activity Using Box–Behnken Design. J. Food Sci. Technol. 2019, 56, 995–1007. [Google Scholar] [CrossRef]
- Zampar, G.G.; Zampar, I.C.; Beserra da Silva de Souza, S.; da Silva, C.; Bolanho Barros, B.C. Effect of Solvent Mixtures on the Ultrasound-Assisted Extraction of Compounds from Pineapple by-Product. Food Biosci. 2022, 50, 102098. [Google Scholar] [CrossRef]
- Samarakoon, S.M.G.K.; Rajapakse, C.S.K. Peel of Pineapple (Ananas Comosus) as a Potential Source of Antioxidants and Photo-Protective Agents for Sun Protection Cosmetics. Med. Plants Int. J. Phytomed. Relat. Ind. 2022, 14, 95–104. [Google Scholar] [CrossRef]
- Candrawinata, V.I.; Golding, J.B.; Roach, P.D.; Stathopoulos, C.E. Total Phenolic Content and Antioxidant Activity of Apple Pomace Aqueous Extract: Effect of Time, Temperature and Water to Pomace Ratio. Int. Food Res. J. 2014, 21, 2337–2344. [Google Scholar]
- Xavier, L.; Pisani, J.; Meghirditchian, G.; de Mattos, R.; Vieitez, I.; Barrenengoa, M.; Zecchi, B. Extraction of Phenolic Compounds from Apple Pomace, Process Modeling and Antioxidant Potential Evaluation of Extracts. Int. J. Food Eng. 2022, 18, 547–558. [Google Scholar] [CrossRef]
- Čanadanović-Brunet, J.M.; Savatović, S.S.; Ćetković, G.S.; Vulić, J.J.; Djilas, S.M.; Markov, S.L.; Cvetković, D.D. Antioxidant and Antimicrobial Activities of Beet Root Pomace Extracts. Czech J. Food Sci. 2011, 29, 575–585. [Google Scholar] [CrossRef]
- Sumner, L.W.; Amberg, A.; Barrett, D.; Beale, M.H.; Beger, R.; Daykin, C.A.; Fan, T.W.-M.; Fiehn, O.; Goodacre, R.; Griffin, J.L.; et al. Proposed Minimum Reporting Standards for Chemical Analysis. Metabolomics 2007, 3, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.-Z.; Zhou, Y.; Guo, R.; Fu, Z.-M.; Chen, D.-F. Structure-Antioxidant Activity Relationship of Ferulic Acid Derivatives: Effect of Ester Groups at the End of the Carbon Side Chain. LWT 2020, 120, 108932. [Google Scholar] [CrossRef]
- Phuong, N.T.M.; Cuong, T.T.; Quang, D.N. Anti-Inflammatory Activity of Methyl Ferulate Isolated from Stemona Tuberosa Lour. Asian Pac. J. Trop. Med. 2014, 7, S327–S331. [Google Scholar] [CrossRef]
- Górecki, M.; Hallmann, E. The Antioxidant Content of Coffee and Its In Vitro Activity as an Effect of Its Production Method and Roasting and Brewing Time. Antioxidants 2020, 9, 308. [Google Scholar] [CrossRef]
- Tan, Y.; Wu, H.; Shi, L.; Barrow, C.; Dunshea, F.R.; Suleria, H.A.R. Impacts of Fermentation on the Phenolic Composition, Antioxidant Potential, and Volatile Compounds Profile of Commercially Roasted Coffee Beans. Fermentation 2023, 9, 918. [Google Scholar] [CrossRef]
