Abstract
Garambullo is a plant with little industrial application. However, garambullo contains betalains, photosensitive phytochemical compounds, which through microencapsulation can be used in chitosan–polyvinyl alcohol (PVOH) films for application in tomato coatings. These biopackages were characterized by physical tests, water vapor permeability, puncture tests, extension, color, differential scanning calorimetry (DCS), Fourier transform infrared (FTIR) spectroscopy, and antioxidant and antimicrobial activity analyses. The influence of the biopackages on the tomato coatings was measured using parameters such as minimum weight loss close to 2% at day 9, pH of 4.6, Brix of 5.5, titratable acidity of 1 g acid/100 mL sample, and shelf life of up to 18 days. The biopackages containing betalain microcapsules had a water vapor permeability of 2 × 10−14 g/h·m·Pa and an elongation of 5 ± 0.5%, indicating that the package did not stretch. The deformation at the breaking point for the package without and with microcapsules was 0.569 and 1.620, respectively. With respect to color, adding white microcapsules and betalains can cause the material to darken, resulting in a yellowish color. Furthermore, the phenolic content was greater for the biopackages with betalains, while there was no significant difference in the antioxidant activity since the active compounds were not released. According to the in vitro results, the inhibition of B. cinerea was achieved on the eighth day when the active compounds were released from the microcapsules. The tomato with betalains lost 2% of its weight, and B. cinerea was inhibited, extending its shelf life to 18 days. The proposed biopackages have good properties as biopolymers and inhibit the presence of B. cinerea.
1. Introduction
Garambullo (Myrtillocactus geometrizans) is a cactus distributed in central Mexico, which can be considered as functional food due to its content of bioactive compounds that are hypoglycemic and hypocholesterolemic [1,2]. Among these phytochemical compounds are betalains, which are used for pigmentation. The betalains are natural colorants derived from indoles [3] and composed of a central nitrogenous structure known as betalamic acid [4-(2-oxoethylidene)-1,2,3,4-tetrahydropyridin-2,6 dicarboxylic acid]. Betalains are classified according to their composition into betaxanthins and betacyanins, representing yellow and purple-red colors, respectively [4]. In addition, several researchers [5] have reported the betalains antioxidant properties because the phenolic groups within betalains are electron donors, obtaining a high capacity to scavenge free radicals. Unfortunately, these compounds are unstable with light, temperature, pH, enzymatic activity, and the presence or absence of oxygen and metals. Therefore, an optimal process is required to preserve the properties of the betalains [6]. For instance, microencapsulation by spray drying can be used to conserve the properties of the betalains. Microencapsulated materials are considered miniature packaging, which can be sealed, and their content can be released at controlled rates under specific conditions [7]. On the other hand, drying involves rapidly evaporating moisture and maintaining a low particle temperature. Furthermore, microcapsule formation consists of homogenizing the core materials and encapsulating materials, generating an emulsion that is atomized in the drying chamber [8]. Food packaging using plastic materials can cause a global pollution problem. Biopackaging can be an alternative solution to reduce this pollution problem. Biopackaging involves packaging that protects the food from the environment and other physical and chemical factors, ensuring the quality and safety of food products and allowing their transport and prolonged storage [9].
The biopackages can be fabricated from edible or biodegradable polymers such as chitosan and polyvinyl alcohol (PVOH), which could reduce plastic waste and environmental impact [10]. Chitosan is the main derivative of chitin, which is an amino-polysaccharide composed mainly of repetitive units of 2-amino-2-deoxy-D-glucopyranose [11]. Barik et al. [12] reported that chitosan-based films can extend the shelf life of foods, including good properties such as color, gloss, resistance to UV light, resistance to water, water permeability, resistance to traction, elongation, and breakage [12]. On the other hand, polyvinyl alcohol is a synthetic and biodegradable polymer containing hydroxyl groups that promote its interaction with proteins, forming a structurally stable polymeric network with a high-water absorption capacity and water vapor permeability [13].
Antimicrobial activity can reduce the presence of microorganisms, such as bacteria and fungi [14]. For the betalains, the antimicrobial activity is due to polyphenolic extracts. For instance, a phenolic content of 5.26 ± 0.28 mg GEA/g−1 was obtained for the garambullo, a stage of consumption maturity with dark purple coloration [15], associating this content with its antimicrobial activity. Botrytis cinerea is a phytopathogenic, necrotrophic, saprophytic, and parasitic-pathogenic fungus that is known to attack more than 200 species of plants, especially horticultural and fruit plants, and numerous wild plants, mainly dicotyledonous [16]. This disease can infect tomato plants at any vegetative stage, including postharvest [17].
In this work, we propose an effective method to preserve the main components in garambullo juice using a microencapsulation process. We present the influence of microencapsulated betalains based on garambullo in the fabrication of chitosan–PVOH films and their application in tomato coating. The performance of these biopackages was characterized using physical tests, water vapor permeability, puncture tests, extension, color, differential scanning calorimetry (DCS), Fourier transform infrared (FTIR) spectroscopy, and antioxidant and antimicrobial activity analyses. This process can enable the long-time delivery throughout biopolymeric films to generate a novel biocomposite with application in the food industry.
2. Materials and Methods
2.1. Chemicals and Reagents
Mucilage (Aloe vera), glycerol (J.T. Baker Lot: M16C52, CDMX, Mexico), globe maltodextrin 10 (Ingredion code: 10520015, Guadalajara, Mexico), silicon dioxide (Agroin, Celaya, Mexico), starch (Ingredion Batch: FKI6714, Guadalajara, Mexico), garambullo juice, Tween 20 (azumex universal code: 07503031232370, CDMX, Mexico), Tween 80 (HYCEL lote: 263534, CDMX, Mexico), chitosan medium molecular weight Lot: STBF4197V Sigma-Aldrich and Poly (vinyl alcohol) Lot: MKBH1410V Sigma-Aldrich, St. Louis, MO, USA.
2.2. Microcapsules Production and Physico-Chemical Characterization
Microcapsules were formed by preparing an emulsion with a maximum content of 30% soluble solids, which were accepted in the spray-drying process, composed by 11.03% garambullo juice, 0.95% Aloe vera mucilage, 9.48% starch, 0.1% Tween 20, 7.11% maltodextrin and 1.42% SiO2 (MBT), and the control microcapsules were prepared by the same formulation without garambullo juice (MB) using a spray-drying temperature of 160 °C.
2.2.1. Determination of the Antioxidant Capacity of Microcapsules and Films
Quantification of Betalains in Microcapsules
Quantification of betalains in microcapsules was performed according to the process reported in the literature [18,19]; 10 mg of microcapsules was weighed and placed in a flask and dispersed with 40 mL of distilled water under vortex agitation for 20 min, then centrifuged at 490 rpm for 5 min and filtered, then centrifuged at 490 rpm for 5 min and filtered on Whatman paper No. 4 to obtain the absorbance reading for betacyanin at 538 nm and for betaxanthin and betaxanthins at 472 nm; the determinations were performed in triplicate. The following equation was used:
where A is the absorbance at 476 nm for betaxanthins and 538 for betacyanins and Fd is the dilution factor that for this case is 0.25, PM is the molecular weight of the pigment, betacyanins (550.5 g/mol) and betaxanthins (339.3 g/mol), E is the molar extinction coefficient, betacyanins (1120 L/mol·cm) and betaxanthins (750 L/mol·cm).
(mg of pigmento)/(100 g of sample (b.h.)) = (A)(Fd)(PM)/E
Phenolic Content
The phenolic content was determined according to the previous method reported in the literature [20] with some modifications; 25 μL of the sample, 25 μL of Folin–Ciocalteau reagent, and 25 μL of sodium carbonate were added to a microplate. The mixture was incubated for 30 min at 40 °C, 200 μL of distilled water was added, and the absorbance was read at 750 nm. The calibration curve was generated with a gallic acid (GA) solution. The results are expressed in mg gallic acid equivalents per gram of dry weight of the sample (mg GAE/g sample). The following equation was used:
(GAE mg)/(100 g) = ((mg/mL)(v extraction))/(g sample)
ABTS ((Ácido 2,2′-Azino-bis-(3-etillbenzotiazolin-6-sulfonic)) Antioxidant Activity
The ABTS method was evaluated according to the procedure proposed by [21]. The reading was performed in a 96-well plate, in which 20 µL of Trolox + 230 µL of the working solution was added for the sample measurement. The mixture was allowed to stand for 6 min, after which the absorbance was read at 734 nm. The samples were read at 4, 10, 30, 60, and 90 min. The calibration curve was generated with a Trolox 800 µM solution. The results are expressed in mg Trolox equivalents per gram of dry weight of the sample (mg Trolox/g sample). The following equation was used:
(µM Eq. of Trolox)/g = ((mg/mL)(v extraction))/(g sample)
DPPH (2,2-Difenil-1-picrilhidrazil) Determination
The DPPH method was assessed according to the procedure proposed by [22]. The reading was performed in a 96-well plate, where 20 µL of extract and 280 µL of radical were added for sample measurement. In addition, 20 µL of absolute methanol and 280 µL of radical were used as blanks; after 30 min, the mixture was allowed to stand in the absence of light, and the reading was obtained at 515 nm. The calibration curve was generated with a Trolox 800 µM solution. The results are expressed in mg Trolox equivalents per gram of dry weight of the sample (mg Trolox/g sample); Equation (3) was used.
FRAP (Ferric Reducing Antioxidant Power) Determination
The FRAP method was evaluated according to the procedure proposed by [23]. In a 96-well microplate, 20 µL of extract and 280 µL of radical were added, and the mixture was allowed to stand for 30 min in the absence of light; the absorbance was read at 593 nm. The calibration curve was generated with a Trolox 800 µM solution. The results are expressed as mg Trolox equivalents per gram of dry weight of the sample (mg Trolox/g sample); Equation (3) was used.
2.3. Scanning Electron Microscopy (SEM) Analysis
The surface morphology of the films was examined by high-resolution scanning electron microscopy (SEM) (XL30-SFEG, Philips/FEI, Hillsboro, OR, USA). Before examination, the microcapsules were coated with a thin layer of gold and inspected at an acceleration voltage of 10 kV.
