An Update on Pentacyclic Triterpenoids Ursolic and Oleanolic Acids and Related Derivatives as Anticancer Candidates
Abstract
1. Introduction
2. Ursolic Acid
2.1. Structural Modifications
2.2. In Vitro, In Vivo, and Clinical Trials
3. Oleanolic Acid
3.1. Structural Modifications
3.2. In Vitro, In Vivo, and Clinical Trials
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Panda, S.S.; Thangaraju, M.; Lokeshwar, B.L. Ursolic Acid Analogs as Potential Therapeutics for Cancer. Molecules 2022, 27, 8981. [Google Scholar] [CrossRef] [PubMed]
- Chhikara, B.S.; Parang, K. Global Cancer Statistics 2022: The Trends Projection Analysis. Chem. Biol. Lett. 2023, 10, 451. [Google Scholar]
- Yazdani, M.; Béni, Z.; Dékány, M.; Szemerédi, N.; Spengler, G.; Hohmann, J.; Ványolós, A. Triterpenes from Pholiota Populnea as Cytotoxic Agents and Chemosensitizers to Overcome Multidrug Resistance of Cancer Cells. J. Nat. Prod. 2022, 85, 910–916. [Google Scholar] [CrossRef] [PubMed]
- Zafar, S.; Khan, K.; Hafeez, A.; Irfan, M.; Armaghan, M.; Rahman, A.; Gürer, E.S.; Sharifi-Rad, J.; Butnariu, M.; Bagiu, I.C.; et al. Ursolic Acid: A Natural Modulator of Signaling Networks in Different Cancers. Cancer Cell Int. 2022, 22, 399. [Google Scholar] [CrossRef] [PubMed]
- Nitika, S.; Priyankul, P.; Inder, K. A Review on Pharmacological Activities of Lupeol and Its Triterpene Derivatives. J. Drug Deliv. Ther. 2020, 10, 325–332. [Google Scholar]
- Dallavalle, S.; Dobričić, V.; Lazzarato, L.; Gazzano, E.; Machuqueiro, M.; Pajeva, I.; Tsakovska, I.; Zidar, N.; Fruttero, R. Improvement of Conventional Anti-Cancer Drugs as New Tools against Multidrug Resistant Tumors. Drug Resist. Updates 2020, 50, 100682. [Google Scholar] [CrossRef]
- Huang, M.; Lu, J.J.; Ding, J. Natural Products in Cancer Therapy: Past, Present and Future. Nat. Prod. Bioprospect. 2021, 11, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Isaković-Vidović, S.; Dariš, B.; Knez, Ž.; Vidović, K.; Oprić, D.; Ferk, P. Antiproliferative Activity of Selected Triterpene Acids from Rosemary on Metastatic Melanoma Cell Line Wm-266-4. Open Access Maced. J. Med. Sci. 2021, 9, 515–521. [Google Scholar] [CrossRef]
- Gupta, N.; Verma, K.; Nalla, S.; Kulshreshtha, A.; Lall, R.; Prasad, S. Free Radicals as a Double-Edged Sword: The Cancer Preventive and Therapeutic Roles of Curcumin. Molecules 2020, 25, 5390. [Google Scholar] [CrossRef]
- Majid Rasheed, H.; Farooq, U.; Bashir, K.; Wahid, F.; Khan, T.; Khusro, A.; Gajdács, M.; Alghamdi, S.; Amer Alsaiari, A.; Almehmadi, M.; et al. Isolation of Oleanolic Acid from Lavandula Stoechas and Its Potent Anticancer Properties against MCF-7 Cancer Cells via Induced Apoptosis. J. King Saud Univ.—Sci. 2023, 35, 102454. [Google Scholar] [CrossRef]
- Cragg, G.M.; Pezzuto, J.M. Natural Products as a Vital Source for the Discovery of Cancer Chemotherapeutic and Chemopreventive Agents. Med. Princ. Pract. 2016, 25, 41–59. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, D.; Tuli, H.S.; Sharma, A.K. Ursolic Acid (UA): A Metabolite with Promising Therapeutic Potential. Life Sci. 2016, 146, 201–213. [Google Scholar] [CrossRef] [PubMed]
- Shanmugam, M.K.; Dai, X.; Kumar, A.P.; Tan, B.K.H.; Sethi, G.; Bishayee, A. Ursolic Acid in Cancer Prevention and Treatment: Molecular Targets, Pharmacokinetics and Clinical Studies. Biochem. Pharmacol. 2013, 85, 1579–1587. [Google Scholar] [CrossRef] [PubMed]
- Chudzik, M.; Korzonek-Szlacheta, I.; Król, W. Triterpenes as Potentially Cytotoxic Compounds. Molecules 2015, 20, 1610–1625. [Google Scholar] [CrossRef] [PubMed]
- Gill, B.S.; Kumar, S.; Navgee. Triterpenes in Cancer: Significance and Their Influence. Mol. Biol. Rep. 2016, 43, 881–896. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.M.; Huang, J.Y.; Sheng, L.X.; Wen, X.A.; Cheng, K.G. Synthesis and Antitumor Activity of Fluorouracil-Oleanolic Acid/Ursolic Acid/Glycyrrhetinic Acid Conjugates. Medchemcomm 2019, 10, 1370–1378. [Google Scholar] [CrossRef] [PubMed]
- Chan, E.W.C.; Soon, C.Y.; Tan, J.B.L.; Wong, S.K.; Hui, Y.W. Ursolic Acid: An Overview on Its Cytotoxic Activities against Breast and Colorectal Cancer Cells. J. Integr. Med. 2019, 17, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Furtado, N.A.J.C.; Pirson, L.; Edelberg, H.; Miranda, L.M.; Loira-Pastoriza, C.; Preat, V.; Larondelle, Y.; André, C.M. Pentacyclic Triterpene Bioavailability: An Overview of in Vitro and in Vivo Studies. Molecules 2017, 22, 400. [Google Scholar] [CrossRef] [PubMed]
- Bian, M.; Sun, Y.; Liu, Y.; Xu, Z.; Fan, R.; Liu, Z.; Liu, W. A Gold(I) Complex Containing an Oleanolic Acid Derivative as a Potential Anti-Ovarian-Cancer Agent by Inhibiting TrxR and Activating ROS-Mediated ERS. Chem.—A Eur. J. 2020, 26, 7092–7108. [Google Scholar] [CrossRef]
- Mlala, S.; Oyedeji, A.O.; Gondwe, M.; Oyedeji, O.O. Ursolic Acid and Its Derivatives as Bioactive Agents. Molecules 2019, 24, 2751. [Google Scholar] [CrossRef]
- Lombrea, A.; Scurtu, A.D.; Avram, S.; Pavel, I.Z.; Turks, M.; Lugiņina, J.; Peipiņš, U.; Dehelean, C.A.; Soica, C.; Danciu, C. Anticancer Potential of Betulonic Acid Derivatives. Int. J. Mol. Sci. 2021, 22, 3676. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Lei, L.; Liu, Z.; Wang, H.; Meng, Q. Design, Synthesis, and Biological Evaluation of Novel Nitrogen Heterocycle-Containing Ursolic Acid Analogs as Antitumor Agents. Molecules 2019, 24, 877. [Google Scholar] [CrossRef] [PubMed]
- Ayeleso, T.B.; Matumba, M.G.; Mukwevho, E. Oleanolic Acid and Its Derivatives: Biological Activities and Therapeutic Potential in Chronic Diseases. Molecules 2017, 22, 1915. [Google Scholar] [CrossRef] [PubMed]
- Seo, D.Y.; Lee, S.R.; Heo, J.W.; No, M.H.; Rhee, B.D.; Ko, K.S.; Kwak, H.B.; Han, J. Ursolic Acid in Health and Disease. Korean J. Physiol. Pharmacol. 2018, 22, 235–248. [Google Scholar] [CrossRef]
- Srinivasan, R.; Aruna, A.; Lee, J.S.; Kim, M.; Shivakumar, M.S.; Natarajan, D. Antioxidant and Antiproliferative Potential of Bioactive Molecules Ursolic Acid and Thujone Isolated from Memecylon Edule and Elaeagnus Indica and Their Inhibitory Effect on Topoisomerase II by Molecular Docking Approach. Biomed Res. Int. 2020, 2020, 8716927. [Google Scholar] [CrossRef]
