Resveratrol Mitigates Cognitive Impairments and Cholinergic Cell Loss in the Medial Septum in a Mouse Model of Gradual Cerebral Hypoperfusion
Abstract
1. Introduction
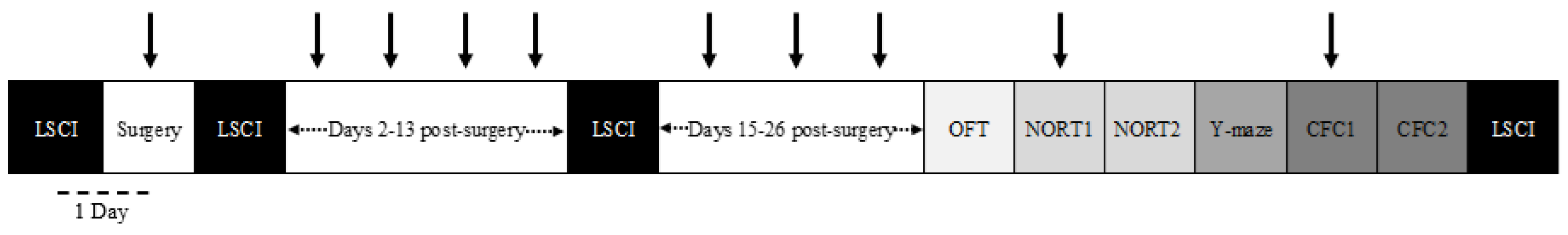
2. Materials and Methods
2.1. Animals
2.2. Surgical Implantation of Ameroid Constrictor
2.3. Resveratrol Treatment
2.4. Laser Speckle Contrast Imaging
2.5. Histological Evaluation
2.6. Open-Field Testing
2.7. Novel Object Recognition Testing
2.8. Spontaneous Alternation
2.9. Contextual Fear Conditioning
2.10. Statistical Analysis
3. Results
3.1. Resveratrol Does Not Ameliorate Reductions in Cerebral Blood Flow in the VCID Model
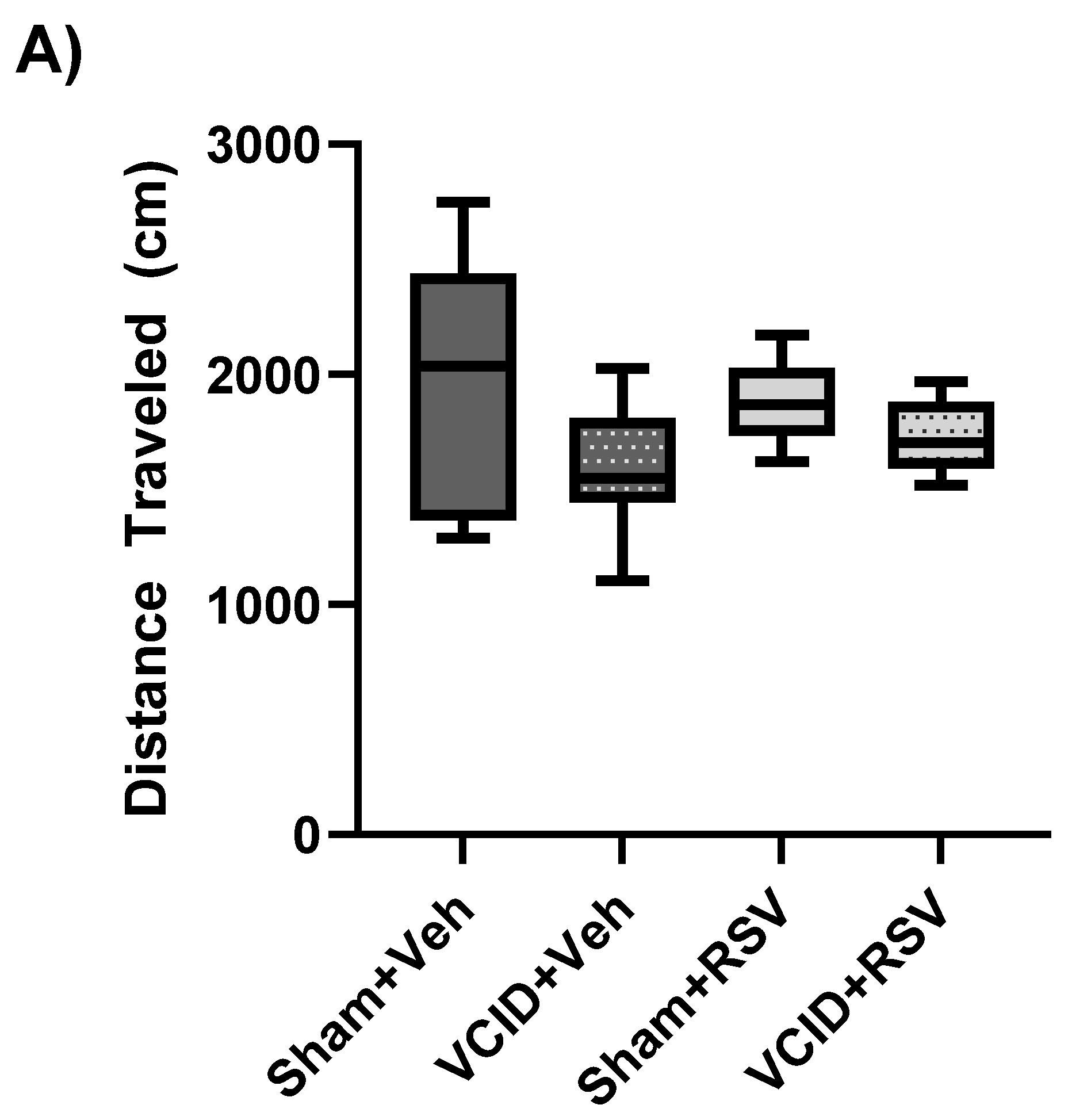
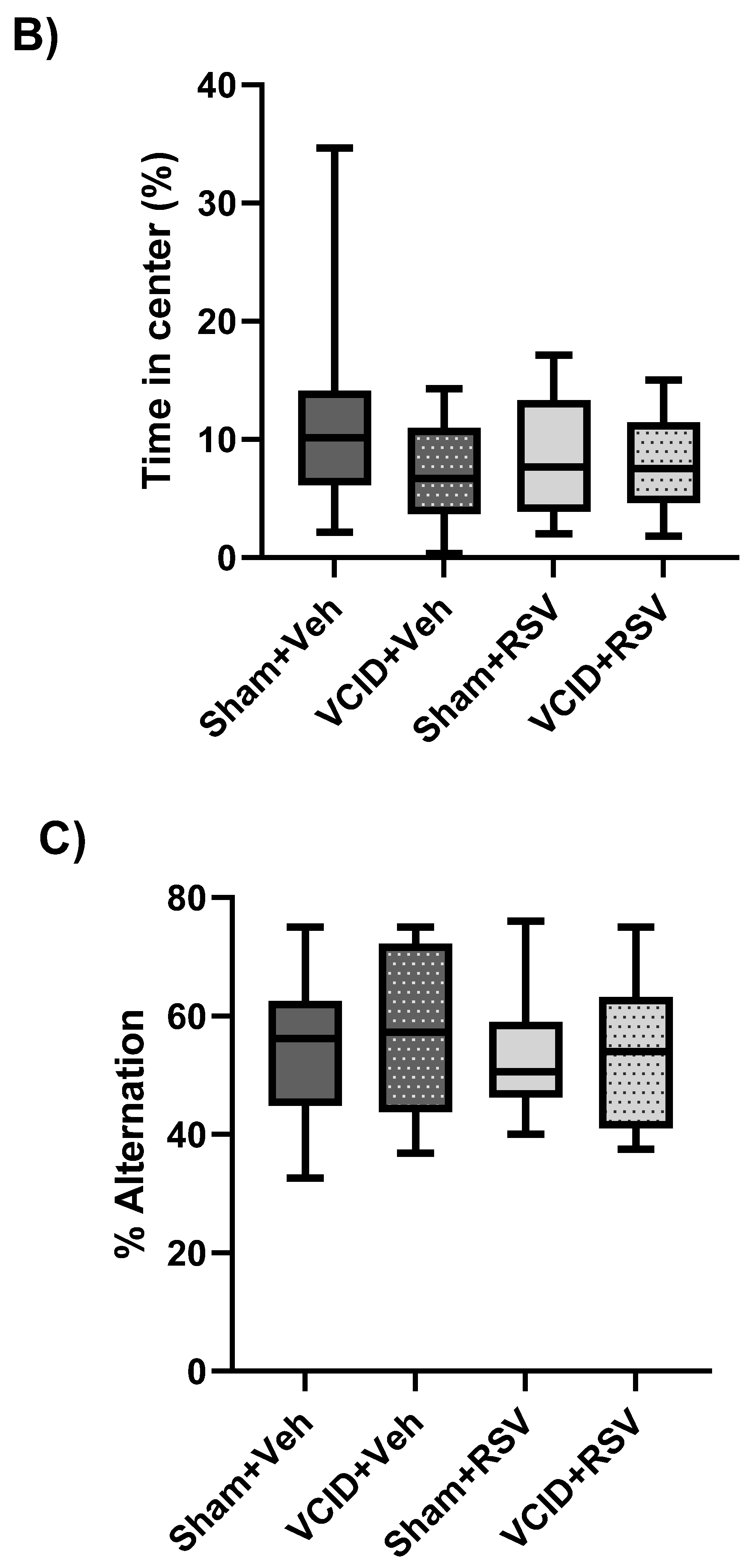
3.2. General Activity/Locomotor Function, Anxiety-Related Behavior, and Spatial Working Memory Are Not Affected by VCID
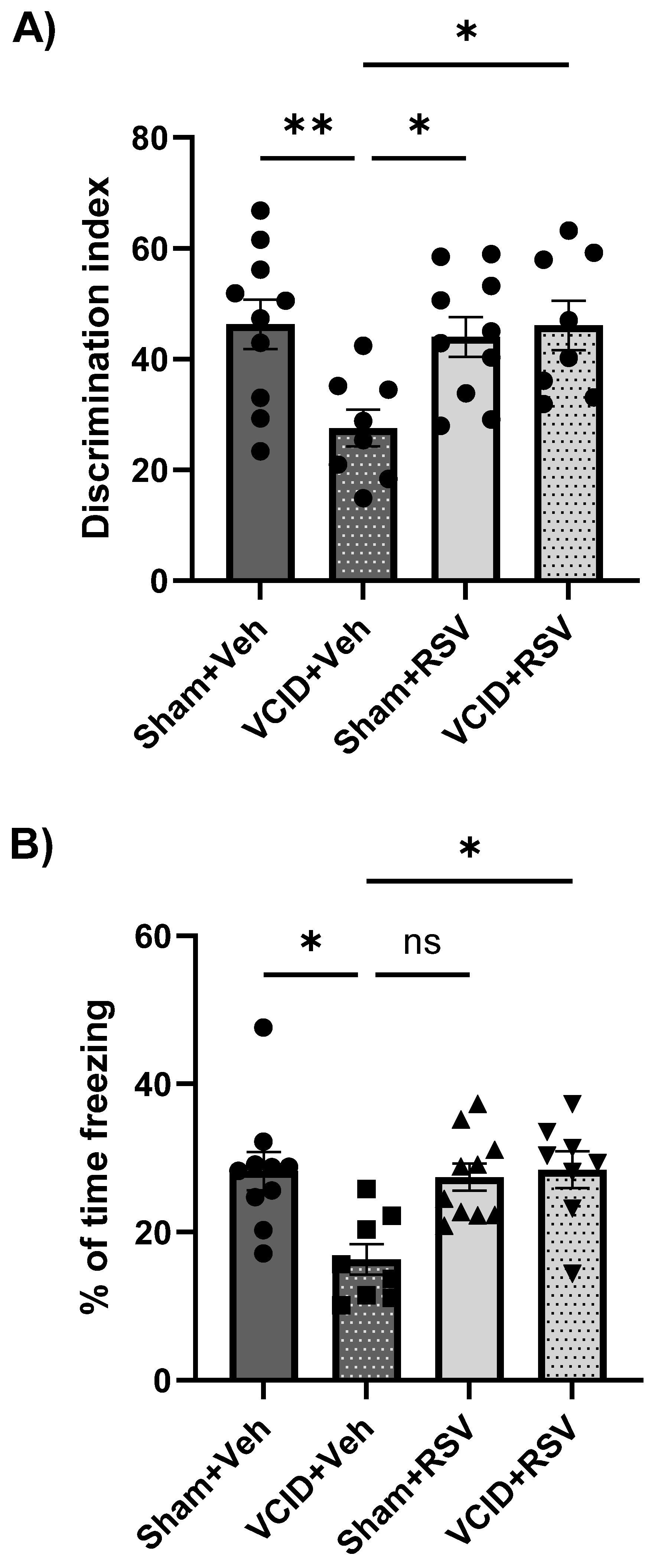
3.3. Resveratrol Mitigates Impairments in Long-Term Object Recognition Memory and in Contextual Fear Memory That Arise from VCID
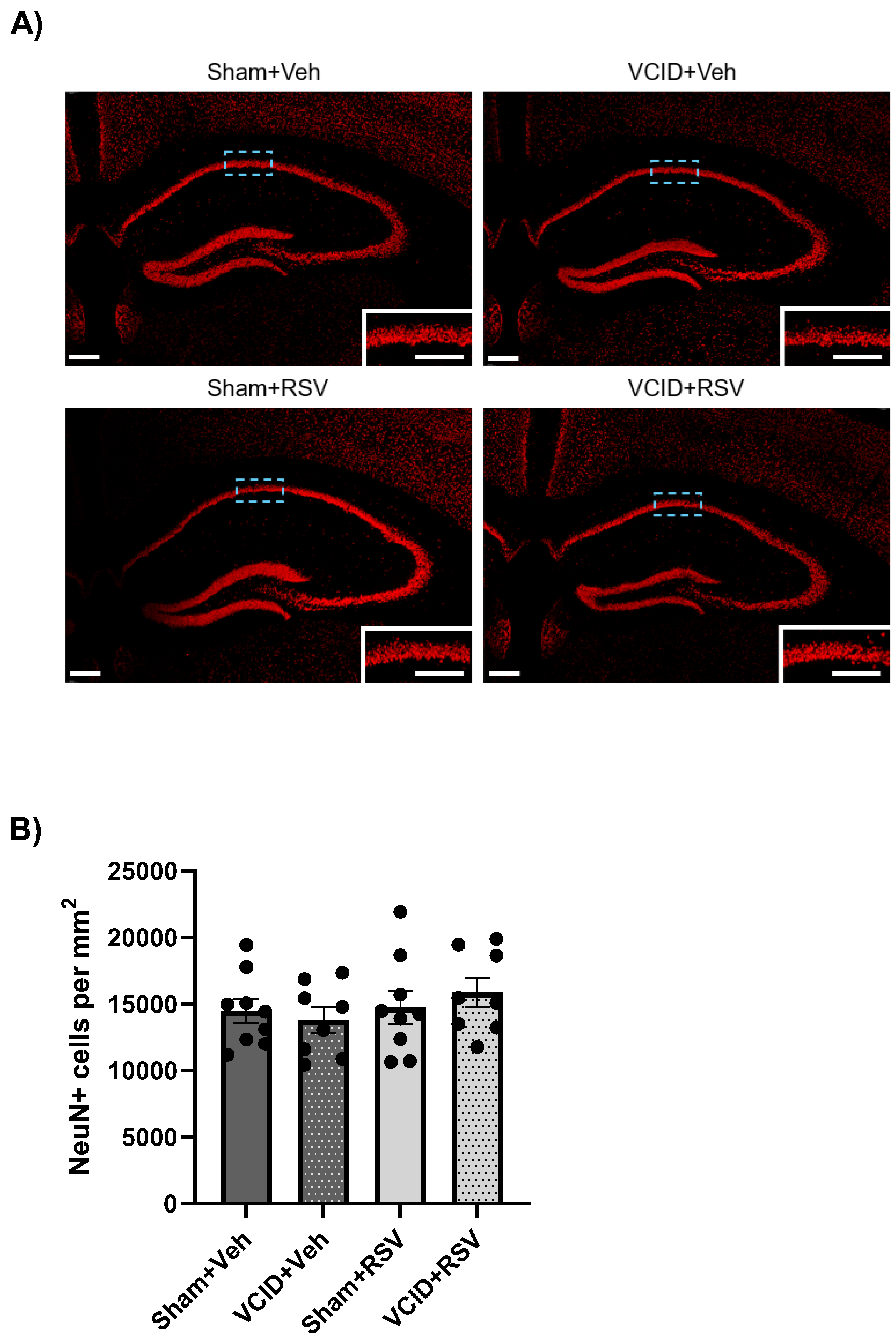
3.4. Hippocampal CA1 Pyramidal Cells Are Unaffected in the VCID Model
3.5. Resveratrol Mitigates VCID-Induced Medial Septal Cholinergic Neuronal Loss
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| VCID | Vascular cognitive impairment and dementia |
| RSV | Resveratrol |
| GBCCAS | Gradual bilateral common carotid artery stenosis |
| NOR | Novel object recognition |
| CFC | Contextual fear conditioning |
| AD | Alzheimer’s disease |
| CCA | Common carotid artery |
| CBF | Cerebral blood flow |
| CFC | Contextual fear conditioning |
| AC | Ameroid constrictor |
| eNOS | Endothelial nitric oxide synthase |
| MCAO | Middle cerebral artery occlusion |
| BF | Basal forebrain |
| ChAT | Choline acetyltransferase |
| OFT | Open field testing |
| NORT | Novel object recognition testing |
References
- Rundek, T.; Tolea, M.; Ariko, T.; Fagerli, E.A.; Camargo, C.J. Vascular Cognitive Impairment (VCI). Neurotherapeutics 2022, 19, 68–88. [Google Scholar] [CrossRef] [PubMed]
- van der Flier, W.M.; Skoog, I.; Schneider, J.A.; Pantoni, L.; Mok, V.; Chen, C.L.H.; Scheltens, P. Vascular cognitive impairment. Nat. Rev. Dis. Primers 2018, 4, 18003. [Google Scholar] [CrossRef] [PubMed]
- Corriveau, R.A.; Bosetti, F.; Emr, M.; Gladman, J.T.; Koenig, J.I.; Moy, C.S.; Pahigiannis, K.; Waddy, S.P.; Koroshetz, W. The Science of Vascular Contributions to Cognitive Impairment and Dementia (VCID): A Framework for Advancing Research Priorities in the Cerebrovascular Biology of Cognitive Decline. Cell Mol. Neurobiol. 2016, 36, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Silva, N.; Bracko, O.; Nelson, A.R.; de Oliveira, F.F.; Robison, L.S.; Shaaban, C.E.; Hainsworth, A.H.; Price, B.R. Vascular cognitive impairment and dementia: An early career researcher perspective. Alzheimers Dement. 2022, 14, e12310. [Google Scholar] [CrossRef] [PubMed]