2.4. Bio-Polymeric Film Fabrication
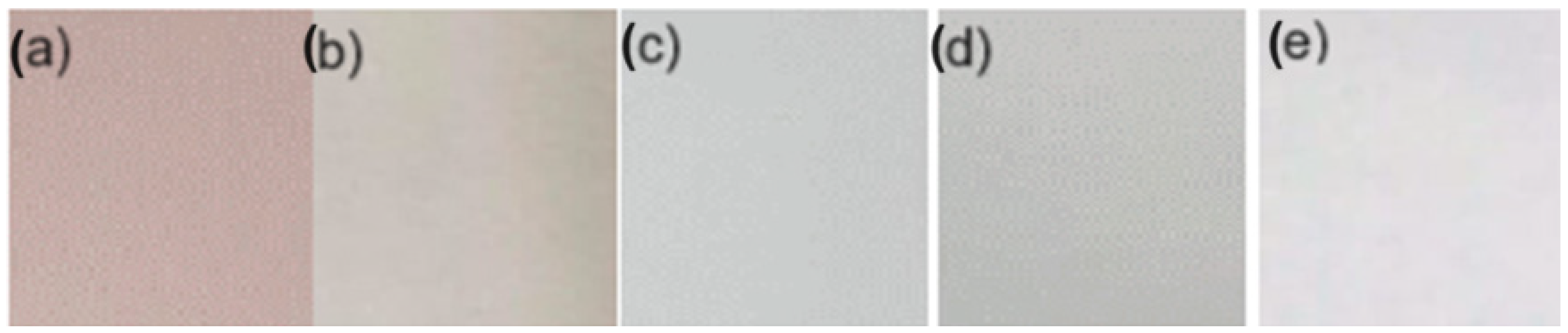
The film-forming chitosan solution was prepared according to the procedure reported by [24]. The resulting solutions were prepared as follows: 7.5 g of chitosan (CS) dissolved in a 1% (w/v) acetic acid solution (500 mL) was constantly stirred and heated for 30 min at 50 °C to improve the solubility of chitosan. A 4% (w/v) polyvinyl alcohol (PVOH) solution was prepared in distilled water at 80 °C. Chitosan and polyvinyl alcohol were mixed at a CS 70%-PVOH 30% ratio at room temperature under magnetic stirring for 30 min. Then, 2% glycerol in the CS base was added and stirred for 10 min. Finally, the betalain microcapsules were incorporated at a 1:1 ratio by weight on a CS basis and kept under magnetic stirring for 30 min. Each forming solution was placed in Petri dishes (5 and 10 cm), dried under laboratory conditions (23–26 °C) for approximately 48 h, and peeled off the plates for further analysis (Figure 1). The negative control was a mixture, and the positive control was a mixture of CS 70%-PVOH 30% with control microcapsules (microcapsule formation without betalains).

Figure 1.
Biopackaging: (a) sample (S) PVOH-CS-MBT based, (b) positive control (C+) PVOH-CS-MBL based, (c) negative control 1 (C-1) PVOH-CS based, (d) negative control 2 (C-2) CS based, and (e) negative control 3 (C-3) PVOH based.
2.5. Physical Characterization of Films
The weight of the films was measured to determine the density and surface density of the material using an analytical balance (Sartorius-Werke AG, Gottingen, Germany) with an accuracy of 0.0001. The surface density was calculated as the ratio of the weight of the sample with respect to the area of 1.26 × 10−3 m2. The density of the material was obtained from the relationship between the surface density and the thickness of the material. The water vapor permeability of the films was determined according to the method presented in the literature [25]. Permeability cells formed by a glass container were used. Furthermore, a perforated screw cap that allowed an exposure area of 9.079 × 10−4 m2 and two Teflon gaskets ensured an airtight seal, between which the film was placed. The permeability cell containing 15 mL of distilled water (RH1 = 100%) was placed in a vacuum desiccator with silica gel as a desiccant that maintained an external relative humidity (RH2) of 34%. The evaluated films were cut with a diameter of 4 cm, and the thickness was determined in 5 points measured randomly using a micrometer (Foil Dial Thickness Gauge F 1101/30, KAFER GmbH of Villingen-Schwenningen, Germany). The jars were weighed at regular time intervals. The kinetics of cell weight variation as a function of time until reaching equilibrium were recorded.
2.6. Mechanical Properties
It was determined on a texture analyzer TA-XT2i coupled to Texture Expert Exceed version 2.63 software. To determine the puncture stress, the samples were cut in a circular shape with a diameter of 4 cm and placed in a cell. These samples were well secured in the texturometer perpendicular to the film’s surface, and the P/2 cylindrical probe of 2 mm diameter of stainless steel was used. The speed of the head was 2 mm/s, a distance of 40 mm, and an activation force of 5 g. Calculating the maximum stress at the film puncture rupture according to the following equation:
where D is the probe displacement and lo is the initial length of the film
The tensile behavior was measured using the following procedure: the samples were trimmed, leaving a length of 8 cm between the edges, and the tape was placed to hold the probe. For the elongation process in the texturometer, the tensile grips probe was used with a uniaxial velocity of the head of 0.05 mm/s, a distance of 30 mm, and a force of 15 g. Thus, tensile strength (MPa), Young’s modulus (MPa), and elongation (%) can be measured [26].
2.7. Color Determination
Color determination was carried out using the Chroma Meter CR-400 (konica minolta Morristown, NJ, USA), and the color coordinates were evaluated according to the scale proposed by the CIE L a b chromaticity and hue degrees.
2.8. Fourier Transform Infrared Spectroscopy (FTIR)
The sample was placed in a cell closed and isolated from the environment. In addition, infrared patterns were recorded on ThermoFisher Scientific Nicolet FTIR 6700 spectrometer, Markham, ON, Canada with a DRIFT Spectra-Tech collector equipped with a high-temperature heating cell. Scanning from 400 to 4000 cm−1.
2.9. Differential Scanning Calorimetry (DSC) Analysis
DSC was used to carry out isothermal crystallization for the iPP and its composites. The equipment used was a TA Instruments DSC (New Castle, DE, USA), model Q2000, with temperature ranges from −70 to 400 °C. Approximately 10 mg of sample was subjected to temperature cycles, performing a heating ramp from 30 °C/min to 270 °C, isotherm for 5 min with a ramp of 10 °C/min under nitrogen atmosphere.
2.10. Antimicrobial Activity
B. cinerea was obtained from the “Instituto Nacional de Investigaciones Forestales Agrícolas y Pecuarias” (INIFAP) provided by Dr. Luis Antonio Mariscal Amaro, extracted from strawberries.
They were grown for 6 days at 19 °C on potato-dextrose agar plates (PDA, MCD LAB, San Jacinto Amilpas, Mexico) acidified with 10% p/v tartaric acid. They were used to recover fungal spores by pouring 25 mL of sterile distilled water over the surface of the agar plate, followed by gentle scraping with a sterile rake to remove the maximum number of spores. Spore suspensions were transferred to tubes containing 9 mL of sterile PDA using the puncture methodology. They were cultured for 6 days at 19 °C. They were subsequently used to recover fungal spores by pouring 10 mL of sterile distilled water with 0.01% Tween over the agar surface, followed by gentle scraping using a sterile rake to remove the maximum number of spores. The number of spores in the suspension was determined using a hemocytometer and an optical microscope, which can be expressed as the number of spores per milliliter (spores/mL). Thus, suspensions were serially diluted to approximately 1 × 106 spores/mL [27].
Disc Contact Assay
The inverted lid technique [28] was used; 100 μL of spore suspension (1 × 106 spores/mL) was placed in the center of the PDA plate and dried in a laminar flow hood under aseptic conditions. The PDA plate was dried in a laminar flow hood under aseptic conditions at room temperature for 30 min, then film discs corresponding to antimicrobial film to cover the plate with growth agar were measured every 24 h for 8 days of incubation at 20 °C. Each assay was performed in triplicate.
2.11. Application on Tomato
The raw material was used in ball tomatoes, which were selected by size, maturity, absence of physical damage, and fungal infections. Next, the tomato pedicle was cut and sanitized using a 0.35% p/v colloidal silver solution (Microdyn, CDMX, Mexico).
2.12. Evaluation of the Coating of CS-PVOH and Microcapsules of Betalains on Tomato
Preliminary tests were carried out on tomatoes to evaluate the behavior of the coating on tomato physicochemical parameters.
The filmogenic suspension was prepared according to Section 2.2. The tomatoes were coated with direct applications of the filmogenic suspension, dipping and adding them with a brush on the stalk side. The coating was dried with Lakewood fans (250 V, 50/60 Hz). After coating, they were kept refrigerated at a temperature range from 4 ± 1 °C for the period necessary for each test.
2.13. Physico-Chemical Properties of Coated Tomatoes
2.13.1. Weight Loss (WL)
The fruit weight of each replicate was recorded on the day of treatment and subsequent days of sampling.
2.13.2. pH Determination
The pH was determined according to [29], which was measured in the tomato juice of each sample using a pH-meter (Oakton, pH/Mv/°C meter, Singapore)
2.13.3. Soluble Solids (SS)
The determination of soluble solids was carried out on juice samples of tomato for each treatment at 25 °C in triplicate, in a refractometer (Pocket pal-1, Atago (0–53%, Shanghai, China)); the results are expressed as Brix index (°Brix).
2.13.4. Titratable Acidity (TA)
The titratable acidity (TA) was obtained according to [29], based on titration of approximately 3 g of fruit puree. AOAC (1996), based on the titration of approximately 3 g of fruit puree with 0.1 N NaOH solution, pre-titrated with 3 drops of 1% phenolphthalein and using a potentiometer to a pH value of 8.2 ± 0.2. The result is expressed as a percentage of citric acid and calculated according to according to the equation:
where % acidity is the g of acid/100 mL of sample, V is the volume (mL) of NaOH used for titration, N is the normality of NaOH, and milliequivalent is the quantity of the predominant acid in the food equivalent to 0.001 mL of 0.1 N NaOH 0.1 N, which in the case of citric acid is equivalent to 6.4 g
2.14. Shelf Life Determination
Tomatoes dip-coated with the different biopackages were subjected to refrigeration (4 °C) for 18 days, visually assessing the growth of B. cinerea on the surface of the samples.
2.15. Statistical Analysis
The results are expressed as the mean ± standard deviation of the experiments with three replicates. The statistical evaluation was carried out by analysis of variance (ANOVA), comparing means by Tukey’s method with a significance level of p < 0.05, using STATGRAPHICS centurion XVI.I software V. 16 no.
3. Results and Discussion
The microcapsules with betalains from garambullo were characterized by color, total betalains, betaxanthins, betacyanins, water activity, total phenols, and antioxidant activity (DPPH, ABTS and FRAP). These experimental results are summarized in Table 1, Table 2 and Table 3.

Table 1.
Color characterization of microcapsules with garambullo juice.

Table 2.
Betalain content, betacyanin content, betaxanthin content, water activity, and condensed tannins in microcapsules with garambullo juice.

Table 3.
Antioxidant characterization of microcapsules.
