Exploration of the Synergistic Regulation Mechanism in Cerebral Ganglion and Heart of Eriocheir sinensis on Energy Metabolism and Antioxidant Homeostasis Maintenance under Alkalinity Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Crabs and Alkalinity Stress
2.2. Measurement of Serum Biochemical Parameters
2.3. Proteomics Analysis on Cerebral Ganglion of E. sinensis
2.3.1. Proteins Extraction, Separation, Enzymolysis, and Desalination
2.3.2. LC-MS/MS Analysis and Database Query
2.3.3. Gene Ontology (GO) Annotation on Proteomic
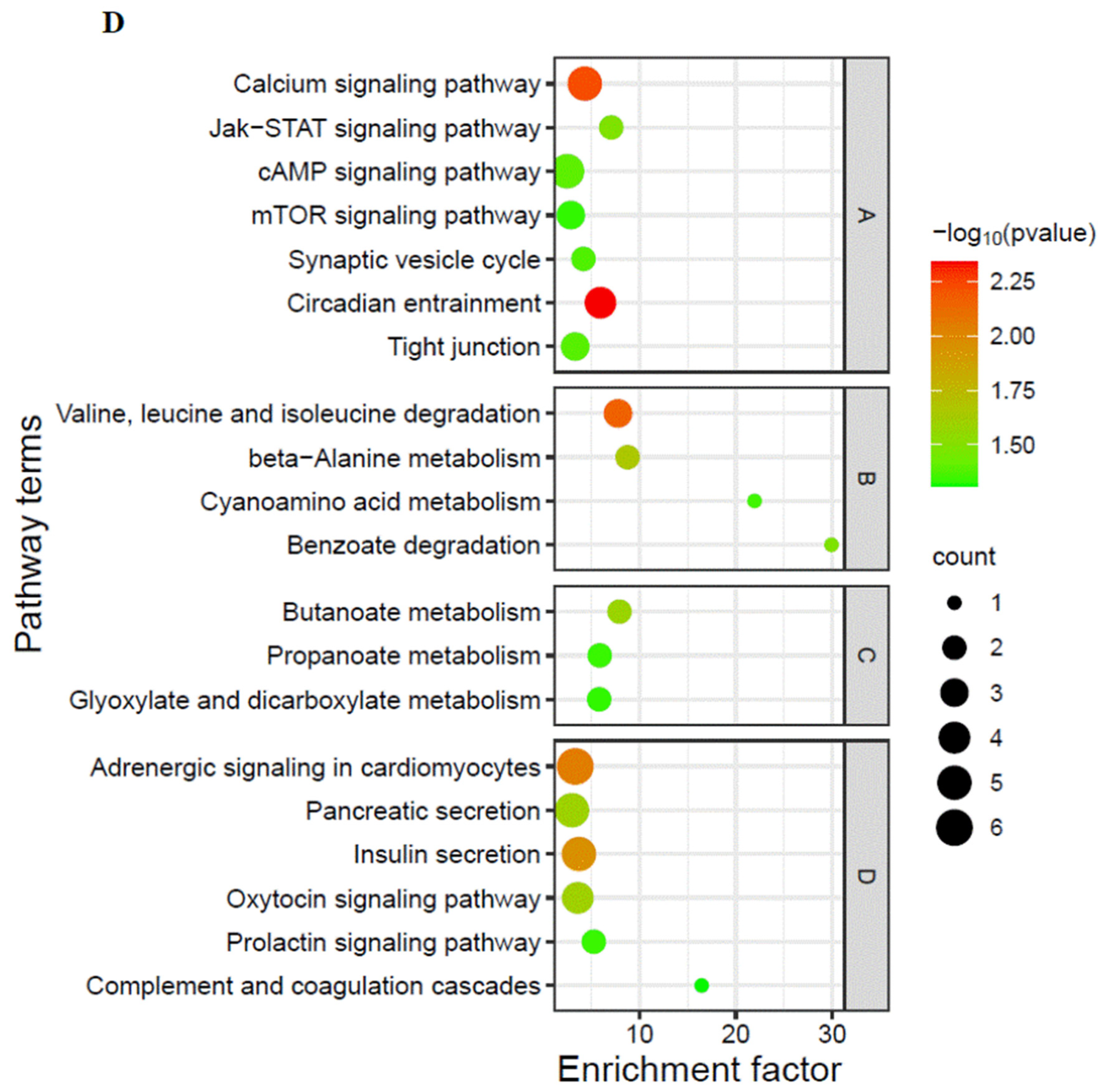
2.3.4. Enrichment Analysis on Differentially Expressed Proteins (DEPs)
2.4. Metabolome Analysis on Heart of E. sinensis
2.4.1. Extraction of Metabolites from Heart of E. sinensis and UPLC-MS Analysis
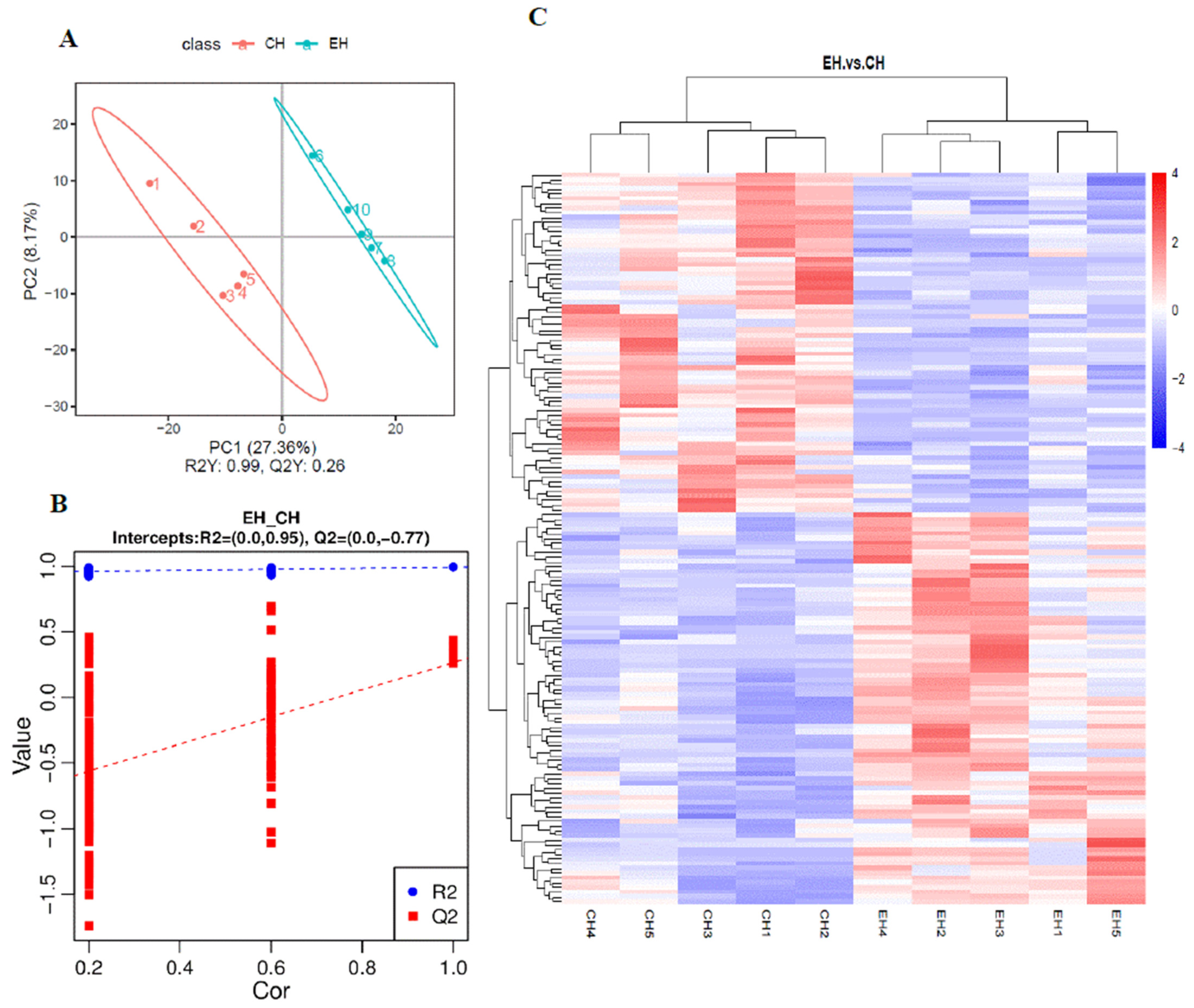
2.4.2. PCA and Partial Least-Squares Discrimination Analysis (PLS-DA) on Differentially Expressed Metabolites (DEMs)
2.4.3. KEGG Enrichment Analysis on DEMs
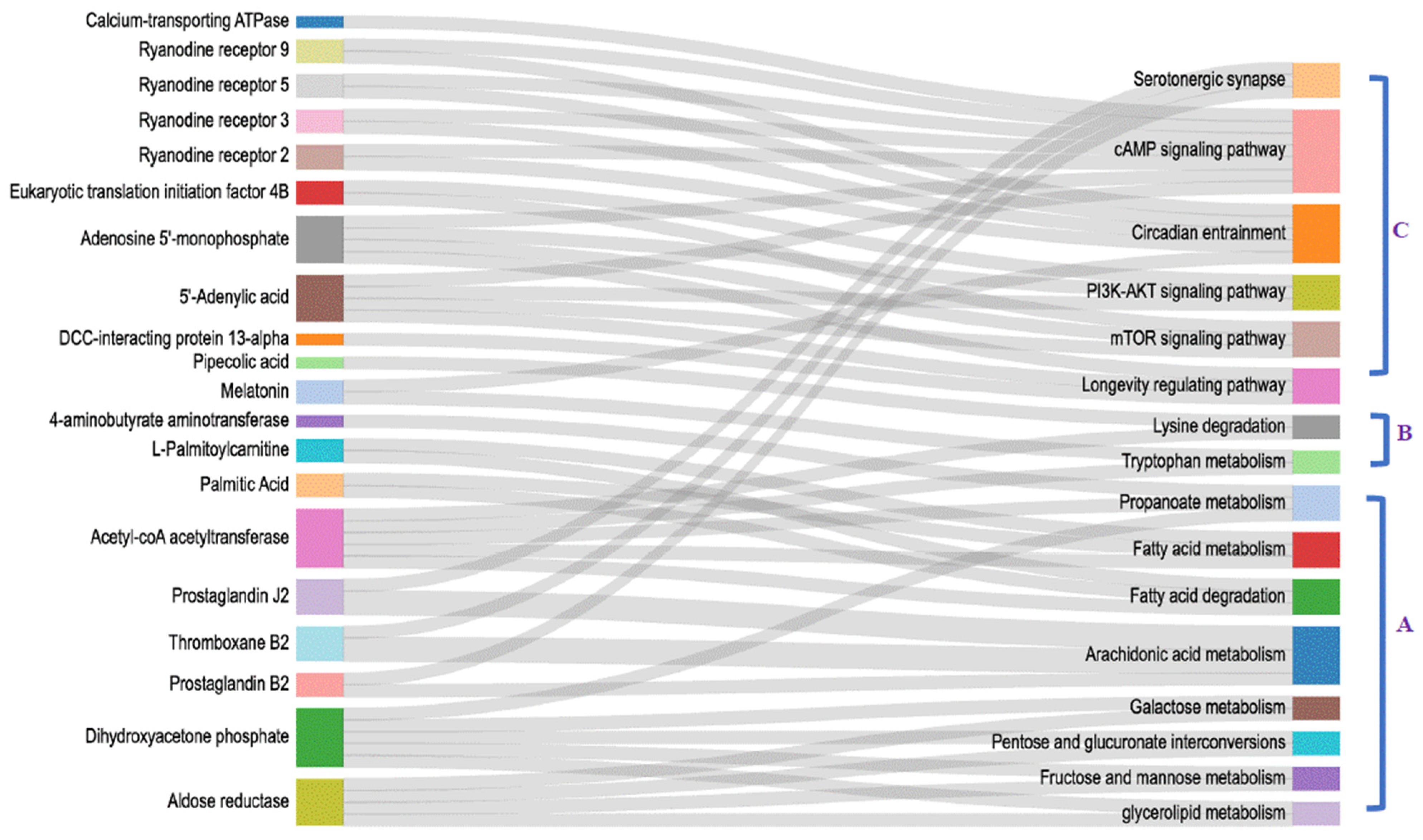
2.5. Combined Analysis on Cerebral Ganglion Proteomic and Heart Metabolome
3. Results
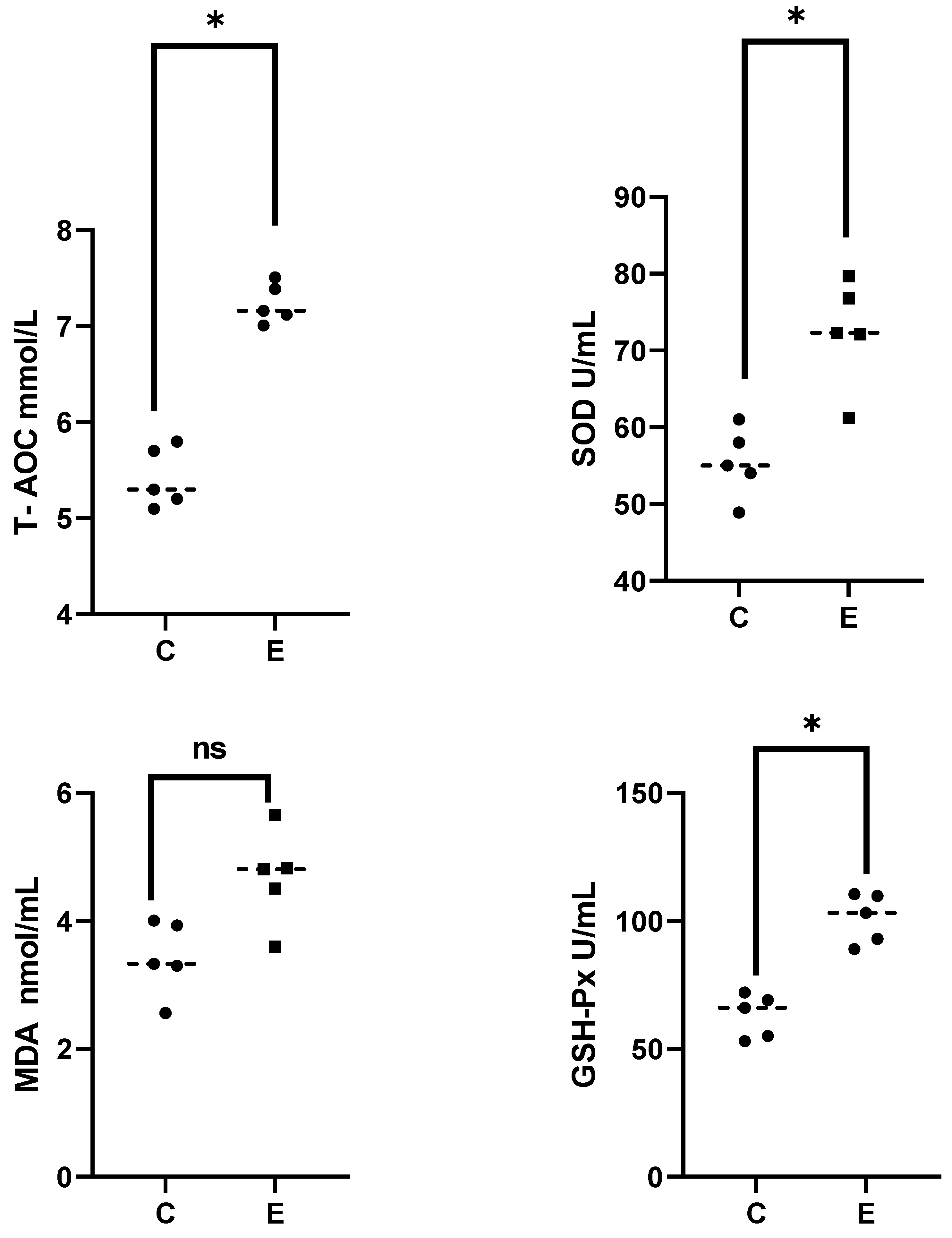
3.1. Analysis on Serum Biochemical Parameters of E. sinensis under Alkalinity Stress
3.2. Proteomic Analysis on Cerebral Ganglion under Alkalinity Stress
3.3. Heart Metabolomics Analysis under Alkalinity Stress
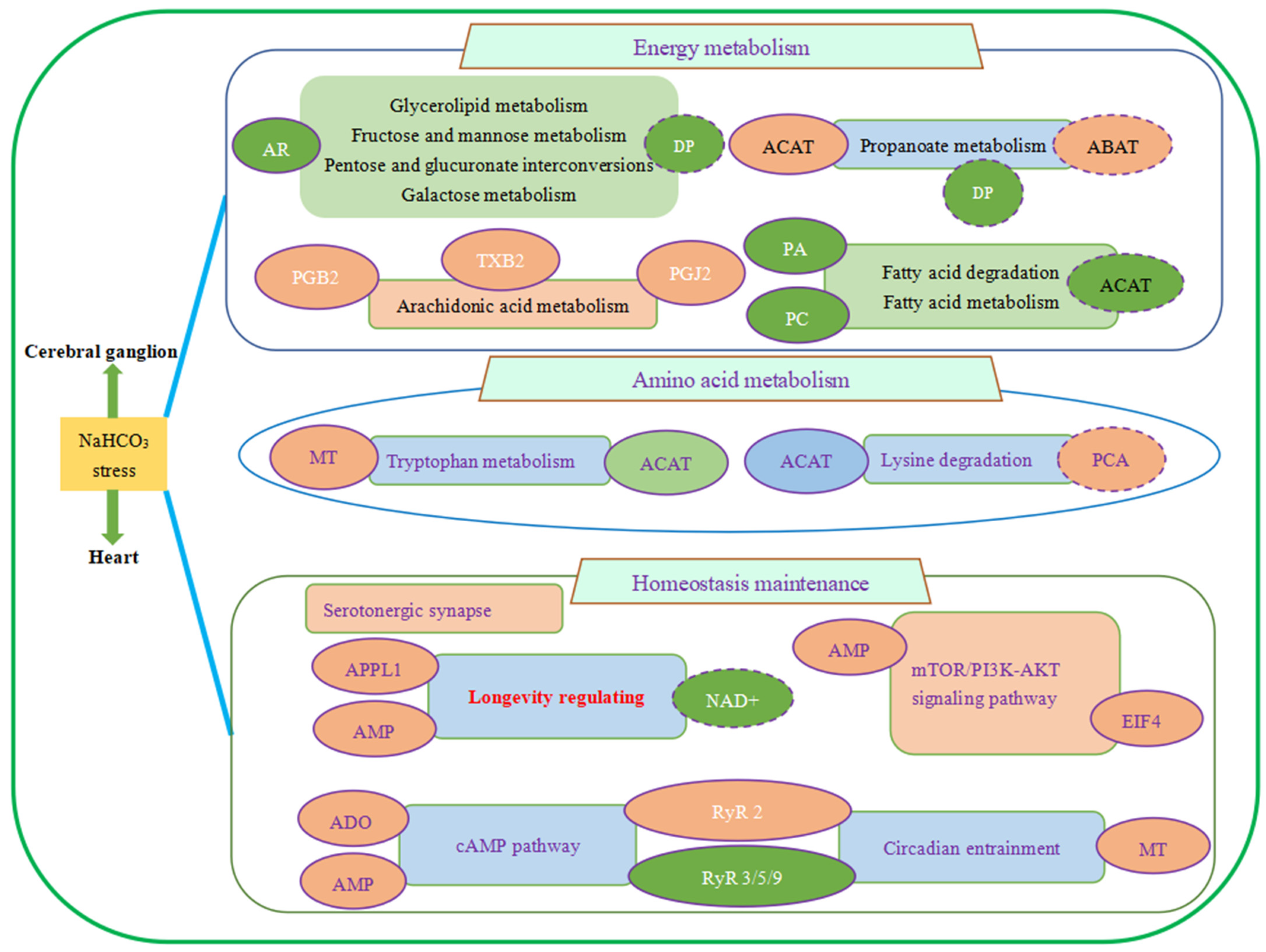
3.4. Integrated Analysis on Cerebral Ganglion Proteomics and Heart Metabolomics under Alkalinity Stress
4. Discussion
4.1. Regulation on Energy Metabolism and Signal Transduction in Cerebral Ganglion and Heart under Acute Alkalinity Stress
4.2. The Co-Regulation of Cerebral Ganglion and Heart on Signal Transduction under Alkalinity Stress
4.3. Synergistic Regulation on Longevity and Antioxidant Homeostasis Maintenance under Alkalinity Stress
