Crude Blueberry Phenolic Extracts Improve Gut Barrier Integrity and Exert Anti-Inflammatory and Antimicrobial Activity in an In Vitro Weaning Stress Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Crude Phenolic Extraction of Blueberries
2.2. Total Monomeric Anthocyanin Content Determination
2.3. Characterization of Blueberry Phenolic Extract
2.4. Cell Culture and Treatments
2.5. Cytotoxicity Assay
2.6. Catalase Activity Assay
2.7. Paracellular Permeability Assay
2.8. RNA Extraction and Real-Time Polymerase Chain Reaction (RT-PCR) Analysis
2.9. Western Blot Analyses of Tight Junction Proteins
2.10. Minimum Inhibitory Concentration Assay
2.11. Statistical Analysis
3. Results
3.1. Determination of BPE Phenolic Compounds
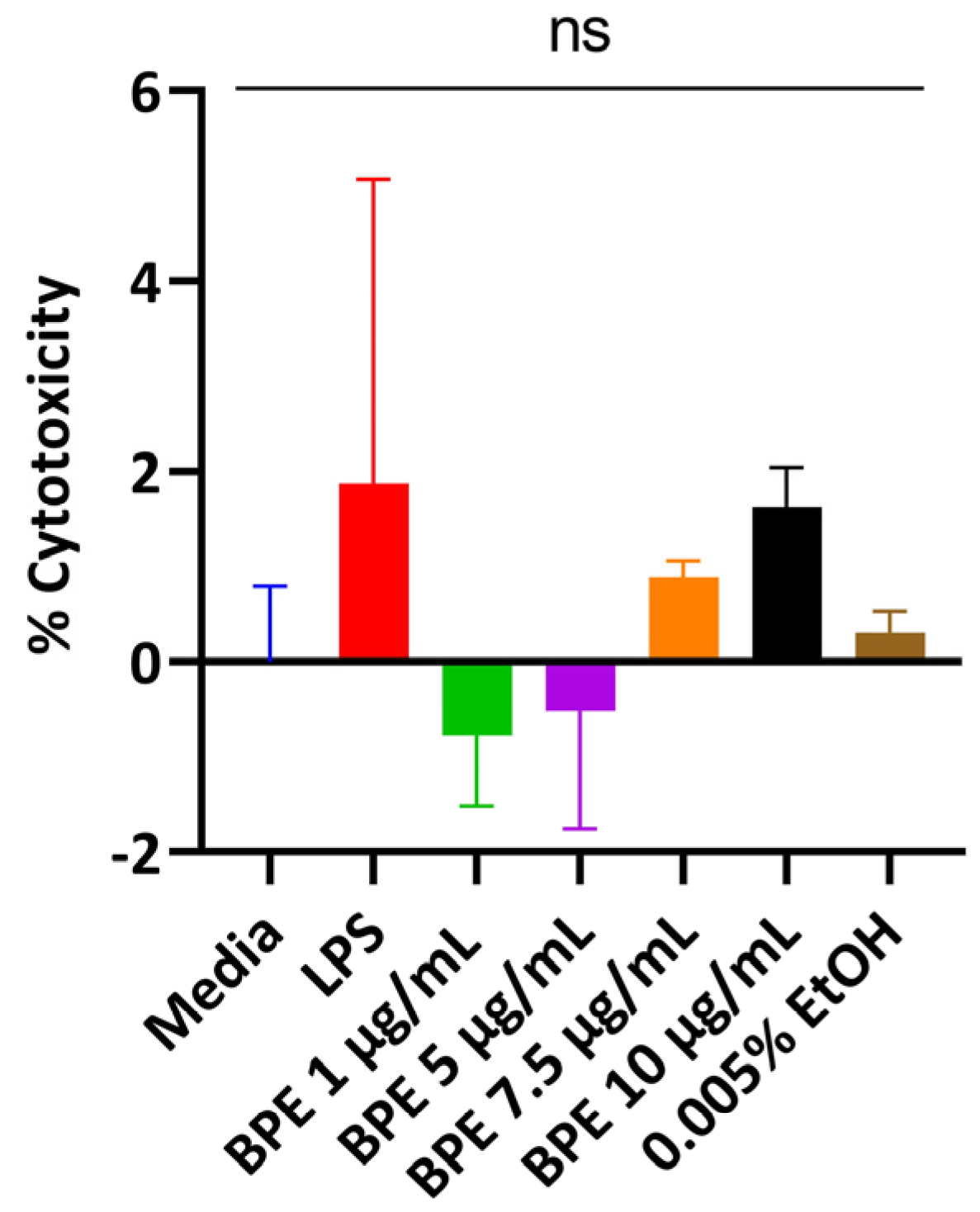
3.2. BPE Is Non-Cytotoxic to IPEC-J2 Cells at Low Concentrations
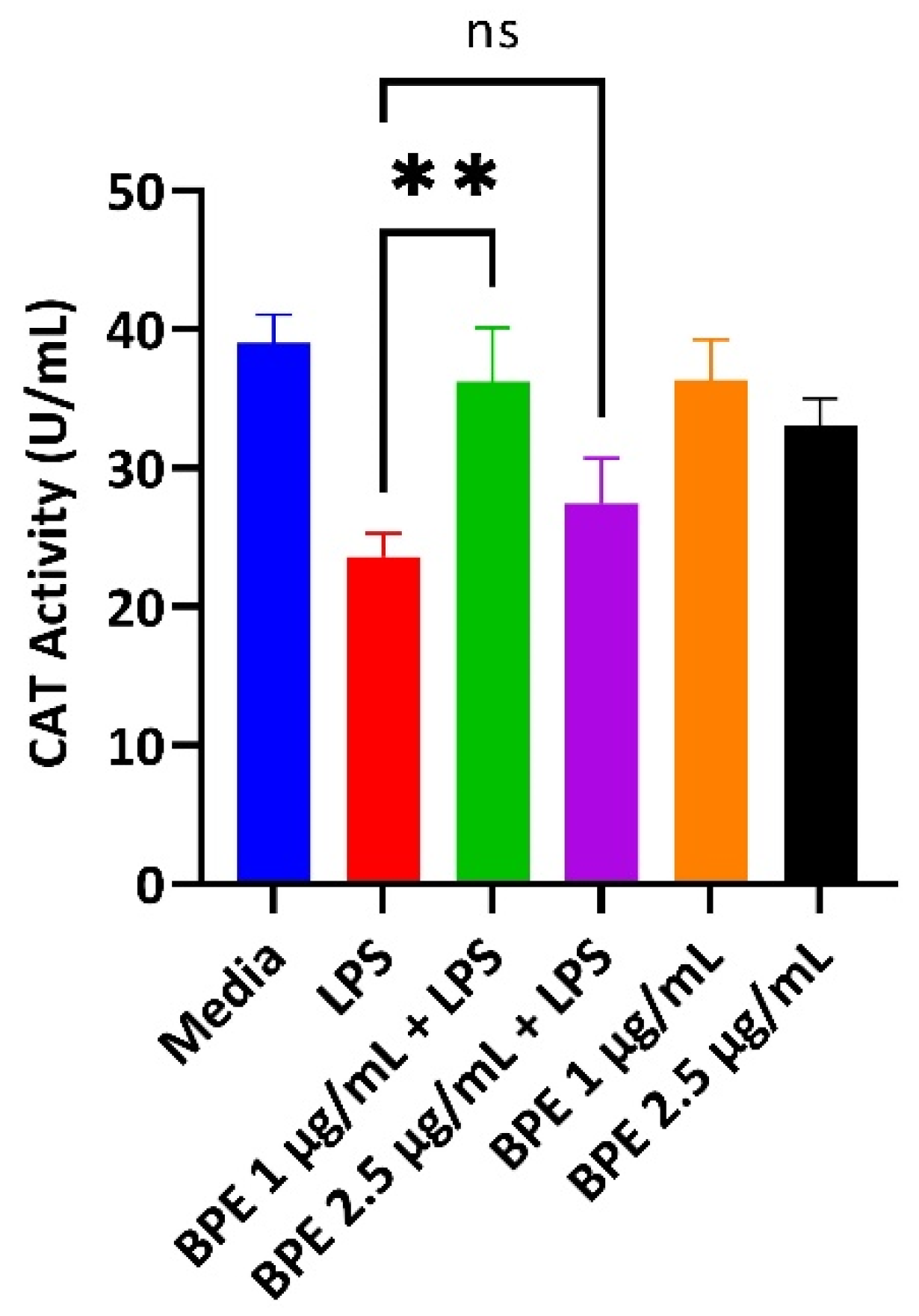
3.3. BPE Pretreatment Conserves Antioxidant Status after LPS Challenge at Low Concentrations
3.4. BPE Pretreatment Reduces Paracellular Permeability after LPS Challenge
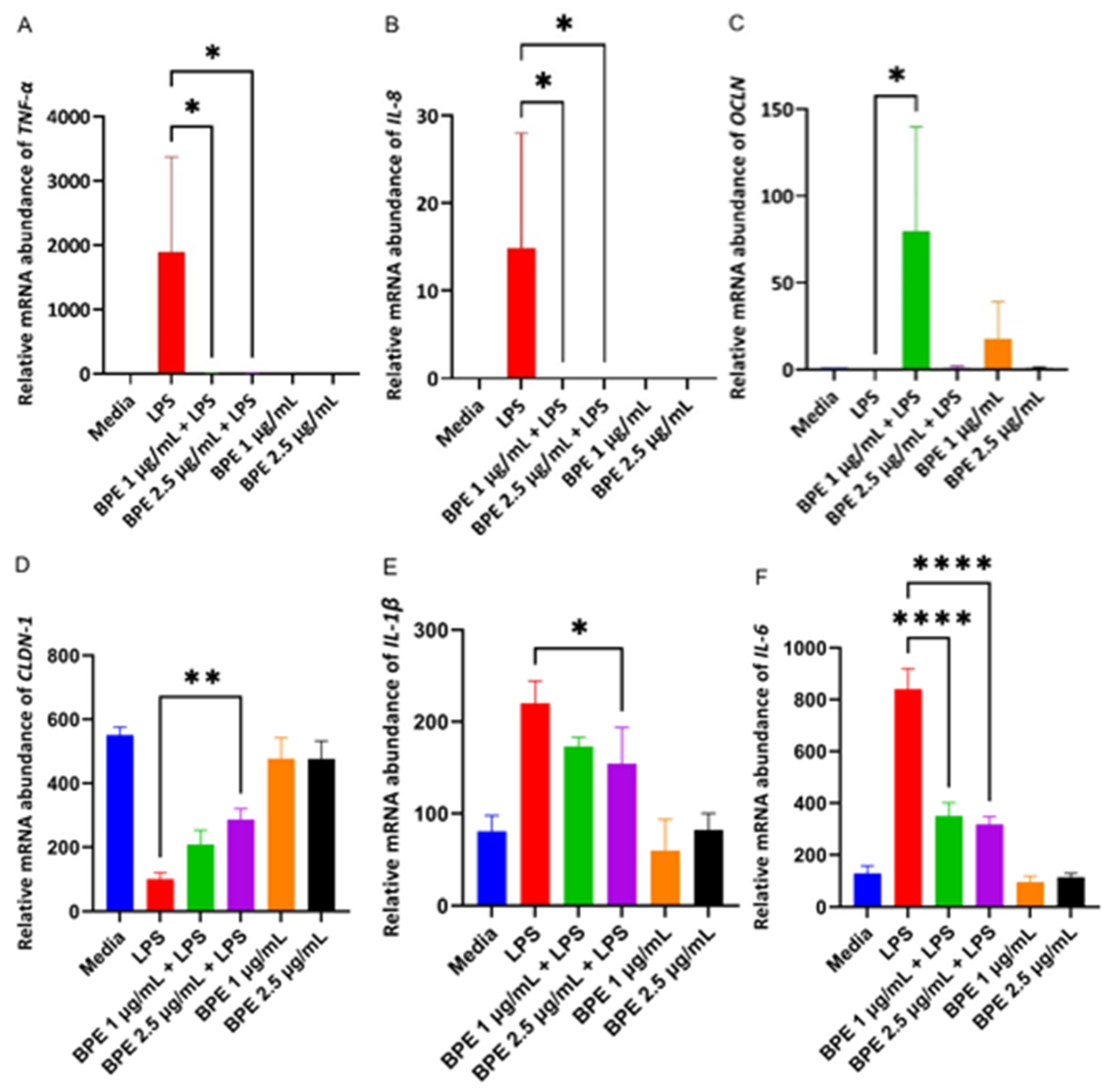
3.5. BPE Pretreatment Decreases Expression of Inflammatory Markers and Upregulates Expression of Tight Junction Proteins
3.6. Pretreatment with BPE Is Able to Preserve the Expression of Tight Junction Protein OCLN
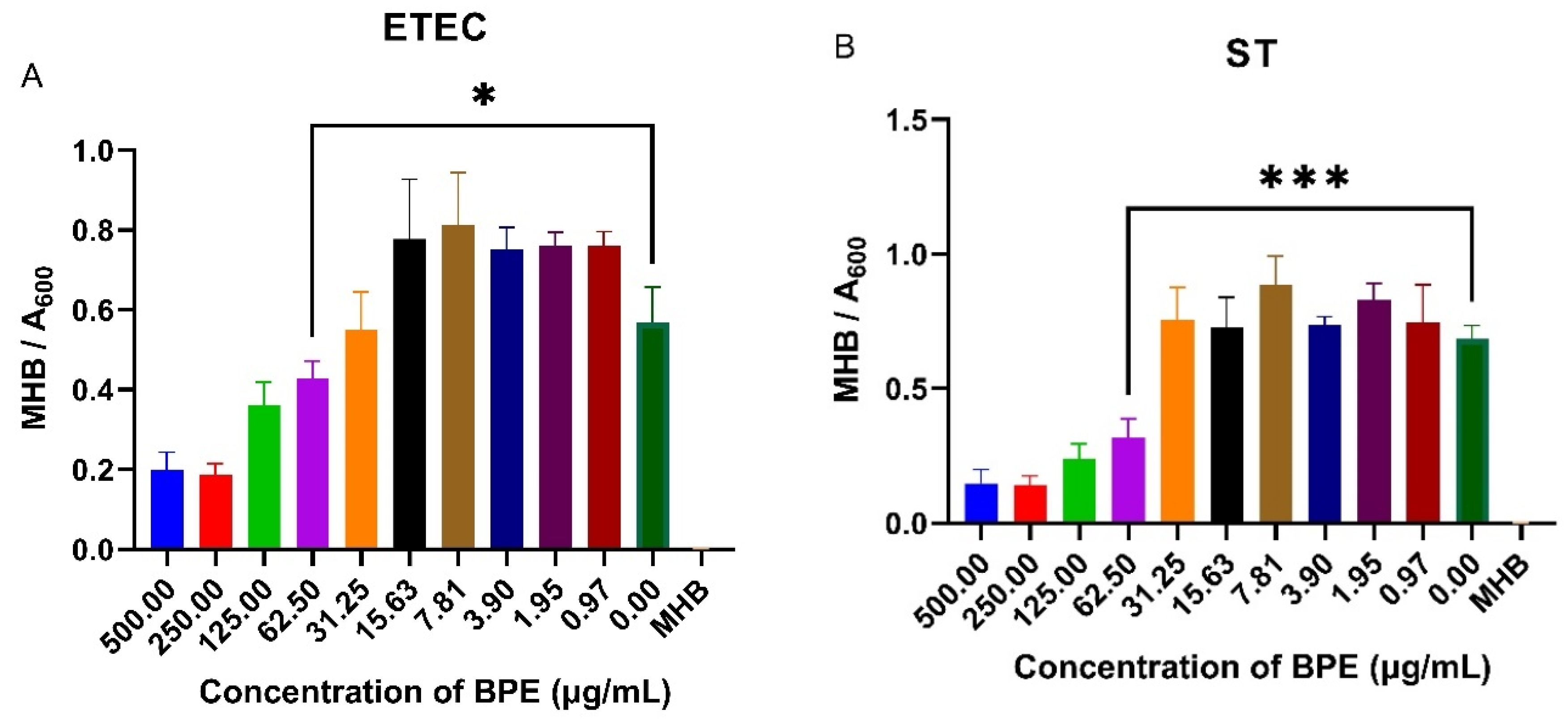
3.7. BPE Exhibits Antimicrobial Activity against Common PWD Pathogens
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jarvis, S.; Moinard, C.; Robson, S.K.; Sumner, B.E.H.; Douglas, A.J.; Seckl, J.R.; Russell, J.A.; Lawrence, A.B. Effects of Weaning Age on the Behavioural and Neuroendocrine Development of Piglets. Appl. Anim. Behav. Sci. 2008, 110, 166–181. [Google Scholar] [CrossRef]
- Ming, D.; Wang, W.; Huang, C.; Wang, Z.; Shi, C.; Ding, J.; Liu, H.; Wang, F. Effects of Weaning Age at 21 and 28 Days on Growth Performance, Intestinal Morphology and Redox Status in Piglets. Animals 2021, 11, 2169. [Google Scholar] [CrossRef]
