Oxidative State in Cutaneous Melanoma Progression: A Question of Balance
Abstract
1. Introduction
2. Redox Mediators and Sources
2.1. ROS Produced by Cellular Organelles
2.2. Exogenous Sources of ROS
2.3. Endogenous Sources of RNS
2.4. Exogenous Sources of RNS
3. Role of ROS in Physiological and Pathological Processes: A Matter of Balance between ROS Production and Efficiency of Antioxidant Cellular System
4. Redox Sensitive Pathways and Cancer Progression
4.1. Involvement of ROS in Cancer Cells Proliferation
4.2. Involvement of ROS in Cancer Metastasis
5. Redox Sensitive Pathways Involved in Cancer Prevention
6. Melanoma Characteristics and Pathophysiology
7. Mutated Driver Genes and Downstream Signal Pathways Involved in Melanoma Progression
8. Melanoma Redox Regulation
9. Nrf2 and NF-kB: The Two Key Factors Involved in Melanoma Redox State
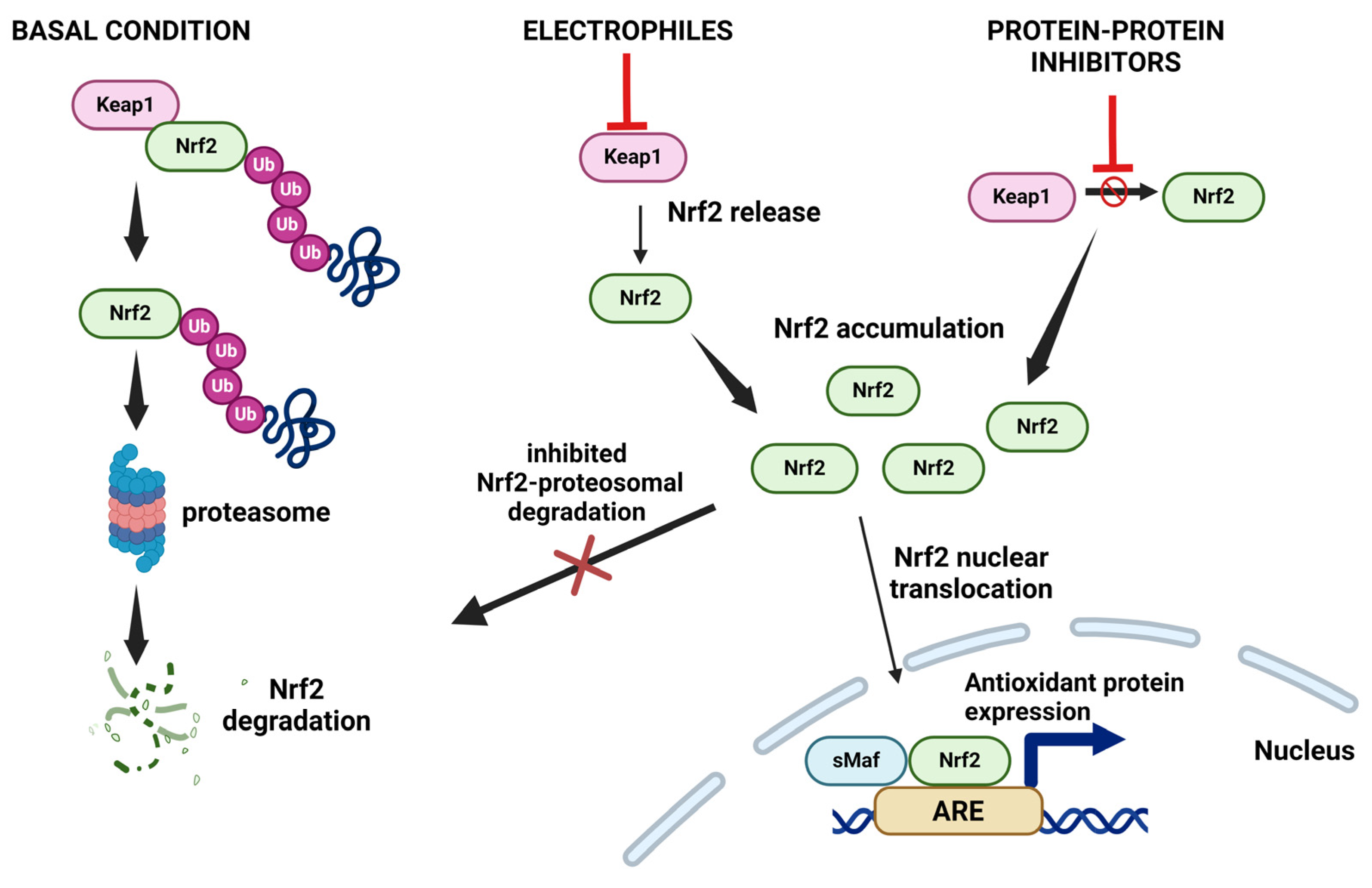
9.1. Nrf2 and Oxidative Stress
9.2. The Role of Nrf2 in Melanoma Development
9.3. The Role of Nrf2 in Melanoma Progression
9.4. The Role of Nrf2 in Melanoma Resistance to Immunotherapy and Targeted Therapy
9.5. NF-κB and Oxidative Stress
9.6. NF-κB in Melanoma
9.7. The Role of NF-κB in Melanoma Resistance to Immunotherapy and Targeted Therapy
9.8. Nrf2-NF-κB Crosstalk in Melanoma Progression
10. Antioxidant vs. Pro-Oxidant Therapeutic Approach: What Is More Effective?
10.1. Pro-Oxidant Therapeutical Approaches
10.1.1. Radiotherapy
10.1.2. Photodynamic Therapy
10.1.3. Hyperthermia
10.1.4. Gas Plasma Technology
10.1.5. Nanotechnology Approaches
10.2. The Antioxidant Therapeutical Approaches
Author Contributions
Funding
Conflicts of Interest
References
- Davalli, P.; Mitic, T.; Caporali, A.; Lauriola, A.; D’Arca, D. ROS, Cell Senescence, and Novel Molecular Mechanisms in Aging and Age-Related Diseases. Oxid. Med. Cell. Longev. 2016, 2016, 3565127. [Google Scholar] [CrossRef] [PubMed]
- Phaniendra, A.; Jestadi, D.B.; Periyasamy, L. Free Radicals: Properties, Sources, Targets, and Their Implication in Various Diseases. Indian J. Clin. Biochem. 2015, 30, 11–26. [Google Scholar] [CrossRef]
- Jomova, K.; Raptova, R.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Reactive oxygen species, toxicity, oxidative stress, and antioxidants: Chronic diseases and aging. Arch. Toxicol. 2023, 97, 2499–2574. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Chen, H.; Liang, J.; Li, Y.; Yang, J.; Luo, C.; Tang, Y.; Ding, Y.; Liu, X.; Yuan, Q.; et al. Dual role of reactive oxygen species and their application in cancer therapy. J. Cancer 2021, 12, 5543–5561. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, H.; Takada, K. Reactive oxygen species in cancer: Current findings and future directions. Cancer Sci. 2021, 112, 3945–3952. [Google Scholar] [CrossRef]
- Fried, L.; Arbiser, J.L. The reactive oxygen-driven tumor: Relevance to melanoma. Pigment Cell Melanoma Res. 2008, 21, 117–122. [Google Scholar] [CrossRef]
- De Almeida, A.J.P.O.; De Oliveira, J.C.P.L.; Da Silva Pontes, L.V.; De Souza Júnior, J.F.; Gonçalves, T.A.F.; Dantas, S.H.; De Almeida Feitosa, M.S.; Silva, A.O.; De Medeiros, I.A. ROS: Basic Concepts, Sources, Cellular Signaling, and its Implications in Aging Pathways. Oxid. Med. Cell. Longev. 2022, 2022, 1225578. [Google Scholar] [CrossRef] [PubMed]
- Skonieczna, M.; Hejmo, T.; Poterala-Hejmo, A.; Cieslar-Pobuda, A.; Buldak, R.J. NADPH Oxidases (NOX): Insights into Selected Functions and Mechanisms of Action in Cancer and Stem Cells. Oxid. Med. Cell. Longev. 2017, 2017, 9420539. [Google Scholar] [CrossRef]
- Hernansanz-Agustín, P.; Enríquez, J.A. Generation of reactive oxygen species by mitochondria. Antioxidants 2021, 10, 415. [Google Scholar] [CrossRef]
- Shadel, G.S.; Horvath, T.L. Mitochondrial ROS Signaling in Organismal Homeostasis. Cell 2015, 163, 560–569. [Google Scholar] [CrossRef]
- Chance, B.; Sies, H.; Boveris, A. Hydroperoxide metabolism in mammalian organs. Physiol. Rev. 1979, 59, 527–605. [Google Scholar] [CrossRef] [PubMed]
- Rimessi, A.; Previati, M.; Nigro, F.; Wieckowski, M.R.; Pinton, P. Mitochondrial reactive oxygen species and inflammation: Molecular mechanisms, diseases and promising therapies. Int. J. Biochem. Cell Biol. 2016, 81, 281–293. [Google Scholar] [CrossRef] [PubMed]
- Venditti, P.; Di Stefano, L.; Di Meo, S. Mitochondrial metabolism of reactive oxygen species. Mitochondrion 2013, 13, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.C.; Borutaite, V. There is no evidence that mitochondria are the main source of reactive oxygen species in mammalian cells. Mitochondrion 2012, 12, 1–4. [Google Scholar] [CrossRef]
- Schwarz, D.S.; Blower, M.D. The endoplasmic reticulum: Structure, function and response to cellular signaling. Cell. Mol. Life Sci. 2016, 73, 79–94. [Google Scholar] [CrossRef]
- Zeeshan, H.M.A.; Lee, G.H.; Kim, H.R.; Chae, H.J. Endoplasmic reticulum stress and associated ROS. Int. J. Mol. Sci. 2016, 17, 327. [Google Scholar] [CrossRef]
- Bhattarai, K.R.; Riaz, T.A.; Kim, H.R.; Chae, H.J. The aftermath of the interplay between the endoplasmic reticulum stress response and redox signaling. Exp. Mol. Med. 2021, 53, 151–167. [Google Scholar] [CrossRef]
- Nikitaki, Z.; Hellweg, C.E.; Georgakilas, A.G.; Ravanat, J.L. Stress-induced DNA damage biomarkers: Applications and limitations. Front. Chem. 2015, 3, 35. [Google Scholar] [CrossRef]
- Santibáñez-Andrade, M.; Quezada-Maldonado, E.M.; Rivera-Pineda, A.; Chirino, Y.I.; García-Cuellar, C.M.; Sánchez-Pérez, Y. The Road to Malignant Cell Transformation after Particulate Matter Exposure: From Oxidative Stress to Genotoxicity. Int. J. Mol. Sci. 2023, 24, 1782. [Google Scholar] [CrossRef] [PubMed]
- Crivellari, I.; Sticozzi, C.; Belmonte, G.; Muresan, X.M.; Cervellati, F.; Pecorelli, A.; Cavicchio, C.; Maioli, E.; Zouboulis, C.C.; Benedusi, M.; et al. SRB1 as a new redox target of cigarette smoke in human sebocytes. Free Radic. Biol. Med. 2017, 102, 47–56. [Google Scholar] [CrossRef]
- Van Loenhout, J.; Peeters, M.; Bogaerts, A.; Smits, E.; Deben, C. Oxidative stress-inducing anticancer therapies: Taking a closer look at their immunomodulating effects. Antioxidants 2020, 9, 1188. [Google Scholar] [CrossRef] [PubMed]
- Araki, S.; Osuka, K.; Takata, T.; Tsuchiya, Y.; Watanabe, Y. Coordination between calcium/calmodulin-dependent protein kinase II and neuronal nitric oxide synthase in neurons. Int. J. Mol. Sci. 2020, 21, 7997. [Google Scholar] [CrossRef] [PubMed]
- Persinger, R.L.; Poynter, M.E.; Ckless, K.; Janssen-Heininger, Y.M. Molecular mechanisms of nitrogen dioxide induced epithelial injury in the lung. Mol. Cell. Biochem. 2002, 234–235, 71–80. [Google Scholar] [CrossRef]
- Yang, S.; Lian, G. ROS and diseases: Role in metabolism and energy supply. Mol. Cell. Biochem. 2020, 467, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Kasai, S.; Shimizu, S.; Tatara, Y.; Mimura, J.; Itoh, K. Regulation of Nrf2 by mitochondrial reactive oxygen species in physiology and pathology. Biomolecules 2020, 10, 320. [Google Scholar] [CrossRef]