- Lasalvia, A.; Cairone, F.; Cesa, S.; Maccelli, A.; Crestoni, M.E.; Menghini, L.; Carradori, S.; Marinacci, B.; Gallorini, M.; Elsallabi, O.; et al. Characterization and Valorization of ‘Sulmona Red Garlic’ Peels and Small Bulbs. Antioxidants 2022, 11, 2088. [Google Scholar] [CrossRef] [PubMed]
- Gorinstein, S.; Leontowicz, H.; Leontowicz, M.; Namiesnik, J.; Najman, K.; Drzewiecki, J.; Cvikrová, M.; Martincová, O.; Katrich, E.; Trakhtenberg, S. Comparison of the Main Bioactive Compounds and Antioxidant Activities in Garlic and White and Red Onions after Treatment Protocols. J. Agric. Food Chem. 2008, 56, 4418–4426. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Li, Y.; Yang, J.; Pu, X.; Du, J.; Yang, X.; Yang, T.; Yang, S. Therapeutic Role of Functional Components in Alliums for Preventive Chronic Disease in Human Being. Evid.-Based Complement. Altern. Med. 2017, 2017, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Melough, M.M.; Lee, S.G.; Cho, E.; Kim, K.; Provatas, A.; Perkins, C.; Park, M.K.; Qureshi, A.; Chun, O.K. Identification and Quantitation of Furocoumarin Contents in Popularly Consumed Foods in the U.S. Using UPLC-MS/MS. FASEB J. 2017, 31, 790.15. [Google Scholar] [CrossRef]
- Peroutka, R.; Schulzová, V.; Botek, P.; Hajšlová, J. Analysis of Furanocoumarins in Vegetables (Apiaceae) and Citrus Fruits (Rutaceae). J. Sci. Food Agric. 2007, 87, 2152–2163. [Google Scholar] [CrossRef]
- Brown, A.E.; Swinburne, T.R. Benzoic Acid: An Antifungal Compound Formed in Bramley’s Seedling Apple Fruits Following Infection by Nectria Galligena Bres. Physiol. Plant Pathol. 1971, 1, 469–475. [Google Scholar] [CrossRef]
- del Olmo, A.; Calzada, J.; Nuñez, M. Benzoic Acid and Its Derivatives as Naturally Occurring Compounds in Foods and as Additives: Uses, Exposure, and Controversy. Crit. Rev. Food Sci. Nutr. 2017, 57, 3084–3103. [Google Scholar] [CrossRef]
- Borjan, D.; Šeregelj, V.; Andrejč, D.C.; Pezo, L.; Šaponjac, V.T.; Knez, Ž.; Vulić, J.; Marevci, M.K. Green Techniques for Preparation of Red Beetroot Extracts with Enhanced Biological Potential. Antioxidants 2022, 11, 805. [Google Scholar] [CrossRef]
- Vulić, J.J.; Ćebović, T.N.; Čanadanović-Brunet, J.M.; Ćetković, G.S.; Čanadanović, V.M.; Djilas, S.M.; Tumbas Šaponjac, V.T. In Vivo and in Vitro Antioxidant Effects of Beetroot Pomace Extracts. J. Funct. Foods 2014, 6, 168–175. [Google Scholar] [CrossRef]
- Chhikara, N.; Kushwaha, K.; Sharma, P.; Gat, Y.; Panghal, A. Bioactive Compounds of Beetroot and Utilization in Food Processing Industry: A Critical Review. Food Chem. 2019, 272, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Maqbool, H.; Safeena, M.P.; Abubacker, Z.; Azhar, M.; Kumar, S. Effect of Beetroot Peel Dip Treatment on the Quality Preservation of Deccan Mahseer (Tor Khudree) Steaks during Frozen Storage (−18 °C). LWT 2021, 151, 112222. [Google Scholar] [CrossRef]