- Lallès, J.-P.; Bosi, P.; Smidt, H.; Stokes, C.R. Nutritional Management of Gut Health in Pigs around Weaning. Proc. Nutr. Soc. 2007, 66, 260–268. [Google Scholar] [CrossRef]
- Campbell, J.M.; Crenshaw, J.D.; Polo, J. The Biological Stress of Early Weaned Piglets. J. Anim. Sci. Biotechnol. 2013, 4, 19. [Google Scholar] [CrossRef]
- Luppi, A.; Gibellini, M.; Gin, T.; Vangroenweghe, F.; Vandenbroucke, V.; Bauerfeind, R.; Bonilauri, P.; Labarque, G.; Hidalgo, Á. Prevalence of Virulence Factors in Enterotoxigenic Escherichia Coli Isolated from Pigs with Post-Weaning Diarrhoea in Europe. Porc. Health Manag. 2016, 2, 20. [Google Scholar] [CrossRef] [PubMed]
- Won, Y.-K.; Kim, S.-J.; Han, J.-H. The Protective Effect of Dietary Supplementation of Salmonella-Specific Bacteriophages in Post-Weaning Piglets Challenged with Salmonella Typhimurium. J. Adv. Vet. Anim. Res. 2021, 8, 440–447. [Google Scholar] [CrossRef]
- Heo, J.M.; Opapeju, F.O.; Pluske, J.R.; Kim, J.C.; Hampson, D.J.; Nyachoti, C.M. Gastrointestinal Health and Function in Weaned Pigs: A Review of Feeding Strategies to Control Post-weaning Diarrhoea without Using In-feed Antimicrobial Compounds. J. Anim. Physiol. Anim. Nutr. 2013, 97, 207–237. [Google Scholar] [CrossRef]
- Groschwitz, K.R.; Hogan, S.P. Intestinal Barrier Function: Molecular Regulation and Disease Pathogenesis. J. Allergy Clin. Immunol. 2009, 124, 3–20. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H. Intestinal Permeability Regulation by Tight Junction: Implication on Inflammatory Bowel Diseases. Intest. Res. 2015, 13, 11. [Google Scholar] [CrossRef] [PubMed]
- Modina, S.C.; Polito, U.; Rossi, R.; Corino, C.; Di Giancamillo, A. Nutritional Regulation of Gut Barrier Integrity in Weaning Piglets. Animals 2019, 9, 1045. [Google Scholar] [CrossRef]
- Celi, P.; Cowieson, A.J.; Fru-Nji, F.; Steinert, R.E.; Kluenter, A.-M.; Verlhac, V. Gastrointestinal Functionality in Animal Nutrition and Health: New Opportunities for Sustainable Animal Production. Anim. Feed Sci. Technol. 2017, 234, 88–100. [Google Scholar] [CrossRef]
