The Antitumour Mechanisms of Carotenoids: A Comprehensive Review
Abstract
1. Introduction
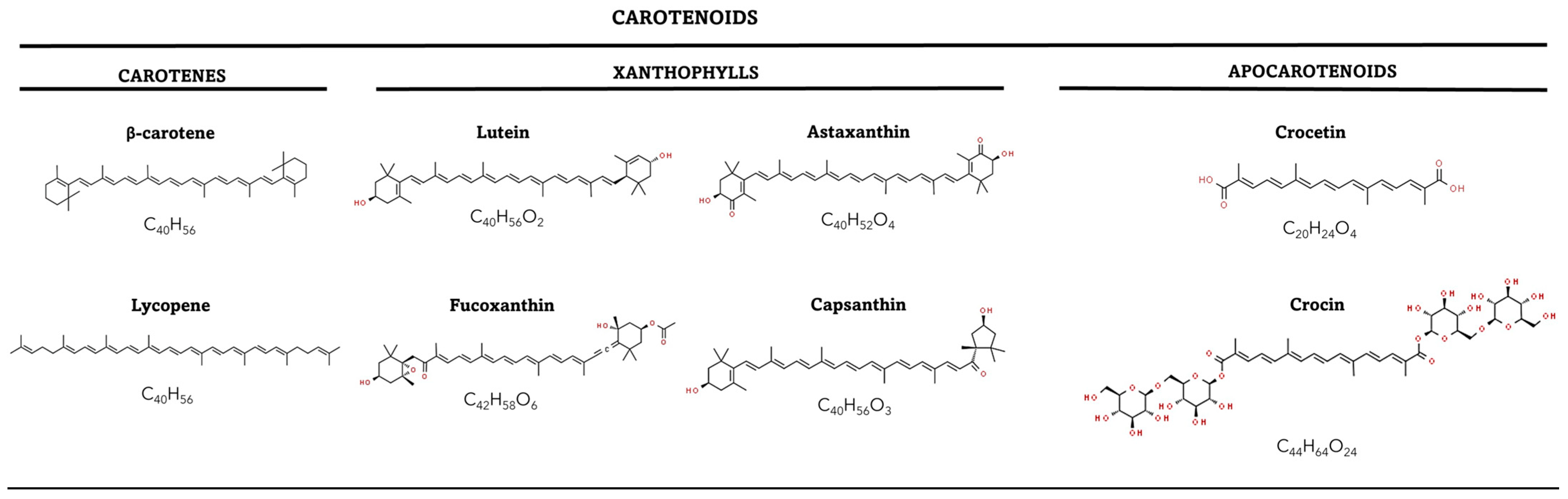
2. Biochemistry of the Carotenoids
2.1. Structure and Diversity
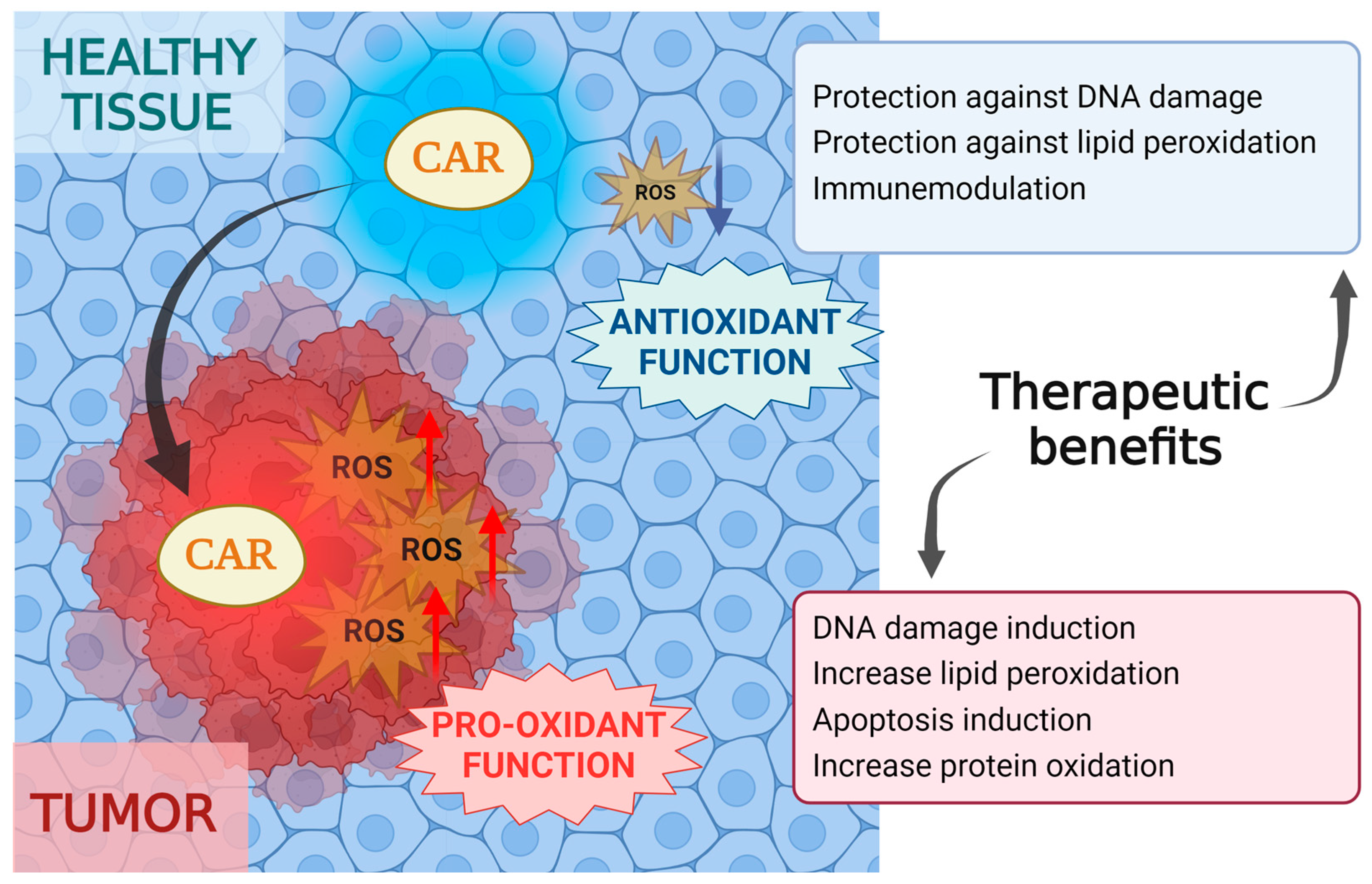
2.2. Antioxidant and Pro-Oxidant Function
- Electron transfer (oxidation/reduction): CAR + R· → CAR·+ + R-;
- Hydrogen abstraction: CAR + R· → CAR· + RH;
- Addition: CAR + R· → R-CAR·.
2.3. Bioavailability and Biotransformation
3. Oxidative Stress and Cancer
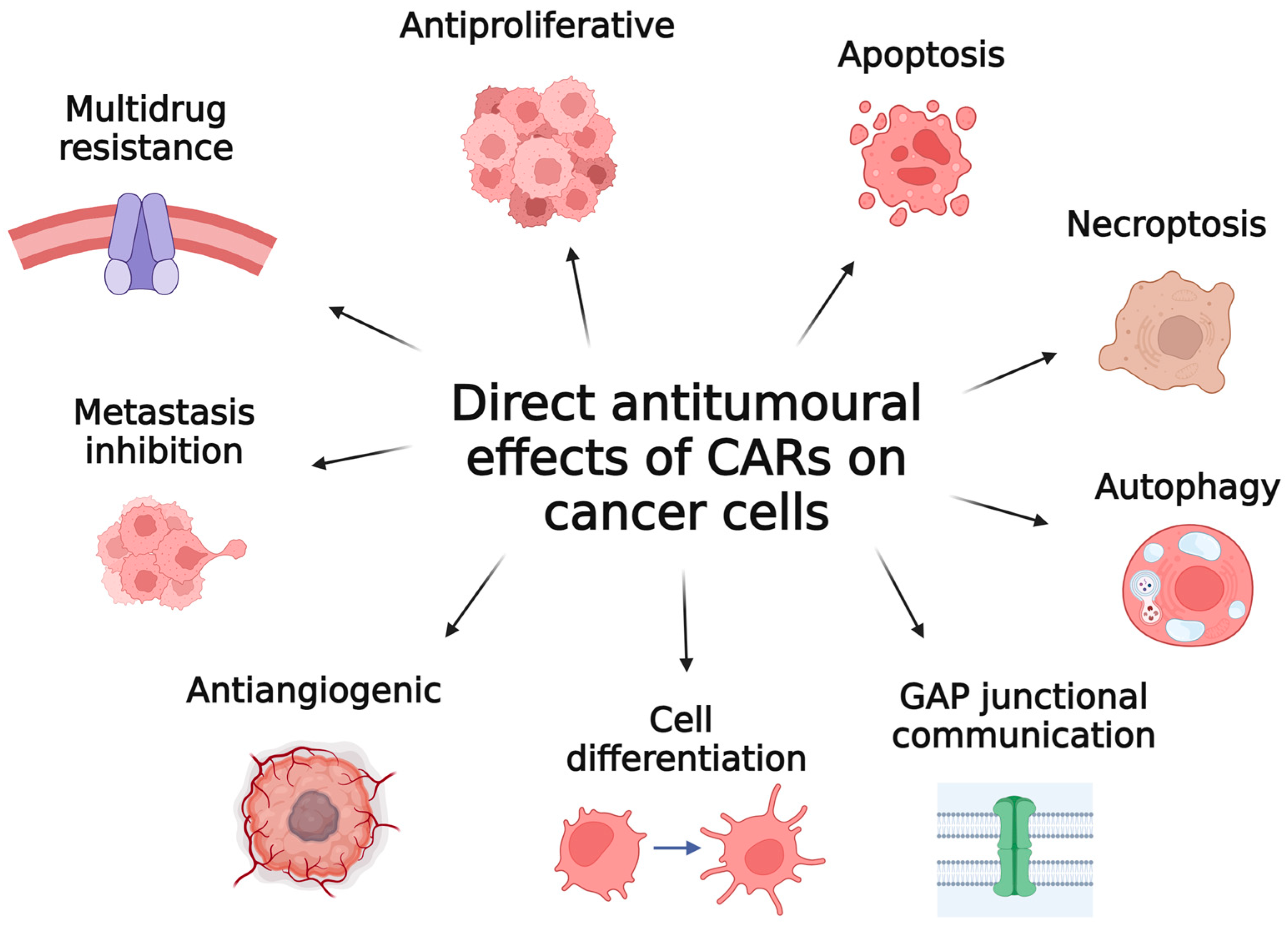
4. Direct Antitumoural Effects of Carotenoids on Cancer Cells
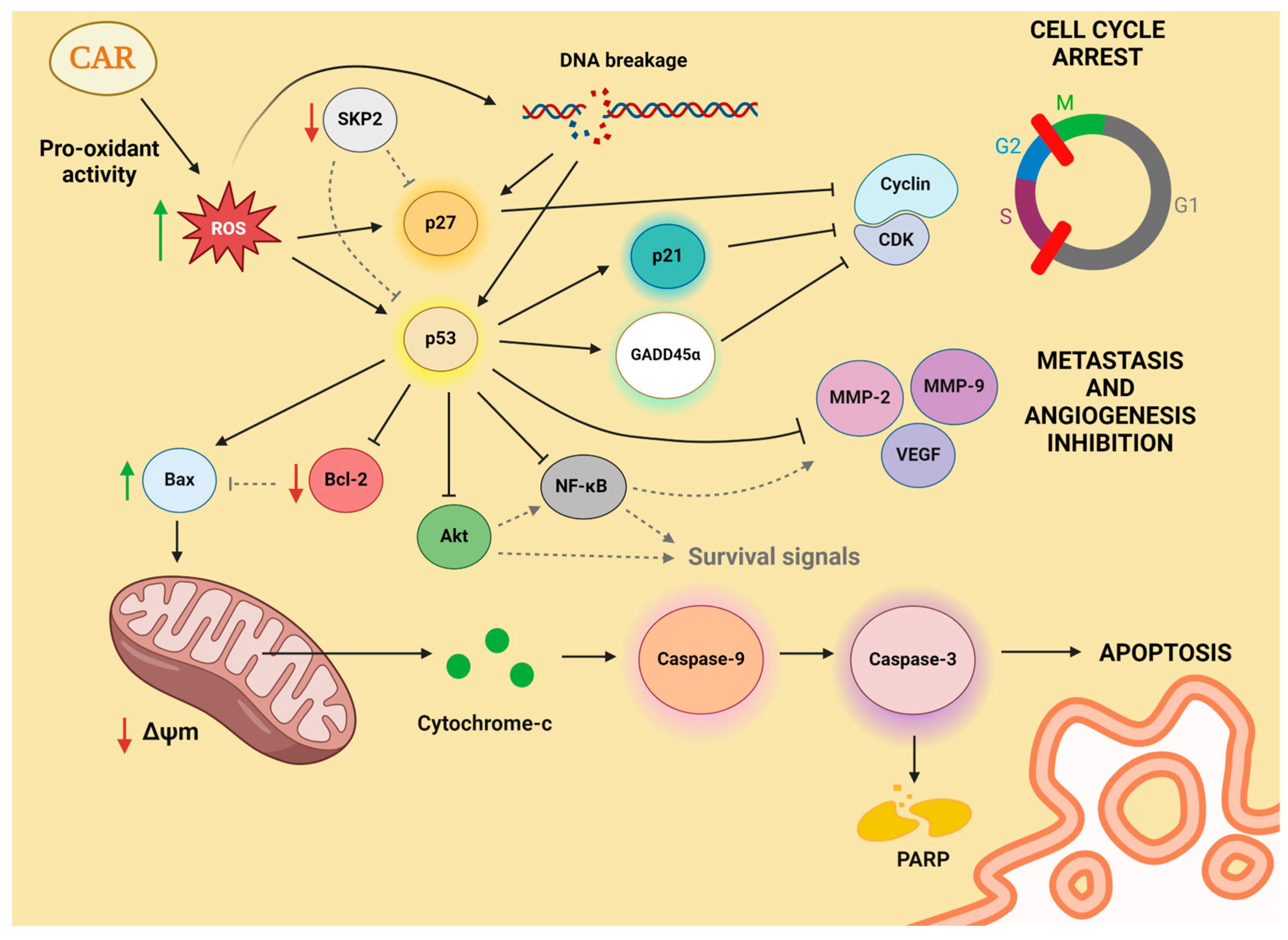
4.1. Cell Cycle Progression and Antiproliferation
4.1.1. Role of β-Carotene in Cell Cycle Regulation and Antiproliferative Effects on Cancer Cells
4.1.2. Role of Lycopene in Cell Cycle Regulation and Antiproliferative Effects on Cancer Cells
4.1.3. Role of Lutein in Cell Cycle Regulation and Antiproliferative Effects on Cancer Cells
4.1.4. Role of Astaxanthin in Cell Cycle Regulation and Antiproliferative Effects on Cancer Cells
4.1.5. Role of Fucoxanthin in Cell Cycle Regulation and Antiproliferative Effects on Cancer Cells
4.1.6. Role of Capsanthin in Cell Cycle Regulation and Antiproliferative Effects on Cancer Cells
4.1.7. Role of Crocetin and Crocin in Cell Cycle Regulation and Antiproliferative Effects on Cancer Cells
4.2. Apoptosis Induction
4.2.1. Role of β-Carotene in Inducing Apoptosis in Cancer Cells
4.2.2. Role of Lycopene in Inducing Apoptosis in Cancer Cells
4.2.3. Role of Lutein in Inducing Apoptosis in Cancer Cells
4.2.4. Role of Astaxanthin in Inducing Apoptosis in Cancer Cells
4.2.5. Role of Fucoxanthin in Inducing Apoptosis in Cancer Cells
4.2.6. Role of Capsanthin in Inducing Apoptosis in Cancer Cells
4.2.7. Role of Crocetin and Crocin in Inducing Apoptosis in Cancer Cells
4.3. Inhibition of Metastasis and Antiangiogenic Effect
4.3.1. Inhibition of Metastasis and Antiangiogenic Effects of β-Carotene in Cancer Cells
4.3.2. Inhibition of Metastasis and Antiangiogenic Effects of Lycopene in Cancer Cells
4.3.3. Inhibition of Metastasis and Antiangiogenic Effects of Lutein in Cancer Cells
4.3.4. Inhibition of Metastasis and Antiangiogenic Effects of Astaxanthin in Cancer Cells
4.3.5. Inhibition of Metastasis and Antiangiogenic Effects of Fucoxanthin in Cancer Cells
4.3.6. Inhibition of Metastasis and Antiangiogenic Effects of Capsanthin in Cancer Cells
4.3.7. Inhibition of Metastasis and Antiangiogenic Effects of Crocetin and Crocin in Cancer Cells
4.4. Other Effects
4.4.1. Autophagy and Necroptosis
4.4.2. Induction of Cell Differentiation
4.4.3. Enhancement of Gap Junctional Communication
4.4.4. Multidrug Resistance
5. Clinical Trials
6. Conclusions and Future Directions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Ighodaro, O.M.; Akinloye, O.A. First Line Defence Antioxidants-Superoxide Dismutase (SOD), Catalase (CAT) and Glutathione Peroxidase (GPX): Their Fundamental Role in the Entire Antioxidant Defence Grid. AJM 2018, 54, 287–293. [Google Scholar] [CrossRef]
- Cauli, O. Oxidative Stress and Cognitive Alterations Induced by Cancer Chemotherapy Drugs: A Scoping Review. Antioxidants 2021, 10, 1116. [Google Scholar] [CrossRef] [PubMed]
- Langi, P.; Kiokias, S.; Varzakas, T.; Proestos, C. Carotenoids: From Plants to Food and Feed Industries. In Methods in Molecular Biology; Springer: Berlin/Heidelberg, Germany, 2018; Volume 1852. [Google Scholar] [CrossRef]
- Johnson, E.J. Role of Lutein and Zeaxanthin in Visual and Cognitive Function throughout the Lifespan. Nutr. Rev. 2014, 72, 605–612. [Google Scholar] [CrossRef]
- Li, C.; Schluesener, H. Health-Promoting Effects of the Citrus Flavanone Hesperidin. Crit. Rev. Food Sci. Nutr. 2017, 57, 613–631. [Google Scholar] [CrossRef]
- Lian, F.; Xiang-Dong, W. Enzymatic Metabolites of Lycopene Induce Nrf2-Mediated Expression of Phase II Detoxifying/Antioxidant Enzymes in Human Bronchial Epithelial Cells. Int. J. Cancer 2008, 123, 1262–1268. [Google Scholar] [CrossRef]
- Thimmulappa, R.K.; Mai, K.H.; Srisuma, S.; Kensler, T.W.; Yamamoto, M.; Biswal, S. Identification of Nrf2-Regulated Genes Induced by the Chemopreventive Agent Sulforaphane by Oligonucleotide Microarray. Cancer Res. 2002, 62, 5196–5203. [Google Scholar]
- Kryston, T.B.; Georgiev, A.B.; Pissis, P.; Georgakilas, A.G. Role of Oxidative Stress and DNA Damage in Human Carcinogenesis. Mutat. Res. 2011, 711, 193–201. [Google Scholar] [CrossRef]
- Qu, M.; Li, L.; Chen, C.; Li, M.; Pei, L.; Chu, F.; Yang, J.; Yu, Z.; Wang, D.; Zhou, Z. Protective Effects of Lycopene against Amyloid β-Induced Neurotoxicity in Cultured Rat Cortical Neurons. Neurosci. Lett. 2011, 505, 286–290. [Google Scholar] [CrossRef]
- Saito, T.; Miyabe, Y.; Ide, H.; Yamamoto, O. Hydroxyl Radical Scavenging Ability of Bacterioruberin. Radiat. Phys. Chem. 1997, 50, 267–269. [Google Scholar] [CrossRef]
- Palozza, P.; Simone, R.; Catalano, A.; Monego, G.; Barini, A.; Mele, M.C.; Parrone, N.; Trombino, S.; Picci, N.; Ranelletti, F.O. Lycopene Prevention of Oxysterol-Induced Proinflammatory Cytokine Cascade in Human Macrophages: Inhibition of NF-ΚB Nuclear Binding and Increase in PPARγ Expression. J. Nutr. Biochem. 2011, 22, 259–268. [Google Scholar] [CrossRef]
- Tanaka, T.; Shnimizu, M.; Moriwaki, H. Cancer Chemoprevention by Carotenoids. Molecules 2012, 17, 3202–3242. [Google Scholar] [CrossRef]
- Bjelakovic, G.; Nikolova, D.; Simonetti, R.G.; Gluud, C. Antioxidant Supplements for Prevention of Gastrointestinal Cancers: A Systematic Review and Meta-Analysis. Lancet 2004, 364, 1219–1228. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Zhang, H.; Chen, J.; Shi, Y.; Cai, J.; Yang, J.; Wu, Y. Association between Dietary Antioxidant Vitamins Intake/Blood Level and Risk of Gastric Cancer. Int. J. Cancer 2014, 135, 1444–1453. [Google Scholar] [CrossRef] [PubMed]
- Astorg, P. Food Carotenoids and Cancer Prevention: An Overview of Current Research. Trends Food Sci. Technol. 1997, 8, 406–413. [Google Scholar] [CrossRef]
- Stahl, W.; Sies, H. Bioactivity and Protective Effects of Natural Carotenoids. Biochim. Biophys. Acta 2004, 1740, 101–107. [Google Scholar] [CrossRef]
- Saini, R.K.; Nile, S.H.; Park, S.W. Carotenoids from Fruits and Vegetables: Chemistry, Analysis, Occurrence, Bioavailability and Biological Activities. Food Res. Int. 2015, 76, 735–750. [Google Scholar] [CrossRef]
- Namitha, K.; Negi, P. Chemistry and Biotechnology of Carotenoids. Crit. Rev. Food Sci. Nutr. 2010, 50, 728–760. [Google Scholar] [CrossRef]
- Sandmann, G. Antioxidant Protection from UV-and Light-Stress Related to Carotenoid Structures. Antioxidants 2019, 8, 219. [Google Scholar] [CrossRef]
- Britton, G. Structure and Properties of Carotenoids in Relation to Function. FASEB J. 1995, 9, 1551–1558. [Google Scholar] [CrossRef] [PubMed]
- Jomova, K.; Valko, M. Health Protective Effects of Carotenoids and Their Interactions with Other Biological Antioxidants. Eur. J. Med. Chem. 2013, 70, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Krinsky, N.I.; Yeum, K.J. Carotenoid-Radical Interactions. Biochem. Biophys. Res. Commun. 2003, 305, 754–760. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Cui, H.L. In Vitro Antioxidant, Antihemolytic, and Anticancer Activity of the Carotenoids from Halophilic Archaea. Curr. Microbiol. 2018, 75, 266–271. [Google Scholar] [CrossRef]
- Yatsunami, R.; Ando, A.; Yang, Y.; Takaichi, S.; Kohno, M.; Matsumura, Y.; Ikeda, H.; Fukui, T.; Nakasone, K.; Fujita, N.; et al. Identification of Carotenoids from the Extremely Halophilic Archaeon Haloarcula Japonica. Front. Microbiol. 2014, 5, 79824. [Google Scholar] [CrossRef]
- Young, A.J.; Lowe, G.M. Antioxidant and Prooxidant Properties of Carotenoids. Arch. Biochem. Biophys. 2001, 385, 20–27. [Google Scholar] [CrossRef]
- El-Agamey, A.; Lowe, G.M.; McGarvey, D.J.; Mortensen, A.; Phillip, D.M.; Truscott, T.G.; Young, A.J. Carotenoid Radical Chemistry and Antioxidant/pro-Oxidant Properties. Arch. Biochem. Biophys. 2004, 430, 37–48. [Google Scholar] [CrossRef]
- Ribeiro, D.; Freitas, M.; Silva, A.M.S.; Carvalho, F.; Fernandes, E. Antioxidant and Pro-Oxidant Activities of Carotenoids and Their Oxidation Products. Food Chem. Toxicol. 2018, 120, 681–699. [Google Scholar] [CrossRef]
