Vitamin C-Dependent Uptake of Non-Heme Iron by Enterocytes, Its Impact on Erythropoiesis and Redox Capacity of Human Erythrocytes
Abstract
:1. Introduction
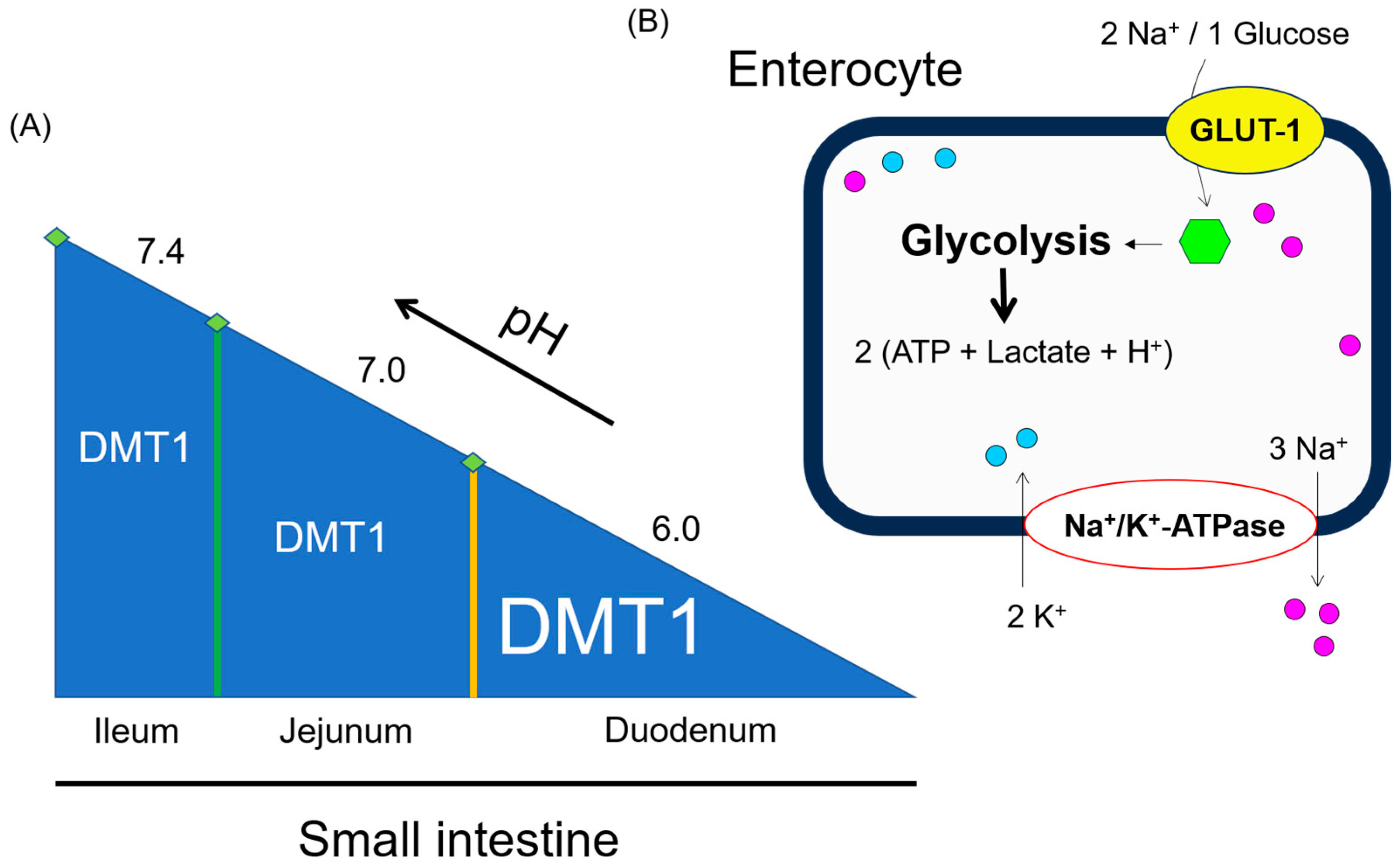
2. pH-Dependent Solubility and Uptake of Vitamin C and Dietary Non-Heme Iron
3. Roles of Copper Ion (Cu+), Regulatory Proteins, and Vitamin C in Non-Heme Iron Absorption across Human Enterocytes
4. Intracellular Enterocytes’ Lactate Production by Glutamine and Glucose Metabolism
5. Lactate-Induced Activation of Hypoxia-Inducible Factor 1-Alpha (Hif-1α) in Enterocytes and Other Cell Types
6. Hif-1α Mediated Control of pH Regulating Pathways and Their Interplay with Divalent Metal Transporter 1 (DMT1) for Non-Heme Iron Transport
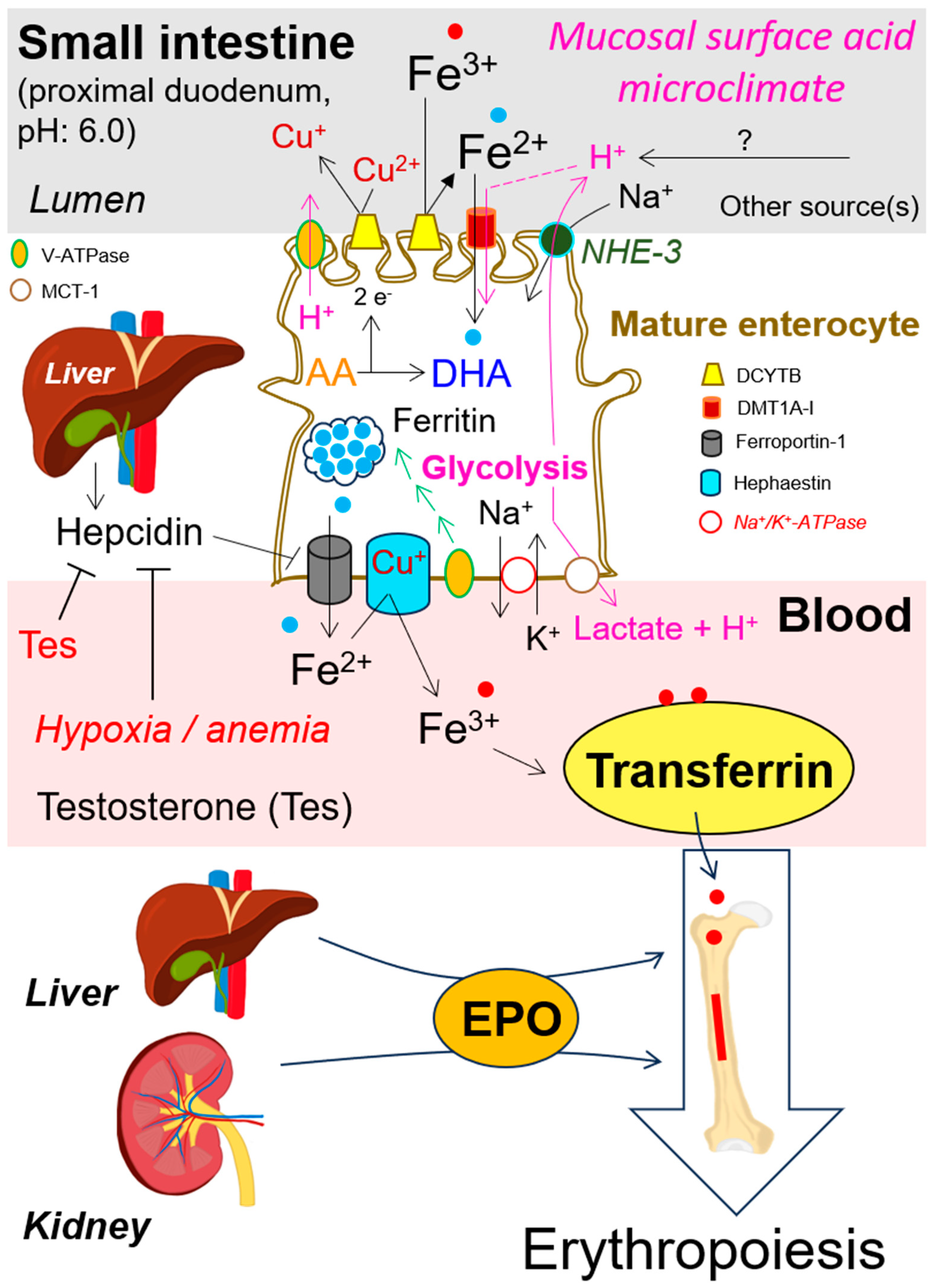
7. Iron Flux across Enterocytes Membrane, Its Release into the Blood and Distribution by Plasma Transferrin
8. Roles of Folates, Vitamin B12, Ferrous Iron (Fe2+), Erythropoietin, Testosterone and Hepcidin in Erythropoiesis
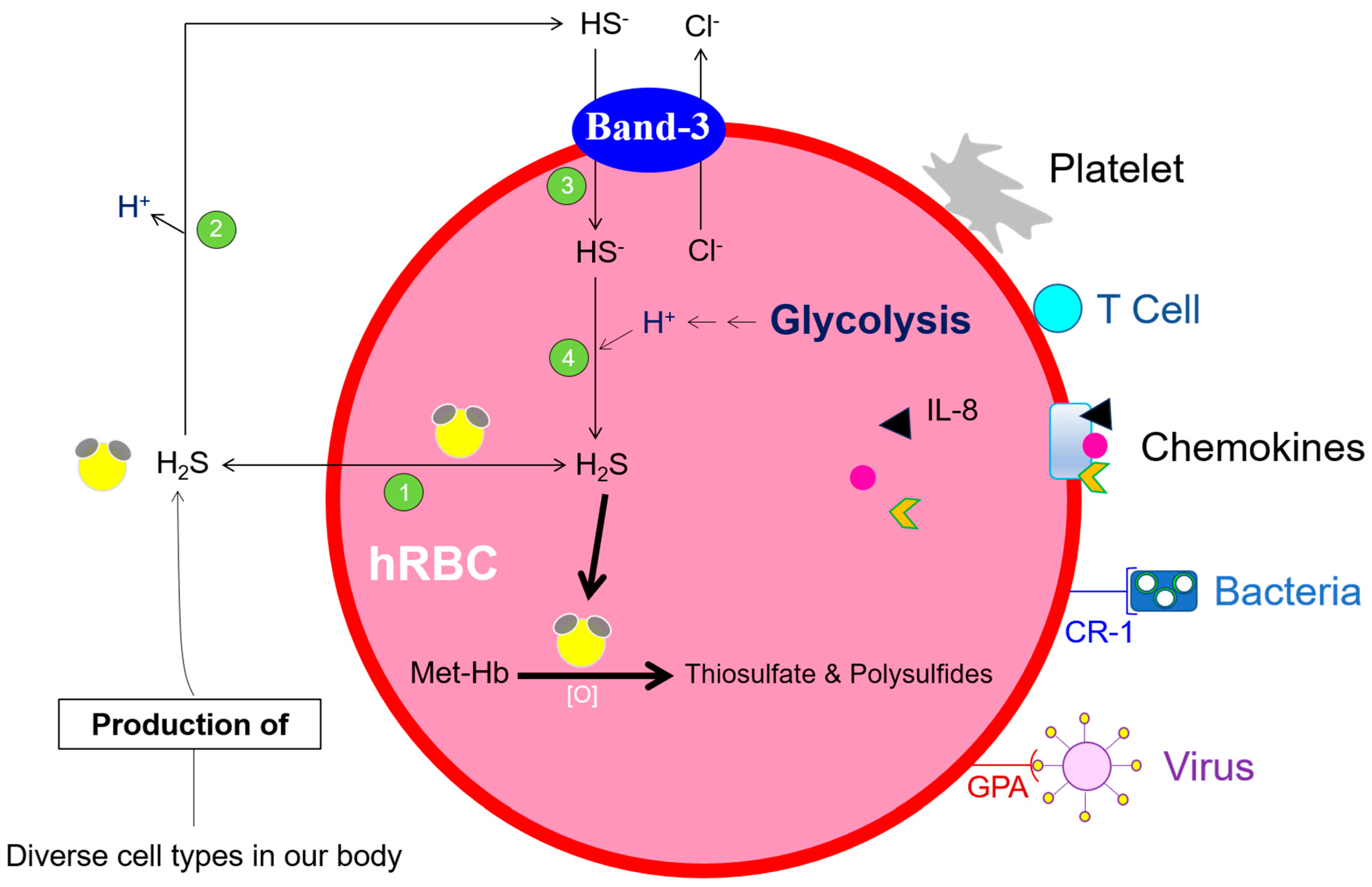
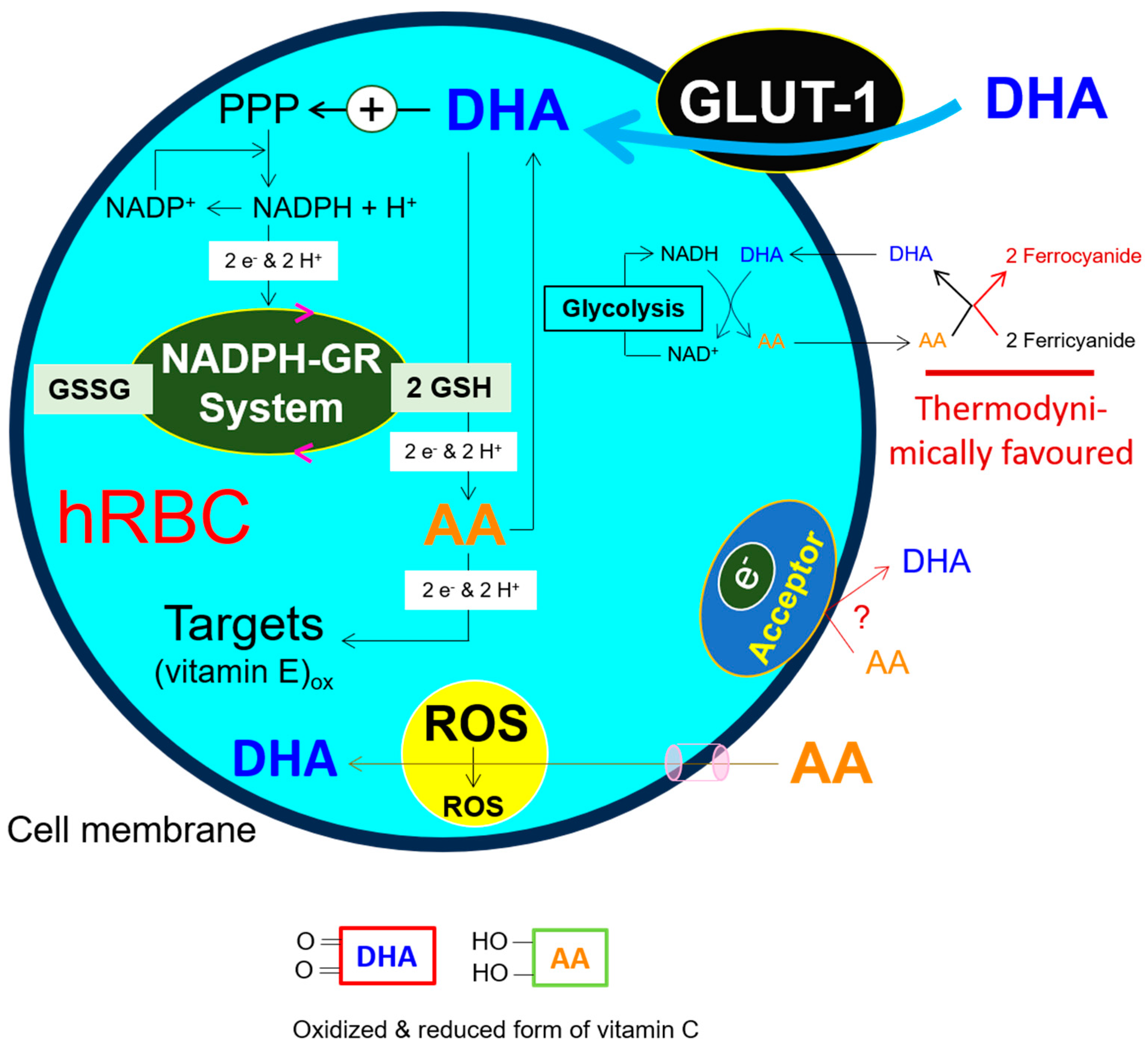
9. Glutathione and NADH-Dependent Vitamin C Reduction, Essential Contributors to Maintaining the Redox Capacity of hRBCs
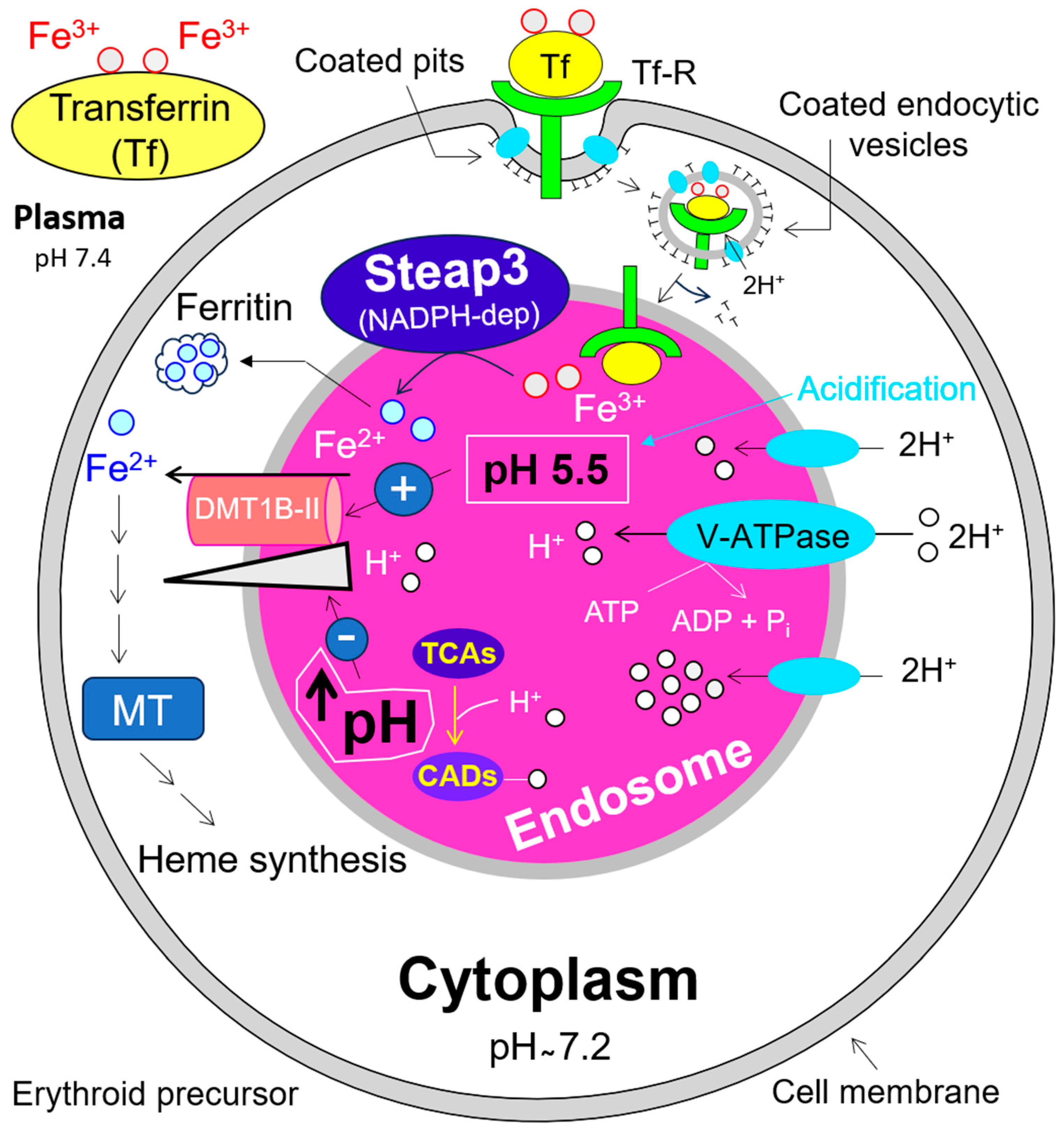
10. Impact of V-ATPase on Endosomal pH and DMT1B-II Mediated Iron (Fe2+) Release into the Cytosol, and Its Relevance for Erythropoiesis
11. Disruption of Lysosomal pH by Tricyclic Antidepressant Desipramine, Its Possible Negative Effect on Endosomal pH, Iron Supply and Erythropoiesis: A High-Risk Drug during Pregnancy?
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stipanuk, M.H.; Beck, P.W. Characterization of the enzymic capacity for cysteine desulphhydration in liver and kidney of the rat. Biochem. J. 1982, 206, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Shibuya, N.; Mikami, Y.; Kimura, Y.; Nagahara, N.; Kimura, H. Vascular endothelium expresses 3-mercaptopyruvate sulfurtransferase and produces hydrogen sulfide. J. Biochem. 2009, 146, 623–626. [Google Scholar] [CrossRef] [PubMed]
- Tanizawa, K. Production of H2S by 3-mercaptopyruvate sulphurtransferase. J. Biochem. 2011, 149, 357–359. [Google Scholar] [CrossRef] [PubMed]
- Vitvitsky, V.; Yadav, P.K.; Kurthen, A.; Banerjee, R. Sulfide oxidation by a noncanonical pathway in red blood cells generates thiosulfate and polysulfides. J. Biol. Chem. 2015, 290, 8310–8320. [Google Scholar] [CrossRef] [PubMed]
- Mathai, J.C.; Missner, A.; Kugler, P.; Saparov, S.M.; Zeidel, M.L.; Lee, J.K.; Pohl, P. No facilitator required for membrane transport of hydrogen sulfide. Proc. Natl. Acad. Sci. USA 2009, 106, 16633–16638. [Google Scholar] [CrossRef] [PubMed]
- Allaway, G.P.; Burness, A.T. Site of attachment of encephalomyocarditis virus on human erythrocytes. J. Virol. 1986, 59, 768–770. [Google Scholar] [CrossRef] [PubMed]
- Craig, M.L.; Bankovich, A.J.; Taylor, R.P. Visualization of the transfer reaction: Tracking immune complexes from erythrocyte complement receptor 1 to macrophages. Clin. Immunol. 2002, 105, 36–47. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, A.M.; Pereira, C.F.; Porto, G.; Arosa, F.A. Red blood cells promote survival and cell cycle progression of human peripheral blood T cells independently of CD58/LFA-3 and heme compounds. Cell Immunol. 2003, 224, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Beck, Z.; Brown, B.K.; Wieczorek, L.; Peachman, K.K.; Matyas, G.R.; Polonis, V.R.; Rao, M.; Alving, C.R. Human erythrocytes selectively bind and enrich infectious HIV-1 virions. PLoS ONE 2009, 4, e8297. [Google Scholar] [CrossRef]
- Darbonne, W.C.; Rice, G.C.; Mohler, M.A.; Apple, T.; Hebert, C.A.; Valente, A.J.; Baker, J.B. Red blood cells are a sink for interleukin 8, a leukocyte chemotaxin. J. Clin. Investig. 1991, 88, 1362–1369. [Google Scholar] [CrossRef]
- Horuk, R.; Colby, T.J.; Darbonne, W.C.; Schall, T.J.; Neote, K. The human erythrocyte inflammatory peptide (chemokine) receptor. Biochemical characterization, solubilization, and development of a binding assay for the soluble receptor. Biochemistry 1993, 32, 5733–5738. [Google Scholar] [CrossRef]
- de Winter, R.J.; Manten, A.; de Jong, Y.P.; Adams, R.; van Deventer, S.J.; Lie, K.I. Interleukin 8 released after acute myocardial infarction is mainly bound to erythrocytes. Heart 1997, 78, 598–602. [Google Scholar] [CrossRef] [PubMed]