- Hachimura, S.; Totsuka, M.; Hosono, A. Immunomodulation by Food: Impact on Gut Immunity and Immune Cell Function. Biosci. Biotechnol. Biochem. 2018, 82, 584–599. [Google Scholar] [CrossRef]
- Cuevas, A.; Saavedra, N.; Salazar, L.; Abdalla, D. Modulation of Immune Function by Polyphenols: Possible Contribution of Epigenetic Factors. Nutrients 2013, 5, 2314–2332. [Google Scholar] [CrossRef]
- Bernal-Gallardo, J.O.; Molina-Torres, J.; Angoa-Pérez, M.V.; Cárdenas-Valdovinos, J.G.; García-Ruíz, I.; Ceja-Díaz, J.A.; Mena-Violante, H.G. Phenolic Compound Content and the Antioxidant and Antimicrobial Activity of Wild Blueberries (Vaccinium stenophyllum Steud.) Fruits Extracts during Ripening. Horticulturae 2021, 8, 15. [Google Scholar] [CrossRef]
- Proca, A.C.; Horodincu, L.; Solcan, C.; Solcan, G. The Potential of Grape Polyphenols Additive in Pig Nutrition: Chemical Structure, Bioavailability and Their Effect on Intestinal Health of Pigs. Agriculture 2024, 14, 1142. [Google Scholar] [CrossRef]
- Chedea, V.S.; Palade, L.M.; Pelmus, R.S.; Dragomir, C.; Taranu, I. Red Grape Pomace Rich in Polyphenols Diet Increases the Antioxidant Status in Key Organs—Kidneys, Liver, and Spleen of Piglets. Animals 2019, 9, 149. [Google Scholar] [CrossRef] [PubMed]
- Ming, D.; Wang, J.; Yin, C.; Chen, Y.; Li, Y.; Sun, W.; Pi, Y.; Monteiro, A.; Li, X.; Jiang, X. Porous Zinc Oxide and Plant Polyphenols as a Replacement for High-Dose Zinc Oxide on Growth Performance, Diarrhea Incidence, Intestinal Morphology and Microbial Diversity of Weaned Piglets. Animals 2024, 14, 523. [Google Scholar] [CrossRef] [PubMed]
- Wan, M.L.Y.; Ling, K.H.; Wang, M.F.; El-Nezami, H. Green Tea Polyphenol Epigallocatechin-3-gallate Improves Epithelial Barrier Function by Inducing the Production of Antimicrobial Peptide PBD-1 and PBD-2 in Monolayers of Porcine Intestinal Epithelial IPEC-J2 Cells. Mol. Nutr. Food Res. 2016, 60, 1048–1058. [Google Scholar] [CrossRef]