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Boyd, C.E.; Tucker, C.S.; Somridhivej, B. Alkalinity and hardness: Critical but elusive concepts in aquaculture. J. World Aquacult. Soc. 2016, 47, 6–41. [Google Scholar]
- Yu, F.; Huang, C.; Wang, X.; Ge, J.; Li, Z. Effects of prior acclimation on the survival, feed intake and salinity preference of a freshwater-adapted euryhaline crab. Crustaceana 2023, 96, 1–17. [Google Scholar] [CrossRef]
- An, H.E.; Choi, T.J.; Kim, C.B. Comparative Transcriptome Analysis of Eriocheir sinensis from wild habitats in Han River, Korea. Life 2022, 12, 2027. [Google Scholar] [CrossRef] [PubMed]
- Song, Q.H.; Zhao, Y.F. Analysis on status and standard for Eriocheir sinensis breeding industry in 2018. Sci. Fish Farming. 2018, 10, 13–16. [Google Scholar]
- Li, C. Preference of Chinese Mitten Crab (Eriocheir sinensis) Based on Some Habitat Factors. Master’s Thesis, Shanghai Ocean University, Shanghai, China, 2022. [Google Scholar]
- Wang, S.; Guo, K.; Luo, L.; Zhang, R.; Xu, W.; Song, Y.; Zhao, Z. Fattening in saline and alkaline water improves the color, nutritional and taste quality of adult Chinese mitten crab Eriocheir sinensis. Foods 2022, 11, 2573. [Google Scholar] [CrossRef] [PubMed]
- Ge, Q.; Wang, J.; Li, J.; Li, J. Effect of high alkalinity on shrimp gills: Histopathological alternations and cell specific responses. Ecotoxicol. Environ. Saf. 2023, 256, 114902. [Google Scholar] [CrossRef] [PubMed]
- Qin, Z.; Ge, Q.; Wang, J.; Li, M.; Liu, P.; Li, J.; Li, J. Comparative Transcriptomic and proteomic analysis of Exopalaemon carinicauda in response to alkalinity stress. Front. Mar. Sci. 2021, 8, 759923. [Google Scholar] [CrossRef]
- Zhang, R.; Shi, X.; Liu, Z.; Sun, J.; Sun, T.; Lei, M. Histological, Physiological and Transcriptomic Analysis Reveal the Acute Alkalinity Stress of the Gill and Hepatopancreas of Litopenaeus vannamei. Mar. Biotechnol. 2023, 25, 588–602. [Google Scholar] [CrossRef] [PubMed]
