Papain Suppresses Atopic Skin Inflammation through Anti-Inflammatory Activities Using In Vitro and In Vivo Models
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Dfb-Induced AD Model
2.3. Dermatitis Severity Score
2.4. Measurement of Transepidermal Water Loss
2.5. IgE Measurement
2.6. Quantification of Cytokine Levels
2.7. Histological Analysis and Immunohistochemical Analysis
2.8. RT-qPCR Analysis
2.9. Cell Viability
2.10. Cell Culture and Sample Treatment
2.11. Western Blot Analysis
2.12. Intracellular Reactive Oxygen Species Assay
2.13. Statistical Analysis
3. Results
3.1. PN Alleviated the Severity of AD-like Skin Lesions and Inflammatory Response in Dfb-Induced AD Mice
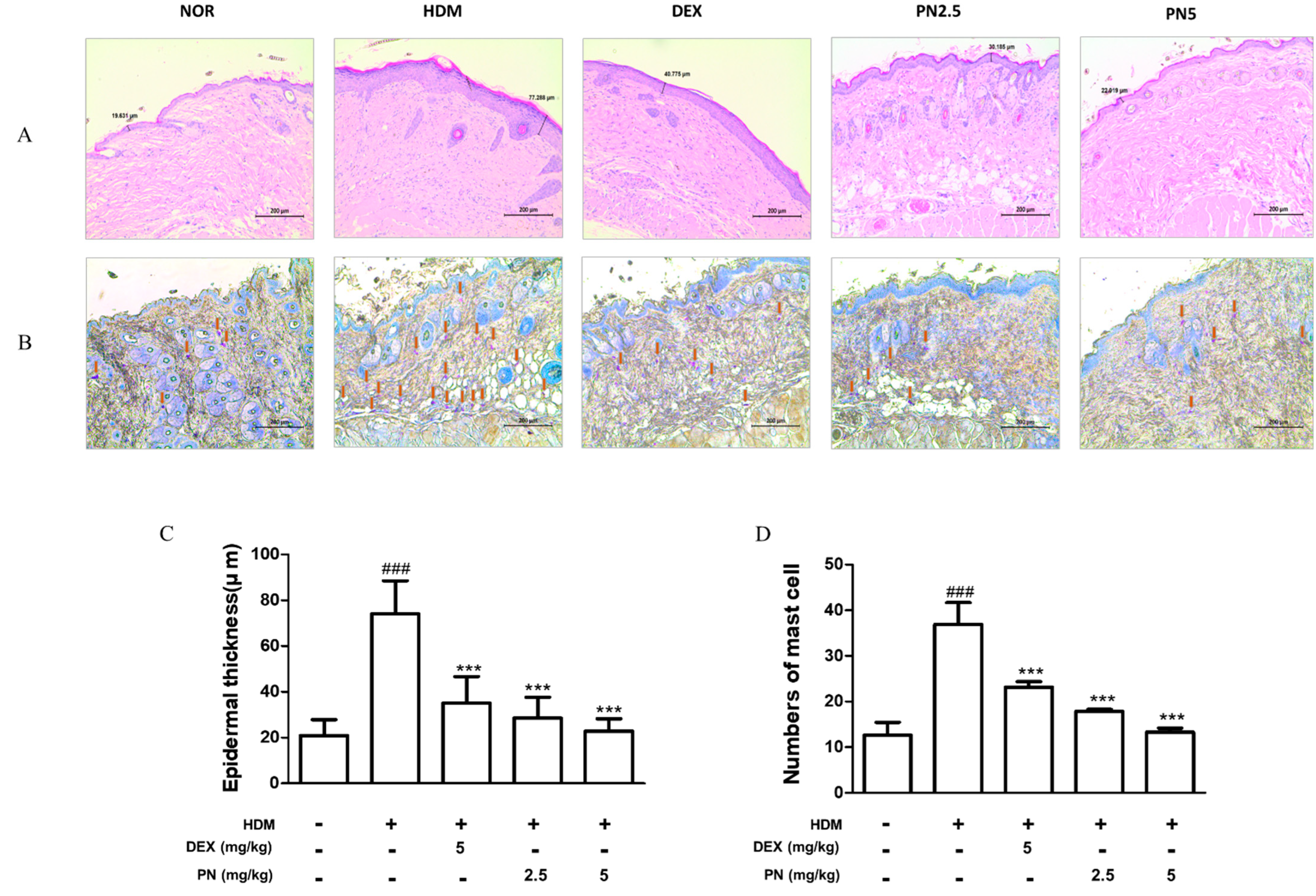
3.2. PN Improved Histological Alterations in the Skin of Dfb-Induced AD Mice
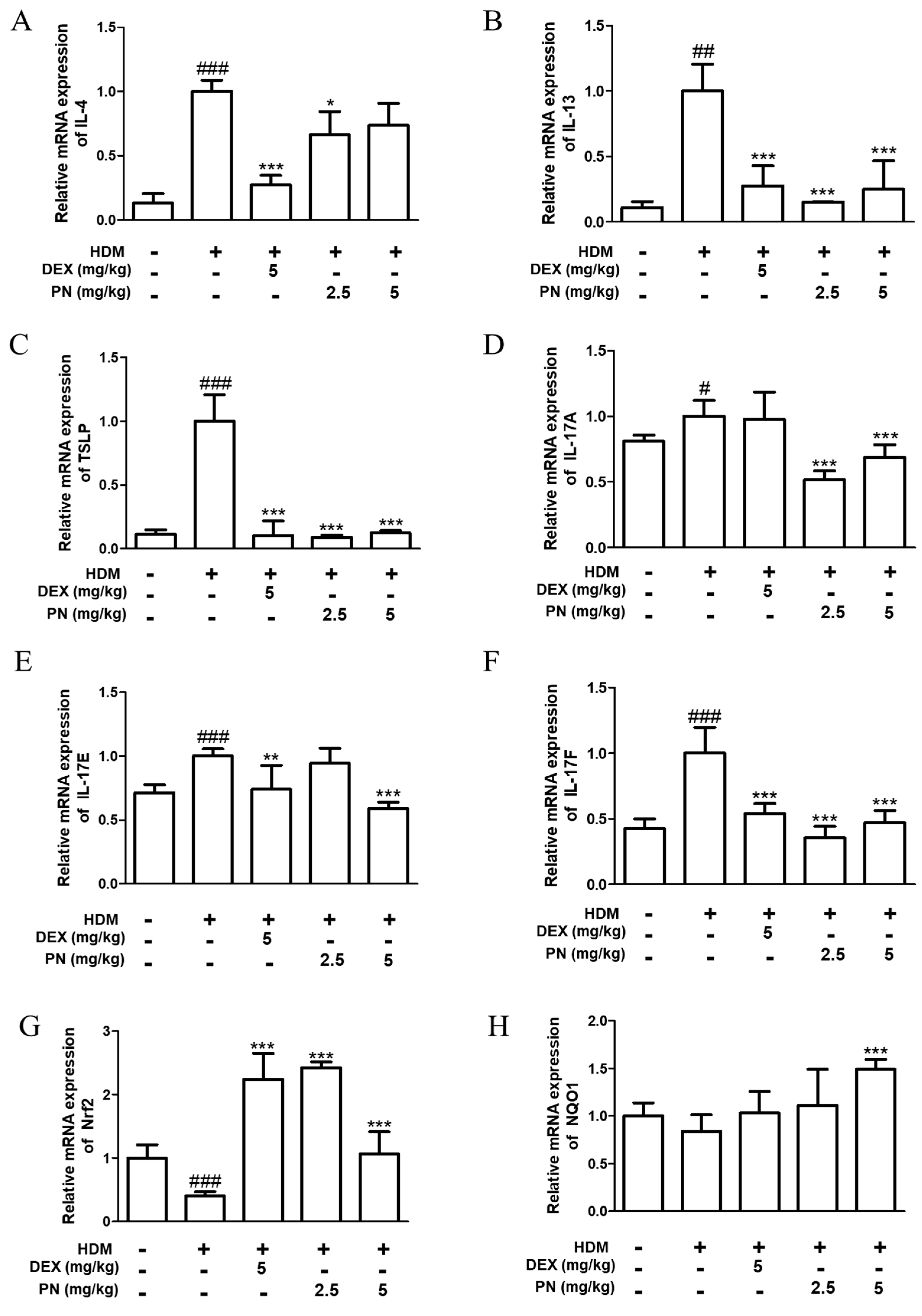
3.3. PN Downregulated AD-Related Cytokines and Upregulated Anti-Oxidative Markers in Dfb-Induced AD Mice
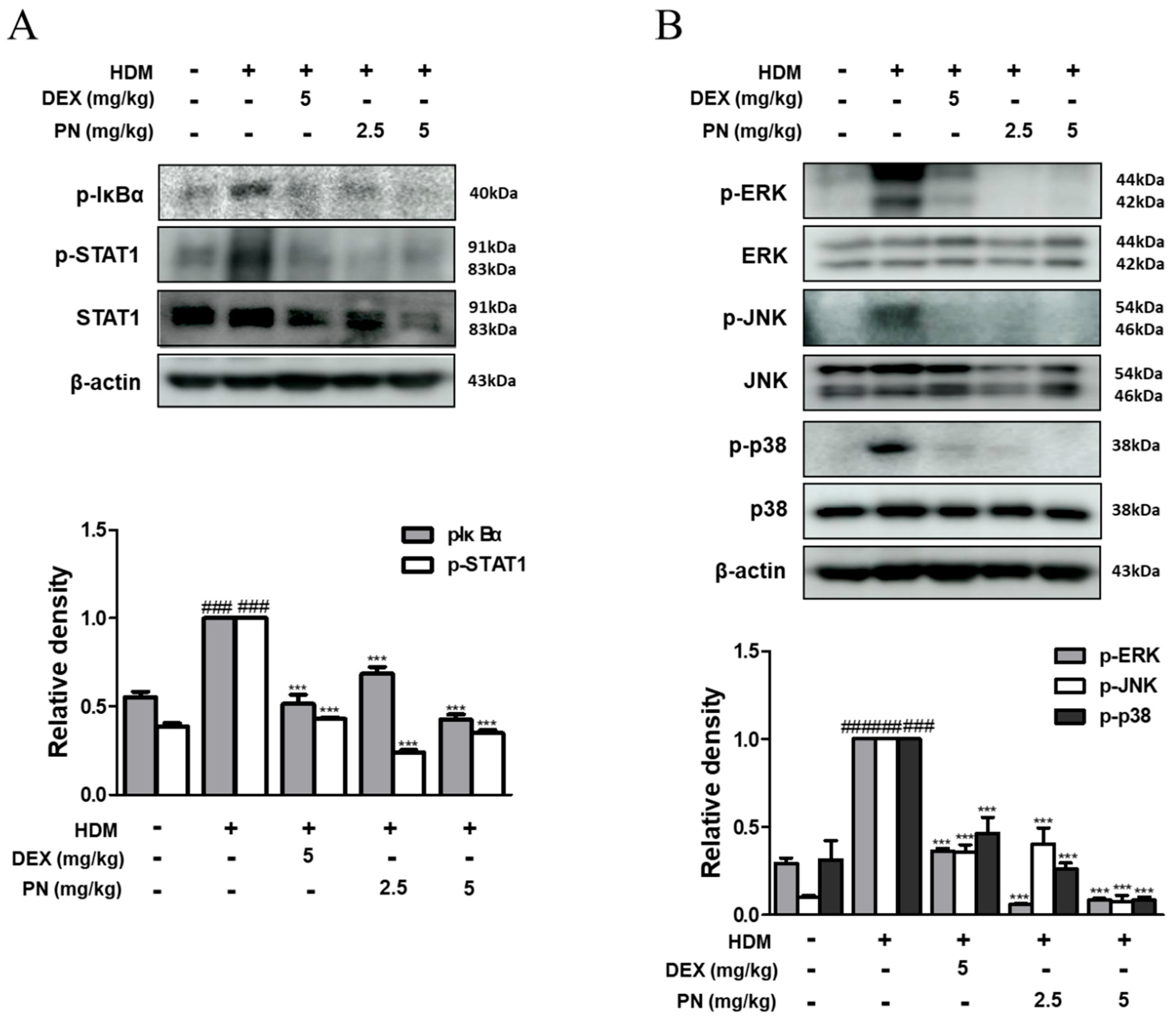
3.4. PN Inhibited the Activation of STAT1 and MAPKs in Dfb-Induced AD Mice
3.5. PN Inhibited the NF-κB Signaling Pathway in TNF-α/IFN-γ-Induced HaCaT Keratinocytes
3.6. PN Inhibited the Activation of STATs and MAPKs in TNF-α/IFN-γ-Stimulated HaCaT Keratinocytes
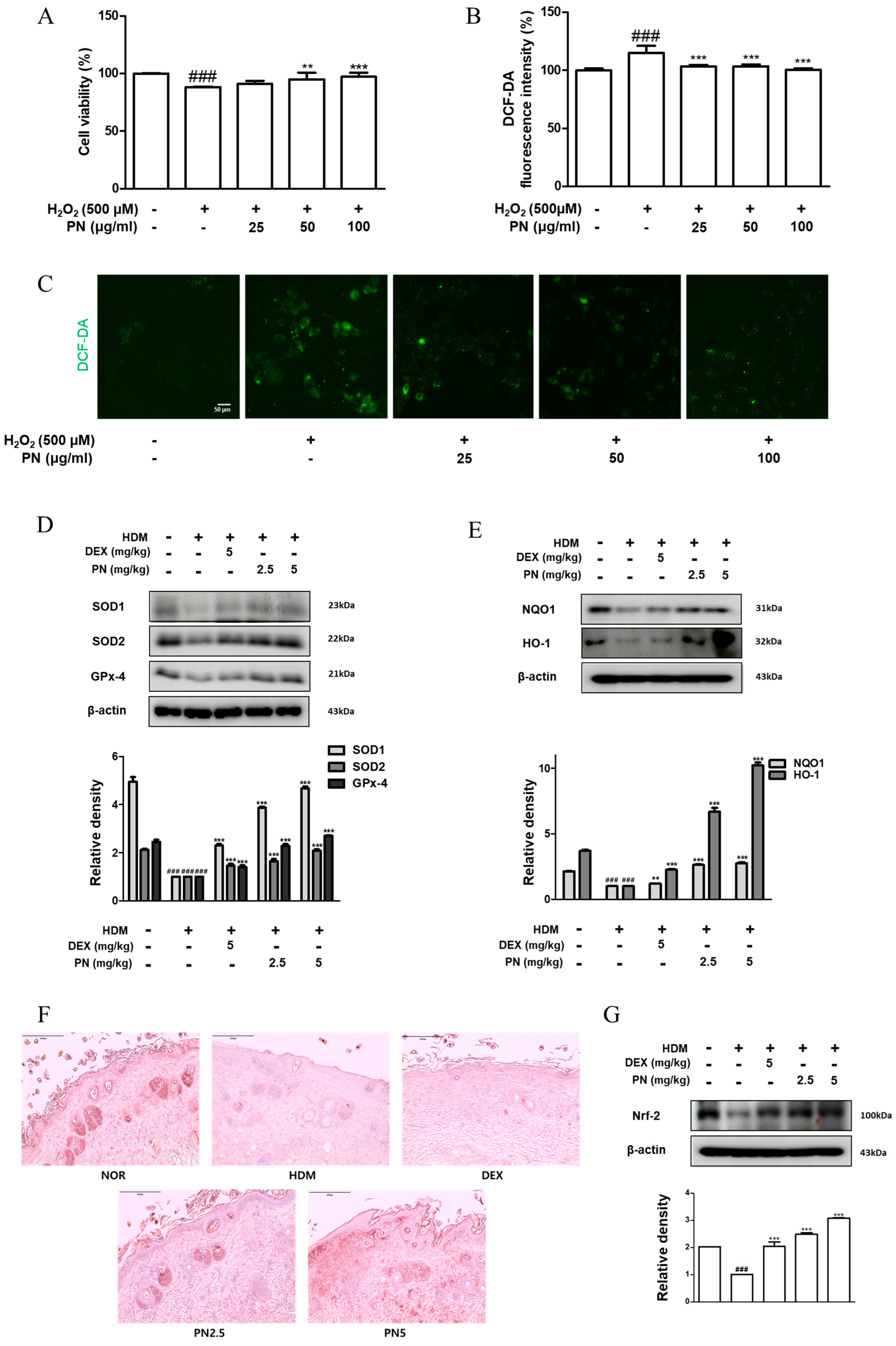
3.7. PN Reduced Oxidative Damage in HaCaT Keratinocytes and Dfb-Induced AD Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Weidinger, S.; Beck, L.A.; Bieber, T.; Kabashima, K.; Irvine, A.D. Atopic dermatitis. Nat. Rev. Dis. Primers 2018, 4, 1. [Google Scholar] [CrossRef]