- Pomothy, J.M.; Barna, R.F.; Pászti, E.A.; Babiczky, Á.; Szóládi, Á.; Jerzsele, Á.; Gere, E.P. Beneficial Effects of Rosmarinic Acid on IPEC-J2 Cells Exposed to the Combination of Deoxynivalenol and T-2 Toxin. Mediat. Inflamm. 2020, 2020, 8880651. [Google Scholar] [CrossRef]
- Madiwale, G.P.; Reddivari, L.; Stone, M.; Holm, D.G.; Vanamala, J. Combined Effects of Storage and Processing on the Bioactive Compounds and Pro-Apoptotic Properties of Color-Fleshed Potatoes in Human Colon Cancer Cells. J. Agric. Food Chem. 2012, 60, 11088–11096. [Google Scholar] [CrossRef]
- Yan, H.; Ajuwon, K.M. Butyrate Modifies Intestinal Barrier Function in IPEC-J2 Cells through a Selective Upregulation of Tight Junction Proteins and Activation of the Akt Signaling Pathway. PLoS ONE 2017, 12, e0179586. [Google Scholar] [CrossRef]
- Sandoval-Ramírez, B.A.; Catalán, Ú.; Pedret, A.; Valls, R.M.; Motilva, M.J.; Rubió, L.; Solà, R. Exploring the Effects of Phenolic Compounds to Reduce Intestinal Damage and Improve the Intestinal Barrier Integrity: A Systematic Review of in Vivo Animal Studies. Clin. Nutr. 2021, 40, 1719–1732. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Sharifi-Rad, J.; Cappellini, F.; Reiner, Ž.; Zorzan, D.; Imran, M.; Sener, B.; Kilic, M.; El-Shazly, M.; Fahmy, N.M.; et al. The Therapeutic Potential of Anthocyanins: Current Approaches Based on Their Molecular Mechanism of Action. Front. Pharmacol. 2020, 11, 1300. [Google Scholar] [CrossRef]
- Chen, X.; Qiao, T.; Mao, Z.; Jia, G.; Zhao, H.; Liu, G.; Huang, Z. Caffeic Acid Improves Intestinal Barrier Functions by Regulating Colonic Bacteria and Tight Junction Protein Expression and Alleviating Inflammation in Weaning Piglets. Anim. Biotechnol. 2023, 34, 3693–3699. [Google Scholar] [CrossRef]