- 8Middlemiss, K.L.; Urbina, M.A.; Wilson, R.W. Effects of seawater alkalinity on calcium and acid-base regulation in juvenile European lobster (Homarus gammarus) during a moult cycle. Comp. Biochem. Phys. A 2016, 193, 22–28. [Google Scholar] [CrossRef]
- Ge, Q.L.; Li, J.L.; Wang, J.L.; Li, Z.L.; Li, J. Characterization, functional analysis, and expression levels of three carbonic anhydrases in response to pH and saline-alkaline stresses in the ridgetail white prawn Exopalaemon carinicauda. Cell Stress Chaperones 2019, 24, 503–515. [Google Scholar] [CrossRef]
- Chen, T.; Lin, T.; Li, H.; Lu, T.; Li, J.; Huang, W.; Sun, H.; Jiang, X.; Zhang, J.; Yan, A.; et al. Heat Shock Protein 40 (HSP40) in Pacific White Shrimp (Litopenaeus vannamei): Molecular Cloning, tissue distribution and ontogeny, response to temperature, acidity/alkalinity and salinity stresses, and potential role in ovarian development. Front. Physiol. 2018, 9, 1784. [Google Scholar] [CrossRef]
- Zhang, R.; Zhao, Z.; Li, M.; Luo, L.; Wang, S.; Guo, K.; Xu, W. Metabolomics analysis reveals the response mechanism to carbonate alkalinity toxicity in the gills of Eriocheir sinensis. Comp. Biochem. Phys. C 2023, 263, 109487. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.B.; Pellicer, A.M.; Brüggmann, A.; Kiggen, M.; Behrens, S.; Good, C.; Straus, D.L.; Meinelt, T. Effect of water hardness/alkalinity and humic substances on the toxicity of peracetic acid to zebrafish embryos and pathogenic isolates. Aquacult. Rep. 2021, 21, 100900. [Google Scholar] [CrossRef]
- Liu, Y.; Li, E.; Xu, C.; Su, Y.; Qin, J.G.; Chen, L.; Wang, X. Brain Transcriptome Profiling Analysis of Nile Tilapia (Oreochromis niloticus) Under Long-Term Hypersaline Stress. Front. Physiol. 2018, 9, 219. [Google Scholar] [CrossRef]
- Li, M.; Niu, C.; Chen, Y. Diverse response pattern to anoxia in three freshwater turtle species. Biology 2023, 12, 50. [Google Scholar] [CrossRef] [PubMed]