- Tumbas Šaponjac, V.; Čanadanović-Brunet, J.; Ćetković, G.; Jakišić, M.; Djilas, S.; Vulić, J.; Stajčić, S.D.S. Encapsulation of Beetroot Pomace Extract: RSM Optimization, Storage and Gastrointestinal Stability. Molecules 2016, 21, 584. [Google Scholar] [CrossRef] [PubMed]
- Suleria, H.A.R.; Barrow, C.J.; Dunshea, F.R. Screening and Characterization of Phenolic Compounds and Their Antioxidant Capacity in Different Fruit Peels. Foods 2020, 9, 1206. [Google Scholar] [CrossRef] [PubMed]
- Nagasampagi, B.A.; Rowe, J.W.; Simpson, R.; Goad, L.J. Sterols of Coffee. Phytochemistry 1971, 10, 1101–1107. [Google Scholar] [CrossRef]
- Alstadhaug, K.B.; Tzoulis, C.; Simonsen, A.M. Beverages, Caffeine, and Parkinson’s Disease. In Diet and Nutrition in Neurological Disorders; Elsevier: Amsterdam, The Netherlands, 2023; pp. 699–715. [Google Scholar]
- Erny, G.L.; Moeenfard, M.; Alves, A. Liquid Chromatography with Diode Array Detection Combined with Spectral Deconvolution for the Analysis of Some Diterpene Esters in Arabica Coffee Brew. J. Sep. Sci. 2015, 38, 612–620. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Meng, Q.; Peng, X.; Hu, G.; Qiu, M. Identification of New Diterpene Esters from Green Arabica Coffee Beans, and Their Platelet Aggregation Accelerating Activities. Food Chem. 2018, 263, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Esquivel, P.; Viñas, M.; Steingass, C.B.; Gruschwitz, M.; Guevara, E.; Carle, R.; Schweiggert, R.M.; Jiménez, V.M. Coffee (Coffea arabica L.) by-Products as a Source of Carotenoids and Phenolic Compounds—Evaluation of Varieties With Different Peel Color. Front. Sustain. Food Syst. 2020, 4, 590597. [Google Scholar] [CrossRef]
- Tian, Z.; Dong, T.; Wang, S.; Sun, J.; Chen, H.; Zhang, N.; Wang, S. A Comprehensive Review on Botany, Chemical Composition and the Impacts of Heat Processing and Dehydration on the Aroma Formation of Fresh Carrot. Food Chem. X 2024, 22, 101201. [Google Scholar] [CrossRef]
- Yahyaa, M.; Tholl, D.; Cormier, G.; Jensen, R.; Simon, P.W.; Ibdah, M. Identification and Characterization of Terpene Synthases Potentially Involved in the Formation of Volatile Terpenes in Carrot (Daucus carota L.) Roots. J. Agric. Food Chem. 2015, 63, 4870–4878. [Google Scholar] [CrossRef]
- Nile, S.H.; Nile, A.; Liu, J.; Kim, D.H.; Kai, G. Exploitation of Apple Pomace towards Extraction of Triterpenic Acids, Antioxidant Potential, Cytotoxic Effects, and Inhibition of Clinically Important Enzymes. Food Chem. Toxicol. 2019, 131, 110563. [Google Scholar] [CrossRef] [PubMed]
- Butkevičiūtė, A.; Janulis, V.; Kviklys, D. Triterpene Content in Flesh and Peel of Apples Grown on Different Rootstocks. Plants 2022, 11, 1247. [Google Scholar] [CrossRef] [PubMed]