- Mishra, R.; Yuan, L.; Patel, H.; Karve, A.S.; Zhu, H.; White, A.; Alanazi, S.; Desai, P.; Merino, E.J.; Garrett, J.T. Phosphoinositide 3-kinase (Pi3k) reactive oxygen species (ros)-activated prodrug in combination with anthracycline impairs pi3k signaling, increases dna damage response and reduces breast cancer cell growth. Int. J. Mol. Sci. 2021, 22, 2088. [Google Scholar] [CrossRef]
- Irato, P.; Santovito, G. Enzymatic and non-enzymatic molecules with antioxidant function. Antioxidants 2021, 10, 579. [Google Scholar] [CrossRef]
- Klran, T.R.; Otlu, O.; Karabulut, A.B. Oxidative stress and antioxidants in health and disease. J. Lab. Med. 2023, 47, 1–11. [Google Scholar] [CrossRef]
- Wang, Y.; Qi, H.; Liu, Y.; Duan, C.; Liu, X.; Xia, T.; Chen, D.; Piao, H.L.; Liu, H.X. The double-edged roles of ROS in cancer prevention and therapy. Theranostics 2021, 11, 4839–4857. [Google Scholar] [CrossRef]
- ArulJothi, K.N.; Kumaran, K.; Senthil, S.; Nidhu, A.B.; Munaff, N.; Janitri, V.B.; Kirubakaran, R.; Singh, S.K.; Gupt, G.; Dua, K.; et al. Implications of reactive oxygen species in lung cancer and exploiting it for therapeutic interventions. Med. Oncol. 2023, 40, 1–19. [Google Scholar] [CrossRef]
- Moloney, J.N.; Cotter, T.G. ROS signalling in the biology of cancer. Semin. Cell Dev. Biol. 2018, 80, 50–64. [Google Scholar] [CrossRef] [PubMed]
- Behrend, L.; Henderson, G.; Zwacka, R.M. Reactive oxygen species in oncogenic transformation. Biochem. Soc. Trans. 2003, 31, 1441–1444. [Google Scholar] [CrossRef] [PubMed]
- Weinberg, F.; Ramnath, N.; Nagrath, D. Reactive Oxygen Species in the Tumor Microenvironment: An Overview. Cancers 2019, 11, 1191. [Google Scholar] [CrossRef]
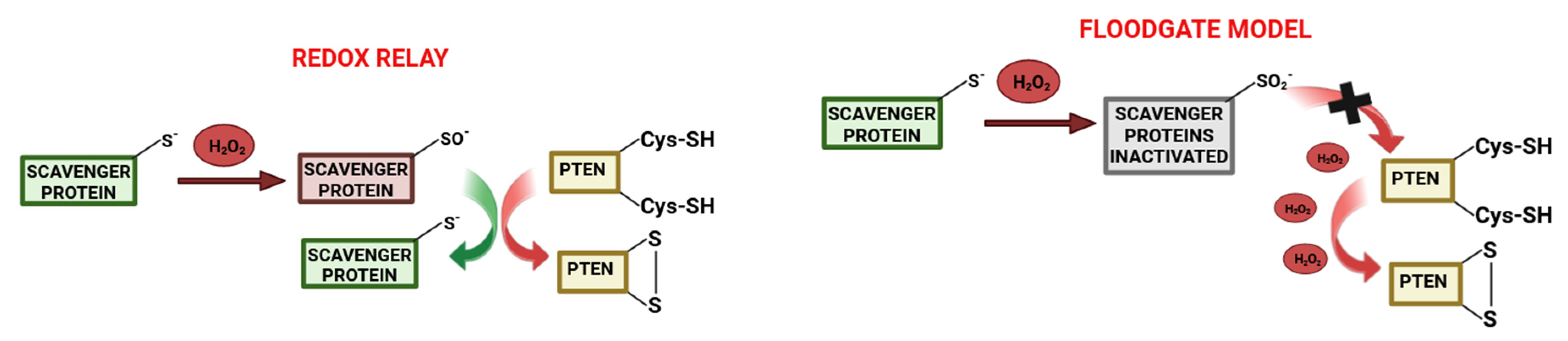
- Lee, S.R.; Yang, K.S.; Kwon, J.; Lee, C.; Jeong, W.; Rhee, S.G. Reversible inactivation of the tumor suppressor PTEN by H2O2. J. Biol. Chem. 2002, 277, 20336–20342. [Google Scholar] [CrossRef] [PubMed]
- Reczek, C.R.; Chandel, N.S. The two faces of reactive oxygen species in cancer. Annu. Rev. Cancer Biol. 2017, 1, 79–98. [Google Scholar] [CrossRef]
- Winterbourn, C.C. The Biological Chemistry of Hydrogen Peroxide. Methods Enzymol. 2013, 528, 3–25. [Google Scholar] [CrossRef]
- Wood, Z.A.; Poole, L.B.; Karplus, P.A. Peroxiredoxin Evolution and the Signaling. Science 2003, 300, 650–653. [Google Scholar] [CrossRef]
- Taniguchi, K.; Karin, M. NF-B, inflammation, immunity and cancer: Coming of age. Nat. Rev. Immunol. 2018, 18, 309–324. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Shi, K.; Fu, M.; Chen, F. Melatonin indirectly decreases gastric cancer cell proliferation and invasion via effects on cancer-associated fibroblasts. Life Sci. 2021, 277, 119497. [Google Scholar] [CrossRef]
- Chang, H.; Li, J.; Qu, K.; Wan, Y.; Liu, S.; Zheng, W.; Zhang, Z.; Liu, C. CRIF1 overexpression facilitates tumor growth and metastasis through inducing ROS/NFκB pathway in hepatocellular carcinoma. Cell Death Dis. 2020, 11, 332. [Google Scholar] [CrossRef]
- Lu, T.X.; Rothenberg, M.E. MicroRNA. J. Allergy Clin. Immunol. 2018, 141, 1202–1207. [Google Scholar] [CrossRef]
- Shademan, B.; Karamad, V.; Nourazarian, A.; Masjedi, S.; Isazadeh, A.; Sogutlu, F.; Avci, C.B. MicroRNAs as Targets for Cancer Diagnosis: Interests and Limitations. Adv. Pharm. Bull. 2023, 13, 435–445. [Google Scholar] [CrossRef] [PubMed]
- Weber, J.A.; Baxter, D.H.; Zhang, S.; Huang, D.Y.; Huang, K.H.; Lee, M.J.; Galas, D.J.; Wang, K. The MicroRNA Spectrum in 12 Body Fluids. Clin. Chem. 2010, 56, 1733–1741. [Google Scholar] [CrossRef]
- Gilad, S.; Meiri, E.; Yogev, Y.; Benjamin, S.; Lebanony, D.; Yerushalmi, N.; Benjamin, H.; Kushnir, M.; Cholakh, H.; Melamed, N.; et al. Serum microRNAs are promising novel biomarkers. PLoS ONE 2008, 3, e3148. [Google Scholar] [CrossRef]
- Calin, G.A.; Croce, C.M. MicroRNA signatures in human cancers. Nat. Rev. Cancer 2006, 6, 857–866. [Google Scholar] [CrossRef]
- Smolarz, B.; Durczyński, A.; Romanowicz, H.; Szyłło, K.; Hogendorf, P. miRNAs in Cancer (Review of Literature). Int. J. Mol. Sci. 2022, 23, 2805. [Google Scholar] [CrossRef] [PubMed]
- Babu, K.R.; Tay, Y. The Yin-Yang regulation of reactive oxygen species and microRNAs in cancer. Int. J. Mol. Sci. 2019, 20, 5335. [Google Scholar] [CrossRef] [PubMed]
- Baylin, S.B.; Herman, J.G. DNA hypermethylation in tumorigenesis: Epigenetics joins genetics. Trends Genet. 2000, 16, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Morales, S.; Monzo, M.; Navarro, A. Epigenetic regulation mechanisms of microRNA expression. Biomol. Concepts 2017, 8, 203–212. [Google Scholar] [CrossRef]
- He, J.; Xu, Q.; Jing, Y.; Agani, F.; Qian, X.; Carpenter, R.; Li, Q.; Wang, X.R.; Peiper, S.S.; Lu, Z.; et al. Reactive oxygen species regulate ERBB2 and ERBB3 expression via miR-199a/125b and DNA methylation. EMBO Rep. 2012, 13, 1116–1122. [Google Scholar] [CrossRef]
- Zhao, Y.; Garcia, B.A. Comprehensive catalog of currently documented histone modifications. Cold Spring Harb. Perspect. Biol. 2015, 7, a025064. [Google Scholar] [CrossRef]
- Gallinari, P.; Di Marco, S.; Jones, P.; Pallaoro, M.; Steinkühler, C. HDACs, histone deacetylation and gene transcription: From molecular biology to cancer therapeutics. Cell Res. 2007, 17, 195–211. [Google Scholar] [CrossRef]
- Ago, T.; Liu, T.; Zhai, P.; Chen, W.; Li, H.; Molkentin, J.D.; Vatner, S.F.; Sadoshima, J. A Redox-Dependent Pathway for Regulating Class II HDACs and Cardiac Hypertrophy. Cell 2008, 133, 978–993. [Google Scholar] [CrossRef]
- Han, C.; Shen, J.K.; Hornicek, F.J.; Kan, Q.; Duan, Z. Regulation of microRNA-1 (miR-1) expression in human cancer. Biochim. Biophys. Acta Gene Regul. Mech. 2017, 1860, 227–232. [Google Scholar] [CrossRef]
- Khalilian, S.; Hosseini Imani, S.Z.; Ghafouri-Fard, S. Emerging roles and mechanisms of miR-206 in human disorders: A comprehensive review. Cancer Cell Int. 2022, 22, 412. [Google Scholar] [CrossRef]
- Singh, A.; Happel, C.; Manna, S.K.; Acquaah-Mensah, G.; Carrerero, J.; Kumar, S.; Nasipuri, P.; Krausz, K.W.; Wakabayashi, N.; Dewi, R.; et al. Transcription factor NRF2 regulates miR-1 and miR-206 to drive tumorigenesis. J. Clin. Investig. 2013, 123, 2921–2934. [Google Scholar] [CrossRef]
- Ebrahimi, S.O.; Reiisi, S.; Shareef, S. miRNAs, oxidative stress, and cancer: A comprehensive and updated review. J. Cell. Physiol. 2020, 235, 8812–8825. [Google Scholar] [CrossRef]
- Liu, C.; Rokavec, M.; Huang, Z.; Hermeking, H. Curcumin activates a ROS/KEAP1/NRF2/miR-34a/b/c cascade to suppress colorectal cancer metastasis. Cell Death Differ. 2023, 30, 1771–1785. [Google Scholar] [CrossRef]