- Bahl, N.; Du, R.; Winarsih, I.; Ho, B.; Tucker-Kellogg, L.; Tidor, B.; Ding, J.L. Delineation of lipopolysaccharide (LPS)-binding sites on hemoglobin: From in silico predictions to biophysical characterization. J. Biol. Chem. 2011, 286, 37793–37803. [Google Scholar] [CrossRef] [PubMed]
- Liepke, C.; Baxmann, S.; Heine, C.; Breithaupt, N.; Standker, L.; Forssmann, W.G. Human hemoglobin-derived peptides exhibit antimicrobial activity: A class of host defense peptides. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2003, 791, 345–356. [Google Scholar] [CrossRef]
- Ghashghaeinia, M.; Mrowietz, U. Human erythrocytes, nuclear factor kappaB (NFkappaB) and hydrogen sulfide (H(2)S)—From non-genomic to genomic research. Cell Cycle 2021, 20, 2091–2101. [Google Scholar] [CrossRef]
- Perutz, M.F. Submicroscopic structure of the red cell. Nature 1948, 161, 204. [Google Scholar] [CrossRef] [PubMed]
- Su, D.; Asard, H. Three mammalian cytochromes b561 are ascorbate-dependent ferrireductases. FEBS J. 2006, 273, 3722–3734. [Google Scholar] [CrossRef]
- Lloyd, R.V.; Hanna, P.M.; Mason, R.P. The origin of the hydroxyl radical oxygen in the Fenton reaction. Free Radic. Biol. Med. 1997, 22, 885–888. [Google Scholar] [CrossRef]
- Turnbull, A.; Cleton, F.; Finch, C.A. Iron absorption. IV. The absorption of hemoglobin iron. J. Clin. Investig. 1962, 41, 1897–1907. [Google Scholar] [CrossRef]
- Young, G.P.; Rose, I.S.; St John, D.J. Haem in the gut. I. Fate of haemoproteins and the absorption of haem. J. Gastroenterol. Hepatol. 1989, 4, 537–545. [Google Scholar] [CrossRef]
- Raffin, S.B.; Woo, C.H.; Roost, K.T.; Price, D.C.; Schmid, R. Intestinal absorption of hemoglobin iron-heme cleavage by mucosal heme oxygenase. J. Clin. Investig. 1974, 54, 1344–1352. [Google Scholar] [CrossRef]
- Conrad, M.E.; Schade, S.G. Ascorbic acid chelates in iron absorption: A role for hydrochloric acid and bile. Gastroenterology 1968, 55, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Lynch, S.R.; Cook, J.D. Interaction of vitamin C and iron. Ann. N. Y. Acad. Sci. 1980, 355, 32–44. [Google Scholar] [CrossRef] [PubMed]
- May, J.M.; Qu, Z.C.; Whitesell, R.R. Ascorbate is the major electron donor for a transmembrane oxidoreductase of human erythrocytes. Biochim. Biophys. Acta 1995, 1238, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Oakhill, J.S.; Marritt, S.J.; Gareta, E.G.; Cammack, R.; McKie, A.T. Functional characterization of human duodenal cytochrome b (Cybrd1): Redox properties in relation to iron and ascorbate metabolism. Biochim. Biophys. Acta 2008, 1777, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Lane, D.J.; Bae, D.H.; Merlot, A.M.; Sahni, S.; Richardson, D.R. Duodenal cytochrome b (DCYTB) in iron metabolism: An update on function and regulation. Nutrients 2015, 7, 2274–2296. [Google Scholar] [CrossRef] [PubMed]
- Ludwiczek, S.; Rosell, F.I.; Ludwiczek, M.L.; Mauk, A.G. Recombinant expression and initial characterization of the putative human enteric ferric reductase Dcytb. Biochemistry 2008, 47, 753–761. [Google Scholar] [CrossRef] [PubMed]
- McKie, A.T.; Barrow, D.; Latunde-Dada, G.O.; Rolfs, A.; Sager, G.; Mudaly, E.; Mudaly, M.; Richardson, C.; Barlow, D.; Bomford, A.; et al. An iron-regulated ferric reductase associated with the absorption of dietary iron. Science 2001, 291, 1755–1759. [Google Scholar] [CrossRef]
- Gunshin, H.; Mackenzie, B.; Berger, U.V.; Gunshin, Y.; Romero, M.F.; Boron, W.F.; Nussberger, S.; Gollan, J.L.; Hediger, M.A. Cloning and characterization of a mammalian proton-coupled metal-ion transporter. Nature 1997, 388, 482–488. [Google Scholar] [CrossRef]
- Mackenzie, B.; Ujwal, M.L.; Chang, M.H.; Romero, M.F.; Hediger, M.A. Divalent metal-ion transporter DMT1 mediates both H+ -coupled Fe2+ transport and uncoupled fluxes. Pflugers Arch. 2006, 451, 544–558. [Google Scholar] [CrossRef]
- Ehrnstorfer, I.A.; Manatschal, C.; Arnold, F.M.; Laederach, J.; Dutzler, R. Structural and mechanistic basis of proton-coupled metal ion transport in the SLC11/NRAMP family. Nat. Commun. 2017, 8, 14033. [Google Scholar] [CrossRef] [PubMed]
- Newsholme, E.A.; Carrie, A.L. Quantitative aspects of glucose and glutamine metabolism by intestinal cells. Gut 1994, 35, S13–S17. [Google Scholar] [CrossRef] [PubMed]
- Trejdosiewicz, L.K. What is the role of human intestinal intraepithelial lymphocytes? Clin. Exp. Immunol. 1993, 94, 395–397. [Google Scholar] [CrossRef] [PubMed]
- Dahan, S.; Roth-Walter, F.; Arnaboldi, P.; Agarwal, S.; Mayer, L. Epithelia: Lymphocyte interactions in the gut. Immunol. Rev. 2007, 215, 243–253. [Google Scholar] [CrossRef] [PubMed]