- Yogeeswari, P.; Sriram, D. Betulinic Acid and Its Derivatives: A Review on Their Biological Properties. Curr. Med. Chem. 2005, 12, 657–666. [Google Scholar] [CrossRef] [PubMed]
- Mikołajczyk-Bator, K.; Błaszczyk, A.; Czyżniejewski, M.; Kachlicki, P.; Stochmal, A. Metabolite Profiling of Triterpene Saponins from Different Red Beetroot Cultivars Using Ultra-High Performance Liquid Chromatography High-Resolution Mass Spectrometry. J. Food Compos. Anal. 2024, 130, 106141. [Google Scholar] [CrossRef]
- González-Peña, M.A.; Ortega-Regules, A.E.; Anaya de Parrodi, C.; Lozada-Ramírez, J.D. Chemistry, Occurrence, Properties, Applications, and Encapsulation of Carotenoids—A Review. Plants 2023, 12, 313. [Google Scholar] [CrossRef]
- Saini, R.K.; Nile, S.H.; Park, S.W. Carotenoids from Fruits and Vegetables: Chemistry, Analysis, Occurrence, Bioavailability and Biological Activities. Food Res. Int. 2015, 76, 735–750. [Google Scholar] [CrossRef]
- Steingass, C.B.; Vollmer, K.; Lux, P.E.; Dell, C.; Carle, R.; Schweiggert, R.M. HPLC-DAD-APCI-MS Analysis of the Genuine Carotenoid Pattern of Pineapple (Ananas comosus [L.] Merr.) Infructescence. Food Res. Int. 2020, 127, 108709. [Google Scholar] [CrossRef]
- Meriga, B.; Mopuri, R.; MuraliKrishna, T. Insecticidal, Antimicrobial and Antioxidant Activities of Bulb Extracts of Allium Sativum. Asian Pac. J. Trop. Med. 2012, 5, 391–395. [Google Scholar] [CrossRef]
- Volk, R.-B. A Newly Developed Assay for the Quantitative Determination of Antimicrobial (Anticyanobacterial) Activity of Both Hydrophilic and Lipophilic Test Compounds without Any Restriction. Microbiol. Res. 2008, 163, 161–167. [Google Scholar] [CrossRef]
- Martínez, S.; Fuentes, C.; Carballo, J. Antioxidant Activity, Total Phenolic Content and Total Flavonoid Content in Sweet Chestnut (Castanea sativa Mill.) Cultivars Grown in Northwest Spain under Different Environmental Conditions. Foods 2022, 11, 3519. [Google Scholar] [CrossRef]
- Karapandzova, M.; Stefkov, G.; Cvetkovikj, I.; Stanoeva, J.P.; Stefova, M.; Kulevanova, S. Flavonoids and Other Phenolic Compounds in Needles of Pinus Peuce and Other Pine Species from the Macedonian Flora. Nat. Prod. Commun. 2015, 10, 987–990. [Google Scholar] [CrossRef] [PubMed]
- Seeram, N.P.; Heber, D. Impact of Berry Phytochemicals on Human Health: Effects beyond Antioxidation. J. Nutr. Food Sci. 2007, 06, 326–336. [Google Scholar]
- Hassanpour, S.H.; Doroudi, A. Review of the Antioxidant Potential of Flavonoids as a Subgroup of Polyphenols and Partial Substitute for Synthetic Antioxidants. Avicenna J. Phytomed. 2023, 13, 354–376. [Google Scholar] [PubMed]