- Radcliffe, J.S.; Brito, L.F.; Reddivari, L.; Schmidt, M.; Herman, E.M.; Schinckel, A.P. A Swine Model of Soy Protein–Induced Food Allergenicity: Implications in Human and Swine Nutrition. Anim. Front. 2019, 9, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Rhouma, M.; Fairbrother, J.M.; Beaudry, F.; Letellier, A. Post Weaning Diarrhea in Pigs: Risk Factors and Non-Colistin-Based Control Strategies. Acta Vet. Scand. 2017, 59, 31. [Google Scholar] [CrossRef]
- Shahidi, F.; Yeo, J. Bioactivities of Phenolics by Focusing on Suppression of Chronic Diseases: A Review. Int. J. Mol. Sci. 2018, 19, 1573. [Google Scholar] [CrossRef]
- Pap, N.; Fidelis, M.; Azevedo, L.; do Carmo, M.A.V.; Wang, D.; Mocan, A.; Pereira, E.P.R.; Xavier-Santos, D.; Sant’Ana, A.S.; Yang, B.; et al. Berry Polyphenols and Human Health: Evidence of Antioxidant, Anti-Inflammatory, Microbiota Modulation, and Cell-Protecting Effects. Curr. Opin. Food Sci. 2021, 42, 167–186. [Google Scholar] [CrossRef]
- Vergauwen, H. The IPEC-J2 Cell Line. In The Impact of Food Bioactives on Health; Springer International Publishing: Cham, Switzerland, 2015; pp. 125–134. [Google Scholar]
- Husain, A.; Chanana, H.; Khan, S.A.; Dhanalekshmi, U.M.; Ali, M.; Alghamdi, A.A.; Ahmad, A. Chemistry and Pharmacological Actions of Delphinidin, a Dietary Purple Pigment in Anthocyanidin and Anthocyanin Forms. Front. Nutr. 2022, 9, 746881. [Google Scholar] [CrossRef]
- Oteiza, P.I.; Cremonini, E.; Fraga, C.G. Anthocyanin Actions at the Gastrointestinal Tract: Relevance to Their Health Benefits. Mol. Aspects Med. 2023, 89, 101156. [Google Scholar] [CrossRef]
- Wang, L.; Pan, X.; Jiang, L.; Chu, Y.; Gao, S.; Jiang, X.; Zhang, Y.; Chen, Y.; Luo, S.; Peng, C. The Biological Activity Mechanism of Chlorogenic Acid and Its Applications in Food Industry: A Review. Front. Nutr. 2022, 9, 943911. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Chen, D.; Yu, B.; Luo, Y.; Zheng, P.; Mao, X.; Yu, J.; Luo, J.; Huang, Z.; Yan, H.; et al. Chlorogenic Acid Attenuates Oxidative Stress-Induced Intestinal Mucosa Disruption in Weaned Pigs. Front. Vet. Sci. 2022, 9, 806253. [Google Scholar] [CrossRef] [PubMed]
