Role of Oxidative Stress Signaling, Nrf2, on Survival and Stemness of Human Adipose-Derived Stem Cells Exposed to X-rays, Protons and Carbon Ions
Abstract
1. Introduction
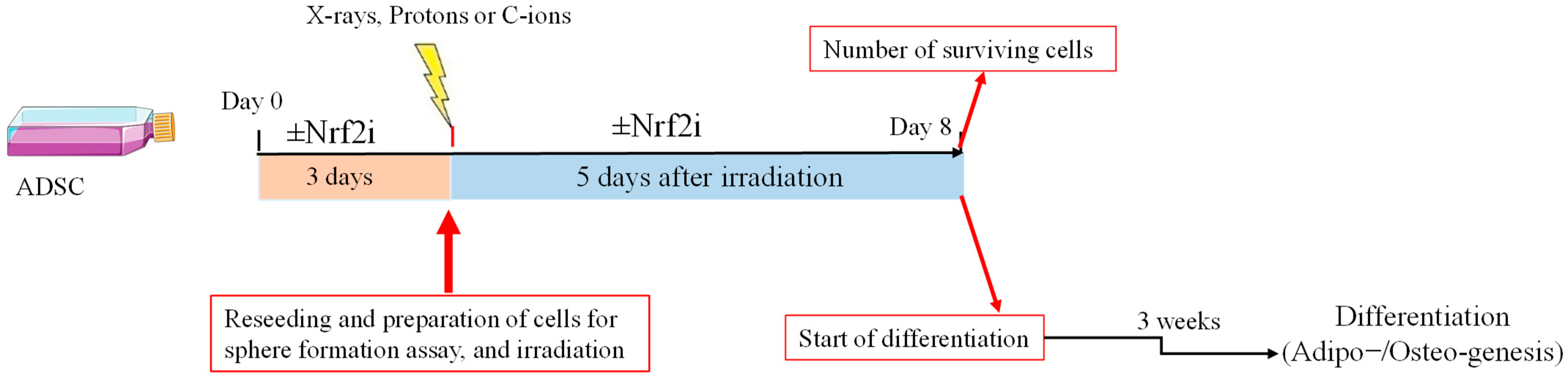
2. Materials and Methods
2.1. Cell Culture
2.2. Nrf2 Inhibitor
2.3. Cell Preparation
2.4. ADSC Differentiation
2.5. Irradiations
2.6. Preparation of Cells for Western Blotting (WB)
2.7. Statistics
3. Results
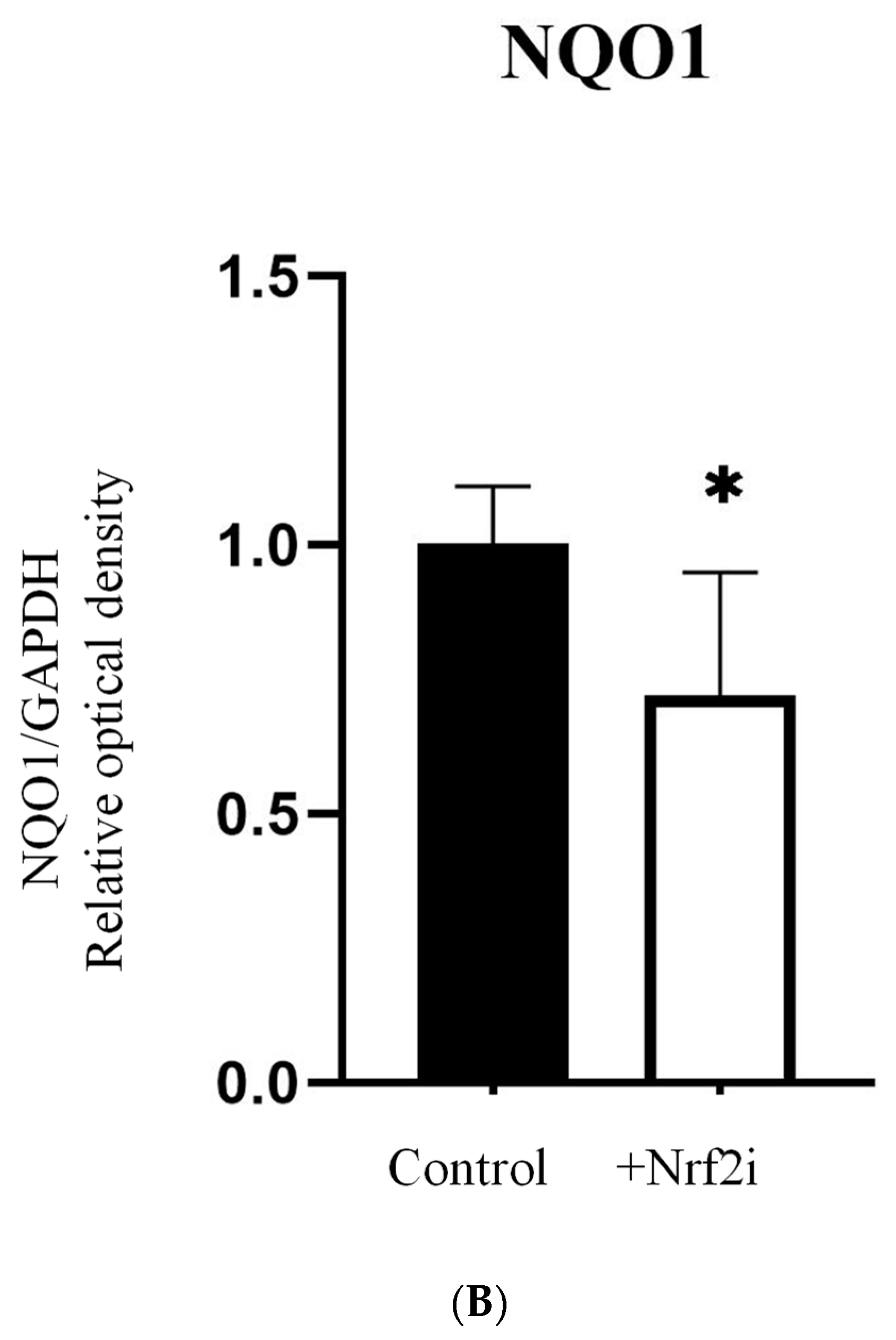
3.1. Efficiency of the Nrf2 Inhibitor
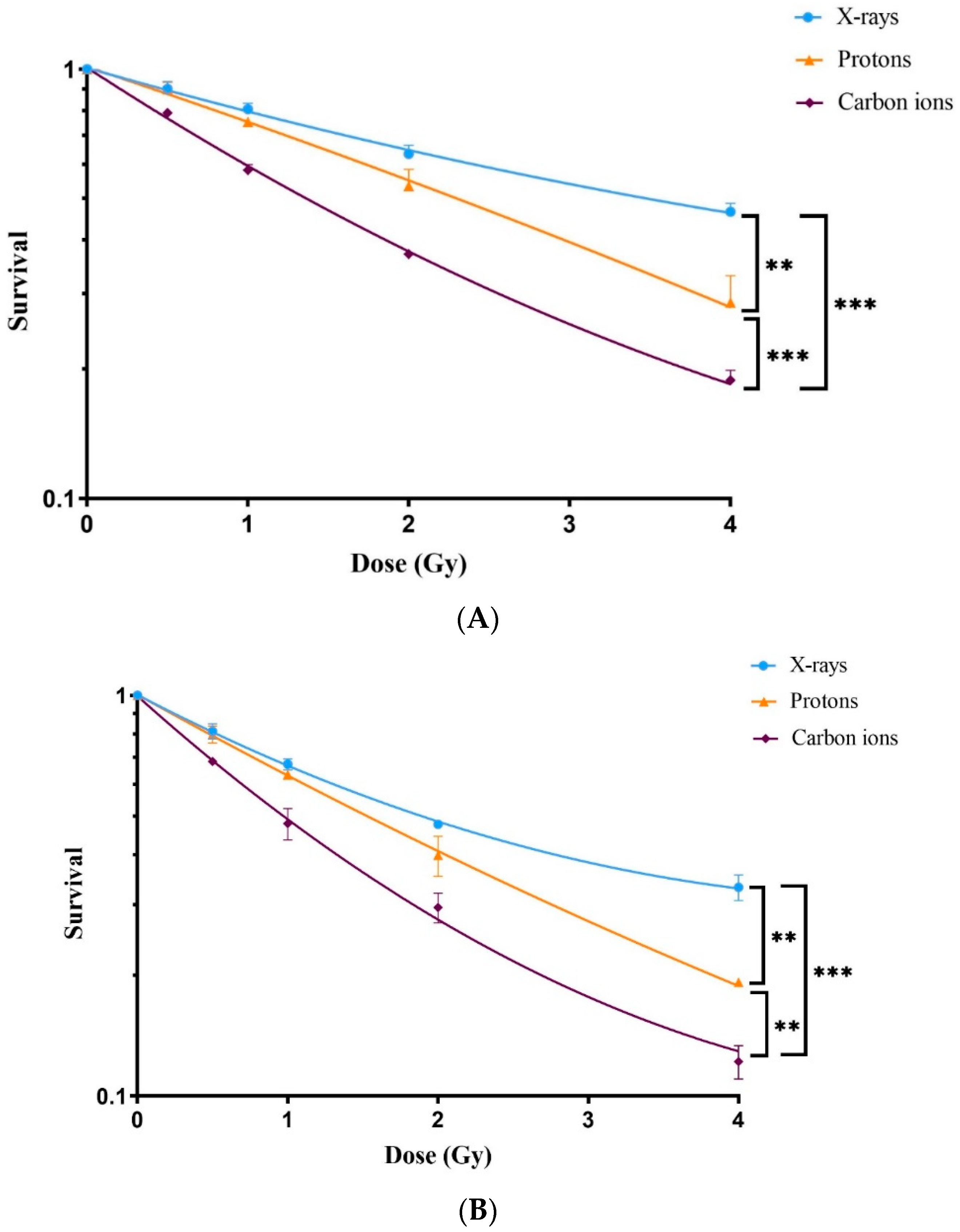
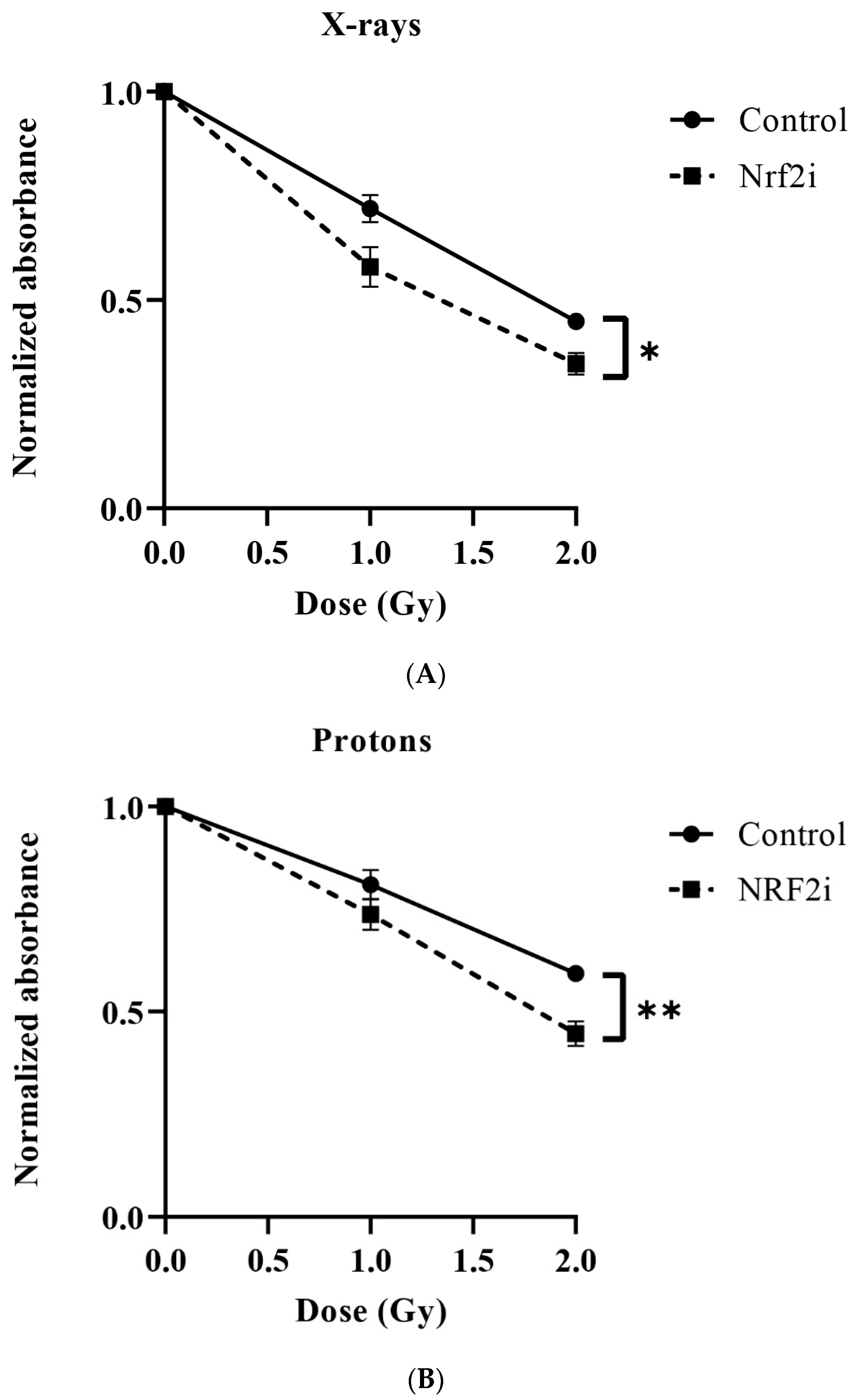
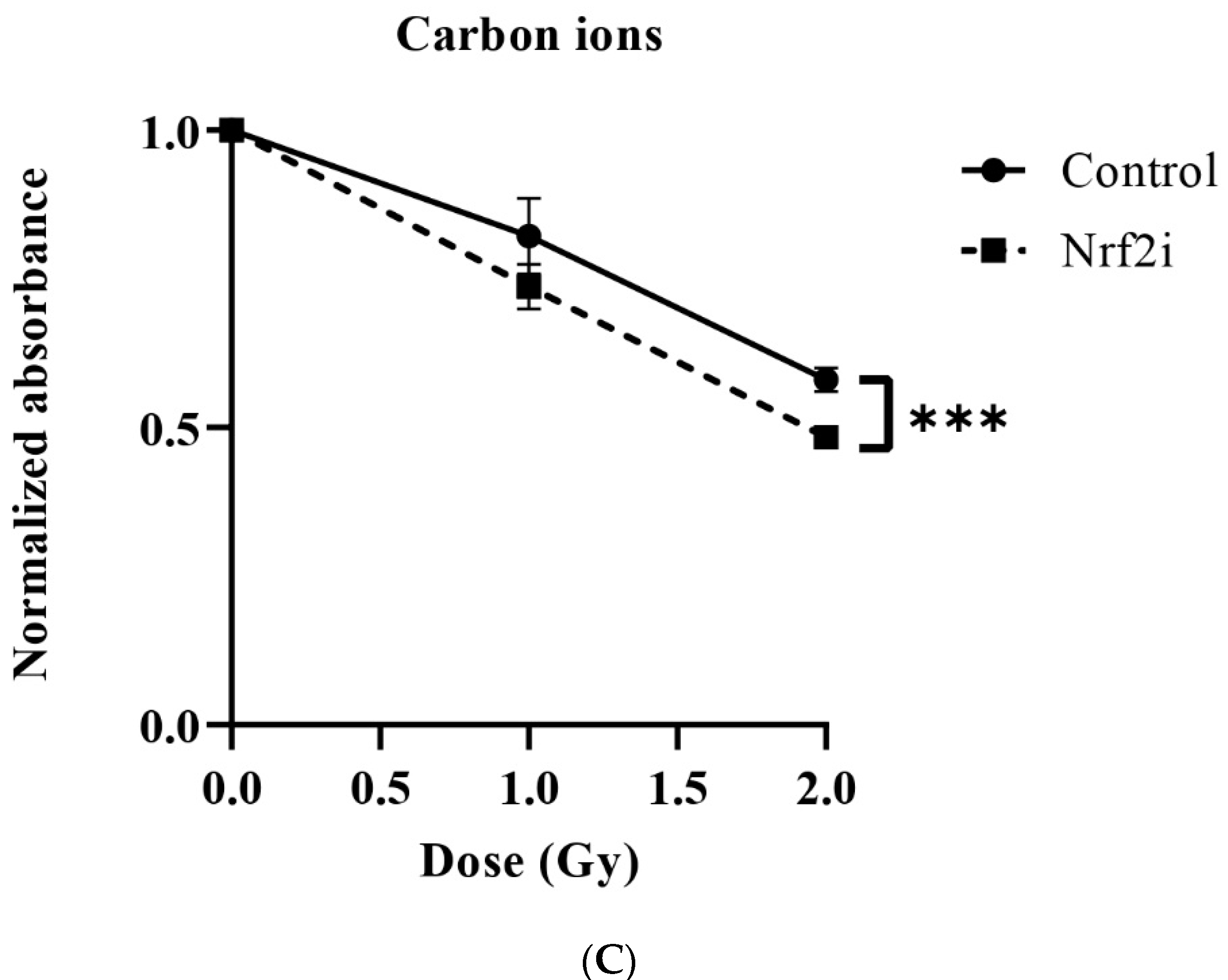
3.2. Dose–Response Relationship of Survival after Exposure to Radiation Quality
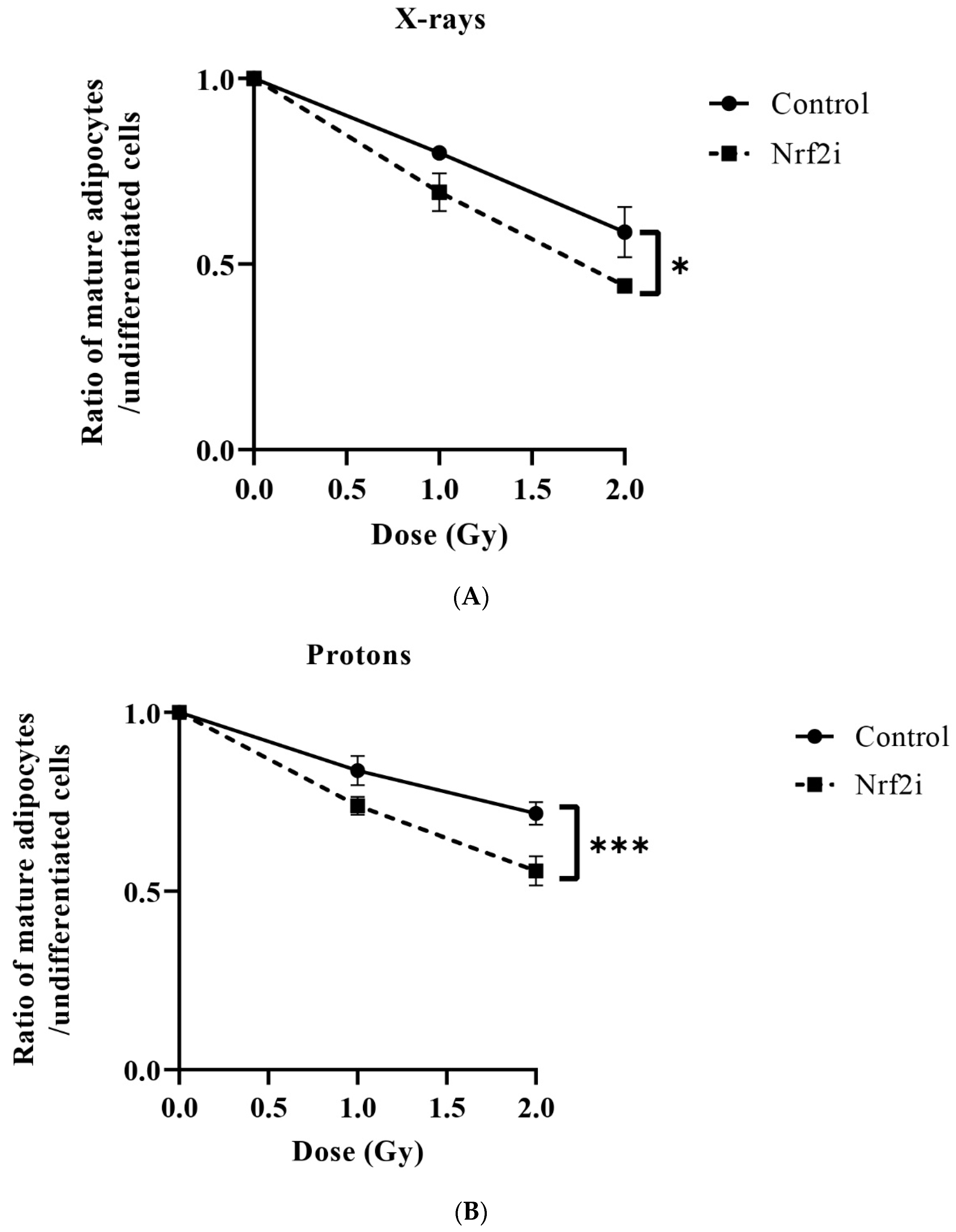
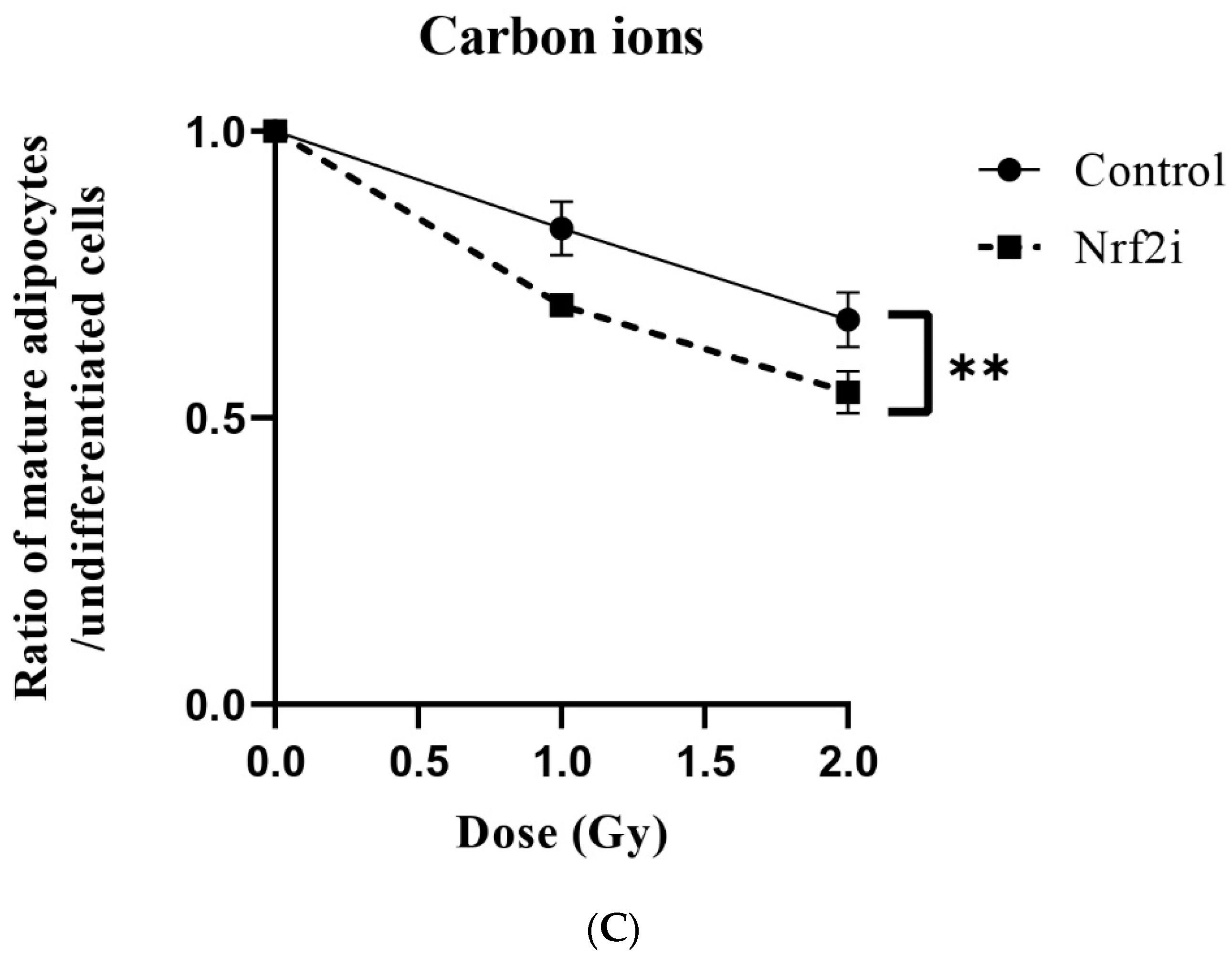
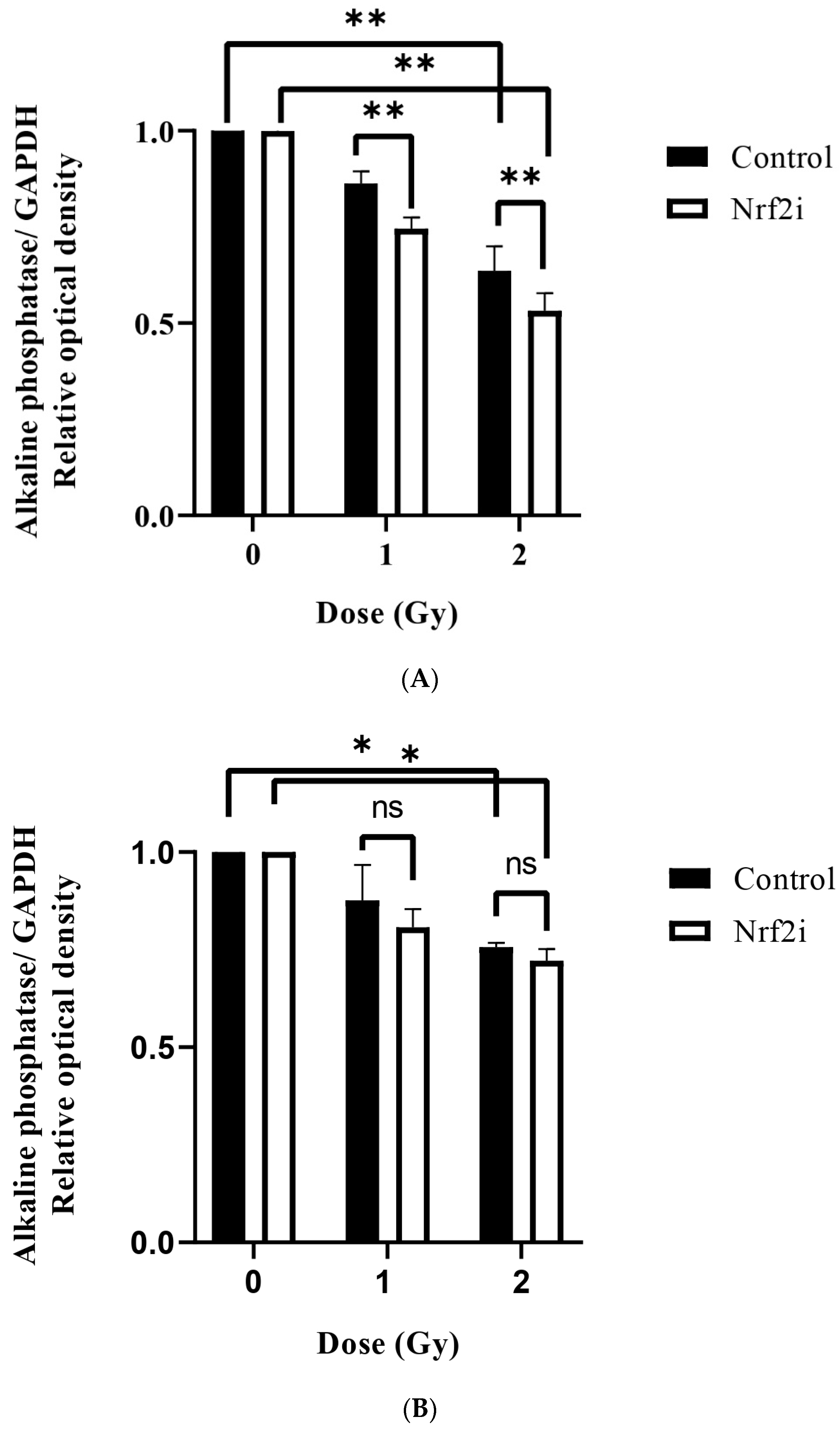
3.3. ADSC Differentiation after Radiation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bhattacharya, S.; Asaithamby, A. Repurposing DNA repair factors to eradicate tumor cells upon radiotherapy. Transl. Cancer Res. 2017, 6, S822–S839. [Google Scholar] [CrossRef] [PubMed]
- Vicar, T.; Gumulec, J.; Kolar, R.; Kopecna, O.; Pagacova, E.; Falkova, I.; Falk, M. DeepFoci: Deep learning-based algorithm for fast automatic analysis of DNA double-strand break ionizing radiation-induced foci. Comput. Struct. Biotechnol. J. 2021, 19, 6465–6480. [Google Scholar] [CrossRef] [PubMed]
- Reisz, J.A.; Bansal, N.; Qian, J.; Zhao, W.; Furdui, C.M. Effects of ionizing radiation on biological molecules—Mechanisms of damage and emerging methods of detection. Antioxid. Redox Signal. 2014, 21, 260–292. [Google Scholar] [CrossRef]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive oxygen species in inflammation and tissue injury. Antioxid. Redox Signal. 2014, 20, 1126–1167. [Google Scholar] [CrossRef]
- Kaur, P.; Sandhu, K.S.; Bangar, S.P.; Purewal, S.S.; Kaur, M.; Ilyas, R.A.; Asyraf, M.R.M.; Razman, M.R. Unraveling the Bioactive Profile, Antioxidant and DNA Damage Protection Potential of Rye (Secale cereale) Flour. Antioxidants 2021, 10, 1214. [Google Scholar] [CrossRef]
- Kasai, S.; Shimizu, S.; Tatara, Y.; Mimura, J.; Itoh, K. Regulation of Nrf2 by Mitochondrial Reactive Oxygen Species in Physiology and Pathology. Biomolecules 2020, 10, 320. [Google Scholar] [CrossRef]
- Godoy, P.; Pour Khavari, A.; Rizzo, M.; Sakamoto-Hojo, E.T.; Haghdoost, S. Targeting NRF2, Regulator of Antioxidant System, to Sensitize Glioblastoma Neurosphere Cells to Radiation-Induced Oxidative Stress. Oxidative Med. Cell. Longev. 2020, 2020, 2534643. [Google Scholar] [CrossRef] [PubMed]
- Baird, L.; Yamamoto, M. The Molecular Mechanisms Regulating the KEAP1-NRF2 Pathway. Mol. Cell. Biol. 2020, 40, e00099-20. [Google Scholar] [CrossRef]
- Deshmukh, P.; Unni, S.; Krishnappa, G.; Padmanabhan, B. The Keap1-Nrf2 pathway: Promising therapeutic target to counteract ROS-mediated damage in cancers and neurodegenerative diseases. Biophys. Rev. 2017, 9, 41–56. [Google Scholar] [CrossRef]