- Black, L.E.; Longo, J.F.; Carroll, S.L. Mechanisms of Receptor Tyrosine-Protein Kinase ErbB-3 (ERBB3) Action in Human Neoplasia. Am. J. Pathol. 2019, 189, 1898–1912. [Google Scholar] [CrossRef]
- Meng, F.; Henson, R.; Wehbe-Janek, H.; Ghoshal, K.; Jacob, S.T.; Patel, T. MicroRNA-21 Regulates Expression of the PTEN Tumor Suppressor Gene in Human Hepatocellular Cancer. Gastroenterology 2007, 133, 647–658. [Google Scholar] [CrossRef]
- Weidemann, A.; Johnson, R.S. Biology of HIF-1α. Cell Death Differ. 2008, 15, 621–627. [Google Scholar] [CrossRef]
- Calvani, M.; Comito, G.; Giannoni, E.; Chiarugi, P. Time-Dependent Stabilization of Hypoxia Inducible Factor-1α by Different Intracellular Sources of Reactive Oxygen Species. PLoS ONE 2012, 7, e38388. [Google Scholar] [CrossRef]
- Comito, G.; Calvani, M.; Giannoni, E.; Bianchini, F.; Calorini, L.; Torre, E.; Migliore, C.; Giordano, S.; Chiarugi, P. HIF-1α stabilization by mitochondrial ROS promotes Met-dependent invasive growth and vasculogenic mimicry in melanoma cells. Free Radic. Biol. Med. 2011, 51, 893–904. [Google Scholar] [CrossRef] [PubMed]
- Xia, C.; Meng, Q.; Liu, L.Z.; Rojanasakul, Y.; Wang, X.R.; Jiang, B.H. Reactive oxygen species regulate angiogenesis and tumor growth through vascular endothelial growth factor. Cancer Res. 2007, 67, 10823–10830. [Google Scholar] [CrossRef]
- Liu, W.-J.; Huang, Y.-X.; Wang, W.; Zhang, Y.; Liu, B.-J.; Qiu, J.-G.; Jiang, B.-H.; Liu, L.-Z. NOX4 Signaling Mediates Cancer Development and Therapeutic Resistance through HER3 in Ovarian Cancer Cells. Cells 2021, 10, 1647. [Google Scholar] [CrossRef]
- Brabletz, T.; Kalluri, R.; Nieto, M.A.; Weinberg, R.A. EMT in cancer. Nat. Rev. Cancer 2018, 18, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Nikitovic, D.; Corsini, E.; Kouretas, D.; Tsatsakis, A.; Tzanakakis, G. ROS-major mediators of extracellular matrix remodeling during tumor progression. Food Chem. Toxicol. 2013, 61, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Díaz, B.; Courtneidge, S.A. Redox signaling at invasive microdomains in cancer cells. Free Radic. Biol. Med. 2012, 52, 247–256. [Google Scholar] [CrossRef]
- Brabletz, S.; Schuhwerk, H.; Brabletz, T.; Stemmler, M.P. Dynamic EMT: A multi-tool for tumor progression. EMBO J. 2021, 40, 1–22. [Google Scholar] [CrossRef]
- Dongre, A.; Weinberg, R.A. New insights into the mechanisms of epithelial–mesenchymal transition and implications for cancer. Nat. Rev. Mol. Cell Biol. 2019, 20, 69–84. [Google Scholar] [CrossRef]
- Cannito, S.; Novo, E.; Di Bonzo, L.V.; Busletta, C.; Colombatto, S.; Parola, M. Epithelial-mesenchymal transition: From molecular mechanisms, redox regulation to implications in human health and disease. Antioxid. Redox Signal. 2010, 12, 1383–1430. [Google Scholar] [CrossRef]
- Farahzadi, R.; Valipour, B.; Fathi, E.; Pirmoradi, S.; Molavi, O.; Montazersaheb, S.; Sanaat, Z. Oxidative stress regulation and related metabolic pathways in epithelial–mesenchymal transition of breast cancer stem cells. Stem Cell Res. Ther. 2023, 14, 342. [Google Scholar] [CrossRef]
- Cencioni, C.; Comunanza, V.; Middonti, E.; Vallariello, E.; Bussolino, F. The role of redox system in metastasis formation. Angiogenesis 2021, 24, 435–450. [Google Scholar] [CrossRef]
- Ashkenazi, A.; Salvesen, G. Regulated cell death: Signaling and mechanisms. Annu. Rev. Cell Dev. Biol. 2014, 30, 337–356. [Google Scholar] [CrossRef]
- Kagan, V.E.; Tyurin, V.A.; Jiang, J.; Tyurina, Y.Y.; Ritov, V.B.; Amoscato, A.A.; Osipov, A.N.; Belikova, N.A.; Kapralov, A.A.; Kini, V.; et al. Cytochrome C Acts As A Cardiolipin Oxygenase Required for Release of Proapoptotic Factors. Nat. Chem. Biol. 2005, 1, 223–232. [Google Scholar] [CrossRef]
- Li, Z.; Guo, D.; Yin, X.; Ding, S.; Shen, M.; Zhang, R.; Wang, Y.; Xu, R. Zinc oxide nanoparticles induce human multiple myeloma cell death via reactive oxygen species and Cyt-C/Apaf-1/Caspase-9/Caspase-3 signaling pathway in vitro. Biomed. Pharmacother. 2020, 122, 109712. [Google Scholar] [CrossRef]
- Tu, Y.; Zhou, Y.; Zhang, D.; Yang, J.; Li, X.; Ji, K.; Wu, X.; Liu, R.; Zhang, Q. Light-Induced Reactive Oxygen Species (ROS) Generator for Tumor Therapy through an ROS Burst in Mitochondria and AKT-Inactivation-Induced Apoptosis. ACS Appl. Bio Mater. 2021, 4, 5222–5230. [Google Scholar] [CrossRef]
- Wilkie-Grantham, R.P.; Matsuzawa, S.I.; Reed, J.C. Novel phosphorylation and ubiquitination sites regulate reactive oxygen species-dependent degradation of anti-apoptotic c-FLIP protein. J. Biol. Chem. 2013, 288, 12777–12790. [Google Scholar] [CrossRef]
- Seo, S.U.; Kim, T.H.; Kim, D.E.; Jin Min, K.; Kwon, T.K. NOX4-mediated ROS production induces apoptotic cell death via down-regulation of c-FLIP and Mcl-1 expression in combined treatment with thioridazine and curcumin. Redox Biol. 2017, 13, 608–622. [Google Scholar] [CrossRef]
- Degterev, A.; Huang, Z.; Boyce, M.; Li, Y.; Jagtap, P.; Mizushima, N.; Cuny, G.D.; Mitchison, T.J.; Moskowitz, M.A.; Yuan, J. Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat. Chem. Biol. 2005, 1, 112–119. [Google Scholar] [CrossRef]
- Zhang, Y.; Su, S.S.; Zhao, S.; Yang, Z.; Zhong, C.Q.; Chen, X.; Cai, Q.; Yang, Z.H.; Huang, D.; Wu, R.; et al. RIP1 autophosphorylation is promoted by mitochondrial ROS and is essential for RIP3 recruitment into necrosome. Nat. Commun. 2017, 8. [Google Scholar] [CrossRef]
- Li, Y.; Gong, P.; Kong, C.; Tian, X. Bufalin engages in RIP1-dependent and ROS-dependent programmed necroptosis in breast cancer cells by targeting the RIP1/RIP3/PGAM5 pathway. Anticancer. Drugs 2019, 30, 706–713. [Google Scholar] [CrossRef]
- Mann, J.; Yang, N.; Montpetit, R.; Kirschenman, R.; Lemieux, H.; Goping, I.S. BAD sensitizes breast cancer cells to docetaxel with increased mitotic arrest and necroptosis. Sci. Rep. 2020, 10, 355. [Google Scholar] [CrossRef]
- Han, Q.; Ma, Y.; Wang, H.; Dai, Y.; Chen, C.; Liu, Y.; Jing, L.; Sun, X. Resibufogenin suppresses colorectal cancer growth and metastasis through RIP3-mediated necroptosis. J. Transl. Med. 2018, 16, 1–13. [Google Scholar] [CrossRef]
- Liu, X.; Liu, L.; Wang, X.; Jin, Y.; Wang, S.; Xie, Q.; Jin, Y.; Zhang, M.; Liu, Y.; Li, J.; et al. Necroptosis inhibits autophagy by regulating the formation of RIP3/p62/Keap1 complex in shikonin-induced ROS dependent cell death of human bladder cancer. Phytomedicine 2023, 118, 154943. [Google Scholar] [CrossRef]
- Yang, R.; Li, Y.; Wang, X.; Yan, J.; Pan, D.; Xu, Y.; Wang, L.; Yang, M. Doxorubicin loaded ferritin nanoparticles for ferroptosis enhanced targeted killing of cancer cells. RSC Adv. 2019, 9, 28548–28553. [Google Scholar] [CrossRef]
- Wang, X.; Chen, Y.; Wang, X.; Tian, H.; Wang, Y.; Jin, J.; Shan, Z.; Liu, Y.; Cai, Z.; Tong, X.; et al. Stem cell factor SOX2 confers ferroptosis resistance in lung cancer via upregulation of SLC7A11. Cancer Res. 2021, 81, 5217–5229. [Google Scholar] [CrossRef]
