Mechanism of Mitochondrial Kinetic Imbalance and Nrf2 Signaling Pathway-Mediated Oxidative Stress in Nickel and/or Chromium-Induced Kidney Injury in Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Experimental Design
2.2. Sample Collection and Administration
2.3. Observation of Kidney Microstructure
2.4. Ultrastructural Observation of the Kidney
2.5. Measurement of Kidney Function
2.6. Determination of Antioxidant Indicators
2.7. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
2.8. Western Blot Analysis
2.9. Statistical Analysis
3. Results
3.1. Impairments of Renal Function in Mice by Nickel and/or Chromium
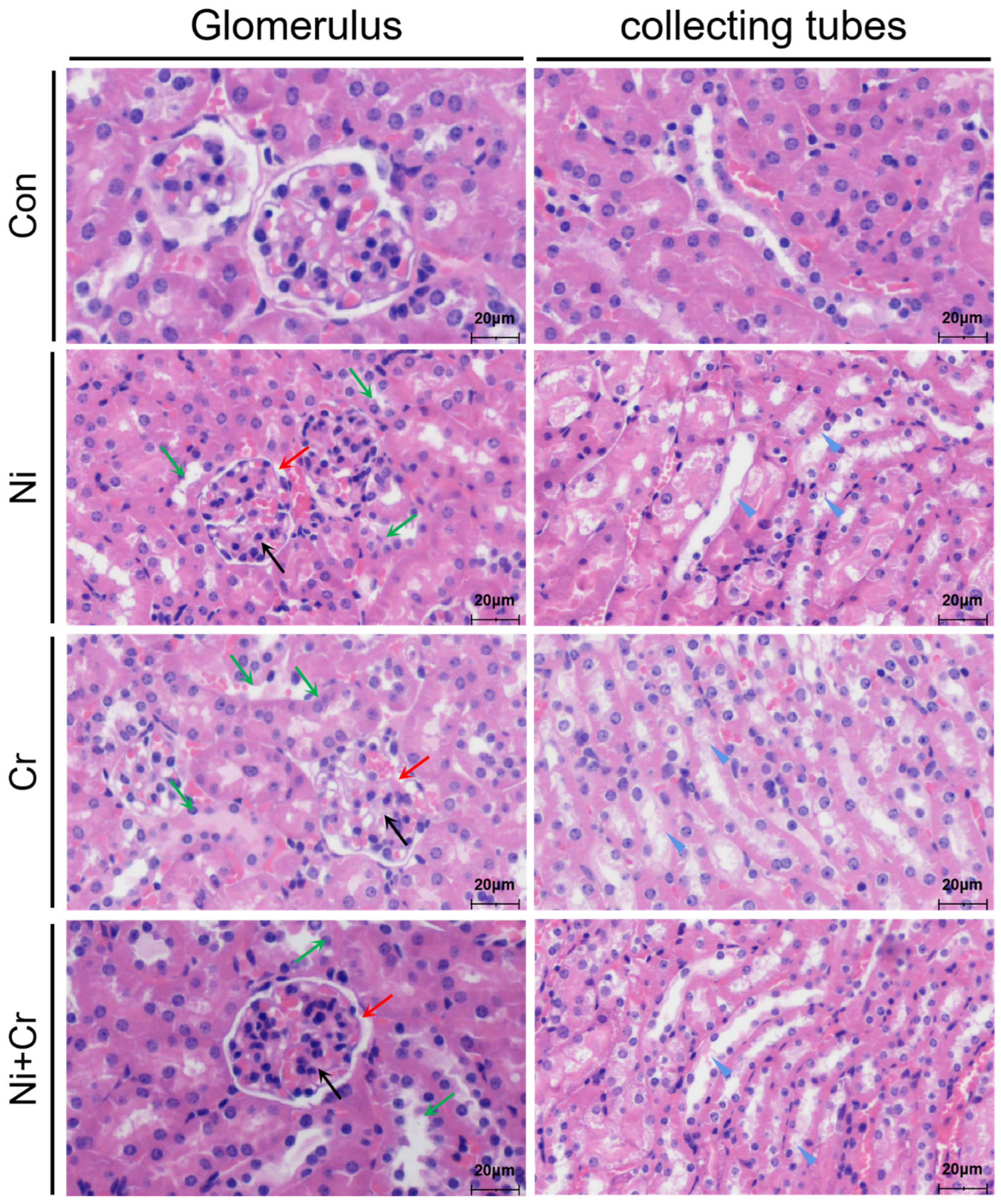
3.2. Effects of Nickel and/or Chromium Exposure on the Pathologic Histology of the Mouse Kidney
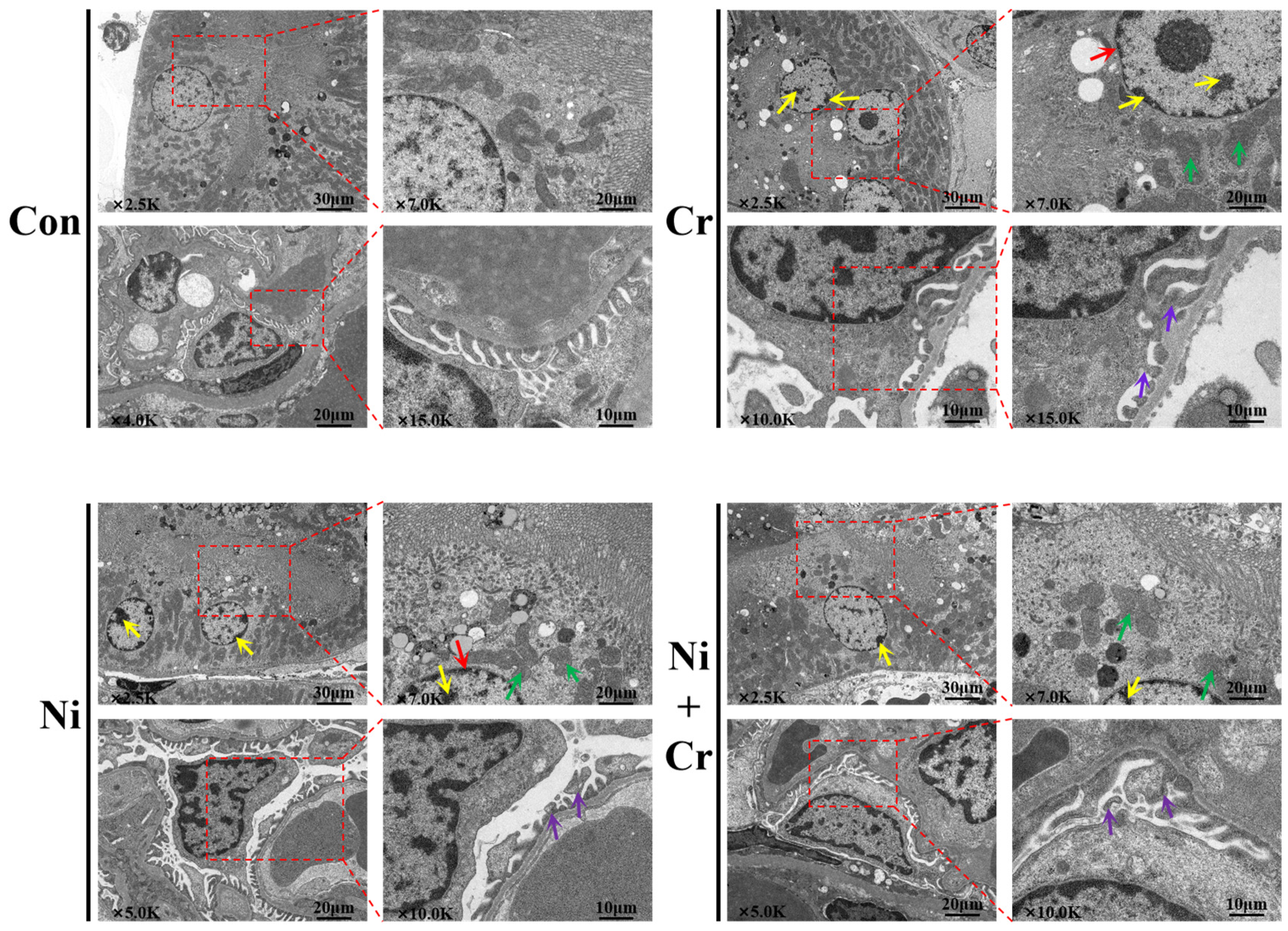
3.3. Effects of Nickel and/or Chromium Exposure on the Ultrastructure of the Mouse Kidney
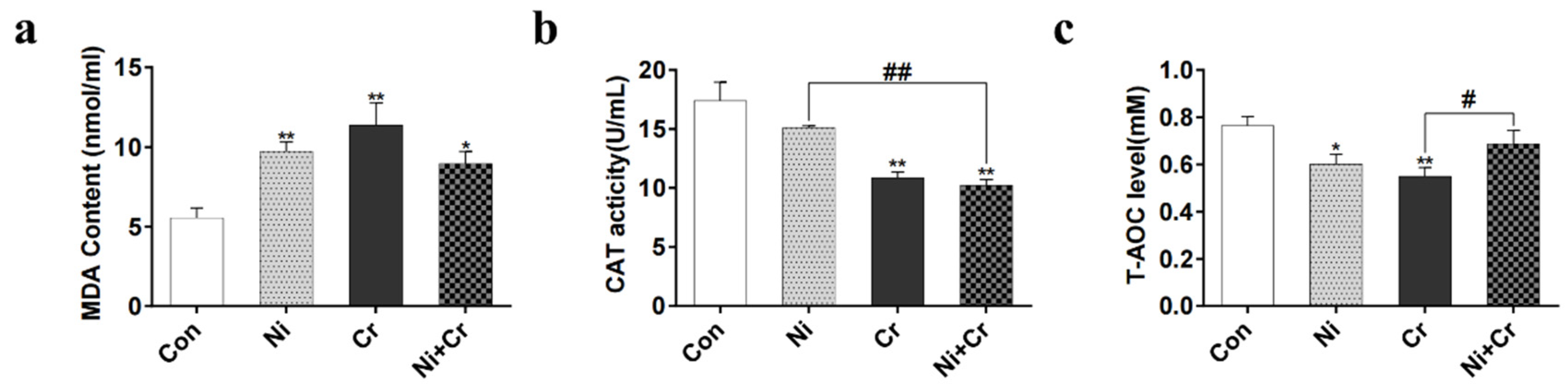
3.4. Effects of Nickel and/or Chromium Exposure on Oxidative Stress in Mice
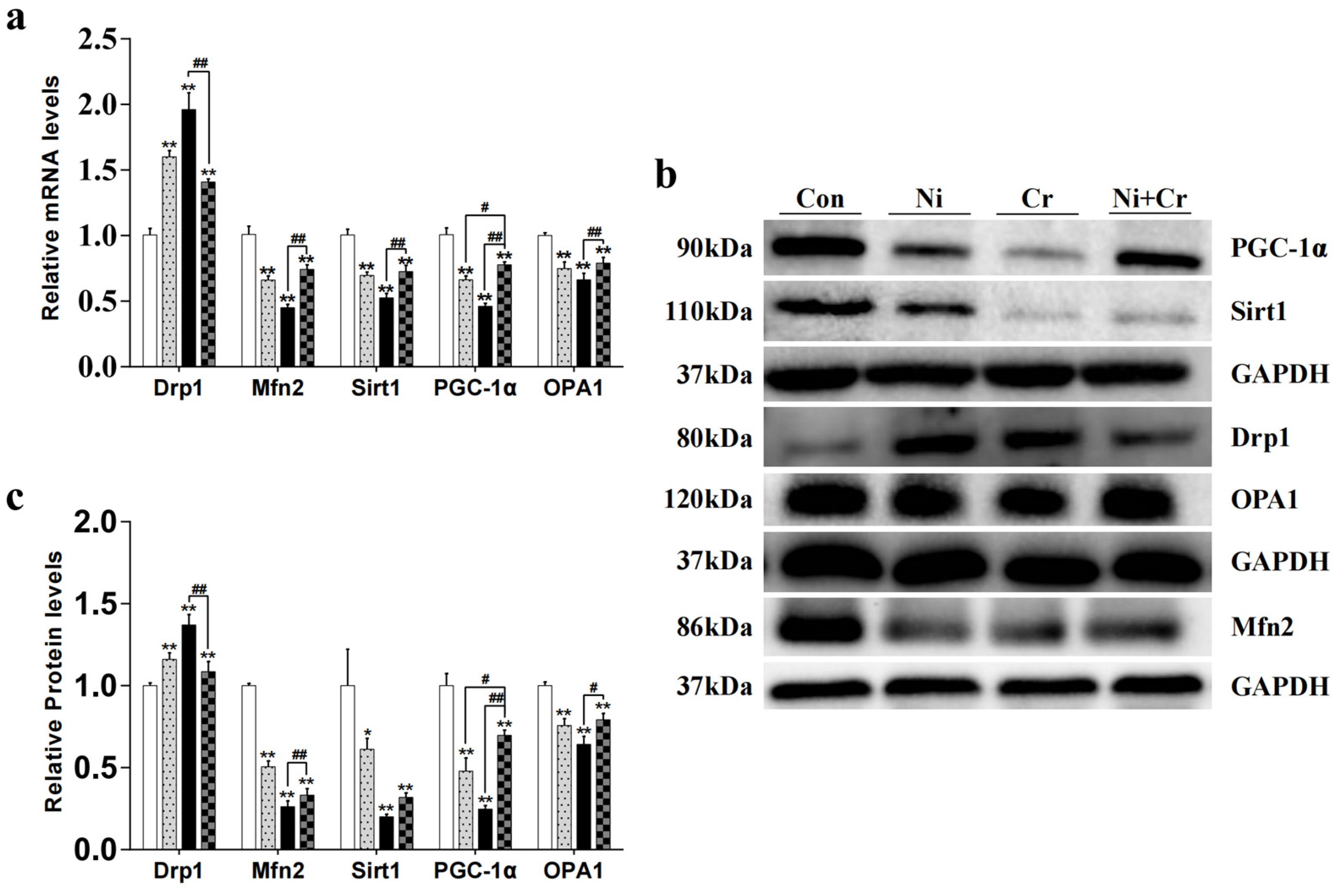
3.5. Effects of Nickel and/or Chromium Exposure on the Relative Expression of Genes and Proteins Associated with Mitochondrial Dynamics
3.6. Effects of Nickel and/or Chromium Exposure on the Relative Expression of Key Molecular Genes and Proteins of the Nrf2 Signaling Pathway
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Arita, A.; Niu, J.; Qu, Q.; Zhao, N.; Ruan, Y.; Nadas, A.; Chervona, Y.; Wu, F.; Sun, H.; Hayes, R.B.; et al. Global levels of histone modifications in peripheral blood mononuclear cells of subjects with exposure to nickel. Environ. Health Perspect. 2012, 120, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Greenwood, N.N.; Earnshaw, A. Chemistry of the Elements; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Genchi, G.; Carocci, A.; Lauria, G.; Sinicropi, M.S.; Catalano, A. Nickel: Human health and environmental toxicology. Int. J. Environ. Res. Public Health 2020, 17, 679. [Google Scholar] [CrossRef] [PubMed]