- Jayakumar, S.; Pal, D.; Sandur, S.K. Nrf2 facilitates repair of radiation induced DNA damage through homologous recombination repair pathway in a ROS independent manner in cancer cells. Mutat. Res. 2015, 779, 33–45. [Google Scholar] [CrossRef]
- Hammad, M.; Raftari, M.; Cesario, R.; Salma, R.; Godoy, P.; Emami, S.N.; Haghdoost, S. Roles of Oxidative Stress and Nrf2 Signaling in Pathogenic and Non-Pathogenic Cells: A Possible General Mechanism of Resistance to Therapy. Antioxidants 2023, 12, 1371. [Google Scholar] [CrossRef]
- Wei, Y.; Zhang, J.; Yan, X.; Peng, X.; Xu, S.; Chang, H.; Wang, H.; Gao, Y. Remarkable Protective Effects of Nrf2-Mediated Antioxidant Enzymes and Tissue Specificity in Different Skeletal Muscles of Daurian Ground Squirrels over the Torpor-Arousal Cycle. Front. Physiol. 2019, 10, 1449. [Google Scholar] [CrossRef]
- Miura, S.; Yamaguchi, M.; Yoshino, H.; Nakai, Y.; Kashiwakura, I. Dose-Dependent Increase of Nrf2 Target Gene Expression in Mice Exposed to Ionizing Radiation. Radiat. Res. 2019, 191, 176–188. [Google Scholar] [CrossRef] [PubMed]
- Kato, K.; Takahashi, K.; Monzen, S.; Yamamoto, H.; Maruyama, A.; Itoh, K.; Kashiwakura, I. Relationship between radiosensitivity and Nrf2 target gene expression in human hematopoietic stem cells. Radiat. Res. 2010, 174, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Lister, A.; Nedjadi, T.; Kitteringham, N.R.; Campbell, F.; Costello, E.; Lloyd, B.; Copple, I.M.; Williams, S.; Owen, A.; Neoptolemos, J.P.; et al. Nrf2 is overexpressed in pancreatic cancer: Implications for cell proliferation and therapy. Mol. Cancer 2011, 10, 37. [Google Scholar] [CrossRef] [PubMed]
- Durante, M.; Loeffler, J.S. Charged particles in radiation oncology. Nat. Rev. Clin. Oncol. 2010, 7, 37–43. [Google Scholar] [CrossRef]
- Spiotto, M.T.; McGovern, S.L.; Gunn, G.B.; Grosshans, D.; McAleer, M.F.; Frank, S.J.; Paulino, A.C. Proton Radiotherapy to Reduce Late Complications in Childhood Head and Neck Cancers. Int. J. Part. Ther. 2021, 8, 155–167. [Google Scholar] [CrossRef]
- Mizumoto, M.; Fuji, H.; Miyachi, M.; Soejima, T.; Yamamoto, T.; Aibe, N.; Demizu, Y.; Iwata, H.; Hashimoto, T.; Motegi, A.; et al. Proton beam therapy for children and adolescents and young adults (AYAs): JASTRO and JSPHO Guidelines. Cancer Treat. Rev. 2021, 98, 102209. [Google Scholar] [CrossRef]
- Mohamad, O.; Imai, R.; Kamada, T.; Nitta, Y.; Araki, N.; the Working Group for Bone and Soft Tissue Sarcoma. Carbon ion radiotherapy for inoperable pediatric osteosarcoma. Oncotarget 2018, 9, 22976–22985. [Google Scholar] [CrossRef]
- Hu, J.; Hu, W.; Gao, J.; Yang, J.; Qiu, X.; Huang, Q.; Kong, L.; Lu, J.J. The role of carbon-ion radiotherapy in the treatment of adenoid cystic carcinoma of the nasopharynx. Ann. Transl. Med. 2022, 10, 1198. [Google Scholar] [CrossRef]
- Fabbrizi, M.R.; Parsons, J.L. Radiotherapy and the cellular DNA damage response: Current and future perspectives on head and neck cancer treatment. Cancer Drug Resist. 2020, 3, 775–790. [Google Scholar] [CrossRef] [PubMed]