3.1. Microparticle Morphology (SEM)
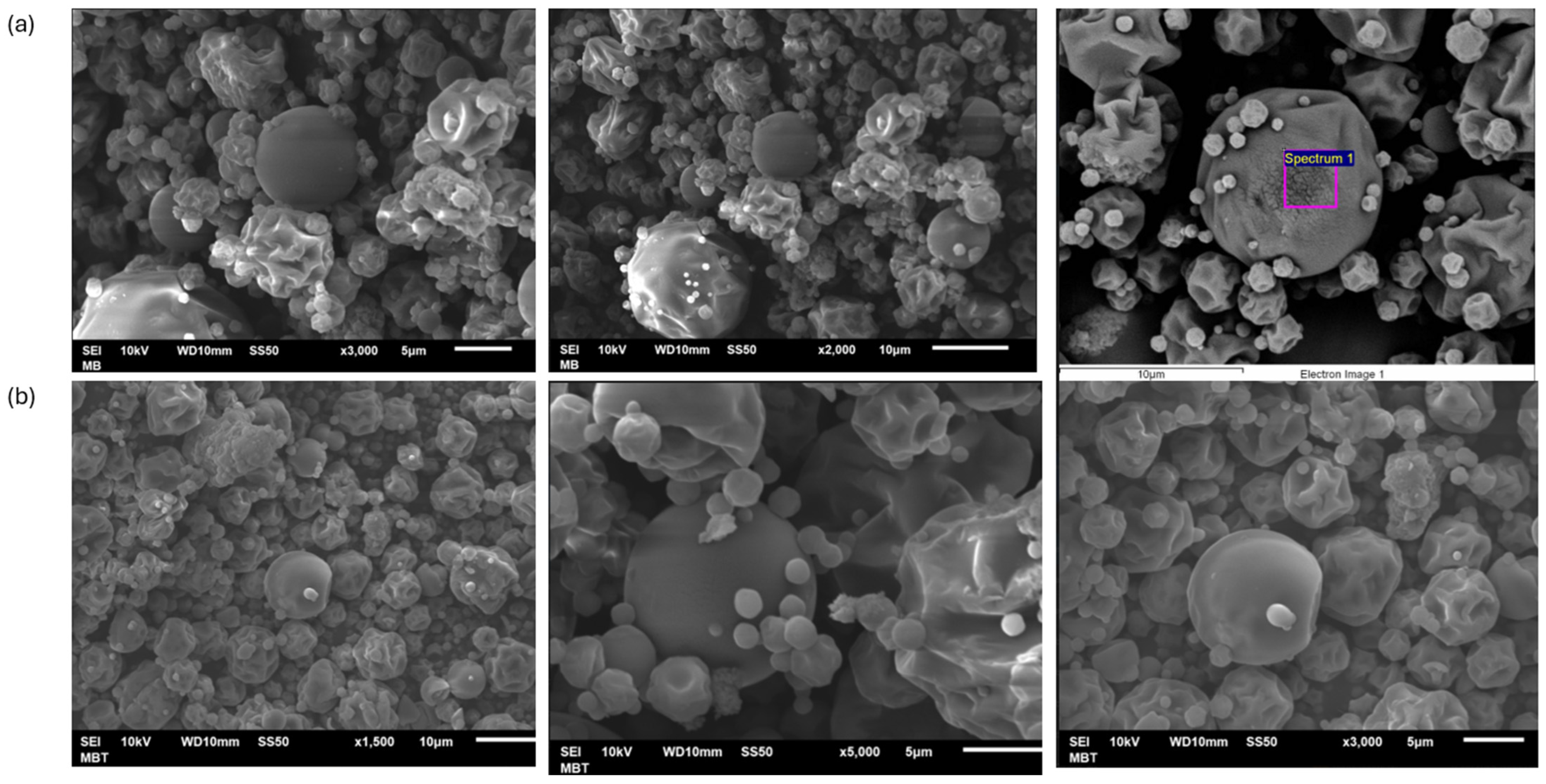
The morphology of the control (white) and betalain microcapsules were observed in SEM images, as shown in Figure 2.
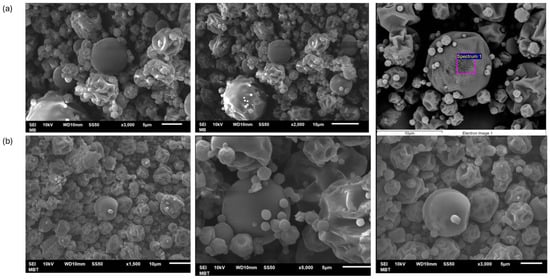
Figure 2.
SEM images of microcapsules. (a) control microcapsules and (b) Microcapsules with juice of garambullo (Myrtillocactus geometrizans).
Figure 2 shows the microcapsules containing betalains from the garambullo treatment and the control at 160 °C. These microcapsules depict variable shapes, such as spherical-shaped capsules with smooth surfaces and capsules with irregular surfaces (sizes between 5 and 10 μm). Generally, the formation of dents on the capsule surface is caused by the shrinkage of the particles during drying. It is due to the drastic loss of moisture, followed by cooling [30]. Ref. [31] investigated that the spherical morphology is due to maltodextrin in the formulations. Low-molecular-weight sugars (e.g., maltodextrin) can act as plasticizers that reduce the number of polymer chain contacts, reducing the rigidity of the three-dimensional layered structure. In addition, Fernández-Repetto et al. [32] reported that higher solids content caused more roundness in the capsule shape. It is due to the combination of the encapsulating agents and the encapsulated material, which is shown in microcapsules with garambullo juice inside.
3.2. Mechanical Characterization of Biopackages
Table 4 depicts the parameters of a group of samples, including the negative control (C-1) and the positive control (C+). The parameters of both control samples do not have significant difference. However, the samples with microcapsules of betalains registered a significant difference.

Table 4.
Physical parameters of biopackages based on CS-PVOH, control microcapsules, and microcapsules with garambullo juice.
3.3. Water Vapor Permeability
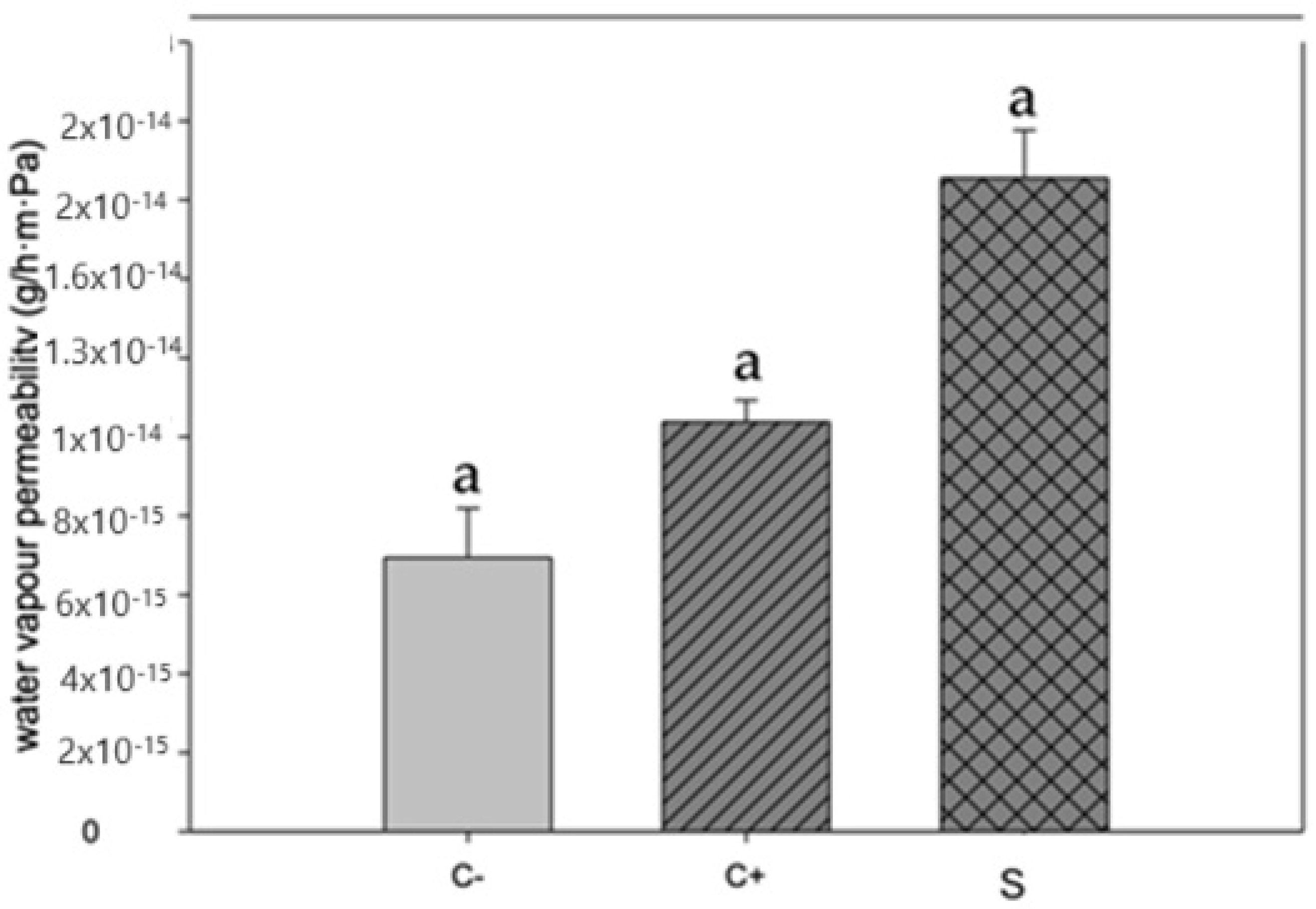
Figure 3 depicts the values of water vapor permeability of the proposed biopackages, according to [33]. This parameter must decrease to avoid the moisture transfer between food and the environment, which can increase the shelf life of the food. The results of water vapor permeability of our biopackages are lower than those reported by [33,34], which were based on chitosan and betalains.
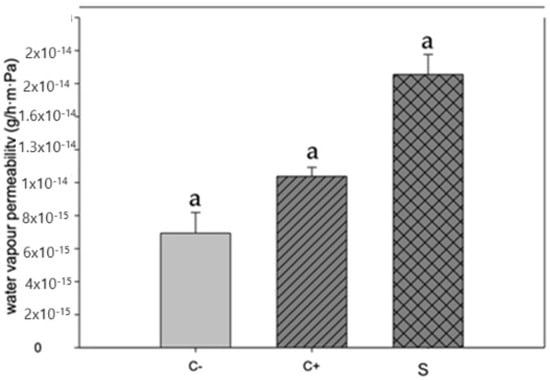
Figure 3.
Water vapor permeability in biopackage based on CS-PVOH, control microcapsules, and microcapsules with garambullo juice. Letter (a) within each column indicate significant differences for each variable (p = 0.05).
3.4. Puncture Stress and Strain at Extension
Table 5 shows the characteristics of the puncture stress and tensile strain. The samples containing betalain microcapsules showed no significant difference in Young’s modulus and tensile strength. However, there was a significant difference in the % elongation, and the values were very close, which indicates that the packaging did not stretch, as there was cohesion between the chains. According to the literature [35], the same behavior was observed for samples of chitosan–gelatin films supplemented with tyrosol and encapsulated ferulic acid, while the tensile strength decreased without microcapsules. On the other hand, Kurek et al. [36] studied a functional packaging of chitosan and pectin with encapsulated Opuntia-ficus indica residues, reporting that variations in the results of the biopackages can be due to the nature and source of the film-forming polymer (impurities, degree of deacetylation of chitosan, esterification of pectin, and so on). These parameters vary according to the study, the type and content of the plasticizer, and the intermolecular forces; at the time of puncture, there is a difference as it is in a direction perpendicular to the manufactured film, in which the microcapsules are all over the packaging.

Table 5.
Mechanical properties of biopackaging based on CS-PVOH, control microcapsules, and microcapsules with garambullo juice.
3.5. Determination of Color in Biopackaging
Table 6 indicates the results of evaluating the color parameters in the biopackaging films. The statistical analysis shows significant differences for the parameters measured. These differences indicate that the addition of both white and betalain microcapsules tends to darken, acquiring a yellowish color. This result agrees with the literature [37], in which the chitosan biopackage tends to have a yellowish tone because the microcapsules have betalains, divided into betacyanins and betaxanthins. It gives the yellow colorations; furthermore, the value for the parameter a* is positive and higher for those with microcapsules. It is due to the microcapsules with betalains, and the tone would be associated with the presence of betacyanins, which are associated with the red-violet colors [38], presenting a higher saturation for the samples with betalains.

Table 6.
Color parameters in biopackages based on CS-PVOH films, control microcapsules, and microcapsules with garambullo juice.