- Hossein Maleki, M.; Daneshniya, M.; Latifi, Z.; Pirouz Zarrin, Y.; Behzadinia, M.; Morakabati, N. Evaluating the Potential of Phytochemicals as Natural Substitute for Synthetic Antioxidant: A Review. Asian J. Res. Biochem. 2022, 36–62. [Google Scholar] [CrossRef]
- Attard, K.; Oztop, M.H.; Lia, F. The Effect of Hydrolysis on the Antioxidant Activity of Olive Mill Waste. Appl. Sci. 2022, 12, 12187. [Google Scholar] [CrossRef]
- Jia, R.; Tang, C.; Chen, J.; Zhang, X.; Wang, Z. Total Phenolics and Anthocyanins Contents and Antioxidant Activity in Four Different Aerial Parts of Leafy Sweet Potato (Ipomoea batatas L.). Molecules 2022, 27, 3117. [Google Scholar] [CrossRef] [PubMed]
- Saini, S. In Vitro Antioxidant Activity and Total Phenolic Content of Digera Muricata Leaves. World J. Biol. Pharm. Health Sci. 2023, 14, 105–112. [Google Scholar] [CrossRef]
- Limmongkon, A.; Janhom, P.; Amthong, A.; Kawpanuk, M.; Nopprang, P.; Poohadsuan, J.; Somboon, T.; Saijeen, S.; Surangkul, D.; Srikummool, M.; et al. Antioxidant Activity, Total Phenolic, and Resveratrol Content in Five Cultivars of Peanut Sprouts. Asian Pac. J. Trop. Biomed. 2017, 7, 332–338. [Google Scholar] [CrossRef]
- Russo, R.; Reggiani, R. Phenolics and Antioxidant Activity in Flax Varieties with Different Productive Attitude. Int. Food Res. J. 2015, 22, 1736–1739. [Google Scholar]
- Terpinc, P.; Čeh, B.; Ulrih, N.P.; Abramovič, H. Studies of the Correlation between Antioxidant Properties and the Total Phenolic Content of Different Oil Cake Extracts. Ind. Crops Prod. 2012, 39, 210–217. [Google Scholar] [CrossRef]



| Ionization Mode | ESI+ | ESI− | ESI+ | ESI− |
|---|---|---|---|---|
| Phytochemicals | Matched with Databases | Mass Error < 20 ppm | ||
| Alkaloids | 28 | 44 | 8 | 15 |
| Carbohydrates | 9 | 16 | 5 | 8 |
| Organosulfur and n-containing compounds | 13 | 34 | 3 | 14 |
| Polyphenols | 97 | 197 | 29 | 71 |
| Terpenes | 95 | 86 | 34 | 33 |
| Miscellaneous phytochemicals | 19 | 44 | 7 | 6 |
| Other organic compounds | 23 | 29 | 13 | 13 |
| Total | 284 | 450 | 99 | 160 |
| ESI Mode | Measured m/z | Error (ppm) | Molecular Formula | Putative Annotation | Subclass | SCG | CBCG | GP | BCP | AP | BP | PP |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| − | 386.1259 | 11.85 | C17H22O10 | 1-O-Sinapoyl-Beta-D-Glucose | Phenolic acids | 7.04 | 6.40 | 0.00 | 20.15 | 0.00 | 0.00 | 6.64 |
| + | 362.3189 | 1.18 | C24H42O2 | 5-Octadecylresorcinol | Alkylresorcinols | 17.26 | 9.79 | 0.00 | 8.91 | 0.00 | 0.00 | 2.56 |
| − | 488.1390 | 14.54 | C24H24O11 | 6-O-Acetylglycitin | Flavonoids | 73.47 | 214.33 | 0.00 | 0.00 | 0.71 | 22.56 | 5.53 |
| − | 374.1364 | −0.32 | C20H22O7 | 7-Hydroxymatairesinol | Alkylresorcinols | 0.00 | 0.00 | 0.00 | 0.00 | 7.70 | 21.06 | 0.00 |
| − | 122.0362 | −4.98 | C7H6O2 | Benzoic Acid | Phenolic acids | 8.69 | 0.00 | 6.01 | 3.43 | 16.58 | 0.00 | 11.95 |