- Nickoloff, J.A.; Sharma, N.; Taylor, L. Clustered DNA Double-Strand Breaks: Biological Effects and Relevance to Cancer Radiotherapy. Genes 2020, 11, 99. [Google Scholar] [CrossRef] [PubMed]
- Nakano, T.; Akamatsu, K.; Tsuda, M.; Tujimoto, A.; Hirayama, R.; Hiromoto, T.; Tamada, T.; Ide, H.; Shikazono, N. Formation of clustered DNA damage in vivo upon irradiation with ionizing radiation: Visualization and analysis with atomic force microscopy. Proc. Natl. Acad. Sci. USA 2022, 119, e2119132119. [Google Scholar] [CrossRef] [PubMed]
- Ezashi, T.; Das, P.; Roberts, R.M. Low O2 tensions and the prevention of differentiation of hES cells. Proc. Natl. Acad. Sci. USA 2005, 102, 4783–4788. [Google Scholar] [CrossRef]
- Juntilla, M.M.; Patil, V.D.; Calamito, M.; Joshi, R.P.; Birnbaum, M.J.; Koretzky, G.A. AKT1 and AKT2 maintain hematopoietic stem cell function by regulating reactive oxygen species. Blood 2010, 115, 4030–4038. [Google Scholar] [CrossRef]
- Owusu-Ansah, E.; Banerjee, U. Reactive oxygen species prime Drosophila haematopoietic progenitors for differentiation. Nature 2009, 461, 537–541. [Google Scholar] [CrossRef]
- Sauer, H.; Wartenberg, M. Reactive oxygen species as signaling molecules in cardiovascular differentiation of embryonic stem cells and tumor-induced angiogenesis. Antioxid. Redox Signal. 2005, 7, 1423–1434. [Google Scholar] [CrossRef]
- Chen, X.; Vega, V.B.; Ng, H.H. Transcriptional regulatory networks in embryonic stem cells. Cold Spring Harb. Symp. Quant. Biol. 2008, 73, 203–209. [Google Scholar] [CrossRef][Green Version]
- Chen, X.; Xu, H.; Yuan, P.; Fang, F.; Huss, M.; Vega, V.B.; Wong, E.; Orlov, Y.L.; Zhang, W.; Jiang, J.; et al. Integration of external signaling pathways with the core transcriptional network in embryonic stem cells. Cell 2008, 133, 1106–1117. [Google Scholar] [CrossRef]
- Gan, B.; Sahin, E.; Jiang, S.; Sanchez-Aguilera, A.; Scott, K.L.; Chin, L.; Williams, D.A.; Kwiatkowski, D.J.; DePinho, R.A. mTORC1-dependent and -independent regulation of stem cell renewal, differentiation, and mobilization. Proc. Natl. Acad. Sci. USA 2008, 105, 19384–19389. [Google Scholar] [CrossRef]
- Miyamoto, K.; Araki, K.Y.; Naka, K.; Arai, F.; Takubo, K.; Yamazaki, S.; Matsuoka, S.; Miyamoto, T.; Ito, K.; Ohmura, M.; et al. Foxo3a is essential for maintenance of the hematopoietic stem cell pool. Cell Stem Cell 2007, 1, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Tothova, Z.; Kollipara, R.; Huntly, B.J.; Lee, B.H.; Castrillon, D.H.; Cullen, D.E.; McDowell, E.P.; Lazo-Kallanian, S.; Williams, I.R.; Sears, C.; et al. FoxOs are critical mediators of hematopoietic stem cell resistance to physiologic oxidative stress. Cell 2007, 128, 325–339. [Google Scholar] [CrossRef] [PubMed]
- Baasse, A.; Machoy, F.; Juerss, D.; Baake, J.; Stang, F.; Reimer, T.; Krapohl, B.D.; Hildebrandt, G. Radiation Sensitivity of Adipose-Derived Stem Cells Isolated from Breast Tissue. Int. J. Mol. Sci. 2018, 19, 1988. [Google Scholar] [CrossRef] [PubMed]