- Ratko, J.; da Silva, N.G.; da Silva, D.O.; Corre, A.P.N.; Pereira, D.M.C.; Schleger, I.C.; Neundorf, A.K.A.; Herrerias, T.; Corso, C.R.; de Souza, M.R.D.P.; et al. Can high- and low-temperature thermal stress modulate the antioxidant defense response of Astyanax lacustris brain? Brain. Res. 2022, 1797, 148118. [Google Scholar] [CrossRef]
- Diwan, A.D.; Harke, S.N.; Panche, A.N. Application of proteomics in shrimp and shrimp aquaculture. Comp. Biochem. Phys. D 2022, 43, 101015. [Google Scholar] [CrossRef]
- Zhang, L.J.; Qian, L.; Ding, L.Y.; Wang, L.; Wong, M.H.; Tao, H.C. Ecological and toxicological assessments of anthropogenic contaminants based on environmental metabolomics. Environ. Sci. Technol. 2021, 5, 100081. [Google Scholar] [CrossRef]
- Conesa, A.; Gotz, S. Blast2GO: A comprehensive suite for functional analysis in plant genomics. Int. J. Plant Genomics. 2008, 2008, 619832. [Google Scholar] [CrossRef]
- Zhao, Z.; Wu, F.; Ding, S.; Sun, L.; Liu, Z.; Ding, K.; Lu, J. Label-free quantitative proteomic analysis reveals potential biomarkers and pathways in renal cell carcinoma. Tumor. Biol. 2015, 36, 939–951. [Google Scholar] [CrossRef]
- Want, E.J.; Masson, P.; Michopoulos, F.; Wilson, I.D.; Theodoridis, G.; Plumb, R.S.; Shockcor, J.; Loftus, N.; Holmes, E.; Nicholson, J.K. Global metabolic profiling of animal and human tissues via UPLC-MS. Nat. Protoc. 2013, 8, 17–32. [Google Scholar] [CrossRef] [PubMed]
- Boulesteix, A.L.; Strimmer, K. Partial least squares: A versatile tool for the analysis of high-dimensional genomic data. Brief. Bioinform. 2007, 8, 32–44. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.B.; Pu, S.B.; Sun, Y.; Li, Z.F.; Niu, M.; Yan, X.Z.; Zhao, Y.L.; Wang, L.F.; Qin, X.M.; Ma, Z.J.; et al. Metabolomic profiling of autoimmune hepatitis: The diagnostic utility of nuclear magnetic resonance spectroscopy. J. Proteome. Res. 2014, 13, 3792–3801. [Google Scholar] [CrossRef] [PubMed]