- Lutter, L.; Hoytema van Konijnenburg, D.P.; Brand, E.C.; Oldenburg, B.; van Wijk, F. The elusive case of human intraepithelial T cells in gut homeostasis and inflammation. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 637–649. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Tao, W.; Zhu, S. T lymphocytes in the intestinal mucosa: Defense and tolerance. Cell Mol. Immunol. 2019, 16, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Newsholme, E.A.; Crabtree, B.; Ardawi, M.S.M. Glutamine metabolism in lymphocytes: Its biochemical, physiological and clinical importance. Q. J. Exp. Physiol. Transl. Integr. 1985, 70, 473–489. [Google Scholar] [CrossRef] [PubMed]
- Duée, P.-H.; Darcy-Vrillon, B.; Blachier, F.; Morel, M.-T. Fuel selection in intestinal cells. Proc. Nutr. Soc. 1995, 54, 83–94. [Google Scholar] [CrossRef]
- Kight, C.E.; Fleming, S.E. Transamination processes promote incomplete glutamine oxidation in small intestine epithelial cells. J. Nutr. Biochem. 1995, 6, 27–37. [Google Scholar] [CrossRef]
- Fallingborg, J. Intraluminal pH of the human gastrointestinal tract. Dan. Med. Bull. 1999, 46, 183–196. [Google Scholar]
- Fallingborg, J.; Christensen, L.A.; Ingeman-Nielsen, M.; Jacobsen, B.A.; Abildgaard, K.; Rasmussen, H.H. pH-profile and regional transit times of the normal gut measured by a radiotelemetry device. Aliment. Pharmacol. Ther. 1989, 3, 605–613. [Google Scholar] [CrossRef] [PubMed]
- Cham, C.M.; Gajewski, T.F. Glucose availability regulates IFN-gamma production and p70S6 kinase activation in CD8+ effector T cells. J. Immunol. 2005, 174, 4670–4677. [Google Scholar] [CrossRef] [PubMed]
- Kondoh, H.; Lleonart, M.E.; Nakashima, Y.; Yokode, M.; Tanaka, M.; Bernard, D.; Gil, J.; Beach, D. A high glycolytic flux supports the proliferative potential of murine embryonic stem cells. Antioxid. Redox Signal. 2007, 9, 293–299. [Google Scholar] [CrossRef]
- Spencer, T.L.; Lehninger, A.L. L-lactate transport in Ehrlich ascites-tumour cells. Biochem. J. 1976, 154, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Broer, S.; Rahman, B.; Pellegri, G.; Pellerin, L.; Martin, J.L.; Verleysdonk, S.; Hamprecht, B.; Magistretti, P.J. Comparison of lactate transport in astroglial cells and monocarboxylate transporter 1 (MCT 1) expressing Xenopus laevis oocytes. Expression of two different monocarboxylate transporters in astroglial cells and neurons. J. Biol. Chem. 1997, 272, 30096–30102. [Google Scholar] [CrossRef] [PubMed]
- Kozlov, A.M.; Lone, A.; Betts, D.H.; Cumming, R.C. Lactate preconditioning promotes a HIF-1alpha-mediated metabolic shift from OXPHOS to glycolysis in normal human diploid fibroblasts. Sci. Rep. 2020, 10, 8388. [Google Scholar] [CrossRef] [PubMed]
- Sonveaux, P.; Copetti, T.; De Saedeleer, C.J.; Vegran, F.; Verrax, J.; Kennedy, K.M.; Moon, E.J.; Dhup, S.; Danhier, P.; Frerart, F.; et al. Targeting the lactate transporter MCT1 in endothelial cells inhibits lactate-induced HIF-1 activation and tumor angiogenesis. PLoS ONE 2012, 7, e33418. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wang, M.; Feng, H.; Peng, Y.; Sun, J.; Qu, X.; Li, C. Lactate induces osteoblast differentiation by stabilization of HIF1alpha. Mol. Cell Endocrinol. 2017, 452, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Semenza, G.L. Hypoxia-inducible factor 1: Master regulator of O2 homeostasis. Curr. Opin. Genet. Dev. 1998, 8, 588–594. [Google Scholar] [CrossRef]
- Goldberg, M.A.; Dunning, S.P.; Bunn, H.F. Regulation of the erythropoietin gene: Evidence that the oxygen sensor is a heme protein. Science 1988, 242, 1412–1415. [Google Scholar] [CrossRef]
- Shimoda, L.A.; Fallon, M.; Pisarcik, S.; Wang, J.; Semenza, G.L. HIF-1 regulates hypoxic induction of NHE1 expression and alkalinization of intracellular pH in pulmonary arterial myocytes. Am. J. Physiol. Lung Cell Mol. Physiol. 2006, 291, L941–L949. [Google Scholar] [CrossRef] [PubMed]
- Ghashghaeinia, M.; Koberle, M.; Mrowietz, U.; Bernhardt, I. Proliferating tumor cells mimick glucose metabolism of mature human erythrocytes. Cell Cycle 2019, 18, 1316–1334. [Google Scholar] [CrossRef] [PubMed]
- Donovan, A.; Brownlie, A.; Zhou, Y.; Shepard, J.; Pratt, S.J.; Moynihan, J.; Paw, B.H.; Drejer, A.; Barut, B.; Zapata, A.; et al. Positional cloning of zebrafish ferroportin1 identifies a conserved vertebrate iron exporter. Nature 2000, 403, 776–781. [Google Scholar] [CrossRef] [PubMed]