- Michaelidesova, A.; Vachelova, J.; Cupal, L.; Konirova, J.; Brabcova, K.P.; Bacakova, L.; Vandrovcova, M.; Davidkova, M. Comparison of the Radiation Sensitivity of the Breast Cancer Cell Line Mcf7 and Human Adipose-Derived Stem Cells. Radiat. Prot. Dosim. 2019, 186, 155–158. [Google Scholar] [CrossRef]
- Sangsuwan, T.; Pour Khavari, A.; Blomberg, E.; Romell, T.; Godoy, P.; Harms-Ringdahl, M.; Haghdoost, S. Oxidative Stress Levels and DNA Repair Kinetics in Senescent Primary Human Fibroblasts Exposed to Chronic Low Dose Rate of Ionizing Radiation. Front. Biosci. (Landmark Ed) 2023, 28, 296. [Google Scholar] [CrossRef]
- Aldridge, A.; Kouroupis, D.; Churchman, S.; English, A.; Ingham, E.; Jones, E. Assay validation for the assessment of adipogenesis of multipotential stromal cells—A direct comparison of four different methods. Cytotherapy 2013, 15, 89–101. [Google Scholar] [CrossRef]
- McMahon, S.J. The linear quadratic model: Usage, interpretation and challenges. Phys. Med. Biol. 2018, 64, 01TR01. [Google Scholar] [CrossRef]
- Schwenke, K.; Peterson, H.P.; von Wangenheim, K.H.; Feinendegen, L.E. Radiation-enhanced differentiation of erythroid progenitor cells and its relation to reproductive cell death. Int. J. Radiat. Biol. 1996, 69, 309–317. [Google Scholar] [CrossRef]
- von Wangenheim, K.H.; Peterson, H.P.; Schwenke, K. Review: A major component of radiation action: Interference with intracellular control of differentiation. Int. J. Radiat. Biol. 1995, 68, 369–388. [Google Scholar] [CrossRef]
- Liang, D.; Ning, M.; Xie, H.; He, X.; Ren, P.; Lei, X.; Zhang, X. Radiotherapy Side Effects: Comprehensive Proteomic Study Unraveled Neural Stem Cell Degenerative Differentiation upon Ionizing Radiation. Biomolecules 2022, 12, 1759. [Google Scholar] [CrossRef]
- Berry, C.E.; Abbas, D.B.; Lintel, H.A.; Churukian, A.A.; Griffin, M.; Guo, J.L.; Cotterell, A.C.; Parker, J.B.L.; Downer, M.A.; Longaker, M.T.; et al. Adipose-Derived Stromal Cell-Based Therapies for Radiation-Induced Fibrosis. Adv. Wound Care 2024, 13, 235–252. [Google Scholar] [CrossRef] [PubMed]
- Goodhead, D.T. Energy deposition stochastics and track structure: What about the target? Radiat. Prot. Dosim. 2006, 122, 3–15. [Google Scholar] [CrossRef]
- Georgakilas, A.G.; O’Neill, P.; Stewart, R.D. Induction and repair of clustered DNA lesions: What do we know so far? Radiat. Res. 2013, 180, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Lopez Perez, R.; Nicolay, N.H.; Wolf, J.C.; Frister, M.; Schmezer, P.; Weber, K.J.; Huber, P.E. DNA damage response of clinical carbon ion versus photon radiation in human glioblastoma cells. Radiother. Oncol. 2019, 133, 77–86. [Google Scholar] [CrossRef]
- Chaudhary, P.; Marshall, T.I.; Perozziello, F.M.; Manti, L.; Currell, F.J.; Hanton, F.; McMahon, S.J.; Kavanagh, J.N.; Cirrone, G.A.; Romano, F.; et al. Relative biological effectiveness variation along monoenergetic and modulated Bragg peaks of a 62-MeV therapeutic proton beam: A preclinical assessment. Int. J. Radiat. Oncol. Biol. Phys. 2014, 90, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chen, X.; Li, G.; Han, X.; Gao, T.; Liu, W.; Tang, X. Application of Carbon Ion and Its Sensitizing Agent in Cancer Therapy: A Systematic Review. Front. Oncol. 2021, 11, 708724. [Google Scholar] [CrossRef]