- Bednarczyk-Cwynar, B.; Leśków, A.; Szczuka, I.; Zaprutko, L.; Diakowska, D. The Effect of Oleanolic Acid and Its Four New Semisynthetic Derivatives on Human MeWo and A375 Melanoma Cell Lines. Pharmaceuticals 2023, 16, 746. [Google Scholar] [CrossRef]
- Zhang, X.; Li, T.; Gong, E.S.; Liu, R.H. Antiproliferative Activity of Ursolic Acid in MDA-MB-231 Human Breast Cancer Cells through Nrf2 Pathway Regulation. J. Agric. Food Chem. 2020, 68, 7404–7415. [Google Scholar] [CrossRef]
- Lin, W.; Ye, H. Anticancer Activity of Ursolic Acid on Human Ovarian Cancer Cells via ROS and MMP Mediated Apoptosis, Cell Cycle Arrest and Downregulation of PI3K/AKT Pathway. J. BUON 2020, 25, 750–756. [Google Scholar]
- Liao, W.L.; Liu, Y.F.; Ying, T.H.; Shieh, J.C.; Hung, Y.T.; Lee, H.J.; Shen, C.Y.; Cheng, C.W. Inhibitory Effects of Ursolic Acid on the Stemness and Progression of Human Breast Cancer Cells by Modulating Argonaute-2. Int. J. Mol. Sci. 2023, 24, 366. [Google Scholar] [CrossRef]
- Zhou, D.; Bao, Q.; Fu, S. Anticancer Activity of Ursolic Acid on Retinoblastoma Cells Determined by Bioinformatics Analysis and Validation. Ann. Transl. Med. 2021, 9, 1548. [Google Scholar] [CrossRef]
- Majhi, S.; Das, D. Chemical Derivatization of Natural Products: Semisynthesis and Pharmacological Aspects- A Decade Update. Tetrahedron 2021, 78, 131801. [Google Scholar] [CrossRef]
- Isika, D.K.; Sadik, O.A. Selective Structural Derivatization of Flavonoid Acetamides Significantly Impacts Their Bioavailability and Antioxidant Properties. Molecules 2022, 27, 8133. [Google Scholar] [CrossRef]
- Baer-Dubowska, W.; Narożna, M.; Krajka-Kuźniak, V. Anti-Cancer Potential of Synthetic Oleanolic Acid Derivatives and Their Conjugates with Nsaids. Molecules 2021, 26, 4957. [Google Scholar] [CrossRef]
- Yadav, D.; Nath Mishra, B.; Khan, F. 3D-QSAR and Docking Studies on Ursolic Acid Derivatives for Anticancer Activity Based on Bladder Cell Line T24 Targeting NF-KB Pathway Inhibition. J. Biomol. Struct. Dyn. 2019, 37, 3822–3837. [Google Scholar] [CrossRef] [PubMed]
- Ou, K.; Xu, X.; Guan, S.; Zhang, R.; Zhang, X.; Kang, Y.; Wu, J. Nanodrug Carrier Based on Poly(Ursolic Acid) with Self-Anticancer Activity against Colorectal Cancer. Adv. Funct. Mater. 2020, 30, 1907857. [Google Scholar] [CrossRef]
- Liu, H.-R.; Ahmad, N.; Lv, B.; Li, C. Advances in Production and Structural Derivatization of the Promising Molecule Ursolic Acid. Proteomics 2021, 16, 2000657. [Google Scholar] [CrossRef]
- Qian, Z.; Wang, X.; Song, Z.; Zhang, H.; Zhou, S.; Zhao, J.; Wang, H. A Phase i Trial to Evaluate the Multiple-Dose Safety and Antitumor Activity of Ursolic Acid Liposomes in Subjects with Advanced Solid Tumors. Biomed. Res. Int. 2015, 2015, 809714. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.H.; Zhou, S.Y.; Qian, Z.Z.; Zhang, H.L.; Qiu, L.H.; Song, Z.; Zhao, J.; Wang, P.; Hao, X.S.; Wang, H.Q. Evaluation of Toxicity and Single-Dose Pharmacokinetics of Intravenous Ursolic Acid Liposomes in Healthy Adult Volunteers and Patients with Advanced Solid Tumors. Expert Opin. Drug Metab. Toxicol. 2013, 9, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Khwaza, V.; Oyedeji, O.O.; Aderibigbe, B.A. Ursolic Acid-Based Derivatives as Potential Anti-Cancer Agents: An Update. Int. J. Mol. Sci. 2020, 21, 5920. [Google Scholar] [CrossRef]
- Mancha-Ramirez, A.M.; Slaga, T.J. Ursolic Acid and Chronic Disease: An Overview of UA’s Effects on Prevention and Treatment of Obesity and Cancer. Adv. Exp. Med. Biol. 2016, 928, 75–96. [Google Scholar] [CrossRef]
- Pathak, A.K.; Bhutani, M.; Nair, A.S.; Kwang, S.A.; Chakraborty, A.; Kadara, H.; Guha, S.; Sethi, G.; Aggarwal, B.B. Ursolic Acid Inhibits STAT3 Activation Pathway Leading to Suppression of Proliferation and Chemosensitization of Human Multiple Myeloma Cells. Mol. Cancer Res. 2007, 5, 943–955. [Google Scholar] [CrossRef] [PubMed]
- Prasad, S.; Yadav, V.R.; Sung, B.; Reuter, S.; Kannappan, R.; Deorukhkar, A.; Diagaradjane, P.; Wei, C.; Baladandayuthapani, V.; Krishnan, S.; et al. Ursolic Acid Inhibits Growth and Metastasis of Human, Colorectal Cancer in an Orthotopic Nude Mouse Model by Targeting Multiple Cell Signaling Pathways: Chemosensitization with Capecitabine. Clin. Cancer Res. 2012, 18, 4942–4953. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Chu, X.W.; Liu, C.; Sheng, L.X.; Chen, Z.X.; Cheng, K.G. Antiproliferative Activity of Ursolic Acid/Glycyrrhetinic Acid-Uracil/Thymine Hybrids. Med. Chem. Res. 2019, 28, 892–899. [Google Scholar] [CrossRef]
- Wang, S.; Meng, X.; Dong, Y. Ursolic Acid Nanoparticles Inhibit Cervical Cancer Growth in Vitro and in Vivo via Apoptosis Induction. Int. J. Oncol. 2017, 50, 1330–1340. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B. Understanding Mechanisms of Antioxidant Action in Health and Disease. Nat. Rev. Mol. Cell Biol. 2024, 25, 13–33. [Google Scholar] [CrossRef] [PubMed]
- Didier, A.J.; Stiene, J.; Fang, L.; Watkins, D.; Dworkin, L.D.; Creeden, J.F. Antioxidant and Anti-Tumor Effects of Dietary Vitamins A, C, and E. Antioxidants 2023, 12, 632. [Google Scholar] [CrossRef]
- Luo, M.; Zhou, L.; Huang, Z.; Li, B.; Nice, E.C.; Xu, J.; Huang, C. Antioxidant Therapy in Cancer: Rationale and Progress. Antioxidants 2022, 11, 1128. [Google Scholar] [CrossRef] [PubMed]
- Yin, M.C.; Chan, K.C. Nonenzymatic Antioxidative and Antiglycative Effects of Oleanolic Acid and Ursolic Acid. J. Agric. Food Chem. 2007, 55, 7177–7181. [Google Scholar] [CrossRef] [PubMed]
- Samsonowicz, M.; Kalinowska, M.; Gryko, K. Enhanced Antioxidant Activity of Ursolic Acid by Complexation with Copper (II): Experimental and Theoretical Study. Materials 2021, 14, 264. [Google Scholar] [CrossRef]
- Do Nascimento, P.G.G.; Lemos, T.L.G.; Bizerra, A.M.C.; Arriaga, A.M.C.; Ferreira, D.A.; Santiago, G.M.P.; Braz-Filho, R.; Costa, J.G.M. Antibacterial and Antioxidant Activities of Ursolic Acid and Derivatives. Molecules 2014, 19, 1317–1327. [Google Scholar] [CrossRef]
