Oxidative Stress in Transthyretin-Mediated Amyloidosis: An Exploratory Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients’ Recruitment
2.2. FORD (Free Oxygen Radicals Defense) and FORT (Free Oxygen Radicals Test) Analyses
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Buraczynska, M.; Mears, A.J.; Zareparsi, S.; Farjo, R.; Filippova, E.; Yuan, Y.; MacNee, S.P.; Hughes, B.; Swaroop, A. Gene Expression Profile of Native Human Retinal Pigment Epithelium. Investig. Ophthalmol. Vis. Sci. 2002, 43, 603–607. [Google Scholar] [PubMed]
- Jaworowski, A.; Fang, Z.; Khong, T.F.; Augusteyn, R.C. Protein Synthesis and Secretion by Cultured Retinal Pigment Epithelia. Biochim. Biophys. Acta 1995, 1245, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Aimo, A.; Vergaro, G.; Castiglione, V.; Fabiani, I.; Barison, A.; Gentile, F.; Ferrari Chen, Y.F.; Giorgetti, A.; Genovesi, D.; Buda, G.; et al. Wild-Type Transthyretin Cardiac Amyloidosis Is Not Rare in Elderly Subjects: The CATCH Screening Study. Eur. J. Prev. Cardiol. 2024, zwae093. [Google Scholar] [CrossRef] [PubMed]
- Russo, M.; Obici, L.; Bartolomei, I.; Cappelli, F.; Luigetti, M.; Fenu, S.; Cavallaro, T.; Chiappini, M.G.; Gemelli, C.; Pradotto, L.G.; et al. ATTRv Amyloidosis Italian Registry: Clinical and Epidemiological Data. Amyloid 2020, 27, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Luigetti, M.; Guglielmino, V.; Antonini, G.; Casali, C.; Ceccanti, M.; Chiappini, M.G.; De Giglio, L.; Di Lazzaro, V.; Di Muzio, A.; Goglia, M.; et al. ATTRv in Lazio-Italy: A High-Prevalence Region in a Non-Endemic Country. Genes 2021, 12, 829. [Google Scholar] [CrossRef] [PubMed]
- Adams, D.; Koike, H.; Slama, M.; Coelho, T. Hereditary Transthyretin Amyloidosis: A Model of Medical Progress for a Fatal Disease. Nat. Rev. Neurol. 2019, 15, 387–404. [Google Scholar] [CrossRef] [PubMed]
- Ruberg, F.L.; Grogan, M.; Hanna, M.; Kelly, J.W.; Maurer, M.S. Transthyretin Amyloid Cardiomyopathy: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2019, 73, 2872–2891. [Google Scholar] [CrossRef] [PubMed]