- Guttman-Yassky, E.; Bissonnette, R.; Ungar, B.; Suárez-Fariñas, M.; Ardeleanu, M.; Esaki, H.; Suprun, M.; Estrada, Y.; Xu, H.; Peng, X.; et al. Dupilumab progressively improves systemic and cutaneous abnormalities in patients with atopic dermatitis. J. Allergy Clin. Immunol. 2019, 143, 155–172. [Google Scholar] [CrossRef] [PubMed]
- Kwon, D.J.; Bae, Y.S.; Ju, S.M.; Goh, A.R.; Youn, G.S.; Choi, S.Y.; Park, J. Casuarinin suppresses TARC/CCL17 and MDC/CCL22 production via blockade of NF-kappaB and STAT1 activation in HaCaT cells. Biochem. Biophys. Res. Commun. 2012, 417, 1254–1259. [Google Scholar] [CrossRef] [PubMed]
- Gil, T.Y.; Hong, C.H.; An, H.J. Anti-Inflammatory Effects of Ellagic Acid on Keratinocytes via MAPK and STAT Pathways. Int. J. Mol. Sci. 2021, 22, 1277. [Google Scholar] [CrossRef]
- Howell, M.D.; Kuo, F.I.; Smith, P.A. Targeting the Janus Kinase Family in Autoimmune Skin Diseases. Front. Immunol. 2019, 10, 2342. [Google Scholar] [CrossRef] [PubMed]
- Tan, Q.; Yang, H.; Liu, E.; Wang, H. P38/ERK MAPK signaling pathways are involved in the regulation of filaggrin and involucrin by IL17. Mol. Med. Rep. 2017, 16, 8863–8867. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Sayama, K.; Tohyama, M.; Shirakata, Y.; Yang, L.; Hirakawa, S.; Tokumaru, S.; Hashimoto, K. The NF-kappaB, p38 MAPK and STAT1 pathways differentially regulate the dsRNA-mediated innate immune responses of epidermal keratinocytes. Int. Immunol. 2008, 20, 901–909. [Google Scholar] [CrossRef] [PubMed]