- Buters, J.; Biedermann, T. Chromium(VI) Contact Dermatitis: Getting Closer to Understanding the Underlying Mechanisms of Toxicity and Sensitization! J. Investig. Dermatol. 2017, 137, 274–277. [Google Scholar] [CrossRef] [PubMed]
- Kotyzova, D.; Hodkova, A.; Bludovska, M.; Eybl, V. Effect of chromium (VI) exposure on antioxidant defense status and trace element homeostasis in acute experiment in rat. Toxicol. Ind. Health 2015, 31, 1044–1050. [Google Scholar] [CrossRef] [PubMed]
- Ogunbileje, J.; Sadagoparamanujam, V.-M.; Anetor, J.; Farombi, E.; Akinosun, O.; Okorodudu, A. Lead, mercury, cadmium, chromium, nickel, copper, zinc, calcium, iron, manganese and chromium (VI) levels in Nigeria and United States of America cement dust. Chemosphere 2013, 90, 2743–2749. [Google Scholar] [CrossRef] [PubMed]
- Skjelkvåle, B.L.; Andersen, T.; Fjeld, E.; Mannio, J.; Wilander, A.; Johansson, K.; Jensen, J.P.; Moiseenko, T. Heavy metal surveys in Nordic lakes; concentrations, geographic patterns and relation to critical limits. AMBIO A J. Hum. Environ. 2001, 30, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Krishna, A.K.; Mohan, K.R.; Murthy, N.; Periasamy, V.; Bipinkumar, G.; Manohar, K.; Rao, S.S. Assessment of heavy metal contamination in soils around chromite mining areas, Nuggihalli, Karnataka, India. Environ. Earth Sci. 2013, 70, 699–708. [Google Scholar] [CrossRef]
- Liao, X.; Chen, T.; Wu, B.; Yan, X.; Nie, C.; Xie, H.; Zhai, L.; Xiao, X. Mining urban soil pollution: Concentrations and patterns of heavy metals in the soils of Jinchang, China. Geogr. Res. 2006, 25, 843–852. [Google Scholar]
- Wang, S.; Nan, Z.; Liu, X.; Li, Y.; Qin, S.; Ding, H. Accumulation and bioavailability of copper and nickel in wheat plants grown in contaminated soils from the oasis, northwest China. Geoderma 2009, 152, 290–295. [Google Scholar] [CrossRef]
- Wang, X.; Li, L.; Yan, X.; Meng, X.; Chen, Y. Processes of chromium (VI) migration and transformation in chromate production site: A case study from the middle of China. Chemosphere 2020, 257, 127282. [Google Scholar] [CrossRef]
- Li, S.; Wu, J.; Huo, Y.; Zhao, X.; Xue, L. Profiling multiple heavy metal contamination and bacterial communities surrounding an iron tailing pond in Northwest China. Sci. Total Environ. 2021, 752, 141827. [Google Scholar] [CrossRef] [PubMed]