- Russo, G.L.; Moccia, S.; Russo, M.; Spagnuolo, C. Redox Regulation by Carotenoids: Evidence and Conflicts for Their Application in Cancer. Biochem. Pharmacol. 2021, 194, 114838. [Google Scholar] [CrossRef]
- Eroglu, A.; Harrison, E.H. Carotenoid Metabolism in Mammals, Including Man: Formation, Occurrence, and Function of Apocarotenoids. J. Lipid Res. 2013, 54, 1719–1730. [Google Scholar] [CrossRef]
- Carbonell-Capella, J.M.; Buniowska, M.; Barba, F.J.; Esteve, M.J.; Frígola, A. Analytical Methods for Determining Bioavailability and Bioaccessibility of Bioactive Compounds from Fruits and Vegetables: A Review. Compr. Rev. Food Sci. Food Saf. 2014, 13, 155–171. [Google Scholar] [CrossRef]
- Molteni, C.; La Motta, C.; Valoppi, F. Improving the Bioaccessibility and Bioavailability of Carotenoids by Means of Nanostructured Delivery Systems: A Comprehensive Review. Antioxidants 2022, 11, 1931. [Google Scholar] [CrossRef]
- Erdman, J.W.; Bierer, T.L.; Gugger, E.T. Absorption and Transport of Carotenoids. Ann. N. Y. Acad. Sci. 1993, 691, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Furr, H.C.; Clark, R.M. Transport, Uptake, and Target Tissue Storage of Carotenoids. Carotenoids Health Dis. 2004, 13, 229–278. [Google Scholar] [CrossRef]
- Granado-Lorencio, F.; Olmedilla-Alonso, B.; Herrero-Barbudo, C.; Blanco-Navarro, I.; Pérez-Sacristán, B.; Blázquez-García, S. In Vitro Bioaccessibility of Carotenoids and Tocopherols from Fruits and Vegetables. Food Chem. 2007, 102, 641–648. [Google Scholar] [CrossRef]
- von Lintig, J.; Moon, J.; Lee, J.; Ramkumar, S. Carotenoid metabolism at the intestinal barrier. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2020, 1865, 158580. [Google Scholar] [CrossRef] [PubMed]
- Goodman, D.S.; Blomstrand, R.; Werner, B.; Huang, H.S.; Shiratori, T. The Intestinal Absorption and Metabolism of Vitamin A and Beta-Carotene in Man. J. Clin. Investig. 1966, 45, 1615–1623. [Google Scholar] [CrossRef]
- Klaunig, J.E.; Kamendulis, L.M.; Hocevar, B.A. Oxidative Stress and Oxidative Damage in Carcinogenesis. Toxicol. Pathol. 2010, 38, 96–109. [Google Scholar] [CrossRef]
- Tafani, M.; Sansone, L.; Limana, F.; Arcangeli, T.; De Santis, E.; Polese, M.; Fini, M.; Russo, M.A. The Interplay of Reactive Oxygen Species, Hypoxia, Inflammation, and Sirtuins in Cancer Initiation and Progression. Oxid. Med. Cell Longev. 2016, 2016, 3907147. [Google Scholar] [CrossRef]
- Sullivan, L.B.; Chandel, N.S. Mitochondrial Reactive Oxygen Species and Cancer. Cancer Metab. 2014, 2, 17. [Google Scholar] [CrossRef]
- Murata, M.; Thanan, R.; Ma, N.; Kawanishi, S. Role of Nitrative and Oxidative DNA Damage in Inflammation-Related Carcinogenesis. J. Biomed. Biotechnol. 2012, 2012, 623019. [Google Scholar] [CrossRef]
- Liberti, M.V.; Locasale, J.W. The Warburg Effect: How Does It Benefit Cancer Cells? Trends Biochem. Sci. 2016, 41, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Tuy, K.; Rickenbacker, L.; Hjelmeland, A.B. Reactive oxygen species produced by altered tumor metabolism impacts cancer stem cell maintenance. Redox Biol. 2021, 44, 101953. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, H.; Takada, K. Reactive oxygen species in cancer: Current findings and future directions. Cancer Sci. 2021, 112, 3945–3952. [Google Scholar] [CrossRef]
- Weinberg, F.; Ramnath, N.; Nagrath, D. Reactive Oxygen Species in the Tumor Microenvironment: An Overview. Cancers 2019, 11, 1191. [Google Scholar] [CrossRef]
- Dizdaroglu, M.; Jaruga, P.; Birincioglu, M.; Rodriguez, H. Free Radical-Induced Damage to DNA: Mechanisms and Measurement. Free Radic. Biol. Med. 2002, 32, 1102–1115. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Pant, M.C.; Singh, H.S.; Khandelwal, S. Determinants of Oxidative Stress and DNA Damage (8-OhdG) in Squamous Cell Carcinoma of Head and Neck. Indian. J. Cancer 2012, 49, 309–315. [Google Scholar] [CrossRef]
- Matsuzawa, A.; Ichijo, H. Redox Control of Cell Fate by MAP Kinase: Physiological Roles of ASK1-MAP Kinase Pathway in Stress Signaling. Biochim. Biophys. Acta Gen. Subj. 2008, 1780, 1325–1336. [Google Scholar] [CrossRef]
- Huo, L.; Li, C.-W.; Huang, T.-H.; Lam, Y.C.; Xia, W.; Tu, C.; Chang, W.-C.; Hsu, J.L.; Lee, D.-F.; Nie, L.; et al. Activation of Keap1/Nrf2 Signaling Pathway by Nuclear Epidermal Growth Factor Receptor in Cancer Cells. Am. J. Transl. Res. 2014, 6, 649–663. [Google Scholar]
- Xia, L.; Tan, S.; Zhou, Y.; Lin, J.; Wang, H.; Oyang, L.; Tian, Y.; Liu, L.; Su, M.; Wang, H.; et al. Role of the NFκB-Signaling Pathway in Cancer. OncoTargets Ther. 2018, 11, 2063–2073. [Google Scholar] [CrossRef]
- Anderson, N.M.; Simon, M.C. The Tumor Microenvironment. Current Biology 2020, 30, 921–925. [Google Scholar] [CrossRef] [PubMed]
- Nam, T.G. Lipid Peroxidation and Its Toxicological Implications. Toxicol. Res. 2011, 27, 1. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, N.; Das, A.; Chaffee, S.; Roy, S.; Sen, C.K. Reactive Oxygen Species, Oxidative Damage and Cell Death. In Immunity and Inflammation in Health and Disease: Emerging Roles of Nutraceuticals and Functional Foods in Immune Support; Academic Press: Cambridge, MA, USA, 2018; pp. 45–55. [Google Scholar] [CrossRef]
- Shin, J.; Song, M.; Oh, J.; Keum, Y.; Saini, R. Pro-Oxidant Actions of Carotenoids in Triggering Apoptosis of Cancer Cells: A Review of Emerging Evidence. Antioxidants 2020, 9, 532. [Google Scholar] [CrossRef]
- Schumacker, P.T. Reactive Oxygen Species in Cancer Cells: Live by the Sword, Die by the Sword. Cancer Cell 2006, 10, 175–176. [Google Scholar] [CrossRef] [PubMed]
- Antunes, A.; Carmo, F.; Pinto, S.; Andrade, N.; Martel, F. The Anti-Proliferative Effect of β-Carotene against a Triple-Negative Breast Cancer Cell Line Is Cancer Cell-Specific and JNK-Dependent. PharmaNutrition 2022, 22, 100320. [Google Scholar] [CrossRef]
- Wu, Q.; Wu, W.; Jacevic, V.; Franca, T.C.C.; Wang, X.; Kuca, K. Selective Inhibitors for JNK Signalling: A Potential Targeted Therapy in Cancer. J. Enzyme Inhib. Med. Chem. 2020, 35, 574–583. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, W.-E.; Hu, L.; Zhao, L.; Huang, J. Carotenoids Inhibit Proliferation and Regulate Expression of Peroxisome Proliferators-Activated Receptor Gamma (PPARγ) in K562 Cancer Cells. Arch. Biochem. Biophys. 2011, 512, 96–106. [Google Scholar] [CrossRef]
- Sporn, M.B.; Suh, N.; Mangelsdorf, D.J. Prospects for Prevention and Treatment of Cancer with Selective PPARγ Modulators (SPARMs). Trends Mol. Med. 2001, 7, 395–400. [Google Scholar] [CrossRef]
- Yu, X.; Kensler, T. Nrf2 as a Target for Cancer Chemoprevention. Mutat. Res. 2005, 591, 93–102. [Google Scholar] [CrossRef]
- Stivala, L.A.; Savio, M.; Quarta, S.; Scotti, C.; Cazzalini, O.; Rossi, L.; Scovassi, I.A.; Pizzala, R.M.; Bianchi, L.; Vannini, V.; et al. The Antiproliferative Effect of Beta-Carotene Requires P21waf1/Cip1 in Normal Human Fibroblasts. Eur. J. Biochem. 2000, 267, 2290–2296. [Google Scholar] [CrossRef]
- Palozza, P. Can Beta-Carotene Regulate Cell Growth by a Redox Mechanism? An Answer from Cultured Cells. Biochim. Biophys. Acta 2005, 1740, 215–221. [Google Scholar] [CrossRef]
- Palozza, P.; Serini, S.; Maggiano, N.; Angelini, M.; Boninsegna, A.; Nicuolo, F.D.; Ranelletti, F.O.; Calviello, G. Induction of Cell Cycle Arrest and Apoptosis in Human Colon Adenocarcinoma Cell Lines by β-Carotene through down-Regulation of Cyclin A and Bcl-2 Family Proteins. Carcinogenesis 2002, 23, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Yam, C.H.; Fung, T.K.; Poon, R.Y.C. Cyclin A in Cell Cycle Control and Cancer. Cell Mol. Life Sci. 2002, 59, 1317–1326. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.E.; Kwon, M.; Kim, Y.S.; Kim, Y.; Chung, M.G.; Heo, S.C.; Kim, Y. β-carotene regulates cancer stemness in colon cancer in vivo and in vitro. Nutr. Res. Pract. 2022, 16, 161–172. [Google Scholar] [CrossRef] [PubMed]
- Haddad, N.F.; Teodoro, A.J.; Leite de Oliveira, F.; Soares, N.; de Mattos, R.M.; Hecht, F.; Dezonne, R.S.; Vairo, L.; Goldenberg, R.C.S.; Gomes, F.C.A.; et al. Lycopene and Beta-Carotene Induce Growth Inhibition and Proapoptotic Effects on ACTH-Secreting Pituitary Adenoma Cells. PLoS ONE 2013, 8, e62773. [Google Scholar] [CrossRef]