| − | 216.0436 | 6.14 | C12H8O4 | Bergapten | Flavonoids | 4.79 | 6.09 | 111.24 | 44.09 | 5.54 | 0.00 | 84.05 |
| + | 576.4152 | −4.66 | C38H56O4 | Campesteryl Ferulate | Phenolic acids | 74.19 | 28.29 | 0.00 | 221.27 | 0.46 | 44.88 | 7.30 |
| + | 302.2595 | −4.78 | C21H34O | Cardanol | Misc. polyphenols | 160.71 | 141.77 | 6.57 | 0.00 | 0.30 | 62.93 | 5.41 |
| + | 449.1119 | 7.89 | C21H21O11 | Cyanidin-3-Glucoside | Flavonoids | 0.00 | 0.00 | 9.45 | 5.11 | 0.00 | 23.52 | 1.13 |
| − | 1317.3600 | −9.17 | C60H69O33 | Cyanidin3-(Feruloyl)(Sinapoyl)-Triglucoside-5-Glucoside | Flavonoids | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 70.25 | 0.00 |
| − | 979.2775 | 5.69 | C44H51O25 | Cyanidin3-(Sinapoyl)-Diglucoside-5-Glucoside | Flavonoids | 0.00 | 0.00 | 9.21 | 0.00 | 0.69 | 39.04 | 2.40 |
| − | 491.1259 | 14.22 | C23H23O12 | Cyanidin3-O-(6-Acetyl-Glucoside) | Flavonoids | 38.10 | 90.41 | 0.00 | 0.00 | 0.00 | 11.72 | 4.17 |
| − | 435.0945 | 4.08 | C20H19O11 | Delphinidin-3-Arabinoside | Flavonoids | 107.76 | 160.34 | 0.00 | 0.00 | 0.00 | 0.00 | 4.18 |
| − | 270.0906 | 5.15 | C16H14O4 | Echinatin | Flavonoids | 0.00 | 0.00 | 0.00 | 3.75 | 9.70 | 6.38 | 0.00 |
| + | 706.1948 | 7.12 | C36H34O15 | Epicatechin-(2a-7)(4a-8)-Epicatechin 3-O-Galactoside | Flavonoids | 0.00 | 150.64 | 21.15 | 0.00 | 0.00 | 322.55 | 0.00 |
| − | 594.1696 | 18.77 | C27H30O15 | Kaempferol-3-O-Galactoside7-O-Rhamnoside | Flavonoids | 0.00 | 0.00 | 28.91 | 0.00 | 0.00 | 0.00 | 0.00 |
| − | 534.1010 | 0.05 | C24H22O14 | Kaempferol3-O-(6-Malonyl-Glucoside) | Flavonoids | 795.20 | 1443.82 | 13.68 | 0.00 | 3.26 | 619.05 | 195.86 |
| + | 578.4275 | −10.43 | C38H58O4 | Methylcholestanol Ferulate | Phenolic acids | 307.56 | 159.82 | 10.51 | 73.57 | 0.51 | 65.69 | 39.51 |
| − | 178.0608 | −12.24 | C10H10O3 | Methyl-p-Coumaric Acid | Phenolic acids | 36.25 | 92.10 | 4.50 | 0.00 | 0.00 | 5.74 | 6.94 |
| − | 402.1316 | 0.24 | C21H22O8 | Nobiletin | Flavonoids | 4.23 | 5.83 | 0.00 | 56.73 | 1.24 | 24.79 | 8.28 |
| + | 370.2875 | 0.76 | C25H38O2 | Nonadecatrienyl-Resorcinol | Alkylresorcinols | 668.90 | 354.55 | 64.46 | 60.91 | 0.00 | 71.81 | 142.05 |
| − | 378.1295 | −5.09 | C19H22O8 | Oleuropein-Aglycone | Misc. polyphenols | 5.02 | 0.00 | 47.17 | 13.78 | 72.85 | 81.61 | 129.33 |
| − | 726.2045 | 5.22 | C32H38O19 | Pelargonidin-3-Sambubioside-5-Glucoside | Flavonoids | 53.27 | 155.38 | 0.00 | 0.00 | 0.00 | 8.89 | 0.00 |
| − | 898.1918 | −4.31 | C45H38O20 | Prodelphinidintrimergc-Gc-C | Flavonoids | 20.95 | 25.65 | 19.06 | 0.00 | 0.00 | 16.62 | 0.00 |
| − | 506.1129 | 13.63 | C23H22O13 | Quercetin-3-(6-Acetylglucoside) | Flavonoids | 41.99 | 98.28 | 0.00 | 0.00 | 1.07 | 11.94 | 5.11 |