- Britten, R.A.; Nazaryan, V.; Davis, L.K.; Klein, S.B.; Nichiporov, D.; Mendonca, M.S.; Wolanski, M.; Nie, X.; George, J.; Keppel, C. Variations in the RBE for cell killing along the depth-dose profile of a modulated proton therapy beam. Radiat. Res. 2013, 179, 21–28. [Google Scholar] [CrossRef]
- Schroder, A.; Kriesen, S.; Hildebrandt, G.; Manda, K. First Insights into the Effect of Low-Dose X-ray Irradiation in Adipose-Derived Stem Cells. Int. J. Mol. Sci. 2019, 20, 6075. [Google Scholar] [CrossRef]
- Legzdina, D.; Romanauska, A.; Nikulshin, S.; Kozlovska, T.; Berzins, U. Characterization of Senescence of Culture-expanded Human Adipose-derived Mesenchymal Stem Cells. Int. J. Stem Cells 2016, 9, 124–136. [Google Scholar] [CrossRef]
- Zheng, Y.; Huang, C.; Liu, F.; Lin, H.; Yang, X.; Zhang, Z. Comparison of the neuronal differentiation abilities of bone marrow-derived and adipose tissue-derived mesenchymal stem cells. Mol. Med. Rep. 2017, 16, 3877–3886. [Google Scholar] [CrossRef][Green Version]
- Esfandiary, E.; Valiani, A.; Hashemibeni, B.; Moradi, I.; Narimani, M. The evaluation of toxicity of carbon nanotubes on the human adipose-derived-stem cells in-vitro. Adv. Biomed. Res. 2014, 3, 40. [Google Scholar] [CrossRef]
- Karger, C.P.; Peschke, P. RBE and related modeling in carbon-ion therapy. Phys. Med. Biol. 2017, 63, 01TR02. [Google Scholar] [CrossRef] [PubMed]
- Willers, H.; Allen, A.; Grosshans, D.; McMahon, S.J.; von Neubeck, C.; Wiese, C.; Vikram, B. Toward A variable RBE for proton beam therapy. Radiother. Oncol. 2018, 128, 68–75. [Google Scholar] [CrossRef]
- Leduc, A.; Chaouni, S.; Pouzoulet, F.; De Marzi, L.; Megnin-Chanet, F.; Corre, E.; Stefan, D.; Habrand, J.L.; Sichel, F.; Laurent, C. Differential normal skin transcriptomic response in total body irradiated mice exposed to scattered versus scanned proton beams. Sci. Rep. 2021, 11, 5876. [Google Scholar] [CrossRef]
- Chandna, S.; Dagur, R.S.; Mathur, A.; Natarajan, A.T.; Harms-Ringdahl, M.; Haghdoost, S. Agarose overlay selectively improves macrocolony formation and radiosensitivity assessment in primary fibroblasts. Int. J. Radiat. Biol. 2014, 90, 401–406. [Google Scholar] [CrossRef]
- Singh, A.; Bodas, M.; Wakabayashi, N.; Bunz, F.; Biswal, S. Gain of Nrf2 function in non-small-cell lung cancer cells confers radioresistance. Antioxid. Redox Signal. 2010, 13, 1627–1637. [Google Scholar] [CrossRef]
- Sun, C.; Chu, A.; Song, R.; Liu, S.; Chai, T.; Wang, X.; Liu, Z. PARP inhibitors combined with radiotherapy: Are we ready? Front. Pharmacol. 2023, 14, 1234973. [Google Scholar] [CrossRef] [PubMed]
- Kishi, Y.; Fujihara, H.; Kawaguchi, K.; Yamada, H.; Nakayama, R.; Yamamoto, N.; Fujihara, Y.; Hamada, Y.; Satomura, K.; Masutani, M. PARP Inhibitor PJ34 Suppresses Osteogenic Differentiation in Mouse Mesenchymal Stem Cells by Modulating BMP-2 Signaling Pathway. Int. J. Mol. Sci. 2015, 16, 24820–24838. [Google Scholar] [CrossRef]