3.6. Fourier Transform Infrared (FTIR) Spectroscopy
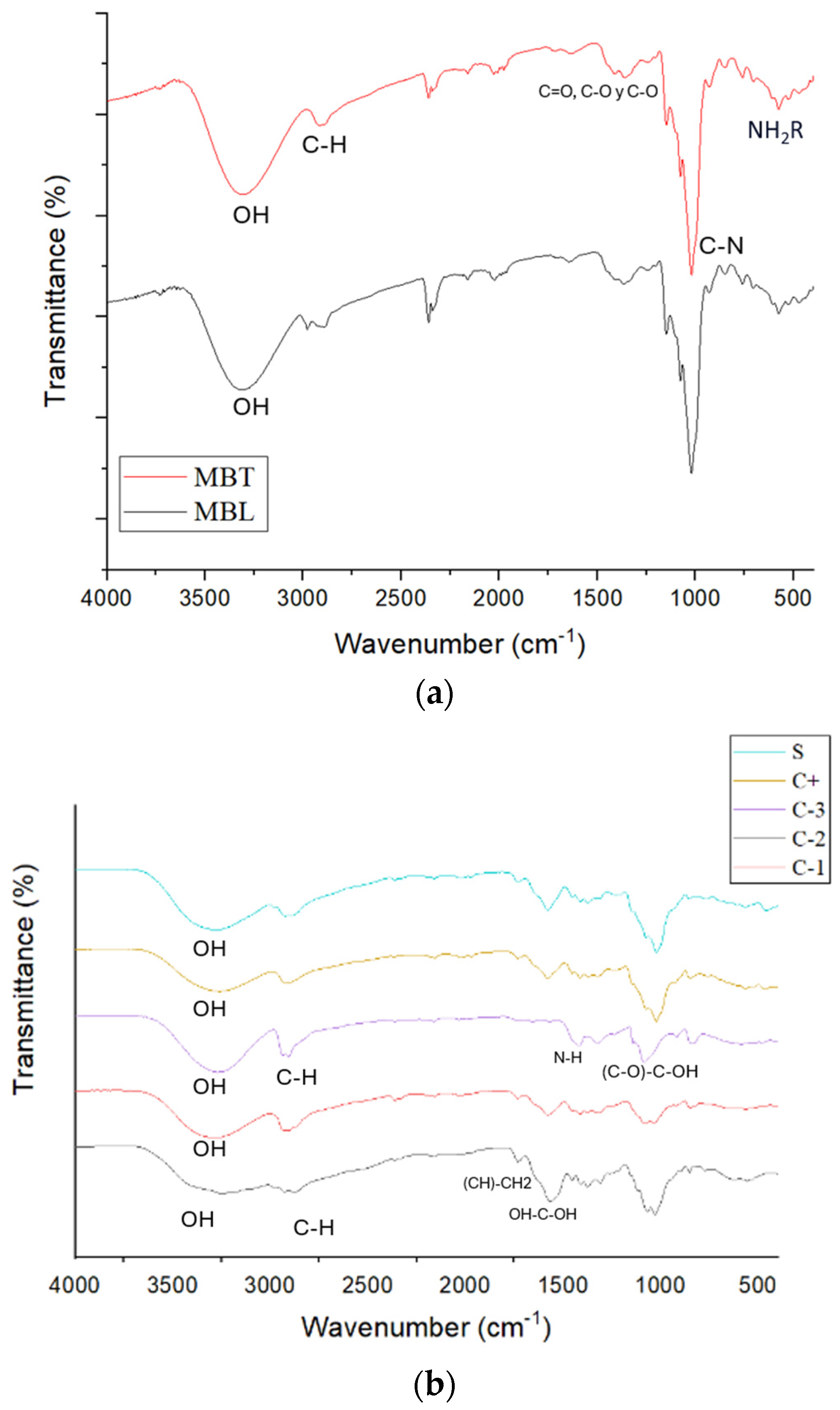
Figure 4 depicts the FTIR spectrum of the biopackaging films, in which the characteristic absorption bands of the OH, C-O, C-H, CH2, and C=O functional groups of the chitosan (CS) and PVOH are observed. The band at 3274 cm−1 corresponding to -OH is more pronounced for PVOH than for CS. CS has a pronounced band at 2915 corresponding to -C-H; while for PVOH, it is at 2940 cm−1. At 1745 cm−1, a band corresponding to (CH)-CH2 is observed for CS. At 1573 cm−1, the band in CS corresponding to OH-C-OH is visualized, while for PVOH, at 1409 cm−1, the band is of N-H. Also, for PVOH at 1020 cm−1, a peak corresponding to (C-O)-C-OH is illustrated and was identified at 1087 cm−1, -C-O-C in PVOH at 1328 cm−1, and in CS at 1382 cm−1, in the CS/PVOH mixture the OH band was more pronounced than for CS. The OH bands in the PVOH-CS system increase in size with a lower intensity, indicating a reduction in the crystallinity of PVOH to shift the position and intensity of the O-H peak, the hydrogen atoms of PVOH and the oxygen atom of CS establish hydrogen bonds with each other [39]. For (CH)-CH2, the peak is found at 1729 cm−1, which is less pronounced than for CS but higher than for that of PVOH. The (C-O)-C-OH is found at 1596 cm−1. In microcapsules, there was no change between microcapsules with betalains and those without betalains, according to [40]. Betalains show a band at 3400 cm−1 corresponding to O-H stretching, and at 2921 cm−1 associated with symmetric C-H stretching, at 1646 cm−1, 1411 cm−1, and 1253 cm−1 corresponding to asymmetric C=O, C-O, and C-O stretching of the acid, respectively. The band at 1023 cm−1 corresponds to the C-N stretching and the band at 754 cm−1 corresponds to the presence of the amide group. These changes in the bands could not be identified in the microcapsules with betalains, probably because the microencapsulation was properly performed, avoiding the betalains being exposed to be able to visualize them. When added to the packages in both cases, there was an extension of the (CH)-CH2 band and a decrease in the band corresponding to (C-O)-C-OH. There were no modifications concerning the incorporation of betalains when they were microencapsulated.
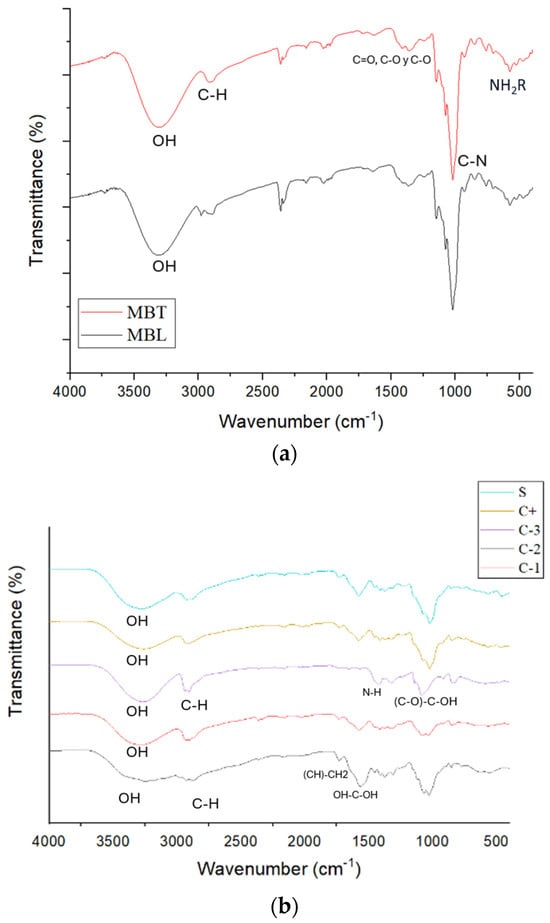
Figure 4.
FTIR spectrum of (a) MBL and MBT microcapsules and (b) C-1, C-2, C-3, C+, and S.
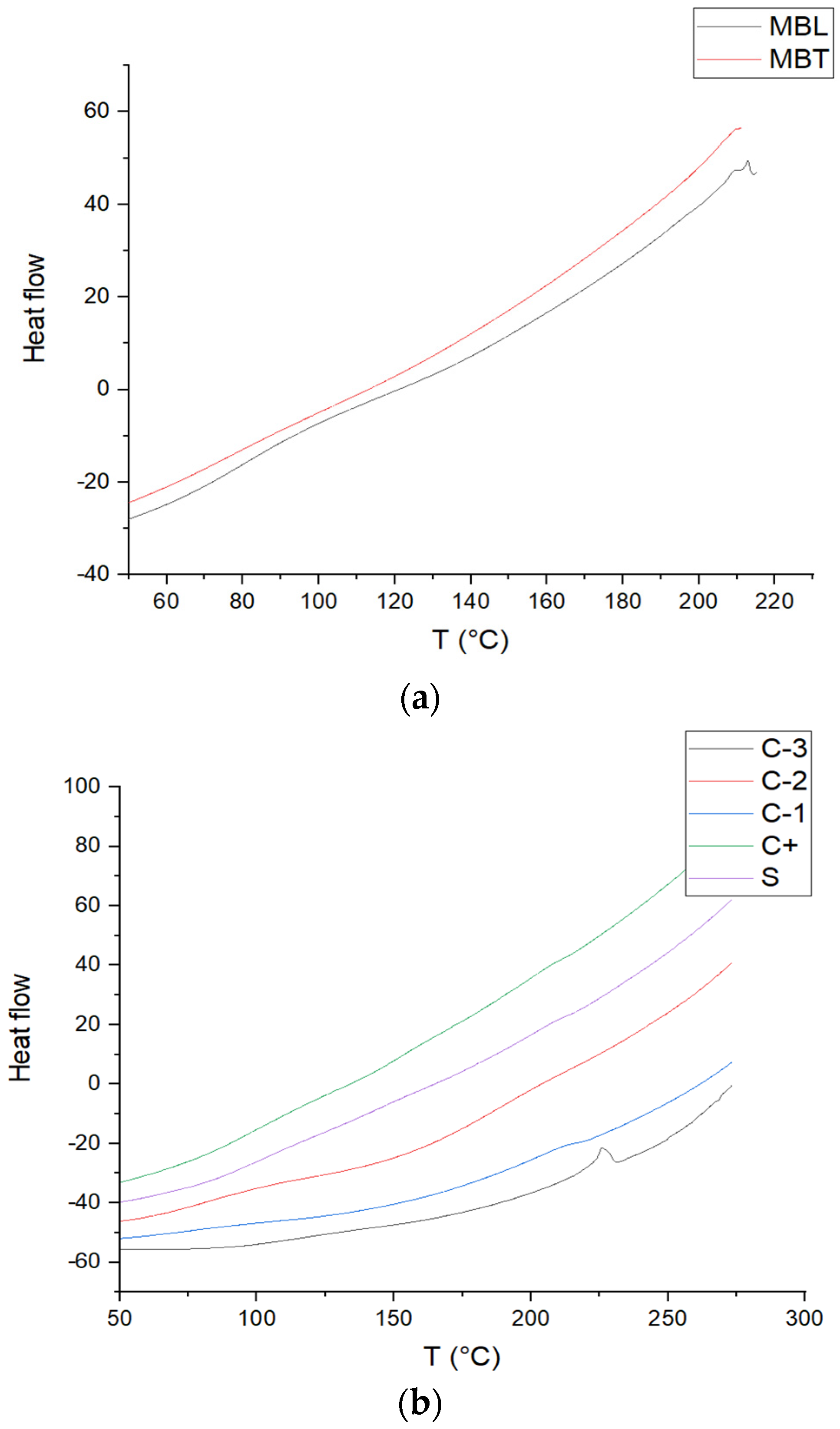
3.7. Determination DSC
Figure 5 shows the DSC thermograms of the batches of chitosan films, PVOH, the CS-PVOH blend, CS-PVOH-MB, CS-PVOH-MBT, MB, and MBT, giving a glass transition (Tg) of 80 °C for PVOH, as reported by the literature [41] which was 52.4 °C with a melting temperature (Tm) of 225 °C. This temperature is similar to that reported by [42] of 219.5 °C. For CS, the glass transition is 118 °C that is similar to that reported by [43] of 123 °C; also, this result agrees with this research since it does not show melting behavior in the lactic acid films, suggesting that the chitosan molecules were in an amorphous state, making that for the other biopackages did not show melting behavior. The value for the CS-PVOH mixture was 110 °C, for CS-PVOH-MB, it was 154 °C, and for CS-PVOH-MBT, it was 80 °C. The change in the glass transition of the latter two was modified due to the addition of the microcapsules. For the CS-PVOH-MB packaging, it is modified since the white microcapsules have a glass transition of 120 °C and have a degradation at 217 °C, while for MBT, these behaviors are not perceptible except for the degradation, which is similar to that of MB with 211 °C.