- Iadecola, C.; Smith, E.E.; Anrather, J.; Gu, C.; Mishra, A.; Misra, S.; Perez-Pinzon, M.A.; Shih, A.Y.; Sorond, F.A.; van Veluw, S.J.; et al. The Neurovasculome: Key Roles in Brain Health and Cognitive Impairment: A Scientific Statement from the American Heart Association/American Stroke Association. Stroke 2023, 54, e251–e271. [Google Scholar] [CrossRef] [PubMed]
- Roman, G.C. Vascular dementia: Distinguishing characteristics, treatment, and prevention. J. Am. Geriatr. Soc. 2003, 51 (Suppl. S5), S296–S304. [Google Scholar] [CrossRef] [PubMed]
- Jiwa, N.S.; Garrard, P.; Hainsworth, A.H. Experimental models of vascular dementia and vascular cognitive impairment: A systematic review. J. Neurochem. 2010, 115, 814–828. [Google Scholar] [CrossRef] [PubMed]
- Hattori, Y.; Enmi, J.; Iguchi, S.; Saito, S.; Yamamoto, Y.; Tsuji, M.; Nagatsuka, K.; Kalaria, R.N.; Iida, H.; Ihara, M. Gradual Carotid Artery Stenosis in Mice Closely Replicates Hypoperfusive Vascular Dementia in Humans. J. Am. Heart Assoc. 2016, 5, e002757. [Google Scholar] [CrossRef] [PubMed]
- Hattori, Y.; Kakino, Y.; Hattori, Y.; Iwashita, M.; Uchiyama, H.; Noda, K.; Yoshimoto, T.; Iida, H.; Ihara, M. Long-Term Resveratrol Intake for Cognitive and Cerebral Blood Flow Impairment in Carotid Artery Stenosis/Occlusion. J. Stroke 2024, 26, 64–74. [Google Scholar] [CrossRef]
- Hattori, Y.; Minami, M.; Omae, K.; Yoshimoto, T.; Abe, S.; Yamamoto, H.; Iida, H.; Ihara, M. REsveratrol for VAscular cognitive impairment investigating cerebral Metabolism and Perfusion (REVAMP trial): A study protocol for a randomized, double-blind, placebo-controlled trial. Front. Nutr. 2024, 11, 1359330. [Google Scholar] [CrossRef]
- Smith, E.E. Introduction to Focused Update Vascular Cognitive Impairment and Dementia: Better Diagnosis, More Avenues for Prevention. Stroke 2024, 55, 788–790. [Google Scholar] [CrossRef] [PubMed]
- Pyo, I.S.; Yun, S.; Yoon, Y.E.; Choi, J.W.; Lee, S.J. Mechanisms of Aging and the Preventive Effects of Resveratrol on Age-Related Diseases. Molecules 2020, 25, 4649. [Google Scholar] [CrossRef] [PubMed]
- Caruso, F.; Tanski, J.; Villegas-Estrada, A.; Rossi, M. Structural basis for antioxidant activity of trans-resveratrol: Ab initio calculations and crystal and molecular structure. J. Agric. Food Chem. 2004, 52, 7279–7285. [Google Scholar] [CrossRef]