- Denkhaus, E.; Salnikow, K. Nickel essentiality, toxicity, and carcinogenicity. Crit. Rev. Oncol./Hematol. 2002, 42, 35–56. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-M.; Ma, J.-Q.; Liu, S.-S.; Feng, Z.-J.; Wang, A.-M. Puerarin protects mouse liver against nickel-induced oxidative stress and inflammation associated with the TLR4/p38/CREB pathway. Chem.-Biol. Interact. 2016, 243, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Templeton, D.M.; Sunderman, F.W., Jr.; Herber, R.F. Tentative reference values for nickel concentrations in human serum, plasma, blood, and urine: Evaluation according to the TRACY protocol. Sci. Total Environ. 1994, 148, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Zuo, Z.; Yang, Z.; Guo, H.; Fang, J.; Cui, H.; Ouyang, P.; Chen, X.; Chen, J.; Geng, Y. Nickel induces autophagy via PI3K/AKT/mTOR and AMPK pathways in mouse kidney. Ecotoxicol. Environ. Saf. 2021, 223, 112583. [Google Scholar] [CrossRef] [PubMed]
- Elangovan, P.; Ramakrishnan, R.; Amudha, K.; Jalaludeen, A.M.; Sagaran, G.K.; Babu, F.R.; Pari, L. Beneficial protective effect of troxerutin on nickel-induced renal dysfunction in wistar rats. J. Environ. Pathol. Toxicol. Oncol. 2018, 37, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Amudha, K.; Pari, L. Beneficial role of naringin, a flavanoid on nickel induced nephrotoxicity in rats. Chem.-Biol. Interact. 2011, 193, 57–64. [Google Scholar] [CrossRef]
- Kotyk, B.; Iskra, R.; Lubunets, V. Antioxidant Effect of the Complex Action of Vitamin E and Ethylthiosulfanylate in the Liver and Kidneys of Rats under Conditions of Chrome (VI)-Induced Oxidative Stress. Biointerface Res. Appl. Chem. 2021, 12, 1405–1420. [Google Scholar]
- Suljević, D.; Sulejmanović, J.; Fočak, M.; Halilović, E.; Pupalović, D.; Hasić, A.; Alijagic, A. Assessing hexavalent chromium tissue-specific accumulation patterns and induced physiological responses to probe chromium toxicity in Coturnix japonica quail. Chemosphere 2021, 266, 129005. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, X.; Wang, L.; Cheng, G.; Zhang, M.; Xing, Y.; Zhao, X.; Liu, Y.; Liu, J. Mitophagy induced by mitochondrial function damage in chicken kidney exposed to Cr (VI). Biol. Trace Elem. Res. 2021, 199, 703–711. [Google Scholar] [CrossRef]