- Nshinaka, T.; Shimizu, K.; Miura, T.; Yabe-Nishimura, C.; Terada, T. Cooperative regulation of mouse aldose reductase (AKR1B3) gene transcription by Nrf2, TonEBP, and c-jun. Chem-Biol. Interact. 2019, 302, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Wu, B.; Tu, K.; Zhou, L.; Yang, A.; Liu, Z. Transcriptome and metabolome analyses provide insights into the salinity adaptation of clam Ruditapes philippinarum. Aquac. Rep. 2022, 27, 101368. [Google Scholar] [CrossRef]
- Baratta, F.; Pastori, D.; Delben, M.; Carnevale, R.; Novo, M.; Labbadia, G.; Angelico, F.; Violi, F. Enhanced urinary excretion of thromboxane B2 in non-alcoholic fatty liver disease. implication for antiplatelet treatment. Atherosclerosis 2018, 275, E65–E66. [Google Scholar] [CrossRef]
- Lv, L.; Wang, X.; Wu, H. Assessment of palmitic acid toxicity to animal hearts and other major organs based on acute toxicity, network pharmacology, and molecular docking. Comput. Biol. Med. 2023, 158, 106899. [Google Scholar] [CrossRef] [PubMed]
- Berezhnov, A.V.; Fedotova, E.I.; Nenov, M.N.; Kasymov, V.A.; Pimenov, O.Y.; Dynnik, V.V. Dissecting cellular mechanisms of long-chain acylcarnitines-driven cardiotoxicity: Disturbance of calcium homeostasis, activation of Ca2+-dependent phospholipases, and mitochondrial energetics collapse. Int. J. Mol. Sci. 2020, 21, 7461. [Google Scholar] [CrossRef] [PubMed]
- Gao, K.; Mu, C.L.; Farzi, A.; Zhu, W.Y. Tryptophan Metabolism: A link between the gut microbiota and brain. Adv. Nutr. 2020, 11, 709–723. [Google Scholar] [CrossRef]
- Tobeiha, M.; Jafari, A.; Fadaei, S.; Mirazimi, S.M.A.; Dashti, F.; Amiri, A.; Khan, H.; Asemi, Z.; Reiter, R.J.; Hamblin, M.R.; et al. Evidence for the benefits of melatonin in cardiovascular disease. Front. Cardiovasc. Med. 2022, 9, 888319. [Google Scholar] [CrossRef]