- Hong, T.; Lei, G.; Chen, X.; Li, H.; Zhang, X.; Wu, N.; Zhao, Y.; Zhang, Y.; Wang, J. PARP inhibition promotes ferroptosis via repressing SLC7A11 and synergizes with ferroptosis inducers in BRCA-proficient ovarian cancer. Redox Biol. 2021, 42, 101928. [Google Scholar] [CrossRef]
- Wang, Z.; Shu, W.; Zhao, R.; Liu, Y.; Wang, H. Sodium butyrate induces ferroptosis in endometrial cancer cells via the RBM3/SLC7A11 axis. Apoptosis 2023, 28, 1168–1183. [Google Scholar] [CrossRef]
- Eddy, K.; Shah, R.; Chen, S. Decoding Melanoma Development and Progression: Identification of Therapeutic Vulnerabilities. Front. Oncol. 2021, 10, 626129. [Google Scholar] [CrossRef]
- Arnold, M.; Singh, D.; Laversanne, M.; Vignat, J.; Vaccarella, S.; Meheus, F.; Cust, A.E.; De Vries, E.; Whiteman, D.C.; Bray, F. Global Burden of Cutaneous Melanoma in 2020 and Projections to 2040. JAMA Dermatol. 2022, 158, 495–503. [Google Scholar] [CrossRef] [PubMed]
- Tsao, H.; Bevona, C.; Goggins, W.Q.T. The Transformation Rate of Moles (Melanocytic Nevi) Into Cutaneous Melanoma. Arch. Dermatol. 2003, 139, 282–288. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro Moura Brasil Arnaut, J.; dos Santos Guimarães, I.; Evangelista dos Santos, A.C.; de Moraes Lino da Silva, F.; Machado, J.R.; de Melo, A.C. Molecular landscape of Hereditary Melanoma. Crit. Rev. Oncol. Hematol. 2021, 164. [Google Scholar] [CrossRef]
- Berger, M.F.; Levin, J.Z.; Vijayendran, K.; Sivachenko, A.; Adiconis, X.; Maguire, J.; Johnson, L.A.; Robinson, J.; Verhaak, R.G.; Sougnez, C.; et al. Integrative analysis of the melanoma transcriptome. Genome Res. 2010, 20, 413–427. [Google Scholar] [CrossRef] [PubMed]
- Akbani, R.; Akdemir, K.C.; Aksoy, B.A.; Albert, M.; Ally, A.; Amin, S.B.; Arachchi, H.; Arora, A.; Auman, J.T.; Ayala, B.; et al. Genomic Classification of Cutaneous Melanoma. Cell 2015, 161, 1681–1696. [Google Scholar] [CrossRef]
- Parkman, G.L.; Foth, M.; Kircher, D.A.; Holmen, S.L.; Mcmahon, M. The role of PI3′-lipid signalling in melanoma initiation, progression and maintenance. Exp. Dermatol. 2023, 31, 43–56. [Google Scholar] [CrossRef]
- Kreuger, I.Z.M.; Slieker, R.C.; van Groningen, T.; van Doorn, R. Therapeutic Strategies for Targeting CDKN2A Loss in Melanoma. J. Investig. Dermatol. 2023, 143, 18–25.e1. [Google Scholar] [CrossRef]
- Kawakami, A.; Fisher, D.E. The master role of microphthalmia-associated transcription factor in melanocyte and melanoma biology. Lab. Investig. 2017, 97, 649–656. [Google Scholar] [CrossRef]
- Richmond, A.; Ueda, Y. NF-κB activation in melanoma. Pigment Cell Res. 2006, 19, 112–124. [Google Scholar] [CrossRef]
- Xu, W.; McArthur, G. Cell Cycle Regulation and Melanoma. Curr. Oncol. Rep. 2016, 18, 34. [Google Scholar] [CrossRef]
- Teixido, C.; Castillo, P.; Martinez-Vila, C.; Arance, A.; Alos, L. Molecular markers and targets in melanoma. Cells 2021, 10, 2320. [Google Scholar] [CrossRef] [PubMed]
- Raimondi, S.; Sera, F.; Gandini, S.; Iodice, S.; Caini, S.; Maisonneuve, P.; Fargnoli, M.C. MC1R variants, melanoma and red hair color phenotype: A meta-analysis. Int. J. Cancer 2008, 122, 2753–2760. [Google Scholar] [CrossRef]
- García-Borrón, J.C.; Sánchez-Laorden, B.L.; Jiménez-Cervantes, C. Melanocortin-1 receptor structure and functional regulation. Pigment Cell Res. 2005, 18, 393–410. [Google Scholar] [CrossRef]
- Li, X.; Zou, H.; Tang, M.; Wang, Y.; Tang, X.; Tian, C. Optimization analysis of linear compressor using R290 for capacity-modulation performance improvement. Int. J. Refrig. 2021, 127, 111–119. [Google Scholar] [CrossRef]
- Guida, S.; Guida, G.; Goding, C.R. MC1R Functions, Expression, and Implications for Targeted Therapy. J. Investig. Dermatol. 2022, 142, 293–302.e1. [Google Scholar] [CrossRef] [PubMed]
- Black, J.O. Xeroderma Pigmentosum. Head Neck Pathol. 2016, 10, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Kamb, A.; Shattuck-Eidens, D.; Eeles, R.; Liu, Q.; Gruis, N.A.; Ding, W.; Hussey, C.; Tran, T.; Miki, Y.; Weaver-Feldhaus, J.; et al. Analysis of the p16 gene (CDKN2) as a candidate for the chromosome 9p melanoma susceptibility locus. Nat. Genet. 1994, 8, 22–26. [Google Scholar] [CrossRef]
- Emanuelli, M.; Sartini, D.; Molinelli, E.; Campagna, R.; Pozzi, V.; Salvolini, E.; Simonetti, O.; Campanati, A.; Offidani, A. The Double-Edged Sword of Oxidative Stress in Skin Damage and Melanoma: From Physiopathology to Therapeutical Approaches. Antioxidants 2022, 11, 612. [Google Scholar] [CrossRef]
- Becker, A.L.; Indra, A.K. Oxidative Stress in Melanoma: Beneficial Antioxidant and Pro-Oxidant Therapeutic Strategies. Cancers 2023, 15, 3038. [Google Scholar] [CrossRef]
- Diffey, B.L. Human exposure to solar ultraviolet radiation. J. Cosmet. Dermatol. 2002, 1, 124–130. [Google Scholar] [CrossRef]
- Umar, S.A.; Tasduq, S.A. Ozone Layer Depletion and Emerging Public Health Concerns—An Update on Epidemiological Perspective of the Ambivalent Effects of Ultraviolet Radiation Exposure. Front. Oncol. 2022, 12, 866733. [Google Scholar] [CrossRef] [PubMed]
- Roy, S. Impact of UV radiation on genome stability and human health. Adv. Exp. Med. Biol. 2017, 996, 207–219. [Google Scholar] [CrossRef]
- Schuch, A.P.; Moreno, N.C.; Schuch, N.J.; Menck, C.F.M.; Garcia, C.C.M. Sunlight damage to cellular DNA: Focus on oxidatively generated lesions. Free Radic. Biol. Med. 2017, 107, 110–124. [Google Scholar] [CrossRef]
- Wondrak, G.T.; Jacobson, M.K.; Jacobson, E.L. Endogenous UVA-photosensitizers: Mediators of skin photodamage and novel targets for skin photoprotection. Photochem. Photobiol. Sci. 2006, 5, 215–237. [Google Scholar] [CrossRef]
- de Jager, T.L.; Cockrell, A.E.; Du Plessis, S.S. Ultraviolet light induced generation of reactive oxygen species. Adv. Exp. Med. Biol. 2017, 996, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Cadet, J.; Douki, T.; Ravanat, J.L. Oxidatively generated damage to cellular DNA by UVB and UVA radiation. Photochem. Photobiol. 2015, 91, 140–155. [Google Scholar] [CrossRef]
- Cadet, J.; Douki, T.; Ravanat, J.L.; Di Mascio, P. Sensitized formation of oxidatively generated damage to cellular DNA by UVA radiation. Photochem. Photobiol. Sci. 2009, 8, 903–911. [Google Scholar] [CrossRef]
- Brenner, M.; Hearing, V.J. Review The Protective Role of Melanin Against UV Damage in Human Skin. Photochem. Photobiol. 2008, 84, 539–549. [Google Scholar] [CrossRef]
- Gloster, H.M.; Neal, K. Skin cancer in skin of color. J. Am. Acad. Dermatol. 2006, 55, 741–760. [Google Scholar] [CrossRef] [PubMed]
- Heymann, W.R. Skin cancer in African Americans. J. Am. Acad. Dermatol. 2005, 53, 484–485. [Google Scholar] [CrossRef]
- Simon, J.D.; Peles, D.; Wakamatsu, K.; Ito, S. Current challenges in understanding melanogenesis: Bridging chemistry, biological control, morphology, and function. Pigment Cell Melanoma Res. 2009, 22, 563–579. [Google Scholar] [CrossRef] [PubMed]