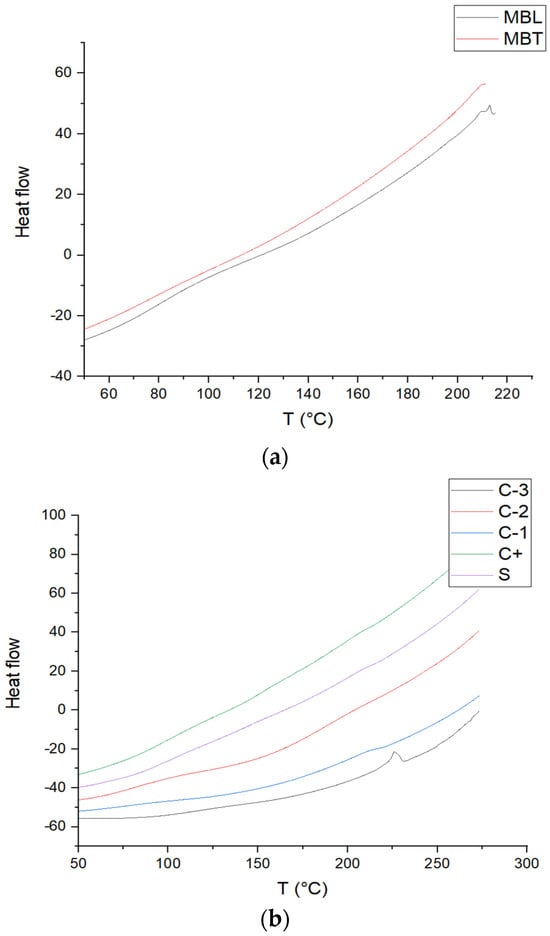
Figure 5.
DSC curves of (a) MBL and MBT microcapsules, and (b) C-1, C-2, C-3, C+, and S.
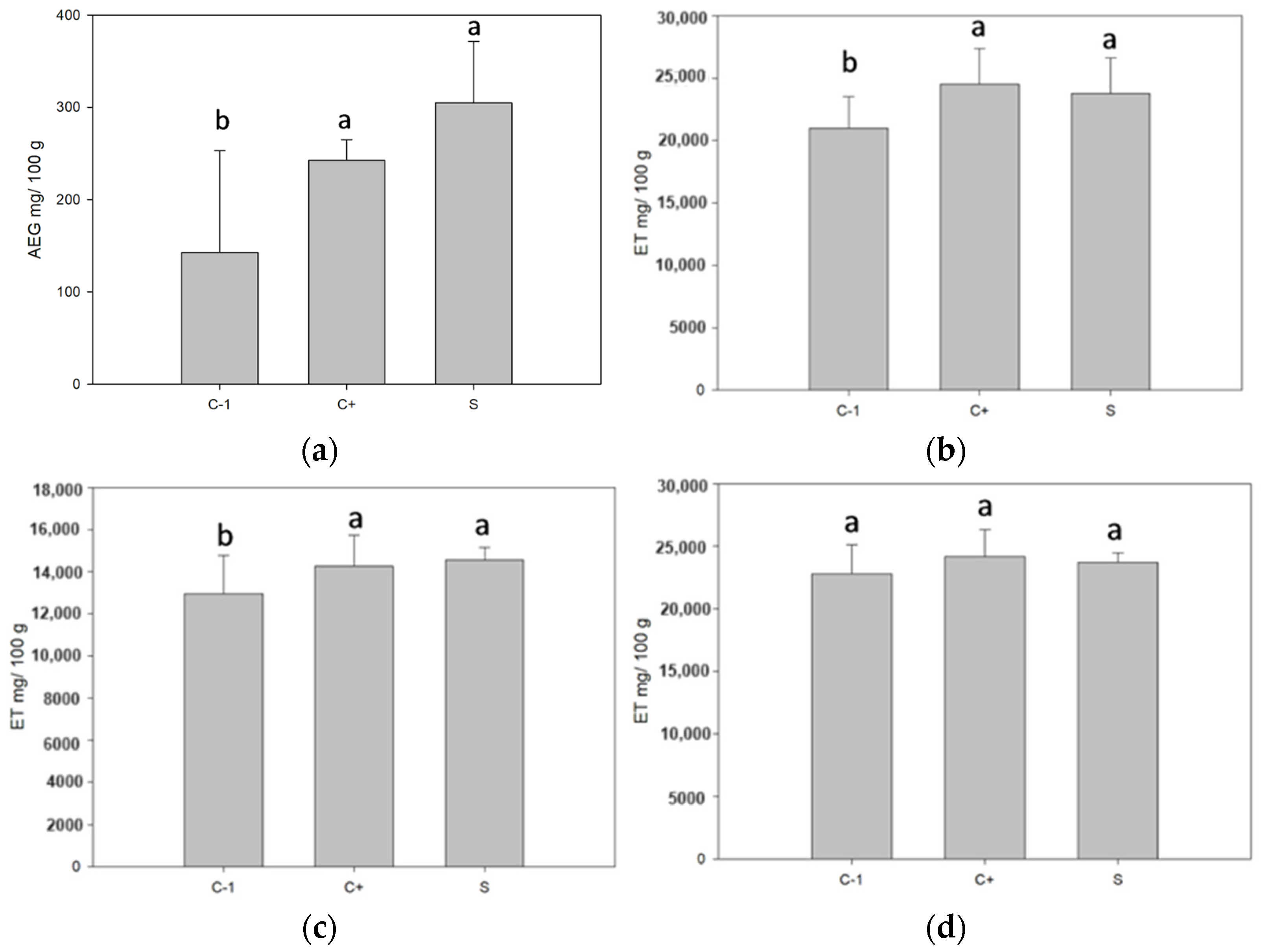
3.8. Determination of the Antioxidant Capacity of the Filmogenic Suspension
The phenolic content in the biopackages significantly increased in the samples with microcapsules. Both white ones, without active nuclei, showed a greater phenolic content than those with nuclei (see Figure 6). It may be due to the added concentration of betalains, which contain phenolic compounds [44]. Antioxidant activity is performed by these three determinations since DPPH shows antioxidant activity against lipophilic compounds, ABTS against hydrophilic compounds, and antioxidant activity by FRAP is against lipophilic and hydrophilic compounds. As a result, for the determinations DPPH and ABTS, there was no significant difference for the samples with control microcapsules and microcapsules with betalains, which was probably because the release of the microcapsules was due to dissolution or swelling. In the first one, the wall material dissolved due to the influence of external factors, and in the second one, it is due to the absorption of the surrounding liquid [8]. The core was released by placing the microcapsules in the packaging [45]. Before packaging formation, the microcapsules had values above 4000 GAE mg/100 g of the sample. After the packaging and dissolution of the same for the measurement, the degradation of the compounds took place when in contact with the environment and probably left microcapsules that would only be released by the fracture mechanism, in which the encapsulant breaks due to external or internal forces. When the microcapsules do not break, they do not release the compound [8], releasing the nucleus at the moment of placing the microcapsules in the packaging. The equation for the calibration curve is as follows: for total phenols, Y = 0.004x + 0.0663 and an R2 = 0.9833, for DPPH, Y = −0.0007 + 0.8446 and R2 = 0.9946, for ABTS, Y = −0.001x + 1.552 and R2 = 0.9519, and for FRAP, Y = 0.0018x + 1.3584 and R2 = 0.9805.
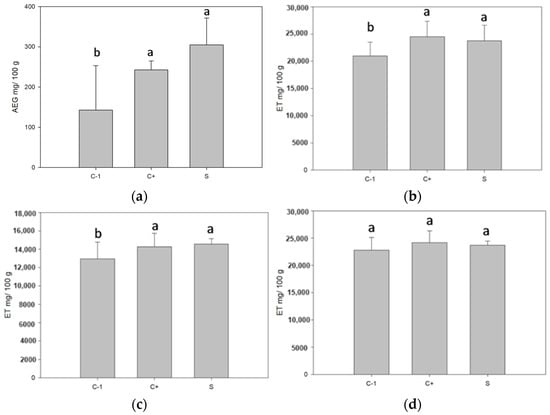
Figure 6.
Phenolic content and antioxidant activity of biopackages from CS-PVOH, control microcapsules, and microcapsules with garambullo juice: (a) total phenols, (b) DPPH determination, (c) ABTS determination, and (d) FRAP determination. Different letters (a, b) within each column indicate significant differences for each variable (p = 0.05).