- Jacquet, A. The role of innate immunity activation in house dust mite allergy. Trends Mol. Med. 2011, 17, 604–611. [Google Scholar] [CrossRef]
- Post, S.; Nawijn, M.C.; Hackett, T.L.; Baranowska, M.; Gras, R.; van Oosterhout, A.J.M.; Heijink, I.H. The composition of house dust mite is critical for mucosal barrier dysfunction and allergic sensitisation. Thorax 2012, 67, 488–495. [Google Scholar] [CrossRef]
- Siracusa, M.C.; Kim, B.S.; Spergel, J.M.; Artis, D. Basophils and allergic inflammation. J. Allergy Clin. Immunol. 2013, 132, 789–801, quiz 788. [Google Scholar] [CrossRef]
- Ying, S.; O’connor, B.; Ratoff, J.; Meng, Q.; Mallett, K.; Cousins, D.; Robinson, D.; Zhang, G.; Zhao, J.; Lee, T.H.; et al. Thymic stromal lymphopoietin expression is increased in asthmatic airways and correlates with expression of Th2-attracting chemokines and disease severity. J. Immunol. 2005, 174, 8183–8190. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Hener, P.; Li, J.; Li, M. Skin thymic stromal lymphopoietin promotes airway sensitization to inhalant house dust mites leading to allergic asthma in mice. Allergy 2012, 67, 1078–1082. [Google Scholar] [CrossRef] [PubMed]
- Kanemaru, K.; Noguchi, E.; Tahara-Hanaoka, S.; Mizuno, S.; Tateno, H.; Fujisawa, Y.; Nakamura, Y.; Denda-Nagai, K.; Irimura, T.; Matsuda, H.; et al. Clec10a regulates mite-induced dermatitis. Sci. Immunol. 2019, 4, eaax6908. [Google Scholar] [CrossRef]