- Munoz-Munoz, J.L.; García-Molina, F.; Varón, R.; Tudela, J.; García-Cánovas, F.; Rodríguez-López, J.N. Generation of hydrogen peroxide in the melanin biosynthesis pathway. Biochim. Biophys. Acta Proteins Proteom. 2009, 1794, 1017–1029. [Google Scholar] [CrossRef]
- Noonan, F.P.; Zaidi, M.R.; Wolnicka-Glubisz, A.; Anver, M.R.; Bahn, J.; Wielgus, A.; Cadet, J.; Douki, T.; Mouret, S.; Tucker, M.A.; et al. Melanoma induction by ultraviolet A but not ultraviolet B radiation requires melanin pigment. Nat. Commun. 2012, 3, 884. [Google Scholar] [CrossRef]
- Sarna, M.; Krzykawska-Serda, M.; Jakubowska, M.; Zadlo, A.; Urbanska, K. Melanin presence inhibits melanoma cell spread in mice in a unique mechanical fashion. Sci. Rep. 2019, 9, 9280. [Google Scholar] [CrossRef]
- Liu-Smith, F.; Dellinger, R.; Meyskens, F.L. Updates of reactive oxygen species in melanoma etiology and progression. Arch. Biochem. Biophys. 2014, 563, 51–55. [Google Scholar] [CrossRef]
- Liu, F.; Gomez Garcia, A.M.; Meyskens, F.L. NADPH oxidase 1 overexpression enhances invasion via matrix metalloproteinase-2 and epithelial-mesenchymal transition in melanoma cells. J. Investig. Dermatol. 2012, 132, 2033–2041. [Google Scholar] [CrossRef]
- Govindarajan, B.; Sligh, J.E.; Vincent, B.J.; Li, M.; Canter, J.A.; Nickoloff, B.J.; Rodenburg, R.J.; Smeitink, J.A.; Oberley, L.; Zhang, Y.; et al. Overexpression of Akt converts radial growth melanoma to vertical growth melanoma. J. Clin. Investig. 2007, 117, 719–729. [Google Scholar] [CrossRef] [PubMed]
- Yamaura, M.; Mitsushita, J.; Furuta, S.; Kiniwa, Y.; Ashida, A.; Goto, Y.; Shang, W.H.; Kubodera, M.; Kato, M.; Takata, M.; et al. NADPH oxidase 4 contributes to transformation phenotype of melanoma cells by regulating G2-M cell cycle progression. Cancer Res. 2009, 69, 2647–2654. [Google Scholar] [CrossRef] [PubMed]
- Meitzler, J.L.; Makhlouf, H.R.; Antony, S.; Wu, Y.; Butcher, D.; Jiang, G.; Juhasz, A.; Lu, J.; Dahan, I.; Jansen-Dürr, P.; et al. Decoding NADPH oxidase 4 expression in human tumors. Redox Biol. 2017, 13, 182–195. [Google Scholar] [CrossRef]
- Beretti, F.; Farnetani, F.; Bonetti, L.R.; Fabbiani, L.; Zavatti, M.; Maiorana, A.; Pellacani, G.; Maraldi, T. The interplay between HGF/C-MET axis and NOX4 in braf mutated melanoma. Int. J. Mol. Sci. 2021, 22, 761. [Google Scholar] [CrossRef]
- Antony, S.; Jiang, G.; Wu, Y.; Meitzler, J.L.; Makhlouf, H.R.; Haines, D.C.; Butcher, D.; Hoon, D.S.; Ji, J.; Zhang, Y.; et al. NADPH oxidase 5 (NOX5)—Induced reactive oxygen signaling modulates normoxic HIF-1α and p27Kip1 expression in malignant melanoma and other human tumors. Mol. Carcinog. 2017, 56, 2643–2662. [Google Scholar] [CrossRef] [PubMed]
- Piskounova, E.; Agathocleous, M.; Murphy, M.M.; Hu, Z.; Huddlestun, S.E.; Zhao, Z.; Leitch, A.M.; Johnson, T.M.; DeBerardinis, R.J.; Morrison, S.J. Oxidative stress inhibits distant metastasis by human melanoma cells. Nature 2015, 527, 186–191. [Google Scholar] [CrossRef]
- Sander, C.S.; Hamm, F.; Elsner, P.; Thiele, J.J. Oxidative stress in malignant melanoma and non-melanoma skin cancer. Br. J. Dermatol. 2003, 148, 913–922. [Google Scholar] [CrossRef]
- Xian, D.; Lai, R.; Song, J.; Xiong, X.; Zhong, J. Emerging Perspective: Role of Increased ROS and Redox Imbalance in Skin Carcinogenesis. Oxid. Med. Cell. Longev. 2019, 2019. [Google Scholar] [CrossRef]
- Glaviano, A.; Foo, A.S.C.; Lam, H.Y.; Yap, K.C.H.; Jacot, W.; Jones, R.H.; Eng, H.; Nair, M.G.; Makvandi, P.; Geoerger, B.; et al. PI3K/AKT/mTOR signaling transduction pathway and targeted therapies in cancer. Mol. Cancer 2023, 22, 1–37. [Google Scholar] [CrossRef]
- Wardyn, J.D.; Ponsford, A.H.; Sanderson, C.M. Dissecting molecular cross-talk between Nrf2 and NF-κB response pathways. Biochem. Soc. Trans. 2015, 43, 621–626. [Google Scholar] [CrossRef]
- He, F.; Ru, X.; Wen, T. NRF2, a Transcription Factor for Stress Response and Beyond. Int. J. Mol. Sci. 2020, 21, 4777. [Google Scholar] [CrossRef] [PubMed]
- Baird, L.; Yamamoto, M. The Molecular Mechanisms Regulating the KEAP1-NRF2 Pathway. Mol. Cell. Biol. 2020, 40, 1–23. [Google Scholar] [CrossRef]
- Bloom, D.A.; Jaiswal, A.K. Phosphorylation of Nrf2 at Ser40 by Protein Kinase C in Response to Antioxidants Leads to the Release of Nrf2 from INrf2, but Is Not Required for Nrf2 Stabilization/Accumulation in the Nucleus and Transcriptional Activation of Antioxidant Response Element. J. Biol. Chem. 2003, 278, 44675–44682. [Google Scholar] [CrossRef]
- Lee, J.M.; Hanson, J.M.; Chu, W.A.; Johnson, J.A. Phosphatidylinositol 3-Kinase, Not Extracellular Signal-regulated Kinase, Regulates Activation of the Antioxidant-Responsive Element in IMR-32 Human Neuroblastoma Cells. J. Biol. Chem. 2001, 276, 20011–20016. [Google Scholar] [CrossRef] [PubMed]
- Yu, R.; Chen, C.; Mo, Y.Y.; Hebbar, V.; Owuor, E.D.; Tan, T.H.; Kong, A.N.T. Activation of mitogen-activated protein kinase pathways induces antioxidant response element-mediated gene expression via a Nrf2-dependent mechanism. J. Biol. Chem. 2000, 275, 39907–39913. [Google Scholar] [CrossRef] [PubMed]
- Thiruvengadam, R.; Venkidasamy, B.; Samynathan, R.; Govindasamy, R.; Thiruvengadam, M.; Kim, J.H. Association of nanoparticles and Nrf2 with various oxidative stress-mediated diseases. Chem. Biol. Interact. 2023, 380, 110535. [Google Scholar] [CrossRef] [PubMed]
- George, M.; Tharakan, M.; Culberson, J.; Reddy, A.P.; Reddy, P.H. Role of Nrf2 in aging, Alzheimer’s and other neurodegenerative diseases. Ageing Res. Rev. 2022, 82, 101756. [Google Scholar] [CrossRef]
- Liu, Q.; Gao, Y.; Ci, X. Role of Nrf2 and its activators in respiratory diseases. Oxid. Med. Cell. Longev. 2019, 2019. [Google Scholar] [CrossRef] [PubMed]
- Tanase, D.M.; Gosav, E.M.; Anton, M.I.; Floria, M.; Seritean Isac, P.N.; Hurjui, L.L.; Tarniceriu, C.C.; Costea, C.F.; Ciocoiu, M.; Rezus, C. Oxidative Stress and NRF2/KEAP1/ARE Pathway in Diabetic Kidney Disease (DKD): New Perspectives. Biomolecules 2022, 12, 1227. [Google Scholar] [CrossRef]
- Bourgonje, A.R.; Kloska, D.; Grochot-Przęczek, A.; Feelisch, M.; Cuadrado, A.; van Goor, H. Personalized redox medicine in inflammatory bowel diseases: An emerging role for HIF-1α and NRF2 as therapeutic targets. Redox Biol. 2023, 60, 102603. [Google Scholar] [CrossRef]
- Hämälaïnen, M.; Teppo, H.R.; Skarp, S.; Haapasaari, K.M.; Porvari, K.; Vuopala, K.; Kietzmann, T.; Karihtala, P. NRF1 and NRF2 mRNA and Protein Expression Decrease Early during Melanoma Carcinogenesis: An Insight into Survival and MicroRNAs. Oxid. Med. Cell. Longev. 2019, 2019, 2647068. [Google Scholar] [CrossRef]
- Knatko, E.V.; Ibbotson, S.H.; Zhang, Y.; Higgins, M.; Fahey, J.W.; Talalay, P.; Dawe, R.S.; Ferguson, J.; Huang, J.T.J.; Clarke, R.; et al. Nrf2 activation protects against solar-simulated ultraviolet radiation in mice and humans. Cancer Prev. Res. 2015, 8, 475–486. [Google Scholar] [CrossRef]