3.9. Antimicrobial Activity
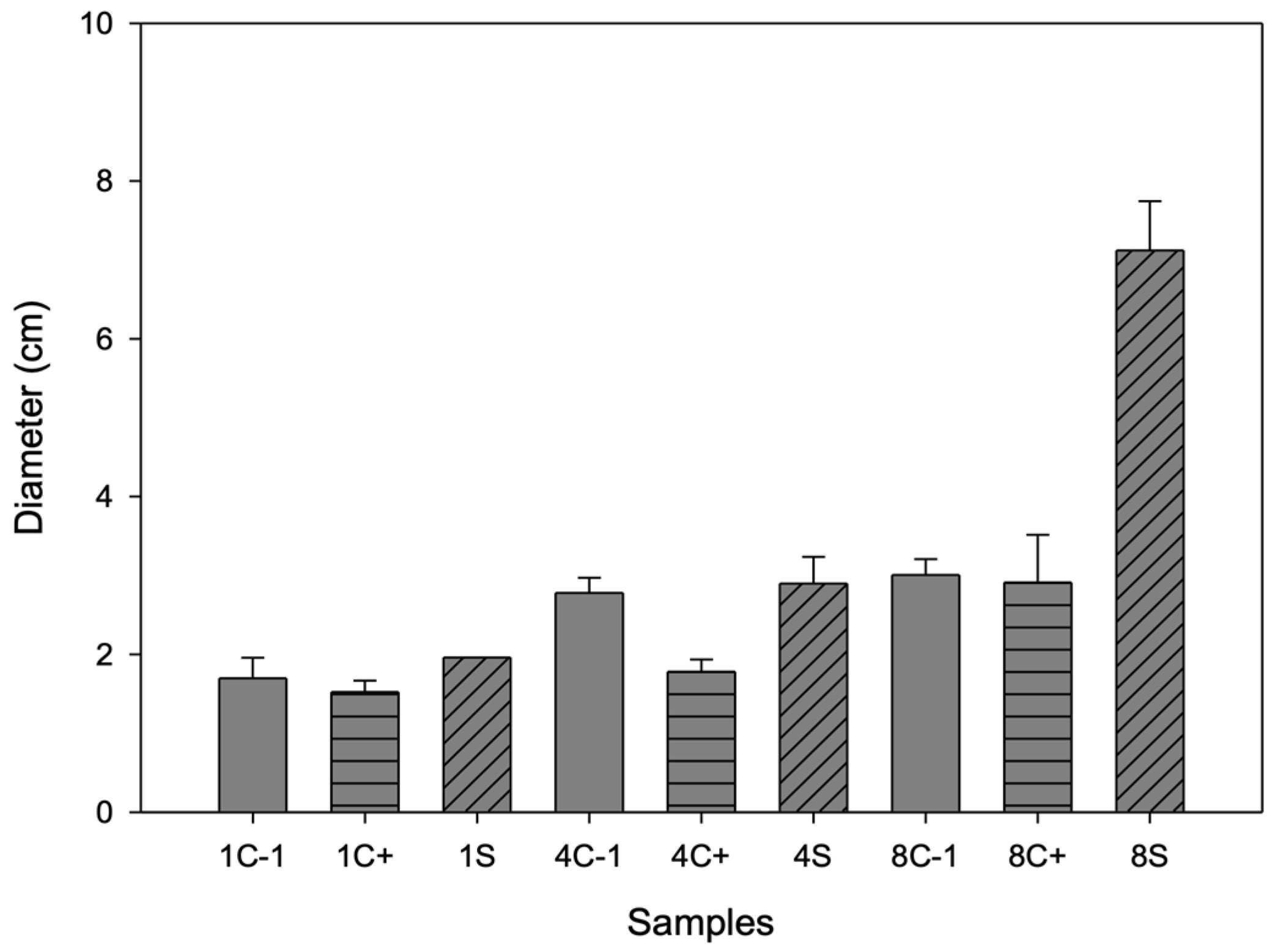
On day 1, no growth of B. cinerea was observed for any of the samples (see Figure 7); on day 4, growth was inhibited, mainly for the sample, and on day 8, for C- and C+, the fungus completely covered the box. In the first days, the antimicrobial effect could be attributed to the antimicrobial activity of chitosan since it has only cationic (positive) charges. These charges are responsible for binding to anionic (negative) components in bacterial membranes, such as phosphoryl groups of phospholipid components, proteins, amino acids, and various lipopolysaccharides, through electrostatic attractions leading to the breakdown of cell membrane permeability [46]. However, the main active compound promoting the antimicrobial activity of this container is betalains, which are released on day 8 when a larger halo of inhibition occurs because the biopolymer decomposes, and microcapsules release the betalains, which have aromatic rings in their chemical structure that include elements in common with phenolic compounds [47]. These phytochemicals have been shown to have antimicrobial activity, as lipophilicity allows active phenols to penetrate biological membranes, while hydroxyl groups can act by uncoupling.
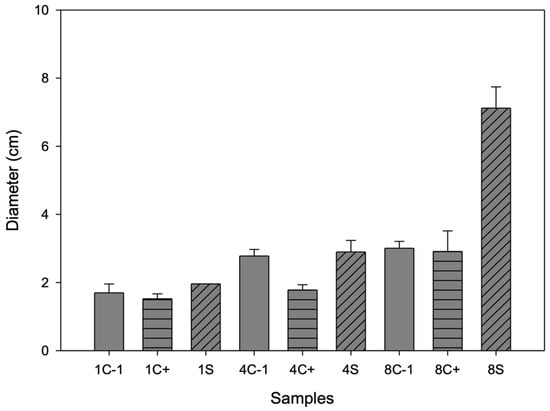
Figure 7.
Inhibition halo diameter of biopackages from CS-PVOH, control microcapsules, and microcapsules with garambullo juice against Botrytis cinerea on days 1, 4, and 8.
3.10. Coating Application on Tomato
3.10.1. Physico-Chemical Properties of Tomato
The titratable acidity, °Brix and pH, is related to the degree of maturity of the product. For instance, a product with a greater °Brix value can have a lower % of citric acid [48]. According to previous research [49], the reported °Brix of tomatoes is 4.94 and 0.35% of citric acid. The °Brix values are close to the those obtained in this research, exhibiting variation in titratable acidity. For both products, as the number of days passed, the °Brix increased. There was a greater increase in the C- samples that had no biopackaging. Furthermore, the C- samples had greater maturity, which affected their shelf life, while those with the coating had a lower content of °Brix. The results are shown in Table 7.

Table 7.
Physicochemical properties of tomatoes on days 1 and 9 after coating with CS-PVOH, control microcapsules, and microcapsules.
3.10.2. Weight Loss (WL)
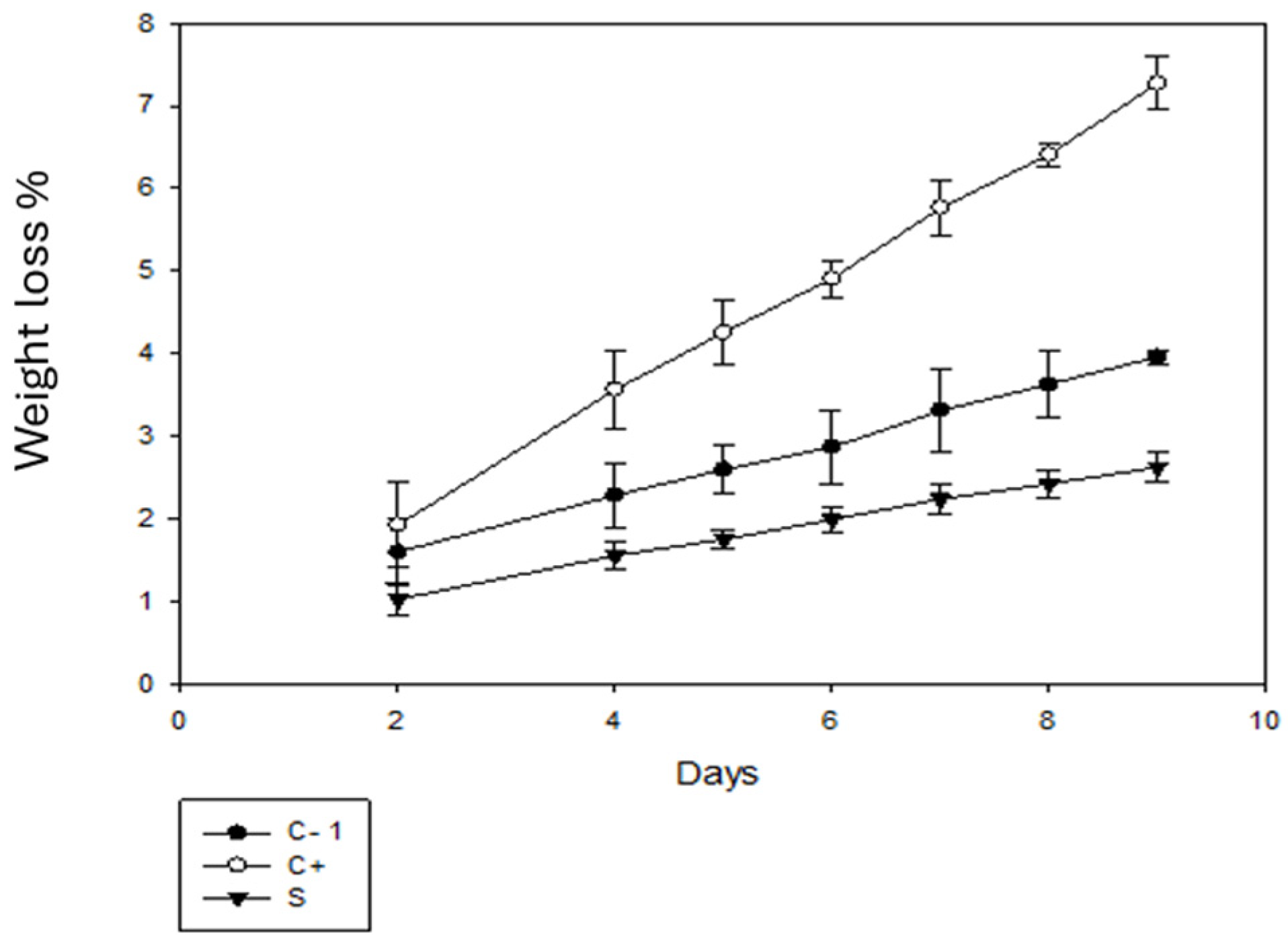
Figure 8 shows the weight loss of the tomato fruits. In comparison with day 1, on day 9, there was a loss of tomato weight due to the dehydration process due to respiration, which is considered the main cause of the decrease in fruit and vegetable fruits and vegetables. This water loss is related to the pressure difference between the surrounding atmosphere and the surface of the fruit [50]. A greater loss percentage was observed for the C+ group, with a percentage of more than 7%. On the other hand, for the C- group, the percentage was less than 4% and for the samples with packaging using betalains, the percentage of loss was less than 3%, which is the lowest percentage of loss. According to [51], the use of biodegradable bags resulted in a loss of 2.5%, which is similar to the results obtained for the sample with coating.
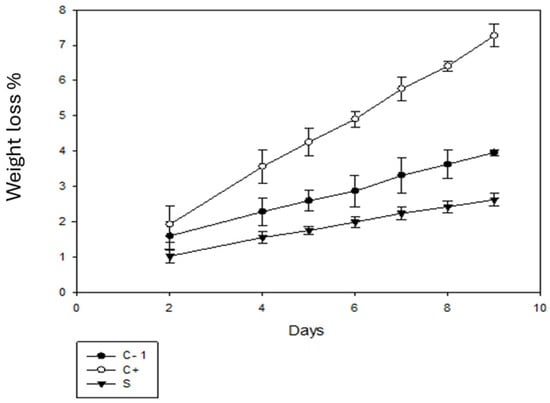
Figure 8.
Percentage weight loss of tomato without packaging (C-), and with C+ and S packaging.