- Fuqua, B.K.; Lu, Y.; Darshan, D.; Frazer, D.M.; Wilkins, S.J.; Wolkow, N.; Bell, A.G.; Hsu, J.; Yu, C.C.; Chen, H.; et al. The multicopper ferroxidase hephaestin enhances intestinal iron absorption in mice. PLoS ONE 2014, 9, e98792. [Google Scholar] [CrossRef] [PubMed]
- Kuo, Y.M.; Su, T.; Chen, H.; Attieh, Z.; Syed, B.A.; McKie, A.T.; Anderson, G.J.; Gitschier, J.; Vulpe, C.D. Mislocalisation of hephaestin, a multicopper ferroxidase involved in basolateral intestinal iron transport, in the sex linked anaemia mouse. Gut 2004, 53, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Reeves, P.G.; Demars, L.C.; Johnson, W.T.; Lukaski, H.C. Dietary copper deficiency reduces iron absorption and duodenal enterocyte hephaestin protein in male and female rats. J. Nutr. 2005, 135, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Stearman, R.; Yuan, D.S.; Yamaguchi-Iwai, Y.; Klausner, R.D.; Dancis, A. A permease-oxidase complex involved in high-affinity iron uptake in yeast. Science 1996, 271, 1552–1557. [Google Scholar] [CrossRef] [PubMed]
- Doguer, C.; Ha, J.H.; Collins, J.F. Intersection of Iron and Copper Metabolism in the Mammalian Intestine and Liver. Compr. Physiol. 2018, 8, 1433–1461. [Google Scholar] [CrossRef] [PubMed]
- Petrak, J.; Vyoral, D. Hephaestin—A ferroxidase of cellular iron export. Int. J. Biochem. Cell Biol. 2005, 37, 1173–1178. [Google Scholar] [CrossRef]
- Anderson, G.J.; Frazer, D.M.; McKie, A.T.; Vulpe, C.D. The ceruloplasmin homolog hephaestin and the control of intestinal iron absorption. Blood Cells Mol. Dis. 2002, 29, 367–375. [Google Scholar] [CrossRef]
- Helman, S.L.; Zhou, J.; Fuqua, B.K.; Lu, Y.; Collins, J.F.; Chen, H.; Vulpe, C.D.; Anderson, G.J.; Frazer, D.M. The biology of mammalian multi-copper ferroxidases. Biometals 2023, 36, 263–281. [Google Scholar] [CrossRef]
- Vashchenko, G.; MacGillivray, R.T. Multi-copper oxidases and human iron metabolism. Nutrients 2013, 5, 2289–2313. [Google Scholar] [CrossRef]
- Mackenzie, B.; Hediger, M.A. SLC11 family of H+-coupled metal-ion transporters NRAMP1 and DMT1. Pflugers Arch. 2004, 447, 571–579. [Google Scholar] [CrossRef]
- Gkouvatsos, K.; Papanikolaou, G.; Pantopoulos, K. Regulation of iron transport and the role of transferrin. Biochim. Biophys. Acta 2012, 1820, 188–202. [Google Scholar] [CrossRef]
- Nandakumar, S.K.; Ulirsch, J.C.; Sankaran, V.G. Advances in understanding erythropoiesis: Evolving perspectives. Br. J. Haematol. 2016, 173, 206–218. [Google Scholar] [CrossRef]
- Palis, J. Primitive and definitive erythropoiesis in mammals. Front. Physiol. 2014, 5, 3. [Google Scholar] [CrossRef]
- Dzierzak, E.; Philipsen, S. Erythropoiesis: Development and differentiation. Cold Spring Harb. Perspect. Med. 2013, 3, a011601. [Google Scholar] [CrossRef]
- Koury, M.J.; Ponka, P. New insights into erythropoiesis: The roles of folate, vitamin B12, and iron. Annu. Rev. Nutr. 2004, 24, 105–131. [Google Scholar] [CrossRef]
- Visentin, M.; Diop-Bove, N.; Zhao, R.; Goldman, I.D. The intestinal absorption of folates. Annu. Rev. Physiol. 2014, 76, 251–274. [Google Scholar] [CrossRef]
- Baker, H.; Thomson, A.D.; Feingold, S.; Frank, O. Role of the jejunum in the absorption of folic acid and its polyglutamates. Am. J. Clin. Nutr. 1969, 22, 124–132. [Google Scholar] [CrossRef]
- Kozyraki, R.; Cases, O. Vitamin B12 absorption: Mammalian physiology and acquired and inherited disorders. Biochimie 2013, 95, 1002–1007. [Google Scholar] [CrossRef]
- Cooper, B.A. Complex of intrinsic factor and B12 in human ileum during vitamin B12 absorption. Am. J. Physiol. 1968, 214, 832–835. [Google Scholar] [CrossRef]
- Valman, H.B.; Roberts, P.D. Vitamin B12 absorption after resection of ileum in childhood. Arch. Dis. Child. 1974, 49, 932–935. [Google Scholar] [CrossRef]
- Jacobson, L.O.; Goldwasser, E.; Fried, W.; Plzak, L. Role of the kidney in erythropoiesis. Nature 1957, 179, 633–634. [Google Scholar] [CrossRef]
- Bachmann, S.; Le Hir, M.; Eckardt, K.U. Co-localization of erythropoietin mRNA and ecto-5′-nucleotidase immunoreactivity in peritubular cells of rat renal cortex indicates that fibroblasts produce erythropoietin. J. Histochem. Cytochem. 1993, 41, 335–341. [Google Scholar] [CrossRef]
- Koury, S.T.; Bondurant, M.C.; Koury, M.J. Localization of erythropoietin synthesizing cells in murine kidneys by in situ hybridization. Blood 1988, 71, 524–527. [Google Scholar] [CrossRef]