- Robledinos-Antón, N.; Fernández-Ginés, R.; Manda, G.; Cuadrado, A. Activators and Inhibitors of NRF2: A Review of Their Potential for Clinical Development. Oxid. Med. Cell. Longev. 2019, 2019, 9372182. [Google Scholar] [CrossRef]
- Takasaki, T.; Hamabe, Y.; Touchi, K.; Khandakar, G.I.; Ueda, T.; Okada, H.; Sakai, K.; Nishio, K.; Tanabe, G.; Sugiura, R. ACA-28, an ERK MAPK Signaling Modulator, Exerts Anticancer Activity through ROS Induction in Melanoma and Pancreatic Cancer Cells. Oxid. Med. Cell. Longev. 2024, 2024, 7683793. [Google Scholar] [CrossRef]
- Yu, B.; Liang, J.; Li, X.; Liu, L.; Yao, J.; Chen, X.; Chen, R. Renieramycin T Inhibits Melanoma B16F10 Cell Metastasis and Invasion via Regulating Nrf2 and STAT3 Signaling Pathways. Molecules 2022, 27, 5337. [Google Scholar] [CrossRef] [PubMed]
- Kuo, H.C.D.; Wu, R.; Li, S.; Yang, A.Y.; Kong, A.N. Anthocyanin Delphinidin Prevents Neoplastic Transformation of Mouse Skin JB6 P+ Cells: Epigenetic Re-activation of Nrf2-ARE Pathway. AAPS J. 2019, 21. [Google Scholar] [CrossRef]
- Zhu, H.; Jia, Z.; Trush, M.A.; Li, Y.R. Nrf2 Deficiency Promotes Melanoma Growth and Lung Metastasis. React Oxyg Species 2016, 2, 308–314. [Google Scholar] [CrossRef][Green Version]
- De Cicco, P.; Busà, R.; Ercolano, G.; Formisano, C.; Allegra, M.; Taglialatela-Scafati, O.; Ianaro, A. Inhibitory effects of cynaropicrin on human melanoma progression by targeting MAPK, NF-κB, and Nrf-2 signaling pathways in vitro. Phyther. Res. 2021, 35, 1432–1442. [Google Scholar] [CrossRef]
- Luke, J.J.; Flaherty, K.T.; Ribas, A.; Long, G.V. Target agents and immunotherapies: Optimizing outcomes in melanoma. Nat. Rev. Clin. Oncol. 2017, 14, 463–482. [Google Scholar] [CrossRef] [PubMed]
- Lorusso, P.M.; Schalper, K.; Sosman, J. Target therapy and immunotherapy:emerging biomarkers in metastatic melanoma. Pigment Cell Melanoma Res. 2019, 33, 390–402. [Google Scholar] [CrossRef] [PubMed]
- Malakoutikhah, Z.; Mohajeri, Z.; Dana, N.; Haghjooy Javanmard, S. The dual role of Nrf2 in melanoma: A systematic review. BMC Mol. Cell Biol. 2023, 24, 5. [Google Scholar] [CrossRef]
- Zhu, B.; Tang, L.; Chen, S.; Yin, C.; Peng, S.; Li, X.; Liu, T.; Liu, W.; Han, C.; Stawski, L.; et al. Targeting the upstream transcriptional activator of PD-L1 as an alternative strategy in melanoma therapy. Oncogene 2018, 37, 4941–4954. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, E.L.; Becker, A.L.; Indra, A.K. NRF2 and Key Transcriptional Targets in Melanoma Redox Manipulation. Cancers 2022, 14, 1531. [Google Scholar] [CrossRef]
- Coleman, D.J.; Garcia, G.; Hyter, S.; Jang, H.S.; Chagani, S.; Liang, X.; Larue, L.; Ganguli-indra, G.; Indra, A.K. Retinoid-X-Receptors (a/b) in Melanocytes Modulate Innate Immune Responses and Differentially Regulate Cell Survival following UV Irradiation. PLoS Genet. 2014, 10, e1004321. [Google Scholar] [CrossRef]
- Cucci, M.A.; Grattarola, M.; Monge, C.; Roetto, A.; Barrera, G.; Caputo, E.; Dianzani, C.P.S. Nrf2 as a Therapeutic Target in the Resistance to Targeted Therapies in Melanoma. Antioxidants 2023, 12, 1313. [Google Scholar] [CrossRef] [PubMed]
- Khamari, R.; Trinh, A.; Gabert, P.E.; Corazao-rozas, P.; Riveros-cruz, S.; Balayssac, S.; Malet-martino, M.; Dekiouk, S.; Joncquel, M.; Curt, C.; et al. Glucose metabolism and NRF2 coordinate the antioxidant response in melanoma resistant to MAPK inhibitors. Cell Death Dis. 2018, 9, 325. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef] [PubMed]
- Schreck, R.; Rieber, P.; Baeuerle, P.A. Reactive oxygen intermediates as apparently widely used messengers in the activation of the NF-kappa B transcription factor and HIV-1. EMBO J. 1991, 10, 2247–2258. [Google Scholar] [CrossRef]
- Takada, Y.; Mukhopadhyay, A.; Kundu, G.C.; Mahabeleshwar, G.H.; Singh, S.; Aggarwal, B.B. Hydrogen peroxide activates NF-κB through tyrosine phosphorylation of IκBα and serine phosphorylation of p65. Evidence for the involvement of IκBα kinase and Syk protein-tyrosine kinase. J. Biol. Chem. 2003, 278, 24233–24241. [Google Scholar] [CrossRef]
- Schoonbroodt, S.; Best-belpomme, M.; Boelaert, J.R. Crucial Role of the Amino-Terminal Tyrosine Residue 42 and the Carboxyl- Terminal PEST Domain of I kappa B alpha in NF-kappa B activation by an oxidative stress. J. Immunol. 2000, 164, 4292–4300. [Google Scholar] [CrossRef]
- Béraud, C.; Henzel, W.J.; Baeuerle, P.A. Involvement of regulatory and catalytic subunits of phosphoinositide 3-kinase in NF-κB activation. Proc. Natl. Acad. Sci. USA 1999, 96, 429–434. [Google Scholar] [CrossRef]
- Morgan, M.J.; Liu, Z.G. Crosstalk of reactive oxygen species and NF-κB signaling. Cell Res. 2011, 21, 103–115. [Google Scholar] [CrossRef]
- McNulty, S.E.; Tohidian, N.B.; Meyskens, F.L. RelA, p50 and inhibitor of kappa B alpha are elevated in human metastatic melanoma cells and respond aberrantly to ultraviolet light B. Pigment Cell Res. 2001, 14, 456–465. [Google Scholar] [CrossRef] [PubMed]
- Meyskens, F.L.; McNulty, S.E.; Buckmeier, J.A.; Tohidian, N.B.; Spillane, T.J.; Kahlon, R.S.; Gonzalez, R.I. Aberrant redox regulation in human metastatic melanoma cells compared to normal melanocytes. Free Radic. Biol. Med. 2001, 31, 799–808. [Google Scholar] [CrossRef]
- McNulty, S.E.; Del Rosario, R.; Cen, D.; Meyskens, F.L.; Yang, S. Comparative Expression of NFκB Proteins in Melanocytes of Normal Skin vs. Benign Intradermal Naevus and Human Metastatic Melanoma Biopsies. Pigment Cell Res. 2004, 17, 173–180. [Google Scholar] [CrossRef]
- Wang, C.Y.; Mayo, M.W.; Korneluk, R.G.; Goeddel, D.V.; Baldwin, A.S. NF-≃B antiapoptosis: Induction of TRAF1 and TRAF2 and c-IAP1 and c- IAP2 to suppress caspase-8 activation. Science 1998, 281, 1680–1683. [Google Scholar] [CrossRef] [PubMed]
- Alonso, S.R.; Ortiz, P.; Pollán, M.; Pérez-Gómez, B.; Sánchez, L.; Acuña, M.J.; Pajares, R.; Martínez-Tello, F.J.; Hortelano, C.M.; Piris, M.A.; et al. Progression in Cutaneous Malignant Melanoma Is Associated with Distinct Expression Profiles: A Tissue Microarray-Based Study. Am. J. Pathol. 2004, 164, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Guttridge, D.C.; Albanese, C.; Reuther, J.Y.; Pestell, R.G.; Baldwin, A.S. NF-κB Controls Cell Growth and Differentiation through Transcriptional Regulation of Cyclin D1. Mol. Cell. Biol. 1999, 19, 5785–5799. [Google Scholar] [CrossRef]
- Marín, Y.E.; Wall, B.A.; Wang, S.; Namkoong, J.; Martino, J.J.; Suh, J.; Lee, H.J.; Rabson, A.B.; Yang, C.S.; Chen, S.; et al. Curcumin downregulates the constitutive activity of NF-κB and induces apoptosis in novel mouse melanoma cells. Melanoma Res. 2007, 17, 274–283. [Google Scholar] [CrossRef]