- Mota Vde, S.; Turrini, R.N.; Poveda Vde, B. Antimicrobial activity of Eucalyptus globulus oil, xylitol and papain: A pilot study. Rev. Esc. Enferm. USP 2015, 49, 216–220. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.-M.; Kang, H.-A.; Cominguez, D.C.; Kim, S.-H.; An, H.-J. Papain Ameliorates Lipid Accumulation and Inflammation in High-Fat Diet-Induced Obesity Mice and 3T3-L1 Adipocytes via AMPK Activation. Int. J. Mol. Sci. 2021, 22, 9885. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.R.; Jong, Y.X.; Balakrishnan, M.; Bok, Z.K.; Weng, J.K.K.; Tay, K.C.; Goh, B.H.; Ong, Y.S.; Chan, K.G.; Lee, L.H.; et al. Beneficial Role of Carica papaya Extracts and Phytochemicals on Oxidative Stress and Related Diseases: A Mini Review. Biology 2021, 10, 287. [Google Scholar] [CrossRef] [PubMed]
- Udod, V.M.; Storozhuk, V.T.; Trofimenko, S.P.; Shabash, E.G.; Markelov, S.I. Effect of the proteolytic enzyme papain on the body organs and systems of experimental animals. Farmakol. Toksikol. 1983, 46, 95–98. [Google Scholar]
- Chopra, R.; Vakharia, P.P.; Sacotte, R.; Patel, N.; Immaneni, S.; White, T.; Kantor, R.; Hsu, D.Y.; Silverberg, J.I. Severity strata for Eczema Area and Severity Index (EASI), modified EASI, Scoring Atopic Dermatitis (SCORAD), objective SCORAD, Atopic Dermatitis Severity Index and body surface area in adolescents and adults with atopic dermatitis. Br. J. Dermatol. 2017, 177, 1316–1321. [Google Scholar] [CrossRef]