- Tsai, S.J.; Yin, M.C. Antioxidative and Anti-Inflammatory Protection of Oleanolic Acid and Ursolic Acid in PC12 Cells. J. Food Sci. 2008, 73, 174–178. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.Q.; Ding, J.; Zhang, L.; Liu, C.M. Protective Effects of Ursolic Acid in an Experimental Model of Liver Fibrosis through Nrf2/ARE Pathway. Clin. Res. Hepatol. Gastroenterol. 2015, 39, 188–197. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, S.; Prasad, N.R. Effect of Ursolic Acid, a Triterpenoid Antioxidant, on Ultraviolet-B Radiation-Induced Cytotoxicity, Lipid Peroxidation and DNA Damage in Human Lymphocytes. Chem. Biol. Interact. 2008, 176, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, S.; Rajendra Prasad, N.; Pugalendi, K.V.; Menon, V.P. Modulation of UVB-Induced Oxidative Stress by Ursolic Acid in Human Blood Lymphocytes. Asian J. Biochem. 2008, 3, 11–18. [Google Scholar] [CrossRef]
- Jin, H.; Pi, J.; Yang, F.; Jiang, J.; Wang, X.; Bai, H.; Shao, M.; Huang, L.; Zhu, H.; Yang, P.; et al. Folate-Chitosan Nanoparticles Loaded with Ursolic Acid Confer Anti-Breast Cancer Activities in Vitro and in Vivo. Sci. Rep. 2016, 6, 30782. [Google Scholar] [CrossRef] [PubMed]
- Gou, W.; Luo, N.; Wei, H.; Wu, H.; Yu, X.; Duan, Y.; Bi, C.; Ning, H.; Hou, W.; Li, Y. Ursolic Acid Derivative UA232 Evokes Apoptosis of Lung Cancer Cells Induced by Endoplasmic Reticulum Stress. Pharm. Biol. 2020, 58, 707–715. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.Y.; Chen, H.; Li, D.D.; Li, A.L.; Wang, W.Y.; Gu, W. Design, Synthesis, and Anticancer Evaluation of Novel Quinoline Derivatives of Ursolic Acid with Hydrazide, Oxadiazole, and Thiadiazole Moieties as Potent MEK Inhibitors. J. Enzyme Inhib. Med. Chem. 2019, 34, 955–972. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Kang, S.; Chen, H.; Bahetjan, Y.; Zhang, J.; Lu, R.; Zheng, N.; Yang, G.; Yang, X. A Composition of Ursolic Acid Derivatives from Ludwigia Hyssopifolia Induces Apoptosis in Throat Cancer Cells via the Akt/MTOR and Mitochondrial Signaling Pathways and by Modulating Endoplasmic Reticulum Stress. J. Ethnopharmacol. 2024, 319, 117351. [Google Scholar] [CrossRef] [PubMed]
- da Silva, E.F.; de Vargas, A.S.; Willig, J.B.; de Oliveira, C.B.; Zimmer, A.R.; Pilger, D.A.; Buffon, A.; Gnoatto, S.C.B. Synthesis and Antileukemic Activity of an Ursolic Acid Derivative: A Potential Co-Drug in Combination with Imatinib. Chem. Biol. Interact. 2021, 344, 109535. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.Q.; Xu, C.D.; Yu, T.T.; Li, W.; Li, Q.W.; Li, X.X. Synthesis and Antitumor Activity Evaluation of Ursolic Acid Derivatives. J. Asian Nat. Prod. Res. 2020, 22, 359–369. [Google Scholar] [CrossRef]
- Li, W.; Zhang, H.; Nie, M.; Wang, W.; Liu, Z.; Chen, C.; Chen, H.; Liu, R.; Baloch, Z.; Ma, K. A Novel Synthetic Ursolic Acid Derivative Inhibits Growth and Induces Apoptosis in Breast Cancer Cell Lines. Oncol. Lett. 2018, 15, 2323–2329. [Google Scholar] [CrossRef] [PubMed]
- Fontana, G.; Bruno, M.; Notarbartolo, M.; Labbozzetta, M.; Poma, P.; Spinella, A.; Rosselli, S. Cytotoxicity of Oleanolic and Ursolic Acid Derivatives toward Hepatocellular Carcinoma and Evaluation of NF-ΚB Involvement. Bioorg. Chem. 2019, 90, 103054. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zhang, Z.H.; Zhang, L.H.; Jin, X.J.; Ma, J.; Piao, H.R. Design, Synthesis, and Screening of Novel Ursolic Acid Derivatives as Potential Anti-Cancer Agents That Target the HIF-1α Pathway. Bioorganic Med. Chem. Lett. 2019, 29, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.Y.; Wu, W.Y.; Li, A.L.; Liu, Q.S.; Sun, Y.; Gu, W. Synthesis, Anticancer Evaluation and Mechanism Studies of Novel Indolequinone Derivatives of Ursolic Acid. Bioorg. Chem. 2021, 109, 104705. [Google Scholar] [CrossRef] [PubMed]
- Rashid, S.; Dar, B.A.; Majeed, R.; Hamid, A.; Bhat, B.A. Synthesis and Biological Evaluation of Ursolic Acid-Triazolyl Derivatives as Potential Anti-Cancer Agents. Eur. J. Med. Chem. 2013, 66, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Dar, B.A.; Lone, A.M.; Shah, W.A.; Qurishi, M.A. Synthesis and Screening of Ursolic Acid-Benzylidine Derivatives as Potential Anti-Cancer Agents. Eur. J. Med. Chem. 2016, 111, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.Y.; Sp, N.; Lee, J.M.; Jang, K.J. Antitumor Effects of Ursolic Acid through Mediating the Inhibition of STAT3/PD-L1 Signaling in Non-Small Cell Lung Cancer Cells. Biomedicines 2021, 9, 297. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Chang, X.; Zhang, J.; Li, J.; Wang, N.; Yang, B.; Pan, B.; Zheng, Y.; Wang, X.; Ou, H.; et al. Ursolic Acid Inhibits Breast Cancer Metastasis by Suppressing Glycolytic Metabolism via Activating SP1/Caveolin-1 Signaling. Front. Oncol. 2021, 11, 745584. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Xu, B.; Wang, X.; Zheng, B.; Du, J.; Liu, S. The Analysis of the Anti-Tumor Mechanism of Ursolic Acid Using Connectively Map Approach in Breast Cancer Cells Line MCF-7. Cancer Manag. Res. 2020, 12, 3469–3476. [Google Scholar] [CrossRef]
- Lin, C.W.; Chin, H.K.; Lee, S.L.; Chiu, C.F.; Chung, J.G.; Lin, Z.Y.; Wu, C.Y.; Liu, Y.C.; Hsiao, Y.T.; Feng, C.H.; et al. Ursolic Acid Induces Apoptosis and Autophagy in Oral Cancer Cells. Environ. Toxicol. 2019, 34, 983–991. [Google Scholar] [CrossRef]
- Liu, M.C.; Yang, S.J.; Jin, L.H.; Hu, D.Y.; Xue, W.; Song, B.A.; Yang, S. Synthesis and Cytotoxicity of Novel Ursolic Acid Derivatives Containing an Acyl Piperazine Moiety. Eur. J. Med. Chem. 2012, 58, 128–135. [Google Scholar] [CrossRef]
- Gu, W.; Jin, X.Y.; Li, D.D.; Wang, S.F.; Tao, X.B.; Chen, H. Design, Synthesis and in Vitro Anticancer Activity of Novel Quinoline and Oxadiazole Derivatives of Ursolic Acid. Bioorganic Med. Chem. Lett. 2017, 27, 4128–4132. [Google Scholar] [CrossRef]
- Ma, C.M.; Cai, S.Q.; Cui, J.R.; Wang, R.Q.; Tu, P.F.; Hattori, M.; Daneshtalab, M. The Cytotoxic Activity of Ursolic Acid Derivatives. Eur. J. Med. Chem. 2005, 40, 582–589. [Google Scholar] [CrossRef]
- Xu, J.; Wang, X.; Zhang, H.; Yue, J.; Sun, Y.; Zhang, X.; Zhao, Y. Synthesis of Triterpenoid Derivatives and Their Anti-Tumor and Anti-Hepatic Fibrosis Activities. Nat. Prod. Res. 2020, 34, 766–772. [Google Scholar] [CrossRef] [PubMed]
- Tian, T.; Liu, X.; Lee, E.S.; Sun, J.; Feng, Z.; Zhao, L.; Zhao, C. Synthesis of Novel Oleanolic Acid and Ursolic Acid in C-28 Position Derivatives as Potential Anticancer Agents. Arch. Pharm. Res. 2017, 40, 458–468. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Chen, Y.; Wei, L.; Hong, Z.; Sferra, T.J.; Peng, J. Ursolic Acid Inhibits Colorectal Cancer Angiogenesis through Suppression of Multiple Signaling Pathways. Int. J. Oncol. 2013, 43, 1666–1674. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Men, X.; Lei, P. Review on Anti-Tumor Effect of Triterpene Acid Compounds. J. Cancer Res. Ther. 2014, 10, C14–C19. [Google Scholar] [CrossRef]