- Hume, S.; Grou, C.P.; Lascaux, P.; D’Angiolella, V.; Legrand, A.J.; Ramadan, K.; Dianov, G.L. The NUCKS1-SKP2-P21/P27 Axis Controls S Phase Entry. Nat. Commun. 2021, 12, 6959. [Google Scholar] [CrossRef]
- Razavipour, S.F.; Harikumar, K.B.; Slingerland, J.M. P27 as a Transcriptional Regulator: New Roles in Development and Cancer. Cancer Res. 2020, 80, 3451–3458. [Google Scholar] [CrossRef]
- Kacar, S.; Sariisik, E.; Sahinturk, V. Beta-Carotene Exerted Anti-Proliferative and Apoptotic Effect on Malignant Mesothelioma Cells. Naunyn Schmiedebergs Arch. Pharmacol. 2022, 395, 407–415. [Google Scholar] [CrossRef]
- Gloria, N.F.; Soares, N.; Brand, C.; Oliveira, F.L.; Borojevic, R.; Teodoro, A.J. Lycopene and Beta-Carotene Induce Cell-Cycle Arrest and Apoptosis in Human Breast Cancer Cell Lines. Anticancer. Res. 2014, 34, 1377–1386. [Google Scholar]
- Huang, H.-C.; Lin, C.-L.; Lin, J.-K. 1,2,3,4,6-Penta- O -Galloyl-β- d -Glucose, Quercetin, Curcumin and Lycopene Induce Cell-Cycle Arrest in MDA-MB-231 and BT474 Cells through Downregulation of Skp2 Protein. J. Agric. Food Chem. 2011, 59, 6765–6775. [Google Scholar] [CrossRef]
- Karas, M.; Amir, H.; Fishman, D.; Danilenko, M.; Segal, S.; Nahum, A.; Koifmann, A.; Giat, Y.; Levy, J.; Sharoni, Y. Lycopene Interferes with Cell Cycle Progression and Insulin-like Growth Factor I Signaling in Mammary Cancer Cells. Nutr. Cancer 2000, 36, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Anisimov, V.N. Insulin/IGF-1 Signaling Pathway Driving Aging and Cancer as a Target for Pharmacological Intervention. Exp. Gerontol. 2003, 38, 1041–1049. [Google Scholar] [CrossRef] [PubMed]
- Nahum, A.; Hirsch, K.; Danilenko, M.; Watts, C.K.W.; Prall, O.W.J.; Levy, J.; Sharoni, Y. Lycopene Inhibition of Cell Cycle Progression in Breast and Endometrial Cancer Cells Is Associated with Reduction in Cyclin D Levels and Retention of P27Kip1 in the Cyclin E-Cdk2 Complexes. Oncogene 2001, 20, 3428–3436. [Google Scholar] [CrossRef] [PubMed]
- Sherr, C.J. Cancer Cell Cycles. Science 1996, 274, 1672–1677. [Google Scholar] [CrossRef]
- Buckley, M.F.; Sweeney, K.J.E.; Hamilton, J.A.; Sini, R.L.; Manning, D.L.; Nicholson, R.I.; DeFazio, A.; Watts, C.K.W.; Musgrove, E.A.; Sutherland, R.L. Expression and Amplification of Cyclin Genes in Human Breast Cancer. Oncogene 1993, 8, 2127–2133. [Google Scholar]
- Musgrove, E.; Hui, R.; Seweeney, K.J.E.; Watts, C.K.W.; Sutherland, R.L. Cyclins and Breast Cancer. J. Mammary Gland. Biol. Neoplasia 1996, 1, 153–162. [Google Scholar] [CrossRef]
- Assar, E.A.; Vidalle, M.C.; Chopra, M.; Hafizi, S. Lycopene Acts through Inhibition of IκB Kinase to Suppress NF-ΚB Signaling in Human Prostate and Breast Cancer Cells. Tumor Biol. 2016, 37, 9375–9385. [Google Scholar] [CrossRef]
- Orlowski, R.Z.; Baldwin, A.S. NF-KappaB as a Therapeutic Target in Cancer. Trends Mol. Med. 2002, 8, 385–389. [Google Scholar] [CrossRef]
- Teodoro, A.J.; Oliveira, F.L.; Martins, N.B.; Maia, G.D.A.; Martucci, R.B.; Borojevic, R. Effect of Lycopene on Cell Viability and Cell Cycle Progression in Human Cancer Cell Lines. Cancer Cell Int. 2012, 12, 36. [Google Scholar] [CrossRef]
- Tang, F.Y.; Pai, M.H.; Wang, X.D. Consumption of lycopene inhibits the growth and progression of colon cancer in a mouse xenograft model. J. Agric. Food Chem. 2011, 59, 9011–9021. [Google Scholar] [CrossRef]
- Amir, H.; Karas, M.; Giat, J.; Danilenko, M.; Levy, R.; Yermiahu, T.; Levy, J.; Sharoni, Y. Lycopene and 1,25-Dihydroxyvitamin D3 Cooperate in the Inhibition of Cell Cycle Progression and Induction of Differentiation in HL-60 Leukemic Cells. Nutr. Cancer 1999, 33, 105–112. [Google Scholar] [CrossRef]
- Zhou, Y.; Fu, R.; Yang, M.; Liu, W.; Tong, Z. Lycopene Suppresses Gastric Cancer Cell Growth without Affecting Normal Gastric Epithelial Cells. J. Nutr. Biochem. 2023, 116, 109313. [Google Scholar] [CrossRef]
- Xing, R.; Zhou, Y.; Yu, J.; Yu, Y.; Nie, Y.; Luo, W.; Yang, C.; Xiong, T.; Wu, W.K.K.; Li, Z.; et al. Whole-Genome Sequencing Reveals Novel Tandem-Duplication Hotspots and a Prognostic Mutational Signature in Gastric Cancer. Nat. Commun. 2019, 10, 2037. [Google Scholar] [CrossRef] [PubMed]
- Akkewar, A.S.; Mishra, K.A.; Kamble, M.G.; Kumar, S.; Dey, J.; Sethi, K.K. A Mechanistic Review on Growing Multiple Therapeutic Applications of Lutein and Its Global Market Research. Phytother. Res. 2024, 38, 3190–3217. [Google Scholar] [CrossRef]
- Gong, X.; Smith, J.R.; Swanson, H.M.; Rubin, L.P. Carotenoid Lutein Selectively Inhibits Breast Cancer Cell Growth and Potentiates the Effect of Chemotherapeutic Agents through ROS-Mediated Mechanisms. Molecules 2018, 23, 905. [Google Scholar] [CrossRef]
- Ozaki, T.; Nakagawara, A. Role of P53 in Cell Death and Human Cancers. Cancers 2011, 3, 994. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.Y.; Lu, Y.I.; He, X.; Liang, S.Y.; Hu, F.; Wei, X.S.; Pan, M.J.; Zhou, Q.; Yang, W. Lutein Inhibits Tumor Progression through the ATR/Chk1/P53 Signaling Pathway in Non-Small Cell Lung Cancer. Phytother. Res. 2023, 37, 1260–1273. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Park, J.-J.; Lee, B.J.; Joo, M.K.; Chun, H.J.; Lee, S.W.; Bak, Y.-T. Astaxanthin Inhibits Proliferation of Human Gastric Cancer Cell Lines by Interrupting Cell Cycle Progression. Gut Liver 2016, 10, 369–374. [Google Scholar] [CrossRef]
- Adachi, S.; Natsume, H.; Yamauchi, J.; Matsushima-Nishiwaki, R.; Joe, A.K.; Moriwaki, H.; Kozawa, O. P38 MAP Kinase Controls EGF Receptor Downregulation via Phosphorylation at Ser1046/1047. Cancer Lett. 2009, 277, 108–113. [Google Scholar] [CrossRef]
- Kim, M.S.; Ahn, Y.T.; Lee, C.W.; Kim, H.; An, W.G. Astaxanthin Modulates Apoptotic Molecules to Induce Death of SKBR3 Breast Cancer Cells. Mar. Drugs 2020, 18, 266. [Google Scholar] [CrossRef]
- Liu, X.; Song, M.; Gao, Z.; Cai, X.; Dixon, W.; Chen, X.; Cao, Y.; Xiao, H. Stereoisomers of Astaxanthin Inhibit Human Colon Cancer Cell Growth by Inducing G2/M Cell Cycle Arrest and Apoptosis. J. Agric. Food Chem. 2016, 64, 7750–7759. [Google Scholar] [CrossRef] [PubMed]
- Shokrian, Z.M.; Pakravesh, S.M.; Jalili, K.S.M.; Soghala, S.; Dabbagh, O.M.A.; Ghanbar, A.A.H.; Sayyahi, Z.; Mahya, L.; Jahani, S.; Shojaei, B.S.; et al. Astaxanthin as an Anticancer Agent against Breast Cancer: An In Vivo and In Vitro Investigation. Curr. Med. Chem. 2024, 17. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, C.; Tafuku, S.; Kadekaru, T.; Sawada, S.; Tomita, M.; Okudaira, T.; Nakazato, T.; Toda, T.; Uchihara, J.-N.; Taira, N.; et al. Antiadult T-Cell Leukemia Effects of Brown Algae Fucoxanthin and Its Deacetylated Product, Fucoxanthinol. Int. J. Cancer 2008, 123, 2702–2712. [Google Scholar] [CrossRef]
- Tamura, R.E.; Ferreira De Vasconcellos, J.; Sarkar, D.; Libermann, T.A.; Fisher, P.B.; Zerbini, L.F. GADD45 Proteins: Central Players in Tumorigenesis. Curr. Mol. Med. 2012, 12, 634–651. [Google Scholar] [CrossRef] [PubMed]
- Rokkaku, T.; Kimura, R.; Ishikawa, C.; Yasumoto, T.; Senba, M.; Kanaya, F.; Mori, N. Anticancer Effects of Marine Carotenoids, Fucoxanthin and Its Deacetylated Product, Fucoxanthinol, on Osteosarcoma. Int. J. Oncol. 2013, 43, 1176–1186. [Google Scholar] [CrossRef] [PubMed]
- Yu, R.X.; Hu, X.M.; Xu, S.Q.; Jiang, Z.J.; Yang, W. Effects of Fucoxanthin on Proliferation and Apoptosis in Human Gastric Adenocarcinoma MGC-803 Cells via JAK/STAT Signal Pathway. Eur. J. Pharmacol. 2011, 657, 10–19. [Google Scholar] [CrossRef]
- Yu, H.; Lee, H.; Herrmann, A.; Buettner, R.; Jove, R. Revisiting STAT3 Signalling in Cancer: New and Unexpected Biological Functions. Nat. Rev. Cancer 2014, 14, 736–746. [Google Scholar] [CrossRef]
- Yu, R.-X.; Yu, R.-T.; Liu, Z. Inhibition of Two Gastric Cancer Cell Lines Induced by Fucoxanthin Involves Downregulation of Mcl-1 and STAT3. Hum. Cell 2018, 31, 50–63. [Google Scholar] [CrossRef]
- Yoshiko, S.; Hoyoko, N. Fucoxanthin, a Natural Carotenoid, Induces G1 Arrest and GADD45 Gene Expression in Human Cancer Cells. In Vivo 2007, 21, 305–309. [Google Scholar]
- Das, S.K.; Hashimoto, T.; Kanazawa, K. Growth Inhibition of Human Hepatic Carcinoma HepG2 Cells by Fucoxanthin Is Associated with Down-Regulation of Cyclin D. Biochim. Biophys. Acta Gen. Subj. 2008, 1780, 743–749. [Google Scholar] [CrossRef]
