The SOD1 Inhibitor, LCS-1, Oxidizes H2S to Reactive Sulfur Species, Directly and Indirectly, Through Conversion of SOD1 to an Oxidase
Abstract
:1. Introduction
2. Methods
2.1. H2S and Polysulfide Measurements
2.2. Liquid Chromatography Mass Spectrometry (LC-MS/MS)
2.3. Colloidal Sulfur (S8)
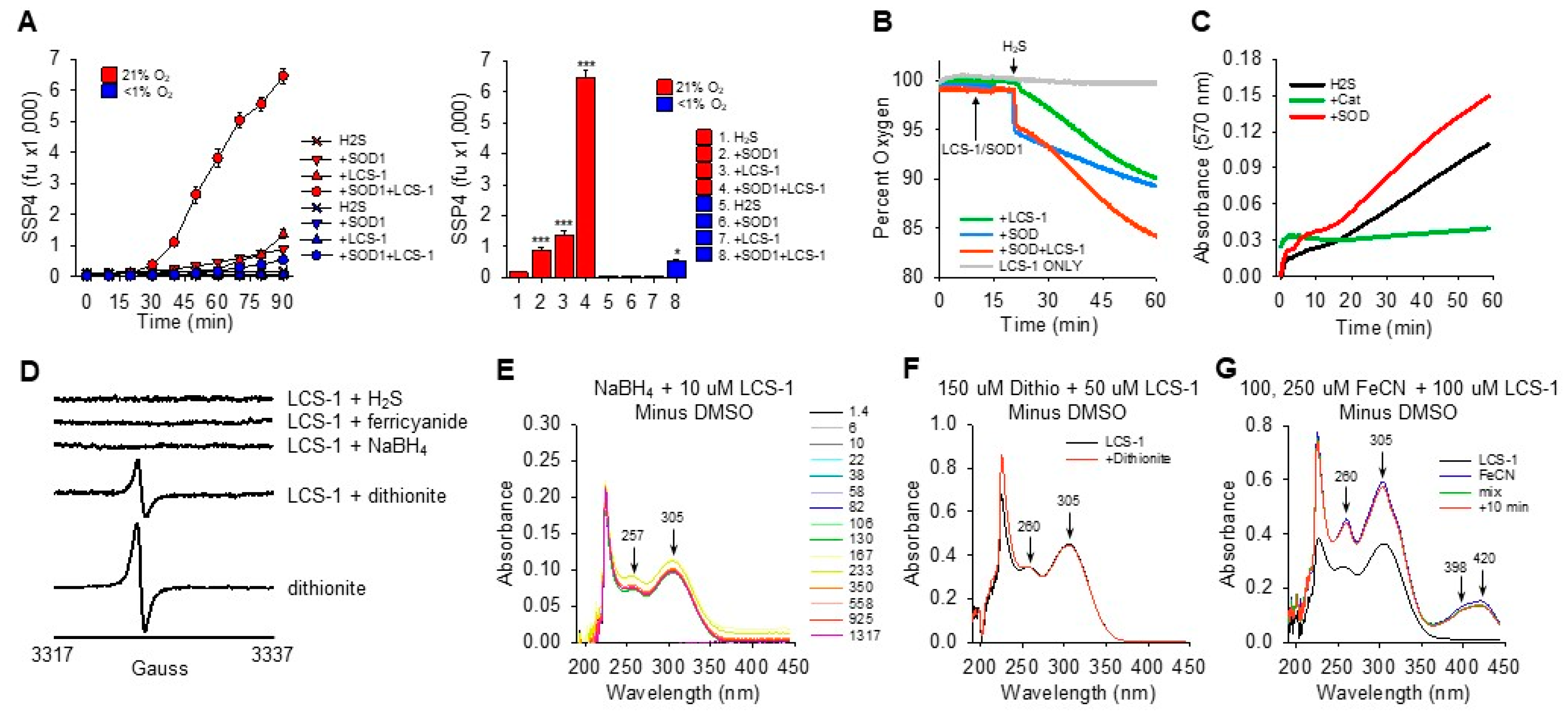
2.4. Oxygen Consumption
2.5. H2O2 Detection with Amplex Red
2.6. SOD1 Assay
2.7. Electron Paramagnetic Resonance (EPR) Spectrometry
2.8. Absorbance Spectra
2.9. Preparation of Thiol Adducts
2.10. Speciation of Inorganic RSS
2.11. Chemicals
2.12. Statistical Analysis
3. Results
3.1. LCS-1 Synergizes with SOD1 but Not SOD2 to Oxidize H2S to Polysulfides
3.2. Oxidation of H2S by LCS-1 Does Not Produce Colloidal Sulfur (S8)
3.3. Effects of SOD1 and LCS-1 Concentrations on Synergistic Oxidation of H2S
3.4. H2S Oxidation by SOD1 and LCS-1 Is Oxygen-Dependent and Produces Hydrogen Peroxide
3.5. SOD1-LCS-1 Oxidation of H2S Does Not Appear to Involve Redox Cycling of LCS-1
3.6. Catalase Only Slightly Inhibits LCS-1-SOD1 Oxidation of H2S
3.7. LCS-1 Forms Monothiol Adducts with SOD1 That Affect H2S Oxidation
3.8. SOD Inhibition by LCS-1 and ATN-244
3.9. Effects of LCS-1 on RSS in HEK293T Cells
4. Discussion
4.1. H2S Oxidation by LCS-1
4.2. Proposed Mechanism of LCS-1/SOD1 Synergism
4.3. Chemical Reactivity of Products from H2S Oxidation
4.4. Biological Significance of H2S Oxidation by LCS-1
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Somwar, R.; Shum, D.; Djaballah, H.; Varmus, H. Identification and preliminary characterization of novel small molecules that inhibit growth of human lung adenocarcinoma cells. J. Biomol. Screen. 2009, 14, 1176–1184. [Google Scholar] [CrossRef] [PubMed]
- Somwar, R.; Erdjument-Bromage, H.; Larsson, E.; Shum, D.; Lockwood, W.W.; Yang, G.; Sander, C.; Ouerfelli, O.; Tempst, P.J.; Djaballah, H.; et al. Superoxide dismutase 1 (SOD1) is a target for a small molecule identified in a screen for inhibitors of the growth of lung adenocarcinoma cell lines. Proc. Natl. Acad. Sci. USA 2011, 108, 16375–16380. [Google Scholar] [CrossRef] [PubMed]
- Du, T.; Song, Y.; Ray, A.; Chauhan, D.; Anderson, K.C. Proteomic analysis identifies mechanism(s) of overcoming bortezomib resistance via targeting ubiquitin receptor Rpn13. Leukemia 2021, 35, 550–561. [Google Scholar] [CrossRef] [PubMed]
- Ling, M.; Liu, Q.; Wang, Y.; Liu, X.; Jiang, M.; Hu, J. LCS-1 inhibition of superoxide dismutase 1 induces ROS-dependent death of glioma cells and degradates PARP and BRCA1. Front. Oncol. 2022, 12, 937444. [Google Scholar] [CrossRef]
- McAndrew, E.N.; Lepage, C.C.; McManus, K.J. The synthetic lethal killing of RAD54B-deficient colorectal cancer cells by PARP1 inhibition is enhanced with SOD1 inhibition. Oncotarget 2016, 7, 87417–87430. [Google Scholar] [CrossRef]