- Moustafa-Farag, M.; Elkelish, A.; Dafea, M.; Khan, M.; Arnao, M.B.; Abdelhamid, M.T.; Abu El-Ezz, A.; Almoneafy, A.; Mahmoud, A.; Awad, M.; et al. Role of melatonin in plant tolerance to soil stressors: Salinity, pH and heavy metals. Molecules 2020, 25, 5359. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Wu, M.; Pang, Y.; Song, X.; Shi, A.; Shi, X.; Niu, C.; Cheng, Y.; Yang, X. Effects of melatonin feed on the changes of hemolymph immune parameters, antioxidant capacity, and mitochondrial functions in Chinese mitten crab (Eriocheir sinensis) caused by acute hypoxia. Aquaculture 2021, 535, 736374. [Google Scholar] [CrossRef]
- Dalazen, G.R.; Terra, M.; Diaz Jacques, C.E.; Coelho, J.G.; Freitas, R.; Mazzola, P.N.; Dutra-Filho, C.S. Pipecolic acid induces oxidative stress in vitro in cerebral cortex of young rats and the protective role of lipoic acid. Metab. Brain Dis. 2014, 29, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Deepa, S.S.; Dong, L.Q. APPL1: Role in adiponectin signaling and beyond. Am. J. Physiol.-Endoc. M. 2009, 296, E22–E36. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Anand, S.K.; Singh, N.; Dwivedi, U.N.; Kakkar, P. AMP-activated protein kinase: An energy sensor and survival mechanism in the reinstatement of metabolic homeostasis. Exp. Cell Res. 2023, 428, 113614. [Google Scholar] [CrossRef]
- Wiersema, J.M.; Kamphuis, A.E.P.; Rohling, J.H.T.; Kervezee, L.; Akintola, A.A.; Jansen, S.W.; Slagboom, P.E.; van Heemst, D.; van der Spoel, E. The association between continuous ambulatory heart rate, heart rate variability, and 24h rhythms of heart rate with familial longevity and aging. Aging-Us 2022, 14, 7223–7239. [Google Scholar] [CrossRef] [PubMed]
- Atli, G.; Canli, E.G.; Eroglu, A.; Canli, M. Characterization of antioxidant system parameters in four freshwater fish species. Ecoto. Environ. Safe. 2016, 126, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.D.; Si, M.R.; Jiang, S.G.; Yang, Q.B.; Jiang, S.; Yang, L.S.; Huang, J.H.; Chen, X.; Zhou, F.L.; Li, E. Transcriptome and molecular regulatory mechanisms analysis of gills in the black tiger shrimp Penaeus monodon under chronic low-salinity stress. Front. Physiol. 2023, 14, 1118341. [Google Scholar] [CrossRef] [PubMed]