- Stewart, R. Vascular dementia: A diagnosis running out of time. Br. J. Psychiatry 2002, 180, 152–156. [Google Scholar] [CrossRef] [PubMed]
- Aliev, G.; Palacios, H.H.; Gasimov, E.; Obrenovich, M.E.; Morales, L.; Leszek, J.; Bragin, V.; Herrera, A.S.; Gokhman, D. Oxidative Stress Induced Mitochondrial Failure and Vascular Hypoperfusion as a Key Initiator for the Development of Alzheimer Disease. Pharmaceuticals 2010, 3, 158–187. [Google Scholar] [CrossRef] [PubMed]
- Tewari, D.; Sah, A.N.; Bawari, S.; Nabavi, S.F.; Dehpour, A.R.; Shirooie, S.; Braidy, N.; Fiebich, B.L.; Vacca, R.A.; Nabavi, S.M. Role of Nitric Oxide in Neurodegeneration: Function, Regulation, and Inhibition. Curr. Neuropharmacol. 2021, 19, 114–126. [Google Scholar] [CrossRef]
- Zhu, H.Y.; Hong, F.F.; Yang, S.L. The Roles of Nitric Oxide Synthase/Nitric Oxide Pathway in the Pathology of Vascular Dementia and Related Therapeutic Approaches. Int. J. Mol. Sci. 2021, 22, 4540. [Google Scholar] [CrossRef]
- Li, H.; Xia, N.; Hasselwander, S.; Daiber, A. Resveratrol and Vascular Function. Int. J. Mol. Sci. 2021, 20, 2155. [Google Scholar] [CrossRef]
- Khoury, N.; Xu, J.; Stegelmann, S.D.; Jackson, C.W.; Koronowski, K.B.; Dave, K.R.; Young, J.I.; Perez-Pinzon, M.A. Resveratrol Preconditioning Induces Genomic and Metabolic Adaptations within the Long-Term Window of Cerebral Ischemic Tolerance Leading to Bioenergetic Efficiency. Mol. Neurobiol. 2019, 56, 4549–4565. [Google Scholar] [CrossRef]
- Escobar, I.; Xu, J.; Jackson, C.W.; Stegelmann, S.D.; Fagerli, E.A.; Dave, K.R.; Perez-Pinzon, M.A. Resveratrol Preconditioning Protects Against Ischemia-Induced Synaptic Dysfunction and Cofilin Hyperactivation in the Mouse Hippocampal Slice. Neurotherapeutics 2023, 20, 1177–1197. [Google Scholar] [CrossRef]
- Koronowski, K.B.; Dave, K.R.; Saul, I.; Camarena, V.; Thompson, J.W.; Neumann, J.T.; Young, J.I.; Perez-Pinzon, M.A. Resveratrol Preconditioning Induces a Novel Extended Window of Ischemic Tolerance in the Mouse Brain. Stroke 2015, 46, 2293–2298. [Google Scholar] [CrossRef] [PubMed]
- Lopez, M.S.; Dempsey, R.J.; Vemuganti, R. Resveratrol preconditioning induces cerebral ischemic tolerance but has minimal effect on cerebral microRNA profiles. J. Cereb. Blood Flow Metab. 2016, 36, 1644–1650. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, S.V.; Dave, K.R.; Saul, I.; Perez-Pinzon, M.A. Resveratrol Preconditioning Protects Against Cerebral Ischemic Injury via Nuclear Erythroid 2-Related Factor 2. Stroke 2015, 46, 1626–1632. [Google Scholar] [CrossRef] [PubMed]