3.10.3. Shelf Life
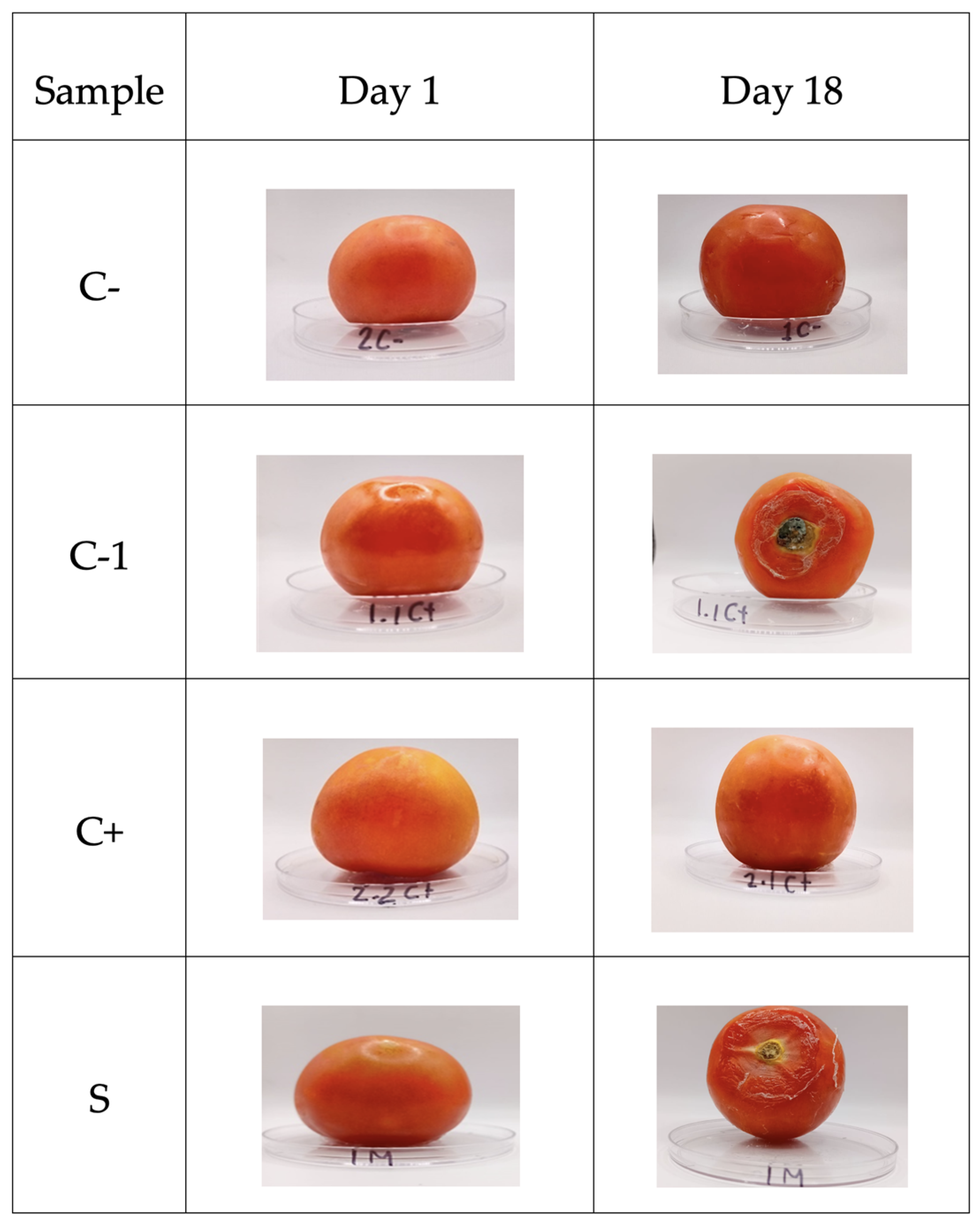
The determination of the shelf life of the tomato fruits without (C-) and with (1C+) biopackage showed growth of B. cinerea, and 2C+ increased only its maturity (Figure 9). However, it inhibited the growth of the fungus, while the sample containing microcapsules with betalains inhibited the fungus, resulting in a lower maturity index and less dehydration.
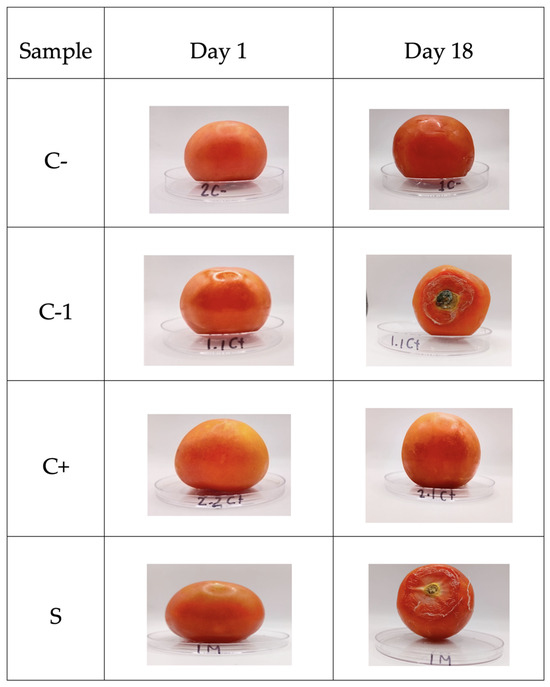
Figure 9.
Shelf-life of tomatoes. C- refers to samples without biopackaging, C-1 refers to samples with bi-opackaging, C+ refers to samples with biopackaging and control microcapsules, and S refers to biopackaging samples with microcapsules of betalains.
Based on [52], the shelf life of ball tomatoes is between 10 and 12 days, if a temperature of 10 °C is applied. The shelf life of coated tomatoes increased by up to 18 days when they were stored at a temperature of 4–5 °C. According to [53,54], the delayed deterioration of the fruits could be attributed to the antimicrobial activity of the tested films, showing inhibition of B. cinerea by the packages. The release of betalains occurs from day 8, which is the common shelf life of this tomato, which can help to protect tomato from the growth of this fungus. Thus, the biopackages are a good alternative for extending the shelf life of tomatoes (see Figure 8).
4. Conclusions
The influence of microencapsulated betalains based on garambullo in the preparation of chitosan–PVOH films was investigated. In addition, the application of these biopackages for tomato coating was reported. The behavior of the biopackages was characterized using physical tests, water vapor permeability, puncture tests, extension, color, differential scanning calorimetry (DCS), and Fourier transform infrared (FTIR) spectroscopy. Also, the antioxidant and antimicrobial activities of the biopackages were studied. The proposed biopackages exhibited good water vapor permeability characteristics and good mechanical properties. The biopackages registered a greater content of total phenols in comparison with the controls due to the presence of betalains that registered phenolic compounds, allowing the in vitro inhibition of B. cinerea.
The application of the proposed coating on the tomatoes increased their shelf life by 80%. Thus, the proposed biopackages have potential application in the coating of fruits and vegetables.
Author Contributions
Conceptualization, D.G.-E. and M.C.I.P.-P.; methodology, J.A.G.-C. and R.R.-V.; validation, D.G.-E. and M.C.I.P.-P.; investigation, C.L.A.-M.; resources, M.C.I.P.-P.; writing—original draft preparation, M.C.I.P.-P. and J.A.G.-C.; writing—review and editing, J.A.G.-C., E.D.-A. and A.L.H.-M.; visualization, E.D.-A.; supervision, M.C.I.P.-P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Tecnológico Nacional de México, grant number 17317.23-P Efecto de la encapsulación de betalaínas de garambullo sobre la elaboración de películas de quitosano para alargar la vida de anaquel de productos hortofrutícolas.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Acknowledgments
This research was developed at “TecNM en Celaya”.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Moreno-Ley, C.M.; Osorio-Revilla, G.; Hernández-Martínez, D.M.; Ramos-Monroy, O.A.; Gallardo-Velázquez, T. Anti-inflammatory activity of betalains: A comprehensive review. Hum. Nutr. Metab. 2021, 25, 200126. [Google Scholar] [CrossRef]
- Ramírez-Rodríguez, Y.; Martínez-Huélamo, M.; Pedraza-Chaverri, J.; Ramírez, V.; Martínez-Tagüeña, N.; Trujillo, J. Ethnobotanical, nutritional and medicinal properties of Mexican drylands Cactaceae Fruits: Recent findings and research opportunities. Food Chem. 2020, 312, 126073. [Google Scholar] [CrossRef]
- Rahimi, P.; Abedimanesh, S.; Mesbah-Namin, S.A.; Ostadrahimi, A. Betalains, the nature-inspired pigments, in health and diseases. Crit. Rev. Food Sci. Nutr. 2019, 59, 2949–2978. [Google Scholar] [CrossRef]
- Villaño, D.; Garcia-Viguera, C.; Mena, P. Colors: Health Effects. In The Encyclopedia of Food and Health; Caballero, B., Finglas, P., Toldra, F., Eds.; Oxford Academic Press: Oxford, UK, 2016; Volume 2, pp. 265–272. [Google Scholar]
- Mancha MA, F.; Monterrubio, A.L.R.; Vega, R.S.; Martínez, A.C. Estructura y estabilidad de las betalaínas. Interciencia 2019, 44, 318–325. [Google Scholar]
- Otálora, M.C.; Carriazo, J.G.; Iturriaga, L.; Nazareno, M.A.; Osorio, C. Microencapsulation of betalains obtained from cactus fruit (Opuntia ficus-indica) by spray drying using cactus cladode mucilage and maltodextrin as encapsulating agents. Food Chem. 2015, 187, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Bhosale, S.; Desale, R.J.; Fulpagare, Y.G. Microencapsulation: Applications in the different dairy products. Int. J. Pharm. Biomed. Eng. 2020, 6, 7–11. [Google Scholar] [CrossRef]
- Choudhury, N.; Meghwal, M.; Das, K. Microencapsulación: Una visión general de conceptos, métodos, propiedades y aplicaciones en alimentos. Front. Aliment. 2021, 2, 426–442. [Google Scholar] [CrossRef]
- de Sousa, M.S.; Schlogl, A.E.; Estanislau, F.R.; Souza, V.G.L.; dos Reis Coimbra, J.S.; Santos, I.J.B. Nanotecnología en envases para la industria alimentaria: Pasado, presente y futuro. Recubrimientos 2023, 13, 1411. [Google Scholar]
- Asgher, M.; Qamar, S.A.; Bilal, M.; Iqbal, H.M. Bio-based active food packaging materials: Sustainable alternative to conventional petrochemical-based packaging materials. Food Res. Int. 2020, 137, 109625. [Google Scholar] [CrossRef]
- Román-Doval, R.; Torres-Arellanes, S.P.; Tenorio-Barajas, A.Y.; Gómez-Sánchez, A.; Valencia-Lazcano, A.A. Chitosan: Properties and its application in agriculture in context of molecular weight. Polymers 2023, 15, 2867. [Google Scholar] [CrossRef]
- Barik, M.; BhagyaRaj GV, S.; Dash, K.K.; Shams, R. A thorough evaluation of chitosan-based packaging film and coating for food product shelf-life extension. J. Agric. Food Res. 2024, 16, 101164. [Google Scholar] [CrossRef]
- Elango, J.; Zamora-Ledezma, C.; Alexis, F.; Wu, W.; Maté-Sánchez de Val, J.E. Protein adsorption, calcium-binding ability, and biocompatibility of silver nanoparticle-loaded polyvinyl alcohol (PVA) hydrogels using bone marrow-derived mesenchymal stem cells. Pharmaceutics 2023, 15, 1843. [Google Scholar] [CrossRef] [PubMed]
- Tenore, G.C.; Novellino, E.; Basile, A. Nutraceutical potential and antioxidant benefits of red pitaya (Hylocereus polyrhizus) extracts. J. Funct. Foods 2012, 4, 129–136. [Google Scholar] [CrossRef]
- Arredondo, J.M.F.; López, A.P. 2020 Caracterización Física y Bioquímica de Frutos de Garambullo (Myrtillocactus geometrizans). Química, Etnobotánica, Economía y Finanzas, 8. Available online: https://dicea.chapingo.mx/wp-content/uploads/2021/02/Quimica-etno-eco-y-Finanzas.pdf (accessed on 18 March 2023).
- Mathan, L.; Dubey, N.; Verma, S.; Singh, K. Factores de transcripción asociados a la respuesta de defensa contra hongos necrótrofos. In En Factores de Transcripción Para la Tolerancia al Estrés Biótico en Plantas; Editorial Internacional Springer: Cham, Switzerland, 2022; pp. 61–78. [Google Scholar]
- Chaouachi, M.; Marzouk, T.; Jallouli, S.; Elkahoui, S.; Gentzbittel, L.; Ben, C.; Djébali, N. Activity assessment of tomato endophytic bacteria bioactive compounds for the postharvest biocontrol of Botrytis cinerea. Postharvest Biol. Technol. 2021, 172, 111389. [Google Scholar] [CrossRef]
- Castellanos Santiago, E.; Yahia, E.M. Identificationand quantificationo of betalains from the fruits of 10 mexicanpricly pear cultivars by High Performance Liquid Cromatography and Electrospray Ionization Mass Spectometry. J. Agric. Food Chem. 2008, 56, 5758–5764. [Google Scholar] [CrossRef]
- Robles, A.M.V. Contenido de Betalainas y Actividad Antioxidante en Brácteas de Bougainvillea Glabra Choisy. Universidad Técnica de Machala. 2016. Available online: http://repositorio.utmachala.edu.ec/handle/48000/7795 (accessed on 18 March 2023).