- Mohindra, V.; Chowdhury, L.M.; Chauhan, N.; Paul, A.; Singh, R.K.; Kushwaha, B.; Maurya, R.K.; Lal, K.K.; Jena, J.K. Transcriptome analysis revealed osmoregulation related regulatory networks and hub genes in the gills of Hilsa shad, Tenualosa ilisha, during the migratory osmotic stress. Mar. Biotechnol. 2023, 25, 161–173. [Google Scholar] [CrossRef]
- Fu, W.; Wu, G. Targeting mTOR for anti-aging and anti-cancer therapy. Molecules 2023, 28, 3157. [Google Scholar] [CrossRef]
- Nicolini, C.; Ahn, Y.; Michalski, B.; Rho, J.M.; Fahnestock, M. Decreased mTOR signaling pathway in human idiopathic autism and in rats exposed to valproic acid. Acta Neuropathol. Commun. 2015, 3, 3. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Fan, X.; Zhao, X.; Shen, Y.; Xu, X.; Wei, L.; Wang, W.; Wei, D. cAMP activates calcium signalling via phospholipase C to regulate cellulase production in the filamentous fungus Trichoderma reesei. Biotechnol. Biofuels. 2021, 14, 62. [Google Scholar] [CrossRef] [PubMed]
- Willis, J.R.; Jeffs, A.; Hickey, A.J.R. Adenosine modulates heart rate and tissue glycogen of the giant freshwater prawn Macrobrachium rosenbergii (De Man, 1879). Aquac. Rep. 2022, 27, 101359. [Google Scholar] [CrossRef]
- Liu, J.; Zhao, Y.; Zhu, Y.; Wang, Y.; Liu, X.; Nie, X.; Zhao, J.; Wang, W.; Cheng, J. Rhynchophylline regulates calcium homeostasis by antagonizing ryanodine receptor 2 phosphorylation to improve diabetic cardiomyopathy. Front. Pharmacol. 2022, 13, 882198. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Li, Q.; Xu, Z.; Chen, Z.; Tao, Y.; Tong, Y.; Wang, T.; Chen, S.; Wang, P. Detection of the role of intestinal flora and tryptophan metabolism involved in antidepressant-like actions of crocetin based on a multi-omics approach. Psychopharmacology 2022, 239, 3657–3677. [Google Scholar] [CrossRef] [PubMed]