- Jackson, C.W.; Xu, J.; Escobar, I.; Saul, I.; Fagerli, E.; Dave, K.R.; Perez-Pinzon, M.A. Resveratrol Preconditioning Downregulates PARP1 Protein to Alleviate PARP1-Mediated Cell Death Following Cerebral Ischemia. Transl. Stroke Res. 2024, 15, 165–178. [Google Scholar] [CrossRef] [PubMed]
- Jackson, C.W.; Escobar, I.; Xu, J.; Perez-Pinzon, M.A. Effects of ischemic preconditioning on mitochondrial and metabolic neuroprotection: 5′ adenosine monophosphate-activated protein kinase and sirtuins. Brain Circ. 2018, 4, 54–61. [Google Scholar] [PubMed]
- Davies, D.A.; Adlimoghaddam, A.; Albensi, B.C. Role of Nrf2 in Synaptic Plasticity and Memory in Alzheimer’s Disease. Cells 2021, 10, 1884. [Google Scholar] [CrossRef] [PubMed]
- Gureev, A.P.; Popov, V.N.; Starkov, A.A. Crosstalk between the mTOR and Nrf2/ARE signaling pathways as a target in the improvement of long-term potentiation. Exp. Neurol. 2020, 328, 113285. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Morales, M.A.; Escobar, I.; Saul, I.; Jackson, C.W.; Ferrier, F.J.; Fagerli, E.A.; Raval, A.P.; Dave, K.R.; Perez-Pinzon, M.A. Resveratrol Preconditioning Mitigates Ischemia-Induced Septal Cholinergic Cell Loss and Memory Impairments. Stroke 2023, 54, 1099–1109. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, N.Y.; Knowles, R.; Dehorter, N. New Insights into Cholinergic Neuron Diversity. Front. Mol. Neurosci. 2019, 12, 204. [Google Scholar] [CrossRef]
- Chen, X.Q.; Mobley, W.C. Exploring the Pathogenesis of Alzheimer Disease in Basal Forebrain Cholinergic Neurons: Converging Insights from Alternative Hypotheses. Front. Neurosci. 2019, 13, 446. [Google Scholar] [CrossRef]
- Winek, K.; Soreq, H.; Meisel, A. Regulators of cholinergic signaling in disorders of the central nervous system. J. Neurochem. 2021, 158, 1425–1438. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, Y.; Wang, Y.; Wang, G.; Mao, L.; Zhang, D.; Wang, J. Effects of resveratrol on learning and memory in rats with vascular dementia. Mol. Med. Rep. 2019, 20, 4587–4593. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Sun, Z.; Liu, Y.; Jia, Y.; Zhang, B.; Zhang, J. Resveratrol improves cognition and reduces oxidative stress in rats with vascular dementia. Neural Regen. Res. 2013, 8, 2050–2059. [Google Scholar] [PubMed]
- Gocmez, S.S.; Sahin, T.D.; Yazir, Y.; Duruksu, G.; Eraldemir, F.C.; Polat, S.; Utkan, T. Resveratrol prevents cognitive deficits by attenuating oxidative damage and inflammation in rat model of streptozotocin diabetes induced vascular dementia. Physiol. Behav. 2019, 201, 198–207. [Google Scholar] [CrossRef] [PubMed]