- Sajesh, B.V.; McManus, K.J. Targeting SOD1 induces synthetic lethal killing in BLM- and CHEK2-deficient colorectal cancer cells. Oncotarget 2015, 6, 27907–27922. [Google Scholar] [CrossRef]
- Papa, L.; Hahn, M.; Marsh, E.L.; Evans, B.S.; Germain, D. SOD2 to SOD1 switch in breast cancer. J. Biol. Chem. 2014, 289, 5412–5416. [Google Scholar] [CrossRef]
- Guo, T.; Wang, X.; Zhang, G.; Xia, T.; Zhu, R.; Tou, J. Dihydromyricetin functions as a tumor suppressor in hepatoblastoma by regulating SOD1/ROS pathway. Front. Oncol. 2023, 13, 1160548. [Google Scholar] [CrossRef]
- Steverding, D.; Barcelos, Y. Cytotoxic Activity of LCS-1 is not Only due to Inhibition of SOD1. Drug Res. 2020, 70, 57–60. [Google Scholar] [CrossRef]
- Switzer, C.H.; Kasamatsu, S.; Ihara, H.; Eaton, P. SOD1 is an essential H2S detoxifying enzyme. Proc. Natl. Acad. Sci. USA 2023, 120, e2205044120. [Google Scholar] [CrossRef]
- DeLeon, E.R.; Gao, Y.; Huang, E.; Arif, M.; Arora, N.; Divietro, A.; Patel, S.; Olson, K.R. A case of mistaken identity: Are reactive oxygen species actually reactive sulfide species? Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2016, 310, R549–R560. [Google Scholar] [CrossRef] [PubMed]
- Greiner, R.; Palinkas, Z.; Basell, K.; Becher, D.; Antelmann, H.; Nagy, P.; Dick, T.P. Polysulfides link H2S to protein thiol oxidation. Antioxid. Redox Signal 2013, 19, 1749–1765. [Google Scholar] [CrossRef]
- Olson, K.R. Are Reactive Sulfur Species the New Reactive Oxygen Species? Antioxid. Redox Signal. 2020, 33, 1125–1142. [Google Scholar] [CrossRef] [PubMed]
- Olson, K.R.; Clear, K.J.; Gao, Y.; Ma, Z.; Cieplik, N.M.; Fiume, A.R.; Gaziano, D.J.; Kasko, S.M.; Luu, J.; Pfaff, E.; et al. Redox and Nucleophilic Reactions of Naphthoquinones with Small Thiols and Their Effects on Oxidization of H2S to Inorganic and Organic Hydropolysulfides and Thiosulfate. Int. J. Mol. Sci. 2023, 24, 7516. [Google Scholar] [CrossRef] [PubMed]
- Olson, K.R.; Clear, K.J.; Takata, T.; Gao, Y.; Ma, Z.; Pfaff, E.; Travlos, A.; Luu, J.; Wilson, K.; Joseph, Z.; et al. Reaction Mechanisms of H2S Oxidation by Naphthoquinones. Antioxidants 2024, 13, 619. [Google Scholar] [CrossRef] [PubMed]
- Bibli, S.I.; Luck, B.; Zukunft, S.; Wittig, J.; Chen, W.; Xian, M.; Papapetropoulos, A.; Hu, J.; Fleming, I. A selective and sensitive method for quantification of endogenous polysulfide production in biological samples. Redox Biol. 2018, 18, 295–304. [Google Scholar] [CrossRef]
- Olson, K.R.; Gao, Y. Effects of inhibiting antioxidant pathways on cellular hydrogen sulfide and polysulfide metabolism. Free Radic. Biol. Med. 2019, 135, 1–14. [Google Scholar] [CrossRef]