- Campbell, C.M.; LoRusso, S.; Dispenzieri, A.; Kristen, A.V.; Maurer, M.S.; Rapezzi, C.; Lairez, O.; Drachman, B.; Garcia-Pavia, P.; Grogan, M.; et al. Sex Differences in Wild-Type Transthyretin Amyloidosis: An Analysis from the Transthyretin Amyloidosis Outcomes Survey (THAOS). Cardiol. Ther. 2022, 11, 393–405. [Google Scholar] [CrossRef]
- Rapezzi, C.; Riva, L.; Quarta, C.C.; Perugini, E.; Salvi, F.; Longhi, S.; Ciliberti, P.; Pastorelli, F.; Biagini, E.; Leone, O.; et al. Gender-Related Risk of Myocardial Involvement in Systemic Amyloidosis. Amyloid 2008, 15, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Maurer, M.S.; Soman, P.; Hernandez, A.; Garcia-Pavia, P.; Signorovitch, J.; Wei, L.J.; Hanna, M.; Ruberg, F.L.; Kittleson, M.; Kazi, D.; et al. Advancing Transthyretin Amyloidosis Drug Development in an Evolving Treatment Landscape: Amyloidosis Forum Meeting Proceedings. Adv. Ther. 2024, 14, 2723–2742. [Google Scholar] [CrossRef]
- Sekijima, Y. Hereditary Transthyretin Amyloidosis. In GeneReviews®; Adam, M.P., Mirzaa, G.M., Pagon, R.A., Wallace, S.E., Bean, L.J., Gripp, K.W., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 1993. [Google Scholar]
- Bulawa, C.E.; Connelly, S.; Devit, M.; Wang, L.; Weigel, C.; Fleming, J.A.; Packman, J.; Powers, E.T.; Wiseman, R.L.; Foss, T.R.; et al. Tafamidis, a Potent and Selective Transthyretin Kinetic Stabilizer That Inhibits the Amyloid Cascade. Proc. Natl. Acad. Sci. USA 2012, 109, 9629–9634. [Google Scholar] [CrossRef] [PubMed]
- Maurer, M.S.; Schwartz, J.H.; Gundapaneni, B.; Elliott, P.M.; Merlini, G.; Waddington-Cruz, M.; Kristen, A.V.; Grogan, M.; Witteles, R.; Damy, T.; et al. Tafamidis Treatment for Patients with Transthyretin Amyloid Cardiomyopathy. N. Engl. J. Med. 2018, 379, 1007–1016. [Google Scholar] [CrossRef] [PubMed]
- Maurer, M.S. Overview of Current and Emerging Therapies for Amyloid Transthyretin Cardiomyopathy. Am. J. Cardiol. 2022, 185 (Suppl. S1), S23–S34. [Google Scholar] [CrossRef] [PubMed]