- Smith, M.L.; Chen, I.T.; Zhan, Q.; Bae, I.; Chen, C.Y.; Gilmer, T.M.; Kastan, M.B.; O’Connor, P.M.; Fornace, A.J. Interaction of the P53-Regulated Protein GADD45 with Proliferating Cell Nuclear Antigen. Science 1994, 266, 7973727. [Google Scholar] [CrossRef] [PubMed]
- Vairapandi, M.; Balliet, A.G.; Fornace, A.J.; Hoffman, B.; Liebermann, D.A. The Differentiation Primary Response Gene MyD118, Related to GADD45, Encodes for a Nuclear Protein Which Interacts with PCNA and P21(WAF1/CIP1). Oncogene 1996, 12, 2579–2594. [Google Scholar] [PubMed]
- Kim, K.N.; Ahn, G.; Heo, S.J.; Kang, S.M.; Kang, M.C.; Yang, H.M.; Kim, D.; Roh, S.W.; Kim, S.K.; Jeon, B.T.; et al. Inhibition of Tumor Growth in Vitro and in Vivo by Fucoxanthin against Melanoma B16F10 Cells. Environ. Toxicol. Pharmacol. 2013, 35, 39–46. [Google Scholar] [CrossRef]
- Wang, L.; Zeng, Y.; Liu, Y.; Hu, X.; Li, S.; Wang, Y.; Li, L.; Lei, Z.; Zhang, Z. Fucoxanthin Induces Growth Arrest and Apoptosis in Human Bladder Cancer T24 Cells by Up-Regulation of P21 and down-Regulation of Mortalin. Acta Biochim. Biophys. Sin. 2014, 46, 877–884. [Google Scholar] [CrossRef]
- Wu, J.-Y.; Chien, Y.-C.; Tsai, I.-C.; Hung, C.-C.; Huang, W.-C.; Liu, L.-C.; Yu, Y.-L. Capsanthin Induces G1/S Phase Arrest, Erlotinib-Sensitivity and Inhibits Tumor Progression by Suppressing EZH2-Mediated Epigenetically Silencing of P21 in Triple-Negative Breast Cancer Cells. Aging 2021, 13, 12514–12525. [Google Scholar] [CrossRef] [PubMed]
- Hock, H. A Complex Polycomb Issue: The Two Faces of EZH2 in Cancer. Genes. Dev. 2012, 26, 751–755. [Google Scholar] [CrossRef] [PubMed]
- Varambally, S.; Dhanasekaran, S.M.; Zhou, M.; Barrette, T.R.; Kumar-Sinha, C.; Sanda, M.G.; Ghosh, D.; Pienta, K.J.; Sewalt, R.G.A.B.; Rubin, M.A.; et al. The Polycomb Group Protein EZH2 Is Involved in Progression of Prostate Cancer. Nature 2002, 419, 624–629. [Google Scholar] [CrossRef]
- Mohamadpour, A.H.; Ayati, Z.; Parizadeh, M.R.; Rajbai, O.; Hosseinzadeh, H. Safety Evaluation of Crocin (a Constituent of Saffron) Tablets in Healthy Volunteers. Iran. J. Basic. Med. Sci. 2013, 16, 39. [Google Scholar]
- Ashrafi, M.; Bathaie, S.Z.; Abroun, S.; Azizian, M. Effect of Crocin on Cell Cycle Regulators in N-Nitroso-N-Methylurea-Induced Breast Cancer in Rats. DNA Cell Biol. 2015, 34, 684–691. [Google Scholar] [CrossRef]
- Hosseini, S.S.; Reihani, R.Z.; Doustvandi, M.A.; Amini, M.; Zargari, F.; Baradaran, B.; Yari, A.H.; Hashemi, M.; Tohidast, M.; Mokhtarzadeh, A. Synergistic Anticancer Effects of Curcumin and Crocin on Human Colorectal Cancer Cells. Mol. Biol. Rep. 2022, 49, 8741–8752. [Google Scholar] [CrossRef]
- Luo, Y.; Yu, P.; Zhao, J.; Guo, Q.; Fan, B.; Diao, Y.; Jin, Y.; Wu, J.; Zhang, C. Inhibitory Effect of Crocin Against Gastric Carcinoma via Regulating TPM4 Gene. OncoTargets Ther. 2021, 14, 111–122. [Google Scholar] [CrossRef]
- Dhanasekaran, D.N.; Reddy, E.P. JNK Signaling in Apoptosis. Oncogene 2008, 27, 6245–6251. [Google Scholar] [CrossRef] [PubMed]
- Redza-Dutordoir, M.; Averill-Bates, D.A. Activation of Apoptosis Signalling Pathways by Reactive Oxygen Species. Biochim. Biophys. Acta Mol. Cell Res. 2016, 1863, 2977–2992. [Google Scholar] [CrossRef] [PubMed]
- Takeshima, M.; Ono, M.; Higuchi, T.; Chen, C.; Hara, T.; Nakano, S. Anti-Proliferative and Apoptosis-Inducing Activity of Lycopene against Three Subtypes of Human Breast Cancer Cell Lines. Cancer Sci. 2014, 105, 252–257. [Google Scholar] [CrossRef]
- Jain, A.; Sharma, G.; Kushwah, V.; Thakur, K.; Ghoshal, G.; Singh, B.; Jain, S.; Shivhare, U.S.; Katare, O.P. Fabrication and Functional Attributes of Lipidic Nanoconstructs of Lycopene: An Innovative Endeavour for Enhanced Cytotoxicity in MCF-7 Breast Cancer Cells. Colloids Surf. B Biointerfaces 2017, 152, 482–491. [Google Scholar] [CrossRef] [PubMed]
- Do, K.; Chen, A.P. Molecular Pathways: Targeting PARP in Cancer Treatment. Clin. Cancer Res. 2013, 19, 977–984. [Google Scholar] [CrossRef]
- Tian, X.; Chen, B.; Liu, X. Telomere and Telomerase as Targets for Cancer Therapy. Appl. Biochem. Biotechnol. 2010, 160, 1460–1472. [Google Scholar] [CrossRef]
- Harley, C.B. Telomerase and Cancer Therapeutics. Nat. Rev. Cancer 2008, 8, 167–179. [Google Scholar] [CrossRef]
- Gharib, A.; Faezizadeh, Z. In Vitro Anti-Telomerase Activity of Novel Lycopene-Loaded Nanospheres in the Human Leukemia Cell Line K562. Pharmacogn. Mag. 2014, 10, S157–S163. [Google Scholar] [CrossRef]
- Huang, R.-F.S.; Wei, Y.-J.; Inbaraj, B.S.; Chen, B.-H. Inhibition of Colon Cancer Cell Growth by Nanoemulsion Carrying Gold Nanoparticles and Lycopene. Int. J. Nanomed. 2015, 10, 2823–2846. [Google Scholar] [CrossRef]
- Edlich, F. BCL-2 Proteins and Apoptosis: Recent Insights and Unknowns. Biochem. Biophys. Res. Commun. 2018, 500, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Chen, L.; Zhao, W.; Hao, J.; An, R. MicroRNA-Let-7f-1 Is Induced by Lycopene and Inhibits Cell Proliferation and Triggers Apoptosis in Prostate Cancer. Mol. Med. Rep. 2016, 13, 2708–2714. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Lu, S.; Chen, Y.; Zheng, L.; Chen, L.; Ding, H.; Ding, J.; Lou, D.; Liu, F.; Zheng, B. AKT2 Phosphorylation of Hexokinase 2 at T473 Promotes Tumorigenesis and Metastasis in Colon Cancer Cells via NF-ΚB, HIF1α, MMP2, and MMP9 Upregulation. Cell Signal 2019, 58, 99–110. [Google Scholar] [CrossRef]
- Liu, T.; Zhu, J.; Du, W.; Ning, W.; Zhang, Y.; Zeng, Y.; Liu, Z.; Huang, J.A. AKT2 Drives Cancer Progression and Is Negatively Modulated by MiR-124 in Human Lung Adenocarcinoma. Respir. Res. 2020, 21, 227. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Xu, F.; Wu, K.; Li, L.; Qiao, T.; Li, Z.; Chen, T.; Sun, C. Anticancer Effects and Possible Mechanisms of Lycopene Intervention on N-Methylbenzylnitrosamine Induced Esophageal Cancer in F344 Rats Based on PPARγ1. Eur. J. Pharmacol. 2020, 881, 173230. [Google Scholar] [CrossRef]
- Viatour, P.; Bentires-Alj, M.; Chariot, A.; Deregowski, V.; de Leval, L.; Merville, M.P.; Bours, V. NF-ΚB2/P100 Induces Bcl-2 Expression. Leukemia 2003, 17, 1349–1356. [Google Scholar] [CrossRef]
- Cha, J.H.; Kim, W.K.; Ha, A.W.; Kim, M.H.; Chang, M.J. Anti-Inflammatory Effect of Lycopene in SW480 Human Colorectal Cancer Cells. Nutr. Res. Pract. 2017, 11, 90–96. [Google Scholar] [CrossRef]
- Arathi, B.P.; Sowmya, P.R.-R.; Kuriakose, G.C.; Vijay, K.; Baskaran, V.; Jayabaskaran, C.; Lakshminarayana, R. Enhanced Cytotoxic and Apoptosis Inducing Activity of Lycopene Oxidation Products in Different Cancer Cell Lines. Food Chem. Toxicol. 2016, 97, 265–276. [Google Scholar] [CrossRef]
- Chandra, D.; Choy, G.; Tang, D.G. Cytosolic Accumulation of HSP60 during Apoptosis with or without Apparent Mitochondrial Release: Evidence That Its pro-Apoptotic or pro-Survival Functions Involve Differential Interactions with Caspase-3. J. Biol. Chem. 2007, 282, 31289–31301. [Google Scholar] [CrossRef]
- Sumantran, V.N.; Zhang, R.; Lee, D.S.; Wicha, M.S. Differential Regulation of Apoptosis in Normal versus Transformed Mammary Epithelium by Lutein and Retinoic Acid. CEBP 2000, 9, 257–263. [Google Scholar]
- Kavalappa, Y.P.; Gopal, S.S.; Ponesakki, G. Lutein Inhibits Breast Cancer Cell Growth by Suppressing Antioxidant and Cell Survival Signals and Induces Apoptosis. J. Cell Physiol. 2021, 236, 1798–1809. [Google Scholar] [CrossRef] [PubMed]
- Chew, B.P.; Brown, C.M.; Park, J.S.; Mixter, P.F. Dietary lutein inhibits mouse mammary tumor growth by regulating angiogenesis and apoptosis. Anticancer. Res. 2003, 23, 3333–3339. [Google Scholar]
- Zhang, W.-L.; Zhao, Y.-N.; Shi, Z.-Z.; Cong, D.; Bai, Y.-S. Lutein Inhibits Cell Growth and Activates Apoptosis via the PI3K/AKT/MTOR Signaling Pathway in A549 Human Non-Small-Cell Lung Cancer Cells. J. Environ. Pathol. Toxicol. Oncol. 2018, 37, 341–350. [Google Scholar] [CrossRef]
- Eom, J.W.; Lim, J.W.; Kim, H. Lutein Induces Reactive Oxygen Species-Mediated Apoptosis in Gastric Cancer AGS Cells via NADPH Oxidase Activation. Molecules 2023, 28, 1178. [Google Scholar] [CrossRef] [PubMed]