- Lacombe, C.; Da Silva, J.L.; Bruneval, P.; Fournier, J.G.; Wendling, F.; Casadevall, N.; Camilleri, J.P.; Bariety, J.; Varet, B.; Tambourin, P. Peritubular cells are the site of erythropoietin synthesis in the murine hypoxic kidney. J. Clin. Investig. 1988, 81, 620–623. [Google Scholar] [CrossRef]
- Tojo, Y.; Sekine, H.; Hirano, I.; Pan, X.; Souma, T.; Tsujita, T.; Kawaguchi, S.; Takeda, N.; Takeda, K.; Fong, G.H.; et al. Hypoxia Signaling Cascade for Erythropoietin Production in Hepatocytes. Mol. Cell Biol. 2015, 35, 2658–2672. [Google Scholar] [CrossRef]
- Erslev, A.J.; Caro, J.; Kansu, E.; Silver, R. Renal and extrarenal erythropoietin production in anaemic rats. Br. J. Haematol. 1980, 45, 65–72. [Google Scholar] [CrossRef]
- Maxwell, P.H.; Ferguson, D.J.; Osmond, M.K.; Pugh, C.W.; Heryet, A.; Doe, B.G.; Johnson, M.H.; Ratcliffe, P.J. Expression of a homologously recombined erythopoietin-SV40 T antigen fusion gene in mouse liver: Evidence for erythropoietin production by Ito cells. Blood 1994, 84, 1823–1830. [Google Scholar] [CrossRef]
- Suzuki, N.; Yamamoto, M. Roles of renal erythropoietin-producing (REP) cells in the maintenance of systemic oxygen homeostasis. Pflugers Arch. 2016, 468, 3–12. [Google Scholar] [CrossRef]
- Weidemann, A.; Johnson, R.S. Nonrenal regulation of EPO synthesis. Kidney Int. 2009, 75, 682–688. [Google Scholar] [CrossRef]
- Moritz, K.M.; Lim, G.B.; Wintour, E.M. Developmental regulation of erythropoietin and erythropoiesis. Am. J. Physiol. 1997, 273, R1829–R1844. [Google Scholar] [CrossRef]
- Latour, C.; Kautz, L.; Besson-Fournier, C.; Island, M.L.; Canonne-Hergaux, F.; Loreal, O.; Ganz, T.; Coppin, H.; Roth, M.P. Testosterone perturbs systemic iron balance through activation of epidermal growth factor receptor signaling in the liver and repression of hepcidin. Hepatology 2014, 59, 683–694. [Google Scholar] [CrossRef]
- Hennigar, S.R.; Berryman, C.E.; Harris, M.N.; Karl, J.P.; Lieberman, H.R.; McClung, J.P.; Rood, J.C.; Pasiakos, S.M. Testosterone Administration during Energy Deficit Suppresses Hepcidin and Increases Iron Availability for Erythropoiesis. J. Clin. Endocrinol. Metab. 2020, 105, e1316–e1321. [Google Scholar] [CrossRef]
- Coviello, A.D.; Kaplan, B.; Lakshman, K.M.; Chen, T.; Singh, A.B.; Bhasin, S. Effects of graded doses of testosterone on erythropoiesis in healthy young and older men. J. Clin. Endocrinol. Metab. 2008, 93, 914–919. [Google Scholar] [CrossRef]
- Park, C.H.; Valore, E.V.; Waring, A.J.; Ganz, T. Hepcidin, a urinary antimicrobial peptide synthesized in the liver. J. Biol. Chem. 2001, 276, 7806–7810. [Google Scholar] [CrossRef]
- Pigeon, C.; Ilyin, G.; Courselaud, B.; Leroyer, P.; Turlin, B.; Brissot, P.; Loreal, O. A new mouse liver-specific gene, encoding a protein homologous to human antimicrobial peptide hepcidin, is overexpressed during iron overload. J. Biol. Chem. 2001, 276, 7811–7819. [Google Scholar] [CrossRef]
- Katsarou, A.; Pantopoulos, K. Hepcidin Therapeutics. Pharmaceuticals 2018, 11, 127. [Google Scholar] [CrossRef]
- Nemeth, E.; Tuttle, M.S.; Powelson, J.; Vaughn, M.B.; Donovan, A.; Ward, D.M.; Ganz, T.; Kaplan, J. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science 2004, 306, 2090–2093. [Google Scholar] [CrossRef]
- De Domenico, I.; Ward, D.M.; Langelier, C.; Vaughn, M.B.; Nemeth, E.; Sundquist, W.I.; Ganz, T.; Musci, G.; Kaplan, J. The molecular mechanism of hepcidin-mediated ferroportin down-regulation. Mol. Biol. Cell 2007, 18, 2569–2578. [Google Scholar] [CrossRef]
- Nicolas, G.; Chauvet, C.; Viatte, L.; Danan, J.L.; Bigard, X.; Devaux, I.; Beaumont, C.; Kahn, A.; Vaulont, S. The gene encoding the iron regulatory peptide hepcidin is regulated by anemia, hypoxia, and inflammation. J. Clin. Investig. 2002, 110, 1037–1044. [Google Scholar] [CrossRef]
- Tsukaguchi, H.; Tokui, T.; Mackenzie, B.; Berger, U.V.; Chen, X.Z.; Wang, Y.; Brubaker, R.F.; Hediger, M.A. A family of mammalian Na+-dependent L-ascorbic acid transporters. Nature 1999, 399, 70–75. [Google Scholar] [CrossRef]
- Ulloa, V.; Garcia-Robles, M.; Martinez, F.; Salazar, K.; Reinicke, K.; Perez, F.; Godoy, D.F.; Godoy, A.S.; Nualart, F. Human choroid plexus papilloma cells efficiently transport glucose and vitamin C. J. Neurochem. 2013, 127, 403–414. [Google Scholar] [CrossRef]
- Amano, A.; Aigaki, T.; Maruyama, N.; Ishigami, A. Ascorbic acid depletion enhances expression of the sodium-dependent vitamin C transporters, SVCT1 and SVCT2, and uptake of ascorbic acid in livers of SMP30/GNL knockout mice. Arch. Biochem. Biophys. 2010, 496, 38–44. [Google Scholar] [CrossRef]