- Yang, D.; Yang, Q.; Fu, N.; Li, S.; Han, B.; Liu, Y.; Tang, Y.; Guo, X.; Lv, Z.; Zhang, Z. Hexavalent chromium induced heart dysfunction via Sesn2-mediated impairment of mitochondrial function and energy supply. Chemosphere 2021, 264, 128547. [Google Scholar] [CrossRef] [PubMed]
- Pagliarini, D.J.; Calvo, S.E.; Chang, B.; Sheth, S.A.; Vafai, S.B.; Ong, S.-E.; Walford, G.A.; Sugiana, C.; Boneh, A.; Chen, W.K. A mitochondrial protein compendium elucidates complex I disease biology. Cell 2008, 134, 112–123. [Google Scholar] [CrossRef] [PubMed]
- Chandhok, G.; Lazarou, M.; Neumann, B. Structure, function, and regulation of mitofusin-2 in health and disease. Biol. Rev. Camb. Philos. Soc. 2018, 93, 933–949. [Google Scholar] [CrossRef] [PubMed]
- Wai, T.; Langer, T. Mitochondrial Dynamics and Metabolic Regulation. Trends Endocrinol. Metab. 2016, 27, 105–117. [Google Scholar] [CrossRef]
- Chan, D.C. Fusion and fission: Interlinked processes critical for mitochondrial health. Annu. Rev. Genet. 2012, 46, 265–287. [Google Scholar] [CrossRef] [PubMed]
- Otera, H.; Mihara, K. Molecular mechanisms and physiologic functions of mitochondrial dynamics. J. Biochem. 2011, 149, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Stefanatos, R.; Sanz, A. The role of mitochondrial ROS in the aging brain. FEBS Lett. 2018, 592, 743–758. [Google Scholar] [CrossRef]
- Haemmerle, G.; Moustafa, T.; Woelkart, G.; Büttner, S.; Schmidt, A.; Van De Weijer, T.; Hesselink, M.; Jaeger, D.; Kienesberger, P.C.; Zierler, K. ATGL-mediated fat catabolism regulates cardiac mitochondrial function via PPAR-α and PGC-1. Nat. Med. 2011, 17, 1076–1085. [Google Scholar] [CrossRef] [PubMed]
- Fang, E.F.; Lautrup, S.; Hou, Y.; Demarest, T.G.; Croteau, D.L.; Mattson, M.P.; Bohr, V.A. NAD+ in aging: Molecular mechanisms and translational implications. Trends Mol. Med. 2017, 23, 899–916. [Google Scholar] [CrossRef]