- Dunn, A.K.; Bolay, H.; Moskowitz, M.A.; Boas, D.A. Dynamic imaging of cerebral blood flow using laser speckle. J. Cereb. Blood Flow Metab. 2001, 21, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Lipkind, D.; Sakov, A.; Kafkafi, N.; Elmer, G.I.; Benjamini, Y.; Golani, I. New replicable anxiety-related measures of wall vs center behavior of mice in the open field. J. Appl. Physiol. (1985) 2004, 97, 347–359. [Google Scholar] [CrossRef] [PubMed]
- Cheon, S.Y.; Koo, B.N.; Kim, S.Y.; Kam, E.H.; Nam, J.; Kim, E.J. Scopolamine promotes neuroinflammation and delirium-like neuropsychiatric disorder in mice. Sci. Rep. 2021, 11, 8376. [Google Scholar] [CrossRef] [PubMed]
- Tahamtan, M.; Allahtavakoli, M.; Abbasnejad, M.; Roohbakhsh, A.; Taghipour, Z.; Taghavi, M.; Khodadadi, H.; Shamsizadeh, A. Exercise preconditioning improves behavioral functions following transient cerebral ischemia induced by 4-vessel occlusion (4-VO) in rats. Arch. Iran. Med. 2013, 16, 697–704. [Google Scholar]
- Denninger, J.K.; Smith, B.M.; Kirby, E.D. Novel Object Recognition and Object Location Behavioral Testing in Mice on a Budget. J. Vis. Exp. 2018. [Google Scholar] [CrossRef]
- Leger, M.; Quiedeville, A.; Bouet, V.; Haelewyn, B.; Boulouard, M.; Schumann-Bard, P.; Freret, T. Object recognition test in mice. Nat. Protoc. 2013, 8, 2531–2537. [Google Scholar] [CrossRef]
- Prieur, E.A.K.; Jadavji, N.M. Assessing Spatial Working Memory Using the Spontaneous Alternation Y-maze Test in Aged Male Mice. Bio Protoc. 2019, 9, e3162. [Google Scholar] [CrossRef] [PubMed]
- Faul, F.; Erdfelder, E.; Lang, A.G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Simao, F.; Pagnussat, A.S.; Seo, J.H.; Navaratna, D.; Leung, W.; Lok, J.; Guo, S.; Waeber, C.; Salbego, C.G.; Lo, E.H. Pro-angiogenic effects of resveratrol in brain endothelial cells: Nitric oxide-mediated regulation of vascular endothelial growth factor and metalloproteinases. J. Cereb. Blood Flow Metab. 2012, 32, 884–895. [Google Scholar] [CrossRef] [PubMed]
- Almatroodi, S.A.; Alsahli, M.A.; Aljohani, A.S.M.; Alhumaydhi, F.A.; Babiker, A.Y.; Khan, A.A.; Rahmani, A.H. Potential Therapeutic Targets of Resveratrol, a Plant Polyphenol, and Its Role in the Therapy of Various Types of Cancer. Molecules 2022, 27, 2665. [Google Scholar] [CrossRef] [PubMed]
- Muller, C.; Remy, S. Septo-hippocampal interaction. Cell Tissue Res. 2018, 373, 565–575. [Google Scholar] [CrossRef] [PubMed]
- Fraga, E.; Medina, V.; Cuartero, M.I.; Garcia-Culebras, A.; Bravo-Ferrer, I.; Hernandez-Jimenez, M.; Garcia-Segura, J.M.; Hurtado, O.; Pradillo, J.M.; Lizasoain, I.; et al. Defective hippocampal neurogenesis underlies cognitive impairment by carotid stenosis-induced cerebral hypoperfusion in mice. Front. Cell Neurosci. 2023, 17, 1219847. [Google Scholar] [CrossRef] [PubMed]