- Alessandrello, C.; Sanfilippo, S.; Minciullo, P.L.; Gangemi, S. An Overview on Atopic Dermatitis, Oxidative Stress, and Psychological Stress: Possible Role of Nutraceuticals as an Additional Therapeutic Strategy. Int. J. Mol. Sci. 2024, 25, 5020. [Google Scholar] [CrossRef]
- Klisic, A.; Bakic, M.; Karanikolic, V. Comparative Analysis of Redox Homeostasis Biomarkers in Patients with Psoriasis and Atopic Dermatitis. Antioxidants 2023, 12, 1875. [Google Scholar] [CrossRef]
- Liu, L.; Lian, N.; Shi, L.; Hao, Z.; Chen, K. Ferroptosis: Mechanism and connections with cutaneous diseases. Front. Cell Dev. Biol. 2022, 10, 1079548. [Google Scholar] [CrossRef] [PubMed]
- Shim, K.S.; Park, M.; Yang, W.K.; Lee, H.; Kim, S.H.; Choo, B.K.; Chae, S.; Kim, H.K.; Kim, T.; Kim, K.M. Veronica persica Ethanol Extract Ameliorates Dinitrochlorobenzene-Induced Atopic Dermatitis-like Skin Inflammation in Mice, Likely by Inducing Nrf2/HO-1 Signaling. Antioxidants 2023, 12, 1267. [Google Scholar] [CrossRef]
- Ogawa, T.; Ishitsuka, Y. The Role of KEAP1-NRF2 System in Atopic Dermatitis and Psoriasis. Antioxidants 2022, 11, 1397. [Google Scholar] [CrossRef] [PubMed]
- Schmid-Schonbein, G.W. 2008 Landis Award lecture. Inflammation and the autodigestion hypothesis. Microcirculation 2009, 16, 289–306. [Google Scholar] [CrossRef] [PubMed]
- Mayerle, J.; Sendler, M.; Hegyi, E.; Beyer, G.; Lerch, M.M.; Sahin-Tóth, M. Genetics, Cell Biology, and Pathophysiology of Pancreatitis. Gastroenterology 2019, 156, 1951–1968.e1. [Google Scholar] [CrossRef] [PubMed]