- Sołtys, A.; Galanty, A.; Zagrodzki, P.; Grabowska, K.; Malarz, J.; Podolak, I. Sorbus Intermedia (EHRH.) PERS. Fruits as a Novel Source of Biologically Active Triterpenoids—Comparative Studies of Ursolic Acid Derivatives with Cytotoxic Potential. Biomed. Pharmacother. 2022, 154, 113592. [Google Scholar] [CrossRef]
- Prasad, S.; Yadav, V.R.; Sung, B.; Gupta, S.C.; Tyagi, A.K.; Aggarwal, B.B. Ursolic Acid Inhibits the Growth of Human Pancreatic Cancer and Enhances the Antitumor Potential of Gemcitabine in an Orthotopic Mouse Model through Suppression of the Inflammatory Microenvironment. Oncotarget 2016, 7, 13182–13196. [Google Scholar] [CrossRef]
- Shan, J.; Xuan, Y.; Zhang, Q.; Zhu, C.; Liu, Z.; Zhang, S. Ursolic Acid Synergistically Enhances the Therapeutic Effects of Oxaliplatin in Colorectal Cancer. Protein Cell 2016, 7, 571–585. [Google Scholar] [CrossRef][Green Version]
- Sen, A. Prophylactic and Therapeutic Roles of Oleanolic Acid and Its Derivatives in Several Diseases. World J. Clin. Cases 2020, 8, 1767–1792. [Google Scholar] [CrossRef] [PubMed]
- Duan, L.; Yang, Z.; Jiang, X.; Zhang, J.; Guo, X. Oleanolic Acid Inhibits Cell Proliferation Migration and Invasion and Induces SW579 Thyroid Cancer Cell Line Apoptosis by Targeting Forkhead Transcription Factor A. Anticancer Drugs 2019, 30, 812–820. [Google Scholar] [CrossRef] [PubMed]
- Kazakova, O.; Rubanik, L.; Smirnova, I.; Poleschuk, N.; Petrova, A.; Kapustsina, Y.; Baikova, I.; Tret’yakova, E.; Khusnutdinova, E. Synthesis and in Vitro Activity of Oleanolic Acid Derivatives against Chlamydia Trachomatis and Staphylococcus Aureus. Med. Chem. Res. 2021, 30, 1408–1418. [Google Scholar] [CrossRef]
- Yang, Y.H.; Dai, S.Y.; Deng, F.H.; Peng, L.H.; Li, C.; Pei, Y.H. Recent Advances in Medicinal Chemistry of Oleanolic Acid Derivatives. Phytochemistry 2022, 203, 113397. [Google Scholar] [CrossRef] [PubMed]
- An, J.Y.; Yang, H.S.; Park, N.R.; Koo, T.; Shin, B.; Lee, E.H.; Cho, S.H. Development of Polymeric Micelles of Oleanolic Acid and Evaluation of Their Clinical Efficacy. Nanoscale Res. Lett. 2020, 15, 133. [Google Scholar] [CrossRef] [PubMed]
- Sasikumar, K.; Dubey, V.; Ghosh, A.R. Oleanolic Acid from Black Raisins, Vitis Vinifera with Antioxidant and Antiproliferative Potentials on Hct 116 Colon Cancer Cell Line. Brazilian J. Pharm. Sci. 2020, 56, e17158. [Google Scholar] [CrossRef]
- García-González, A.; Espinosa-Cabello, J.M.; Cerrillo, I.; Montero-Romero, E.; Rivas-Melo, J.J.; Romero-Báez, A.; Jiménez-Andreu, M.D.; Ruíz-Trillo, C.A.; Rodríguez-Rodríguez, A.; Martínez-Ortega, A.J.; et al. Bioavailability and Systemic Transport of Oleanolic Acid in Humans, Formulated as a Functional Olive Oil. Food Funct. 2023, 14, 9681–9694. [Google Scholar] [CrossRef] [PubMed]
- Castellano, J.M.; Ramos-Romero, S.; Perona, J.S. Oleanolic Acid: Extraction, Characterization and Biological Activity. Nutrients 2022, 14, 623. [Google Scholar] [CrossRef] [PubMed]
- Nie, H.; Wang, Y.; Qin, Y.; Gong, X.G. Oleanolic Acid Induces Autophagic Death in Human Gastric Cancer Cells in Vitro and in Vivo. Cell Biol. Int. 2016, 40, 770–778. [Google Scholar] [CrossRef] [PubMed]
- Mo, W.; Su, C.; Huang, J.; Liu, J.; Chen, Z.; Cheng, K. Synthesis of Acyl Oleanolic Acid-Uracil Conjugates and Their Anti-Tumor Activity. Chem. Cent. J. 2016, 10, 69. [Google Scholar] [CrossRef]
- Lin, C.; Wen, X.; Sun, H. Oleanolic Acid Derivatives for Pharmaceutical Use: A Patent Review. Expert Opin. Ther. Pat. 2016, 26, 643–655. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.K.; Wang, Q.W.; Xu, Y.J.; Sun, H.M.; Wang, L.; Zhang, L.X. Co-Delivery of Cisplatin and Oleanolic Acid by Silica Nanoparticles-Enhanced Apoptosis and Reverse Multidrug Resistance in Lung Cancer. Kaohsiung J. Med. Sci. 2021, 37, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Hu, Z.; Xing, H.; Kang, L.; Chen, X.; Liu, B.; Niu, K. Renoprotective Effects of Oleanolic Acid and Its Possible Mechanisms in Rats with Diabetic Kidney Disease. Biochem. Biophys. Res. Commun. 2022, 636, 1–9. [Google Scholar] [CrossRef]
- Mallavadhani, U.V.; Vanga, N.R.; Jeengar, M.K.; Naidu, V.G.M. Synthesis of Novel Ring-A Fused Hybrids of Oleanolic Acid with Capabilities to Arrest Cell Cycle and Induce Apoptosis in Breast Cancer Cells. Eur. J. Med. Chem. 2014, 74, 398–404. [Google Scholar] [CrossRef] [PubMed]
- Khwaza, V.; Oyedeji, O.O.; Aderibigbe, B.A. Antiviral Activities of Oleanolic Acid and Its Analogues. Molecules 2018, 23, 2300. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.J.; Jo, H.J.; Lee, K.J.; Choi, J.W.; An, J.H. Oleanolic Acid Induces P53-Dependent Apoptosis via the ERK/JNK/AKT Pathway in Cancer Cell Lines in Prostatic Cancer Xenografts in Mice. Oncotarget 2018, 9, 26370–26386. [Google Scholar] [CrossRef] [PubMed]
- Jo, H.; Oh, J.H.; Park, D.W.; Lee, C.; Min, C.K. Oleanolic Acid 3-Acetate, a Minor Element of Ginsenosides, Induces Apoptotic Cell Death in Ovarian Carcinoma and Endometrial Carcinoma Cells via the Involvement of a Reactive Oxygen Species–Independent Mitochondrial Pathway. J. Ginseng Res. 2020, 44, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.M.; Alvarado, H.L.; Abrego, G.; Martins-Gomes, C.; Garduño-Ramirez, M.L.; García, M.L.; Calpena, A.C.; Souto, E.B. In Vitro Cytotoxicity of Oleanolic/Ursolic Acids-Loaded in PLGA Nanoparticles in Different Cell Lines. Pharmaceutics 2019, 11, 362. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ye, X.; Liu, R.; Chen, H.L.; Bai, H.; Liang, X.; Zhang, X.; Wang, Z.; Li, W.; Hai, C.X. Antioxidant Activities of Oleanolic Acid in Vitro: Possible Role of Nrf2 and MAP Kinases. Chem. Biol. Interact. 2010, 184, 328–337. [Google Scholar] [CrossRef]
- Ghafoor, K. Antioxidant Properties of Oleanolic Acid from Grape Peel. Agro Food Ind. Hi. Tech. 2014, 25, 54–57. [Google Scholar]
- Feng, A.; Yang, S.; Sun, Y.; Zhang, L.; Bo, F.; Li, L. Development and Evaluation of Oleanolic Acid Dosage Forms and Its Derivatives. Biomed. Res. Int. 2020, 2020, 1308749. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Yang, W.; Fan, Y.; Dehaen, W.; Li, Y.; Li, H.; Wang, W.; Zheng, Q.; Huai, Q. Design and Synthesis of the Novel Oleanolic Acid-Cinnamic Acid Ester Derivatives and Glycyrrhetinic Acid-Cinnamic Acid Ester Derivatives with Cytotoxic Properties. Bioorg. Chem. 2019, 88, 102951. [Google Scholar] [CrossRef] [PubMed]