- McDonald, J.T.; Kim, K.; Norris, A.J.; Vlashi, E.; Phillips, T.M.; Lagadec, C.; Della Donna, L.; Ratikan, J.; Szelag, H.; Hlatky, L.; et al. Ionizing radiation activates the Nrf2 antioxidant response. Cancer Res. 2010, 70, 8886–8895. [Google Scholar] [CrossRef]
- Qin, S.; He, X.; Lin, H.; Schulte, B.A.; Zhao, M.; Tew, K.D.; Wang, G.Y. Nrf2 inhibition sensitizes breast cancer stem cells to ionizing radiation via suppressing DNA repair. Free Radic. Biol. Med. 2021, 169, 238–247. [Google Scholar] [CrossRef]
- Journy, N.; Bolle, S.; Brualla, L.; Dumas, A.; Fresneau, B.; Haddy, N.; Haghdoost, S.; Haustermans, K.; Jackson, A.; Karabegovic, S.; et al. Assessing late outcomes of advances in radiotherapy for paediatric cancers: Study protocol of the “HARMONIC-RT” European registry (NCT 04746729). Radiother. Oncol. 2024, 190, 109972. [Google Scholar] [CrossRef] [PubMed]
- Andreassi, M.G.; Haddy, N.; Harms-Ringdahl, M.; Campolo, J.; Borghini, A.; Chevalier, F.; Schwenk, J.M.; Fresneau, B.; Bolle, S.; Fuentes, M.; et al. A Longitudinal Study of Individual Radiation Responses in Pediatric Patients Treated with Proton and Photon Radiotherapy, and Interventional Cardiology: Rationale and Research Protocol of the HARMONIC Project. Int. J. Mol. Sci. 2023, 24, 8416. [Google Scholar] [CrossRef]
- Kurpinski, K.; Jang, D.J.; Bhattacharya, S.; Rydberg, B.; Chu, J.; So, J.; Wyrobek, A.; Li, S.; Wang, D. Differential effects of X-rays and high-energy 56Fe ions on human mesenchymal stem cells. Int. J. Radiat. Oncol. Biol. Phys. 2009, 73, 869–877. [Google Scholar] [CrossRef] [PubMed]
- Arihara, Y.; Takada, K.; Kamihara, Y.; Hayasaka, N.; Nakamura, H.; Murase, K.; Ikeda, H.; Iyama, S.; Sato, T.; Miyanishi, K.; et al. Small molecule CP-31398 induces reactive oxygen species-dependent apoptosis in human multiple myeloma. Oncotarget 2017, 8, 65889–65899. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, H.; Takada, K.; Arihara, Y.; Hayasaka, N.; Murase, K.; Iyama, S.; Kobune, M.; Miyanishi, K.; Kato, J. Six-transmembrane epithelial antigen of the prostate 1 protects against increased oxidative stress via a nuclear erythroid 2-related factor pathway in colorectal cancer. Cancer Gene Ther. 2019, 26, 313–322. [Google Scholar] [CrossRef] [PubMed]
- DeAtley, S.M.; Aksenov, M.Y.; Aksenova, M.V.; Harris, B.; Hadley, R.; Cole Harper, P.; Carney, J.M.; Butterfield, D.A. Antioxidants protect against reactive oxygen species associated with adriamycin-treated cardiomyocytes. Cancer Lett. 1999, 136, 41–46. [Google Scholar] [CrossRef]
- Siomek, A.; Tujakowski, J.; Gackowski, D.; Rozalski, R.; Foksinski, M.; Dziaman, T.; Roszkowski, K.; Olinski, R. Severe oxidatively damaged DNA after cisplatin treatment of cancer patients. Int. J. Cancer. 2006, 119, 2228–2230. [Google Scholar] [CrossRef]