- Haung, H.-Y.; Wang, Y.-C.; Cheng, Y.-C.; Kang, W.; Hu, S.-H.; Liu, D.; Xiao, C.; Wang, H.-M.D.; Ali, D. A Novel Oral Astaxanthin Nanoemulsion from Haematococcus Pluvialis Induces Apoptosis in Lung Metastatic Melanoma. Oxid. Med. Cell Longev. 2020, 2020, 2647670. [Google Scholar] [CrossRef]
- Minella, A.C.; Loeb, K.R.; Knecht, A.; Welcker, M.; Varnum-Finney, B.J.; Bernstein, I.D.; Roberts, J.M.; Clurman, B.E. Cyclin E Phosphorylation Regulates Cell Proliferation in Hematopoietic and Epithelial Lineages in Vivo. Genes. Dev. 2008, 22, 1677–1689. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.; Fang, B.; Liao, Y.; Chresta, C.M.; Smith, P.D.; Roth, J.A. Apoptosis Induction by MEK Inhibition in Human Lung Cancer Cells Is Mediated by Bim. PLoS ONE 2010, 5, 13026. [Google Scholar] [CrossRef]
- Gialeli, C.; Theocharis, A.D.; Karamanos, N.K. Roles of Matrix Metalloproteinases in Cancer Progression and Their Pharmacological Targeting. FEBS J. 2011, 278, 16–27. [Google Scholar] [CrossRef]
- Kavitha, K.; Kowshik, J.; Kishore, T.K.K.; Baba, A.B.; Nagini, S. Astaxanthin Inhibits NF-ΚB and Wnt/β-Catenin Signaling Pathways via Inactivation of Erk/MAPK and PI3K/Akt to Induce Intrinsic Apoptosis in a Hamster Model of Oral Cancer. Biochim. Biophys. Acta Gen. Subj. 2013, 1830, 4433–4444. [Google Scholar] [CrossRef]
- Shanmugapriya, K.; Kim, H.; Kang, H.W. In Vitro Antitumor Potential of Astaxanthin Nanoemulsion against Cancer Cells via Mitochondrial Mediated Apoptosis. Int. J. Pharm. 2019, 560, 334–346. [Google Scholar] [CrossRef]
- Hormozi, M.; Ghoreishi, S.; Baharvand, P. Astaxanthin Induces Apoptosis and Increases Activity of Antioxidant Enzymes in LS-180 Cells. Artif. Cells Nanomed. Biotechnol. 2019, 47, 891–895. [Google Scholar] [CrossRef]
- Papa, L.; Manfredi, G.; Germain, D. SOD1, an Unexpected Novel Target for Cancer Therapy. Genes Cancer 2014, 5, 15. [Google Scholar]
- Chen, W.; Zhang, H.; Liu, Y. Anti-Inflammatory and Apoptotic Signaling Effect of Fucoxanthin on Benzo(A)Pyrene-Induced Lung Cancer in Mice. J. Environ. Pathol. Toxicol. Oncol. 2019, 38, 239–251. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.; Choi, J.; Park, J.-K.; Won, G.; Seol, J.-W. Fucoxanthin Exerts Anti-Tumor Activity on Canine Mammary Tumor Cells via Tumor Cell Apoptosis Induction and Angiogenesis Inhibition. Animals 2021, 11, 1512. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.H.; Han, C.W.; Jeong, M.S.; Jang, S.B. DED Interaction of FADD and Caspase-8 in the Induction of Apoptotic Cell Death. J. Microbiol. Biotechnol. 2022, 32, 1034–1040. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Qiu, S.; Shao, N.; Zheng, J. Fucoxanthin and Tumor Necrosis Factor-Related Apoptosis-Inducing Ligand (TRAIL) Synergistically Promotes Apoptosis of Human Cervical Cancer Cells by Targeting PI3K/Akt/NF-KB Signaling Pathway. Med. Sci. Monit. 2018, 24, 11–18. [Google Scholar] [CrossRef]
- Lopes, F.G.; Oliveira, K.A.; Lopes, R.G.; Poluceno, G.G.; Simioni, C.; Pescador, G.D.S.; Bauer, C.M.; Maraschin, M.; Derner, R.B.; Garcez, R.C.; et al. Anti-Cancer Effects of Fucoxanthin on Human Glioblastoma Cell Line. Anticancer. Res. 2020, 40, 6799–6815. [Google Scholar] [CrossRef]
- Loeffler, M.; Kroemer, G. The Mitochondrion in Cell Death Control: Certainties and Incognita. Exp. Cell Res. 2000, 256, 19–26. [Google Scholar] [CrossRef]
- Wu, H.-L.; Fu, X.-Y.; Cao, W.-Q.; Xiang, W.-Z.; Hou, Y.-J.; Ma, J.-K.; Wang, Y.; Fan, C.-D. Induction of Apoptosis in Human Glioma Cells by Fucoxanthin via Triggering of ROS-Mediated Oxidative Damage and Regulation of MAPKs and PI3K-AKT Pathways. J. Agric. Food Chem. 2019, 67, 2212–2219. [Google Scholar] [CrossRef]
- Erden, Y. Capsanthin Stimulates the Mitochondrial Apoptosis-Mediated Cell Death, Following DNA Damage in MCF-7 Cells. Nutr. Cancer 2020, 73, 662–670. [Google Scholar] [CrossRef]
- Shanmugham, V.; Subban, R. Capsanthin-Loaded Micelles: Preparation, Characterization and in Vitro Evaluation of Cytotoxicity Using MDA-MB-231 Breast Cancer Cell Line. Food Technol. Biotechnol. 2022, 60, 350–360. [Google Scholar] [CrossRef]
- Molnar, J.; Gyémant, N.; Mucsi, I.; Molnar, A.; Szabo, M.; Körtvélyesi, T.; Varga, A.; Molnar, P.; Toth, G. Modulation of Multidrug Resistance and Apoptosis of Cancer Cells by Selected Carotenoids. In Vivo 2004, 18, 237–244. [Google Scholar]
- Bathaie, S.Z.; Hoshyar, R.; Miri, H.; Sadeghizadeh, M. Anticancer Effects of Crocetin in Both Human Adenocarcinoma Gastric Cancer Cells and Rat Model of Gastric Cancer. Biochem. Cell Biol. 2013, 91, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Hoshyar, R.; Bathaie, S.Z.; Sadeghizadeh, M. Crocin Triggers the Apoptosis through Increasing the Bax/Bcl-2 Ratio and Caspase Activation in Human Gastric Adenocarcinoma, AGS, Cells. DNA Cell Biol. 2013, 32, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Colapietro, A.; Mancini, A.; Vitale, F.; Martellucci, S.; Angelucci, A.; Llorens, S.; Mattei, V.; Gravina, G.L.; Alonso, G.L.; Festuccia, C. Crocetin Extracted from Saffron Shows Antitumor Effects in Models of Human Glioblastoma. Int. J. Mol. Sci. 2020, 21, 423. [Google Scholar] [CrossRef]
- Grube, S.; Dünisch, P.; Freitag, D.; Klausnitzer, M.; Sakr, Y.; Walter, J.; Kalff, R.; Ewald, C. Overexpression of Fatty Acid Synthase in Human Gliomas Correlates with the WHO Tumor Grade and Inhibition with Orlistat Reduces Cell Viability and Triggers Apoptosis. J. Neurooncol. 2014, 118, 277–287. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Li, J.; Lu, S.; Su, Y. Crocin Inhibits Proliferation and Induces Apoptosis through Suppressing MYCN Expression in Retinoblastoma. J. Biochem. Mol. Toxicol. 2019, 33, e22292. [Google Scholar] [CrossRef]
- Zhang, S.; Wei, J.S.; Li, S.Q.; Badgett, T.C.; Song, Y.K.; Agarwal, S.; Coarfa, C.; Tolman, C.; Hurd, L.; Liao, H.; et al. MYCN Controls an Alternative RNA Splicing Program in High-Risk Metastatic Neuroblastoma. Cancer Lett. 2016, 371, 214–224. [Google Scholar] [CrossRef]
- Schramm, A.; Lode, H. MYCN-Targeting Vaccines and Immunotherapeutics. Hum. Vaccin. Immunother. 2016, 12, 1171430. [Google Scholar] [CrossRef]
- Li, S.; Qu, Y.; Shen, X.-Y.; Ouyang, T.; Fu, W.-B.; Luo, T.; Wang, H.-Q. Multiple Signal Pathways Involved in Crocetin-Induced Apoptosis in KYSE-150 Cells. Pharmacology 2019, 103, 263–272. [Google Scholar] [CrossRef]
- Mollaei, H.; Safaralizadeh, R.; Babaei, E.; Abedini, M.R.; Hoshyar, R. The Anti-Proliferative and Apoptotic Effects of Crocin on Chemosensitive and Chemoresistant Cervical Cancer Cells. Biomed. Pharmacother. 2017, 94, 307–316. [Google Scholar] [CrossRef]
- Ramos, A.; Sadeghi, S.; Tabatabaeian, H. Battling Chemoresistance in Cancer: Root Causes and Strategies to Uproot Them. Int. J. Mol. Sci. 2021, 22, 9451. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.-J.; Shi, F.; Zheng, X.-L.; Wang, Q.; Yang, L.; Sun, H.; He, F.; Zhang, L.; Lin, Y.; Qin, Y.; et al. Crocetin Induces Cytotoxicity and Enhances Vincristine-Induced Cancer Cell Death via P53-Dependent and -Independent Mechanisms. Acta Pharmacol. Sin. 2011, 32, 1529–1536. [Google Scholar] [CrossRef] [PubMed]
- Moradzadeh, M.; Tabarraei, A.; Ghorbani, A.; Hosseini, A.; Sadeghnia, H.R. Short-Term in Vitro Exposure to Crocetin Promotes Apoptosis in Human Leukemic HL-60 Cells via Intrinsic Pathway. Acta Pol. Pharm.-Drug Res. 2018, 75, 445–451. [Google Scholar]
- Mostafavinia, S.E.; Khorashadizadeh, M.; Hoshyar, R. Antiproliferative and Proapoptotic Effects of Crocin Combined with Hyperthermia on Human Breast Cancer Cells. DNA Cell Biol. 2016, 35, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Schwock, J.; Pham, N.A.; Cao, M.P.; Hedley, D.W. Efficacy of Hsp90 Inhibition for Induction of Apoptosis and Inhibition of Growth in Cervical Carcinoma Cells in Vitro and in Vivo. Cancer Chemother. Pharmacol. 2008, 61, 669–681. [Google Scholar] [CrossRef]
- Nasimian, A.; Farzaneh, P.; Tamanoi, F.; Bathaie, S.Z. Cytosolic and Mitochondrial ROS Production Resulted in Apoptosis Induction in Breast Cancer Cells Treated with Crocin: The Role of FOXO3a, PTEN and AKT Signaling. Biochem. Pharmacol. 2020, 177, 113999. [Google Scholar] [CrossRef]
- Carter, M.E.; Brunet, A. FOXO Transcription Factors. Curr. Biol. 2007, 17, R113–R114. [Google Scholar] [CrossRef]