- Narożna, M.; Krajka-Kuźniak, V.; Bednarczyk-Cwynar, B.; Kucińska, M.; Kleszcz, R.; Kujawski, J.; Piotrowska-Kempisty, H.; Plewiński, A.; Murias, M.; Baer-Dubowska, W. Conjugation of Diclofenac with Novel Oleanolic Acid Derivatives Modulate Nrf2 and Nf-Κb Activity in Hepatic Cancer Cells and Normal Hepatocytes Leading to Enhancement of Its Therapeutic and Chemopreventive Potential. Pharmaceuticals 2021, 14, 688. [Google Scholar] [CrossRef] [PubMed]
- Halil, Ş.; Berre, M.; Rabia Büşra, Ş.; Halil Burak, K.; Ebru, H. Synthesis of Oleanolic Acid Hydrazide-Hydrazone Hybrid Derivatives and Investigation of Their Cytotoxic Effects on A549 Human Lung Cancer Cells. Results Chem. 2022, 4, 100317. [Google Scholar] [CrossRef]
- Şenol, H.; Ghaffari-Moghaddam, M.; Bulut, Ş.; Akbaş, F.; Köse, A.; Topçu, G. Synthesis and Anticancer Activity of Novel Derivatives of α,β-Unsaturated Ketones Based on Oleanolic Acid: In Vitro and in Silico Studies against Prostate Cancer Cells. Chem. Biodivers. 2023, 20, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Zuo, Q.; Jiang, T.; Song, H.; Zhou, J. A Newly Synthesized Oleanolic Acid Derivative Inhibits the Growth of Osteosarcoma Cells in Vitro and in Vivo by Decreasing C-MYC-Dependent Glycolysis. J. Cell. Biochem. 2019, 120, 9264–9276. [Google Scholar] [CrossRef] [PubMed]
- Speranza, G.; Gutierrez, M.E.; Kummar, S.; Strong, J.M.; Parker, R.J.; Collins, J.; Yu, Y.; Cao, L.; Murgo, A.J.; Doroshow, J.H.; et al. Phase i Study of the Synthetic Triterpenoid, 2-Cyano-3, 12-Dioxoolean-1, 9-Dien-28-Oic Acid (CDDO), in Advanced Solid Tumors. Cancer Chemother. Pharmacol. 2012, 69, 431–438. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hong, D.S.; Kurzrock, R.; Supko, J.G.; He, X.; Naing, A.; Wheler, J.; Lawrence, D.; Eder, J.P.; Meyer, C.J.; Ferguson, D.A.; et al. A Phase I First-in-Human Trial of Bardoxolone Methyl in Patients with Advanced Solid Tumors and Lymphomas. Clin. Cancer Res. 2012, 18, 3396–3406. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.Q.; Zhao, Y.W.; Kuai, Z.Y.; Liu, L.W.; Li, W. Synthesis and Antitumor Activity Evaluation of Novel Oleanolic Acid Derivatives. J. Asian Nat. Prod. Res. 2017, 19, 1000–1010. [Google Scholar] [CrossRef]
- Huang, D.; Ding, Y.; Li, Y.; Zhang, W.; Fang, W.; Chen, X. Anti-Tumor Activity of a 3-Oxo Derivative of Oleanolic Acid. Cancer Lett. 2006, 233, 289–296. [Google Scholar] [CrossRef]
- Hao, J.; Liu, J.; Wen, X.; Sun, H. Synthesis and Cytotoxicity Evaluation of Oleanolic Acid Derivatives. Bioorganic Med. Chem. Lett. 2013, 23, 2074–2077. [Google Scholar] [CrossRef] [PubMed]
- Borella, R.; Forti, L.; Gibellini, L.; De Gaetano, A.; De Biasi, S.; Nasi, M.; Cossarizza, A.; Pinti, M. Synthesis and Anticancer Activity of CDDO and CDDO-Me, Two Derivatives of Natural Triterpenoids. Molecules 2019, 24, 4097. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Sun, W.; Peng, W.; Yu, R.; Li, G.; Jiang, T. Pharmacokinetics in Vitro and in Vivo of Two Novel Prodrugs of Oleanolic Acid in Rats and Its Hepatoprotective Effects against Liver Injury Induced by CCl4. Mol. Pharm. 2016, 13, 1699–1710. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Li, X.; Yu, S.; Liang, L. Inhibition of Cancer Cell Growth by Oleanolic Acid in Multidrug Resistant Liver Carcinoma Is Mediated via Suppression of Cancer Cell Migration and Invasion, Mitochondrial Apoptosis, G2/M Cell Cycle Arrest and Deactivation of JNK/P38 Signalling Pathway. J. BUON 2019, 24, 1964–1969. [Google Scholar] [PubMed]
- Woo, J.S.; Yoo, E.S.; Kim, S.H.; Lee, J.H.; Han, S.H.; Jung, S.H.; Jung, G.H.; Jung, J.Y. Anticancer Effects of Oleanolic Acid on Human Melanoma Cells. Chem. Biol. Interact. 2021, 347, 109619. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Wang, P.; Sun, Y.; Jiang, J.; Du, H.; Wang, Z.; Duan, Z.; Lei, H.; Li, H. Induction of Apoptosis by an Oleanolic Acid Derivative in SMMC-7721 Human Hepatocellular Carcinoma Cells Is Associated with Mitochondrial Dysfunction. Oncol. Lett. 2018, 15, 2821–2828. [Google Scholar] [CrossRef] [PubMed]
- Xiaofei, J.; Mingqing, S.; Miao, S.; Yizhen, Y.; Shuang, Z.; Qinhua, X.; Kai, Z. Oleanolic Acid Inhibits Cervical Cancer Hela Cell Proliferation through Modulation of the ACSL4 Ferroptosis Signaling Pathway. Biochem. Biophys. Res. Commun. 2021, 545, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Cheon, S.Y.; Jin, B.R.; Kim, H.J.; An, H.J. Oleanolic Acid Ameliorates Benign Prostatic Hyperplasia by Regulating PCNA-Dependent Cell Cycle Progression in Vivo and in Vitro. J. Nat. Prod. 2020, 83, 1183–1189. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wei, L.; Shen, A.; Chu, J.; Lin, J.; Peng, J. Oleanolic Acid Modulates Multiple Intracellular Targets to Inhibit Colorectal Cancer Growth. Int. J. Oncol. 2015, 47, 2247–2254. [Google Scholar] [CrossRef]
- Furtado, R.A.; Rodrigues, É.P.; Araújo, F.R.R.; Oliveira, W.L.; Furtado, M.A.; Castro, M.B.; Cunha, W.R.; Tavares, D.C. Ursolic Acid and Oleanolic Acid Suppress Preneoplastic Lesions Induced by 1,2-Dimethylhydrazine in Rat Colon. Toxicol. Pathol. 2008, 36, 576–580. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Yang, Y.X.; Zhe, H.; He, Z.X.; Zhou, S.F. Bardoxolone Methyl (CDDO-Me) as a Therapeutic Agent: An Update on Its Pharmacokinetic and Pharmacodynamic Properties. Drug Des. Devel. Ther. 2014, 8, 2075–2088. [Google Scholar] [CrossRef] [PubMed]
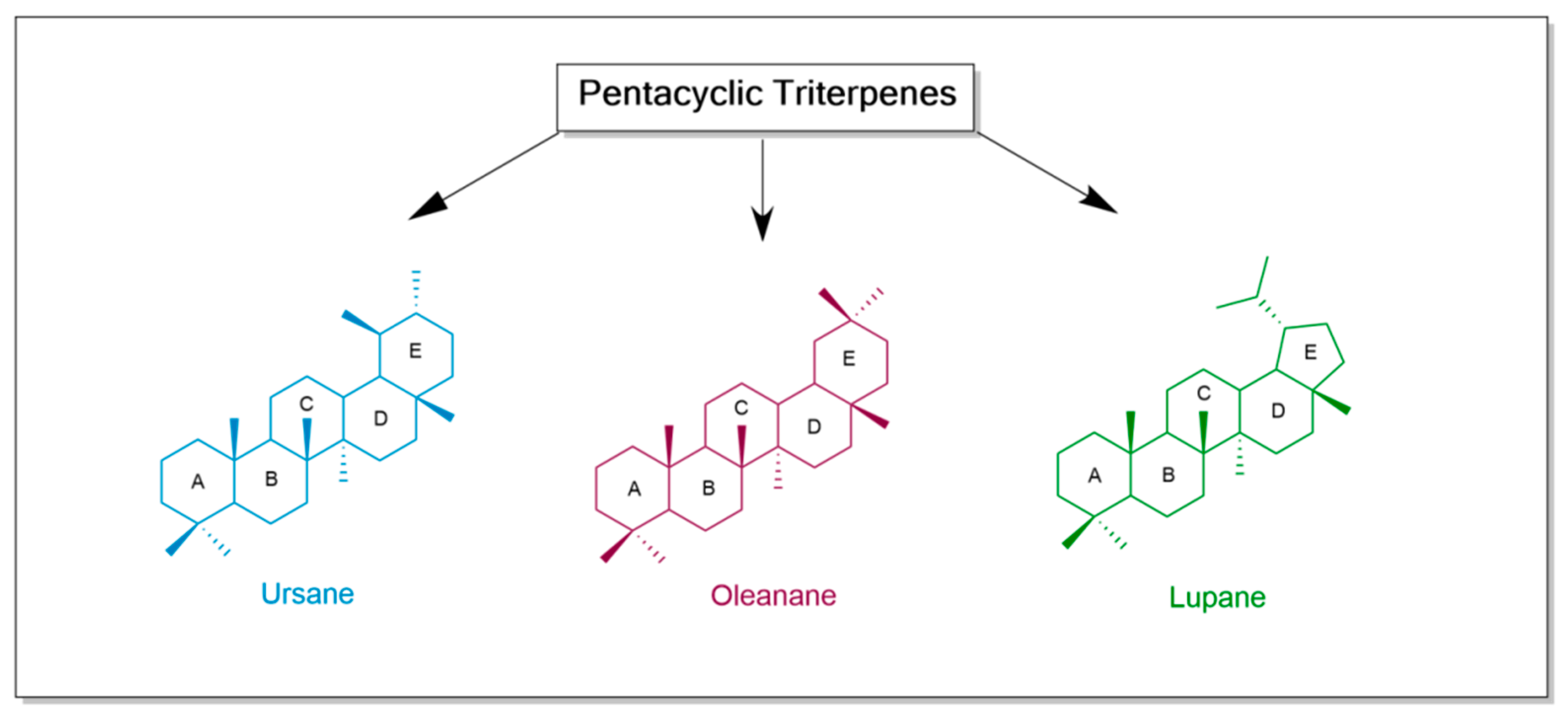

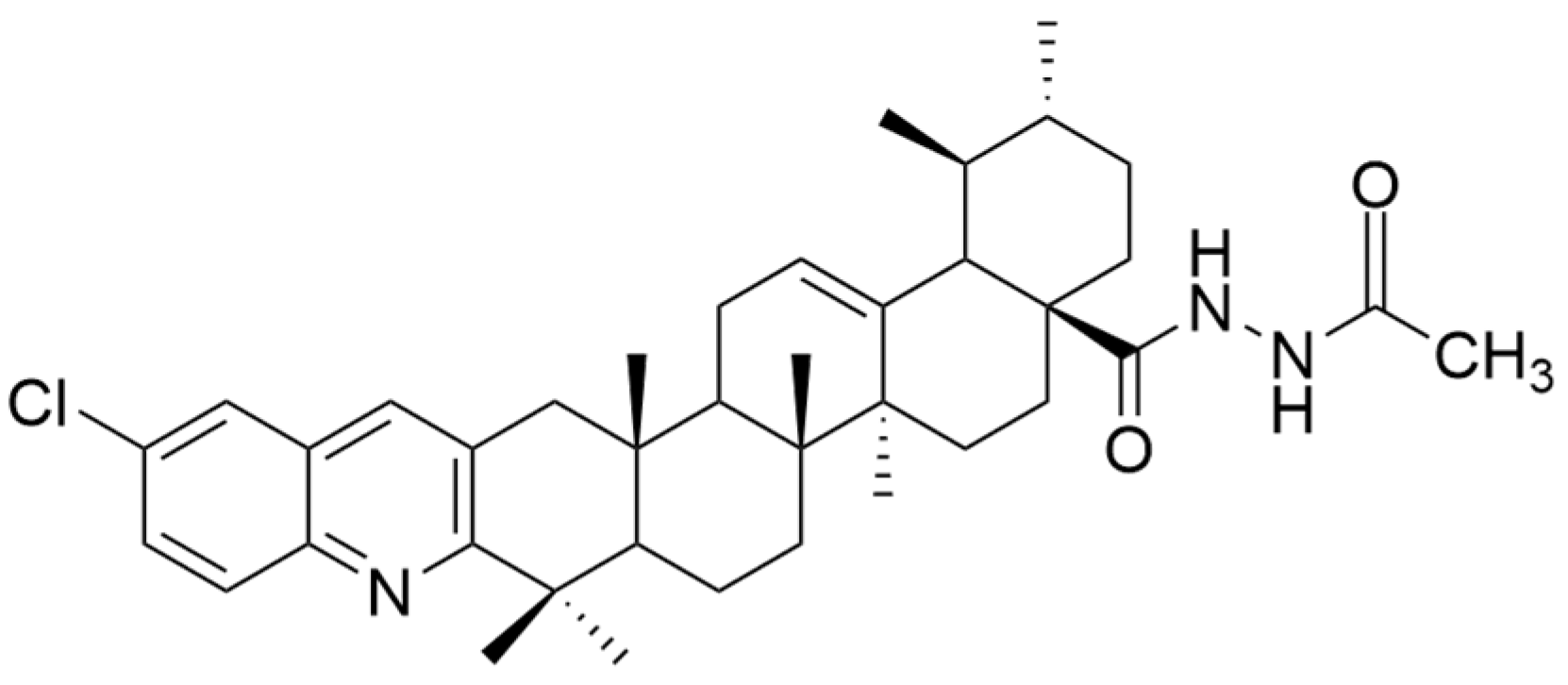
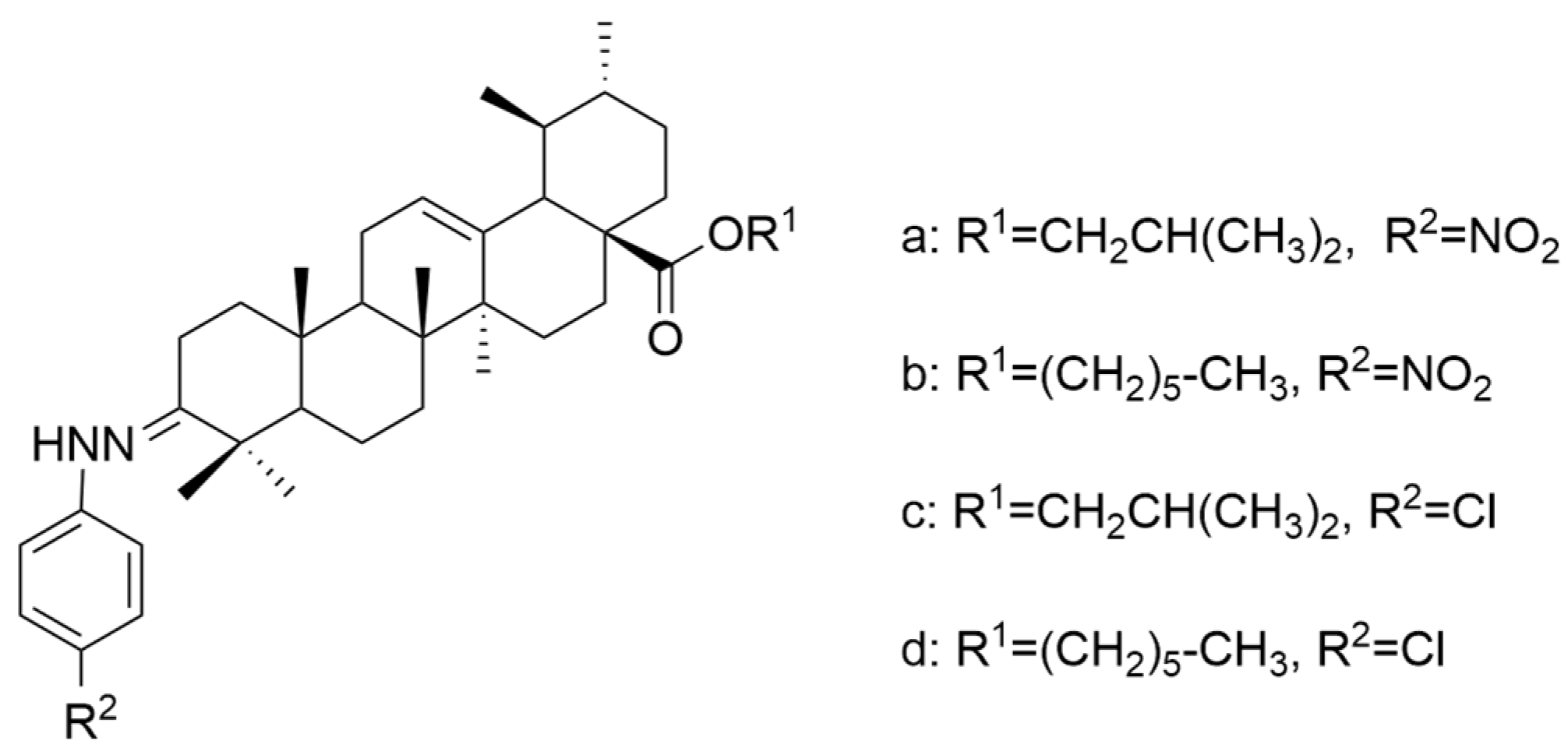
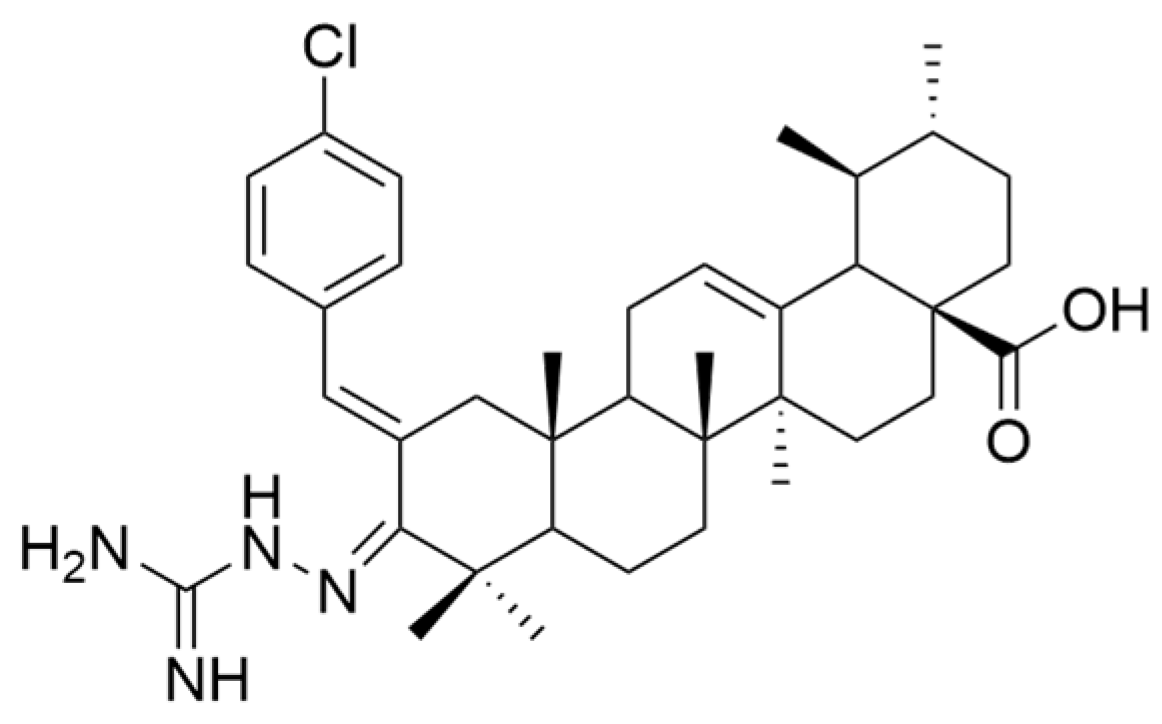
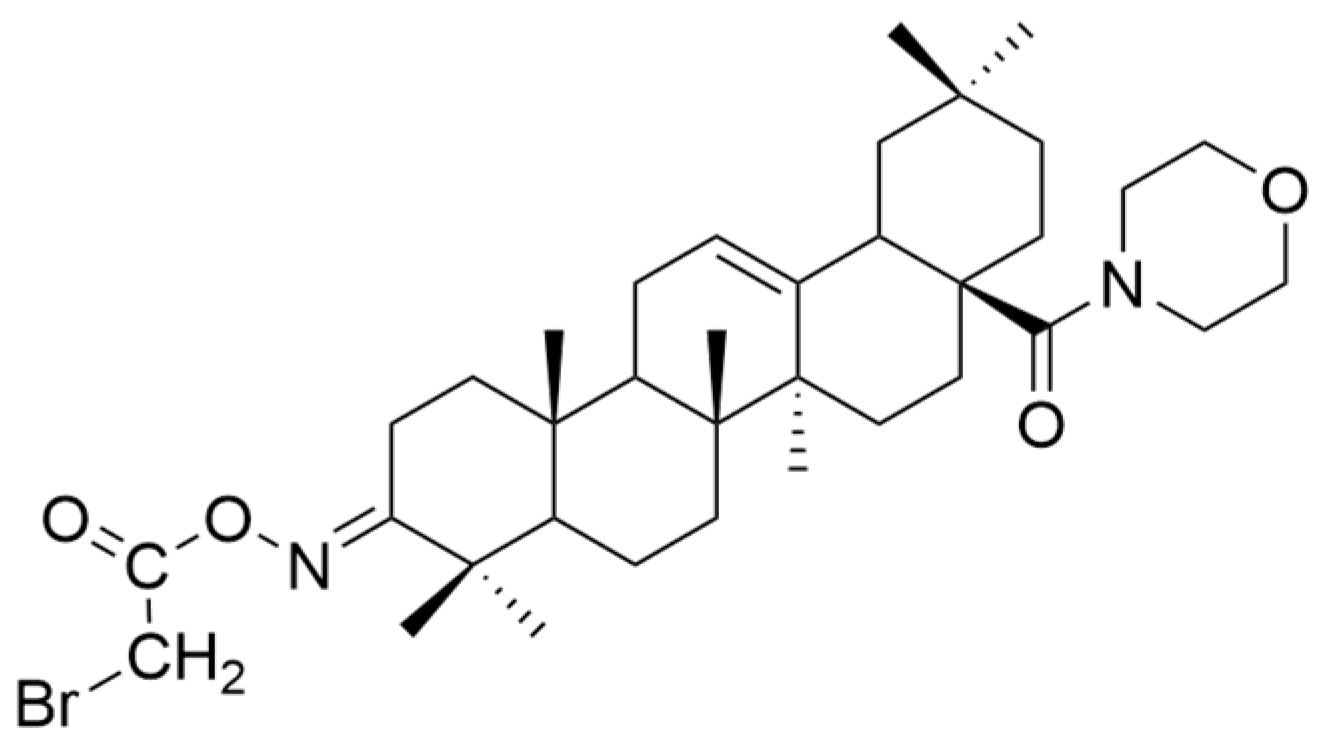
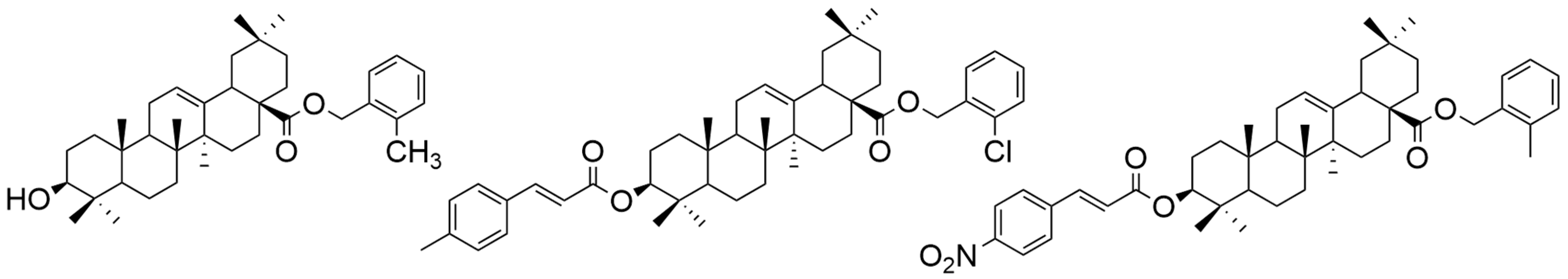

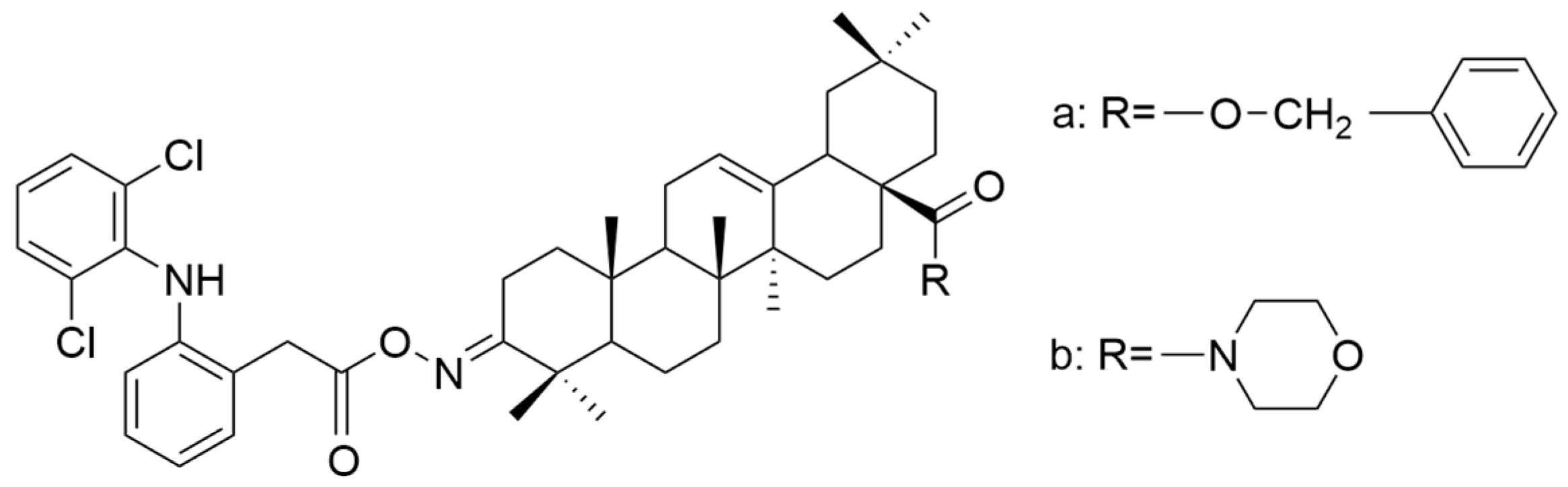

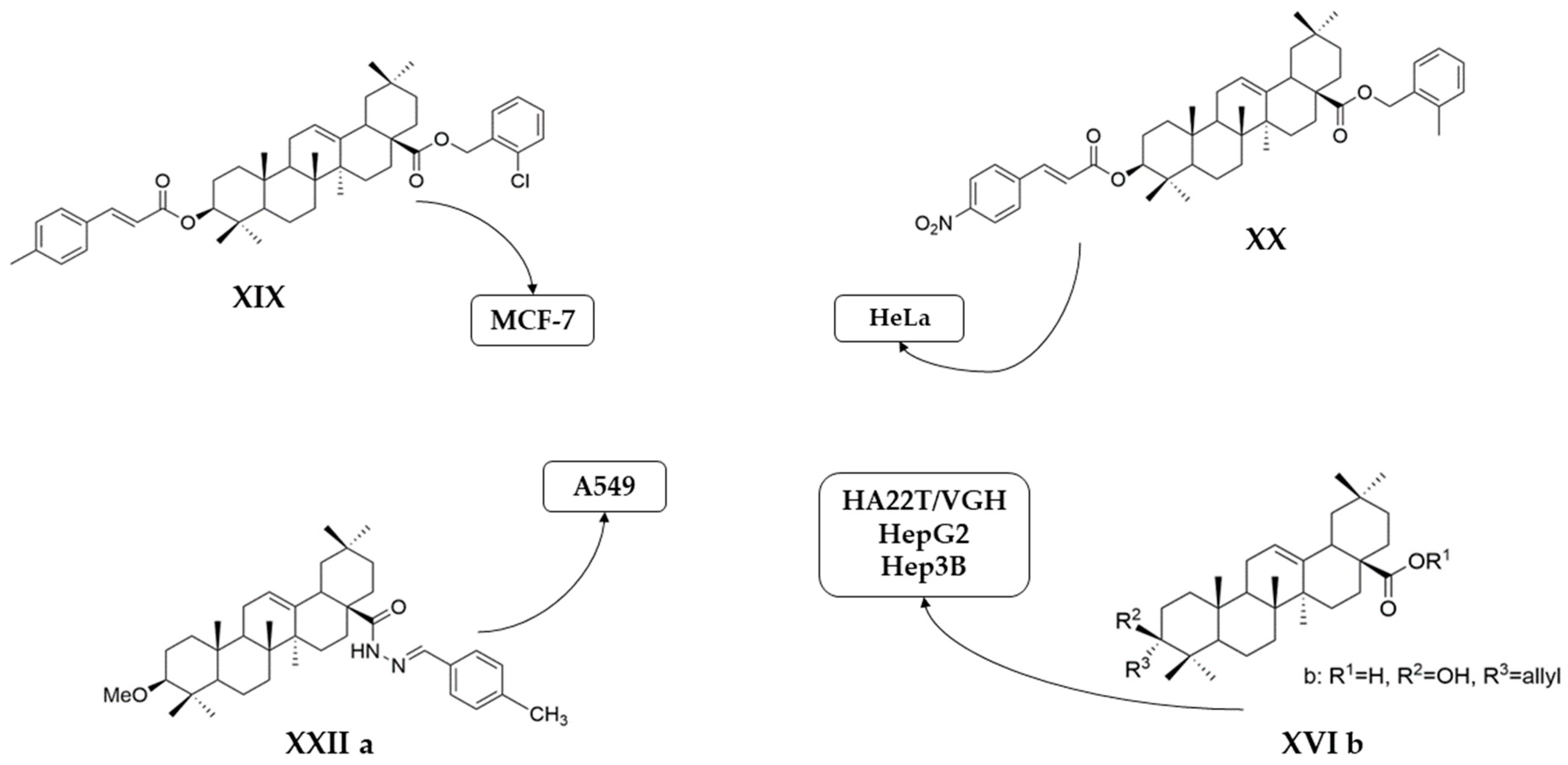
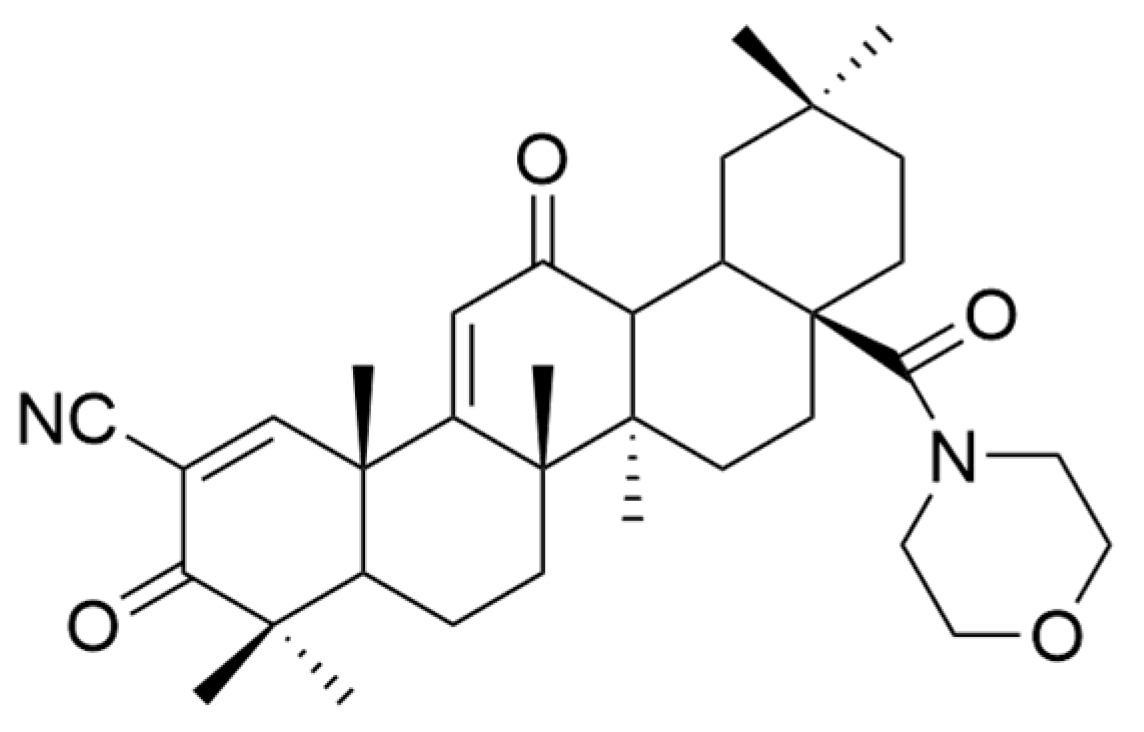
| Number | Chemical Structure | Reference |
|---|---|---|
| I | 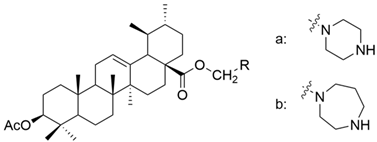 | [22] |
| II | 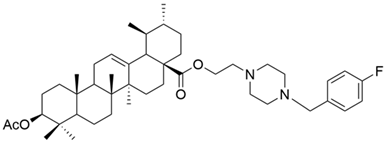 | [22] |
| III |  | [22] |
| IV | 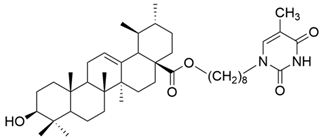 | [43] |
| V | 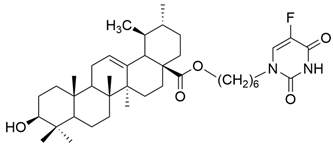 | [16] |
| VI | 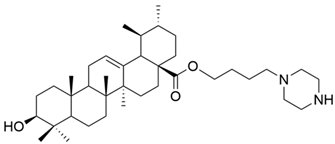 | [56] |