| − | 354.1109 | 1.63 | C20H18O6 | Sesamin | Lignans | 46.18 | 76.68 | 0.00 | 0.00 | 0.31 | 0.00 | 9.96 |
| + | 354.1158 | 15.49 | C20H18O6 | Sesamin | Lignans | 9.56 | 24.80 | 13.00 | 0.00 | 0.33 | 64.94 | 3.22 |
| ESI Mode | Measured m/z | Error (ppm) | Molecular Formula | Putative Annotation | Subclass | SCG | CBCG | GP | BCP | AP | BP | PP |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| + | 540.3652 | −1.86 | C30H52O8 | (+)-Longilene peroxide | Triterpenoids | 19.33 | 30.84 | 4.53 | 99.62 | 1.18 | 13.90 | 7.18 |
| − | 306.2533 | −8.54 | C20H34O2 | Akhdardiol | Diterpenoids | 142.51 | 93.53 | 40.33 | 103.56 | 0.00 | 0.00 | 1.82 |
| + | 306.2568 | 3.13 | C20H34O2 | Akhdardiol | Diterpenoids | 0.00 | 8.15 | 50.95 | 118.74 | 0.31 | 0.00 | 0.00 |
| + | 288.2444 | −3.24 | C20H32O | Akhdarenol | Diterpenoids | 135.62 | 54.79 | 2.53 | 0.00 | 0.00 | 271.64 | 36.03 |
| − | 488.3593 | 18.62 | C30H48O5 | Asiatic Acid | Triterpenoids | 0.00 | 0.00 | 0.00 | 117.34 | 0.00 | 0.00 | 5.96 |
| − | 456.3632 | 6.34 | C30H48O3 | Betulinic Acid | Triterpenoids | 0.00 | 0.00 | 0.00 | 11.25 | 4.86 | 0.00 | 0.00 |
| + | 596.4879 | 12.42 | C39H64O4 | Cafestolstearate | Diterpenoids | 272.83 | 701.28 | 119.11 | 301.06 | 0.44 | 0.00 | 13.32 |
| + | 586.4168 | −11.12 | C36H58O6 | Caffarolide B | Diterpenoids | 18.18 | 24.43 | 5.62 | 0.00 | 0.00 | 0.00 | 24.43 |
| 594.3925 | 0.73 | C37H54O6 | Caffarolide F | Diterpenoids | 22.15 | 35.24 | 9.21 | 42.11 | 0.00 | 0.00 | 0.00 | |
| + | 608.4037 | −6.59 | C38H56O6 | Caffarolide G | Diterpenoids | 96.63 | 207.68 | 7.84 | 250.35 | 0.00 | 203.23 | 0.00 |
| − | 564.3921 | −8.26 | C40H52O2 | Canthaxanthin | Carotenoids | 21.27 | 11.66 | 17.39 | 0.00 | 0.00 | 0.00 | 4.77 |
| + | 424.3662 | −10.07 | C30H48O | Cycloeucalenone | Triterpenoids | 155.94 | 38.04 | 9.16 | 0.00 | 0.00 | 0.00 | 0.00 |
| − | 414.3137 | 0.68 | C27H42O3 | Diosgenin | Saponins | 70.78 | 28.91 | 0.00 | 0.00 | 0.00 | 0.00 | 15.57 |
| + | 584.4227 | −0.34 | C40H56O3 | Flavoxanthin | Carotenoids | 292.32 | 79.93 | 67.99 | 32.03 | 0.49 | 29.53 | 30.97 |
| − | 360.1190 | −5.19 | C19H20O7 | Gibberellin A8-Catabolite | Diterpenoids | 0.00 | 0.00 | 0.00 | 0.00 | 2.33 | 0.00 | 3.29 |
| + | 456.1494 | −3.86 | C22H33BrO5 | Isoparguerol | Diterpenoids | 0.00 | 0.00 | 0.00 | 0.00 | 0.52 | 4.29 | 7.12 |
| − | 240.0813 | 11.10 | C15H12O3 | Lettucenin A | Sesquiterpenoids | 0.00 | 0.00 | 0.00 | 11.38 | 10.27 | 0.00 | 0.00 |
| − | 684.2740 | −6.05 | C36H44O13 | MascarosideIII | Diterpenoids | 13.83 | 36.49 | 0.00 | 0.00 | 6.95 | 232.52 | 6.49 |
| + | 152.1192 | −6.27 | C10H16O | Myrcenol | Monoterpenoids | 0.00 | 0.00 | 0.00 | 8.37 | 0.00 | 98.79 | 0.00 |
| + | 496.0853 | 5.92 | C20H34Br2O4 | Neoirietetraol | Diterpenoids | 12.26 | 40.70 | 0.00 | 8.46 | 0.38 | 44.40 | 0.00 |