- Cornforth, A.N.; Davis, J.S.; Khanifar, E.; Nastiuk, K.L.; Krolewski, J.J. FOXO3a Mediates the Androgen-Dependent Regulation of FLIP and Contributes to TRAIL-Induced Apoptosis of LNCaP Cells. Oncogene 2008, 27, 4422–4433. [Google Scholar] [CrossRef][Green Version]
- Luo, H.; Yang, Y.; Duan, J.; Wu, P.; Jiang, Q.; Xu, C. PTEN-Regulated AKT/FoxO3a/Bim Signaling Contributes to Reactive Oxygen Species-Mediated Apoptosis in Selenite-Treated Colorectal Cancer Cells. Cell Death Dis. 2013, 4, e481. [Google Scholar] [CrossRef]
- Schmidt, M.; Fernandez de Mattos, S.; van der Horst, A.; Klompmaker, R.; Kops, G.J.P.L.; Lam, E.W.-F.; Burgering, B.M.T.; Medema, R.H. Cell Cycle Inhibition by FoxO Forkhead Transcription Factors Involves Downregulation of Cyclin D. Mol. Cell Biol. 2002, 22, 7842–7852. [Google Scholar] [CrossRef]
- Ray, P.; Guha, D.; Chakraborty, J.; Banerjee, S.; Adhikary, A.; Chakraborty, S.; Das, T.; Sa, G. Crocetin Exploits P53-Induced Death Domain (PIDD) and FAS-Associated Death Domain (FADD) Proteins to Induce Apoptosis in Colorectal Cancer. Sci. Rep. 2016, 6, 32979. [Google Scholar] [CrossRef]
- De Palma, M.; Biziato, D.; Petrova, T.V. Microenvironmental Regulation of Tumour Angiogenesis. Nat. Rev. Cancer 2017, 17, 457–474. [Google Scholar] [CrossRef] [PubMed]
- Coban, B.; Bergonzini, C.; Zweemer, A.J.M.; Danen, E.H.J. Metastasis: Crosstalk between Tissue Mechanics and Tumour Cell Plasticity. Br. J. Cancer 2021, 124, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Muz, B.; de la Puente, P.; Azab, F.; Azab, A.K. The Role of Hypoxia in Cancer Progression, Angiogenesis, Metastasis, and Resistance to Therapy. Hypoxia 2015, 83, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Bae, S.; Lim, J.W.; Kim, H. β-Carotene Inhibits Expression of Matrix Metalloproteinase-10 and Invasion in Helicobacter Pylori-Infected Gastric Epithelial Cells. Molecules 2021, 26, 1567. [Google Scholar] [CrossRef]
- Kim, Y.S.; Lee, H.A.; Lim, J.Y.; Kim, Y.; Jung, C.H.; Yoo, S.H.; Kim, Y. β-Carotene Inhibits Neuroblastoma Cell Invasion and Metastasis in Vitro and in Vivo by Decreasing Level of Hypoxia-Inducible Factor-1α. J. Nutr. Biochem. 2014, 25, 655–664. [Google Scholar] [CrossRef]
- Sugiura, Y.; Shimada, H.; Seeger, R.C.; Laug, W.E.; DeClerck, Y.A. Matrix Metalloproteinases-2 and -9 Are Expressed in Human Neuroblastoma: Contribution of Stromal Cells to Their Production and Correlation with Metastasis. Cancer Res. 1998, 58, 2209–2216. [Google Scholar] [CrossRef]
- Carmeliet, P. VEGF as a Key Mediator of Angiogenesis in Cancer. Oncology 2005, 69, 4–10. [Google Scholar] [CrossRef]
- Veys, K.; Fan, Z.; Ghobrial, M.; Bouché, A.; García-Caballero, M.; Vriens, K.; Conchinha, N.V.; Seuwen, A.; Schlegel, F.; Gorski, T.; et al. Role of the GLUT1 Glucose Transporter in Postnatal CNS Angiogenesis and Blood-Brain Barrier Integrity. Circ. Res. 2020, 127, 466–482. [Google Scholar] [CrossRef]
- Guruvayoorappan, C.; Kuttan, G. β-Carotene Inhibits Tumor-Specific Angiogenesis by Altering the Cytokine Profile and Inhibits the Nuclear Translocation of Transcription Factors in B16F-10 Melanoma Cells. Integr. Cancer Ther. 2007, 6, 258–270. [Google Scholar] [CrossRef]
- Pradeep, C.R.; Kuttan, G. Effect of B-Carotene on the Inhibition of Lung Metastasis in Mice. Phytomedicine 2003, 10, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, G.I.; Strongin, A.; Collier, I.E.; Genrich, L.T.; Marmer, B.L. Interaction of 92-KDa Type IV Collagenase with the Tissue Inhibitor of Metalloproteinases Prevents Dimerization, Complex Formation with Interstitial Collagenase, and Activation of the Proenzyme with Stromelysin. J. Biol. Chem. 1992, 267, 4583–4591. [Google Scholar] [CrossRef]
- Bonanno, E.; Lurlaro, M.; Madri, J.A.; Nicosia, R.F. Type IV Colla Gen Modulates Angiogenesis and Neovessel Survival in the Rat Aorta Model. In Vitro Cell Dev. Biol. Anim. 2000, 36, 336–340. [Google Scholar] [CrossRef] [PubMed]
- Ye, M.; Wu, Q.; Zhang, M.; Huang, J. Lycopene Inhibits the Cell Proliferation and Invasion of Human Head and Neck Squamous Cell Carcinoma. Mol. Med. Rep. 2016, 14, 2953–2958. [Google Scholar] [CrossRef]
- Na, T.-Y.; Schecterson, L.; Mendonsa, A.M.; Gumbiner, B.M. The Functional Activity of E-Cadherin Controls Tumor Cell Metastasis at Multiple Steps CELL BIOLOGY. Proc. Natl. Acad. Sci. USA 2020, 117, 5931–5937. [Google Scholar] [CrossRef] [PubMed]
- Mittal, V. Epithelial Mesenchymal Transition in Tumor Metastasis. Annu. Rev. Pathol. 2017, 13, 395–412. [Google Scholar] [CrossRef] [PubMed]
- Elgass, S.; Cooper, A.; Chopra, M. Lycopene Treatment of Prostate Cancer Cell Lines Inhibits Adhesion and Migration Properties of the Cells. Int. J. Med. Sci. 2014, 11, 948–954. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Huang, C.-S.; Shih, M.-K.; Chuang, C.-H.; Hu, M.-L. Lycopene Inhibits Cell Migration and Invasion and Upregulates Nm23-H1 in a Highly Invasive Hepatocarcinoma, SK-Hep-1 Cells. J. Nutr. 2005, 135, 2119–2123. [Google Scholar] [CrossRef]
- Hwang, E.-S.; Hyong, A.; Lee, J. Inhibitory Effects of Lycopene on the Adhesion, Invasion, and Migration of SK-Hep1 Human Hepatoma Cells. Exp. Biol. Med. 2006, 231, 322–327. [Google Scholar] [CrossRef]
- Kim, B.; Lee, K.J. Activation of Nm23-H1 to Suppress Breast Cancer Metastasis via Redox Regulation. Exp. Mol. Med. 2021, 53, 346–357. [Google Scholar] [CrossRef]
- Han, L.; Song, X. Lutein Induces an Inhibitory Effect on the Malignant Progression of Pancreatic Adenocarcinoma by Targeting BAG3/Cholesterol Homeostasis. J. Biochem. Mol. Toxicol. 2022, 36, 22958. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Lv, M.; Zhang, W.; Zhan, Q. Dysregulation of Cholesterol Metabolism in Cancer Progression. Oncogene 2023, 42, 3289–3302. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, Y.; Liu, X.; Wang, M.; Wang, P.; Yang, J.; Zhang, S. Lutein Inhibits Proliferation, Invasion and Migration of Hypoxic Breast Cancer Cells via Downregulation of HES1. Int. J. Oncol. 2018, 52, 2119–2129. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.H.; Dai, X.M.; Du, B. Hes1: A Key Role in Stemness, Metastasis and Multidrug Resistance. Cancer Biol. Ther. 2015, 16, 353–359. [Google Scholar] [CrossRef]
- Kim, H.Y.; Kim, Y.M.; Hong, S. Astaxanthin Suppresses the Metastasis of Colon Cancer by Inhibiting the MYC-Mediated Downregulation of MicroRNA-29a-3p and MicroRNA-200a. Sci. Rep. 2019, 9, 9457. [Google Scholar] [CrossRef]
- Nagendraprabhu, P.; Sudhandiran, G. Astaxanthin Inhibits Tumor Invasion by Decreasing Extracellular Matrix Production and Induces Apoptosis in Experimental Rat Colon Carcinogenesis by Modulating the Expressions of ERK-2, NFkB and COX-2. Investig. New Drugs 2011, 29, 207–224. [Google Scholar] [CrossRef]
- Badak, B.; Aykanat, N.E.B.; Kacar, S.; Sahinturk, V.; Arik, D.; Canaz, F. Effects of Astaxanthin on Metastasis Suppressors in Ductal Carcinoma. A Preliminary Study. Ann. Ital. Chir. 2021, 92, 565–574. [Google Scholar]
- Chen, X.; Xu, Z.; Wang, Y. Recent Advances in Breast Cancer Metastasis Suppressor 1. Int. J. Biol. Markers 2011, 26, 1–8. [Google Scholar] [CrossRef]
- Woo, J.; Lim, J.W.; Kim, H. Astaxanthin Inhibits Integrin A5 Expression by Suppressing Activation of JAK1/STAT3 in Helicobacter Pylori stimulated Gastric Epithelial Cells. Mol. Med. Rep. 2023, 27, 13014. [Google Scholar] [CrossRef]
- Siangcham, T.; Vivithanaporn, P.; Sangpairoj, K. Anti-Migration and Invasion Effects of Astaxanthin against A172 Human Glioblastoma Cell Line. Asian Pac. J. Cancer Prev. 2020, 21, 2029–2033. [Google Scholar] [CrossRef]
- Wang, W.; Fu, C.; Lin, M.; Lu, Y.; Lian, S.; Xie, X.; Zhou, G.; Li, W.; Zhang, Y.; Jia, L.; et al. Fucoxanthin Prevents Breast Cancer Metastasis by Interrupting Circulating Tumor Cells Adhesion and Transendothelial Migration. Front. Pharmacol. 2022, 13, 960375. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Lian, S.; Cheng, Y.; Ye, Y.; Xie, X.; Fu, C.; Zhang, C.; Zhu, Y.; Iqbal Parker, M.; Jia, L. Circulation Patterns and Seed-Soil Compatibility Factors Cooperate to Cause Cancer Organ-Specific Metastasis. Exp. Cell Res. 2019, 375, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ma, Y.; Yang, J.; Jin, L.; Gao, Z.; Xue, L.; Hou, L.; Sui, L.; Liu, J.; Zou, X. Fucoxanthin Inhibits Tumour-Related Lymphangiogenesis and Growth of Breast Cancer. J. Cell Mol. Med. 2019, 23, 2219–2229. [Google Scholar] [CrossRef] [PubMed]