- Rao, R. Oxidative Stress-Induced Disruption of Epithelial and Endothelial Tight Junctions. Front. Biosci. 2008, 13, 7210. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Song, Y.; Yu, B.; He, J.; Zheng, P.; Mao, X.; Huang, Z.; Luo, Y.; Luo, J.; Yan, H.; et al. Tannic Acid Prevents Post-Weaning Diarrhea by Improving Intestinal Barrier Integrity and Function in Weaned Piglets. J. Anim. Sci. Biotechnol. 2020, 11, 87. [Google Scholar] [CrossRef]
- Remenyik, J.; Biró, A.; Klusóczki, Á.; Juhász, K.Z.; Szendi-Szatmári, T.; Kenesei, Á.; Szőllősi, E.; Vasvári, G.; Stündl, L.; Fenyvesi, F.; et al. Comparison of the Modulating Effect of Anthocyanin-Rich Sour Cherry Extract on Occludin and ZO-1 on Caco-2 and HUVEC Cultures. Int. J. Mol. Sci. 2022, 23, 9036. [Google Scholar] [CrossRef]
- Bomba, L.; Minuti, A.; Moisá, S.J.; Trevisi, E.; Eufemi, E.; Lizier, M.; Chegdani, F.; Lucchini, F.; Rzepus, M.; Prandini, A.; et al. Gut Response Induced by Weaning in Piglet Features Marked Changes in Immune and Inflammatory Response. Funct. Integr. Genom. 2014, 14, 657–671. [Google Scholar] [CrossRef] [PubMed]
- Lallès, J.P.; Sève, B.; Pié, S.; Blazy, F.; Laffitte, J.; Oswald, I.P. Weaning Is Associated with an Upregulation of Expression of Inflammatory Cytokines in the Intestine of Piglets. J. Nutr. 2004, 134, 641–647. [Google Scholar] [CrossRef]


| Genes | Genes Accession Number | Primer Sequences |
|---|---|---|
| TNF-α | P23563 | F: 5′-ATGGATGGGTGGATGAGAAA-3′ R: 5′-TGGAAACTGTTGGGGAGAAG-3′ |
| IL-8 | P26894 | F: 5′-CACCTGTCTGTCCACGTTGT-3′ R: 5′-AGAGGTCTGCCTGGACCCCA-3′ |
| OCLN | A0A287AL69 | F: 5′-GAGAGAGTGGACAGCCCCAT-3′ R: 5′-TGCTGCTGTAATGAGGCTGC-3′ |
| IL-1β | P26889 | F: 5′-CCAAAGAGGGACATGGAGAA-3′ R: 5′-GGGCTTTTGTTCTGCTTGAG-3′ |
| IL-6 | P26893 | F: 5′-TCTGGGTTCAATCAGGAGACCTGC-3′ R: 5′-TGCACGGCCTCGACATTTCCC-3′ |
| CLDN-1 | A0A287A1F1 | F: 5′-TTTCCTCAATACAGGAGGGAAGC-3′ R: 5′-CCCTCTCCCCACATTCGAG-3′ |