- Barbieri de Oliveira, I.G.; Ferreira Junior, M.D.; Lopes, P.R.; Taveira Campos, D.B.; Ferreira-Neto, M.L.; Rosa Santos, E.H.; de Freitas Mathias, P.C.; Francisco, F.A.; Koike, B.D.V.; de Castro, C.H.; et al. Forced internal desynchrony induces cardiometabolic alterations in adult rats. J. Endocrinol. 2019, 242, 25–36. [Google Scholar] [CrossRef]
- Javier Sanchez-Vazquez, F.; Fernando Lopez-Olmeda, J.; Vera, L.M.; Migaud, H.; Antonio Lopez-Patino, M.; Miguez, J.M. Environmental cycles, melatonin, and circadian control of stress response in fish. Front. Endocrinol. 2019, 10, 279. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, M.; Zhou, J.; Xu, G.; Tang, Y. Exploration of the Synergistic Regulation Mechanism in Cerebral Ganglion and Heart of Eriocheir sinensis on Energy Metabolism and Antioxidant Homeostasis Maintenance under Alkalinity Stress. Antioxidants 2024, 13, 986. https://doi.org/10.3390/antiox13080986
Wang M, Zhou J, Xu G, Tang Y. Exploration of the Synergistic Regulation Mechanism in Cerebral Ganglion and Heart of Eriocheir sinensis on Energy Metabolism and Antioxidant Homeostasis Maintenance under Alkalinity Stress. Antioxidants. 2024; 13(8):986. https://doi.org/10.3390/antiox13080986
Chicago/Turabian StyleWang, Meiyao, Jun Zhou, Gangchun Xu, and Yongkai Tang. 2024. "Exploration of the Synergistic Regulation Mechanism in Cerebral Ganglion and Heart of Eriocheir sinensis on Energy Metabolism and Antioxidant Homeostasis Maintenance under Alkalinity Stress" Antioxidants 13, no. 8: 986. https://doi.org/10.3390/antiox13080986
APA StyleWang, M., Zhou, J., Xu, G., & Tang, Y. (2024). Exploration of the Synergistic Regulation Mechanism in Cerebral Ganglion and Heart of Eriocheir sinensis on Energy Metabolism and Antioxidant Homeostasis Maintenance under Alkalinity Stress. Antioxidants, 13(8), 986. https://doi.org/10.3390/antiox13080986