- Adams, D.; Gonzalez-Duarte, A.; O’Riordan, W.D.; Yang, C.-C.; Ueda, M.; Kristen, A.V.; Tournev, I.; Schmidt, H.H.; Coelho, T.; Berk, J.L.; et al. Patisiran, an RNAi Therapeutic, for Hereditary Transthyretin Amyloidosis. N. Engl. J. Med. 2018, 379, 11–21. [Google Scholar] [CrossRef]
- Benson, M.D.; Waddington-Cruz, M.; Berk, J.L.; Polydefkis, M.; Dyck, P.J.; Wang, A.K.; Planté-Bordeneuve, V.; Barroso, F.A.; Merlini, G.; Obici, L.; et al. Inotersen Treatment for Patients with Hereditary Transthyretin Amyloidosis. N. Engl. J. Med. 2018, 379, 22–31. [Google Scholar] [CrossRef]
- Wu, D.; Chen, W. Molecular Mechanisms and Emerging Therapies in Wild-Type Transthyretin Amyloid Cardiomyopathy. Heart Fail. Rev. 2024, 29, 511. [Google Scholar] [CrossRef] [PubMed]
- Dittloff, K.T.; Iezzi, A.; Zhong, J.X.; Mohindra, P.; Desai, T.A.; Russell, B. Transthyretin Amyloid Fibrils Alter Primary Fibroblast Structure, Function, and Inflammatory Gene Expression. Am. J. Physiol. Heart Circ. Physiol. 2021, 321, H149–H160. [Google Scholar] [CrossRef]
- Sartiani, L.; Bucciantini, M.; Spinelli, V.; Leri, M.; Natalello, A.; Nosi, D.; Maria Doglia, S.; Relini, A.; Penco, A.; Giorgetti, S.; et al. Biochemical and Electrophysiological Modification of Amyloid Transthyretin on Cardiomyocytes. Biophys. J. 2016, 111, 2024–2038. [Google Scholar] [CrossRef]
- Lim, K.H.; Dasari, A.K.R.; Ma, R.; Hung, I.; Gan, Z.; Kelly, J.W.; Fitzgerald, M.C. Pathogenic Mutations Induce Partial Structural Changes in Native β-Sheet Structure of Transthyretin and Accelerate Aggregation. Biochemistry 2017, 56, 4808–4818. [Google Scholar] [CrossRef]
- Liu, J.P.; Wang, Q.Y.; Zheng, F.; Lu, J.H.; Ge, P.; Gu, Y.J.; Sun, X.G. Effect of MPO/H2O2/NO(-) System on Nitric Oxide-Mediated Modification of TTR Amyloid and Serum TTR in FAP ATTR Val30Met Patients. Genet. Mol. Res. 2014, 13, 2368–2376. [Google Scholar] [CrossRef]
- Fong, V.H.; Vieira, A. Pro-Oxidative Effects of Aggregated Transthyretin in Human Schwannoma Cells. Neurotoxicology 2013, 39, 109–113. [Google Scholar] [CrossRef] [PubMed]