| β-actin | Q80X90 | F: 5′ AGCCATGTACGTAGCCATCC-3′ R: 5′-CTCTCAGCTGTGGTGGTGAA-3′ |
| Metabolite | Area Under Curve (±) SE |
|---|---|
| Hydroxycinnamic acids | |
| 3-Hydroxycinnamic acid | 15,033 ± 188 |
| Caffeic acid | 91,956 ± 215 |
| Flavonoid-3-O-glycosides | |
| Isoquercitrin | 4663 ± 806 |
| Quercetin-3-O-xyloside | 151,812 ± 3697 |
| Isorhamnetin-3-O-beta-D-Glucoside | 328,457 ± 4587 |
| Cyanidin-3-O-alpha-arabinopyranoside * | 1,558,051 ± 3961 |
| Quercetin-3-O-xyloside | 7692 ± 295 |
| 5,7-dihydroxy-2-[4-hydroxy-3-[(2S,3R,4S,5R)-3,4,5-trihydroxyoxan-2-yl]oxyphenyl]-3-methoxychromen-4-one | 119,690 ± 1817 |
| Cyanidin-3-O-alpha-arabinopyranoside * | 5358 ± 278 |
| Peonidin-3-O-alpha-arabinopyranoside * | 70,535 ± 846 |
| Quercetin-3-O-glucosyl-6′′-acetate | 80,661 ± 993 |
| Peonidin-3-O-alpha-arabinoside * | 10,584 ± 465 |
| Myricetin-3-Xyloside | 9074 ± 485 |
| Isoquercetin | 123,995 ± 2503 |
| Quercetin-3-Arabinoside | 29,406 ± 988 |
| Quercetin 3-O-malonylglucoside | 8552 ± 223 |
| Kaempferol-3-glucoside | 5162 ± 49 |
| 5,7-dihydroxy-2-(4-hydroxy-3-methoxyphenyl)-3-[3,4,5-trihydroxy-6-[[(2R,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyloxan-2-yl]oxymethyl]oxan-2-yl]oxychromen-4-one | 4754 ± 266 |
| Quercetin-3-O-alpha-L-rhamnopyranoside | 32,385 ± 1379 |
| Isorhamnetin-3-O-beta-D-Glucoside | 6642 ± 94 |
| Quercetin-3-O-glucosyl-6′′-acetate | 5422 ± 157 |
| Quercetin-3-O-glucosyl-6′′-acetate | 9679 ± 443 |
| 2-(3,4-dihydroxyphenyl)-5,8-dihydroxy-7-methoxy-3-[(2S,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyloxan-2-yl]oxychromen-4-one | 6220 ± 303 |
| Flavonoid-3-O-glucuronides | |
| Quercetin 3-O-glucuronide | 66,122 ± 1386 |
| Kaempferol 3-glucuronide | 6642 ± 94 |
| Flavonoid-7-O-glycosides | |
| Nepetin-7-glucoside | 3270 ± 430 |
| Plantaginin | 240,153 ± 8929 |
| NCGC00380911-01!2-(3,4-dihydroxyphenyl)-3,5-dihydroxy-8-methoxy-7-[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxychromen-4-one | 18,211 ± 321 |