- Agoro, R.; Piotet-Morin, J.; Palomo, J.; Michaudel, C.; Vigne, S.; Maillet, I.; Chenuet, P.; Guillou, N.; Le Bérichel, J.; Kisielow, M.; et al. IL-1R1-MyD88 axis elicits papain-induced lung inflammation. Eur. J. Immunol. 2016, 46, 2531–2541. [Google Scholar] [CrossRef]
- Stremnitzer, C.; Manzano-Szalai, K.; Willensdorfer, A.; Starkl, P.; Pieper, M.; König, P.; Mildner, M.; Tschachler, E.; Reichart, U.; Jensen-Jarolim, E. Papain Degrades Tight Junction Proteins of Human Keratinocytes In Vitro and Sensitizes C57BL/6 Mice via the Skin Independent of its Enzymatic Activity or TLR4 Activation. J. Invest. Dermatol. 2015, 135, 1790–1800. [Google Scholar] [CrossRef] [PubMed]
- Schuler, C.F.t.; Billi, A.C.; Maverakis, E.; Tsoi, L.C.; Gudjonsson, J.E. Novel insights into atopic dermatitis. J. Allergy Clin. Immunol. 2023, 151, 1145–1154. [Google Scholar] [CrossRef] [PubMed]
- Kamijo, S.; Suzuki, M.; Hara, M.; Shimura, S.; Ochi, H.; Maruyama, N.; Matsuda, A.; Saito, H.; Nakae, S.; Suto, H.; et al. Subcutaneous Allergic Sensitization to Protease Allergen Is Dependent on Mast Cells but Not IL-33: Distinct Mechanisms between Subcutaneous and Intranasal Routes. J. Immunol. 2016, 196, 3559–3569. [Google Scholar] [CrossRef]
- Kamijo, S.; Hara, M.; Suzuki, M.; Nakae, S.; Ogawa, H.; Okumura, K.; Takai, T. Innate IL-17A Enhances IL-33-Independent Skin Eosinophilia and IgE Response on Subcutaneous Papain Sensitization. J. Invest. Dermatol. 2021, 141, 105–113.e14. [Google Scholar] [CrossRef]