- Wozny, A.S.; Vares, G.; Alphonse, G.; Lauret, A.; Monini, C.; Magne, N.; Cuerq, C.; Fujimori, A.; Monboisse, J.C.; Beuve, M.; et al. ROS Production and Distribution: A New Paradigm to Explain the Differential Effects of X-ray and Carbon Ion Irradiation on Cancer Stem Cell Migration and Invasion. Cancers 2019, 11, 468. [Google Scholar] [CrossRef]
- Bekaert, L.; Valable, S.; Lechapt-Zalcman, E.; Ponte, K.; Collet, S.; Constans, J.M.; Levallet, G.; Bordji, K.; Petit, E.; Branger, P.; et al. [18F]-FMISO PET study of hypoxia in gliomas before surgery: Correlation with molecular markers of hypoxia and angiogenesis. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 1383–1392. [Google Scholar] [CrossRef]
- Sheppard, A.J.; Barfield, A.M.; Barton, S.; Dong, Y. Understanding Reactive Oxygen Species in Bone Regeneration: A Glance at Potential Therapeutics and Bioengineering Applications. Front. Bioeng. Biotechnol. 2022, 10, 836764. [Google Scholar] [CrossRef]
- Kanda, Y.; Hinata, T.; Kang, S.W.; Watanabe, Y. Reactive oxygen species mediate adipocyte differentiation in mesenchymal stem cells. Life Sci. 2011, 89, 250–258. [Google Scholar] [CrossRef]
- Kohan, K.; Carvajal, R.; Gabler, F.; Vantman, D.; Romero, C.; Vega, M. Role of the transcriptional factors FOXO1 and PPARG on gene expression of SLC2A4 in endometrial tissue from women with polycystic ovary syndrome. Reproduction 2010, 140, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Li, X.; Jia, H.; Wang, H.; Shui, G.; Qin, Y.; Shu, X.; Wang, Y.; Dong, J.; Liu, G.; et al. Nuclear Factor E2-Related Factor 2 Mediates Oxidative Stress-Induced Lipid Accumulation in Adipocytes by Increasing Adipogenesis and Decreasing Lipolysis. Antioxid. Redox Signal. 2020, 32, 173–192. [Google Scholar] [CrossRef] [PubMed]
- Ito, K.; Hirao, A.; Arai, F.; Matsuoka, S.; Takubo, K.; Hamaguchi, I.; Nomiyama, K.; Hosokawa, K.; Sakurada, K.; Nakagata, N.; et al. Regulation of oxidative stress by ATM is required for self-renewal of haematopoietic stem cells. Nature 2004, 431, 997–1002. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Shao, L.; Spitz, D.R. Reactive oxygen species in normal and tumor stem cells. Adv. Cancer Res. 2014, 122, 1–67. [Google Scholar] [CrossRef]
- Yuan, Z.; Zhang, J.; Huang, Y.; Zhang, Y.; Liu, W.; Wang, G.; Zhang, Q.; Wang, G.; Yang, Y.; Li, H.; et al. NRF2 overexpression in mesenchymal stem cells induces stem-cell marker expression and enhances osteoblastic differentiation. Biochem. Biophys. Res. Commun. 2017, 491, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Pakvasa, M.; Alverdy, A.; Mostafa, S.; Wang, E.; Fu, L.; Li, A.; Oliveira, L.; Athiviraham, A.; Lee, M.J.; Wolf, J.M.; et al. Neural EGF-like protein 1 (NELL-1): Signaling crosstalk in mesenchymal stem cells and applications in regenerative medicine. Genes Dis. 2017, 4, 127–137. [Google Scholar] [CrossRef]
- Ma, F.; Luo, S.; Lu, C.; Jiang, X.; Chen, K.; Deng, J.; Ma, S.; Li, Z. The role of Nrf2 in periodontal disease by regulating lipid peroxidation, inflammation and apoptosis. Front. Endocrinol. 2022, 13, 963451. [Google Scholar] [CrossRef]