- Leung, A.; Nah, S.K.; Reid, W.; Ebata, A.; Koch, C.M.; Monti, S.; Genereux, J.C.; Wiseman, R.L.; Wolozin, B.; Connors, L.H.; et al. Induced Pluripotent Stem Cell Modeling of Multisystemic, Hereditary Transthyretin Amyloidosis. Stem Cell Rep. 2013, 1, 451–463. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Buxbaum, J.N.; Reixach, N. Age-Related Oxidative Modifications of Transthyretin Modulate Its Amyloidogenicity. Biochemistry 2013, 52, 1913–1926. [Google Scholar] [CrossRef] [PubMed]
- Olsson, M.; Hellman, U.; Planté-Bordeneuve, V.; Jonasson, J.; Lång, K.; Suhr, O.B. Mitochondrial Haplogroup Is Associated with the Phenotype of Familial Amyloidosis with Polyneuropathy in Swedish and French Patients. Clin. Genet. 2009, 75, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, P.F.; Cerca, F.; Santos, S.D.; Saraiva, M.J. Endoplasmic Reticulum Stress Associated with Extracellular Aggregates. Evidence from Transthyretin Deposition in Familial Amyloid Polyneuropathy. J. Biol. Chem. 2006, 281, 21998–22003. [Google Scholar] [CrossRef] [PubMed]
- Gillmore, J.D.; Maurer, M.S.; Falk, R.H.; Merlini, G.; Damy, T.; Dispenzieri, A.; Wechalekar, A.D.; Berk, J.L.; Quarta, C.C.; Grogan, M.; et al. Nonbiopsy Diagnosis of Cardiac Transthyretin Amyloidosis. Circulation 2016, 133, 2404–2412. [Google Scholar] [CrossRef]
- Maurer, M.S.; Kale, P.; Fontana, M.; Berk, J.L.; Grogan, M.; Gustafsson, F.; Hung, R.R.; Gottlieb, R.L.; Damy, T.; González-Duarte, A.; et al. Patisiran Treatment in Patients with Transthyretin Cardiac Amyloidosis. N. Engl. J. Med. 2023, 389, 1553–1565. [Google Scholar] [CrossRef]
- Damy, T.; Garcia-Pavia, P.; Hanna, M.; Judge, D.P.; Merlini, G.; Gundapaneni, B.; Patterson, T.A.; Riley, S.; Schwartz, J.H.; Sultan, M.B.; et al. Efficacy and Safety of Tafamidis Doses in the Tafamidis in Transthyretin Cardiomyopathy Clinical Trial (ATTR-ACT) and Long-Term Extension Study. Eur. J. Heart Fail. 2021, 23, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Derme, M.; Piccioni, M.G.; Brunelli, R.; Crognale, A.; Denotti, M.; Ciolli, P.; Scomparin, D.; Tarani, L.; Paparella, R.; Terrin, G.; et al. Oxidative Stress in a Mother Consuming Alcohol during Pregnancy and in Her Newborn: A Case Report. Antioxidants 2023, 12, 1216. [Google Scholar] [CrossRef]
- Fiore, M.; Petrella, C.; Coriale, G.; Rosso, P.; Fico, E.; Ralli, M.; Greco, A.; De Vincentiis, M.; Minni, A.; Polimeni, A.; et al. Markers of Neuroinflammation in the Serum of Prepubertal Children with Fetal Alcohol Spectrum Disorders. CNS Neurol. Disord.-Drug Targets 2022, 21, 854–868. [Google Scholar] [CrossRef] [PubMed]
- Koike, H.; Misu, K.; Sugiura, M.; Iijima, M.; Mori, K.; Yamamoto, M.; Hattori, N.; Mukai, E.; Ando, Y.; Ikeda, S.; et al. Pathology of Early- vs Late-Onset TTR Met30 Familial Amyloid Polyneuropathy. Neurology 2004, 63, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Cambieri, C.; Libonati, L.; Moret, F.; Tartaglia, G.; Garibaldi, M.; Chimenti, C.; Inghilleri, M.; Ceccanti, M. The Silent Period for Small Fiber Sensory Neuropathy Assessment in a Mixed Cohort of Transthyretin-Mediated Amyloidosis. Biomedicines 2022, 10, 2073. [Google Scholar] [CrossRef] [PubMed]
- Gertz, M.A.; Dispenzieri, A. Systemic Amyloidosis Recognition, Prognosis, and Therapy: A Systematic Review. JAMA 2020, 324, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Cambieri, C.; Marenco, M.; Colasanti, T.; Mancone, C.; Corsi, A.; Riminucci, M.; Libonati, L.; Moret, F.; Chimenti, C.; Lambiase, A.; et al. Does Patisiran Reduce Ocular Transthyretin Synthesis? A Pilot Study of Two Cases. Curr. Neuropharmacol. 2023. [Google Scholar] [CrossRef] [PubMed]
- Ceccanti, M.; Inghilleri, M. RNA Interference and Neuromuscular Diseases: A Focus on Hereditary Transthyretin Amyloidosis. Curr. Gene Ther. 2024, 24, 6–7. [Google Scholar] [CrossRef] [PubMed]
- Benatar, M.; Wuu, J.; Andersen, P.M.; Lombardi, V.; Malaspina, A. Neurofilament Light: A Candidate Biomarker of Presymptomatic Amyotrophic Lateral Sclerosis and Phenoconversion. Ann. Neurol. 2018, 84, 130–139. [Google Scholar] [CrossRef]