- Jarisarapurin, W.; Sanrattana, W.; Chularojmontri, L.; Kunchana, K.; Wattanapitayakul, S.K. Antioxidant Properties of Unripe Carica papaya Fruit Extract and Its Protective Effects against Endothelial Oxidative Stress. Evid. Based Complement. Alternat. Med. 2019, 2019, 4912631. [Google Scholar] [CrossRef]
- Herrerias, A.; Torres, R.; Serra, M.; Marco, A.; Roca-Ferrer, J.; Picado, C.; de Mora, F. Subcutaneous prostaglandin E(2) restrains airway mast cell activity in vivo and reduces lung eosinophilia and Th(2) cytokine overproduction in house dust mite-sensitive mice. Int. Arch. Allergy Immunol. 2009, 149, 323–332. [Google Scholar] [CrossRef]
- Guggi, D.; Bernkop-Schnurch, A. Improved paracellular uptake by the combination of different types of permeation enhancers. Int. J. Pharm. 2005, 288, 141–150. [Google Scholar] [CrossRef]
- Danielsen, E.M.; Hansen, G.H. Intestinal surfactant permeation enhancers and their interaction with enterocyte cell membranes in a mucosal explant system. Tissue Barriers 2017, 5, e1361900. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yu, J.; Goktepe, I.; Ahmedna, M. The potential of papain and alcalase enzymes and process optimizations to reduce allergenic gliadins in wheat flour. Food Chem. 2016, 196, 1338–1345. [Google Scholar] [CrossRef] [PubMed]
- Ezekiel Amri, F.M. Papain, a Plant Enzyme of Biological Importance: A Review. Am. J. Biochem. Biotechnol. 2012, 8, 99–104. [Google Scholar]