| VII | 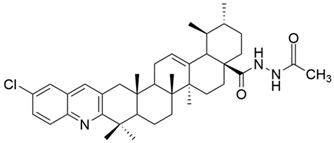 | [57] |
| VIII | 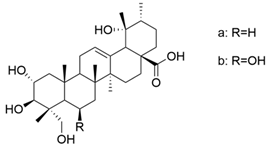 | [58] |
| IX | 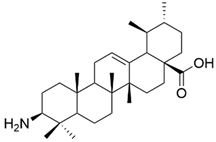 | [59] |
| X | 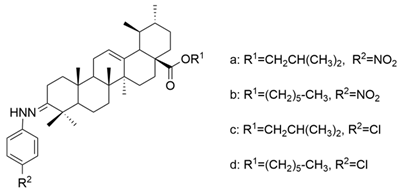 | [60] |
| XI | 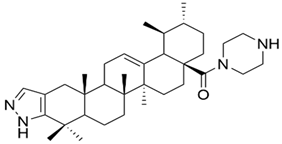 | [61] |
| XII | 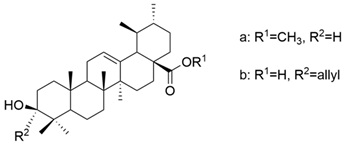 | [62] |
| XIII | 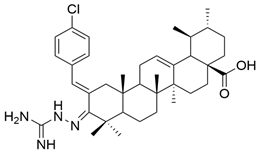 | [63] |
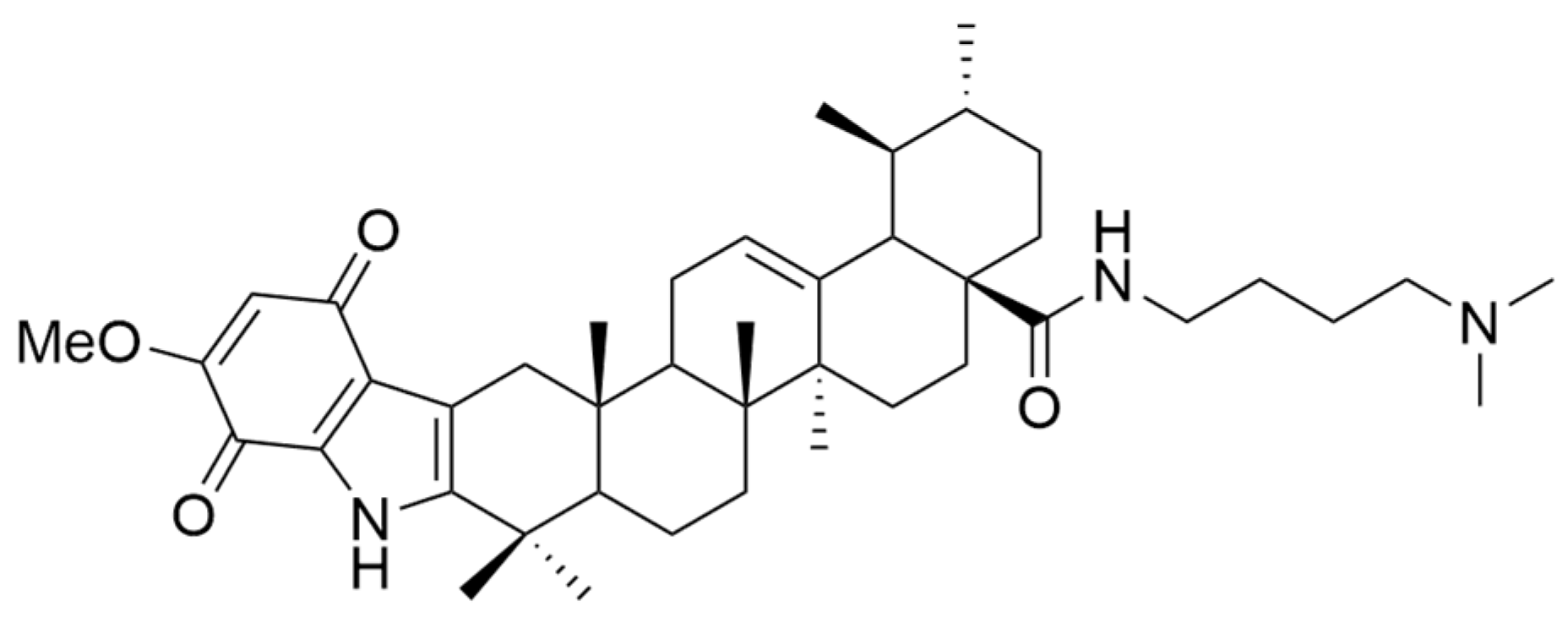
| XIV |  | [64] |
| Compound | Type of Cancer Cell Line | Cell Line | In Vitro | Conclusions | Reference |
|---|---|---|---|---|---|
| UA | human leukemic monocyte lymphoma | U-937 | MTT IC50 = 36.59 μM | HL-60 cell line was more sensitive to the antiproliferative action of UA compared to the U-937 line. | [25] |
| human acute promyelocytic leukemia | HT-60 | MTT IC50 = 26.83 μM | [25] | ||
| human breast cancer | MDA-MB-231 | Methylene blue assay (72 h) (antiproliferative activity) EC50 = 18.12 μM | UA significantly inhibited the proliferation of MCF-7 cells in a dose-dependent manner without being cytotoxic. | [27] | |
| human ovarian cancer | SKOV-3 OC | CCK8 (48 h) (Cell viability assay) IC50 = 35 μM | UA caused a significant decrease in the viability of SKOV-3 cells. | [28] | |
| non-small cell lung cancer | A549 | MTT (24 h) IC50 ≥ 20 μM | UA inhibited cell proliferation in a dose-dependent manner. | [67] | |
| non-small cell lung cancer | H460 | MTT (24 h) IC50 ≥ 20 μM | [67] | ||
| human breast cancer | MDA-MB-231 | CCK8 (48 h) IC50 = 24 μM | UA inhibited cell proliferation in a dose- and time-dependent manner. | [29] | |
| MCF-7 | CCK8 (48 h) IC50 = 29.2 μM | [29] | |||
| CCK8 (48 h) IC50 = 7.96 μM | UA significantly suppressed the proliferation of cancer cells in a doze-time-dependent manner. | [68] | |||
| MDA-MB-231 | CCK8 (48 h) IC50 = 9.02 μM | [68] | |||
| MCF-7 | MTT (24 h) IC50 = 20 μM | UA determined the decrease in the cell viability in a dose-dependent manner. | [69] | ||
| human gingival squamous carcinoma | Ca922 | MTT (48 h) IC50 = 11.5 μM | UA exhibited good antiproliferative effects. | [70] | |
| human oral squamous carcinoma | SCC2095 | MTT (48 h) IC50 = 13.8 μM | [70] | ||
| human breast cancer | SUM149PT | SRB (48 h) IC50 = 8–10 μM | UA significantly inhibited proliferation of cancer cells. | [61] | |
| HCC1937 | SRB (48 h) IC50 = 8–10 μM | ||||
| UA | human skin metastatic melanoma | WM-266-4 | MTT (4, 24, 48 h) The lowest efficient concentration = 10 μM | Both UA and UA+OA decreased cell proliferation. | [8] |
| UA+OA (both 1:1 and 3.5:1) | [8] | ||||
| Ia | human gastric cancer | MKN45 | MTT (72 h) IC50 = 6.4 μM | The inhibition rate was above 75%, which means that the introduction of a piperazine or homopiperazine radical increases the antitumor activity. | [22] |
| Ib | MTT (72 h) IC50 = 6.2 μM | [22] | |||
| II | MTT (72 h) IC50 = 2.1 μM | [22] | |||
| III | MTT (72 h) IC50 = 4.5 μM | [22] | |||
| IV | human liver cancer | HL-7702 | MTT (72 h) IC50 = 42.1 μM | UA thymine hybrids had a slightly lower antiproliferative activity than glycyrrhetinic acid hybrids. | [43] |
| V | non-small cell lung cancer | A549, | MTT (48 h) IC50 = 17.35 μM | This compound presented antiproliferative activity against all cancer cell lines. | [16] |
| human breast cancer | MCF-7 | MTT (48 h) IC50 = 18.86 μM | [16] | ||
| human hepatocellular carcinoma | Bel-7402 | MTT (48 h) IC50 = 32.50 μM | [16] | ||
| human myelogenous leukemia | K562 | MTT (48 h) IC50 = 14.89 μM | [16] | ||
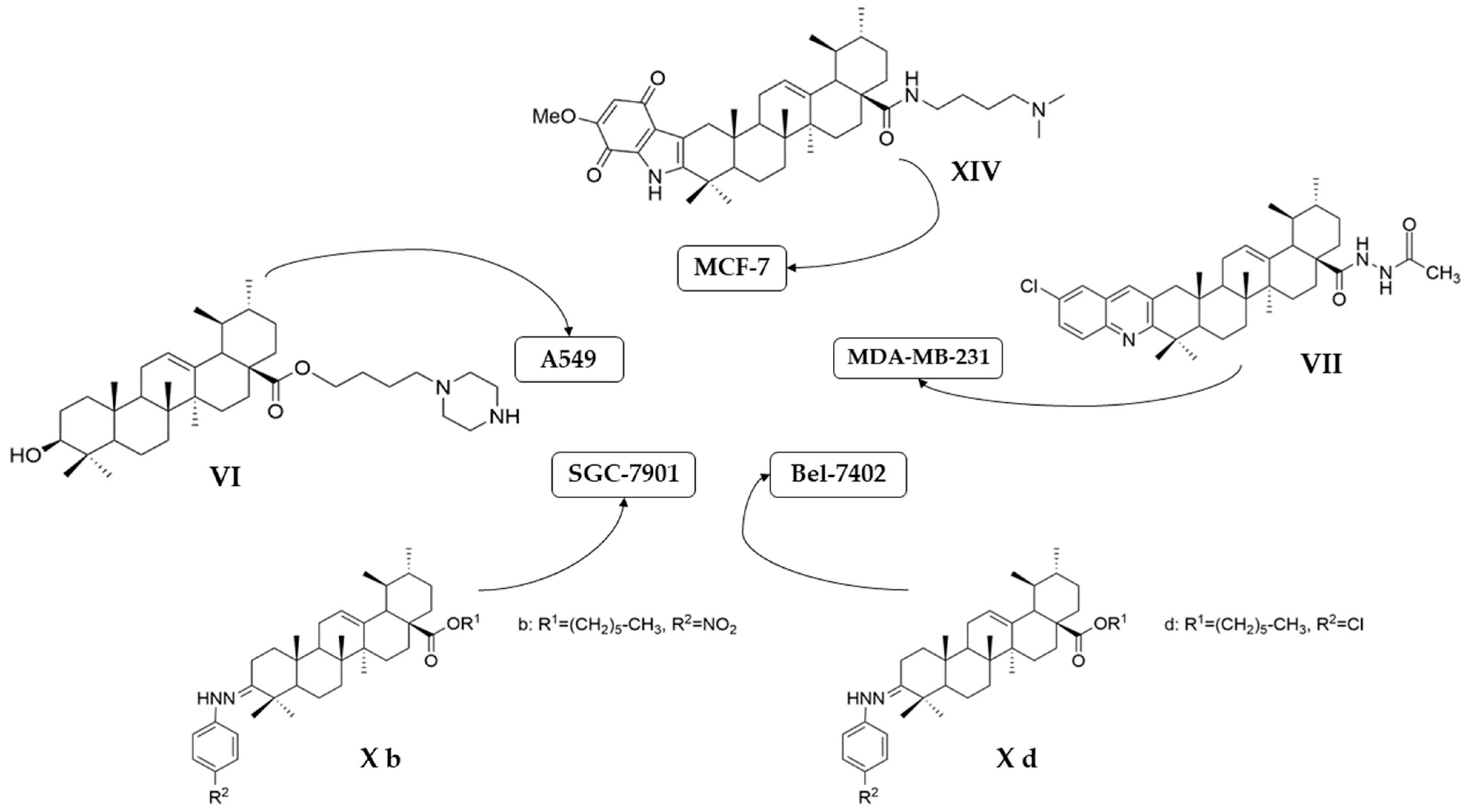
| VI | non-small cell lung cancer | A549 | CCK9 (24 h) IC50 = 6.1 μM | The new derivative exhibited significantly better antiproliferative effect than UA. | [56] |
| CCK9 (48 h) IC50 = 5.5 μM | |||||
| CCK9 (72 h) IC50 = 5.4 μM | |||||
| H460 | CCK9 (24 h) IC50 = 5.7 μM | [56] | |||
| CCK9 (48 h) IC50 = 4.5 μM | |||||
| CCK9 (72 h) IC50 = 3.9 μM | |||||
| VII | human breast cancer | MDA-MB-231 | MTT (72 h) IC50 = 0.12 μM | This acylhydrazine derivative showed the strongest antiproliferative effect against all three cancer cell lines tested, being superior to etoposide. | [57] |
| cervical carcinoma | HeLa | MTT (72 h) IC50 = 0.08 μM | [57] | ||
| hepatocarcinoma | SMMC-7721 | MTT (72 h) IC50 = 0.34 μM | [57] | ||
| VIII (a+b) | human laryngeal carcinoma | Hep-2 | MTT (48 h) IC50 = 6.8 μM | These derivatives presented a significant antiproliferative effect against cancer cells. | [58] |
| human hypolaryngeal carcinoma | FaDu | MTT (48 h) IC50 = 6.78 μM | [58] | ||
| IX | human chronic myelogenous leukemia | K562 | MTT (48 h) IC50 = 5.2 μM | This compound was the most effective antiproliferative agent among all tested derivatives, showing low cytotoxicity against normal cells. | [59] |
| X a | human hepatoma | BEL-7402 | MTT (48 h) IC50 = 7.08 μM | These compounds showed antiproliferative activity that was comparable to stronger than its positive control drugs (VP-16 and adriamycin). | [60] |
| human gastric cancer | SGC-7901 | MTT (48 h) IC50 = 15.62 μM | |||
| X b | human hepatoma | BEL-7402 | MTT (48 h) IC50 = 8.57 μM | [60] | |
| human gastric cancer | SGC-7901 | MTT (48 h) IC50 = 6.30 μM | |||
| X c | human hepatoma | BEL-7402 | MTT (48 h) IC50 = 5.63 μM | [60] | |
| human gastric cancer | SGC-7901 | MTT (48 h) IC50 = 8.73 μM | |||
| X d | human hepatoma | BEL-7402 | MTT (48 h) IC50 = 4.49 μM | [60] | |
| human gastric cancer | SGC-7901 | MTT (48 h) IC50 = 7.01 μM | |||
| XI | human breast cancer | SUM149PT | SRB (48 h) IC50 = 4–6 μM (for both cell lines) | This compound significantly inhibited the proliferation of cancer cells, exhibiting very low toxicity on normal cells. Moreover, this compound showed better antiproliferative activity than UA. | [61] |
| HCC1937 | |||||
| XII a | human hepatocellular carcinoma | Hep3B | MTT (72 h) IC50 = 38.0 μM | These compounds showed a superior antiproliferative effect compared to UA against only one cell line among the three tested (HA22T/VGH, HepG2, and Hep3B) | [62] |
| XII b | MTT (72 h) IC50 = 40.0 μM | [62] | |||
| XIII | HRE (24 h) (luciferase reporter assay) IC50 = 4.0 μM | This compound inhibited the HIF-1α transcriptional activity, which is a very important factor in tumor growth. | [63] | ||