- Maki, T.; Ihara, M.; Fujita, Y.; Nambu, T.; Miyashita, K.; Yamada, M.; Washida, K.; Nishio, K.; Ito, H.; Harada, H.; et al. Angiogenic and vasoprotective effects of adrenomedullin on prevention of cognitive decline after chronic cerebral hypoperfusion in mice. Stroke 2011, 42, 1122–1128. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Zhang, L.; Kong, X.; Liu, K.; Wu, H. Different Exercise Time on 5-HT and Anxiety-like Behavior in the Rat with Vascular Dementia. Am. J. Alzheimers Dis. Other Demen. 2022, 37, 15333175221082743. [Google Scholar] [CrossRef] [PubMed]
- Anagnostaras, S.G.; Gale, G.D.; Fanselow, M.S. Hippocampus and contextual fear conditioning: Recent controversies and advances. Hippocampus 2001, 11, 8–17. [Google Scholar] [CrossRef]
- Ji, J.; Maren, S. Differential roles for hippocampal areas CA1 and CA3 in the contextual encoding and retrieval of extinguished fear. Learn. Mem. 2008, 15, 244–251. [Google Scholar] [CrossRef]
- Shibata, M.; Ohtani, R.; Ihara, M.; Tomimoto, H. White matter lesions and glial activation in a novel mouse model of chronic cerebral hypoperfusion. Stroke 2004, 35, 2598–2603. [Google Scholar] [CrossRef] [PubMed]
- Shibata, M.; Yamasaki, N.; Miyakawa, T.; Kalaria, R.N.; Fujita, Y.; Ohtani, R.; Ihara, M.; Takahashi, R.; Tomimoto, H. Selective impairment of working memory in a mouse model of chronic cerebral hypoperfusion. Stroke 2007, 38, 2826–2832. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.; Zhang, X.; Gao, D.; Li, N. Cerebral angiogenesis induced by resveratrol contributes to relieve cerebral ischemic-reperfusion injury. Med. Hypotheses 2007, 69, 226–227. [Google Scholar] [CrossRef] [PubMed]
- Santabarbara, J.; Lipnicki, D.M.; Olaya, B.; Villagrasa, B.; Gracia-Garcia, P.; Bueno-Notivol, J.; Lobo, A.; Lopez-Anton, R. Association between Anxiety and Vascular Dementia Risk: New Evidence and an Updated Meta-Analysis. J. Clin. Med. 2020, 9, 1368. [Google Scholar] [CrossRef] [PubMed]
- Gomoll, B.P.; Kumar, A. Managing anxiety associated with neurodegenerative disorders. F1000Prime Rep. 2015, 7, 05. [Google Scholar] [CrossRef]
- Hainsworth, A.H.; Allan, S.M.; Boltze, J.; Cunningham, C.; Farris, C.; Head, E.; Ihara, M.; Isaacs, J.D.; Kalaria, R.N.; Lesnik Oberstein, S.A.; et al. Translational models for vascular cognitive impairment: A review including larger species. BMC Med. 2017, 15, 16. [Google Scholar] [CrossRef]
- Wang, Q.; Yu, Q.; Wu, M. Antioxidant and neuroprotective actions of resveratrol in cerebrovascular diseases. Front. Pharmacol. 2022, 13, 948889. [Google Scholar] [CrossRef]