- Kobayashi, T.A.; Shimada, H.; Sano, F.K.; Itoh, Y.; Enoki, S.; Okada, Y.; Kusakizako, T.; Nureki, O. Dimeric transport mechanism of human vitamin C transporter SVCT1. Nat. Commun. 2024, 15, 5569. [Google Scholar] [CrossRef]
- Mann, G.V.; Newton, P. The membrane transport of ascorbic acid. Ann. N. Y. Acad. Sci. 1975, 258, 243–252. [Google Scholar] [CrossRef]
- Ingermann, R.L.; Stankova, L.; Bigley, R.H.; Bissonnette, J.M. Effect of monosaccharide on dehydroascorbic acid uptake by placental membrane vesicles. J. Clin. Endocrinol. Metab. 1988, 67, 389–394. [Google Scholar] [CrossRef]
- Hornung, T.C.; Biesalski, H.K. Glut-1 explains the evolutionary advantage of the loss of endogenous vitamin C-synthesis: The electron transfer hypothesis. Evol. Med. Public Health 2019, 2019, 221–231. [Google Scholar] [CrossRef]
- Goldenberg, H.; Schweinzer, E. Transport of vitamin C in animal and human cells. J. Bioenerg. Biomembr. 1994, 26, 359–367. [Google Scholar] [CrossRef]
- Skou, J.C. Enzymatic Basis for Active Transport of Na+ and K+ across Cell Membrane. Physiol. Rev. 1965, 45, 596–617. [Google Scholar] [CrossRef]
- Cereijido, M.; Shoshani, L.; Contreras, R.G. The polarized distribution of Na+, K+-ATPase and active transport across epithelia. J. Membr. Biol. 2001, 184, 299–304. [Google Scholar] [CrossRef]
- Sage, J.M.; Carruthers, A. Human erythrocytes transport dehydroascorbic acid and sugars using the same transporter complex. Am. J. Physiol. Cell Physiol. 2014, 306, C910–C917. [Google Scholar] [CrossRef]
- Montel-Hagen, A.; Kinet, S.; Manel, N.; Mongellaz, C.; Prohaska, R.; Battini, J.L.; Delaunay, J.; Sitbon, M.; Taylor, N. Erythrocyte Glut1 triggers dehydroascorbic acid uptake in mammals unable to synthesize vitamin C. Cell 2008, 132, 1039–1048. [Google Scholar] [CrossRef]
- Montel-Hagen, A.; Sitbon, M.; Taylor, N. Erythroid glucose transporters. Curr. Opin. Hematol. 2009, 16, 165–172. [Google Scholar] [CrossRef]
- May, J.M.; Qu, Z.C.; Whitesell, R.R.; Cobb, C.E. Ascorbate recycling in human erythrocytes: Role of GSH in reducing dehydroascorbate. Free Radic. Biol. Med. 1996, 20, 543–551. [Google Scholar] [CrossRef]
- Hughes, R.E. Reduction of Dehydroasorbic Acid by Animal Tissues. Nature 1964, 203, 1068–1069. [Google Scholar] [CrossRef]
- Dereven’kov, I.A.; Makarov, S.V.; Bui Thi, T.T.; Makarova, A.S.; Koifman, O.I. Studies on the reduction of dehydroascorbic acid by glutathione in the presence of aquahydroxocobinamide. Eur. J. Inorg. Chem. 2018, 2018, 2987–2992. [Google Scholar] [CrossRef]
- Sasaki, H.; Giblin, F.J.; Winkler, B.S.; Chakrapani, B.; Leverenz, V.; Shu, C.C. A protective role for glutathione-dependent reduction of dehydroascorbic acid in lens epithelium. Investig. Ophthalmol. Vis. Sci. 1995, 36, 1804–1817. [Google Scholar]
- Cisternas, P.; Silva-Alvarez, C.; Martinez, F.; Fernandez, E.; Ferrada, L.; Oyarce, K.; Salazar, K.; Bolanos, J.P.; Nualart, F. The oxidized form of vitamin C, dehydroascorbic acid, regulates neuronal energy metabolism. J. Neurochem. 2014, 129, 663–671. [Google Scholar] [CrossRef]
- Puskas, F.; Gergely, P., Jr.; Banki, K.; Perl, A. Stimulation of the pentose phosphate pathway and glutathione levels by dehydroascorbate, the oxidized form of vitamin C. FASEB J. 2000, 14, 1352–1361. [Google Scholar] [CrossRef]
- Orringer, E.P.; Roer, M.E. An ascorbate-mediated transmembrane-reducing system of the human erythrocyte. J. Clin. Investig. 1979, 63, 53–58. [Google Scholar] [CrossRef]
- D’Alessandro, A.; Keele, G.R.; Hay, A.; Nemkov, T.; Earley, E.J.; Stephenson, D.; Vincent, M.; Deng, X.; Stone, M.; Dzieciatkowska, M.; et al. Ferroptosis regulates hemolysis in stored murine and human red blood cells. bioRxiv 2024. [Google Scholar] [CrossRef]
- Howie, H.L.; Hay, A.M.; de Wolski, K.; Waterman, H.; Lebedev, J.; Fu, X.; Culp-Hill, R.; D’Alessandro, A.; Gorham, J.D.; Ranson, M.S.; et al. Differences in Steap3 expression are a mechanism of genetic variation of RBC storage and oxidative damage in mice. Blood Adv. 2019, 3, 2272–2285. [Google Scholar] [CrossRef]
- Sender, R.; Fuchs, S.; Milo, R. Revised Estimates for the Number of Human and Bacteria Cells in the Body. PLoS Biol. 2016, 14, e1002533. [Google Scholar] [CrossRef]
- Callender, S.T.; Powell, E.; Witts, L. The life-span of the red cell in man. J. Pathol. Bacteriol. 1945, 57, 129–139. [Google Scholar] [CrossRef]