- Grabovac, V.; Schmitz, T.; Föger, F.; Bernkop-Schnürch, A. Papain: An effective permeation enhancer for orally administered low molecular weight heparin. Pharm. Res. 2007, 24, 1001–1006. [Google Scholar] [CrossRef]
- Silva, C.R.D.; Oliveira, M.B.; Motta, E.S.; Almeida, G.S.D.; Varanda, L.L.; Pádula, M.D.; Leitão, A.C.; Caldeira-de-Araújo, A. Genotoxic and Cytotoxic Safety Evaluation of Papain (Carica papaya L.) Using In Vitro Assays. J. Biomed. Biotechnol. 2010, 2010, 197898. [Google Scholar] [CrossRef]
- Kim, H.J.; Zheng, M.; Kim, S.-K.; Cho, J.J.; Shin, C.H.; Joe, Y.; Chung, H.T. CO/HO-1 Induces NQO-1 Expression via Nrf2 Activation. Immune Netw. 2011, 11, 376–382. [Google Scholar] [CrossRef]
- Edamitsu, T.; Taguchi, K.; Okuyama, R.; Yamamoto, M. AHR and NRF2 in Skin Homeostasis and Atopic Dermatitis. Antioxidants 2022, 11, 227. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.-M.; Kang, Y.-M.; Lee, M.; An, H.-J. Papain Suppresses Atopic Skin Inflammation through Anti-Inflammatory Activities Using In Vitro and In Vivo Models. Antioxidants 2024, 13, 928. https://doi.org/10.3390/antiox13080928
Kim H-M, Kang Y-M, Lee M, An H-J. Papain Suppresses Atopic Skin Inflammation through Anti-Inflammatory Activities Using In Vitro and In Vivo Models. Antioxidants. 2024; 13(8):928. https://doi.org/10.3390/antiox13080928
Chicago/Turabian StyleKim, Hye-Min, Yun-Mi Kang, Minho Lee, and Hyo-Jin An. 2024. "Papain Suppresses Atopic Skin Inflammation through Anti-Inflammatory Activities Using In Vitro and In Vivo Models" Antioxidants 13, no. 8: 928. https://doi.org/10.3390/antiox13080928