| XIV | human breast cancer | MCF-7 | MTT (72 h) IC50 = 1.66 μM | This compound displayed a better antiproliferative effect than UA against all tested cell lines and better activity than Etoposide against MCF-7 and HeLa cell lines, with low toxicity against normal cells. | [64] |
| human cervical carcinoma | HeLa | MTT (72 h) IC50 = 3.16 μM | |||
| human hepatocarcinoma | HepG2 | MTT (72 h) IC50 = 10.35 μM |
| Compound | Experimental Animal Model | Injected Tumor Cells | Concentration | Conclusions | Reference |
|---|---|---|---|---|---|
| UA | Male BALB/c athymic nude mice | human colon carcinoma (HT-29) | 12.5 mg/kg, i.p, 6 days/week, 16 days | UA inhibited tumor growth without apparent toxicity. | [76] |
| UA | Chick chorioallantoic membrane (CAM) | - | 10 μL of UA (25 μg/μL), 72 h of incubation | UA inhibited angiogenesis. | [76] |
| UA | Female Balb/c mice | breast cancer (4T1-Luc) | 25 and 50 mg/kg/day i.p., measuring tumor volume every 3 days | UA suppressed the proliferation of cancer cells and prevented the occurrence of lung metastasis without significant body weight loss. | [68] |
| UA | Nude mouse subcutaneous xenograft model | human retinoblastoma (SO-RB50) | 200 mg/kg, i.p., twice a week, 7 weeks | UA suppressed the tumor growth. | [30] |
| VIII (a+b) | Female BALB/c nude mice, 4 weeks old | human laryngeal carcinoma (Hep-2) | 45 and 90 mg/kg/day, 28 days | Administration of VIII (a+b) resulted in inhibition of tumor growth without significant weight loss. | [58] |
| Number | Chemical Structure | Reference |
|---|---|---|
| XV | 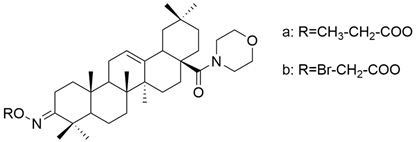 | [26] |
| XVI | 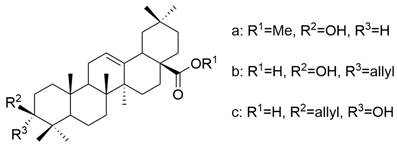 | [62] |
| XVII |  | [97] |
| XVIII | 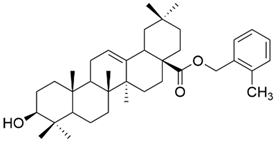 | [102] |
| XIX | 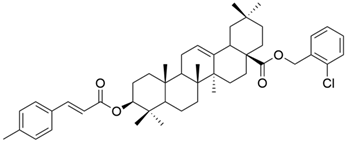 | [102] |
| XX |  | [102] |
| XXI |  | [103] |
| XXII | 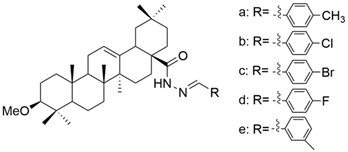 | [104] |
| XXIII | 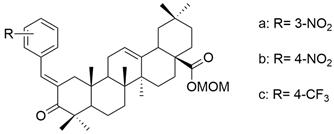 | [105] |
| XXIV | 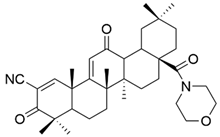 | [106] |
| XXV | 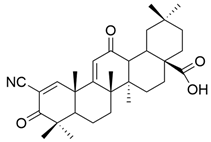 | [107] |
| XXVI |  | [108] |
| XXVII | 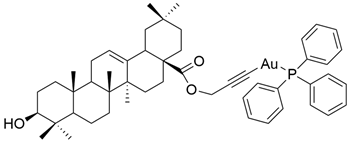 | [19] |
| XXVIII | 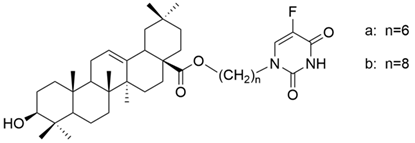 | [16] |
| Compound | Type of Cancer Cell Line | Cell Line | In Vitro | Conclusions | Reference |
|---|---|---|---|---|---|
| OA | human skin metastatic melanoma | WM-244-6 | MTT (24 h, 48 h) The lowest concentration that caused a significant antiproliferative effect was 20 μM. | OA inhibited cancer cell proliferation, showing a maximal effect after 48 h. | [8] |
| OA | human gastric cancer | SGC-7901 | MTT (48 h) IC50 = 25.9 μM MTT (72 h) IC50 = 21.2 μM | OA decreased the viability of cancer cells in a dose-dependent manner, showing slight inhibition against normal cells. | [89] |
| MGC-803 | MTT (48 h) IC50 = 24.0 μM MTT (72 h) IC50 = 20.4 μM | ||||
| BGC-823 | MTT (48 h) IC50 = 30.1 μM MTT (72 h) IC50 = 22.4 μM | ||||
| OA | human breast carcinoma | MCF-7 | MTT (48 h) IC50 = 13.09 μg/mL | OA exhibited dose-dependent antiproliferative activity against cancer cells with negligible toxicity against normal cells. | [10] |
| MDA-MB-231 | MTT (48 h) IC50 = 160.22 μg/mL | ||||
| OA | human colon adenocarcinoma | HCT-116 | MTT (48 h) IC50 = 40.00 μg/mL | OA significantly decreased the cancer cells’ viability. | [86] |
| OA | human thyroid carcinoma | SW579 | MTT (24 h) IC50 = 42.20 μmol/L | OA exhibited a dose-dependent antiproliferative effect against cancer cells. | [82] |
| OA | liver carcinoma | HepG2 | CCK (24 h) IC50 = 30 μM | OA determined a dose-dependent decrease in cancer cells’ viability without significant toxicity against normal cells. | [114] |
| OA | human melanoma | A375SM | MTT (24 h) The 100 μM dose reduced the cell viability to 41.5%. | OA decreased the cell viability in a dose-dependent manner. | [115] |
| A375P | MTT (24 h) The 100 μM dose reduced the cell viability to 46.4%. | [115] | |||
| OA | human prostate cancer | DU145 | MTT (24 h) IC50 = 112.57 μg/mL | OA inhibited the proliferation of cancer cells. | [96] |
| human breast cancer | MCF-7 | MTT (24 h) IC50 = 132.29 μg/mL | |||
| human glioblastoma | U87 | MTT (24 h) IC50 = 163.6 μg/mL | |||
| OA | human melanoma | MeWo | MTT (48 h) OA did not have a significant antiproliferative effect against MeWo cells. | Derivatives XVa and XVb exhibited dose-dependent antiproliferative effects against both cancer cell lines, being superior to OA. | [26] |
| A375 | MTT (48 h) The 100 μM dose reduced the cell viability to 61%. | ||||
| XV a | MeWo | MTT (48 h) The 100 μM dose reduced the cell viability to 37.2%. | [26] | ||
| A375 | MTT (48 h) The 100 μM dose reduced the cell viability to 16.4%. | ||||
| XV b | MeWo | MTT (48 h) The 100 μM dose reduced the cell viability to 10.9%. | [26] | ||
| A375 | MTT (48 h) The 100 μM dose reduced the cell viability to 16%. | ||||
| XVI a | hepatocellular carcinoma | HA22T/VGH | MTT (72 h) IC50 = 42.5 μM | These compounds showed a superior antiproliferative effect compared to OA against all tested cell lines. | [62] |
| HepG2 | MTT (72 h) IC50 = 40.0 μM | ||||
| Hep3B | MTT (72 h) IC50 = 41.5 μM | ||||
| XVI b | HA22T/VGH | MTT (72 h) IC50 = 31.0 μM | [62] | ||
| HepG2 | MTT (72 h) IC50 = 28.0 μM | ||||
| Hep3B | MTT (72 h) IC50 = 32.5 μM | ||||
| XVI c | HA22T/VGH | MTT (72 h) IC50 = 35.8 μM | [62] | ||
| HepG2 | MTT (72 h) IC50 = 44.0 μM | ||||
| Hep3B | MTT (72 h) IC50 = 33.7 μM | ||||
| XVII | human ovarian cancer | SKOV3 | MTT (24 h) IC50 = 8.3 μM | This compound exhibited strong antiproliferative activity against cancer cells without significant toxicity against normal cells. | [97] |
| human endometrial cancer | HEC-1A | MTT (24 h) IC50 = 0.8 μM | [97] | ||
| XVIII | human cervical cancer | HeLa | MTT (48 h) IC50 = 1.55 μM | Compounds XVIII and XX exhibited strong antiproliferative activity against HeLa cells. Compound XIX was the most efficient against MCF-7 cells. | [102] |
| human breast cancer | MCF-7 | MTT (48 h) IC50 = 32.49 μM | |||
| XIX | human cervical cancer | HeLa | MTT (48 h) IC50 > 100 μM | [102] | |
| human breast cancer | MCF-7 | MTT (48 h) IC50 = 1.79 μM | |||
| XX | human cervical cancer | HeLa | MTT (48 h) IC50 = 1.35 μM | [102] | |
| human breast cancer | MCF-7 | MTT (48 h) IC50 > 100 μM | |||
| XXI a | human hepatoma | HepG2 | MTT (24 h) IC50 = 37.0 μM | These compounds presented better antiproliferative activity than diclofenac. | [103] |