- Olson, K.R.; Gao, Y.; Arif, F.; Patel, S.; Yuan, X.; Mannam, V.; Howard, S.; Batinic-Haberle, I.; Fukuto, J.; Minnion, M.; et al. Manganese Porphyrin-Based SOD Mimetics Produce Polysulfides from Hydrogen Sulfide. Antioxidants 2019, 8, 639. [Google Scholar] [CrossRef]
- Kasamatsu, S.; Ida, T.; Koga, T.; Asada, K.; Motohashi, H.; Ihara, H.; Akaike, T. High-Precision Sulfur Metabolomics Innovated by a New Specific Probe for Trapping Reactive Sulfur Species. Antioxid. Redox Signal. 2021, 34, 1407–1419. [Google Scholar] [CrossRef]
- Ewing, J.F.; Janero, D.R. Microplate superoxide dismutase assay employing a nonenzymatic superoxide generator. Anal. Biochem. 1995, 232, 243–248. [Google Scholar] [CrossRef]
- Olson, K.R.; Gao, Y.; Arif, F.; Arora, K.; Patel, S.; DeLeon, E.R.; Sutton, T.R.; Feelisch, M.; Cortese-Krott, M.M.; Straub, K.D. Metabolism of hydrogen sulfide (H2S) and Production of Reactive Sulfur Species (RSS) by superoxide dismutase. Redox Biol. 2017, 15, 74–85. [Google Scholar] [CrossRef]
- Olson, K.R.; Clear, K.J.; Derry, P.J.; Gao, Y.; Ma, Z.; Wu, G.; Kent, T.A.; Straub, K.D. Coenzyme Q10 and related quinones oxidize H2S to polysulfides and thiosulfate. Free Radic. Biol. Med. 2022, 182, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Olson, K.R.; Clear, K.J.; Derry, P.J.; Gao, Y.; Ma, Z.; Cieplik, N.M.; Fiume, A.; Gaziano, D.J.; Kasko, S.M.; Narloch, K.; et al. Naphthoquinones Oxidize H2S to Polysulfides and Thiosulfate, Implications for Therapeutic Applications. Int. J. Mol. Sci. 2022, 23, 13293. [Google Scholar] [CrossRef]
- Lyga, J.W. The reaction of 2-substituted-4,5-dichloro-3(2H)-pyridazinones with alkoxides and alkylthiolates. J. Heterocycl. Chem. 1988, 25, 1757–1760. [Google Scholar] [CrossRef]
- Song, Y.; Buettner, G.R. Thermodynamic and kinetic considerations for the reaction of semiquinone radicals to form superoxide and hydrogen peroxide. Free Radic. Biol. Med. 2010, 49, 919–962. [Google Scholar] [CrossRef]
- Zimpl, M.; Kotoucek, M.; Lemr, K.; Vesela, J.; Skopalova, J. Electrochemical reduction of chloridazon at mercury electrodes, and its analytical application. Fresenius J. Anal. Chem. 2001, 371, 975–982. [Google Scholar] [PubMed]
- Winterbourn, C.C.; Peskin, A.V.; Parsons-Mair, H.N. Thiol oxidase activity of copper, zinc superoxide dismutase. J. Biol. Chem. 2002, 277, 1906–1911. [Google Scholar] [CrossRef]
- Yamazaki, K.; Tahara, S.; Ohyama, T.; Kuroi, K.; Nakabayashi, T. SOD1 gains pro-oxidant activity upon aberrant oligomerization: Change in enzymatic activity by intramolecular disulfide bond cleavage. Sci. Rep. 2022, 12, 11750. [Google Scholar] [CrossRef] [PubMed]
- Bakavayev, S.; Chetrit, N.; Zvagelsky, T.; Mansour, R.; Vyazmensky, M.; Barak, Z.; Israelson, A.; Engel, S. Cu/Zn-superoxide dismutase and wild-type like fALS SOD1 mutants produce cytotoxic quantities of H2O2 via cysteine-dependent redox short-circuit. Sci. Rep. 2019, 9, 10826. [Google Scholar] [CrossRef]