| + | 600.4195 | 2.76 | C40H56O4 | Neoxanthin | Carotenoids | 252.21 | 160.91 | 46.02 | 160.39 | 0.44 | 14.74 | 40.43 |
| − | 600.4260 | 13.48 | C40H56O4 | Neoxanthin | Carotenoids | 8.12 | 0.00 | 21.85 | 17.03 | 0.00 | 0.00 | 6.85 |
| + | 936.4179 | −2.46 | C43H68O22 | Rebaudioside F | Diterpenoids | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 14.32 |
| − | 936.4312 | 11.71 | C43H68O22 | Rebaudioside F | Diterpenoids | 47.28 | 35.85 | 0.00 | 0.00 | 0.00 | 4.43 | 0.00 |
| + | 795.4651 | 15.10 | C42H67O14 | Soyasaponin III | Saponins | 370.27 | 382.64 | 0.00 | 48.25 | 12.00 | 214.64 | 0.00 |
| + | 804.3780 | 0.08 | C38H60O18 | Stevioside | Diterpenoids | 47.23 | 26.60 | 0.00 | 30.66 | 0.63 | 0.00 | 26.41 |
| + | 592.4430 | −10.32 | C39H60O4 | Stigmastanol Ferulate | Triterpenoids | 175.27 | 1674.56 | 155.30 | 293.40 | 0.83 | 623.35 | 4.65 |
| − | 592.4508 | 2.69 | C39H60O4 | Stigmastanol Ferulate | Triterpenoids | 62.48 | 56.20 | 15.32 | 17.02 | 0.00 | 3.12 | 4.33 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández-Montesinos, I.Y.; Carreón-Delgado, D.F.; Lazo-Zamalloa, O.; Tapia-López, L.; Rosas-Morales, M.; Ochoa-Velasco, C.E.; Hernández-Carranza, P.; Cruz-Narváez, Y.; Ramírez-López, C. Exploring Agro-Industrial By-Products: Phenolic Content, Antioxidant Capacity, and Phytochemical Profiling via FI-ESI-FTICR-MS Untargeted Analysis. Antioxidants 2024, 13, 925. https://doi.org/10.3390/antiox13080925
Hernández-Montesinos IY, Carreón-Delgado DF, Lazo-Zamalloa O, Tapia-López L, Rosas-Morales M, Ochoa-Velasco CE, Hernández-Carranza P, Cruz-Narváez Y, Ramírez-López C. Exploring Agro-Industrial By-Products: Phenolic Content, Antioxidant Capacity, and Phytochemical Profiling via FI-ESI-FTICR-MS Untargeted Analysis. Antioxidants. 2024; 13(8):925. https://doi.org/10.3390/antiox13080925
Chicago/Turabian StyleHernández-Montesinos, Itzel Yoali, David Fernando Carreón-Delgado, Oxana Lazo-Zamalloa, Lilia Tapia-López, Minerva Rosas-Morales, Carlos Enrique Ochoa-Velasco, Paola Hernández-Carranza, Yair Cruz-Narváez, and Carolina Ramírez-López. 2024. "Exploring Agro-Industrial By-Products: Phenolic Content, Antioxidant Capacity, and Phytochemical Profiling via FI-ESI-FTICR-MS Untargeted Analysis" Antioxidants 13, no. 8: 925. https://doi.org/10.3390/antiox13080925
APA StyleHernández-Montesinos, I. Y., Carreón-Delgado, D. F., Lazo-Zamalloa, O., Tapia-López, L., Rosas-Morales, M., Ochoa-Velasco, C. E., Hernández-Carranza, P., Cruz-Narváez, Y., & Ramírez-López, C. (2024). Exploring Agro-Industrial By-Products: Phenolic Content, Antioxidant Capacity, and Phytochemical Profiling via FI-ESI-FTICR-MS Untargeted Analysis. Antioxidants, 13(8), 925. https://doi.org/10.3390/antiox13080925