- Berends, M.; Nienhuis, H.L.A.; Adams, D.; Karam, C.; Luigetti, M.; Polydefkis, M.; Reilly, M.M.; Sekijima, Y.; Hazenberg, B.P.C. Neurofilament Light Chains in Systemic Amyloidosis: A Systematic Review. Int. J. Mol. Sci. 2024, 25, 3770. [Google Scholar] [CrossRef]
- Romano, A.; Primiano, G.; Antonini, G.; Ceccanti, M.; Fenu, S.; Forcina, F.; Gentile, L.; Inghilleri, M.; Leonardi, L.; Manganelli, F.; et al. Serum Neurofilament Light Chain: A Promising Early Diagnostic Biomarker for Hereditary Transthyretin Amyloidosis? Eur. J. Neurol. 2024, 31, e16070. [Google Scholar] [CrossRef]
- Ando, Y.; Nyhlin, N.; Suhr, O.; Holmgren, G.; Uchida, K.; el Sahly, M.; Yamashita, T.; Terasaki, H.; Nakamura, M.; Uchino, M.; et al. Oxidative Stress Is Found in Amyloid Deposits in Systemic Amyloidosis. Biochem. Biophys. Res. Commun. 1997, 232, 497–502. [Google Scholar] [CrossRef]
- Sousa, M.M.; Cardoso, I.; Fernandes, R.; Guimarães, A.; Saraiva, M.J. Deposition of Transthyretin in Early Stages of Familial Amyloidotic Polyneuropathy: Evidence for Toxicity of Nonfibrillar Aggregates. Am. J. Pathol. 2001, 159, 1993–2000. [Google Scholar] [CrossRef] [PubMed]
- Aimo, A.; Panichella, G.; Garofalo, M.; Gasparini, S.; Arzilli, C.; Castiglione, V.; Vergaro, G.; Emdin, M.; Maffei, S. Sex Differences in Transthyretin Cardiac Amyloidosis. Heart Fail. Rev. 2024, 29, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Wixner, J.; Westermark, P.; Ihse, E.; Pilebro, B.; Lundgren, H.-E.; Anan, I. The Swedish Open-Label Diflunisal Trial (DFNS01) on Hereditary Transthyretin Amyloidosis and the Impact of Amyloid Fibril Composition. Amyloid 2019, 26, 39–40. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.; Saint Croix, G.R.; Lacy, S.; Fattouh, M.; Barillas-Lara, M.I.; Behrooz, L.; Mechanic, O. The Use of Diflunisal for Transthyretin Cardiac Amyloidosis: A Review. Heart Fail. Rev. 2022, 27, 517–524. [Google Scholar] [CrossRef] [PubMed]