- Kim, J.H.; Singhal, V.; Biswal, S.; Thimmulappa, R.K.; DiGirolamo, D.J. Nrf2 is required for normal postnatal bone acquisition in mice. Bone Res. 2014, 2, 14033. [Google Scholar] [CrossRef]
- Boorman, E.; Killick, R.; Aarsland, D.; Zunszain, P.; Mann, G.E. NRF2: An emerging role in neural stem cell regulation and neurogenesis. Free Radic. Biol. Med. 2022, 193, 437–446. [Google Scholar] [CrossRef]
- Nakamura, H.; Takada, K. Reactive oxygen species in cancer: Current findings and future directions. Cancer Sci. 2021, 112, 3945–3952. [Google Scholar] [CrossRef]


| Radiation Quality | LD50 | p Value | RBE | Sensitizing Effect (%) | |||
|---|---|---|---|---|---|---|---|
| −Nrf2i | +Nrf2i | −Nrf2i | +Nrf2i | −Nrf2i | +Nrf2i | LD50 (+Nrf2i)/LD50 (−Nrf2i) | |
| X-rays | 3.5 ± 0.08 | 2.33 ± 0.13 | X-P < 0.003 ** | X-P 0.003 ** | 1 | 1 | 33 |
| Protons | 2.23 ± 0.27 | 1.60 ± 0.27 | P-C < 0.001 *** | P-C 0.003 ** | 1.57 ± 0.19 | 1.45 ± 0.1 | 28.25 |
| Carbon ions | 1.61 ± 0.05 | 1.24 ± 0.03 | X-C < 0.001 *** | X-C < 0.001 *** | 2.19 ± 0.12 | 1.88 ± 0.09 | 23 |
| Radiation Quality | DI50 | p Value | RBE | Sensitizing Effect (%) | ||
|---|---|---|---|---|---|---|
| −Nrf2i | +Nrf2i | −Nrf2i | +Nrf2i | DI50 (+Nrf2i)/DI50 (−Nrf2i) | ||
| X-rays | 2.69 ± 0.43 | 1.76 ± 0.08 | 0.022 ** | 1 | 1 | 34.57 |
| Protons | 3.95 ± 0.08 | 2.42 ± 0.09 | 0.0018 *** | 0.68 ± 0.08 | 0.72 ± 0.06 | 38.74 |
| Carbon ions | 3.61 ± 0.24 | 2.23 ± 0.13 | 0.019 ** | 0.74 ± 0.11 | 0.78 ± 0.04 | 38.2 |
| Radiation Quality | DI50 | p Value | RBE | Sensitizing Effect (%) | ||
|---|---|---|---|---|---|---|
| −Nrf2i | +Nrf2i | −Nrf2i | +Nrf2i | DI50 (+Nrf2i)/DI50 (−Nrf2i) | ||
| X-rays | 1.79 ± 0.052 | 1.30 ± 0.11 | 0.002 ** | 1 | 1 | 27.37 |
| Protons | 2.76 ± 0.208 | 1.91 ± 0.19 | 0.002 ** | 0.65 ± 0.08 | 0.68 ± 0.06 | 30.72 |
| Carbon ions | 2.69 ± 0.057 | 1.96 ± 0.06 | 0.0001 *** | 0.66 ± 0.11 | 0.66 ± 0.04 | 27.13 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hammad, M.; Salma, R.; Balosso, J.; Rezvani, M.; Haghdoost, S. Role of Oxidative Stress Signaling, Nrf2, on Survival and Stemness of Human Adipose-Derived Stem Cells Exposed to X-rays, Protons and Carbon Ions. Antioxidants 2024, 13, 1035. https://doi.org/10.3390/antiox13091035
Hammad M, Salma R, Balosso J, Rezvani M, Haghdoost S. Role of Oxidative Stress Signaling, Nrf2, on Survival and Stemness of Human Adipose-Derived Stem Cells Exposed to X-rays, Protons and Carbon Ions. Antioxidants. 2024; 13(9):1035. https://doi.org/10.3390/antiox13091035
Chicago/Turabian StyleHammad, Mira, Rima Salma, Jacques Balosso, Mohi Rezvani, and Siamak Haghdoost. 2024. "Role of Oxidative Stress Signaling, Nrf2, on Survival and Stemness of Human Adipose-Derived Stem Cells Exposed to X-rays, Protons and Carbon Ions" Antioxidants 13, no. 9: 1035. https://doi.org/10.3390/antiox13091035
APA StyleHammad, M., Salma, R., Balosso, J., Rezvani, M., & Haghdoost, S. (2024). Role of Oxidative Stress Signaling, Nrf2, on Survival and Stemness of Human Adipose-Derived Stem Cells Exposed to X-rays, Protons and Carbon Ions. Antioxidants, 13(9), 1035. https://doi.org/10.3390/antiox13091035