| XXI b | MTT (24 h) IC50 = 33.5 μM | [103] | |||
| XXII a | human non-small lung cancer | A549 | MTT (24 h) IC50 = 0.08 μM | Compound XXII a had the greatest cytotoxic activity (equivalent to doxorubicin) against cancer cells while being less toxic to normal cells. | [104] |
| XXII b | MTT (24 h) IC50 = 0.35 μM | [104] | |||
| XXII c | MTT (24 h) IC50 = 0.31 μM | [104] | |||
| XXII d | MTT (24 h) IC50 = 1.72 μM | [104] | |||
| XXII e | MTT (24 h) IC50 = 0.22 μM | [104] | |||
| XXIII a | human prostate cancer | PC3 | MTT (24 h) IC50 = 7.79 μM | All of these compounds presented good antiproliferative effects against cancer cells, being superior to OA. | [105] |
| XXIII b | MTT (24 h) IC50 = 8.87 μM | [105] | |||
| XXIII c | MTT (24 h) IC50 = 8.77 μM | [105] | |||
| XXVII | human ovarian cancer | A2780 | MTT (72 h) IC50 = 10.24 μM | This gold alkyne complex was more active than OA against cancer cells. | [19] |
| XXVIII a | non-small cell lung cancer | A549 | MTT (48 h) IC50 = 23.44 μM | The obtained hybrids possess antiproliferative properties. The hybrid XXVIII b presented potential selectivity against cancer cells and moderate antiproliferative potential against both ordinary and multidrug-resistant (MDR) A549/T and Bel-7402/FU cell lines. | [16] |
| human breast cancer | MCF-7 | MTT (48 h) IC50 = 24.33 μM | [16] | ||
| human hepatocellular carcinoma | Bel-7402 | MTT (48 h) IC50 = 25.22 μM | [16] | ||
| human myelogenous leukemia | K562 | MTT (48 h) IC50 = 14.92 μM | [16] | ||
| XXVIII b | non-small cell lung cancer | A549, | MTT (48 h) IC50 = 50.54 μM | [16] | |
| A549/T | MTT (48 h) IC50 = 43.07 μM | [16] | |||
| human breast cancer | MCF-7, | MTT (48 h) IC50 = 53.63 μM | [16] | ||
| MCF-7/ADR | MTT (48 h) IC50 = 166.2 μM | [16] | |||
| human hepatocellular carcinoma | Bel-7402 | MTT (48 h) IC50 = 43.82 μM | [16] | ||
| Bel-7402/FU | MTT (48 h) IC50 = 31.42 μM | [16] | |||
| human myelogenous leukemia | K562 | MTT (48 h) IC50 = 22.99 μM | [16] | ||
| K562/ADR | MTT (48 h) IC50 > 200 μM | [16] |
| Compound | Experimental Animal Model | Injected Tumor Cells | Concentration | Conclusions | Reference |
|---|---|---|---|---|---|
| OA | Female nude BALB/c mice, 6 weeks old | human gastric cancer (MGC-803) | 100 mg/kg/day, orally, measuring the tumor volume every 3 days | OA inhibited tumor growth and delayed the onset of tumor formation. | [89] |
| OA | Female BALB/c nude mice, 4 weeks old | human melanoma (A375SM) | 75 mg/kg and 150 mg/kg, i.p., 5 times/week, 13 days | OA (150 mg/kg dose) caused a significant reduction in the tumor volume. | [115] |
| OA | Male BALB/c nude mice, 5 weeks old | human cervical cancer (Hela) | 40 and 80 mg/kg/day, i.p., 15 days | OA decreased the size of cervical cancer tumors. | [117] |
| OA | Male Sprague-Dawley rats, 8 weeks old | human benign prostate hyperplasia (BHP-1) | 1 and 10 mg/kg/day, i.p., 4 weeks | OA treatment determined the reduction of prostate tissue weight (by 30.91%, and 31.23%) without affecting the body weight. | [118] |
| OA | Male BALB/c mice, 7 weeks old | human prostate cancer (DU145) | 50 μg/mouse, i.m., every 2 days, 4 times | OA significantly decreased the net weight of tumors (about 29.38%). | [96] |
| XVII | Female athymic mice, BALB/c nu/nu, 6–8 weeks old | human ovarian cancer (SKOV3) | 10, 20, and 40 mg/kg/day, i.p., 3 weeks | XVII (OA-3 acetate) exhibited significant tumor growth inhibition rates (32.5, 38.47, and 46.02%) without causing weight loss. | [97] |
| XXIV | Female BALB/c nude mice, 5 weeks old | human osteosarcoma (143B) | 40 mg/kg, i.p., every 2 days, 3 weeks | This compound reduced the tumor volume without affecting the body weight and other organs. | [106] |
| XXVII | Male BALB/c nude mice | human ovarian cancer (A2780) | 20 mg/kg/day, i.p., 15 days | This complex determined a significant tumor inhibition rate (40.2%) without clear weight loss. | [19] |
| Compound | Clinical Trial Phase/Type | Subjects | Type of Cancer | Dose | Results | Conclusions | Reference |
|---|---|---|---|---|---|---|---|
| XXV | open-label, single-arm phase I study | two males, five females, aged 47–62 | colorectal (4), bladder (1), ovarian (1), and uterine (1) cancer | Seven different dose levels (0.6 to 38.4 mg/m2/h) in continuous infusion on days 1–5 of a 28-day cycle | Bardoxolone pharmacokinetics showed no evidence of non-linearity. The desired dose level of 1 µM was reached only after the administration of the highest dose. | The study was discontinued due to the occurrence of thromboembolic events. | [107] |
| XXVI | phase I | 47 patients (34 males and 13 females), aged 24–81 | solid tumors or refractory lymphoid malignancies | 5 mg/d (starting dose) administrated orally for 21 days of a 28-day cycle | The dose-dependent toxicity consists of a reversible increase in ALT serum level. | Compound XXVI was a well-tolerated drug. Thus, the dose of 900 mg/d could be recommended for phase II clinical trials. | [108] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Similie, D.; Minda, D.; Bora, L.; Kroškins, V.; Lugiņina, J.; Turks, M.; Dehelean, C.A.; Danciu, C. An Update on Pentacyclic Triterpenoids Ursolic and Oleanolic Acids and Related Derivatives as Anticancer Candidates. Antioxidants 2024, 13, 952. https://doi.org/10.3390/antiox13080952
Similie D, Minda D, Bora L, Kroškins V, Lugiņina J, Turks M, Dehelean CA, Danciu C. An Update on Pentacyclic Triterpenoids Ursolic and Oleanolic Acids and Related Derivatives as Anticancer Candidates. Antioxidants. 2024; 13(8):952. https://doi.org/10.3390/antiox13080952
Chicago/Turabian StyleSimilie, Diana, Daliana Minda, Larisa Bora, Vladislavs Kroškins, Jevgeņija Lugiņina, Māris Turks, Cristina Adriana Dehelean, and Corina Danciu. 2024. "An Update on Pentacyclic Triterpenoids Ursolic and Oleanolic Acids and Related Derivatives as Anticancer Candidates" Antioxidants 13, no. 8: 952. https://doi.org/10.3390/antiox13080952
APA StyleSimilie, D., Minda, D., Bora, L., Kroškins, V., Lugiņina, J., Turks, M., Dehelean, C. A., & Danciu, C. (2024). An Update on Pentacyclic Triterpenoids Ursolic and Oleanolic Acids and Related Derivatives as Anticancer Candidates. Antioxidants, 13(8), 952. https://doi.org/10.3390/antiox13080952