- Nordlund, A.; Leinartaite, L.; Saraboji, K.; Aisenbrey, C.; Grobner, G.; Zetterstrom, P.; Danielsson, J.; Logan, D.T.; Oliveberg, M. Functional features cause misfolding of the ALS-provoking enzyme SOD1. Proc. Natl. Acad. Sci. USA 2009, 106, 9667–9672. [Google Scholar] [CrossRef]
- Akaike, T.; Morita, M.; Ogata, S.; Yoshitake, J.; Jung, M.; Sekine, H.; Motohashi, H.; Barayeu, U.; Matsunaga, T. New aspects of redox signaling mediated by supersulfides in health and disease. Free Radic. Biol. Med. 2024, 222, 539–551. [Google Scholar] [CrossRef] [PubMed]
- Fukuto, J.M. The chemistry of hydropersulfides (RSSH) as related to possible physiological functions. Arch. Biochem. Biophys. 2023, 743, 109659. [Google Scholar] [CrossRef]
- Switzer, C.H. How super is supersulfide?: Reconsidering persulfide reactivity in cellular biology. Redox Biol. 2023, 67, 102899. [Google Scholar] [CrossRef] [PubMed]
- Kamyshny, A., Jr.; Goifman, A.; Rizkov, D.; Lev, O. Formation of carbonyl sulfide by the reaction of carbon monoxide and inorganic polysulfides. Environ. Sci. Technol. 2003, 37, 1865–1872. [Google Scholar] [CrossRef]
- Benchoam, D.; Semelak, J.A.; Cuevasanta, E.; Mastrogiovanni, M.; Grassano, J.S.; Ferrer-Sueta, G.; Zeida, A.; Trujillo, M.; Moller, M.N.; Estrin, D.A.; et al. Acidity and nucleophilic reactivity of glutathione persulfide. J. Biol. Chem. 2020, 295, 15466–15481. [Google Scholar] [CrossRef]
- Benchoam, D.; Cuevasanta, E.; Roman, J.V.; Banerjee, R.; Alvarez, B. Acidity of persulfides and its modulation by the protein environments in sulfide quinone oxidoreductase and thiosulfate sulfurtransferase. J. Biol. Chem. 2024, 300, 107149. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olson, K.R.; Takata, T.; Clear, K.J.; Gao, Y.; Ma, Z.; Pfaff, E.; Mouli, K.; Kent, T.A.; Jones, P., Jr.; Fukuto, J.; et al. The SOD1 Inhibitor, LCS-1, Oxidizes H2S to Reactive Sulfur Species, Directly and Indirectly, Through Conversion of SOD1 to an Oxidase. Antioxidants 2024, 13, 991. https://doi.org/10.3390/antiox13080991
Olson KR, Takata T, Clear KJ, Gao Y, Ma Z, Pfaff E, Mouli K, Kent TA, Jones P Jr., Fukuto J, et al. The SOD1 Inhibitor, LCS-1, Oxidizes H2S to Reactive Sulfur Species, Directly and Indirectly, Through Conversion of SOD1 to an Oxidase. Antioxidants. 2024; 13(8):991. https://doi.org/10.3390/antiox13080991
Chicago/Turabian StyleOlson, Kenneth R., Tsuyoshi Takata, Kasey J. Clear, Yan Gao, Zhilin Ma, Ella Pfaff, Karthik Mouli, Thomas A. Kent, Prentiss Jones, Jr., Jon Fukuto, and et al. 2024. "The SOD1 Inhibitor, LCS-1, Oxidizes H2S to Reactive Sulfur Species, Directly and Indirectly, Through Conversion of SOD1 to an Oxidase" Antioxidants 13, no. 8: 991. https://doi.org/10.3390/antiox13080991