- Hybertson, B.M.; Gao, B.; Bose, S.K.; McCord, J.M. Oxidative stress in health and disease: The therapeutic potential of Nrf2 activation. Mol. Asp. Med. 2011, 32, 234–246. [Google Scholar] [CrossRef]
- Shelton, L.M.; Park, B.K.; Copple, I.M. Role of Nrf2 in protection against acute kidney injury. Kidney Int. 2013, 84, 1090–1095. [Google Scholar] [CrossRef] [PubMed]
- Saini, S.; Nair, N.; Saini, M.R. Embryotoxic and teratogenic effects of nickel in Swiss albino mice during organogenetic period. BioMed Res. Int. 2013, 2013, 701439. [Google Scholar] [CrossRef] [PubMed]
- Thompson, C.M.; Proctor, D.M.; Haws, L.C.; Hébert, C.D.; Grimes, S.D.; Shertzer, H.G.; Kopec, A.K.; Hixon, J.G.; Zacharewski, T.R.; Harris, M.A. Investigation of the mode of action underlying the tumorigenic response induced in B6C3F1 mice exposed orally to hexavalent chromium. Toxicol. Sci. 2011, 123, 58–70. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Dwivedi, S. A review on accessible techniques for removal of hexavalent Chromium and divalent Nickel from industrial wastewater: Recent research and future outlook. J. Clean. Prod. 2021, 295, 126229. [Google Scholar] [CrossRef]
- Zheng, X.; Li, S.; Li, J.; Lv, Y.; Wang, X.; Wu, P.; Yang, Q.; Tang, Y.; Liu, Y.; Zhang, Z. Hexavalent chromium induces renal apoptosis and autophagy via disordering the balance of mitochondrial dynamics in rats. Ecotoxicol. Environ. Saf. 2020, 204, 111061. [Google Scholar] [CrossRef] [PubMed]
- de Andrade Lima, L.; Bernardez, L.; Barbosa, L.A.D. Characterization and treatment of artisanal gold mine tailings. J. Hazard. Mater. 2008, 150, 747–753. [Google Scholar] [CrossRef] [PubMed]
- Terpilowska, S.; Siwicki, A.K. Interactions between chromium (III) and iron (III), molybdenum (III) or nickel (II): Cytotoxicity, genotoxicity and mutagenicity studies. Chemosphere 2018, 201, 780–789. [Google Scholar] [CrossRef]
- Guo, P.; Liu, A.; Huang, D.; Wu, Q.; Fatima, Z.; Tao, Y.; Cheng, G.; Wang, X.; Yuan, Z. Brain damage and neurological symptoms induced by T-2 toxin in rat brain. Toxicol. Lett. 2018, 286, 96–107. [Google Scholar] [CrossRef]