| Anthocyanidin-3-O-glycosides | |
| Delphinidin 3-glucoside * | 3557 ± 588 |
| Petunidin-3-O-beta-glucoside * | 2141 ± 393 |
| Delphinidin-3-O-beta-glucopyranoside * | 370,983 ± 6428 |
| Peonidin-3-o-beta-d-glucopyranoside * | 148,567 ± 3296 |
| Flavonols | |
| Quercetin | 31,625 ± 245 |
| Kaempferol | 22,206 ± 854 |
| Isorhamnetin | 25,146 ± 999 |
| Myricetin | 9599 ± 225 |
| Isorhamnetin | 6573 ± 357 |
| Limocitrin | 11,597 ± 481 |
| Flavones | |
| Luteolin | 18,168 ± 243 |
| Flavanones | |
| 2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-4H-chromen-4-one | 5354 ± 223 |
| Catechins | |
| Epicatechin | 20,234 ± 562 |
| Epigallocatechins | |
| Epigallocatechin | 4253 ± 217 |
| Quinic acids and derivatives | |
| Chlorogenic acid | 3354 ± 254 |
| Biflavonoids and polyflavonoids | |
| Procyanidin B1 | 25,277 ± 688 |
| Phenolic glycosides | |
| (E)-3-[4-[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxyphenyl]prop-2-enoic acid | 5894 ± 395 |
| 6-O-methylated flavonoids | |
| 4′,5,7-trihydroxy-3,6-dimethoxyflavone | 14,776 ± 448 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nathan, V.B.; Eckrote, S.; Li, S.; Reddivari, L. Crude Blueberry Phenolic Extracts Improve Gut Barrier Integrity and Exert Anti-Inflammatory and Antimicrobial Activity in an In Vitro Weaning Stress Model. Antioxidants 2024, 13, 1044. https://doi.org/10.3390/antiox13091044
Nathan VB, Eckrote S, Li S, Reddivari L. Crude Blueberry Phenolic Extracts Improve Gut Barrier Integrity and Exert Anti-Inflammatory and Antimicrobial Activity in an In Vitro Weaning Stress Model. Antioxidants. 2024; 13(9):1044. https://doi.org/10.3390/antiox13091044
Chicago/Turabian StyleNathan, Vignesh B., Sarah Eckrote, Shiyu Li, and Lavanya Reddivari. 2024. "Crude Blueberry Phenolic Extracts Improve Gut Barrier Integrity and Exert Anti-Inflammatory and Antimicrobial Activity in an In Vitro Weaning Stress Model" Antioxidants 13, no. 9: 1044. https://doi.org/10.3390/antiox13091044