- George, S.; Brat, P.; Alter, P.; Amiot, M.J. Rapid determination of polyphenols and vitamin C in plant-derived products. J. Agric. Food Chem. 2005, 53, 1370–1373. [Google Scholar] [CrossRef]
- González, A.; Guerrero, J. Betalaínas: Importancia, presencia en vegetales y sus aplicaciones en la industria alimentaria. Dep. Ing. Quím. Aliment. 2008, 2008, 1–9. [Google Scholar]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C.L.W.T. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Yingyuad, S.; Ruamsin, S.; Leekprokok, T.; Douglas, S.; Pongamphai, S.; Siripatrawan, U. Effect of chitosan coating and vacuum packaging on the quality of refrigerated grilled pork. Packag. Technol. Sci. 2006, 19, 149–157. [Google Scholar] [CrossRef]
- Sobral PD, A.; Menegalli, F.C.; Hubinger, M.D.; Roques, M.A. Mechanical, water vapor barrier and thermal properties of gelatin-based edible films. Food Hydrocoll. 2001, 15, 423–432. [Google Scholar] [CrossRef]
- ASTM Subcommittee D20; 10 on Mechanical Properties. Standard Test Method for Tensile Properties of Thin Plastic Sheeting. American Society for Testing and Materials: West Conshohocken, PA, USA, 1995.
- Sebti, I.; Martial-Gros, A.; Carnet-Pantiez, A.; Grelier, S.; Coma, V. Chit|osan polymer as bioactive coating and film against Aspergillus niger contamination. J. Food Sci. 2005, 70, M100–M104. [Google Scholar] [CrossRef]
- Du, W.X.; Olsen, C.W.; Avena-Bustillos, R.J.; McHugh, T.H.; Levin, C.E.; Friedman, M. Antibacterial activity against E. coli O157:H7, physical properties, and storage stability of novel carvacrol-containing edible tomato films. J. Food Sci. 2008, 73, 378–383. [Google Scholar] [CrossRef]
- AOAC. 1995 Method 942.15: Acidity (titratable) of fruit products. In Official Method of Analysis, 16th ed.; AOAC: Arlington, TX, USA, 1995. [Google Scholar]
- Moawad, S.; El-Kalyoubi, M.; Khallaf, M.; Abd El Mageed, M.A.; Ali, H.; Farouk, A. Influence of Carriers on the Functional Properties of Spray-Dried Flavors During Storage. Egypt. J. Food Sci. 2021, 49, 231–238. [Google Scholar] [CrossRef]
- Utpott, M.; Assis, R.Q.; Pagno, C.H.; Pereira Krigger, S.; Rodrigues, E.; de Oliveira Rios, A.; Hickmann Flôres, S. Evaluation of the use of industrial wastes on the encapsulation of betalains extracted from red pitaya pulp (Hylocereus polyrhizus) by spray drying: Powder stability and application. Food Bioprocess Technol. 2020, 13, 1940–1953. [Google Scholar] [CrossRef]
- Fernández-Repetto, A.; Gómez-Maqueo, A.; García-Cayuela, T.; Guajardo-Flores, D.; Cano, M.P. Analysis of hydrocolloid excipients for controlled delivery of high-value microencapsulated prickly pear extracts. Food Hydrocoll. Health 2023, 3, 100115. [Google Scholar] [CrossRef]
- Hu, H.; Yao, X.; Qin, Y.; Yong, H.; Liu, J. Development of multifunctional food packaging by incorporating betalains from vegetable amaranth (Amaranthus tricolor L.) into quaternary ammonium chitosan/fish gelatin blend films. Int. J. Biol. Macromol. 2020, 159, 675–684. [Google Scholar] [CrossRef]
- Yao, X.; Hu, H.; Qin, Y.; YLiu, J. Development of antioxidant, antimicrobial and ammonia-sensitive films based on quaternary ammonium chitosan, polyvinyl alcohol and betalains-rich cactus pears (Opuntia ficusindica) extract. Food Hydrocoll. 2020, 106, 105896. [Google Scholar] [CrossRef]
- Benbettaïeb, N.; Assifaoui, A.; Karbowiak, T.; Debeaufort, F.; Chambin, O. Controlled release of tyrosol and ferulic acid encapsulated in chitosan–gelatin films after electron beam irradiation. Radiat. Phys. Chem. 2016, 118, 81–86. [Google Scholar] [CrossRef]
- Kurek, M.; Benbettaieb, N.; Ščetar, M.; Chaudy, E.; Elez-Garofulić, I.; Repajić, M.; Galić, K. Novel functional chitosan and pectin bio-based packaging films with encapsulated Opuntia-ficus indica waste. Food Biosci. 2021, 41, 100980. [Google Scholar] [CrossRef]
- Fernández Hernández, E.; Sandoval-Castilla, O.; Cuevas-Bernardino, J.C.; Pacheco López, N.A. Caracterización de películas bioactivas elaboradas a partir de miel y quitosano. In Factores de la Producción Agrícola; Perez-Soto, F., Figueroa. Hernández, E., Escamilla Garcia, P.E., Garcia-Núñez, R.M., Godínez-Montoya, L., Eds.; Amilla: México City, Mexico, 2022; pp. 9–21. [Google Scholar]
- Jedlińska, A.; Barańska, A.; Witrowa-Rajchert, D.; Ostrowska-Ligęza, E.; Samborska, K. Dehumidified air-assisted spray-drying of cloudy beetroot juice at low temperature. Appl. Sci. 2021, 11, 6578. [Google Scholar] [CrossRef]
- Sadiq, N.M.; Aziz, S.B.; Kadir, M.F. Development of flexible plasticized ion-conducting polymer blend electrolytes based on polyvinyl alcohol (PVA): Chitosan (CS) with high ion transport parameters close to gel-based electrolytes. Gels 2022, 8, 153. [Google Scholar] [CrossRef] [PubMed]
- Chaux-Gutiérrez, A.M. Avaliação da Liofilização Para Produção de Criogéis Como Material de Parede e Sua Aplicação em Encapsulação de Betalaínas. Universidad Estadual Paulista Julio de Mesquita Filho. São José do Rio Preto. 2019. Available online: https://repositorio.unesp.br/server/api/core/bitstreams/41736a2a-dafe-4022-95be-89ce2a41cbc4/content (accessed on 18 March 2023).
- Aslam, M.; Raza, Z.A.; Siddique, A. Fabrication and chemo-physical characterization of CuO/chitosan nanocomposite-mediated tricomponent PVA films. Polym. Bull. 2021, 78, 1955–1965. [Google Scholar] [CrossRef]
- Chopra, H.; Bibi, S.; Kumar, S.; Khan, M.S.; Kumar, P.; Singh, I. Preparation and evaluation of chitosan/PVA based hydrogel films loaded with honey for wound healing application. Gels 2022, 8, 111. [Google Scholar] [CrossRef]
- Qiao, C.; Ma, X.; Wang, X.; Liu, L. Structure and properties of chitosan films: Effect of the type of solvent acid. LWT 2021, 135, 109984. [Google Scholar] [CrossRef]
- Goncalves, L.C.; Lopes, N.B.; Augusto, F.A.; Pioli, R.M.; Machado, C.O.; Freitas-Dörr, B.C.; Suffredini, H.B.; Bastos, E.L. Phenolic betalain as antioxidants: Meta means more. Pure Appl. Chem. 2020, 92, 243–253. [Google Scholar] [CrossRef]
- Gómez-Espinoza, D.; Ríos-Fuentes, B.; Aguirre-Mancilla, C.L.; Villaseñor-Ortega, F.; Pérez-Pérez, M.C.I. Microencapsulation of Betalains Obtained from Garambullo Fruit (Myrtillocactus geometrizans) by Spray Drying Microencapsulación de Betalainas Obtenidas del Fruto de Garambullo (Myrtillocactus geometrizans) por Secado por Aspersión. Available online: https://rmiq.org/iqfvp/Numbers/V23/No2/Alim24247.pdf (accessed on 18 March 2023).
- Yan, D.; Li, Y.; Liu, Y.; Li, N.; Zhang, X.; Yan, C. Antimicrobial properties of chitosan and chitosan derivatives in the treatment of enteric infections. Molecules 2021, 26, 7136. [Google Scholar] [CrossRef]
- Sadowska-Bartosz, I.; Bartosz, G. Biological properties and applications of betalains. Molecules 2021, 26, 2520. [Google Scholar] [CrossRef]
- Vera AE, V.; Vera YA, L.; Mendoza SV, G.; Cedeño SD, R.V.; Chila, C.F.C. Calidad de pitahaya amarilla (Selenicereus megalanthus) en diferentes estados de madurez y temperaturas de conservación. Rev. Espamcienc. 2021, 12, 141–151. [Google Scholar] [CrossRef]
- Machalela, A.A.; Júnior AA, M.; Vatiro, A.; Nanelo, R.F. Production and Characterization of Tomato (Lycopersicon esculentum) Jam. Asian Food Sci. J. 2023, 22, 120–131. [Google Scholar] [CrossRef]
- Candéo, M.; Canteri MH, G.; Macedo DC, D.; Kubaski, E.T.; Tebcherani, S.M. Postharvest durability of tomatoes with PVA covering. Hortic. Bras. 2020, 38, 160–165. [Google Scholar] [CrossRef]
- Kaushal, H. Identifying Possible Quality Deviations and Establishing Critical Quality Points in Perishable Fruits and Vegetables with Bio-Based Packaging Materials; Food Quality and Design (FQD) Wageningen University & Research: Wageningen, The Netherlands, 2020. [Google Scholar]
- Sati, F.; Qubbaj, T. Effect of calcium chloride postharvest treatment in combination with plant natural substance coating on fruit quality and storability of tomato (Solanum lycopersicum) fruits during cold storage. J. Appl. Bot. Food Qual. 2021, 94, 100–107. [Google Scholar]
- Salmas, C.E.; Giannakas, A.E.; Moschovas, D.; Kollia, E.; Georgopoulos, S.; Gioti, C.; Leontiou, A.; Avgeropoulos, A.; Kopsacheili, A.; Avdylaj, L.; et al. Kiwi Fruits Preservation Using Novel Edible Active Coatings Based on Rich Thymol Halloysite Nanostructures and Chitosan/Polyvinyl Alcohol Gels. Gels 2022, 8, 823. [Google Scholar] [CrossRef] [PubMed]
- Salmas, C.E.; Leontiou, A.; Kollia, E.; Zaharioudakis, K.; Kopsacheili, A.; Avdylaj, L.; Georgopoulos, S.; Karabagias, V.K.; Karydis-Messinis, A.; Kehayias, G.; et al. Active Coatings Development Based on Chitosan/Polyvinyl Alcohol Polymeric Matrix Incorporated with Thymol Modified Activated Carbon Nanohybrids. Coatings 2023, 13, 1503. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).