- Patel, A.; Moalem, A.; Cheng, H.; Babadjouni, R.M.; Patel, K.; Hodis, D.M.; Chandegara, D.; Cen, S.; He, S.; Liu, Q.; et al. Chronic cerebral hypoperfusion induced by bilateral carotid artery stenosis causes selective recognition impairment in adult mice. Neurol. Res. 2017, 39, 910–917. [Google Scholar] [CrossRef]
- Zuloaga, K.L.; Zhang, W.; Yeiser, L.A.; Stewart, B.; Kukino, A.; Nie, X.; Roese, N.E.; Grafe, M.R.; Pike, M.M.; Raber, J.; et al. Neurobehavioral and imaging correlates of hippocampal atrophy in a mouse model of vascular cognitive impairment. Transl. Stroke Res. 2015, 6, 390–398. [Google Scholar] [CrossRef]
- Knox, D. The role of basal forebrain cholinergic neurons in fear and extinction memory. Neurobiol. Learn. Mem. 2016, 133, 39–52. [Google Scholar] [CrossRef]
- Knox, D.; Keller, S.M. Cholinergic neuronal lesions in the medial septum and vertical limb of the diagonal bands of Broca induce contextual fear memory generalization and impair acquisition of fear extinction. Hippocampus 2016, 26, 718–726. [Google Scholar] [CrossRef] [PubMed]
- Ferreira-Vieira, T.H.; Guimaraes, I.M.; Silva, F.R.; Ribeiro, F.M. Alzheimer’s disease: Targeting the Cholinergic System. Curr. Neuropharmacol. 2016, 14, 101–115. [Google Scholar] [CrossRef] [PubMed]
- Mehla, J.; Lacoursiere, S.; Stuart, E.; McDonald, R.J.; Mohajerani, M.H. Gradual Cerebral Hypoperfusion Impairs Fear Conditioning and Object Recognition Learning and Memory in Mice: Potential Roles of Neurodegeneration and Cholinergic Dysfunction. J. Alzheimers Dis. 2018, 61, 283–293. [Google Scholar] [CrossRef] [PubMed]
- Tatu, L.; Vuillier, F. Structure and vascularization of the human hippocampus. Front. Neurol. Neurosci. 2014, 34, 18–25. [Google Scholar] [PubMed]
- Michan, S.; Sinclair, D.A. Sirtuins in mammals: Insights into their biological function. Biochem. J. 2007, 404, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Oberdoerffer, P.; Michan, S.; McVay, M.; Mostoslavsky, R.; Vann, J.; Park, S.K.; Hartlerode, A.; Stegmuller, J.; Hafner, A.; Loerch, P.; et al. SIRT1 redistribution on chromatin promotes genomic stability but alters gene expression during aging. Cell 2008, 135, 907–918. [Google Scholar] [CrossRef] [PubMed]
- Haigis, M.C.; Sinclair, D.A. Mammalian sirtuins: Biological insights and disease relevance. Annu. Rev. Pathol. 2010, 5, 253–295. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.L. SIRT1, neuronal cell survival and the insulin/IGF-1 aging paradox. Neurobiol. Aging 2006, 27, 501–505. [Google Scholar] [CrossRef]