- Cardile, A.; Zanrè, V.; Campagnari, R.; Asson, F.; Addo, S.S.; Orlandi, E.; Menegazzi, M. Hyperforin Elicits Cytostatic/Cytotoxic Activity in Human Melanoma Cell Lines, Inhibiting Pro-Survival NF-κB, STAT3, AP1 Transcription Factors and the Expression of Functional Proteins Involved in Mitochondrial and Cytosolic Metabolism. Int. J. Mol. Sci. 2023, 24, 1263. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Liu, G.; Huang, Z.; Zhu, Z.; Yang, K.; Liang, Y.; Xu, Y.; Zhang, L.; Du, Z. Tremella fuciformis Polysaccharide Induces Apoptosis of B16 Melanoma Cells via Promoting the M1 Polarization of Macrophages. Molecules 2023, 28, 4018. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, R.C.; do Nascimento, Y.M.; de Araújo Loureiro, P.B.; Martins, R.X.; de Souza Maia, M.E.; Farias, D.F.; Tavares, J.F.; Gonçalves, J.C.R.; da Silva, M.S.; Sobral, M.V. Chemical Composition, In Vitro Antitumor Effect, and Toxicity in Zebrafish of the Essential Oil from Conyza bonariensis (L.) Cronquist (Asteraceae). Biomolecules 2023, 13, 1439. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, R.C.; Duarte, S.S.; de Sousa, V.M.; de Souza, R.R.M.; Marques, K.K.G.; de Abrantes, R.A.; do Nascimento, Y.M.; de Sousa, N.F.; Scotti, M.T.; Scotti, L.; et al. The Essential Oil from Conyza bonariensis (L.) Cronquist (Asteraceae) Exerts an In Vitro Antimelanoma Effect by Inducing Apoptosis and Modulating the MAPKs, NF-κB, and PKB/AKT Signaling Pathways. Pharmaceuticals 2023, 16, 1553. [Google Scholar] [CrossRef]
- Perkins, N.D.; Gilmore, T.D. Good cop, bad cop: The different faces of NF-κB. Cell Death Differ. 2006, 13, 759–772. [Google Scholar] [CrossRef]
- Parrondo, R.; Pozas, A.D.L.; Reiner, T.; Rai, P.; Perez-Stable, C. NF-κB activation enhances cell death by antimitotic drugs in human prostate cancer cells. Mol. Cancer 2010, 9, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Bellezza, I.; Mierla, A.L.; Minelli, A. Nrf2 and NF-κB and their concerted modulation in cancer pathogenesis and progression. Cancers 2010, 2, 483–497. [Google Scholar] [CrossRef] [PubMed]
- Murgas, K.A.; Elkin, R.; Riaz, N.; Tannenbaum, A.R. Multi-scale geometric network analysis identifies melanoma immunotherapy response gene modules. Sci. Rep. 2024, 14, 6082. [Google Scholar] [CrossRef]
- Lalle, G.; Twardowski, J.; Grinberg-bleyer, Y. NF- κ B in Cancer Immunity: Friend or Foe? Cells 2021, 10, 355. [Google Scholar] [CrossRef] [PubMed]
- Vergani, E.; Dugo, M.; Cossa, M.; Frigerio, S.; Di Guardo, L.; Gallino, G.; Mattavelli, I.; Vergani, B.; Lalli, L.; Tamborini, E.; et al. miR-146a-5p impairs melanoma resistance to kinase inhibitors by targeting COX2 and regulating NFkB-mediated inflammatory mediators. Cell Commun. Signal. 2020, 1, 1–14. [Google Scholar] [CrossRef]
- Konieczkowski, D.J.; Johannessen, C.M.; Abudayyeh, O.; Kim, J.W.; Cooper, Z.A.; Piris, A.; Frederick, D.T.; Barzily-rokni, M.; Straussman, R.; Haq, R.; et al. A Melanoma Cell State Distinction Infl uences Sensitivity to MAPK Pathway Inhibitors. Cancer Discov. 2014, 4, 816–827. [Google Scholar] [CrossRef]
- Bellezza, I.; Tucci, A.; Galli, F.; Grottelli, S.; Mierla, A.L.; Pilolli, F.; Minelli, A. Inhibition of NF-κB nuclear translocation via HO-1 activation underlies α-tocopheryl succinate toxicity. J. Nutr. Biochem. 2012, 23, 1583–1591. [Google Scholar] [CrossRef]
- Sun, Y.Y.; Zhu, H.J.; Zhao, R.Y.; Zhou, S.Y.; Wang, M.Q.; Yang, Y.; Guo, Z.N. Remote ischemic conditioning attenuates oxidative stress and inflammation via the Nrf2/HO-1 pathway in MCAO mice. Redox Biol. 2023, 66, 102852. [Google Scholar] [CrossRef]
- Fang, Z.; Wang, G.; Huang, R.; Liu, C.; Yushanjiang, F.; Mao, T.; Li, J. Astilbin protects from sepsis-induced cardiac injury through the NRF2/HO-1 and TLR4/NF-κB pathway. Phyther. Res. 2024, 38, 1044–1058. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, H.; Li, X.; Li, L.; Li, F.; Li, T.; Peng, R.; Wang, C.; Wang, J.; Liu, X.; et al. NS1619 Alleviate Brain-Derived Extracellular Vesicle-Induced Brain Injury by Regulating BKca Channel and Nrf2/HO-1/NF-ĸB Pathway. Oxid. Med. Cell. Longev. 2022, 2022, 2257427. [Google Scholar] [CrossRef]
- Wakabayashi, N.; Slocum, S.L.; Skoko, J.J.; Shin, S.; Kensler, T.W. When NRF2 talks, who’s listening? Antioxid. Redox Signal. 2010, 13, 1649–1663. [Google Scholar] [CrossRef]
- Brigelius-Flohé, R.; Flohé, L. Basic principles and emerging concepts in the redox control of transcription factors. Antioxid. Redox Signal. 2011, 15, 2335–2381. [Google Scholar] [CrossRef]
- Liu, G.H.; Qu, J.; Shen, X. NF-κB/p65 antagonizes Nrf2-ARE pathway by depriving CBP from Nrf2 and facilitating recruitment of HDAC3 to MafK. Biochim. Biophys. Acta Mol. Cell Res. 2008, 1783, 713–727. [Google Scholar] [CrossRef] [PubMed]
- Long, G.V.; Swetter, S.M.; Menzies, A.M.; Gershenwald, J.E.; Scolyer, R.A. Cutaneous melanoma. Lancet 2023, 402, 485–502. [Google Scholar] [CrossRef]
- de Almeida Gonçalves, V.D.M.; de Almeida Camargo Filho, M.F.; Zaleski, T.; Rodrigues Vilas Boas, R.; Rossi Ribeiro, E.; Saad Vaz, R.; Bridi Cavassin, F. Chemotherapy in focus: A meta-analysis confronts immunotherapy in the treatment of advanced melanoma. Crit. Rev. Oncol. Hematol. 2021, 161, 103304. [Google Scholar] [CrossRef] [PubMed]
- Ralli, M.; Botticelli, A.; Visconti, I.C.; Angeletti, D.; Fiore, M.; Marchetti, P.; Lambiase, A.; De Vincentiis, M.; Greco, A. Immunotherapy in the Treatment of Metastatic Melanoma: Current Knowledge and Future Directions. J. Immunol. Res. 2020, 2020, 9235638. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, M.F.; Choi, J.; Sosman, J. New Approaches to Targeted Therapy in Melanoma. Cancers 2023, 15, 3224. [Google Scholar] [CrossRef]
- Obrador, E.; Liu-Smith, F.; Dellinger, R.W.; Salvador, R.; Meyskens, F.L.; Estrela, J.M. Oxidative stress and antioxidants in the pathophysiology of malignant melanoma. Biol. Chem. 2019, 400, 589–612. [Google Scholar] [CrossRef]
- Venza, I.; Venza, M.; Visalli, M.; Lentini, G.; Teti, D.; D’Alcontres, F.S. ROS as Regulators of Cellular Processes in Melanoma. Oxid. Med. Cell. Longev. 2021, 2021, 1208690. [Google Scholar] [CrossRef]
- Sagwal, S.K.; Bekeschus, S. ROS Pleiotropy in Melanoma and Local Therapy with Physical Modalities. Oxid. Med. Cell. Longev. 2021, 2021, 6816214. [Google Scholar] [CrossRef]
- Krayem, M.; Ghanem, G.E.; Van Gestel, D. Recent advances in radiosensitivity determinants in melanoma. Curr. Opin. Oncol. 2022, 34, 131–138. [Google Scholar] [CrossRef]
- Strojan, P. Role of radiotherapy in melanoma management. Radiol. Oncol. 2010, 44, 1–12. [Google Scholar] [CrossRef]
- Zheng, Z.; Su, J.; Bao, X.; Wang, H.; Bian, C.; Zhao, Q.; Jiang, X. Mechanisms and applications of radiation-induced oxidative stress in regulating cancer immunotherapy. Front. Immunol. 2023, 14, 1247268. [Google Scholar] [CrossRef] [PubMed]
- Matsuoka, Y.; Yoshida, R.; Kawahara, K.; Sakata, J.; Arita, H.; Nkashima, H.; Takahashi, N.; Hirayama, M.; Nagata, M.; Hirosue, A.; et al. The antioxidative stress regulator Nrf2 potentiates radioresistance of oral squamous cell carcinoma accompanied with metabolic modulation. Lab. Investig. 2022, 102, 896–907. [Google Scholar] [CrossRef]