- Szadkowska-Stańczyk, I.; Woźniak, H.; Stroszejn-Mrowca, G. Health effects of occupational exposure among shoe workers. A review. Med. Pract. 2003, 54, 67–71. [Google Scholar]
- Yan, L.-J.; Allen, D.C. Cadmium-induced kidney injury: Oxidative damage as a unifying mechanism. Biomolecules 2021, 11, 1575. [Google Scholar] [CrossRef]
- Nath, K.A.; Croatt, A.J.; Likely, S.; Behrens, T.W.; Warden, D. Renal oxidant injury and oxidant response induced by mercury. Kidney Int. 1996, 50, 1032–1043. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Franco, A.; Fleischer, J.A.; Zhang, L.; Dorn, G.W. Abrogating Mitochondrial Dynamics in Mouse Hearts Accelerates Mitochondrial Senescence. Cell Metab. 2017, 26, 872–883. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Han, B.; Xue, J.; Lv, Y.; Li, S.; Liu, Y.; Wu, P.; Wang, X.; Zhang, Z. Hexavalent chromium induces mitochondrial dynamics disorder in rat liver by inhibiting AMPK/PGC-1α signaling pathway. Environ. Pollut. 2020, 265, 114855. [Google Scholar] [CrossRef] [PubMed]
- Santos, D.; Esteves, A.R.; Silva, D.F.; Januário, C.; Cardoso, S.M. The impact of mitochondrial fusion and fission modulation in sporadic Parkinson’s disease. Mol. Neurobiol. 2015, 52, 573–586. [Google Scholar] [CrossRef] [PubMed]
- Westermann, B. Bioenergetic role of mitochondrial fusion and fission. Biochim. Et Biophys. Acta (BBA)-Bioenerg. 2012, 1817, 1833–1838. [Google Scholar] [CrossRef] [PubMed]
- Yue, W.; Chen, Z.; Liu, H.; Yan, C.; Chen, M.; Feng, D.; Yan, C.; Wu, H.; Du, L.; Wang, Y. A small natural molecule promotes mitochondrial fusion through inhibition of the deubiquitinase USP30. Cell Res. 2014, 24, 482–496. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Baiyun, R.; Lv, Z.; Li, J.; Han, D.; Zhao, W.; Yu, L.; Deng, N.; Liu, Z.; Zhang, Z. Exploring the kidney hazard of exposure to mercuric chloride in mice: Disorder of mitochondrial dynamics induces oxidative stress and results in apoptosis. Chemosphere 2019, 234, 822–829. [Google Scholar] [CrossRef] [PubMed]