- Larkin, J.M.; Donzell, W.C.; Anderson, R.G. Potassium-dependent assembly of coated pits: New coated pits form as planar clathrin lattices. J. Cell Biol. 1986, 103, 2619–2627. [Google Scholar] [CrossRef]
- Song, Q.; Meng, B.; Xu, H.; Mao, Z. The emerging roles of vacuolar-type ATPase-dependent Lysosomal acidification in neurodegenerative diseases. Transl. Neurodegener. 2020, 9, 17. [Google Scholar] [CrossRef]
- Harvey, W.R. Physiology of V-ATPases. J. Exp. Biol. 1992, 172, 1–17. [Google Scholar] [CrossRef]
- Touret, N.; Furuya, W.; Forbes, J.; Gros, P.; Grinstein, S. Dynamic traffic through the recycling compartment couples the metal transporter Nramp2 (DMT1) with the transferrin receptor. J. Biol. Chem. 2003, 278, 25548–25557. [Google Scholar] [CrossRef]
- Eckenroth, B.E.; Steere, A.N.; Chasteen, N.D.; Everse, S.J.; Mason, A.B. How the binding of human transferrin primes the transferrin receptor potentiating iron release at endosomal pH. Proc. Natl. Acad. Sci. USA 2011, 108, 13089–13094. [Google Scholar] [CrossRef]
- Dautry-Varsat, A.; Ciechanover, A.; Lodish, H.F. pH and the recycling of transferrin during receptor-mediated endocytosis. Proc. Natl. Acad. Sci. USA 1983, 80, 2258–2262. [Google Scholar] [CrossRef]
- Ohgami, R.S.; Campagna, D.R.; Greer, E.L.; Antiochos, B.; McDonald, A.; Chen, J.; Sharp, J.J.; Fujiwara, Y.; Barker, J.E.; Fleming, M.D. Identification of a ferrireductase required for efficient transferrin-dependent iron uptake in erythroid cells. Nat. Genet. 2005, 37, 1264–1269. [Google Scholar] [CrossRef]
- Yanatori, I.; Kishi, F. DMT1 and iron transport. Free Radic. Biol. Med. 2019, 133, 55–63. [Google Scholar] [CrossRef]
- Grandchamp, B.; Hetet, G.; Kannengiesser, C.; Oudin, C.; Beaumont, C.; Rodrigues-Ferreira, S.; Amson, R.; Telerman, A.; Nielsen, P.; Kohne, E.; et al. A novel type of congenital hypochromic anemia associated with a nonsense mutation in the STEAP3/TSAP6 gene. Blood 2011, 118, 6660–6666. [Google Scholar] [CrossRef]
- Blanc, L.; Papoin, J.; Debnath, G.; Vidal, M.; Amson, R.; Telerman, A.; An, X.; Mohandas, N. Abnormal erythroid maturation leads to microcytic anemia in the TSAP6/Steap3 null mouse model. Am. J. Hematol. 2015, 90, 235–241. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhen, J.; Karpowich, N.K.; Goetz, R.M.; Law, C.J.; Reith, M.E.; Wang, D.N. LeuT-desipramine structure reveals how antidepressants block neurotransmitter reuptake. Science 2007, 317, 1390–1393. [Google Scholar] [CrossRef]
- Ordway, G.A.; Jia, W.; Li, J.; Zhu, M.Y.; Mandela, P.; Pan, J. Norepinephrine transporter function and desipramine: Residual drug effects versus short-term regulation. J. Neurosci. Methods 2005, 143, 217–225. [Google Scholar] [CrossRef]
- Zhu, M.Y.; Kyle, P.B.; Hume, A.S.; Ordway, G.A. The persistent membrane retention of desipramine causes lasting inhibition of norepinephrine transporter function. Neurochem. Res. 2004, 29, 419–427. [Google Scholar] [CrossRef]
- Andersen, J.; Kristensen, A.S.; Bang-Andersen, B.; Stromgaard, K. Recent advances in the understanding of the interaction of antidepressant drugs with serotonin and norepinephrine transporters. Chem. Commun. 2009, 15, 3677–3692. [Google Scholar] [CrossRef]
- Hyman, S.E.; Nestler, E.J. Initiation and adaptation: A paradigm for understanding psychotropic drug action. Am. J. Psychiatry 1996, 153, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Elojeimy, S.; Holman, D.H.; Liu, X.; El-Zawahry, A.; Villani, M.; Cheng, J.C.; Mahdy, A.; Zeidan, Y.; Bielwaska, A.; Hannun, Y.A.; et al. New insights on the use of desipramine as an inhibitor for acid ceramidase. FEBS Lett. 2006, 580, 4751–4756. [Google Scholar] [CrossRef] [PubMed]
- Hurwitz, R.; Ferlinz, K.; Sandhoff, K. The tricyclic antidepressant desipramine causes proteolytic degradation of lysosomal sphingomyelinase in human fibroblasts. Biol. Chem. Hoppe Seyler 1994, 375, 447–450. [Google Scholar] [CrossRef] [PubMed]
- Kolzer, M.; Werth, N.; Sandhoff, K. Interactions of acid sphingomyelinase and lipid bilayers in the presence of the tricyclic antidepressant desipramine. FEBS Lett. 2004, 559, 96–98. [Google Scholar] [CrossRef] [PubMed]
- Lai, M.; Realini, N.; La Ferla, M.; Passalacqua, I.; Matteoli, G.; Ganesan, A.; Pistello, M.; Mazzanti, C.M.; Piomelli, D. Complete Acid Ceramidase ablation prevents cancer-initiating cell formation in melanoma cells. Sci. Rep. 2017, 7, 7411. [Google Scholar] [CrossRef] [PubMed]