- Zhou, S.; Ye, W.; Shao, Q.; Zhang, M.; Liang, J. Nrf2 is a potential therapeutic target in radioresistance in human cancer. Crit. Rev. Oncol. Hematol. 2013, 88, 706–715. [Google Scholar] [CrossRef]
- Gao, Y.; Zhao, Z.; Meng, X.; Chen, H.; Fu, G. Migration and invasion in b16-f10 mouse melanoma cells are regulated by nrf2 inhibition during treatment with ionizing radiation. Oncol. Lett. 2018, 16, 1959–1966. [Google Scholar] [CrossRef] [PubMed]
- Tagliaferri, L.; Lancellotta, V.; Fionda, B.; Mangoni, M.; Casà, C.; Di Stefani, A.; Pagliara, M.M.; Aviero, D.; Schinzari, G.; Chiesa, S.; et al. Immunotherapy and radiotherapy in melanoma: A multidisciplinary comprehensive review. Hum. Vaccin. Immunother. 2022, 18, 1903827. [Google Scholar] [CrossRef] [PubMed]
- Honors, C.N.; Kruger, C.A.; Abrahamse, H. Photodynamic therapy for metastatic melanoma treatment: A review. Technol. Cancer Res. Treat. 2018, 17, 1–15. [Google Scholar] [CrossRef]
- Civantos, F.J.; Karakullukcu, B.; Biel, M.; Silver, C.E.; Rinaldo, A.; Saba, N.F.; Takes, R.P.; Vander Poorten, V.; Ferlito, A. A Review of Photodynamic Therapy for Neoplasms of the Head and Neck. Adv. Ther. 2018, 35, 324–340. [Google Scholar] [CrossRef]
- Baldea, I.; Giurgiu, L.; Teacoe, I.D.; Olteanu, D.E.; Olteanu, F.C.; Clichici, S.; Filip, G.A. Photodynamic Therapy in Melanoma—Where do we Stand? Curr. Med. Chem. 2017, 25, 5540–5563. [Google Scholar] [CrossRef]
- Algorri, F.; Miguel, L. Advanced Light Source Technologies for Photodynamic Therapy of Skin Cancer Lesions. Pharmaceutics 2023, 15, 2075. [Google Scholar] [CrossRef] [PubMed]
- Schildkopf, P.; Ott, O.J.; Frey, B.; Wadepohl, M.; Sauer, R.; Fietkau, R.; S. Gaipl, U. Biological Rationales and Clinical Applications of Temperature Controlled Hyperthermia—Implications for Multimodal Cancer Treatments. Curr. Med. Chem. 2010, 17, 3045–3057. [Google Scholar] [CrossRef] [PubMed]
- Overgaard, J.; Gonzalez Gonzalez, D.; Hulshof, M.C.C.H.; Arcangeli, G.; Dahl, O.; Mella, O.; Bentzen, S.M. Hyperthermia as an adjuvant to radiation therapy of recurrent or metastatic malignant melanoma. A multicentre randomized trial by the European Society for Hyperthermic Oncology. Int. J. Hyperth. 2009, 25, 323–334. [Google Scholar] [CrossRef]
- Miebach, L.; Melo-Zainzinger, G.; Freund, E.; Clemen, R.; Cecchini, A.L.; Bekeschus, S. Medical Gas Plasma Technology Combines with Antimelanoma Therapies and Promotes Immune-Checkpoint Therapy Responses. Adv. Sci. 2023, 10, 2303183. [Google Scholar] [CrossRef]
- Rahimi, A.; Esmaeili, Y.; Dana, N.; Dabiri, A.; Rahimmanesh, I.; Jandaghian, S.; Vaseghi, G.; Shariati, L.; Zarrabi, A.; Haghjooy Javanmard, S.; et al. A comprehensive review on novel targeted therapy methods and nanotechnology-based gene delivery systems in melanoma. Eur. J. Pharm. Sci. 2023, 187, 106476. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, F.; Zhang, H.X.; Zi, X.Y.; Pan, X.H.; Chen, F.; Luo, W.D.; Li, J.X.; Zhu, H.Y.; Hu, Y.P. Cuprous oxide nanoparticles inhibit the growth and metastasis of melanoma by targeting mitochondria. Cell Death Dis. 2013, 4, e783. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, M.; Kaushik, N.; Ghimire, B.; Adhikari, B.; Baboota, S.; Al-Khedhairy, A.A.; Wahab, R.; Lee, S.J.; Kaushik, N.K.; Choi, E.H. Cold atmospheric plasma and silymarin nanoemulsion synergistically inhibits human melanoma tumorigenesis via targeting HGF/c-MET downstream pathway. Cell Commun. Signal. 2019, 17, 1–14. [Google Scholar] [CrossRef]
- Pereira, I.; Monteiro, C.; Pereira-Silva, M.; Peixoto, D.; Nunes, C.; Reis, S.; Veiga, F.; Hamblin, M.R.; Paiva-Santos, A.C. Nanodelivery systems for cutaneous melanoma treatment. Eur. J. Pharm. Biopharm. 2023, 184, 214–247. [Google Scholar] [CrossRef]
- Miura, K.; Green, A.C. Dietary Antioxidants and Melanoma: Evidence from Cohort and Intervention Studies. Nutr. Cancer 2015, 67, 867–876. [Google Scholar] [CrossRef]
- Asgari, M.M.; Brasky, T.M.; White, E. Association of vitamin a and carotenoid intake with melanoma risk in a large prospective cohort. J. Investig. Dermatol. 2012, 132, 1573–1582. [Google Scholar] [CrossRef]
- Hyeraci, M.; Papanikolau, E.S.; Grimaldi, M.; Ricci, F.; Pallotta, S.; Monetta, R.; Minafò, Y.A.; Di Lella, G.; Galdo, G.; Abeni, D.; et al. Systemic Photoprotection in Melanoma and Non-Melanoma Skin Cancer. Biomolecules 2023, 13, 1067. [Google Scholar] [CrossRef] [PubMed]
- Goodson, A.G.; Cotter, M.A.; Cassidy, P.; Wade, M.; Florell, S.R.; Liu, T.; Boucher, K.M.; Grossman, D. Use of oral N-acetylcysteine for protection of melanocytic nevi against UV-induced oxidative stress: Towards a novel paradigm for melanoma chemoprevention. Clin. Cancer Res. 2009, 15, 7434–7440. [Google Scholar] [CrossRef]
- Obrador, E.; Salvador-palmer, R.; Rafael, L.; Oriol-caballo, M. N-Acetylcysteine Promotes Metastatic Spread of Melanoma in Mice. Cancers 2022, 14, 3614. [Google Scholar] [CrossRef]
- Chhabra, G.; Ndiaye, M.A.; Garcia-Peterson, L.M.; Ahmad, N. Melanoma Chemoprevention: Current Status and Future Prospects. Photochem. Photobiol. 2017, 93, 975–989. [Google Scholar] [CrossRef]
- Kashif, M.; Yao, H.; Schmidt, S.; Chen, X.; Truong, M.; Tüksammel, E.; Liu, Y.; Bergo, M.O. ROS-lowering doses of vitamins C and A accelerate malignant melanoma metastasis. Redox Biol. 2023, 60, 102619. [Google Scholar] [CrossRef]
- Cassidy, P.B.; Fain, H.D.; Cassidy, J.P., Jr.; Tran, S.M.; Moos, P.J.; Leachman, S.A. Selenium for the Prevention of Cutaneous Melanoma. Nutrients 2013, 5, 725–749. [Google Scholar] [CrossRef] [PubMed]
- Arslanbaeva, L.R.; Santoro, M.M. Adaptive redox homeostasis in cutaneous melanoma. Redox Biol. 2020, 37, 101753. [Google Scholar] [CrossRef]
- Pizzimenti, S.; Ribero, S.; Cucci, M.A.; Grattarola, M.; Monge, C.; Dianzani, C.; Barrera, G.; Muzio, G. Oxidative stress-related mechanisms in melanoma and in the acquired resistance to targeted therapies. Antioxidants 2021, 10, 1942. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benedusi, M.; Lee, H.; Lim, Y.; Valacchi, G. Oxidative State in Cutaneous Melanoma Progression: A Question of Balance. Antioxidants 2024, 13, 1058. https://doi.org/10.3390/antiox13091058
Benedusi M, Lee H, Lim Y, Valacchi G. Oxidative State in Cutaneous Melanoma Progression: A Question of Balance. Antioxidants. 2024; 13(9):1058. https://doi.org/10.3390/antiox13091058
Chicago/Turabian StyleBenedusi, Mascia, Heaji Lee, Yunsook Lim, and Giuseppe Valacchi. 2024. "Oxidative State in Cutaneous Melanoma Progression: A Question of Balance" Antioxidants 13, no. 9: 1058. https://doi.org/10.3390/antiox13091058
APA StyleBenedusi, M., Lee, H., Lim, Y., & Valacchi, G. (2024). Oxidative State in Cutaneous Melanoma Progression: A Question of Balance. Antioxidants, 13(9), 1058. https://doi.org/10.3390/antiox13091058