- Sekijima, Y. Transthyretin (ATTR) Amyloidosis: Clinical Spectrum, Molecular Pathogenesis and Disease-Modifying Treatments. J. Neurol. Neurosurg. Psychiatry 2015, 86, 1036–1043. [Google Scholar] [CrossRef]
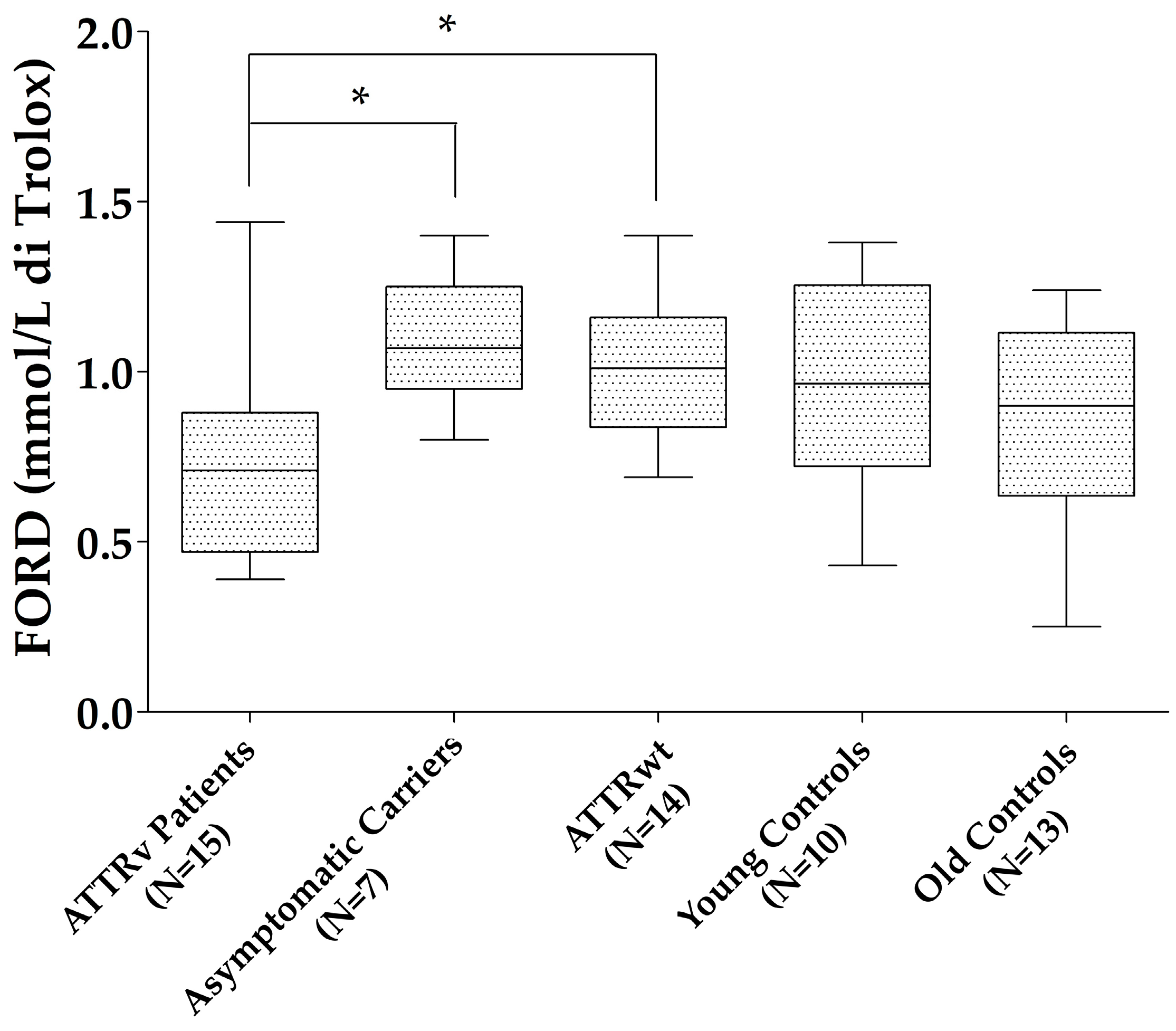
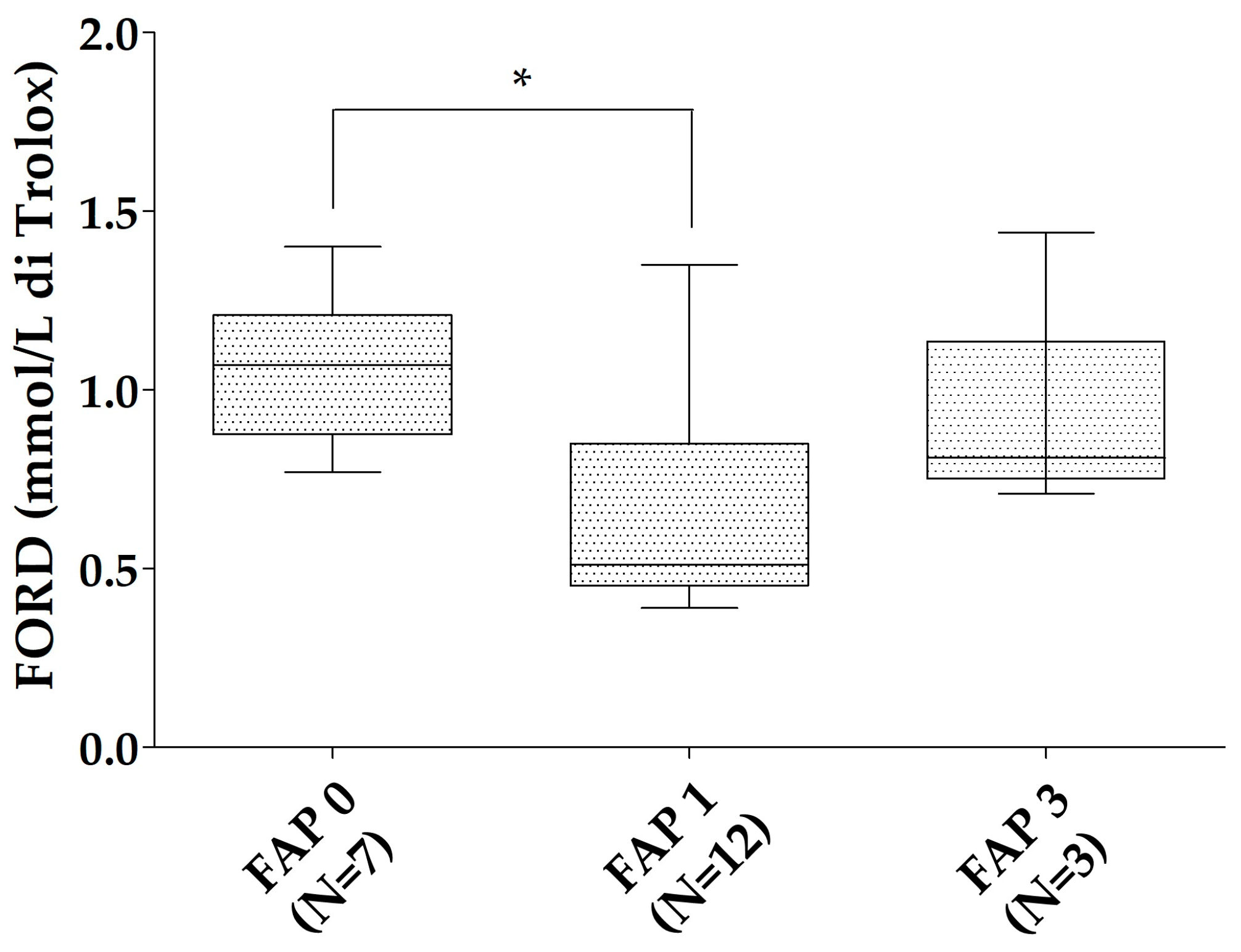
| Patients with ATTRv | ATTRv Asymptomatic Carriers | ATTRwt | Young Controls | Old Controls | |
|---|---|---|---|---|---|
| Age, m (Q1–Q3) | 69 (62−76) | 45 (38−59) | 84 (80.7−86) | 55.5 (41.5−59.7) | 75 (70−81.5) |
| NIS, m (Q1–Q3) | 26 (13.2−33.2) | ||||
| Norfolk QoL, m (Q1–Q3) | 48 (23.7−74.7) | ||||
| BMI, m (Q1–Q3) | 24.2 (22.2−24.6) | 25 (23.9−26.6) | 24.2 (23.2−25) | ||
| FORT Units, m (Q1–Q3) | 1.47 (1.2−2) | 1.9 (1.2−2.7) | 1.8 (1.2−2.3) | 1.7 (1.2−2.2) | 1.2 (1.2−2) |
| FORD (mmo/L), m (Q1–Q3) | 0.7 (0.47−0.88) | 1.07 (0.95−1.25) | 1.01 (0.84−1.16) | 0.96 (0.72−1.25) | 0.9 (0.63−1.11) |
| FORD/FORT, m (Q1–Q3) | 0.38 (0.29−0.66) | 0.56 (0.37−0.95) | 0.53 (0.39−0.8) | 0.52 (0.43−0.76) | 0.56 (0.37−0.68) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fiore, M.; Cambieri, C.; Libonati, L.; Moret, F.; D’Andrea, E.; Di Certo, M.G.; Passananti, C.; Gabanella, F.; Corbi, N.; Garibaldi, M.; et al. Oxidative Stress in Transthyretin-Mediated Amyloidosis: An Exploratory Study. Antioxidants 2024, 13, 998. https://doi.org/10.3390/antiox13080998
Fiore M, Cambieri C, Libonati L, Moret F, D’Andrea E, Di Certo MG, Passananti C, Gabanella F, Corbi N, Garibaldi M, et al. Oxidative Stress in Transthyretin-Mediated Amyloidosis: An Exploratory Study. Antioxidants. 2024; 13(8):998. https://doi.org/10.3390/antiox13080998
Chicago/Turabian StyleFiore, Marco, Chiara Cambieri, Laura Libonati, Federica Moret, Edoardo D’Andrea, Maria Grazia Di Certo, Claudio Passananti, Francesca Gabanella, Nicoletta Corbi, Matteo Garibaldi, and et al. 2024. "Oxidative Stress in Transthyretin-Mediated Amyloidosis: An Exploratory Study" Antioxidants 13, no. 8: 998. https://doi.org/10.3390/antiox13080998