- Benarroch, E.E. Nrf2, cellular redox regulation, and neurologic implications. Neurology 2017, 88, 1942–1950. [Google Scholar] [CrossRef] [PubMed]
- Vomund, S.; Schäfer, A.; Parnham, M.J.; Brüne, B.; Von Knethen, A. Nrf2, the master regulator of anti-oxidative responses. Int. J. Mol. Sci. 2017, 18, 2772. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, J.; Wu, S.; Jin, C.; Lu, X.; Hu, X.; Sun, Y.; Gao, X.; Cai, Y. Activation of Nrf2/ARE signaling pathway attenuates lanthanum chloride induced injuries in primary rat astrocytes. Met. Integr. Biometal Sci. 2017, 9, 1120–1131. [Google Scholar] [CrossRef]
- García-Niño, W.R.; Pedraza-Chaverrí, J. Protective effect of curcumin against heavy metals-induced liver damage. Food Chem. Toxicol. 2014, 69, 182–201. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Qu, W.; Kadiiska, M.B. Role of oxidative stress in cadmium toxicity and carcinogenesis. Toxicol. Appl. Pharmacol. 2009, 238, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Su, P.; Zhang, J.; Wang, S.; Aschner, M.; Cao, Z.; Zhao, F.; Wang, D.; Chen, J.; Luo, W. Genistein alleviates lead-induced neurotoxicity in vitro and in vivo: Involvement of multiple signaling pathways. Neurotoxicology 2016, 53, 153–164. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Pyo, M.C.; Shin, H.S.; Ryu, D.; Lee, K.-W. Renal toxicity through AhR, PXR, and Nrf2 signaling pathway activation of ochratoxin A-induced oxidative stress in kidney cells. Food Chem. Toxicol. 2018, 122, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Liu, F.; Wang, C.; Zhang, J.; Zhu, A.; Zou, L.; Han, A.; Li, J.; Chang, X.; Sun, Y. Role of oxidative stress in liver toxicity induced by nickel oxide nanoparticles in rats. Mol. Med. Rep. 2018, 17, 3133–3139. [Google Scholar] [CrossRef]
- Ryoo, I.-G.; Kwak, M.-K. Regulatory crosstalk between the oxidative stress-related transcription factor Nfe2l2/Nrf2 and mitochondria. Toxicol. Appl. Pharmacol. 2018, 359, 24–33. [Google Scholar] [CrossRef]


| Genes | Accession Number | Primers Sequences (5′-3′) |
|---|---|---|
| β-Actin | NM_007393.5 | F: 5′-GTACTCTGTGTGGATCGGTGG-3′ R: 5′-GCAGCTCAGTAACAGTCCG-3′ |