- Dieterich, L.C.; Detmar, M. Tumor Lymphangiogenesis and New Drug Development. Adv. Drug Deliv. Rev. 2016, 99, 148–160. [Google Scholar] [CrossRef]
- Liu, N.; Liu, M.; Fu, S.; Wang, J.; Tang, H.; Isah, A.D.; Chen, D.; Wang, X. Ang2-Targeted Combination Therapy for Cancer Treatment. Front. Immunol. 2022, 13, 949553. [Google Scholar] [CrossRef]
- Zang, M.; Hou, J.; Huang, Y.; Wang, J.; Ding, X.; Zhang, B.; Wang, Y.; Xuan, Y.; Zhou, Y. Crocetin Suppresses Angiogenesis and Metastasis through Inhibiting Sonic Hedgehog Signaling Pathway in Gastric Cancer. Biochem. Biophys. Res. Commun. 2021, 576, 86–92. [Google Scholar] [CrossRef]
- Di Mauro, C.; Rosa, R.; D’Amato, V.; Ciciola, P.; Servetto, A.; Marciano, R.; Orsini, R.C.; Formisano, L.; De Falco, S.; Cicatiello, V.; et al. Hedgehog Signalling Pathway Orchestrates Angiogenesis in Triple-Negative Breast Cancers. Br. J. Cancer 2017, 116, 1425–1435. [Google Scholar] [CrossRef]
- Zhou, Y.; Xu, Q.; Shang, J.; Lu, L.; Chen, G. Crocin Inhibits the Migration, Invasion, and Epithelial-Mesenchymal Transition of Gastric Cancer Cells via MiR-320/KLF5/HIF-1α Signaling. J. Cell Physiol. 2019, 234, 17876–17885. [Google Scholar] [CrossRef]
- Dong, J.T.; Chen, C. Essential Role of KLF5 Transcription Factor in Cell Proliferation and Differentiation and Its Implications for Human Diseases. Cell Mol. Life Sci. 2009, 66, 2691–2706. [Google Scholar] [CrossRef]
- Farahi, A.; Abedini, M.R.; Javdani, H.; Arzi, L.; Chamani, E.; Farhoudi, R.; Talebloo, N.; Hoshyar, R. Crocin and Metformin Suppress Metastatic Breast Cancer Progression via VEGF and MMP9 Downregulations: In Vitro and in Vivo Studies. Mol. Cell Biochem. 2021, 476, 3341–3351. [Google Scholar] [CrossRef]
- Arzi, L.; Farahi, A.; Jafarzadeh, N.; Riazi, G.; Sadeghizadeh, M.; Hoshyar, R. Inhibitory Effect of Crocin on Metastasis of Triple-Negative Breast Cancer by Interfering with Wnt/β-Catenin Pathway in Murine Model. DNA Cell Biol. 2018, 37, 1068–1075. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Xiao, Q.; Xiao, J.; Niu, C.; Li, Y.; Zhang, X.; Zhou, Z.; Shu, G.; Yin, G. Wnt/β-Catenin Signalling: Function, Biological Mechanisms, and Therapeutic Opportunities. Signal Transduct. Target. Ther. 2022, 7, 3. [Google Scholar] [CrossRef]
- Chryssanthi, D.G.; Dedes, P.G.; Karamanos, N.K.; Cordopatis, P.; Lamari, F.N. Crocetin Inhibits Invasiveness of MDA-MB-231 Breast Cancer Cells via Downregulation of Matrix Metalloproteinases. Planta Med. 2011, 77, 146–151. [Google Scholar] [CrossRef]
- Chen, S.-S.; Gu, Y.; Lu, F.; Qian, D.-P.; Dong, T.-T.; Ding, Z.-H.; Zhao, S.; Yu, Z.-H. Antiangiogenic Effect of Crocin on Breast Cancer Cell MDA-MB-231. J. Thorac. Dis. 2019, 11, 4464–4473. [Google Scholar] [CrossRef] [PubMed]
- Bakshi, H.A.; Hakkim, F.L.; Sam, S.; Javid, F.; Rashan, L. Dietary Crocin Reverses Melanoma Metastasis. J. Biomed. Res. 2018, 32, 39–50. [Google Scholar] [CrossRef]
- Bakshi, H.A.; Quinn, G.A.; Nasef, M.M.; Mishra, V.; Aljabali, A.A.A.; El-Tanani, M.; Serrano-Aroca, Á.; Webba Da Silva, M.; McCarron, P.A.; Tambuwala, M.M. Crocin Inhibits Angiogenesis and Metastasis in Colon Cancer via TNF-α/NF-KB/VEGF Pathways. Cells 2022, 11, 1502. [Google Scholar] [CrossRef]
- Khajeh, E.; Rasmi, Y.; Kheradmand, F.; Malekinejad, H.; Aramwit, P.; Saboory, E.; Daeihassani, B.; Nasirzadeh, M. Crocetin Suppresses the Growth and Migration in HCT-116 Human Colorectal Cancer Cells by Activating the p-38 MAPK Signaling Pathway. Res. Pharm. Sci. 2020, 15, 592. [Google Scholar] [CrossRef]
- Hou, L.-L.; Gao, C.; Chen, L.; Hu, G.-Q.; Xie, S.-Q. Essential Role of Autophagy in Fucoxanthin-Induced Cytotoxicity to Human Epithelial Cervical Cancer HeLa Cells. Acta Pharmacol. Sin. 2013, 34, 1403–1410. [Google Scholar] [CrossRef]
- Long, Y.; Cao, X.; Zhao, R.; Gong, S.; Jin, L.; Feng, C. Fucoxanthin Treatment Inhibits Nasopharyngeal Carcinoma Cell Proliferation through Induction of Autophagy Mechanism. Environ. Toxicol. 2020, 35, 1082–1090. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, S.; Wang, K.; Huang, Y.; Yang, N.; Yang, Z.; Zheng, Z.; Wang, Y. Crocin Induces Autophagic Cell Death and Inhibits Cell Invasion of Cervical Cancer SiHa Cells through Activation of PI3K/AKT. Ann. Transl. Med. 2020, 8, 1180. [Google Scholar] [CrossRef]
- Yun, C.W.; Lee, S.H. The Roles of Autophagy in Cancer. Int. J. Mol. Sci. 2018, 19, 3466. [Google Scholar] [CrossRef] [PubMed]
- Ferraresi, A.; Phadngam, S.; Morani, F.; Galetto, A.; Alabiso, O.; Chiorino, G.; Isidoro, C. Resveratrol Inhibits IL-6-Induced Ovarian Cancer Cell Migration through Epigenetic up-Regulation of Autophagy. Mol. Carcinog. 2017, 56, 1164–1181. [Google Scholar] [CrossRef]
- Bi, S.; Li, L.; Gu, H.; Li, M.; Xu, S.; Bu, W.; Zhang, M.; Zhou, Z.; Chen, X. Lycopene Upregulates ZO-1 and Downregulates Claudin-1 through Autophagy Inhibition in the Human Cutaneous Squamous Cell Carcinoma Cell Line COLO-16. J. Cancer 2019, 10, 510–521. [Google Scholar] [CrossRef]
- Kim, S.; Lee, H.; Lim, J.W.; Kim, H. Astaxanthin Induces NADPH Oxidase Activation and Receptor interacting Protein Kinase 1 mediated Necroptosis in Gastric Cancer AGS Cells. Mol. Med. Rep. 2021, 24, 12477. [Google Scholar] [CrossRef]
- Kim, Y.S.; Gong, X.; Rubin, L.P.; Choi, S.W.; Kim, Y. β-Carotene 15,15′-Oxygenase Inhibits Cancer Cell Stemness and Metastasis by Regulating Differentiation-Related MiRNAs in Human Neuroblastoma. J. Nutr. Biochem. 2019, 69, 31–43. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.A.; Park, S.; Kim, Y. Effect of β-Carotene on Cancer Cell Stemness and Differentiation in SK-N-BE(2)C Neuroblastoma Cells. Oncol. Rep. 2013, 30, 1869–1877. [Google Scholar] [CrossRef]
- Naus, C.C.; Laird, D.W. Implications and Challenges of Connexin Connections to Cancer. Nat. Rev. Cancer 2010, 10, 435–441. [Google Scholar] [CrossRef] [PubMed]
- RUCH, R. Inhibition of Intercellular Communication between Mouse Hepatocytes by Tumor Promoters*1, *2. Toxicol. Appl. Pharmacol. 1987, 87, 111–120. [Google Scholar] [CrossRef]
- Zhou, M.; Zheng, M.; Zhou, X.; Tian, S.; Yang, X.; Ning, Y.; Li, Y.; Zhang, S. The Roles of Connexins and Gap Junctions in the Progression of Cancer. Cell Commun. Signal 2023, 21, 8. [Google Scholar] [CrossRef]
- Liu, C.-L.; Huang, Y.-S.; Hosokawa, M.; Miyashita, K.; Hu, M.-L. Inhibition of Proliferation of a Hepatoma Cell Line by Fucoxanthin in Relation to Cell Cycle Arrest and Enhanced Gap Junctional Intercellular Communication. Chem. Biol. Interact. 2009, 182, 165–172. [Google Scholar] [CrossRef]
- Vinken, M.; Decrock, E.; Leybaert, L.; Bultynck, G.; Himpens, B.; Vanhaecke, T.; Rogiers, V. Non-Channel Functions of Connexins in Cell Growth and Cell Death. Biochimica et Biophysica Acta (BBA)-Biomembranes 2012, 1818, 2002–2008. [Google Scholar] [CrossRef]
- Ford, N.; Elsen, A.; Zuniga, K.; Lindshield, B.; Erdman, J. Lycopene and Apo-12′-Lycopenal Reduce Cell Proliferation and Alter Cell Cycle Progression in Human Prostate Cancer Cells. Nutr. Cancer 2011, 63, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Bukowski, K.; Kciuk, M.; Kontek, R. Mechanisms of Multidrug Resistance in Cancer Chemotherapy. Int. J. Mol. Sci. 2020, 21, 3233. [Google Scholar] [CrossRef]
- Robey, R.W.; Pluchino, K.M.; Hall, M.D.; Fojo, A.T.; Bates, S.E.; Gottesman, M.M. Revisiting the Role of ABC Transporters in Multidrug-Resistant Cancer. Nat. Rev. Cancer 2018, 18, 452–464. [Google Scholar] [CrossRef] [PubMed]
- Gottesman, M.M.; Lavi, O.; Hall, M.D.; Gillet, J.-P. Toward a Better Understanding of the Complexity of Cancer Drug Resistance. Annu. Rev. Pharmacol. Toxicol. 2016, 56, 85–102. [Google Scholar] [CrossRef] [PubMed]
- Teng, Y.-N.; Sheu, M.-J.; Hsieh, Y.-W.; Wang, R.-Y.; Chiang, Y.-C.; Hung, C.-C. β-Carotene Reverses Multidrug Resistant Cancer Cells by Selectively Modulating Human P-Glycoprotein Function. Phytomedicine 2016, 23, 316–323. [Google Scholar] [CrossRef]
- Wang, Q.; Michalak, K.; Wesolowska, O.; Deli, J.; Molnar, P.; Hohmann, J.; Molnar, J.; Engi, H. Reversal of Multidrug Resistance by Natural Substances from Plants. Curr. Top. Med. Chem. 2010, 10, 1757–1768. [Google Scholar] [CrossRef]