- Saad, A.F.; Meacham, W.D.; Bai, A.; Anelli, V.; Elojeimy, S.; Mahdy, A.E.; Turner, L.S.; Cheng, J.; Bielawska, A.; Bielawski, J.; et al. The functional effects of acid ceramidase overexpression in prostate cancer progression and resistance to chemotherapy. Cancer Biol. Ther. 2007, 6, 1455–1460. [Google Scholar] [CrossRef] [PubMed]
- Seelan, R.S.; Qian, C.; Yokomizo, A.; Bostwick, D.G.; Smith, D.I.; Liu, W. Human acid ceramidase is overexpressed but not mutated in prostate cancer. Genes. Chromosomes Cancer 2000, 29, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Beckham, T.H.; Lu, P.; Cheng, J.C.; Zhao, D.; Turner, L.S.; Zhang, X.; Hoffman, S.; Armeson, K.E.; Liu, A.; Marrison, T.; et al. Acid ceramidase-mediated production of sphingosine 1-phosphate promotes prostate cancer invasion through upregulation of cathepsin B. Int. J. Cancer 2012, 131, 2034–2043. [Google Scholar] [CrossRef] [PubMed]
- Awojoodu, A.O.; Keegan, P.M.; Lane, A.R.; Zhang, Y.; Lynch, K.R.; Platt, M.O.; Botchwey, E.A. Acid sphingomyelinase is activated in sickle cell erythrocytes and contributes to inflammatory microparticle generation in SCD. Blood 2014, 124, 1941–1950. [Google Scholar] [CrossRef]
- Lang, P.A.; Schenck, M.; Nicolay, J.P.; Becker, J.U.; Kempe, D.S.; Lupescu, A.; Koka, S.; Eisele, K.; Klarl, B.A.; Rubben, H.; et al. Liver cell death and anemia in Wilson disease involve acid sphingomyelinase and ceramide. Nat. Med. 2007, 13, 164–170. [Google Scholar] [CrossRef]
- Momchilova, A.; Pankov, R.; Alexandrov, A.; Markovska, T.; Pankov, S.; Krastev, P.; Staneva, G.; Vassileva, E.; Krastev, N.; Pinkas, A. Sphingolipid Catabolism and Glycerophospholipid Levels Are Altered in Erythrocytes and Plasma from Multiple Sclerosis Patients. Int. J. Mol. Sci. 2022, 23, 7592. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.Y.; Li, J.; Jo, S.H.; Lee, S.J.; Oh, S.B.; Kim, J.S.; Lee, J.H.; Park, K. Desipramine inhibits Na+/H+ exchanger in human submandibular cells. J. Dent. Res. 2006, 85, 839–843. [Google Scholar] [CrossRef] [PubMed]
- Ellegaard, A.M.; Dehlendorff, C.; Vind, A.C.; Anand, A.; Cederkvist, L.; Petersen, N.H.T.; Nylandsted, J.; Stenvang, J.; Mellemgaard, A.; Osterlind, K.; et al. Repurposing Cationic Amphiphilic Antihistamines for Cancer Treatment. EBioMedicine 2016, 9, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Petersen, N.H.; Olsen, O.D.; Groth-Pedersen, L.; Ellegaard, A.M.; Bilgin, M.; Redmer, S.; Ostenfeld, M.S.; Ulanet, D.; Dovmark, T.H.; Lonborg, A.; et al. Transformation-associated changes in sphingolipid metabolism sensitize cells to lysosomal cell death induced by inhibitors of acid sphingomyelinase. Cancer Cell 2013, 24, 379–393. [Google Scholar] [CrossRef] [PubMed]
- Arimochi, H.; Morita, K. Desipramine induces apoptotic cell death through nonmitochondrial and mitochondrial pathways in different types of human colon carcinoma cells. Pharmacology 2008, 81, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Pakkanen, K.; Salonen, E.; Makela, A.R.; Oker-Blom, C.; Vattulainen, I.; Vuento, M. Desipramine induces disorder in cholesterol-rich membranes: Implications for viral trafficking. Phys. Biol. 2009, 6, 046004. [Google Scholar] [CrossRef] [PubMed]
- Salata, C.; Calistri, A.; Parolin, C.; Baritussio, A.; Palu, G. Antiviral activity of cationic amphiphilic drugs. Expert. Rev. Anti Infect. Ther. 2017, 15, 483–492. [Google Scholar] [CrossRef]
- Pan, X.; Giustarini, D.; Lang, F.; Rossi, R.; Wieder, T.; Koberle, M.; Ghashghaeinia, M. Desipramine induces eryptosis in human erythrocytes, an effect blunted by nitric oxide donor sodium nitroprusside and N-acetyl-L-cysteine but enhanced by Calcium depletion. Cell Cycle 2023, 22, 1827–1853. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, X.; Köberle, M.; Ghashghaeinia, M. Vitamin C-Dependent Uptake of Non-Heme Iron by Enterocytes, Its Impact on Erythropoiesis and Redox Capacity of Human Erythrocytes. Antioxidants 2024, 13, 968. https://doi.org/10.3390/antiox13080968
Pan X, Köberle M, Ghashghaeinia M. Vitamin C-Dependent Uptake of Non-Heme Iron by Enterocytes, Its Impact on Erythropoiesis and Redox Capacity of Human Erythrocytes. Antioxidants. 2024; 13(8):968. https://doi.org/10.3390/antiox13080968
Chicago/Turabian StylePan, Xia, Martin Köberle, and Mehrdad Ghashghaeinia. 2024. "Vitamin C-Dependent Uptake of Non-Heme Iron by Enterocytes, Its Impact on Erythropoiesis and Redox Capacity of Human Erythrocytes" Antioxidants 13, no. 8: 968. https://doi.org/10.3390/antiox13080968