| Keap1 | NM_016679.4 | F: 5′-GGCAGGACCAGTTGAACAGT-3′ R: 5′-GGGTCACCTCACTCCAGGTA-3′ |
| Nrf2 | NM_010902.5 | F: 5′-CAGCCATGACTGATTTAAGCAG-3′ R: 5′-CAGCTGCTTGTTTTCGGTATTA-3′ |
| HO-1 | NM_010442.2 | F: 5′-AGGTACACATCCAAGCCGAGA-3′ R: 5′-CATCACCAGCTTAAAGCCTTCT-3′ |
| NQO1 | NM_008706.5 | F: 5′-GGTGAGCTGAAGGACTCGAA-3′ R: 5′-ACCACTGCAATGGGAACTGAA-3′ |
| Drp1 | NM_001025947.3 | F: 5′-CGTAGTGGGAACTCAGAGCA-3′ R: 5′-TGGACCAGCTGCAGAATAAG-3′ |
| Mfn2 | NM_001285920.1 | F: 5′-GCATTCTTGTGGTCGGAGGAGTG-3′ R: 5′-TGGTCCAGGTCAGTCGCTCATAG-3′ |
| PGC-1α | NM_001402987.1 | F: 5′-GGATATACTTTACGCAGGTCGA-3′ R: 5′-CGTCTGAGTTGGTATCTAGGTC-3′ |
| Sirt1 | NM_019812.3 | F: 5′-CGCTGTGGCAGATTGTTATTAA-3′ R: 5′-TTGATCTGAAGTCAGGAATCCC-3′ |
| OPA1 | NM_001199177.2 | F: 5′-CAGCTGGCAGAAGATCTCAAG-3′ R: 5′-CATGAGCAGGATTTTGACACC-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, J.; Li, Z.; Cao, X.; Qi, Q.; Wang, L.; Liu, P.; Chen, Y.; Hu, G.; Guo, X.; Gao, X. Mechanism of Mitochondrial Kinetic Imbalance and Nrf2 Signaling Pathway-Mediated Oxidative Stress in Nickel and/or Chromium-Induced Kidney Injury in Mice. Antioxidants 2024, 13, 980. https://doi.org/10.3390/antiox13080980
Du J, Li Z, Cao X, Qi Q, Wang L, Liu P, Chen Y, Hu G, Guo X, Gao X. Mechanism of Mitochondrial Kinetic Imbalance and Nrf2 Signaling Pathway-Mediated Oxidative Stress in Nickel and/or Chromium-Induced Kidney Injury in Mice. Antioxidants. 2024; 13(8):980. https://doi.org/10.3390/antiox13080980
Chicago/Turabian StyleDu, Jun, Zhengqing Li, Xianhong Cao, Qiurong Qi, Luqi Wang, Ping Liu, Yifei Chen, Guoliang Hu, Xiaoquan Guo, and Xiaona Gao. 2024. "Mechanism of Mitochondrial Kinetic Imbalance and Nrf2 Signaling Pathway-Mediated Oxidative Stress in Nickel and/or Chromium-Induced Kidney Injury in Mice" Antioxidants 13, no. 8: 980. https://doi.org/10.3390/antiox13080980
APA StyleDu, J., Li, Z., Cao, X., Qi, Q., Wang, L., Liu, P., Chen, Y., Hu, G., Guo, X., & Gao, X. (2024). Mechanism of Mitochondrial Kinetic Imbalance and Nrf2 Signaling Pathway-Mediated Oxidative Stress in Nickel and/or Chromium-Induced Kidney Injury in Mice. Antioxidants, 13(8), 980. https://doi.org/10.3390/antiox13080980