- Lutz, M.I.; Milenkovic, I.; Regelsberger, G.; Kovacs, G.G. Distinct patterns of sirtuin expression during progression of Alzheimer’s disease. Neuromol. Med. 2014, 16, 405–414. [Google Scholar] [CrossRef]
- Manjula, R.; Anuja, K.; Alcain, F.J. SIRT1 and SIRT2 Activity Control in Neurodegenerative Diseases. Front. Pharmacol. 2021, 11, 585821. [Google Scholar] [CrossRef]
- Hussain, A.; Pooryasin, A.; Zhang, M.; Loschek, L.F.; Fortezza, M.L.; Friedrich, A.B.; Blais, C.M.; Üçpunar, H.K.; Yépez, V.A.; Lehmann, M.; et al. Inhibition of oxidative stress in cholinergic projection neurons fully rescues aging-associated olfactory circuit degeneration in Drosophila. eLife 2018, 7, e32018. [Google Scholar] [CrossRef] [PubMed]
- Leung, L.S.; Law, C.S.H. Phasic modulation of hippocampal synaptic plasticity by theta rhythm. Behav. Neurosci. 2020, 134, 595–612. [Google Scholar] [CrossRef] [PubMed]
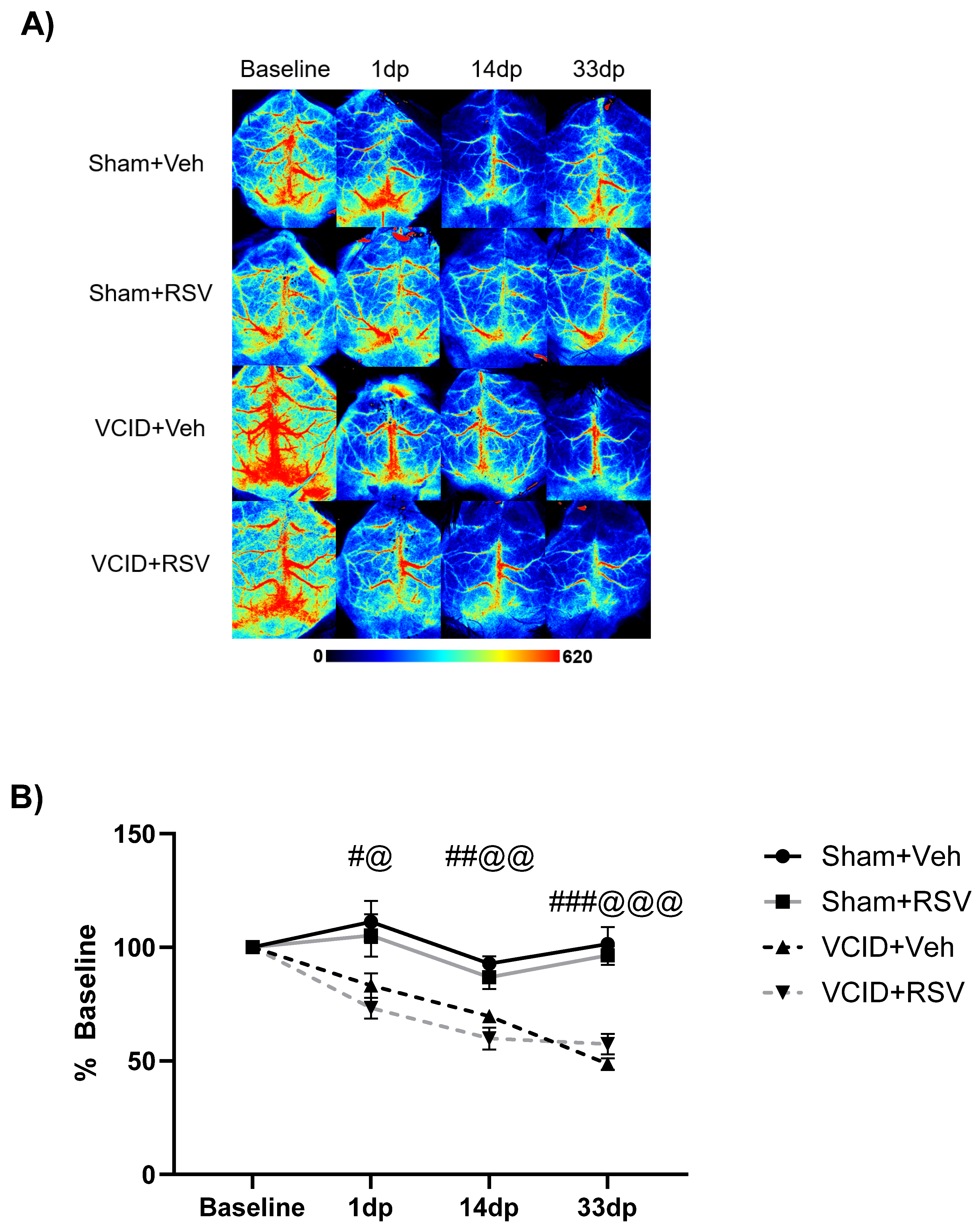

| Sham + Veh | VCID + RSV | Sham + RSV | VCID + Veh | |
|---|---|---|---|---|
| Day 1 | 111 ± 9% | 73 ± 4% | 105 ± 9% | 83 ± 5% |
| Day 14 | 93 ± 3% | 59 ± 4% | 86 ± 5% | 70 ± 2% |
| Day 33 | 101 ± 5% | 57 ± 4% | 96 ± 4% | 49 ± 3% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fagerli, E.; Jackson, C.W.; Escobar, I.; Ferrier, F.J.; Perez Lao, E.J.; Saul, I.; Gomez, J.; Dave, K.R.; Bracko, O.; Perez-Pinzon, M.A. Resveratrol Mitigates Cognitive Impairments and Cholinergic Cell Loss in the Medial Septum in a Mouse Model of Gradual Cerebral Hypoperfusion. Antioxidants 2024, 13, 984. https://doi.org/10.3390/antiox13080984
Fagerli E, Jackson CW, Escobar I, Ferrier FJ, Perez Lao EJ, Saul I, Gomez J, Dave KR, Bracko O, Perez-Pinzon MA. Resveratrol Mitigates Cognitive Impairments and Cholinergic Cell Loss in the Medial Septum in a Mouse Model of Gradual Cerebral Hypoperfusion. Antioxidants. 2024; 13(8):984. https://doi.org/10.3390/antiox13080984
Chicago/Turabian StyleFagerli, Eric, Charles W. Jackson, Iris Escobar, Fernando J. Ferrier, Efrain J. Perez Lao, Isabel Saul, Jorge Gomez, Kunjan R. Dave, Oliver Bracko, and Miguel A. Perez-Pinzon. 2024. "Resveratrol Mitigates Cognitive Impairments and Cholinergic Cell Loss in the Medial Septum in a Mouse Model of Gradual Cerebral Hypoperfusion" Antioxidants 13, no. 8: 984. https://doi.org/10.3390/antiox13080984
APA StyleFagerli, E., Jackson, C. W., Escobar, I., Ferrier, F. J., Perez Lao, E. J., Saul, I., Gomez, J., Dave, K. R., Bracko, O., & Perez-Pinzon, M. A. (2024). Resveratrol Mitigates Cognitive Impairments and Cholinergic Cell Loss in the Medial Septum in a Mouse Model of Gradual Cerebral Hypoperfusion. Antioxidants, 13(8), 984. https://doi.org/10.3390/antiox13080984