- Ugocsai, K.; Varga, A.; Molnár, P.; Antus, S.; Molnár, J. Effects of Selected Flavonoids and Carotenoids on Drug Accumulation and Apoptosis Induction in Multidrug-Resistant Colon Cancer Cells Expressing MDR1/LRP. In Vivo 2005, 19, 433–438. [Google Scholar]
- Luan, R.-L.; Wang, P.-C.; Yan, M.-X.; Chen, J. Effect of Lutein and Doxorubicin Combinatorial Therapy on S180 Cell Proliferation and Tumor Growth. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 1514–1520. [Google Scholar] [CrossRef]
- Liu, C.-L.; Lim, Y.-P.; Hu, M.-L. Fucoxanthin Attenuates Rifampin-Induced Cytochrome P450 3A4 (CYP3A4) and Multiple Drug Resistance 1 (MDR1) Gene Expression through Pregnane X Receptor (PXR)-Mediated Pathways in Human Hepatoma HepG2 and Colon Adenocarcinoma LS174T Cells. Mar. Drugs 2012, 10, 242–257. [Google Scholar] [CrossRef]
- Hofman, J.; Vagiannis, D.; Chen, S.; Guo, L. Roles of CYP3A4, CYP3A5 and CYP2C8 Drug-Metabolizing Enzymes in Cellular Cytostatic Resistance. Chem. Biol. Interact. 2021, 340, 109448. [Google Scholar] [CrossRef] [PubMed]
- Eid, S.Y.; El-Readi, M.Z.; Wink, M. Carotenoids Reverse Multidrug Resistance in Cancer Cells by Interfering with ABC-Transporters. Phytomedicine 2012, 19, 977–987. [Google Scholar] [CrossRef] [PubMed]
- Eid, S.Y.; Althubiti, M.A.; Abdallah, M.E.; Wink, M.; El-Readi, M.Z. The Carotenoid Fucoxanthin Can Sensitize Multidrug Resistant Cancer Cells to Doxorubicin via Induction of Apoptosis, Inhibition of Multidrug Resistance Proteins and Metabolic Enzymes. Phytomedicine 2020, 77, 153280. [Google Scholar] [CrossRef]
- Razavi, S.M.S.; Vaziri, R.M.; Karimi, G.; Arabzadeh, S.; Keyvani, V.; Behravan, J.; Kalalinia, F. Crocin Increases Gastric Cancer Cells’ Sensitivity to Doxorubicin. Asian Pac. J. Cancer Prev. 2020, 21, 1959–1967. [Google Scholar] [CrossRef]
- Neyshaburinezhad, N.; Kalalinia, F.; Hashemi, M. Encapsulation of Crocetin into Poly (Lactic-Co-Glycolic Acid) Nanoparticles Overcomes Drug Resistance in Human Ovarian Cisplatin-Resistant Carcinoma Cell Line (A2780-RCIS). Mol. Biol. Rep. 2019, 46, 6525–6532. [Google Scholar] [CrossRef] [PubMed]
- Mahdizadeh, S.; Karimi, G.; Behravan, J.; Arabzadeh, S.; Lage, H.; Kalalinia, F. Crocin Suppresses Multidrug Resistance in MRP Overexpressing Ovarian Cancer Cell Line. DARU J. Pharm. Sci. 2016, 24, 17. [Google Scholar] [CrossRef]
- Huang, J.; Lu, M.; Fang, Y.; Xu, M.; Huang, W.; Pan, Z.; Chen, Y.; Zhang, C. Serum Carotenoids and Colorectal Cancer Risk: A Case-control Study in Guangdong, China. Mol. Nutr. Food Res. 2017, 61, 1700267. [Google Scholar] [CrossRef]
- Gaziano, J.M.; Sesso, H.D.; Christen, W.G.; Bubes, V.; Smith, J.P.; MacFadyen, J.; Schvartz, M.; Manson, J.E.; Glynn, R.J.; Buring, J.E. Multivitamins in the Prevention of Cancer in Men. JAMA 2012, 308, 1871. [Google Scholar] [CrossRef]
- Hercberg, S.; Galan, P.; Preziosi, P.; Bertrais, S.; Mennen, L.; Malvy, D.; Roussel, A.-M.; Favier, A.; Briançon, S. The SU.VI.MAX Study. Arch. Intern. Med. 2004, 164, 2335. [Google Scholar] [CrossRef]
- He, J.; Gu, Y.; Zhang, S. Vitamin A and Breast Cancer Survival: A Systematic Review and Meta-Analysis. Clin. Breast Cancer 2018, 18, e1389–e1400. [Google Scholar] [CrossRef]
- Heinonen, O.P.; Albanes, D. The Effect of Vitamin E and Beta Carotene on the Incidence of Lung Cancer and Other Cancers in Male Smokers. N. Eng. J. Med. 1994, 330, 1029–1035. [Google Scholar] [CrossRef]
- Lai, G.Y.; Weinstein, S.J.; Taylor, P.R.; McGlynn, K.A.; Virtamo, J.; Gail, M.H.; Albanes, D.; Freedman, N.D. Effects of α-Tocopherol and β-Carotene Supplementation on Liver Cancer Incidence and Chronic Liver Disease Mortality in the ATBC Study. Br. J. Cancer 2014, 111, 2220–2223. [Google Scholar] [CrossRef] [PubMed]
- Mondul, A.M.; Sampson, J.N.; Moore, S.C.; Weinstein, S.J.; Evans, A.M.; Karoly, E.D.; Virtamo, J.; Albanes, D. Metabolomic Profile of Response to Supplementation with β-Carotene in the Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study. Am. J. Clin. Nutr. 2013, 98, 488–493. [Google Scholar] [CrossRef]
- Thorn, C.F.; Oshiro, C.; Marsh, S.; Hernandez-Boussard, T.; McLeod, H.; Klein, T.E.; Altman, R.B. Doxorubicin pathways: Pharmacodynamics and adverse effects. Pharmacogenet. Genomics 2011, 21, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Vijay, K.; Sowmya, P.R.; Arathi, B.P.; Shilpa, S.; Shwetha, H.J.; Raju, M.; Baskaran, V.; Lakshminarayana, R. Low-dose doxorubicin with carotenoids selectively alters redox status and upregulates oxidative stress-mediated apoptosis in breast cancer cells. Food Chem. Toxicol. 2018, 118, 675–690. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Choi, J.; Lim, J.W.; Kim, H. β-Carotene-Induced Apoptosis Is Mediated with Loss of Ku Proteins in Gastric Cancer AGS Cells. Genes. Nutr. 2015, 10, 17. [Google Scholar] [CrossRef]
- Yang, G.; Lee, H.E.; Moon, S.-J.; Ko, K.M.; Koh, J.H.; Seok, J.K.; Min, J.-K.; Heo, T.-H.; Kang, H.C.; Cho, Y.-Y.; et al. Direct Binding to NLRP3 Pyrin Domain as a Novel Strategy to Prevent NLRP3-Driven Inflammation and Gouty Arthritis. Arthritis Rheumatol. 2020, 72, 1192–1202. [Google Scholar] [CrossRef]
- Strzalka-Mrozik, B.; Madej, M.; Kurowska, N.; Kruszniewska-Rajs, C.; Kimsa-Dudek, M.; Adamska, J.; Gola, J.M. Changes in the Expression Profile of Pyroptosis-Related Genes in Senescent Retinal Pigment Epithelial Cells after Lutein Treatment. Curr. Issues Mol. Biol. 2023, 45, 1500–1518. [Google Scholar] [CrossRef]
- Soto, K.M.; Mendoza, S.; López-Romero, J.M.; Gasca-Tirado, J.R.; Manzano-Ramírez, A. Gold nanoparticles: Synthesis, application in colon cancer therapy and new approaches—Review. GCLR 2021, 14, 665–678. [Google Scholar] [CrossRef]
- Rudzińska, M.; Grygier, A.; Knight, G.; Kmiecik, D. Liposomes as Carriers of Bioactive Compounds in Human Nutrition. Foods 2024, 13, 1814. [Google Scholar] [CrossRef]
- Jamroży, M.; Kudłacik-Kramarczyk, S.; Drabczyk, A.; Krzan, M. Advanced Drug Carriers: A Review of Selected Protein, Polysaccharide, and Lipid Drug Delivery Platforms. Int. J. Mol. Sci. 2024, 25, 786. [Google Scholar] [CrossRef]
- Giani, M.; Montero-Lobato, Z.; Garbayo, I.; Vílchez, C.; Vega, J.M.; Martínez-Espinosa, R.M. Haloferax mediterranei Cells as C50 Carotenoid Factories. Mar. Drugs 2021, 19, 100. [Google Scholar] [CrossRef]
- Giani, M.; Martínez-Espinosa, R.M. Carotenoids as a Protection Mechanism against Oxidative Stress in Haloferax mediterranei. Antioxidants 2020, 9, 1060. [Google Scholar] [CrossRef] [PubMed]
- Giani, M.; Montoyo-Pujol, Y.G.; Peiró, G.; Martínez-Espinosa, R.M. Haloarchaeal Carotenoids Exert an in Vitro Antiproliferative Effect on Human Breast Cancer Cell Lines. Sci. Rep. 2023, 13, 7148. [Google Scholar] [CrossRef] [PubMed]
- Shahbazi, S.; Zargar, M.; Zolfaghari, M.R.; Amoozegar, M.A. Carotenoid Pigment of Halophilic Archaeon Haloarcula sp. A15 Induces Apoptosis of Breast Cancer Cells. Cell Biochem. Funct. 2023, 41, 344–354. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baeza-Morales, A.; Medina-García, M.; Martínez-Peinado, P.; Pascual-García, S.; Pujalte-Satorre, C.; López-Jaén, A.B.; Martínez-Espinosa, R.M.; Sempere-Ortells, J.M. The Antitumour Mechanisms of Carotenoids: A Comprehensive Review. Antioxidants 2024, 13, 1060. https://doi.org/10.3390/antiox13091060
Baeza-Morales A, Medina-García M, Martínez-Peinado P, Pascual-García S, Pujalte-Satorre C, López-Jaén AB, Martínez-Espinosa RM, Sempere-Ortells JM. The Antitumour Mechanisms of Carotenoids: A Comprehensive Review. Antioxidants. 2024; 13(9):1060. https://doi.org/10.3390/antiox13091060
Chicago/Turabian StyleBaeza-Morales, Andrés, Miguel Medina-García, Pascual Martínez-Peinado, Sandra Pascual-García, Carolina Pujalte-Satorre, Ana Belén López-Jaén, Rosa María Martínez-Espinosa, and José Miguel Sempere-Ortells. 2024. "The Antitumour Mechanisms of Carotenoids: A Comprehensive Review" Antioxidants 13, no. 9: 1060. https://doi.org/10.3390/antiox13091060
APA StyleBaeza-Morales, A., Medina-García, M., Martínez-Peinado, P., Pascual-García, S., Pujalte-Satorre, C., López-Jaén, A. B., Martínez-Espinosa, R. M., & Sempere-Ortells, J. M. (2024). The Antitumour Mechanisms of Carotenoids: A Comprehensive Review. Antioxidants, 13(9), 1060. https://doi.org/10.3390/antiox13091060






